Abstract
OBJECTIVES:
Lower gastrointestinal (GI) bleeding is a well-known symptom of colorectal cancer (CRC). Whether incident GI bleeding is also a marker of other GI cancers remains unclear.
METHODS:
This nationwide cohort study examined the risk of various GI cancer types in patients with lower GI bleeding. We used Danish medical registries to identify all patients with a first-time hospital diagnosis of lower GI bleeding during 1995–2011 and followed them for 10 years to identify subsequent GI cancer diagnoses. We computed absolute risks of cancer, treating death as a competing risk, and calculated standardized incidence ratios (SIRs) by comparing observed cancer cases with expected cancer incidence rates in the general population.
RESULTS:
Among 58,593 patients with lower GI bleeding, we observed 2,806 GI cancers during complete 10-year follow-up. During the first year of follow-up, the absolute GI cancer risk was 3.6%, and the SIR of any GI cancer was 16.3 (95% confidence interval (CI): 15.6–17.0). Colorectal cancers accounted for the majority of diagnoses, but risks of all GI cancers were increased. During 1–5 years of follow-up, the SIR of any GI cancer declined to 1.36 (95% CI: 1.25–1.49), but risks remained increased for several GI cancers. Beyond 5 years of follow-up, the overall GI cancer risk was close to unity, with reduced risk of rectal cancer and increased risk of liver and pancreatic cancers.
CONCLUSIONS:
A hospital-based diagnosis of lower GI bleeding is a strong clinical marker of prevalent GI cancer, particularly CRC. It also predicts an increased risk of any GI cancer beyond 1 year of follow-up.
INTRODUCTION
Lower gastrointestinal (GI) bleeding, i.e., intestinal bleeding distal to the ligament of Treitz, is a well-known presenting symptom of colorectal cancer (CRC).1, 2 The annual incidence of adult hospitalizations for lower GI bleeding is between 21 and 87 per 100,000 population,3, 4, 5, 6 and cross-sectional studies have reported a prevalence of CRC cancer of 4–12% among persons with this symptom.4, 5, 6, 7 Two cohort studies and one case–control study found greatly increased short-term risk of CRC after lower GI bleeding in primary care settings.8, 9, 10
However, other GI cancers also may be associated with lower GI bleeding.11 Invasive GI cancers either can bleed directly into the intestinal lumen or induce systemic alterations in the coagulation system, increasing the tendency to bleed.12, 13, 14, 15 In addition, excessive alcohol use is a shared risk factor for bleeding and cancer.16, 17, 18 GI cancers are predicted to represent an estimated 18% of all cancers in the USA in 2015.19 Thus recognition of clinical markers of GI cancers is potentially of great importance.
To our knowledge, no previous cohort study has investigated the association between lower GI bleeding and subsequent short- and long-term risk of CRC or other GI cancers in a hospital-based setting. We therefore conducted a nationwide cohort study to examine if a first-time hospital-based diagnosis of lower GI bleeding is a marker of prevalent undiagnosed GI cancer and a predictor of elevated GI cancer risk after >1 year.
METHODS
Data sources and study population
In our cohort study, Danish national medical databases were linked during the 1977–2011 period.
All residents of Denmark have a unique civil registration number,20 which allows linkage between the Danish National Patient Registry (DNPR) and the Danish Cancer Registry (DCR). The DNPR contains 99% of all inpatient discharge diagnoses from Danish hospitals since 1977 and from emergency room and hospital outpatient visits since 1995. DNPR data include dates of inpatient admission and discharge, dates of outpatient visits, surgical procedures performed, and up to 20 discharge diagnoses coded according to the International Classification of Diseases, Eighth Revision (ICD-8) until the end of 1993 and Tenth Revision (ICD-10) thereafter. Surgical procedures were coded according to a Danish classification system until 1995, and thereafter according to a Danish version of the NOMESCO Classification of Surgical Procedures.21 At discharge one diagnosis is coded as primary (the condition that prompted the hospital contact) and the others as secondary.22
The DNPR was used to identify all patients with a first-time hospital-based diagnosis of lower GI bleeding between 1995 and 2011 (See Supplementary Appendix online for diagnosis codes.) We included primary and secondary inpatient, outpatient, and emergency room diagnoses of lower GI bleeding (ICD-10 code K62.5—Hemorrhage of anus and rectum) as documented in the discharge record. Outpatient clinics in Denmark are hospital-based (analogous to outpatient departments). Of note, we did not consider patients with obvious signs of upper GI bleedings such as peptic ulcer bleeding or melena. We excluded patients with a diagnosis of lower GI bleeding before 1995 and patients with a diagnosis of inflammatory bowel disease previous to the lower GI bleeding diagnosis, in order to focus on incident bleeding cases.
We also obtained patients' medical histories from the DNPR, including all types of endoscopic examinations of the GI tract within 3 months prior to the bleeding, as well as hemorrhoids, adenomas, and chronic obstructive pulmonary disease diagnosed at any time before the diagnosis of lower GI bleeding. In addition, to address a priori elevated cancer risk, we obtained data on alcoholism-related disease, and on conditions included in the Charlson Comorbidity Index (CCI). These data allowed us to calculate comorbidity scores (low=CCI score of 0, medium=CCI score of 1–2, and high=CCI score of ≥3), and to identify chronic liver disease prior to the lower GI bleeding episode. Chronic liver disease was categorized in either mild, or moderate–severe according to the CCI (ICD codes are provided in the Supplementary Appendix).
Validation of discharge diagnoses of lower GI bleeding
To evaluate the validity of discharge diagnoses of lower GI bleeding (K62.5) in the DNPR, we reviewed 50 randomly selected records (using the SAS RANUNI function). Owing to limited access to electronic records, only patients diagnosed with lower GI bleeding at Department of Surgery, Aarhus University Hospital, Denmark, in the period 2004–2011 were eligible for inclusion. We considered bleeding per rectum as confirmed when it was described as indication for the outpatient visit, or when it was described in the medical record during the hospital admission. We noted the type of bleeding described in the record (fresh, melena, or other type). We calculated the positive predictive value of the discharge diagnosis code (K62.5) for bleeding per rectum overall, and for fresh bleeding per rectum specifically. The validation was performed by an experienced surgeon.
Cancer
We extracted information on cancer diagnoses from the DCR, which has recorded incident cancers in Denmark since 1943. The DCR classifies cancers according to ICD-10 and ICD-O, including information on cancer stage.23 We linked all individuals who were identified from the DNPR with an episode of lower GI bleeding to the DCR. This allowed us to identify and exclude all patients with a cancer diagnosis (except for non-melanoma skin cancer) prior to the bleeding. We followed the patients for all types of GI cancer (specified in the Supplementary Appendix). Colon cancers were categorized into those proximal and distal to the splenic flexure, as they have been found to differ in regard to etiology, epidemiology, and symptoms on presentation.24, 25
Statistical analysis
We followed each patient from the date of the first hospital contact for lower GI bleeding until the date of his/her first cancer diagnosis, death, emigration, or December 2011, whichever came first. We tabulated the covariates of interest (number and proportion) (Table 1), and computed median age at inclusion and follow-up time.
Table 1. Characteristics of 58,593 patients with a first-time hospital inpatient- or outpatient-based diagnosis of lower GI bleeding.
| No. (%) | |
|---|---|
| All | 58,593 (100.0) |
| Sex | |
| Women | 29,760 (50.8) |
| Men | 28,833 (49.2) |
| Age at GI bleeding | |
| 0–49 years | 19,010(32.4) |
| 50–69 years | 20,383 (34.8) |
| 70+ years | 19,200 (32.8) |
| Place of diagnosisa | |
| Emergency room | 4,661 (8.0) |
| Inpatient unit | 25,836 (44.1) |
| Outpatient clinic | 28,096 (48.0) |
| Type of diagnosis | |
| Primary | 52,576 (89.7) |
| Secondary | 6,017 (10.3) |
| Adenomas | |
| No | 52,020 (88.8) |
| Yes | 6,573 (11.2) |
| Hemorrhoidsa | |
| No | 51,533 (88.0) |
| Yes | 7,060 (12.1) |
| COPDa | |
| No | 55,225 (94.3) |
| Yes | 3,368 (5.8) |
| Charlson scoreb | |
| 0 | 37,456 (63.9) |
| 1–2 | 15,988 (27.3) |
| 3+ | 5,149 (8.8) |
| Alcoholism-related disease | |
| No | 55,853 (95.3) |
| Yes | 2,740 (4.7) |
| Chronic liver diseasec | |
| No | 57,235 (97.7) |
| Mild | 897 (1.5) |
| Moderate-severe | 461 (0.8) |
| Preceding endoscopyd | |
| No | 53,697 (91.6) |
| Yes | 4,896 (8.4) |
| Subsequent lower endoscopye | |
| No | 11,782 (20.1) |
| Yes | 46,811 (79.9) |
COPD, chronic obstructive pulmonary disease; GI, gastrointestinal.
Does not add up to 100% because of rounding.
According to the Charlson Comorbidity Index.
Chronic liver disease according to Charlson Comorbidity Index (specified in the Supplementary Appendix).
Examination during the 3 months prior to bleeding.
Colonoscopic, sigmoidoscopic, or rectoscopic examination up to 6 months following bleeding.
We calculated the absolute risks (or cumulative incidence) of GI cancer in patients with lower GI bleeding during 1, 5, and 10 years of follow-up, considering death as a competing risk. To measure the relative risk of GI cancer among patients with lower GI bleeding compared with the risk in the general Danish population, we computed the observed/expected ratio or the standardized incidence ratio (SIR) of cancer.26 The expected numbers of cancers were estimated based on national general population cancer rates by age, sex, and calendar year. We computed confidence intervals (CIs) for SIRs under the assumption that the observed number of cases in each category followed a Poisson distribution. Exact 95% CIs were used when the observed number was <10; otherwise Byar's approximation was applied.27
The follow-up period was divided into three intervals: <1 year (cancers detected during this period were considered prevalent cancers), 1–<5 years, and 5+ years to a maximum of 10 years. We performed stratified analyses according to sex, age (categorized as ≤49, 50–69, and ≥70 years), place of diagnosis (inpatient, outpatient, and emergency room) and type of diagnosis (primary or secondary). Patients were also stratified according to CCI score, and presence of colorectal adenomas, or alcoholism-related disease diagnosed at any time prior to the hospital contact for lower GI bleeding.
RESULTS
Patient characteristics
We identified a total of 58,593 patients with a first hospital contact for lower GI bleeding, of whom 49% were men. The median age at diagnosis was 60 years (interquartile range: 45–75 years), and median follow-up was 4.3 years (interquartile range: 1.5–8.2 years). The patients were diagnosed with GI bleeding during an inpatient hospital stay (44%), hospital outpatient clinic visit (48%), or emergency room visit (8.0%). Of the 28,096 patients diagnosed in an outpatient clinic, 2,053 (7.3%) were transferred directly to an inpatient department. Most patients had a low CCI score (64%), 27% had a medium score, and 8.8% had a high score. Among the patients, 6,573 (11%) had a previous diagnosis of colon or rectal adenomas, and 4,896 (8.4%) had recently (i.e., within 3 months) undergone an endoscopic examination. During the hospital contact for lower GI bleeding and within 6 months following the bleeding event, 46,811 patients (80%) underwent a lower endoscopy, including colonoscopy (44%), sigmoidoscopy (41%), and/or proctoscopy (17%) (Table 1). For 52,576 (90%) patients, lower GI bleeding was coded as the primary diagnosis. We excluded 1,500 patients with a previous diagnosis of inflammatory bowel disease.
Validation of diagnoses of lower GI bleeding
Among the 50 sampled records of patients registered with a discharge diagnosis of lower GI bleeding, 82% were diagnosed during an outpatient clinic visit and 18% during hospital admission. We confirmed the diagnosis of bleeding per rectum in 48 of 50 patients, corresponding to a positive predictive value of 96% (95% CI: 86–100%). Among 43 of 50 patients the bleeding was described as fresh bleeding per rectum, equivalent to positive predictive value of 86% (95% CI: 73–94%). Importantly, none of the records described melena. Among the five remaining confirmed cases, one had occult bleeding, one had a mechanical injury causing fresh bleeding, and in three cases the bleeding type was not described in detail.
Overall risk of GI cancer
In total, we observed 2,806 GI cancers during complete follow-up of all study patients. The overall 10-year absolute risk of any GI cancer was 5.6% (Table 2). This corresponded to a 3.9-fold increased cancer risk during follow-up (Table 3). Men were at higher risk of cancer than women, and increasing age was associated with a greatly increased absolute risk of all GI cancers (Table 2). In all follow-up periods, patients younger than 50 years had a substantially higher relative GI cancer risk than older patients (Table 3). We found markedly increased absolute and relative risks of cancer among patients diagnosed with lower GI bleeding in the emergency room compared to hospital inpatient and outpatient settings (Table 3 and Supplementary Table 3 online).
Table 2. Absolute risk of GI cancers after 1, 5, and 10 years of follow-up, by age group and cancer type.
|
Absolute risk in % (95% CI) |
|||
|---|---|---|---|
| 1 year | 5 years | 10 years | |
| Overall GI cancer | |||
| All age groups | 3.63 (3.48–3.79) | 4.73 (4.55–4.91) | 5.60 (5.39–5.81) |
| 0–49 years | 0.42 (0.34–0.52) | 0.61 (0.50–0.74) | 0.89 (0.74–1.07) |
| 50–69 years | 4.01 (3.75–4.29) | 5.11 (4.80–5.43) | 6.47 (6.07–6.88) |
| 70+ years | 6.42 (6.08–6.78) | 8.39 (7.98–8.80) | 9.33 (8.89–9.79) |
| Colorectal cancera | |||
| 0–49 years | 0.37 (0.29–0.46) | 0.46 (0.37–0.57) | 0.57 (0.45–0.70) |
| 50–69 years | 3.61 (3.36–3.87) | 4.20 (3.92–4.49) | 4.95 (4.61–5.30) |
| 70+ years | 5.87 (5.54–6.21) | 7.19 (6.81–7.57) | 7.75 (7.35–8.16) |
| Other GI cancers combinedb | |||
| 0–49 years | 0.05 (0.03–0.10) | 0.15 (0.10–0.23) | 0.33 (0.23–0.45) |
| 50–69 years | 0.42 (0.34–0.52) | 0.95 (0.81–1.11) | 1.60 (1.38–1.84) |
| 70+ years | 0.59 (0.49–0.71) | 1.30 (1.13–1.49) | 1.74 (1.52–1.98) |
CI, confidence interval; GI, gastrointestinal.
Including cancer of the colon and rectum.
Including cancer of the esophagus, stomach, small intestines, anus, liver, gall bladder, and pancreas.
Table 3. SIRs for GI cancers after lower GI bleeding (n=58,593), by follow-up period and patient characteristics.
|
Total follow-up |
<1 year |
1–<5 years |
5+ years |
|||||
|---|---|---|---|---|---|---|---|---|
| O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | |
| All GI cancers | 2806 | 3.94 (3.79–4.08) | 2098 | 16.3 (15.6–17.0) | 491 | 1.36 (1.25–1.49) | 217 | 0.97 (0.84–1.10) |
| Sex | ||||||||
| Women | 1208 | 3.65 (3.45–3.86) | 874 | 14.8 (13.8–15.8) | 225 | 1.34 (1.17–1.53) | 109 | 1.04 (0.86–1.26) |
| Men | 1598 | 4.18 (3.98–4.39) | 1224 | 17.6 (16.6–18.6) | 266 | 1.38 (1.22–1.56) | 108 | 0.90 (0.74–1.09) |
| Age | ||||||||
| 0–49 years | 128 | 4.01 (3.35–4.77) | 79 | 26.1 (20.6–32.5) | 27 | 2.04 (1.34–2.96) | 22 | 1.41 (0.88–2.14) |
| 50–69 years | 1086 | 3.80 (3.57–4.03) | 806 | 21.2 (19.7–22.7) | 169 | 1.26 (1.08–1.46) | 111 | 0.98 (0.80–1.18) |
| 70+ years | 1592 | 4.03 (3.84–4.23) | 1213 | 13.9 (13.1–14.7) | 295 | 1.39 (1.24–1.56) | 84 | 0.88 (0.70–1.09) |
| Calendar year | ||||||||
| 1995–2000 | 741 | 2.91 (2.70–3.13) | 472 | 14.4 (13.1–15.7) | 160 | 1.46 (1.25–1.71) | 109 | 0.97 (0.79–1.17) |
| 2001–2006 | 1264 | 3.60 (3.40–3.80) | 914 | 17.0 (15.9–18.2) | 242 | 1.30 (1.14–1.48) | 108 | 0.97 (0.79–1.17) |
| 2007–2011 | 801 | 7.47 (6.97–8.01) | 712 | 16.9 (15.7–18.2) | 89 | 1.37 (1.10–1.68) | 0 | — |
| Place of diagnosis | ||||||||
| Emergency room | 303 | 5.97 (5.32–6.69) | 241 | 24.9 (21.9–28.3) | 42 | 1.69 (1.22–2.28) | 20 | 1.24 (0.75–1.91) |
| Inpatient unit | 1448 | 3.95 (3.75–4.16) | 1044 | 14.6 (13.7–15.5) | 296 | 1.61 (1.43–1.80) | 108 | 0.97 (0.80–1.18) |
| Outpatient clinic | 1055 | 3.57 (3.36–3.79) | 813 | 17.1 (16.0–18.4) | 153 | 1.02 (0.86–1.19) | 89 | 0.91 (0.73–1.13) |
| Type of diagnosis | ||||||||
| Primary | 2448 | 3.82 (3.67–3.97) | 1816 | 15.9 (15.1–16.6) | 434 | 1.34 (1.22–1.47) | 198 | 0.97 (0.84–1.12) |
| Secondary | 358 | 5.02 (4.51–5.57) | 282 | 19.9 (17.7–22.4) | 57 | 1.58 (1.20–2.05) | 19 | 0.90 (0.54–1.41) |
| Adenomas | ||||||||
| No | 2331 | 3.85 (3.69–4.01) | 1753 | 16.20 (15.5–17.0) | 394 | 1.30 (1.17–1.43) | 184 | 0.95 (0.82–1.10) |
| Yes | 475 | 4.44 (4.05–4.85) | 345 | 16.9 (15.1–18.7) | 97 | 1.74 (1.41–2.12) | 33 | 1.07 (0.74–1.51) |
| Charlson scorea | ||||||||
| Low (0) | 1527 | 3.69 (3.51–3.88) | 1163 | 18.8 (17.7–19.9) | 234 | 1.16 (1.02–1.32) | 130 | 0.86 (0.72–1.02) |
| Medium (1–2) | 984 | 4.10 (3.85–4.36) | 708 | 14.2 (13.2–15.3) | 204 | 1.61 (1.40–1.85) | 72 | 1.13 (0.88–1.42) |
| High (3+) | 295 | 4.99 (4.44–5.59) | 227 | 13.3 (11.6–15.2) | 53 | 1.64 (1.23–2.14) | 15 | 1.55 (0.87–2.55) |
| Alcoholism-related disease | ||||||||
| No | 2669 | 3.86 (3.71–4.01) | 2004 | 16.1 (15.4–16.9) | 461 | 1.32 (1.20–1.45) | 204 | 0.93 (0.81–1.07) |
| Yes | 137 | 6.48 (5.44–7.67) | 94 | 20.9 (16.9–25.6) | 30 | 2.74 (1.85–3.92) | 13 | 2.28 (1.21–3.90) |
CI, confidence interval; GI, gastrointestinal; O, observed number of patients; SIR, standardized incidence ratio.
According to the Charlson Comorbidity Index.
During the first year of follow-up, 2,098 patients (3.6%) were diagnosed with a GI cancer, corresponding to a SIR of 16.3 (95% CI: 15.6–17.0). Although all GI cancers occurred more frequently than expected during the first year of follow-up (Table 4), colon and rectal cancers accounted for most (91%) of the diagnosed GI cancers (Table 2). During the first year of follow-up, patients aged 0–49 years had an absolute risk of GI cancer of 0.4%, patients aged 50–69 years had an absolute risk of 4.0%, and patients aged 70 years or more had an absolute risk of 6.4% (Table 2).
Table 4. SIRs for GI cancer after lower GI bleeding (n=58,593), by follow-up period and cancer type.
|
Total follow-up |
<1 year |
1–<5 years |
5+ years |
|||||
|---|---|---|---|---|---|---|---|---|
| O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | O | SIR (95% CI) | |
| All GI cancers | 2806 | 3.94 (3.79–4.08) | 2098 | 16.3 (15.6–17.0) | 491 | 1.36 (1.25–1.49) | 217 | 0.97 (0.84–1.10) |
| Cancer type | ||||||||
| Esophagus | 67 | 1.55 (1.20–1.97) | 13 | 1.67 (0.89–2.85) | 37 | 1.70 (1.20–2.34) | 17 | 1.25 (0.73–2.01) |
| Stomach | 76 | 1.31 (1.03–1.63) | 33 | 3.04 (2.09–4.27) | 32 | 1.08 (0.74–1.53) | 11 | 0.62 (0.31–1.11) |
| Small intestine | 35 | 4.54 (3.16–6.31) | 19 | 14.0 (8.44–21.9) | 15 | 3.90 (2.18–6.44) | 1 | 0.40 (0.01–2.21) |
| Colona | 1419 | 4.70 (4.46–4.95) | 1100 | 20.1 (19.0–21.4) | 229 | 1.50 (1.31–1.71) | 90 | 0.95 (0.76–1.17) |
| Proximalb | 511 | 3.77 (3.45–4.12) | 351 | 14.5 (13.1–16.2) | 113 | 1.66 (1.37–2.00) | 47 | 1.09 (0.80–1.44) |
| Distalc | 793 | 5.63 (5.24–6.03) | 672 | 25.9 (24.0–27.9) | 86 | 1.20 (0.96–1.48) | 35 | 0.81 (0.56–1.12) |
| Rectum | 899 | 6.13 (5.74–6.55) | 801 | 30.4 (28.4–32.6) | 73 | 0.99 (0.77–1.24) | 25 | 0.54 (0.35–0.80) |
| Anal canal | 51 | 5.35 (3.98–7.03) | 36 | 21.6 (15.1–29.9) | 8 | 1.67 (0.72–3.29) | 7 | 2.27 (0.91–4.68) |
| Liverd | 86 | 2.80 (2.24–3.46) | 37 | 6.74 (4.74–9.29) | 33 | 2.14 (1.47–3.00) | 16 | 1.64 (0.93–2.66) |
| Gall bladdere | 29 | 1.37 (0.91–1.96) | 12 | 3.09 (1.60–5.40) | 9 | 0.84 (0.38–1.59) | 8 | 1.21 (0.52–2.39) |
| Pancreas | 144 | 1.53 (1.29–1.80) | 47 | 2.82 (2.07–3.75) | 55 | 1.16 (0.88–1.51) | 42 | 1.40 (1.01–1.89) |
CI, confidence interval; GI, gastrointestinal; O, observed number of patients; SIR, standardized incidence ratio.
Including proximal and distal colon, colon cancer at multiple sites, colon cancer NOS, and cancer in the recto-sigmoid junction.
Including cecum, appendix, ascending colon, right flexure, and transverse colon.
Including left flexure, descending colon, sigmoid colon, and recto-sigmoid junction.
The majority of liver cancers were hepatocellular carcinomas (n=57, 66%), followed by unspecified liver cancer (n=19, 22%), intrahepatic bile duct cancer (n=8, 9%), sarcoma (n=1, 1%), and other specified carcinomas of liver (n=1, 1%).
Including biliary tract.
The overall relative cancer risk decreased markedly throughout the follow-up period; during years 1–5 of follow-up, the overall SIR was 1.36 (95% CI: 1.25–1.49) and beyond 5 years the SIR was 0.97 (95% CI: 0.84–1.10). Still, we found increased risks of all types of GI cancer other than rectal and gall bladder cancer during the 1–5 year follow-up period.
After 5 years of follow-up, the absolute risk of GI cancer was 0.6% in patients aged 0–49 years, 5.1% in patients aged 50–69 years, and 8.4% in patients aged 70 years or more (Table 2).
Risk of upper GI cancers
During the first year of follow-up, we found increased risks of all upper GI cancers including cancers of the esophagus (SIR=1.67 (95% CI: 0.89–2.85)), stomach (SIR=3.04 (95% CI: 2.09–4.27)), liver (SIR=6.74 (95% CI: 4.74–9.29)), gall bladder (SIR=3.09 (95% CI: 1.60–5.40)), and pancreas (SIR=2.82 (95% CI: 2.07–3.75)). Risk of esophagus and liver cancer remained increased 1–5 years after the lower GI bleeding, whereas we found no or weak associations with stomach, gall bladder and pancreatic cancer (Table 4). Beyond 5 years of follow-up, the risks of liver cancer (SIR=1.64 (95% CI: 0.93–2.66)) and pancreatic cancer (SIR=1.40 (95% CI: 1.01–1.89)) remained elevated (Table 4), whereas stomach cancer risk (SIR=0.62 (95% CI: 0.31–1.11)) was decreased. Lower GI bleeding was only weakly associated with an increased risk of esophagus and gall bladder cancer beyond 5 years of follow-up (Table 4).
Risk of lower GI cancers
All individual lower GI cancers occurred more frequently than expected during the first year of follow-up, including cancer of the small intestine (SIR=14.0 (95% CI: 8.44–21.9)), proximal colon (SIR=14.5 (95% CI: 13.1–16.2)), distal colon (SIR=25.9 (95% CI: 24.0–27.9)), rectum (SIR=30.4 (95% CI: 28.4–32.6)), and anal canal (SIR=21.6 (95% CI: 15.1–29.9)). The 1-year absolute risks of distal colon cancer (1.2%) and rectal cancer (1.4%) were higher than for proximal colon cancer (0.6%). During 1–5 years of follow-up, the relative cancer risk remained increased for the small intestines (SIR=3.90 (95% CI: 2.18–6.44)), proximal colon (SIR=1.66 (95% CI: 1.37–2.00)), and anal canal (SIR=1.67 (95% CI: 0.72–3.29)), whereas risk of distal colon and rectal cancer risk dropped markedly beyond 1 year of follow-up (Table 4). The relative risks of rectum cancer (SIR=0.54 (95% CI: 0.35–0.80)) and distal colon cancer (SIR=0.81 (95% CI: 0.56–1.12)) were lower than expected after 5 or more years of follow-up. For small intestinal, proximal colon, and anal cancers, we found only weak associations with lower GI bleeding beyond 5 years (Table 4).
SIR analyses stratified by comorbidities
During the first year of follow-up, patients with low comorbidity had a higher relative risk of GI cancer (SIR=18.8 (95% CI: 17.7–19.9)) than patients with medium (SIR=14.2 (95% CI: 13.2–15.3)) or high comorbidity (SIR=13.3 (95% CI: 11.6–15.2)) (Table 3). In contrast, beyond 1 year of follow-up a higher level of comorbidity was associated with a higher relative GI cancer risk (Table 3). We found an elevated risk of liver cancer 5+ years after the lower GI bleeding, primarily in patients with alcoholism-related disease (SIR=16.1 (95% CI: 5.22–37.5), and mild chronic liver disease (SIR=31.5 (95% CI: 6.49–92.1)), or moderate–severe chronic liver disease (SIR=34.8 (95% CI: 0.88–194)) (Supplementary Table 1). Similarly, pancreas cancer risk beyond 5 years was more increased in patients with alcoholism-related disease (SIR=5.33 (95% CI: 1.45–13.6)), than in patients without (SIR=1.30 (95% CI: 0.92–1.78)) (Supplementary Table 2).
DISCUSSION
We found that a hospital inpatient- or outpatient-based diagnosis of lower GI bleeding was associated with an increased risk of a subsequent GI cancer diagnosis. As expected, lower GI bleeding was a strong marker of prevalent CRC; however, during the first year of follow-up, the occurrence of any GI cancer was more frequent than in the general population. Although the increased risk of colon cancer persisted 1 year after the bleeding diagnosis, we observed no excess risk of rectal cancer beyond 1 year of follow-up, and even a reduced risk after 5 or more years. Of note, increased risks of all GI cancers other than rectal and gall bladder cancers persisted beyond 1 year of follow-up. However, only risks of liver and pancreatic cancers remained increased beyond 5 years of follow-up.
CRC cancer risk after lower GI bleeding diagnosed in primary care settings has been examined previously. Three British studies (two cohort studies8, 9 including ~60,000 persons with rectal bleeding and one case–control study10 including 5,477 CRC cases) compared CRC risk among persons presenting to their general practitioner with rectal bleeding to CRC risk in the general population. CRC risk was >70-fold increased during the first 6 months after the rectal bleeding episode,8 and remained 16-fold increased after 1 year,9 20-fold increased after 2 years,10 and 17-fold increased after 3 years of follow-up.8
We observed slightly higher relative risks of CRC during the first year after lower GI bleeding diagnosed in the hospital setting, compared with the studies restricted to primary care.9 The different study populations in hospital settings and primary care settings could explain this disparity. Also, inclusion in our study of patients aged less than 40 years may have contributed to the higher overall relative risk of CRC.
No previous studies have provided disaggregated risk estimates of CRC more than 1 year beyond the bleeding episode. We found a stronger association between lower GI bleeding and distal CRC than for proximal CRC, consistent with symptomatology and findings in previous studies.25, 28 This finding may be explained in part by easier examination of the rectum and distal colon than the proximal colon, or by underreporting of bleeding from proximal tumors due to darker color and/or mixing with stool. The persistently increased proximal colon cancer risk throughout the follow-up period could be due to interval cancers or insufficient examination of the proximal colon in patients with lower GI bleeding.29, 30 In contrast, removal of adenomas, or negative lower endoscopic findings most likely explain the decreased risk of distal colon and rectal cancer after 5 years of follow-up.31, 32, 33
Our findings of increased risk of non-CRC GI cancers may have several explanations. First, an invasive GI tumor may bleed into the intestinal lumen.11 Second, some cancers can increase the tendency to bleed by causing systemic alterations in the coagulation system (e.g., thrombocytopenia or decreased hepatic synthesis of coagulation factors).12, 13, 14, 15 Finally, alcohol intake may both induce bleeding16, 17 and increase the long-term risk of liver, pancreatic, esophageal, and CRCs.18, 34, 35
Our study has several strengths. The Danish health care system provides free hospital treatment to all Danish residents, permitting the conduct of studies with nationwide coverage and complete follow-up, thus minimizing risks of referral and selection biases. Both the DNPR22 and the DCR23 are of high quality, as assessed by the validity and completeness of diagnoses and procedure codes. We found the ICD-10 code for lower GI bleeding in the DNPR to be highly predictive for fresh bleeding per rectum. Our study is the first to investigate long-term (up to 10 years) risk of all types of GI cancer following a hospital-based diagnosis of lower GI bleeding. Owing to the long follow-up period, we believe that we detected close to all patients with GI cancer in our cohort. Also, our study separately examined risks of proximal and distal CRC after lower GI bleeding.
Several potential study limitations also should be kept in mind when interpreting our results. Over the short term, heightened diagnostic effort potentially explains some of the associations. Our finding of an increased risk of all GI cancers during the first year following a GI bleeding episode is consistent with this explanation. However, the increased risk was remarkably persistent years after the bleeding episode, suggesting that diagnostic bias did not play a prominent role. Even in the short term, diagnostic bias seems unlikely, as the period of heightened cancer surveillance would be followed by a compensatory deficit. We did not see such a pattern except for rectal and gall bladder cancer.
Diagnosis of lower GI bleeding can sometimes be difficult, and the recorded diagnoses we relied on may not have been entirely accurate. In 10–15% of patients with hematochezia, the bleeding source is located above the ligament of Treitz (36). However, diagnostic misclassification would tend to minimize the strength of the associations we recorded.
Also, our study was limited by lack of some clinical detail. Patients with dark rectal bleeding and with bleeding combined with other GI symptoms are more likely to be referred from primary care to hospital care than patients with mono-symptomatic fresh rectal bleeding (37). We lacked information about the severity of the lower GI bleeding, additional GI symptoms, or about lifestyle factors related to bleeding tendency and cancer. Hence our study probably overrepresented patients with dark lower GI bleeding and patients with bleeding combined with other cancer-related symptoms (anemia, weight loss, change in bowel habits, and so on).
Our data did not allow us to make firm recommendations for treatment of patients presenting with lower GI bleeding in a hospital setting. The clinical implications of our findings are therefore not entirely clear. However, our study emphasizes the importance of considering prevalent GI cancers of all types in patients diagnosed with lower GI bleeding in the hospital inpatient or outpatient setting. Future studies are needed to elucidate the increased risk of almost all types of GI cancer beyond 1 year of follow-up, and the weakly increased risk of several types of GI cancer beyond 5 years.
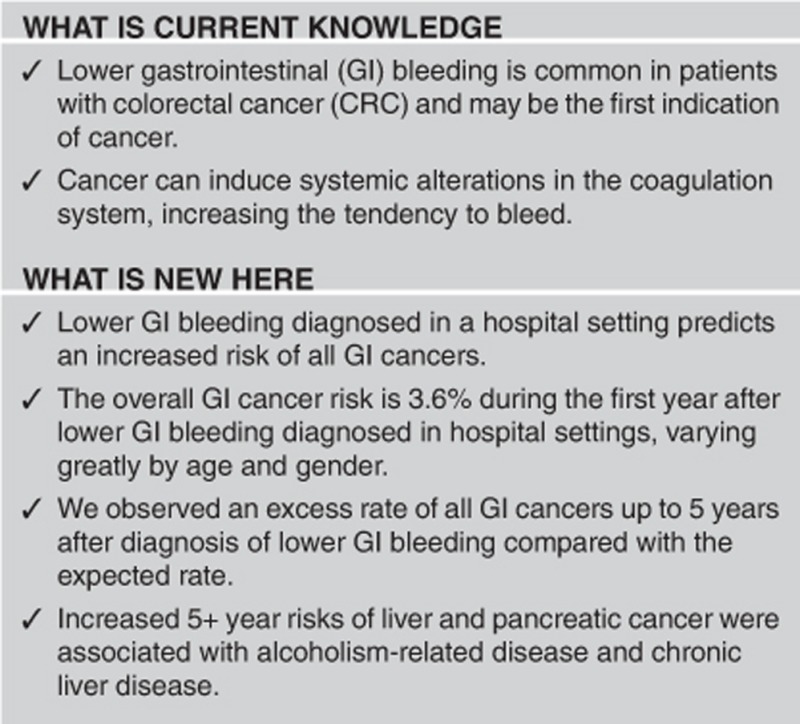
Study Highlights
Acknowledgments
We thank Rune Erichsen, MD, PhD, for skillful assistance in the validation of the diagnostic codes.
Guarantor of the article: Henrik Toft Sørensen, MD, PhD.
Specific author contributions: H.T. Sørensen conceived the idea for the study; S. Viborg, K.K. Søgaard, D.K. Farkas, and H.T. Sørensen designed the study. Data collection, management, and analysis were conducted by L. Pedersen and D.K. Farkas. Interpretation of the analyses and drafting of the article was performed by S. Viborg, K.K. Søgaard, H. Nørrelund, and H.T. Sørensen.
Financial support: The study was supported by a grant from the Danish Cancer Society (R73-A4284–13-S17); a grant from Aarhus University Research Foundation, and by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation. The sponsors did not influence the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Astin M, Griffin T, Neal RD et al. The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review. Br J Gen Pract 2011; 61: e231–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein BA, Macaskill P, Chan SF et al. Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review. BMC Gastroenterol 2011; 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanas A, Garcia-Rodriguez LA, Polo-Tomas M et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol 2009; 104: 1633–1641. [DOI] [PubMed] [Google Scholar]
- Longstreth GF. Epidemiology and outcomeofpatients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol 1997; 92: 419–424. [PubMed] [Google Scholar]
- Hreinsson JP, Gumundsson S, Kalaitzakis E et al. Lower gastrointestinal bleeding: incidence, etiology, and outcomes in a population-based setting. Eur J Gastroenterol Hepatol 2013; 25: 37–43. [DOI] [PubMed] [Google Scholar]
- Ahsberg K, Hoglund P, Kim WH et al. Impact of aspirin, NSAIDs, warfarin, corticosteroids and SSRIs on the site and outcome of non-variceal upper and lower gastrointestinal bleeding. Scand J Gastroenterol 2010; 45: 1404–1415. [DOI] [PubMed] [Google Scholar]
- Arroja B, Cremers I, Ramos R et al. Acute lower gastrointestinal bleeding management in Portugal: a multicentric prospective 1-year survey. Eur J Gastroenterol Hepatol 2011; 23: 317–322. [DOI] [PubMed] [Google Scholar]
- Jones R, Latinovic R, Charlton J et al. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ 2007; 334: 1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson R, Logie J, Marks C. Risk of colorectal cancer in general practice patients presenting with rectal bleeding, change in bowel habit or anaemia. Eur J Cancer Care (Engl) 2006; 15: 267–271. [DOI] [PubMed] [Google Scholar]
- Hamilton W, Lancashire R, Sharp D et al. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med 2009; 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi JJ, Kurtz RC. A multidisciplinary approach to gastrointestinal bleeding in cancer patients. J Support Oncol 2005; 3: 101–110. [PubMed] [Google Scholar]
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013; 11: 223–233. [DOI] [PubMed] [Google Scholar]
- Johnson MJ. Bleeding, clotting and cancer. Clin Oncol (R Coll Radiol) 1997; 9: 294–301. [DOI] [PubMed] [Google Scholar]
- Pereira J. Control of bleeding in cancerIn:Kwaan HC, Green D (eds). Coagulation in Cancer. Springer: US, 2009, pp 305–326. [Google Scholar]
- Rosen PJ. Bleeding problems in the cancer patient. Hematol Oncol Clin North Am 1992; 6: 1315–1328. [PubMed] [Google Scholar]
- Salem RO, Laposata M. Effects of alcohol on hemostasis. Am J Clin Pathol 2005; 123: Suppl:S96–S105. [DOI] [PubMed] [Google Scholar]
- Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World 1997; 21: 42–52. [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum 2010; 96: 3–1383. [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015Cancer 2015; 65: 5–29. [DOI] [PubMed]
- Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011; 39 (7 Suppl): 22–25. [DOI] [PubMed] [Google Scholar]
- Nordic Medico Statistical Committee. NOMESCO: Classification of Surgical Procedures. 16th ed.: Copenhagen; 2011.
- Andersen TF, Madsen M, Jorgensen J et al. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 1999; 46: 263–268. [PubMed] [Google Scholar]
- Storm HH, Michelsen EV, Clemmensen IH et al. The Danish Cancer Registry—history, content, quality and use. Dan Med Bull 1997; 44: 535–539. [PubMed] [Google Scholar]
- Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer 2002; 101: 403–408. [DOI] [PubMed] [Google Scholar]
- Alexiusdottir KK, Moller PH, Snaebjornsson P et al. Association of symptoms of colon cancer patients with tumor location and TNM tumor stage. Scand J Gastroenterol 2012; 47: 795–801. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology3rd edn.Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol II—The Design and Analysis of Cohort Studies. IARC Sci Publ 1987; 82: 1–406. [PubMed] [Google Scholar]
- Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol 1999; 94: 3039–3045. [DOI] [PubMed] [Google Scholar]
- Samadder NJ, Curtin K, Tuohy TM et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology 2014; 146: 950–960. [DOI] [PubMed] [Google Scholar]
- Brenner H, Chang-Claude J, Seiler CM et al. Interval cancers after negative colonoscopy: population-based case-control study. Gut 2012; 61: 1576–1582. [DOI] [PubMed] [Google Scholar]
- Nishihara R, Wu K, Lochhead P et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013; 369: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Chang-Claude J, Jansen L et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014; 146: 709–717. [DOI] [PubMed] [Google Scholar]
- Brenner H, Chang-Claude J, Rickert A et al. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case-control study. J Clin Oncol 2012; 30: 2969–2976. [DOI] [PubMed] [Google Scholar]
- Grewal P, Viswanathen VA. Liver cancer and alcohol. Clin Liver Dis 2012; 16: 839–850. [DOI] [PubMed] [Google Scholar]
- Tramacere I, Scotti L, Jenab M et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer 2010; 126: 1474–1486. [DOI] [PubMed] [Google Scholar]
- Vernava AM 3rd, Moore BA, Longo WE et al. Lower gastrointestinal bleeding. Dis Colon Rectum 1997; 40: 846–858. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Pond CL, Ellis BG et al. Rectal bleeding in general and hospital practice; 'the tip of the iceberg'. Colorectal Dis 2000; 2: 288–293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


