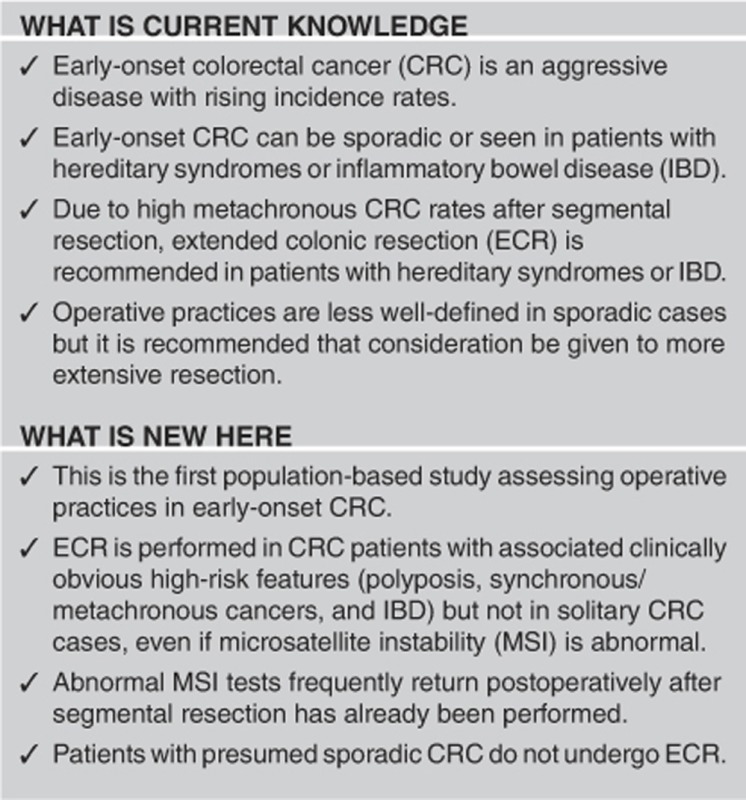
Abstract
OBJECTIVES:
Early-onset colorectal cancer (CRC) incidence rates are rising. This group is susceptible to heritable conditions (i.e., Lynch syndrome (LS)) and inflammatory bowel disease (IBD) with high metachronous CRC rates after segmental resection. Hence, extended colonic resection (ECR) is often performed and considered generally in young patients. As there are no population-based studies analyzing resection extent in early-onset CRC, we used CDC Comparative Effectiveness Research (CER) data to assess state-wide operative practices.
METHODS:
Using CER and Louisiana Tumor Registry data, all CRC patients aged ≤50 years, diagnosed in Louisiana in 2011, who underwent surgery in 2011–2012 were retrospectively analyzed. Prevalence of, and the factors associated with operation type (ECR including subtotal/total/proctocolectomy vs. segmental resection) were evaluated.
RESULTS:
Of 2,427 CRC patients, 274 were aged ≤50 years. In all, 234 underwent surgery at 53 unique facilities and 6.8% underwent ECR. Statistically significant ECR-associated factors included age ≤45 years, polyposis, synchronous/metachronous LS-associated cancers, and IBD. Abnormal microsatellite instability (MSI) was not ECR-associated. ECR was not performed in sporadic CRC.
CONCLUSIONS:
ECR is performed in the setting of clinically obvious associated high-risk features (polyposis, IBD, synchronous/metachronous cancers) but not in isolated/sporadic CRC. However, attention must be paid to patients with seemingly lower risk characteristics (isolated CRC, no polyposis), as LS can still be present. In addition, the presumed sporadic group requires further study as metachronous CRC risk in early-onset sporadic CRC has not been well-defined, and some may harbor undefined/undiagnosed hereditary conditions. Abnormal MSI (LS risk) is not associated with ECR; abnormal MSI results often return postoperatively after segmental resection has already occurred, which is a contributing factor.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in men and women, and the second leading cause of cancer mortality in men and women combined.1 In those aged <50 years, CRC is the second leading cause of cancer in men and third in women.2 Contributing to the substantial CRC burden in young patients are rising incidence rates in this group.3
Early-onset CRC can be divided into sporadic disease and disease associated with a more clearly defined pathogenesis. With regard to the former, sporadic early-onset CRC can be aggressive with frequent metastases.4 Operative practices and long-term metachronous risk in this subset of patients have not been well-studied. However, according to the National Comprehensive Cancer Network (NCCN), more extensive colectomy can be generally considered in young patients, age <50 years.5, 6 The latter subgroup is primarily composed of known hereditary disorders (Lynch syndrome (LS), familial adenomatous polyposis (FAP)) and inflammatory bowel disease (IBD; ulcerative colitis, Crohn's disease). In hereditary disorders and IBD, segmental resection, in contrast to ECR (subtotal/total/proctocolectomy), is associated with increased metachronous CRC risk. Guidelines reflect this and suggest ECR in these groups, with the specific operation depending on the underlying disease type and tumor location.7, 8, 9, 10
With regard to hereditary CRC, LS is the most common syndrome and is associated with up to 5% of all CRC cases with a penetrance of up to 80%.11, 12 In early-onset CRC, ~17% of patients unselected for family history may have LS.13, 14 Owing to metachronous CRC, subtotal or total colectomy decreases subsequent CRC and may increase life expectancy, particularly in younger patients.15, 16, 17 In LS patients undergoing segmental resection, metachronous rates may be 16% at 10 years, 41% at 20 years and 62% at 30 years post operatively, even in those undergoing frequent post-operative surveillance with colonoscopy.15 Studies have demonstrated that the rate of metachronous lesions dropped significantly (post-operative risk 0–3.4%) when the recommended surgical intervention was performed.15, 18 In young patients with synchronous or metachronous LS-associated cancers, mutations may be found in as many as 34% of patients.19 LS testing includes tumor analysis for microsatellite instability (MSI) and/or immunohistochemistry (IHC) staining for mismatch repair proteins to stratify for germline genetic testing.6, 10, 20, 21 Ideally, tumor analysis results should be available preoperatively to facilitate surgical planning.10 Mutations in four mismatch repair genes (MLH1, MSH2, MSH6, and PMS2) and epithelial cell adhesion molecule are most common.22, 23 Current guidelines recommend colectomy with ileorectal anastamosis in LS patients with CRC.10
FAP and IBD account for up to 3 and 4%, respectively, of early-onset CRC.24 ECR (frequently proctocoletomy) is recommended with both due to very high progression to CRC (FAP) and high synchronous/metachronous CRC rates (FAP and IBD).25, 26 MutY DNA glycosylase-associated polyposis accounts for 2–4% of all CRC.27 In patients unable to be managed with endoscopic polypectomy, surgery is suggested.28, 29
Despite metachronous CRC risk in early-onset patients with clearly defined heritable syndromes and IBD undergoing limited colonic resection, there are no population-based studies examining operative practices in these subgroups. Similarly, population-based analyses examining operative practices in presumed sporadic early-onset CRC patients have never been conducted. Hence, the aim of this study is to utilize population-based state-wide Louisiana Tumor Registry (LTR) data supplemented with data from a Centers for Disease Control and Prevention (CDC)-funded Comparative Effectiveness Research (CER) project to assess factors associated with the performance of ECR vs. segmental colonic resection in early-onset CRC. Analyzed variables include socio-demographics, health-care facility type, tumor characteristics, and CRC family history. An analysis of this type enables us to assess how patients with well-defined risks for metachronous CRC are operated upon. It also allows us to better understand the operative practices in subgroups with less-defined risk, particularly the presumed “sporadic group.” This group is likely heterogeneous, consisting of true sporadic patients, and those with undiagnosed LS or hereditary predispositions that have yet to be defined. MSI was an important variable to study as we previously demonstrated a low-MSI and/or IHC testing rate of 23% for LS screening in this 2011 state-wide CRC cohort and that results were available preoperatively in only 16.9%.30 Both of these factors may limit the ability to risk stratify patients preoperatively for germline genetic testing to determine the resection extent.
The LTR is a National Cancer Institute's (NCI) Surveillance Epidemiology and End Results (SEER) program participant and a specialized registry of the CDC's National Program of Cancer Registries (NPCR). The LTR is one of only 10 states participating in CDC CER, which allows for detailed epidemiologic analysis of large populations. The utilization of population-based CER surgical and MSI data, which were only available in 2011–2012, are unique to this study.
METHODS
Data source/study population
Eligible patients were aged ≤50 years, diagnosed with primary CRC in 2011 in Louisiana (ICD-03 codes: C18.0-18.9, C19.9, C20.9, and C26.0), and underwent resection. Surgeries took place in 2011–2012. Death certificate only, non-adenocarcinomatous, and autopsy cases were excluded (n=4). The study was IRB approved.
The LTR has legislative authorization to assess data from hospital and non-hospital settings, including gastroenterology, surgery, and oncology private practice groups. Treatment information up to 1 year from diagnosis was obtained from the LTR supplemented with CER project data. Source records included, but were not limited to, operative and colonoscopy reports, pathology reports, and consultation/progress notes. Information was collated, abstracted, and coded as per the national standards for cancer registries, and reviewed by LTR researchers and physicians. For patients undergoing surgery, operative pathology reports or consolidated medical abstractions were available in 99% of cases. For those undergoing colonoscopy, pathology reports or abstracted reports were available in 93% of cases.
Variables
The number of patients undergoing surgery and operation type including segmental resection vs. ECR (including subtotal, total, or proctocolectomy) was determined. Segmental resection included hemicolectomy, low anterior, and abdominoperineal resection. Socio-demographic, tumor, and health-care facility variables were correlated with operative extent. Socio-demographic variables included diagnosis age, race, sex, parish (county) of residence (urban vs. rural), and health insurance status. Tumor-associated variables included concurrent polyposis (≥10 adenomas combined on colonoscopy and/or surgery), proximal vs. distal CRC, tumor histology, IBD history or histological IBD features, MSI (high, low, and stable) or IHC, and synchronous or metachronous LS-associated cancers. Proximal tumors were defined as proximal to the splenic flexure. For synchronous or metachronous CRC, lesions demonstrating high-grade dysplasia (n=4) were also considered as LS-associated malignancy, because high-grade dysplasia is synonymous with carcinoma in situ which is registry reportable as stage 0 CRC.31 Health-care facilities were categorized by American College of Surgery standards.32 Family history of CRC in a first-degree relative was also analyzed.
Statistical analyses
Colonic resection extent was correlated with the aforementioned variables using Pearson's χ2-test analysis. When cell counts were <5, the Fisher's exact test was used to obtain P-values. Univariate logistic regression was used to estimate unadjusted odds ratios and 95% confidence intervals. Multivariate logistic regression was used to estimate adjusted odds ratios and 95% confidence intervals. Because rare events occurred in our data set (16 ECR cases), we used Firth's penalized likelihood method in logistic regression analysis as an approach to reduce the small-sample bias in maximum likelihood estimation. Statistically significant predictors in the univariate analysis were utilized for multivariate analysis.
RESULTS
Of 2,427 all-age, state-wide CRC patients in 2011, 274 were aged ≤50 years. In all, 234 underwent surgical resection at 53 distinct health-care facilities and had complete surgical data available. A total of 37 out of 274 patients did not undergo surgery (lesions removed during colonoscopy or wide-spread disease). In 3 patients surgical status was unknown. Socio-demographic, tumor, and health-care facility characteristics are recorded in Table 1. Proximal tumors were seen in 30.3%, distal in 65.0%, and synchronous CRCs in 4.7%. Of distal tumors, 40.1% (61/152) were rectal. Synchronous/metachronous LS-associated cancers were noted in 17 patients (19 lesions) including CRC (n=11), polyps with high-grade dysplasia/carcinoma in situ (n=4), bladder cancer (n=2), gastric cancer (n=1), and medulloblastoma (n=1).
Table 1. Descriptive characteristics of Louisiana residents aged ≤50 years diagnosed with CRC in 2011 who underwent surgery in 2011–2012, stratified by age.
| Age ≤40 years (n=46), % | Age 41–50 years (n=188), % | Total (n=234), % | P-value | |
|---|---|---|---|---|
| Sex | 0.7377 | |||
| Male | 45.7 | 48.4 | 47.9 | |
| Female | 54.3 | 51.6 | 52.1 | |
| Race | 0.8137 | |||
| White | 63.0 | 67.0 | 66.2 | |
| Black | 37.0 | 31.9 | 32.9 | |
| Other | 0.0 | 1.1 | 0.9 | |
| Health insurance | 0.9206 | |||
| Uninsured | 10.9 | 13.8 | 23.3 | |
| Insured | 86.9 | 84.0 | 84.6 | |
| Unknown | 2.2 | 2.2 | 2.1 | |
| Geographic area | 0.9601 | |||
| Urban (metro) | 71.8 | 69.1 | 69.7 | |
| Rural (non-metro) | 21.7 | 24.5 | 23.9 | |
| Non-Louisiana | 6.5 | 6.4 | 6.4 | |
| Colectomy | 0.0001 | |||
| No | 80.4 | 96.3 | 93.2 | |
| Yes | 19.6 | 3.7 | 6.8 | |
| AJCC stage | 0.7026 | |||
| 0 | 0.0 | 3.7 | 3.0 | |
| I | 13.0 | 18.1 | 17.1 | |
| II | 21.7 | 20.2 | 20.5 | |
| III | 32.6 | 35.7 | 35.0 | |
| IV | 32.7 | 21.8 | 24.0 | |
| Unknown | 0.0 | 0.5 | 0.4 | |
| Tumora | 0.5903 | |||
| Proximal | 26.1 | 31.4 | 30.3 | |
| Distal | 67.4 | 64.4 | 65.0 | |
| Synchronous | 6.5 | 4.2 | 4.7 | |
| MSI feature seen on histologyb | 0.4826 | |||
| No | 13.0 | 10.1 | 10.7 | |
| Yes | 28.3 | 21.8 | 23.1 | |
| Unknown | 58.7 | 68.1 | 66.2 | |
| Lynch syndrome-associated cancer, synchronous or metachronous | 0.7508 | |||
| No | 91.3 | 93.1 | 92.7 | |
| Yes | 8.7 | 6.9 | 7.3 | |
| First-degree relative with CRC | 0.1848 | |||
| No | 52.2 | 38.3 | 41.0 | |
| Yes | 4.3 | 10.6 | 9.4 | |
| Unknown | 43.5 | 51.1 | 49.6 | |
| Hospital type | 0.9717 | |||
| THCP | 17.4 | 19.2 | 18.8 | |
| COMP | 23.9 | 21.8 | 22.2 | |
| CHCP | 19.6 | 18.6 | 18.8 | |
| Public | 13.0 | 10.6 | 11.1 | |
| Non-CoC/non-public | 26.1 | 29.8 | 29.1 |
AJCC, American Joint Committee on Cancer; CHCP, community hospital cancer program; CoC, commission on cancer; COMP, community hospital comprehensive cancer program; CRC, colorectal cancer; MSI, microsatellite instability; THCP, teaching hospital cancer program.
Proximal represents tumors proximal to the splenic flexure; distal represents tumors distal to the transverse colon.
MSI features as per the revised Bethesda Criteria (i.e., mucinous features etc.).42
The Pearson's χ2-test was used for the univariate analyses. For any cells with counts <5, the Fisher's exact test was used to test significance. Data with zero cell frequencies were analyzed with Firth's penalized maximum likelihood estimator for bias correction (Firth's method). SAS version 9.4 (SA institute, Cary, NC) was used to perform the analyses.
The overall ECR rate was 6.8% (16/234). Factors associated with ECR are presented in Table 2. Age ≤45 years, synchronous/metachronous LS-associated cancers, polyposis, IBD, and distal CRC location were significantly associated. All except IBD remained significant in multivariate analyses. Neither undergoing colonoscopy prior to surgery (85.9% of patients), nor a family history of CRC in a first-degree relative was ECR associated. Tumor histology (i.e., mucinous changes that can be seen in LS) was also not associated with ECR.
Table 2. Variables associated with extended colonic resection, univariate and multivariate analyses.
| Variables |
Extended resection (subtotal/total or proctocolectomy) |
P-value | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
|
No (n=218) |
Yes (n=16) |
Total (n=234) | ||||||
| Count | % | Count | % | |||||
| Sex | 0.4866 | |||||||
| Male | 103 | 92.0 | 9 | 8.0 | 112 | 0.70 (0.25, 1.94) | ||
| Female | 115 | 94.3 | 7 | 5.7 | 122 | Ref | ||
| Race | 0.1796 | |||||||
| White | 145 | 93.6 | 10 | 6.4 | 155 | Ref | ||
| Black | 72 | 93.5 | 5 | 6.5 | 77 | 1.05 (0.36, 3.08) | ||
| Other | 1 | 50.0 | 1 | 50.0 | 2 | 13.85 (0.81, 237.6) | ||
| Age at diagnosis (years) | <0.0001 | |||||||
| ≤40 | 37 | 80.4 | 9 | 19.6 | 46 | 22.38 (3.82, 131.2) | 1.97 (1.91, 2.03) | |
| 41–45 | 49 | 89.1 | 6 | 10.9 | 55 | 11.60 (1.89, 71.15) | 1.88 (1.83, 1.93) | |
| 46–50 | 132 | 99.3 | 1 | 0.7 | 133 | Ref | Ref | |
| Health insurance | 0.8291 | |||||||
| Uninsured | 30 | 96.8 | 1 | 3.2 | 31 | Ref | ||
| Insured | 183 | 92.4 | 15 | 7.6 | 198 | 1.72 (0.30, 9.83) | ||
| Unknown | 5 | 100 | 0 | 0.0 | 5 | 1.85 (0.05, 66.45) | ||
| Geographic area | 0.0885 | |||||||
| Urban (metro) | 154 | 94.5 | 9 | 5.5 | 163 | Ref | ||
| Rural (non-metro) | 52 | 92.9 | 4 | 7.1 | 56 | 1.39 (0.43, 4.50) | ||
| Non-Louisiana | 12 | 80.0 | 3 | 20.0 | 15 | 4.56 (1.34, 18.27) | ||
| Colonoscopy and surgery | 1.000 | |||||||
| Colonoscopy before surgery | 187 | 93.0 | 14 | 7.0 | 201 | Ref | ||
| Surgery only | 31 | 93.9 | 2 | 6.1 | 33 | 0.97 (0.24, 4.00) | ||
| Tumor locationa | <0.0001 | |||||||
| Proximal | 69 | 97.2 | 2 | 2.8 | 71 | Ref | Ref | |
| Distal | 144 | 94.7 | 6 | 5.3 | 152 | 1.64 (0.38, 6.96) | 1.10 (1.08, 1.12) | |
| Synchronous | 5 | 45.5 | 8 | 54.5 | 11 | 32.84 (5.78, 186.8) | 9.65 (9.30, 10.01) | |
| MSI feature seen on histologyb | 0.7164 | |||||||
| No | 24 | 96.0 | 1 | 4.0 | 25 | Ref | ||
| Yes | 49 | 90.7 | 5 | 9.3 | 54 | 1.18 (0.20, 7.09) | ||
| Unknown | 145 | 93.6 | 10 | 6.4 | 155 | 1.82 (0.27, 12.16) | ||
| Synchronous or metachronous Lynch syndrome-associated cancer | <0.0001 | |||||||
| No | 208 | 95.9 | 9 | 4.1 | 217 | Ref | Ref | |
| Yes | 10 | 58.8 | 7 | 41.2 | 17 | 15.68 (4.90, 50.20) | 2.36 (2.28, 2.44) | |
| First-degree relative with CRC | 0.9271 | |||||||
| No | 90 | 93.8 | 6 | 6.2 | 96 | Ref | ||
| Yes | 21 | 95.5 | 1 | 4.5 | 22 | 1.23 (0.43, 3.49) | ||
| Unknown | 107 | 92.2 | 9 | 7.8 | 116 | 0.91 (0.15, 6.32) | ||
| Polyposis | <0.0001 | |||||||
| No | 218 | 96.5 | 8 | 3.5 | 226 | Ref | Ref | |
| Yes | 0 | 0.0 | 8 | 100 | 8 | NAc | NAc,d | |
| IBD | 0.0241 | |||||||
| No | 218 | 94.0 | 14 | 6.0 | 232 | Ref | Ref | |
| Yes | 0 | 0.0 | 2 | 100 | 2 | NAc | NAc,e | |
| Hospital type | 0.3635 | |||||||
| THCP | 40 | 90.9 | 4 | 9.1 | 44 | 1.59 (0.40, 6.31) | ||
| COMP | 50 | 96.2 | 2 | 3.8 | 52 | 0.71 (0.14, 3.53) | ||
| CHCP | 42 | 95.5 | 2 | 4.5 | 44 | 0.84 (0.17, 4.22) | ||
| Public | 22 | 84.6 | 4 | 15.4 | 26 | 2.87 (0.70, 11.75 | ||
| Non-CoC/non-public | 64 | 94.1 | 4 | 5.9 | 68 | Ref | ||
| MSI and/or IHC abnormal | n=56 | n=4 | Total (n=60) | 0.9200 | ||||
| No | 41 | 93.2 | 3 | 6.8 | 44 | |||
| Yes | 12 | 92.3 | 1 | 7.7 | 13 | |||
| Unknown | 3 | 100 | 0 | 0.0 | 3 | |||
CHCP, community hospital cancer program; CI, confidence interval; CoC, commission on cancer; COMP, community hospital comprehensive cancer program; CRC, colorectal cancer; IBD, inflammatory bowel disease; IHC, immunohistochemistry; MSI, microsatellite instability; NA, not available; Ref, reference; THCP, teaching hospital cancer program.
Proximal represents tumors proximal to the splenic flexure; distal tumors represent tumors distal to the transverse colon.
MSI features as per the revised Bethesda Criteria (i.e., mucinous features, etc.).42
Odds ratio cannot be computed.
Polyposis-adjusted odds ratio P-value <0.0001.
IBD-adjusted odds ratio P-value 0.1147.
The Pearson's χ2-test was used for the univariate analyses. For any cells with counts <5, the Fisher's exact test was used to test significance. Data with zero cell frequencies were analyzed with Firth's penalized maximum likelihood estimator for bias correction (Firth's method). SAS version 9.4 (SA institute) was used to perform the analyses.
MSI and/or IHC were ordered in 56/218 (25.7%) of patients undergoing segmental resection and 4/16 (25%) patients undergoing ECR (Table 2, bottom). Among those tested for MSI/IHC, abnormal MSI and/or IHC were present in 13 patients with similar proportions in the segmental resection (12/56, 21.4%) and ECR group (1/4, 25%). There was no difference in ECR performance based on abnormal vs. normal MSI and/or IHC. In all, 1/13 (7.7%) patients with abnormal MSI and/or IHC underwent ECR vs. 3/44 (6.8%) with normal MSI and/or IHC.
Of the 13 patients with abnormal MSI and/or IHC, 8 had testing only on segmental resection specimens and hence results were not available preoperatively. One patient undergoing segmental resection had abnormal IHC (missing MSH6/PMS2) on colonoscopy that was available preoperatively. Three patients with abnormal IHC had wide-spread metastatic disease and likely had limited resection for palliation. The one patient with abnormal tumor analysis undergoing ECR had MSI-high results, but it is unclear whether this was available preoperatively to aid in resection extent planning. This patient also had polyposis, which may have prompted ECR.
Clinical characteristics of the 16 patients undergoing ECR are listed in Table 3, including specifics regarding resection extent. Seven patients had >10 adenomas, 5 of which had at least 100 adenomas. One had seven adenomas but carried a MutY DNA glycosylase diagnosis. Two patients had longstanding UC. Seven had a history of synchronous or metachronous CRC (five with synchronous CRC, one with metachronous CRC, and one with both synchronous and metachronous CRC). Four patients with synchronous/metachronous CRC also had polyposis. ECRs were performed at 12 distinct hospitals and hence there was no facility clustering for operative type.
Table 3. Clinical characteristics of patients undergoing extended colonic resection.
| Patient | Tumor location | Surgery | Polyposis | IBD | Synchronous/metachronous LS-associated cancer | Comments |
|---|---|---|---|---|---|---|
| 1 | R | Completion colectomy (total proctocolectomy) | Yes | No | Yes | 30–50 adenomas on colectomy, multiple with HGD. Prior history of medulloblastoma and CRC of right colon. |
| 2 | DC | Total colectomy with ileorectal anastamosis | No | Yes | Yes | 16-Year history of ulcerative colitis. Metachronous rectal cancer developed in 2012 after total colectomy. |
| 3 | TC | Subtotal colectomy with ileosigmoid anastamosis | No | No | No | Subtotal colectomy possibly due to obstruction—significantly dilated proximal colon seen during surgery. |
| 4 | R and HF | Total proctocolectomy | Yes | No | Yes | 1,000–2,000 adenomas on surgical pathology. Diagnosed with Gardner's Syndrome. Rectal polyp containing adenocarcinoma removed during colonoscopy. HF CRC on surgical pathology |
| 5 | R | Total colectomy (ileorectal anastomosis) | No | No | No | Reason for subtotal colectomy unknown. |
| 6 | R | Total proctocolectomy | Yes | No | No | 7 adenomas on colonoscopy. Diagnosed with MUTYH. |
| 7 | S | Subtotal colectomy (ileosigmoid anastomosis) | Yes | No | No | 200–300 adenomas on surgical pathology. |
| 8 | C | Total proctocolectomy | No | No | Yes | Multiple polyps (<10) with HGD on surgical pathology. |
| 9 | R | Subtotal (ileodescending anastomosis) | No | No | No | Massive cecal dilation and Clostridium difficile on surgical pathology. Surgery did not include the rectal mass so likely done for palliation. |
| 10 | R | Total proctocolectomy | Yes | No | No | >100 adenomas on colectomy specimen. |
| 11 | RS | Total proctocolectomy | Yes | No | Yes | 1,000's of adenomas on colonoscopy and surgical pathology, multiple with HGD. Diagnosed with FAP. |
| 12 | R | Total proctocolectomy | Yes | No | Yes | Synchronous rectal cancers. 1,000's of adenomas on colonoscopy. |
| 13 | DC and AC | Subtotal (ileosigmoid anastomosis) | No | No | Yes | Synchronous CRC (DC and AC) |
| 14 | S | Subtotal (ileosigmoid anastomosis) | No | No | No | Palliative, extensive peritoneal carcinomatosis |
| 15 | S | Total proctocolectomy | No | Yes | No | 20-Year history of ulcerative colitis. |
| 16 | AC | Subtotal (ileodescending anastomosis) | Yes | No | No | Approximately 10–12 adenomas combined on colonoscopy and surgical specimen. MSI-high tumor (unclear if available preoperatively), normal IHC (on operative specimen). |
AC, ascending colon; C, cecum; CRC, colorectal cancer; DC, descending colon; FAP, familial adenomatous polyposis; HF, hepatic flexure; HGD, high-grade dysplasia; IBD, inflammatory bowel disease; IHC, immunohistochemistry; LS, Lynch syndrome; MUTYH, MutY DNA glycosylase; R, rectum; S, sigmoid; TC, transverse colon.
DISCUSSION
This is, to our knowledge, the first study examining detailed factors associated with colonic resection extent in early-onset CRC using population-based registry data. This analysis allowed us to understand the low state-wide ECR rate of 6.8%. CRC arising in the context of clinically obvious high-risk features (polyposis, synchronous/metachronous LS-associated cancer, IBD, and age ≤45 years) was associated with ECR. However, abnormal MSI and/or IHC were not ECR associated. The low overall MSI/IHC testing rates and infrequent preoperative result availability (only 16.9%), which we previously identified in this population, will lead to lower identification of LS patients who could benefit from more extensive surgery.30 In our current study, the majority of those with abnormal MSI and/or IHC undergoing segmental resection had results available only post operatively, and hence not available for surgical planning. Overall, only 7.7% of those with abnormal MSI and/or IHC underwent ECR. It is possible that the high index of suspicion required to identify LS, especially in solitary CRC cases, combined with multiple, time-dependent steps required for diagnosis (MSI/IHC followed by germline testing), may lead to lower LS identification, particularly preoperatively. In contrast, large numbers of adenomas and multiple cancers, especially in young patients, may be sufficient high-risk indicators leading to ECR, independent of further testing.
With regard to family history, a survey of colorectal surgeons demonstrated that in patients aged <50 years with no CRC family history, 33.1% would proceed with resection without MSI/IHC despite NCCN guidelines recommending pre-operative testing in young patients regardless of family history, and that reliance on family history may have pitfalls due to potential de novo mutations.33, 34, 35, 36 However, if pre-operative testing was performed and indicated LS, 84.9% would perform total colectomy suggesting that testing influences management decisions. In our study, even in patients with a strong family history of CRC, there was a lack of association with ECR. This may be potentially explained by several factors including providers not being attuned to the increased risk that family history confers for hereditary CRC syndromes, timing of MSI/IHC results, or patient/provider preference for resection extent. Of note, family history of malignancy was only available in ~50% of patients in our study. This could signify that providers are not routinely assessing this data point, which would be of concern in this early-onset CRC population. However it is also possible that in some cases this variable may not have been captured during the medical record abstraction process.
Important factors regarding surgical approach include perioperative complications and functional outcomes. The overall complication-free rate has been shown to be highest with segmental resection compared with ileosigmoid/ileorectal anastomoses.37 Patients at risk for hereditary CRC who undergo subtotal colectomy (vs. partial colectomy) are more likely to have functional complications.38 However, in some studies, resection extent has not been shown to differentially impact quality of life.38, 39 This may reflect a sense of ease garnered from the decreased future CRC risk. In addition, patients with FAP who undergo proctocolectomy at younger ages have better gastrointestinal function earlier in the post-operative period.40 It was important to address tumor location in our study as this can impact operative choice and quality of life. We found that distal CRC location was significantly associated with ECR, and that 7/16 patients who underwent ECR had rectal cancer. Of these seven patients, five underwent total proctocolectomy. All five had polyposis, which may explain the aggressive surgical approach despite potential for post-operative functional impairment. Overall, risk of perioperative complications or concern for functional compromise after ECR must be balanced with the significant risk of developing a second CRC if segmental resection is performed.
With regard to presumed sporadic CRC, although NCCN guidelines suggest more extensive resection be considered in patients aged <50 years, which may include sporadic CRC, our results indicate that ECR was performed in patients with obvious explanations for CRC (polyposis, synchronous/metachronous CRC, and IBD) or for obstruction/palliation. In a study examining resection extent in sporadic CRC patients aged <50 years, 3.3% undergoing segmental resection developed metachronous CRC vs. 0% undergoing extended resection, however, this was not statistically significant.20 The authors concluded that segmental resection did not independently increase the risk of recurrent disease or mortality, but that a larger volume of prospective studies are necessary. Importantly, they noted that longer term follow-up is needed because the median follow-up in the segmental and extended resection group was only 2 and 3 years, respectively. In our population-based setting, it appears that ECR is not being performed for presumed sporadic CRC. This group will be important to target for future study. As MSI and/or IHC testing was low in our population, there are likely unidentified LS patients in this presumed sporadic subgroup. In addition, there may be patients with hereditary forms of CRC with genetic pathways that have yet to be defined. In terms of the “true sporadic” cases, these require further study regarding operative type, and in general, as rising rates in early-onset CRC may signify underlying risk factors that are poorly understood.
Study limitations include relatively low numbers of patients undergoing ECR. This can hinder the ability to detect other significant variables associated with extended resection. However, low ECR rates have been seen in other studies as well, thus limiting our understanding of surgical practices in young patients in general.20, 41 In addition, low rates of MSI and/or IHC testing in our population can limit our ability to draw conclusions regarding operative practices in those with abnormal results. Furthermore, as the CER project was not designed to collect germline genetic testing information, specific mutation data was limited. Thus the number of patients with LS is unknown, although studies suggest that the majority of young patients with abnormal MSI/IHC will have LS mutations as opposed to sporadic, methylation-dependent CRC.13 It is likely that the majority of polyposis patients had APC gene mutations given large numbers of polyps (100's or 1,000's) in many and that genetic testing would be unlikely to change operative management due to high-risk phenotypes. Finally, surgical practices that we identified may not necessarily reflect the operative patterns in other regions.
Study strengths include that it is large, population-based (all patients state-wide), and conducted by a high-quality SEER/NPCR registry. The utilization of CDC CER data allowed for a comprehensive analysis that integrated detailed colonoscopy, surgical, pathological, and tumor marker data that is not normally collected by cancer registries, which is a unique strength. Surgeries were performed at 53 distinct institutions representing diverse settings, including teaching hospitals, community hospital comprehensive cancer programs, and public institutions. The study included both insured and uninsured patients. These characteristics can minimize bias seen in smaller, non population-based studies. Further strengths include operative reports and pathology reports being available in a very high percentage of cases.
In conclusion, this is the first population-based study analyzing operative practices in early-onset CRC. ECR is performed in patients with clinically obvious high-risk features (polyposis, synchronous/metachronous cancers, and IBD) but not in solitary CRC cases, even with abnormal MSI. However, attention must be paid to patients with seemingly lower risk characteristics (isolated CRC, lack of polyposis) as they are still at risk for LS and long-term metachronous CRC in sporadic cases is not well-defined. As abnormal MSI frequently returns after segmental resection has already been performed, assurances must be made that results are available preoperatively to assist in operative management decisions. The factors underlying low rates of pre-operative MSI and/or IHC result availability, and MSI/IHC testing in general, require further study. The sporadic group is likely heterogeneous, consisting of undiagnosed LS, those with hereditary predispositions that are poorly understood and a true sporadic group with potentially unknown risk factors. Future analyses will need to focus on this “sporadic group” to better understand how to identify heritable conditions and elucidate long-term metachronous CRC risk in those with true sporadic disease. We plan to utilize the LTR to follow the presumed sporadic group over time to assess the metachronous cancer rates. Further study on patient and provider preference regarding resection extent will also be important as this is a complex decision that balances operative risk, functional outcomes, and metachronous CRC risk.
Study Highlights
Guarantor of the article: Jordan J. Karlitz, MD.
Specific author contributions: Study concept: Jordan J. Karlitz; study design: Jordan J. Karlitz and Vivien W. Chen; acquisition of data: Jordan J. Karlitz, Beth Schmidt, Mei-Chin Hsieh, Xiao-Cheng Wu, and Vivien W. Chen; drafting of the manuscript: Jordan J. Karlitz. All authors performed the analysis and interpretation of the data and critical revision of the manuscript. All the authors have approved the final draft submitted.
Financial Support: The research presented in this manuscript was supported in part by the CDC Cooperative Agreements of the National Program of Cancer Registries: #U58DP003915 and CDC/ICF Comparative Effectiveness Research Subcontract: #635243-10 S-1566 with LSUHSC-NO.
Potential competing interests: None.
References
- American Cancer SocietyCancer Facts & Figures 2015 American Cancer Society: Atlanta, GA, USA, 2015. Available at: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index Accessed December 1, 2015. [Google Scholar]
- American Cancer SocietyCancer Facts & Figures 2015 American Cancer Society: Atlanta, GA, USA, 2015. Available at: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044519.pdf Accessed December 1, 2015. [Google Scholar]
- Bailey CE, Hu CY, You YN et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2014; 150: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirzin S, Marisa L, Guimbaud R et al. Sporadic early-onset colorectal cancer is a specific sub-type of cancer: a morphological, molecular and genetics study. PLoS One 2014; 9: e103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos C, Montenegro G, Jamal N et al. Segmental versus extended resection for sporadic colorectal cancer in young patients. J Surg Oncol 2014; 110: 328–332. [DOI] [PubMed] [Google Scholar]
- Benson AB, Venook AP, Bekaii-Saab T et al (eds). NCCN Colon Cancer Guidelines, Principles of Surgery version 2 2015. 2015 National Comprehensive Cancer Network, Inc. Available at: www.NCCN.org/ Accessed December 15, 2015.
- Burke W, Petersen G, Lynch P et al. Recommendations for follow-up care of individuals to an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies consortium. JAMA 1997; 277: 915–919. [PubMed] [Google Scholar]
- Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol 2010; 101: 706–712. [DOI] [PubMed] [Google Scholar]
- Hrabe J, Byrn J, Button A et al. A matched case-control study of IBD-associated colorectal cancer: IBD portends worse outcome. J Surg Oncol 2014; 109: 117–121. [DOI] [PubMed] [Google Scholar]
- Giardello FM, Allen JI, Axilbund JE et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US multi-society task force of colorectal cancer. Am J Gastroenterol 2014; 109: 1159–1179. [DOI] [PubMed] [Google Scholar]
- Van Lier MG, De Wilt JH, Wagemakers JJ et al. Underutilization of microsatellite instability analysis in colorectal cancer patients at high risk for Lynch Syndrome. Scand J Gastroenterol 2009; 44: 600–604. [DOI] [PubMed] [Google Scholar]
- Jasperson KW, Tuohy TM, Neklason DW et al. Hereditary and familial colon cancer. Gastroenterol 2010; 138: 2044–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southey MC, Jenkins MA, Mead L et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol 2005; 23: 6524–6532. [DOI] [PubMed] [Google Scholar]
- Aaltonen LA, Salovaara R, Kristo P et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 1998; 338: 1481–1487. [DOI] [PubMed] [Google Scholar]
- Parry S, Win AK, Parry B et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011; 60: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win AK, Parry S, Parry B et al. Risk of metachronous colon cancer following surgery for rectal cancer in mismatch repair gene mutation carriers. Ann Surg Oncol 2013; 20: 1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos tot Nederveen Cappel WH, Buskens E, van Duijvendijk P et al. Decision analysis in the surgical treatment of colorectal cancer due to a mismatch repair gene defect. Gut 2003; 52: 1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos tot Nederveen Cappel WH. Surveillance for hereditary nonpolyposis colorectal cancer: a long-term study on 114 families. Dis Colon Rectum 2002; 45: 1588–1594. [DOI] [PubMed] [Google Scholar]
- Niessen RC, Berends MJW, Wu Y et al. Identification of mismatch repair gene mutations in young patients with colorectal cancer and in patients with multiple tumors associated with hereditary non-polyposis colorectal cancer. Gut 2006; 55: 1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos C, Montenegro G, Jamal N et al. Segmental versus extended resection for sporadic colorectal cancer in young patients. J Surg Oncol 2014; 110: 328–332. [DOI] [PubMed] [Google Scholar]
- Umar A, Boland CR, Terdiman JP et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg MJ, Kuiper RP, Geurts van Kessel A et al. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam Cancer 2013; 12: 169–174. [DOI] [PubMed] [Google Scholar]
- Sehgal R, Sheahan K, O'Connell PR et al. Lynch syndrome: an updated review. Genes 2014; 5: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Pai RK, Rybicki LA et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012; 25: 1128–1139. [DOI] [PubMed] [Google Scholar]
- Galiatsatos P, Foulkes W. Familial adenomatous polyposis. Am J Gastroenterol 2006; 101: 385–398. [DOI] [PubMed] [Google Scholar]
- Kulaylat M, Dayton M. Ulcerative colitis and cancer. J Surg Oncol 2010; 101: 706–712. [DOI] [PubMed] [Google Scholar]
- Stigliano V, Sanchez-Mete L, Martayan A et al. Early-onset colorectal cancer: a sporadic or inherited disease? World J Gastroenterol 2014; 20: 12420–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand R, Nielsen M, Lynch H et al. MUTYH-Associated Polyposis. GeneReviews 1993-2014. University of Washington: Seattle, WA, USA. [Google Scholar]
- Nieuwenhuis MH, Vogt S, Jones N et al. Evidence for accelerated colorectal adenoma—carcinoma progression in MUTYH-associated polyposis? Gut 2012; 61: 734–738. [DOI] [PubMed] [Google Scholar]
- Karlitz JJ, Hsieh MC, Liu Y et al. Population-based Lynch Syndrome screening by microsatellite instability in patients ≤50: prevalence, testing determinants, and result availability prior to colon surgery. Am J Gastroenterol 2015; 110: 948–955. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Surveillance, Epidemiology, and End Results ProgramIs high-grade dysplasia of the GI tract reportable? Data Collection Answers from the CoC, NPCR, SEER Technical Workgroup. Available at: http://seer.cancer.gov/registrars/data-collection.html Accessed December 1, 2015.
- American College of Surgeons CoC Accreditation Categories. Available at: https://www.facs.org/quality-programs/cancer/accredited/about/categories Accessed December 1, 2015.
- Warrier SK, Kalady MF, Kiran RP et al. Results from an American Society of Colon and Rectal Surgeons survey on the management of young-onset colorectal cancer. Tech Coloproctol 2014; 18: 265–272. [DOI] [PubMed] [Google Scholar]
- Karlitz J, Provenzale D. Invited comment on Warrier et al: hereditary cancer screening and management practices by colorectal surgeons. Tech Coloproctol 2014; 18: 313–314. [DOI] [PubMed] [Google Scholar]
- Win AK, Jenkins MA, Buchanan DD et al. Colorectal cancer cases with de novo germline mutations in MLH1, MSH2, and MSH6 from the colon cancer family registry. J Clin Oncol 2011; 29: 530–534. [Google Scholar]
- Provenzale D, Jasperson K, Ahnen D et al. NCCN Guidelines Genetic/Familial High-risk Assessment: colorectal version 12015. [Google Scholar]
- You YN, Chua HK, Nelson H et al. Segmental vs. extended colectomy: measurable differences in morbidity, function, and quality of life. Dis Colon Rectum 2008; 51: 1036–1043. [DOI] [PubMed] [Google Scholar]
- Haanstra JF, de Vos Tot Nederveen Cappel WH, Gopie JP et al. Quality of life after surgery for colon cancer in patients with Lynch syndrome: partial versus subtotal colectomy. Dis Colon Rectum 2012; 55: 653–659. [DOI] [PubMed] [Google Scholar]
- Pollett WG, Marion K, Moeslein G et al. Quality of life after surgery in individuals with familial colorectal cancer: does extended surgery have an adverse impact? ANZ J Surg 2014; 84: 359–364. [DOI] [PubMed] [Google Scholar]
- Erkek AB, Church JM, Remzi FH et al. Age-related analysis of functional outcome and quality of life after restorative proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis. J Gastroenterol 2007; 22: 710–714. [DOI] [PubMed] [Google Scholar]
- Myers EA, Feingold DL, Forde KA et al. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions' experience. World J Gastroenterol 2013; 19: 5651–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unar A, Boland CR, Terdiman JP et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]


