Abstract
OBJECTIVES:
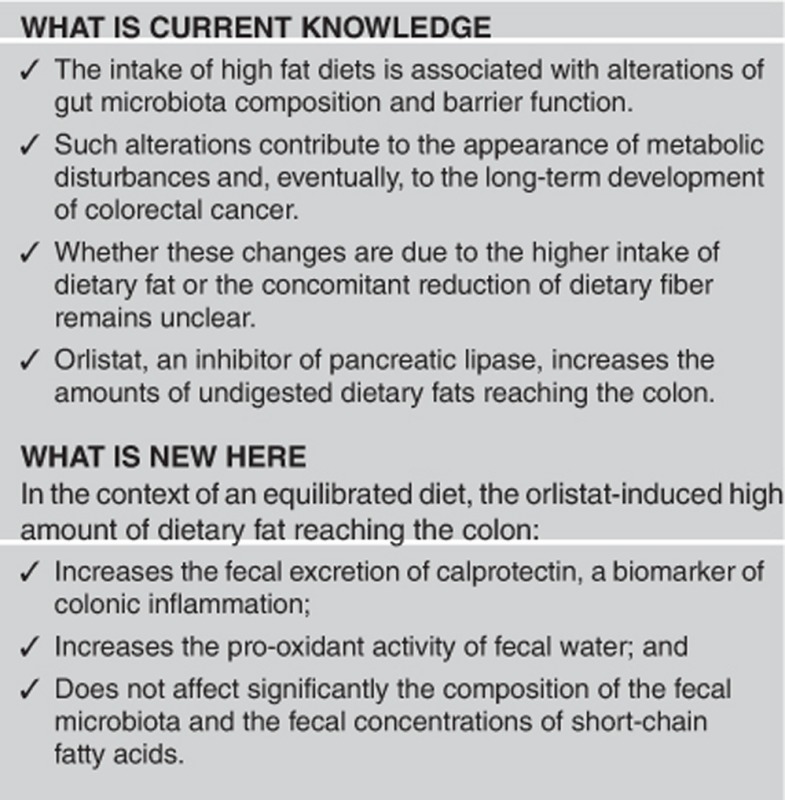
High-fat diets alter gut microbiota and barrier function, inducing metabolic endotoxemia and low-grade inflammation. Whether these effects are due to the high dietary lipid content or to the concomitant decrease of carbohydrate intake is unclear. The aim of this study was to determine whether higher amounts of dietary fat reaching the colon (through orlistat administration) affect the colonic ecosystem in healthy volunteers and the effect of the prebiotic oligofructose (OF) in this model.
METHODS:
Forty-one healthy young subjects were distributed among four groups: Control (C), Prebiotic (P), Orlistat (O), and Orlistat/Prebiotic (OP). They consumed a fat-standardized diet (60 g/day) during Week-1 (baseline) and after 1 week of washout, Week-3. During Week-3, they also received their respective treatment (Orlistat: 2 × 120 mg/day, OF: 16 g/day, and maltodextrin as placebo). A 72-h stool collection was carried out at the end of Week-1 (T0) and Week-3 (T1). Fecal fat, calprotectin, and short-chain fatty acids (SCFAs) as well as the antioxidant activity of fecal waters (ferric-reducing antioxidant power), fecal microbiota composition (by deep sequencing), and gut permeability (Sucralose/Lactulose/Mannitol test) were determined at these times.
RESULTS:
Fecal fat excretion was higher in the O (P=0.0050) and OP (P=0.0069) groups. This event was accompanied, in the O group, by an increased calprotectin content (P=0.047) and a decreased fecal antioxidant activity (P=0.047). However, these alterations did not alter gut barrier function and the changes observed in the composition of the fecal microbiota only affected bacterial populations with low relative abundance (<0.01%); in consequences, fecal SCFA remained mainly unchanged. Part of the colonic alterations induced by orlistat were prevented by OF administration.
CONCLUSIONS:
In the context of an equilibrated diet, the acute exposition of the colonic ecosystem to high amounts of dietary lipids is associated with an incremented excretion of fecal calprotectin and pro-oxidant activity of the colonic content, in the absence of significant changes in the microbiota.
INTRODUCTION
Dietary triglycerides account for approximately 30–35% of the total energy intake in humans. They represent >95% of the dietary lipids consumed daily and are efficiently digested, mainly by the gastric and pancreatic lipase, before being absorbed as free fatty acids by the small intestinal mucosa. It is estimated that only 4–5 g of fat reach the colon daily and that 2–5 g are excreted in stools. Fecal losses >5% of the dietary fat are generally considered as steatorrhea and suggestive of malabsorption.1 The fate of dietary lipids in the colon is poorly understood and the identity of the bacterial populations eventually involved in their metabolism is unclear. Saturated fatty acids may form insoluble soaps with calcium or magnesium in the gut lumen while unsaturated fatty acids may be hydrogenated or oxidized, generating by-products with cathartic and/or pro-oxidant effects.2, 3
Higher input of dietary fat into the colon occurs in patients treated with orlistat (tetrahydrolipstatin), an inhibitor of the pancreatic lipase that is widely used in the management of obesity and dyslipidemia.4 Its administration decreases by approximately 30% the intraluminal hydrolysis of triglycerides, favoring their subsequent excretion in stools. The use of orlistat is frequently associated with side effects, such as bloating, loose stools or diarrhea, steatorrhea, oily stool feeling and/or fecal seeping. Increased fecal fat excretion is detected 24–48 h after orlistat administration and usually returns to baseline levels within 48–72 h after its administration is interrupted.5
Various studies have reported an association between obesity and alterations of the intestinal microbiota in both humans and animals. The main finding initially described was the increase of the Firmicutes/Bacteroidetes ratio.6 However, subsequent studies produced conflicting results and the bacterial populations implicated in this dysbiosis and potentially in the development of obesity remain unclear.7 Hildebrandt et al.8 have suggested that the fat content of the diet, more than the obesity per se, was responsible for the dysbiosis. On the other hand, other authors have proposed bacterial lipopolysaccharide (LPS) as a crucial factor linking high-fat diet consumption with gut microbiota alterations and the subsequent development of low-grade inflammation, insulin resistance, type-2 diabetes and, in some cases, hepatic steatosis.9, 10, 11 High-fat diets have been reported to decrease Gram-positive bacteria and increase the concentrations of LPS in the gut lumen. This latter event was associated with impairment of the gastrointestinal barrier function and the subsequent development of metabolic endotoxemia.9, 10, 11 However, a limitation for the interpretation of these results and their extrapolation to humans are the supra-physiological amounts of fat used in the diets fed to the animals (about 70% of the caloric intake). These amounts of fat are much higher than those provided by the human diet, even in obese subjects; in addition, in these animal studies, the lipids are incorporated to the diet at the expense of the carbohydrates, including the non-digestible polysaccharides/oligosaccharides. In consequence, it is unclear whether the dysbiosis associated with high-fat diets is due to the higher intake of fat or to the lower intake of carbohydrates.
Based on these antecedents, we proposed to use normal-weight, healthy, young subjects consuming a diet containing a standardized, equilibrated fat content and treated with orlistat to determine the impact of a higher amount of dietary fat reaching the colon on the gut barrier function, local and systemic inflammation, and bacterial populations of the colonic microbiota. As a number of studies carried out in animal models and in humans have reported that prebiotic administration improves gut barrier alterations and inflammation and modulates gut microbiota composition,10, 11, 12 we also determined whether dietary supplementation with the prebiotic oligofructose (OF) may interfere with the eventual deleterious effects of dietary fat at the colonic level.
METHODS
Ethics
The study protocol was approved by the Ethics Committee for Research in Humans of the Institute of Nutrition and Food Technology, University of Chile, Santiago, Chile in compliance with the Helsinki Declaration. All subjects were carefully informed about the aims and procedures of the study and those who agreed to participate and met the inclusion and exclusion criteria signed a written informed consent form.
Inclusion and exclusion criteria
Forty-one asymptomatic volunteers of either sex, aged 18–40 years, with a body mass index between 18.5 and 24.9 kg/m2, and non-smokers were recruited for this study. Anthropometric data and biochemical and lipid profiles, as well as the plasma inflammatory markers, interleukin-6 (IL-6), and high-sensitivity C-reactive protein (hsCRP), were determined before initiating the study. Subjects with antecedents of acute or chronic gastrointestinal diseases or with previous gastrointestinal surgery (appendectomy excepted) as well as those with autoimmune or chronic metabolic diseases, dietary treatment for weight loss, and pregnant women were excluded. Subjects treated with drugs that could interfere with the gut microbiota, intestinal permeability, or motility (antibiotics, anti-inflammatory drugs, laxatives, prokinetics) in the past 45 days before the study were also excluded. Subjects were requested to avoid consuming prebiotics and probiotics during 3 weeks previous to the study and during the study.
Experimental design
On admission, subjects were randomly assigned to four groups by using a per block random permutation table: Control (C), Orlistat (O), Prebiotic (P), and Orlistat/Prebiotic (OP). Every day for 1 week, subjects of the O group received 2 × 120 mg Orlistat capsules (Xenical, Roche, Santiago, Chile) and 16 g of placebo, those from the P group 2 × 120 mg of placebo and 16 g of OF (Raftilin, Orafti Chile, Santiago, Chile), those from the OP group 2 × 120 mg of Orlistat and 16 g of OF, and those from the C group 2 × 120 mg and 16 g of placebo. Maltodextrin was used as placebo for Orlistat as well as for OF.
The study began with a run-in basal period of 1 week (days 1–7) during which the volunteers were counseled by two registered dietitians to standardize their daily intake of dietary fat to about 60 g. With this aim, a list of foodstuffs with their corresponding fat content per serving was provided to the volunteers. At the end of this basal period (T0), a 72-h total stool collection was carried out to determine the fecal fat excretion at baseline. The subjects were allowed to return to their usual diet and for the following 7 days (days 8–14). During the third week (days 15–21), the volunteers had to consume the diet with the standardized fat content and, in addition, their corresponding treatment. A second 72-h total stool collection was carried out at the end of this period (T1).
During the study, the volunteers had to register daily the eventual presence of the digestive symptoms (abdominal pain and/or distension, vomiting/regurgitation, increased borborygmi, increased rectal gas, effort/pain during defecation, fecal emergency, fecal incontinence, evacuation of fatty/oily stools, increased stool emission) and distractors, and their respective intensity (absent (0); low (1); mild (2); high (3)). They also had to register their stool frequency and consistency using an ad-hoc form and the seven-point Bristol stool scale. For statistical analysis, the sum of the digestive symptoms was calculated considering their respective intensity for each subject.
Samples
Blood samples were obtained at T0 and T1 from the subjects while fasted to determine their lipid profile, glycemia, hsCRP, and IL-6. At the same time, a fresh stool was obtained to characterize the microbiota composition and to quantify the concentrations of short-chain fatty acids (SCFAs) and calprotectin. Fecal waters were obtained to determine their antioxidant capacity by FRAP (ferric-reducing antioxidant power). Fecal fat excretion was determined in the 72-h stool collection using the Van de Kamer method13 based on the hydrolysis of the fecal triglycerides in acidic conditions and the titration of the released free fatty acids.
Characterization of the fecal microbiota
Bacterial genomic DNA was extracted from the fecal samples with the QIAmp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Libraries and sequencing were carried out by the Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign, Champaign, IL. The V3–V4 region of the 16S rRNA gene was amplified with the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 785R (GACTACHVGGGTATCTAATCC-3′) using the Fluidigm system (Fluidigm, South San Francisco, CA).14 Amplicons were sequenced on a MiSeq Illumina platform (Illumina, San Diego, CA), generating paired end reads (2 × 300 nt). The paired reads were merged using PandaSeq,15 with a threshold of 0.9 and an estimated fragment size of 460 nucleotides. Chimeric sequences were removed using Vsearch (https://github.com/torognes/vsearch) against the Greengenes 13_8 database.16 Reads were clustered into operational taxonomic units (OTUs) using the open-reference protocol implemented in Qiime17 using the Greengenes 13.8 database as reference.
Quantification of specific fecal bacterial populations
DNAs were amplified by real-time PCR (quantitative PCR) using the Light Cycler Fast Start DNA Master SYBR Green I Kit and a Light Cycler (Roche Diagnostics, Mannheim, Germany). The primers used for the detection of the specific bacterial populations (Bifidobacterium spp. and Lactobacillus spp.) are described in Supplementary Table S1.18, 19 The log of the number of copies/g of stool of the 16S RNA gene was calculated for each bacterial group using the appropriate calibration curve.
Quantification of fecal SCFAs
The analysis was performed as previously described by Zhao et al.20 with some modifications. One gram of stool was homogenized with 5 ml of distilled water for 3 min. The pH was adjusted to 2–3 with 5 m HCl, and the samples were left at room temperature for 10 min, with occasional stirring. The suspension was centrifuged for 20 min at 5000 r.p.m., and the supernatant was removed. Ethyl butyric acid-2 was added as an internal standard at a final concentration of 1 mm. The detection and quantification of the SCFAs were performed in a gas chromatograph (Agilent Technologies, Santa Clara, CA) equipped with a FID detector and a Stabilwax capillary column (Restek, Bellefonte, PA). The separation of the SCFAs was carried out in a temperature range of 100–200 °C with an initial heating at 100 °C for 0.5 min and a rise to 180 °C at 8 °C min and held at 200 °C for 5 min. Nitrogen was used as a gas carrier and the temperature of the injector was 200 °C. For calibration, aqueous solutions of acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids (Restek) were used.
Antioxidant activity of fecal water
Fecal waters were obtained from stool samples as described by Klinder et al.21 with some modifications. Fecal samples were mixed with cold phosphate-buffered saline in a 1:1 (w/v) proportion, homogenized three times for 3 min and centrifuged at 35,000 g for 2 h at 4 °C in a refrigerated Neofuge 23 R (Bio-meditech Health Force, Heal Force Bio-meditech, Shanghai, China) centrifuge. Supernatants were removed and a second centrifugation was performed at 13,000 r.p.m. for 30 min at 4 °C to obtain a clear solution. The supernatants were aliquoted and stored at −20 °C until analysis. The antioxidant capacity of the fecal waters was determined by the FRAP assay as described by Benzie and Strain by measuring the changes in the absorbance at 593 nm in a multimode microplate reader (Synergy HT, BioTek, Winooski, VT) after 30 min of incubation.22 FRAP values were determined using a standard curve made with ferrous sulfate, and results were expressed as μmol of Fe++/g of feces.
Assessment of the intestinal barrier function
The evaluation of the intestinal barrier function was carried out as previously described in a subgroup of six subjects from a group, using lactulose/mannitol and sucralose as markers of the intestinal and colonic permeability, respectively.23 Overnight fasted subjects ingested 300 ml of a solution containing 2 g of mannitol, 7 g of lactulose, and 2 g of sucralose, and the urine voided was collected for 5 h. Urinary sugar concentrations were determined by gas chromatography using a Varian 3600 chromatograph equipped with a split/splitless injector and a flame ionization detector (Varian Instruments, San Fernando, CA), using cellobiose and α-methyl-glucose (Sigma Chemical, St Louis, MO) as internal standards. Results were expressed in mg of each sugar and as lactulose/mannitol and lactulose/sucralose ratios as markers of proximal and distal gut permeability, respectively.
Quantification of fecal calprotectin and plasma IL-6
The concentrations of fecal calprotectin and plasma IL-6 were determined by enzyme-linked immunosorbent assay (ELISA) using the PhiCal Calprotectin Elisa Kit (Immundiagnostik, Bensheim, Germany) and the Human IL-6 ELISA Kit (Thermo Scientific, Waltham, MA), respectively, according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed by using the Statistica software package version 11 (StatSoft, Tulsa, OK). As most data were not normally distributed, statistical analysis was carried out by using non-parametric tests. Comparisons between treatments at T0 and at T1 were made by using Kruskal–Wallis analysis of variance. When necessary, the Wilcoxon signed-rank test was used to assess differences between T1 and T0 within each treatment groups and the Mann–Whitney test to compare groups between different treatments. Variables were expressed as median with interquartile range. Statistical analysis of the microbiota composition and correction for multiple hypothesis testing was performed in R statistical software, including the use of the Phyloseq and ggplot2 packages. Statistical significance was considered at P<0.05.
RESULTS
Forty-one healthy volunteers were recruited and all of them completed the study. The daily intake of energy and of macronutrients (including saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids) during the study is shown in Supplementary Table S2; no differences between groups were detected for these variables. The anthropometric, nutritional, and biochemical characteristics of the subjects from each group at T0 and T1 are described in Table 1. No significant differences were detected for these parameters between groups at T0 nor between T1 and T0. Some changes were observed in the biomarkers of inflammation, hsCRP, and IL-6 between T1 and T0, but they were not significant when the four groups were compared.
Table 1. Characteristics of the volunteers participating in the study (median (IQR)).
|
Control (n=10) |
Orlistat (n=10) |
Orlistat–Prebiotic (n=10) |
Prebiotic (n=11) |
P |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |
| % Female | 50 | 50 | 50 | 45 | 0.99 (X2) | |||||
| Age (years) | 24.0 (20.0–25.0) | 26.5 (21.0–28.0) | 25.5 (23.0–27.0) | 24.0 (23.0–30.3) | 0.39 | |||||
| Height (m) | 1.65 (1.57–1.75) | 1.67 (1.57–1.71) | 1.68 (1.61–1.74) | 1.65 (1.61–1.71) | 0.82 | |||||
| Weight (kg) | 60.5 (55.4–68.2) | 59.7 (54.6–69.1) | 63.3 (56.8–69.2) | 63.6 (57.4–68.4) | 63.7 (57.2–67.5) | 63.5 (57.5–67.7) | 61.8 (60.0–67.5) | 61.2 (60.8–67.9) | 0.97 | 0.95 |
| BMI (kg/m2) | 22.2 (20.4–24.1) | 22.0 (20.3–24.4) | 23.0 (22.2–23.9) | 23.1 (22.5–23.7) | 22.2 (21.3–22.8) | 22.1 (21.2–22.5) | 23.1 (21.4–24.4) | 23.2 (21.4–24.0) | 0.40 | 0.50 |
| Glycemia (mg/dl) | 91.7 (77.9–95.2) | 85.6 (80.1–90.3) | 89.4 (83.2–94.8) | 90.7 (82.2–95.5) | 91.7 (85.6–94.7) | 91.8 (84.4–97.4) | 90.8 (82.0–94.0) | 89.2 (81.3–94.5) | 0.91 | 0.86 |
| Total cholesterol (mg/dl) | 144.5 (139.1–185.3) | 148.0 (128.9–189.6) | 163.6 (132.0–188.5) | 159.6 (140.3–183.0) | 161.5 (154.0–173.9) | 155.4 (146.5–165.1) | 164.3 (156.0–179.0) | 169.0 (152.3–182.0) | 0.87 | 0.59 |
| HDL (mg/dl) | 53.2 (41.4–62.7) | 48.5 (42.1–60.3) | 56.1 (54.1–57.4) | 51.8 (47.6–58.2) | 50.5 (46.7–64.2) | 49.1 (44.3–60.0) | 46.3 (43.5–55.9) | 51.9 (43.0–58.0) | 0.41 | 0.94 |
| LDL (mg/dl) | 82.6 (75.0–99.3) | 88.7 (61.4–102.3) | 90.6 (61.0–112.1) | 85.0 (67.6–103.1) | 88.1 (76.5–110.8) | 86.7 (74.4–96.9) | 99.0 (81.8–110.7) | 103.2 (79.3–114.8) | 0.66 | 0.55 |
| Triglycerides (mg/dl) | 94.0 (73.0–134.6) | 87.8 (71.5–102.6) | 92.0 (68.3–124.8) | 90.5 (60.2–142.9) | 94.3 (82.1–107.3) | 94.8 (55.3–120.9) | 103.4 (88.2–107.5) | 69.2 (59.9–132.3) | 0.97 | 0.97 |
| hsCRP (mg/l) | 0.41 (0.31–0.55) | 0.33 (0.16–0.77) | 1.05 (0.37–2.20) | 0.73 (0.14–2.24) | 1.40 (0.23–2.63) | 0.44 (0.04–1.03)a | 0.54 (0.23–1.69) | 0.60 (0.13–2.12) | 0.50 | 0.83 |
| IL-6 (pg /ml) | 8.42 (6.25–8.65) | 7.38 (5.17–7.67)a | 8.50 (7.50–9.70) | 7.75 (6.00–9.00)a | 8.58 (6.67–9.83) | 7.58 (6.67–8.17) | 7.83 (6.28–8.76) | 5.50 (4.22–7.71)a | 0.40 | 0.16 |
BMI, body mass index; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; IL, interleukin; LDL, low-density lipoprotein. The last column (P) shows the level of significance for the comparisons of the four groups by Kruskal–Wallis analysis of variance at T0 and T1 for all the parameters (except for gender, age, and height that were compared only at T0). No differences were observed between groups at T0 and T1.
Significantly different between T1 and T0 for the same group (P<0.05).
The volunteers registered the digestive symptoms daily during the study. As shown in Supplementary Table S3, their intensity did not differ between the groups at baseline. Digestive symptoms increased significantly during the treatment period in the O, OP, and P groups (P=0.046, 0.004, and 0.004, respectively) but without differences between them.
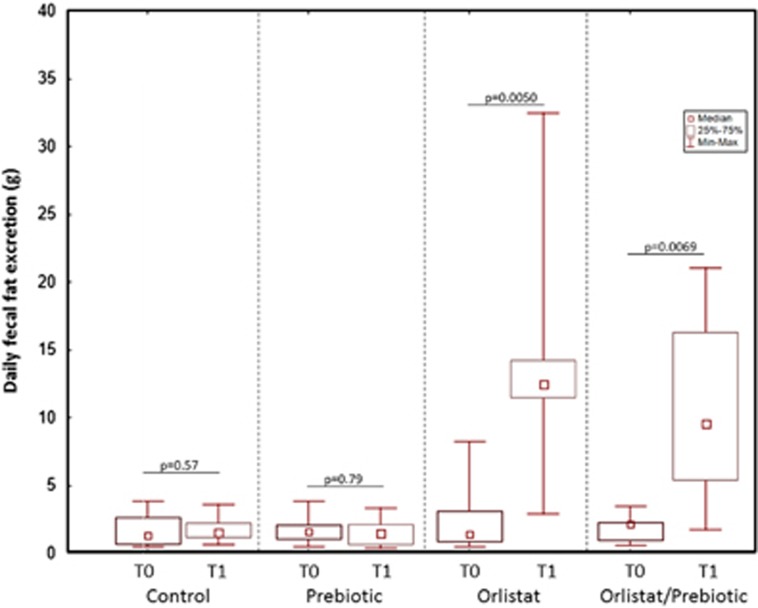
The wet and dry weights of stool excreted in 24 h were comparable in the four groups at T0, and these parameters were not significantly affected by the treatments (data not shown). As shown in Figure 1, at T0, the daily fecal fat excretion determined by the Van de Kamer method was not different between groups (Kruskal–Wallis analysis of variance (ANOVA), P=0.83). However, the fecal fat excretion was strongly affected by the treatments (P<0.0000); it remained unchanged in the C and P groups while it increased by 8.5 and 6.2 times in the O and OP groups (P=0.00078 and P=0.0067, respectively, compared with the C group and P=0.00025 and P=0.0026, respectively, compared with the P group). No difference in the increases of fecal fat excretion was observed between the O and OP groups.
Figure 1.
Changes in daily fecal fat excretion at T0 and T1 in the four treatment groups. Fecal fat was determined by the van de Kamer method. The daily excretion of fecal fat was similar in the four groups at T0 (Kruskal–Wallis analysis of variance, P=0.83). After treatment, this parameter remained unchanged in the control and prebiotic groups, whereas it significantly increased in the Orlistat and Orlistat/Prebiotic groups (Wilcoxon paired test, P=0.005 and P=0.0069). (median, interquartile range, and range).
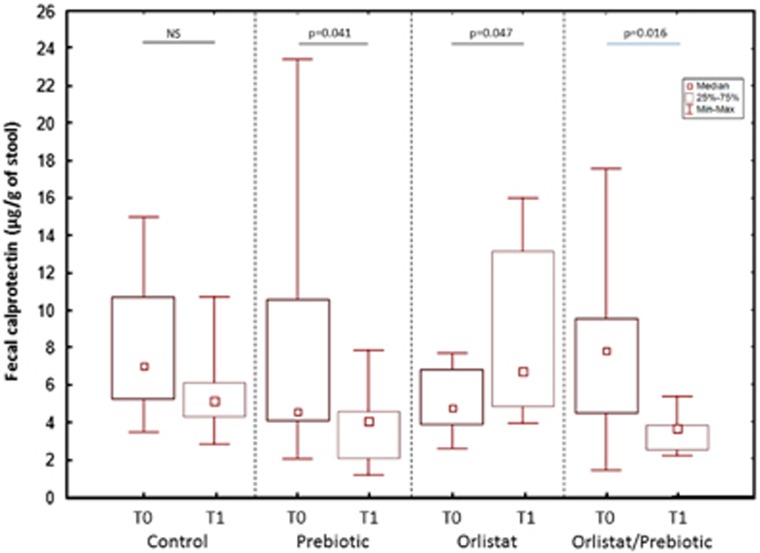
The eventual impact of the increased passage of dietary fat through the colon on the local inflammation was evaluated by determining the fecal concentrations of calprotectin. As shown in Figure 2, these concentrations did not differ between groups at T0 (Kruskal–Wallis ANOVA P=0.21), but that they were significantly affected by the treatments at T1 (Kruskal–Wallis ANOVA, P=0.0012). Fecal calprotectin levels did not change between T1 and T0 in the C group while they significantly decreased in the P group (P=0.041) and increased in the O group (P=0.047). The increase of fecal calprotectin induced by orlistat was prevented by the concomitant administration of prebiotic so that a significant decrease of this parameter was even observed in the OP group at T1 (P=0.016). In consequence, fecal calprotectin was significantly higher at T1 in the O group than in the P and OP groups (P=0.011 and 0.006, respectively). No correlations were observed between fecal calprotectin and fecal fat excretion (P=0.21).
Figure 2.
Changes in fecal calprotectin excretion at T0 and T1 in the four treatment groups. Fecal calprotectin concentrations were similar in the four groups at T0 (Kruskal–Wallis analysis of variance, P=0.21). No change in this parameter was observed at T1 in the Control group while it significantly decreased in the Prebiotic group and increased in the Orlistat group (Wilcoxon paired test). The administration of oligofructose prevented the increase of fecal calprotectin induced by orlistat. (median, interquartile range, and range).
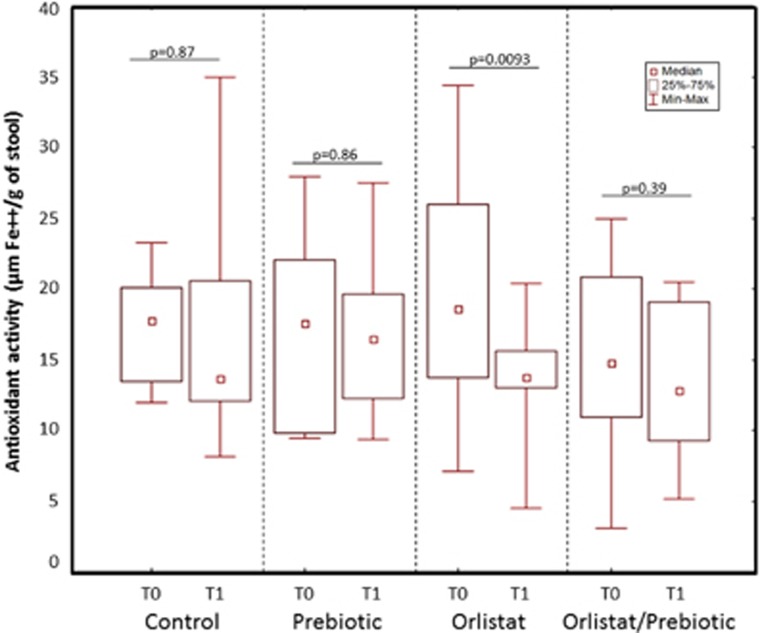
As fat oxidation in the colon may result in the generation of pro-oxidant compounds, in addition to lipid peroxidation products, the antioxidant activity of the fecal water of the volunteers from the four groups was evaluated through the FRAP method. As reported in Figure 3, no differences between groups were detected in the fecal antioxidant activity at T0 (Kruskal–Wallis ANOVA, P=0.93). This parameter remained unchanged at T1 in both the C and P groups but it significantly decreased after orlistat administration. Such decrease was prevented in the OP group.
Figure 3.
Changes in the antioxidant activity (ferric-reducing antioxidant power (FRAP) values) of fecal waters at T0 and T1 in the four treatment groups. No differences of fecal antioxidant activity were observed between groups at T0 (Kruskal–Wallis analysis of variance, P=0.93). Fecal FRAP values remained unchanged at T1 in both the C and P groups, but they significantly decreased after orlistat administration (Wilcoxon paired test). Such decrease was prevented in the OP group. (median, interquartile range, and range).
The evaluation of gut barrier function was evaluated by using the lactulose/mannitol/sucralose test in a subgroup of subjects (six in each group). As shown in Supplementary Table S4, the urinary excretions of lactulose, mannitol, and sucralose were similar in the four groups at T0 and remained unchanged after the treatment period. When the results were expressed as lactulose/mannitol or lactulose/sucralose ratios, as a reflection of the permeability of the proximal and distal gut, respectively, no changes between T1 and T0 or between groups were observed either.
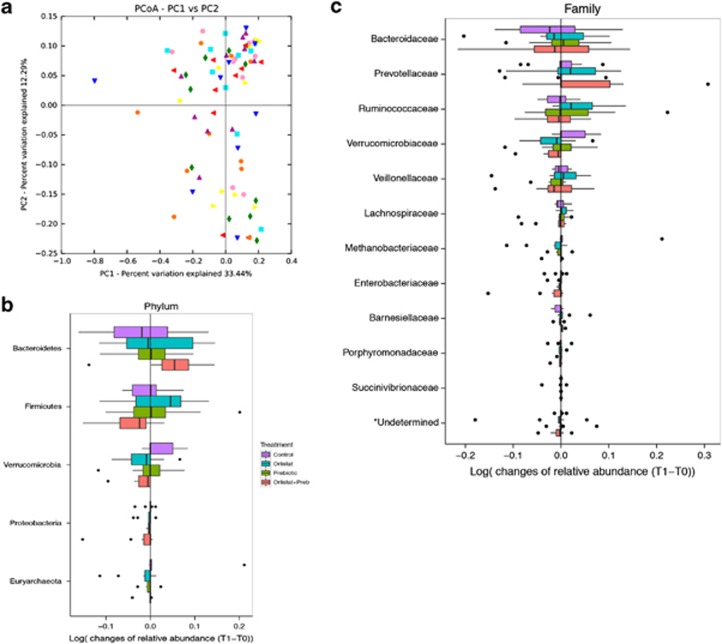
To determine whether the composition of the fecal microbiota was affected by the higher amounts of dietary fat reaching the colon after orlistat administration, we sequenced the V3–V4 region of the 16S rRNA gene. A total of 5,717,914 reads were obtained after trimming, assembly, quality filtering, and chimera checking, with an average of 70,045±11,983 sequences per sample (ranging from 42,308 to 115,406). One of the samples (from control Group, T1) only exhibited 32 sequences and was excluded from the study. Sequences were grouped into OTUs based on a sequence identity of 97%. OTUs with at least 10 sequences were considered for further analysis, resulting in a total of 4,632 OTUs that could be classified into 13 phyla and 64 families. The rarefaction curves tended to the saturation plateau for each group (Supplementary Figure S1). Alpha diversity was not significantly affected by the treatments (Shannon diversity index: Control: 3.67±0.58; Orlistat: 3.71±0.31; Prebiotic: 3.54±0.37; Orlistat–Prebiotic: 3.78±0.36). The analysis of beta-diversity (weighted Unifrac) showed that samples at T1 did not cluster according to treatments; high interindividual variations were detected (Figure 4a). The effect of treatments on the dominant microbial phyla and families (with a relative abundance >0.5%) is depicted in Figures 4b and c. For each treatment, some significant changes in the relative abundance of some phyla and families were detected between T1 and T0 (Supplementary Table S5). However, after correction for multiple comparisons using the Benjamini–Hochberg procedure for controlling false discovery rate, these differences did not remain significant. The specific effect of the OF administration on Lactobacillus and Bifidobacterium populations was also determined by quantitative PCR (Table 2). The fecal counts of Bifidobacterium spp. were similar in the four groups at T0. However, the by-group analysis indicates a significant (P=0.028) and a non-significant (P=0.07) increase of Bifidobacterium at T1 in the P and OP groups, respectively, without changes in the Control and Orlistat groups. The counts of Lactobacillus spp. were similar between groups at T0 and T1 and remained unchanged after treatments.
Figure 4.
Analysis of the fecal microbiota by deep sequencing of the v3–v4 region of the 16S rRNA gene. (a) Analysis of beta-diversity (weighted Unifrac) showing that samples did not cluster according to treatments; high interindividual variations were detected. (b and c) Effect of the different treatments on the dominant phyla and families (>0.5% of relative abundance).
Table 2. Effect of treatments on Bifidobacterium and Lactobacillus populations of the fecal microbiota of the volunteers (median (IQR)).
| Bacterial concentrations (log 16S rRNA gene copies/g) |
Control (n=10) |
Orlistat (n=10) |
Orlistat–Prebiotic (n=10) |
Prebiotic (n=11) |
P (KW) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |
| Lactobacillus spp. | 6.18 (5.96–7.34) | 6.01 (5.83–6.20) | 6.47 (6.01–6.84) | 6.00 (5.76–6.55) | 6.23 (6.06–6.68) | 6.03 (5.87–6.30) | 6.33 (6.08–6.86) | 6.08 (5.71–6.64) | 0.90 | 0.65 |
| Bifidobacterium spp. | 8.39 (8.08–8.88) | 7.97 (7.44–9.02) | 8.00 (7.61–8.30) | 8.02 (6.81–8.52) | 7.87 (7.38–8.31) | 8.35a (7.76–8.92) | 8.07 (7.47–8.25) | 8.61 (7.88–9.01) | 0.12 | 0.54 |
IQR, interquartile range; KW, Kruskal–Wallis. The last column (P) shows the level of significance for the comparisons of the four groups by Kruskal–Wallis analysis of variance at T0 and T1 for both parameters. No differences were observed between groups at T0 and T1.
A significant increase of Bifidobacterium spp. was observed at T1 in the OP group (P=0.028). A similar tendency was observed for the P group (P=0.07).
Results corresponding to the fecal concentrations of SCFAs are described in Table 3. Neither differences between T1 and T0 nor between groups were detected whether for each of them or for the total of SCFAs. However, a significant decrease of the ramified SCFAs, isobutyrate and isovalerate, was observed in the OP group.
Table 3. Effect of the treatments on the fecal concentrations of short-chain fatty acids (median (IQR)).
|
Control (n=10) |
Orlistat (n=10) |
Orlistat–Prebiotic (n=10) |
Prebiotic (n=11) |
P (KW) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |
| Acetate | 36.1 (24.6–38.1) | 33.3 (21.6–38.1) | 29.7 (23.3–35.6) | 28.8 (18.3–40.2) | 29.5 (21.5–42.6) | 44.7 (24.8–54.0) | 37.8 (24.3–40.4) | 32.1 (24.6–48.2) | 0.90 | 0.46 |
| Propionate | 11.2 (8.6–16.4) | 10.2 (7.0–21.4) | 12.0 (7.6–13.5) | 9.9 (5.9–14.6) | 9.3 (7.5–11.1) | 9.0 (5.4–11.6) | 9.9 (6.1–14.4) | 8.5 (7.7–14.7) | 0.62 | 0.74 |
| Butyrate | 9.1 (5.9–12.1) | 9.5 (6.3–14.8) | 9.6 (6.4–14.7) | 10.6 (5.8–15.0) | 8.2 (5.2–10.6) | 11,8 (7.2–21.0) | 11.1 (6.5–15.2) | 8.0 (5.5–10.6) | 0.68 | 0.40 |
| Valerate | 1.6 (1.3–1.9) | 1.2 (0.8–1.5) | 1.9 (1.2–2.7) | 1.6 (1.0–2.5) | 1.2 (1.1–1.6) | 1.1 (0.9–1.4) | 1.5 (1.0–2.0) | 1.3 (0.8–1.6) | 0.52 | 0.35 |
| Isobutyrate | 1.7 (0.9–1.8) | 1.1 (0.8–1.7) | 1.7 (1.1–2.2) | 1.4 (0.9–2.0) | 1.4 (0.8–1.7) | 1.0a (0.7–1.3) | 1.4 (0.8–1.7) | 1.1 (0.9–1.7) | 0.67 | 0.33 |
| Isovalerate | 2.3 (1.4–2.5) | 1.6 (1.2–2.5) | 2.6 (1.7–3.4) | 1.9 (1.6–3.2) | 2.3 (1.2–2.9) | 1.5a (0.9–1.8) | 2.3 (1.2–2.7) | 1.8 (1.2–2.8) | 0.92 | 0.29 |
| Total SCFAs | 67.0 (45.4–77.1) | 70.1 (38.6–84.4) | 60.3 (40.4–80.6) | 51.7 (36.1–83.1) | 51.3 (39.0–71.9) | 73.1 (42.6–91.4) | 62.5 (41.0–74.2) | 53.4 (40.3–79.1) | 0.87 | 0.83 |
IQR, interquartile range; KW, Kruskal–Wallis; SCFA, short-chain fatty acid. The last column (P) shows the level of significance for the comparisons of the four groups by Kruskal–Wallis analysis of variance at T0 and T1 for each parameter. No differences were observed between groups at T0 and T1.
A significant decrease of the fecal concentrations of isobutyrate and isovalerate between T1 and T0 was observed in the OP group (P=0.027 and P=0.022, respectively).
DISCUSSION
The aim of the present study was to assess the way in which increased amounts of dietary fat reaching the colon may alter the colonic ecosystem, including the local microbiota, in healthy, normal-weight volunteers. This was achieved through the administration of orlistat, i.e., without the need to modify the proportion of lipids in the diet at the expense of carbohydrates or fiber.
As expected, we observed that daily intake of orlistat significantly increases fecal fat excretion, compared with the subjects from the C and P groups; this phenomenon was accompanied by adverse effects similar to those reported in other studies.5 Orlistat partially inhibits (by about 30%) triglyceride hydrolysis in the intestinal lumen and the non-absorbed fat finally reaches the colon. Considering that our subjects consumed daily a standardized diet with about 60 g fat, it may be estimated that about 20 g of this fat reached the colon of the orlistat-treated volunteers. To obtain this same amount in the context of a high-fat diet and considering a fat digestion efficiency of 90%, subjects would have to consume about 200 g of dietary fat/day, a high amount but that is compatible with that consumed by obese subjects in some cases.24
In a first time, we evaluated whether the more elevated amount of fat reaching the colon was associated with increased inflammatory processes. We used fecal calprotectin, a biomarker of colonic inflammation currently used in the screening and follow-up of patients with inflammatory bowel diseases25 and that is also associated with physiological changes in the aged gastrointestinal tract and with physical inactivity and obesity.26, 27 In a previous study, we did not detect any changes of this parameter in young “asymptomatic” obese subjects.28 However, our current results show, for the first time, that fecal calprotectin significantly increased after orlistat administration and that, accordingly, fat malabsorption might be associated with increased inflammatory processes in the colonic mucosa. It must be stated, however, that such increase was not accompanied by higher levels of the circulating inflammatory markers, hsCRP and IL-6. Interestingly, the addition of the prebiotic OF prevented the increase of fecal calprotectin induced by orlistat, confirming the observations of Vulevic et al.27 who reported that the administration of transgalacto-oligosaccharides decreased fecal calprotectin in patients with metabolic syndrome.
An explanation for the higher level of colonic inflammation in our subjects is the eventual increase of the pro-oxidant capacity of the intracolonic content resulting from dietary fat oxidation. Accordingly, we determined the antioxidant capacity of the fecal water of our subjects. Our results show a significant decrease of FRAP values between T1 and T0 only in the orlistat-treated subjects, suggesting that the antioxidant capacity of the fecal waters decreased (i.e., its pro-oxidant capacity increased) with fat malabsorption. In agreement with these results, Qiao et al.29 observed that mice fed a high-fat diet had higher contents of reactive oxygen species and malonedialdehyde adducts and exhibited decreased total antioxidant capacity in their colon. Such changes were associated with increased counts of colonic Escherichia coli and Enterococcus and decreased counts of colonic Lactobacillus. High-fat diets have also been shown to increase free radical formation in the human feces30 and to alter the integrity of the intestinal barrier, favoring the entry of LPS into the circulation and the development of metabolic endotoxemia.9, 10, 11, 31
As the increase of inflammation and the decrease of antioxidant capacity have been associated with disturbances of the gut barrier function, we determined the intestinal and colonic permeability to lactulose, mannitol, and sucralose in a subgroup of our subjects. No alterations of the urinary excretion of these biomarkers were detected in our study, suggesting that the increased presence of dietary fat in the colon does not affect the intestinal barrier function. However, it is possible that the duration of the administration of orlistat was not sufficient to induce such alterations. In fact, in most of the animal models of obesity induced by high-fat diet, defects of gut permeability are detected after >1 month of diet consumption.11 However, it is noteworthy that in some genetic models of obesity (ob/ob mice), no alterations of gut barrier function were observed32 and that in obese humans, results are contradictory.28, 33, 34
Another factor possibly implicated in the changes in inflammatory and oxidative status of the colonic ecosystem is the gut microbiota. Some bacterial populations express lipase activities capable of hydrolyzing the dietary triglycerides reaching the colon.35 In our study, the increasing dietary fat reaching the colon after orlistat treatment only provoked minor, not significant, changes in subdominant bacterial populations (with relative abundance <0.01%), suggesting that it is not the fat, per se, which modifies the microbiota composition. In consequence, the shift in the diversity of dominant gut bacterial populations reported after long-term administration of high-fat diets could be due, eventually, to the decreased intake of carbohydrate and dietary fibers.36, 37 It might be argued that in our study, orlistat administration was too short to affect the composition of the microbiota. Nevertheless, our results are supported by the observations of David et al.38 who recently reported significant changes in the composition of the gut microbiota only 1 day after initiating a diet entirely based on animal products (i.e., rich in fat and proteins and poor in dietary fibers). One of the major forces shaping the gut microbiota is the availability of substrates, especially complex dietary polysaccharides.39 In this context, the dominant gut metagenome and proteome is mainly involved in energy production from carbohydrate metabolism even in high-fat diets while genes associated with lipid metabolism are mainly involved in biosynthetic or bioconversion reactions.40 As discussed by other authors, the effect of high-fat diets could also be explained by indirect mechanisms such as the increased secretion of bile acids that they triggered and the increased abundance of bile-tolerant bacteria.38, 41 For example, in mice fed a high-fat diet, the fecal excretion of ursodeoxycholic acid decreased, whereas that of deoxycholic acid increased,42 this bile acid being involved in the disruption of epithelial integrity and in the modification of gut microbiota composition through its strong antibacterial properties.42, 43 On the contrary, the administration of orlistat has been associated with decreased fecal excretion of bile salts,44 meaning a lower potential of modulating the microbiota composition.
The administration of high-fat diets have been reported to decrease Bifidobacterium spp. in mice while increasing LPS-bearing gram-negative bacteria, and the use of probiotics and prebiotics has been proposed as a tool to re-establish the homeostasis of the colonic ecosystem.9, 10, 11, 12, 45, 46 In our study, Bifidobacterium spp. were not affected by orlistat administration but significantly increased in the P and OP groups, confirming the well-known prebiotic effect of OF on this microorganism.46, 47 Bifidobacteria may exert anti-inflammatory and antioxidant activities, which may contribute to prevent the increase of fecal calprotectin and the decrease of antioxidant capacity induced by orlistat, similar to that in our subjects.48, 49, 50
We also evaluated the fecal content of SCFA, as the proportion and concentrations of these bacterial metabolites are known to be strongly influenced by diet.51 However, no significant changes in fecal SCFA were detected in any of the groups, probably reflecting the fact that only small changes were observed in the bacterial populations. In contrast with our results, Brinkworth et al.52 reported decreased fecal butyrate and total SCFAs after the administration of a very low-carbohydrate, high-fat diet, compared with a high-carbohydrate, high-fibre, low-fat diet and Fava et al.53 described higher fecal SCFAs with a diet high in saturated fat in subjects at risk for metabolic syndrome.
In conclusion, increasing the amounts of dietary fat passing through the colon by using orlistat administration results in higher excretion of fecal calprotectin, increased oxidant activity of the fecal water, and small changes in some bacterial populations, without alterations of gut barrier function and fecal SCFA contents. These alterations tend to be prevented by the concomitant administration of the prebiotic OF. The short period of exposition to orlistat must be considered a limitation of this study because it probably does not provide enough time for more intense alterations, particularly on the barrier function. In the context of the regular consumption of high-fat diets, these results suggest that high amounts of dietary lipids could affect the colonic ecosystem and function, with eventual development of inflammatory, oxidative, and metabolic consequences at the systemic level.
Study Highlights
Acknowledgments
We thank the volunteers who participated in the study and Dr Oscar Brunser for revising the manuscript.
Guarantor of the article: Martin Gotteland, PhD.
Specific author contributions: Martin Gotteland and Pamela Morales carried out the experimental design of the study and the statistical analysis of the results and their interpretation. In addition, Pamela Morales was in charge of the recruitment and follow-up of the volunteers, the collection and analysis of fecal samples, and to carry out the gut permeability assay. Carmen Hurtado and Natalia Covarrubias carried out the determination of fecal fat excretion. Catalina Carrasco-Pozo and Karina Tralma participated in the preparation of fecal waters and analysis of antioxidant activity through the FRAP assay. Maria Paz Quezada carried out SCFA analysis. Jerusa Brignardello and Daniela Henriquez were responsible of the dietary counselling of the subjects, the realization of food frequency questionnaires, and the analysis of food consumption. Sayaka Fujio and Paola Navarrete processed the fecal samples for bacterial DNA extraction and purification; they also participated in the bioinformatics analysis of the sequences, together with Fabien Magne and Juan Ugalde. All the authors contributed to the writing of the manuscript.
Financial support: The study was supported in part by grants FONDECYT 1080519, FONDECYT 1120290, and FONDECYT 11140666 from CONICYT Chile. The work was independent of the funding.
Potential competing interest: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Mu H, Høy CE. The digestion of dietary triacylglycerols. Prog Lipid Res 2004; 43: 105–133. [DOI] [PubMed] [Google Scholar]
- Awad AB, Chattopadhyay JP, Danahy ME. Effect of dietary fat composition on rat colon plasma membranes and fecal lipids. J Nutr 1989; 119: 1376–1382. [DOI] [PubMed] [Google Scholar]
- Kato I, Majumdar AP, Land SJ et al. Dietary fatty acids, luminal modifiers, and risk of colorectal cancer. Int J Cancer 2010; 127: 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriére F, Renou C, Ransac S et al. Inhibition of gastrointestinal lipolysis by orlistat during digestion of test meals in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2001; 281: G16–G28. [DOI] [PubMed] [Google Scholar]
- Filippatos TD, Derdemezis CS, Gazi IF et al. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf 2008; 31: 53–65. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S et al. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Sharpton TJ, Laurent TJ et al. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS ONE 2014; 9: e84689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA et al. High fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009; 137: 1716–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P, Amar J, Iglesias M et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- Cani P, Neyrinck A, Fava F et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007; 50: 2374–2383. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470–1481. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Barbara G, Buurman W et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 2014; 14: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kamer JH, Ten Bokkel HH, Weijers HA. Rapid method for the determination of fat in feces. J Biol Chem 1949; 177: 347–355. [PubMed] [Google Scholar]
- Klindworth A, Pruesse E, Schweer T et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013;41:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella AP, Bartram AK, Truszkowski JM et al. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics 2012; 13: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 2012; 6: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Hertel C, Tannock GW et al. Detection of LactobacillusPediococcusLeuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 2001; 67: 2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok RG, de Waal A, Schut F. Specific detection and analysis of a probiotic Bifidobacterium strain in infant feces. Appl Environ Microbiol 1996; 62: 3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Nyman M, Jönsson JA. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr 2006; 20: 674–682. [DOI] [PubMed] [Google Scholar]
- Klinder A, Karlsson PC, Clune Y et al. Fecal water as a non-invasive biomarker in nutritional intervention: comparison of preparation methods and refinement of different endpoints. Nutr Cancer 2007; 57: 158–167. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 1996; 239: 70–76. [DOI] [PubMed] [Google Scholar]
- Gotteland M, Araya M, Pizarro F et al. Effect of acute copper exposure on gastrointestinal permeability in healthy volunteers. Dig Dis Sci 2001; 46: 1909–1914. [DOI] [PubMed] [Google Scholar]
- Lovejoy J, DiGirolamo M. Habitual dietary intake and insulin sensitivity in lean and obese adults. Am J Clin Nutr 1992; 55: 1174–1179. [DOI] [PubMed] [Google Scholar]
- Burri E, Beglinger C. The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert Rev Gastroenterol Hepatol 2014; 8: 197–210. [DOI] [PubMed] [Google Scholar]
- Poullis A, Foster R, Shetty A et al. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2004; 13: 279–284. [DOI] [PubMed] [Google Scholar]
- Vulevic J, Juric A, Tzortzis G et al. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J Nutr 2013; 143: 324–331. [DOI] [PubMed] [Google Scholar]
- Brignardello J, Morales P, Diaz E et al. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther 2010; 32: 1307–1314. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Sun J, Ding Y et al. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl Microbiol Biotechnol 2013; 97: 1689–1697. [DOI] [PubMed] [Google Scholar]
- Erhardt JG, Lim SS, Bode JC et al. A diet rich in fat and poor in dietary fiber increases the in vitro formation of reactive oxygen species in human feces. J Nutr 1997; 127: 706–709. [DOI] [PubMed] [Google Scholar]
- Erridge C, Attina T, Spickett C et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007; 86: 1286–1292. [DOI] [PubMed] [Google Scholar]
- Stenman LK, Holma R, Gylling H et al. Genetically obese mice do not show increased gut permeability or faecal bile acid hydrophobicity. Br J Nutr 2013; 110: 1157–1164. [DOI] [PubMed] [Google Scholar]
- Verdam FJ, Fuentes S, de Jonge C et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013; 21: E607–E615. [DOI] [PubMed] [Google Scholar]
- Gummesson A, Carlsson LM, Storlien LH et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity 2011; 19: 2280–2282. [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Ransac S, Dijkstra BW et al. Bacterial lipases. FEMS Microbiol Rev 1994; 15: 29–63. [DOI] [PubMed] [Google Scholar]
- Lappi J, Salojärvi J, Kolehmainen M et al. Intake of whole-grain and fiber-rich rye bread versus refined wheat bread does not differentiate intestinal microbiota composition in Finnish adults with metabolic syndrome. J Nutr 2013; 143: 648–655. [DOI] [PubMed] [Google Scholar]
- Simões CD, Maukonen J, Kaprio J et al. Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J Nutr 2013; 143: 417–423. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen A, de Vos WM. Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol 2014; 5: 239–262. [DOI] [PubMed] [Google Scholar]
- Daniel H, Moghaddas Gholami A, Berry D et al. High-fat diet alters gut microbiota physiology in mice. ISME J 2014; 8: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BS. Diet and excretion of bile acids. Cancer Res 1981; 41: 3766–3768. [PubMed] [Google Scholar]
- Stenman LK, Holma R, Eggert A et al. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 2013; 304: G227–G234. [DOI] [PubMed] [Google Scholar]
- Islam KB, Fukiya S, Hagio M et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141: 1773–1781. [DOI] [PubMed] [Google Scholar]
- Ahnen DJ, Guerciolini R, Hauptman J et al. Effect of orlistat on fecal fat, fecal biliary acids, and colonic cell proliferation in obese subjects. Clin Gastroenterol Hepatol 2007; 5: 1291–1299. [DOI] [PubMed] [Google Scholar]
- Leber B, Tripolt NJ, Blattl D et al. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: an open label, randomized pilot study. Eur J Clin Nutr 2012; 66: 1110–1115. [DOI] [PubMed] [Google Scholar]
- Brunser O, Gotteland M, Cruchet S et al. Effect of a milk formula with prebiotics on the intestinal microbiota of infants after an antibiotic treatment. Pediatr Res 2006; 59: 451–456. [DOI] [PubMed] [Google Scholar]
- Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther 2006; 24: 701–714. [DOI] [PubMed] [Google Scholar]
- Li S, Huang R, Shah NP et al. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J Dairy Sci 2014; 97: 7334–7343. [DOI] [PubMed] [Google Scholar]
- Mohan R, Koebnick C, Schildt J et al. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res 2008; 64: 418–422. [DOI] [PubMed] [Google Scholar]
- Peña F, Mizgier ML, Morales P et al. Effect of the symbiotics (B. animalis spp. lactis Bb12 and oligofructose) in obese subjects. A randomized, double-blind, controlled clinical trial. J Food Nutr Res 2014; 2: 491–498. [Google Scholar]
- Davila AM, Blachier F, Gotteland M et al. Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 2013; 68: 95–107. [DOI] [PubMed] [Google Scholar]
- Brinkworth GD, Noakes M, Clifton PM et al. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr 2009; 101: 1493–1502. [DOI] [PubMed] [Google Scholar]
- Fava F, Gitau R, Griffin B et al. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk' population. Int J Obesity 2012; 37: 216–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.