Abstract
Objectives:
The safety and efficacy of subcutaneous golimumab through 2 years of maintenance therapy was evaluated in patients with moderate-to-severe ulcerative colitis (UC).
Methods:
Patients completing treatment through week 52 (placebo, golimumab 50, 100, every-4-weeks (q4w)) and evaluations at week 54 were eligible for this long-term extension (LTE) trial. Patients receiving placebo or golimumab 50 mg with worsening disease during the LTE could receive golimumab 100 mg. Efficacy assessments included the Mayo physician's global assessment (PGA) subscore, inflammatory bowel disease questionnaire (IBDQ), and corticosteroid use. Patients who were randomized to golimumab at PURSUIT-Maintenance baseline and continued receiving golimumab during the LTE were analyzed for efficacy (using intention-to-treat and “as observed” analyses; N=195) and safety (N=200). Patients treated with golimumab at any time from induction baseline through week 104 (N=1240) constituted the overall safety population.
Results:
Baseline demographics and disease characteristics of patients entering the LTE receiving golimumab were similar to those of all patients randomized to golimumab maintenance at baseline. At week 104, 80.5% (157/195) of patients had a PGA=0/1 (range weeks 56–104: 80.5–91.8%) and 56.4% (110/195) had a PGA=0 (weeks 56–104: range: 53.8–58.5%). Through week 104, 86% of patients maintained inactive or mild disease activity. Among 174 corticosteroid-free patients at week 54, 88.5% remained corticosteroid-free at week 104. At week 104, 62.2% (120/193) had an IBDQ score ≥170. Tuberculosis, opportunistic infection, and malignancy rates were low, and the overall safety profile was similar to that reported through week 54. Two non-melanoma skin cancers, one metastatic colon cancer, and two deaths (biventricular heart dysfunction, sepsis) occurred between weeks 54 and 104.
Conclusion:
Subcutaneous golimumab q4w through 2 years maintained clinical benefit and reduced corticosteroid use among patients who did well in the maintenance study. No new safety signals were observed.
Introduction
Golimumab is a fully-human monoclonal anti-tumor necrosis factor-α (TNFα) antibody approved for every-4-weeks (q4w) subcutaneous (SC) maintenance treatment of ulcerative colitis (UC). The approval was based on the results of the clinical development program that included patients who participated in the 54-week Program of Ulcerative Colitis Research Studies Utilizing an Investigational Treatment-Maintenance (PURSUIT-M1; ClinialTrials.gov number: NCT00488631) and a 6-week induction study in which golimumab was administered subcutaneously (PURSUIT-SC,2 ClinialTrials.gov number: NCT00487539). A second 6-week induction study in which golimumab was administered intravenously (IV) was supportive (PURSUIT-IV,3 ClinialTrials.gov number: NCT00488774).
The results of the PURSUIT-M main study demonstrated that golimumab therapy maintained clinical response through week 54, as defined by a requirement to be in clinical response at each evaluation time point (q4w), and sustained clinical remission and mucosal healing at weeks 30 and 54. Safety was consistent with that reported for other TNFα antagonists and golimumab in other approved indications.1
As maintenance therapy for UC is required long term, the current study examined the efficacy and safety through 2 years of maintenance golimumab among golimumab induction responders who were randomized to receive golimumab during the maintenance study through week 52 and continued to receive golimumab during the long-term extension (LTE; extension study through 1 year (week 104)).
Methods
Patients
This LTE study comprised patients who participated in the Phase 3, placebo-controlled, randomized-withdrawal maintenance study of golimumab, PURSUIT-M. Patient eligibility criteria have been previously described.1, 2, 3 Patients enrolled were 18 years of age or older with moderate-to-severe UC activity as defined by a Mayo score of 6 to 12, including an endoscopic subscore of ≥2. Patients had an inadequate response to or failed to tolerate at least one of the following therapies: oral 5-aminosalicylates, oral corticosteroids, immunosuppressants (azathioprine or 6-mercaptopurine), or were corticosteroid-dependent. Patients were naive to treatment with anti-TNFα antagonists.
This study was conducted according to the principles of the Declaration of Helsinki and all other applicable national or local laws and regulations. Written informed consent was obtained before any protocol-specific procedure was performed.
LTE study design
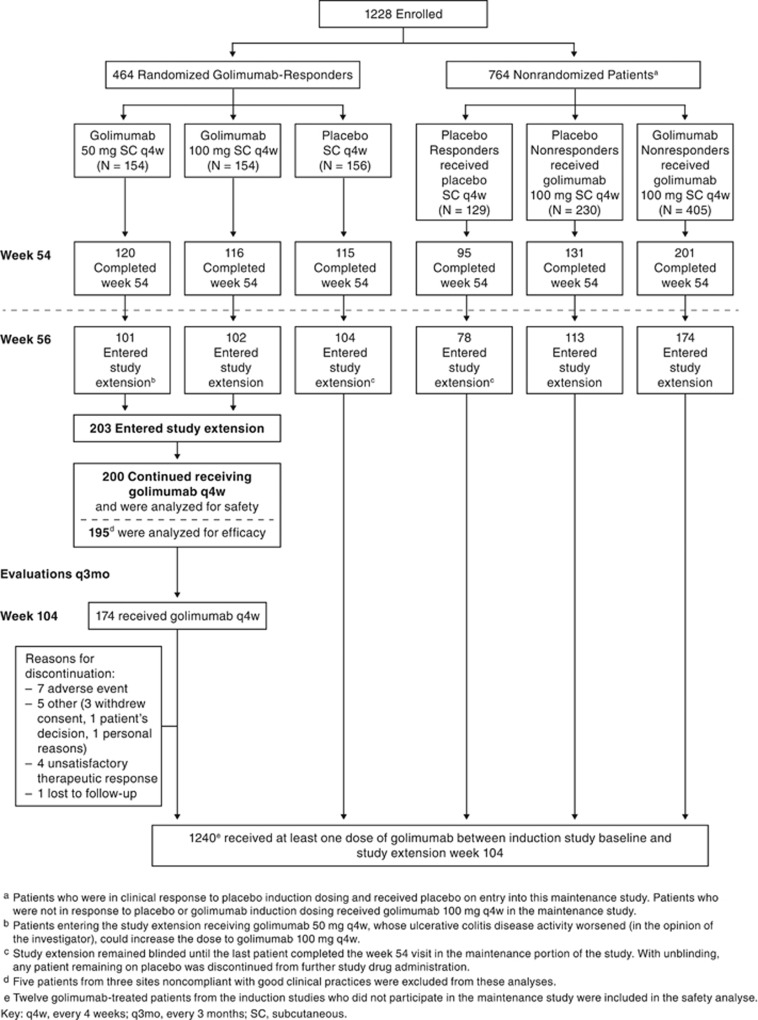
Patients who completed PURSUIT-M through week 52 and final safety and efficacy evaluations at week 54 of PURSUIT-M who, in the opinion of the investigator could benefit from continued treatment, had the opportunity to participate in the LTE study (Figure 1) for a total of approximately 3 years of treatment. No other eligibility criteria were pre-specified by protocol for participation in this study phase. Patients entered the LTE and received the same therapy that they received at week 52 of the maintenance study (placebo or golimumab 50, 100, or 200 mg; Figure 1). As described previously,1 patients who were receiving 200 mg before a protocol amendment eliminated this study dose, had their dose decreased to 100 mg. The first LTE dose was administered at week 56 and continued q4w through week 104 as reported here. In the LTE, q4w dosing continues through week 212 with a final safety evaluation 16 weeks later at week 228. Treatment assignment remained concealed until the last patient completed week 54 evaluations and the main study analyses had been completed. After unblinding, patients who remained on placebo were discontinued from the study.
Figure 1.
Study flow diagram.
During the LTE, patients receiving placebo (before unblinding) or golimumab 50 mg who in the opinion of the investigator experienced disease worsening were permitted to receive a one-time dose increase to golimumab 100 mg. Patients receiving golimumab 100 mg were not eligible for a dose increase. If improvement in their UC disease activity as assessed by the investigator was not observed by 16 weeks following the first administration of the adjusted dose, the patients were discontinued from the study.
Concomitant medications, including UC-specific medications, were administered at the discretion of the investigator and documented.
Efficacy evaluations
Disease activity was evaluated using the Physician's Global Assessment (PGA) at week 56 and every 3 months through week 104. PGA, a subscore of the Mayo score defined on a four-point scale as 0=normal; 1=mild; 2=moderate; and 3=severe, has been used previously.4 Health-related quality of life was assessed with the use of the inflammatory bowel disease questionnaire (IBDQ)5 at week 54, then every 6 months beginning at week 80. The IBDQ is a validated 32-item questionnaire consisting of four dimensions: bowel-related symptoms (e.g., loose stools, abdominal pain), systemic function (e.g., fatigue, sleep pattern), social function (e.g., ability to attend work and social events), and emotional status (e.g., anger, depression, irritability). Each item is scored on a seven-point scale with higher scores indicating better health-related quality of life. A total score of ≥170 points is considered remission.6 Corticosteroid dose was evaluated at week 56 and q4w through week 104.
Safety evaluations
Patients were queried for adverse events (AEs) and concomitant medications, and vital signs were recorded at each study visit. Routine laboratory analyses were done every 3 months.
Data analyses
For efficacy analyses, only the subgroup of patients who responded to golimumab induction therapy and continued to receive golimumab after week 54 were evaluated (Figure 1). Patients who had dose adjustments were included in these analyses as this was considered to be an overall treatment experience.
For safety analyses, the data were summarized for patients who received at least one administration of golimumab at any time from week 0 of induction through week 104 of the LTE. These summaries include data from the time of the first golimumab dose for patients who initially received placebo and subsequently received golimumab. Safety was also summarized for patients randomized to receive golimumab who continued to receive golimumab in the LTE from weeks 54 through 104. Safety was also summarized by maintenance dose (e.g., 50 mg and 100 mg) for patients who received golimumab induction.
Descriptive statistics were used to summarize continuous variables. Counts and percentages were used to summarize categorical variables. Demographic and baseline disease characteristics at week 0 of maintenance were summarized for patients randomized to golimumab at baseline of PURSUIT-M and those who continued to receive golimumab in the LTE.
For most endpoints, two types of analyses were performed (intent-to-treat (ITT) and “as observed”). For ITT analyses, treatment failure rules (patients who initiated oral or parenteral corticosteroids (including budesonide), had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect) and missing data rules (last observation carried forward for continuous variables or treating as non-event for discrete variables) were applied. For the “as observed” analyses, treatment failure rules were not applied and no imputation was made for the missing data. The ITT analyses served to assess the sensitivity of the efficacy results from the “as observed” data analyses.
The patients with PGA scores of 0 or 0/1 were summarized over time using ITT and “as observed” analyses. The ITT analysis was performed for which treatment failure rules described above were applied and patients meeting treatment failure criteria were considered as not having a PGA score of 0/1 after the event and patients with a missing PGA score at any time point were considered as not having a PGA score of 0/1.
A Kaplan–Meier plot, which provides an estimate of the durability of response over time, was generated for the combined golimumab group and by dose group (50 and 100 mg). The patients who had a PGA score of 0/1 upon entry in the LTE were considered responders and were included in this analysis. Treatment failure rules as described previously were applied; in addition, patients with an increase of at least two PGA points were considered to have lost response. An increase of at least two PGA points (i.e., inactive disease to moderate disease activity or mild-to-severe disease activity) was considered a more clinically meaningful definition for loss of response than an incremental change (i.e., inactive disease to mild disease activity, or mild-to-moderate disease activity). In addition to the rules specified above for the Kaplan–Meier analyses, patients whose dose was increased were considered treatment failures for the analyses performed by dose group. The patients who did not lose response through week 104 were censored at week 104.
Patients not receiving corticosteroids at week 54 and who remained corticosteroid-free were summarized through week 104 by ITT analyses. The last observation carried forward was used for patients with a missing corticosteroid value, and the patients who met one of the treatment failure rules were considered to be receiving concomitant corticosteroids.
Patient IBDQ scores were also summarized over time by ITT and observed data analyses. The proportions of patients who achieved IBDQ scores of ≥170 at week 54 are summarized at weeks 80 and 104. Patients in clinical remission typically have IBDQ scores ≥170. Patients with a treatment failure, as described previously, had their week 0 value of an induction study carried forward from the time of the event onward. The last observation carried forward was used for patients with missing data.
Safety was assessed by summarizing the incidences per 100 patient-years of therapy of AEs, serious AEs, and events of special interest such as malignancy, sepsis, pneumonia, tuberculosis, opportunistic infections, cellulitis, demyelination, congestive heart failure, serum sickness and anaphylactic reactions, and hypersensitivity reactions.
Results
Patient characteristics and disposition
The baseline demographics and disease characteristics from week 0 of an induction trial were similar between patients randomized to golimumab at the start of maintenance therapy and those entering the LTE (Table 1A). On entry into the LTE, golimumab-treated patients had a median Mayo score of 2.0, C-reactive protein concentration of 1.6 mg/l and IBDQ score of 191.0 (Table 1B). Of the 200 randomized patients in the maintenance group who continued to receive golimumab in the LTE, 17 (8.5%) discontinued before week 104. The most commonly reported reasons for discontinuation from the LTE were AEs and unsatisfactory therapeutic response (Figure 1).
Table 1A. Baseline demographic and disease characteristics of randomized patients in the maintenance triala, at week 0 of the induction study.
| Total patients | Maintenance group (N=308) | Study extension group (N=203) | |
|---|---|---|---|
| Gender—male | n (%) | 166 (54) | 114 (56) |
| Extensive disease | n (%) | 135 (44) | 87 (43) |
| Age, years | Mean±s.d. | 40.2±13.51 | 40.0±13.13 |
| Median (IQR) | 39.0 (29.0; 50.5) | 39.0 (29.0; 49.0) | |
| Weight, kg | Mean±s.d. | 73.3±17.39 | 73.3±18.09 |
| Median (IQR) | 72.1 (60.0; 84.0) | 72.0 (59.5; 84.0) | |
| UC disease duration, years | Mean±s.d. | 7.0±6.97 | 7.1±6.58 |
| Median (IQR) | 4.6 (2.4; 9.6) | 5.3 (2.7; 10.0) | |
| Hemoglobin, g/dl | Mean±s.d. | 12.9±1.92 | 13.1±1.92 |
| Median (IQR) | 13.2 (11.9; 14.2) | 13.3 (11.9; 14.4) | |
| Albumin, g/dl | Mean±s.d. | 4.2±0.40 | 4.3±0.39 |
| Median (IQR) | 4.3 (4.0; 4.5) | 4.3 (4.1; 4.5) | |
| Fecal calprotectin (mg/kg) | n | 275 | 186 |
| Mean±s.d. | 1719.5±2,813.58 | 1561.8±2,661.22 | |
| Median (IQR) | 760.0 (283.0; 1,683.0) | 689.5 (259.0; 1,514.0) | |
| Mayo scoreb (0–12) | n | 308 | 203 |
| Mean±s.d. | 8.3±1.37 | 8.2±1.37 | |
| Median (IQR) | 8.0 (7.0; 9.0) | 8.0 (7.0; 9.0) | |
| IBDQc | n | 305 | 200 |
| Mean±s.d. | 129.2±32.98 | 131.2±33.50 | |
| Median (IQR) | 129.0 (105.0; 152.0) | 130.0 (109.0; 154.0) | |
| C-reactive protein (mg/l) | n | 301 | 197 |
| Mean±s.d. | 8.7±13.78 | 6.9±10.43 | |
| Median (IQR) | 4.0 (1.3; 10.1) | 3.6 (1.3; 9.0) | |
| Any UC medication | n (%) | 287 (93.2) | 188 (92.6) |
| Corticosteroids (excluding budesonide) | n (%) | 156 (50.6) | 99 (48.8) |
| ≥20 mg/day P.Eq | n (%) | 107 (34.7) | 70 (34.5) |
| <20 mg/day P.Eq | n (%) | 49 (15.9) | 29 (14.3) |
| Budesonide | n (%) | 10 (3.2) | 6 (3.0) |
| Immunomodulatory drugs | n (%) | 95 (30.8) | 58 (28.6) |
| 6-MP/AZA | n (%) | 93 (30.2) | 58 (28.6) |
| Methotrexate | n (%) | 2 (0.6) | 0 |
| Aminosalicylates | n (%) | 247 (80.2) | 165 (81.3) |
6-MP, 6-mercaptopurine; AZA, azathioprine, IBDQ, inflammatory bowel disease questionnaire; IQR, interquartile range; P.Eq, prednisone equivalent; UC, ulcerative colitis.
Golimumab induction responders who received golimumab on entry into maintenance.
Mayo scores range from 0–12, with higher scores indicating more severe disease.
IBDQ score ranges from 32 to 224, with higher scores indicating better quality of life.
Table 1B. Baselinea disease characteristics of the long-term extension study: golimumab induction responders who received golimumab in the maintenance trial and entered the long-term extension.
| n | Mean±s.d. | Median (IQR) | |
|---|---|---|---|
| Mayo scoreb (0–12) | 201 | 2.3±2.05 | 2.0 (1.0; 3.0) |
| Partial Mayo scoreb (0–9) | 203 | 1.5±1.46 | 1.0 (0.0; 2.0) |
| IBDQc | 201 | 181.9±32.06 | 191.0 (162.0; 209.0) |
| C-reactive protein (mg/l) | 202 | 3.6±6.24 | 1.6 (0.5; 3.6) |
IBDQ, inflammatory bowel disease questionnaire; IQR, interquartile range.
Baseline disease characteristics were collected at week 54 of the maintenance trial (i.e., week 0 of long-term extension).
Mayo scores range from 0 to 12, with higher scores indicating more severe disease. The partial Mayo scores (excluding the endoscopy subscore) range from 0 to 9, with higher scores indicating more severe disease.
IBDQ score ranges from 32 to 224, with higher scores indicating better quality of life.
Efficacy
The primary intent of the efficacy analyses was to assess maintenance of efficacy from the end of the main study through the first year of the LTE. Efficacy analyses were to include patients randomized to golimumab at maintenance baseline who continued to receive golimumab in the LTE; however, five patients from three sites noncompliant with good clinical practices were excluded from these analyses (N=195; Figure 1).
Physician's global assessment and corticosteroid use
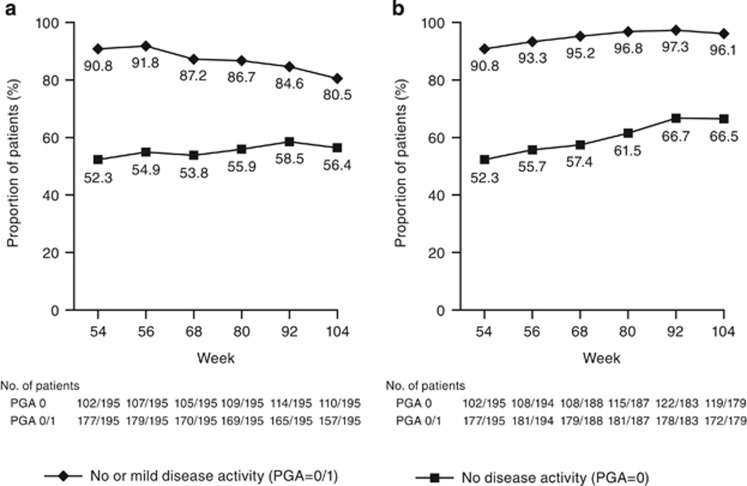
Using ITT analysis (Figure 2a), the proportion of patients who had no disease activity (PGA=0) was maintained during the extension. At week 54, the proportion of patients with no disease activity was 52.3% and ranged from 53.8 to 58.5% between weeks 56 and 104. The proportion of patients with inactive or mild disease activity (PGA=0/1) at week 54 was 90.8% and ranged from 80.5 to 91.8% between weeks 56 and 104.
Figure 2.
Patients with Physician's Global Assessment (PGA) 0 or PGA 0/1 through week 104: golimumab induction responders who received golimumab on entry into the long-term extension for (a) intent-to-treat analysisa,b or (b) observed data analysis. (a) Patients who had a missing PGA score at a time point were considered as not having a PGA score of 0 (no disease activity) or 1 (mild disease activity). (b) Patients who initiated oral or parenteral corticosteroids (including budesonide), had an ostomy or colectomy, or who discontinued study agent due to lack of therapeutic effect before the week 104 visit were considered as not having a PGA score of 0/1 after the event.
Observed data (Figure 2b) results were generally similar to those of ITT analyses. At week 54, the proportion of patients with no disease activity was 52.3%, and ranged from 55.7 to 66.7% between weeks 56 and 104. More than 90% of patients experienced mild or no disease activity including 90.8% at week 54 and ranged from 93.3 to 97.3% between weeks 56 through 104.
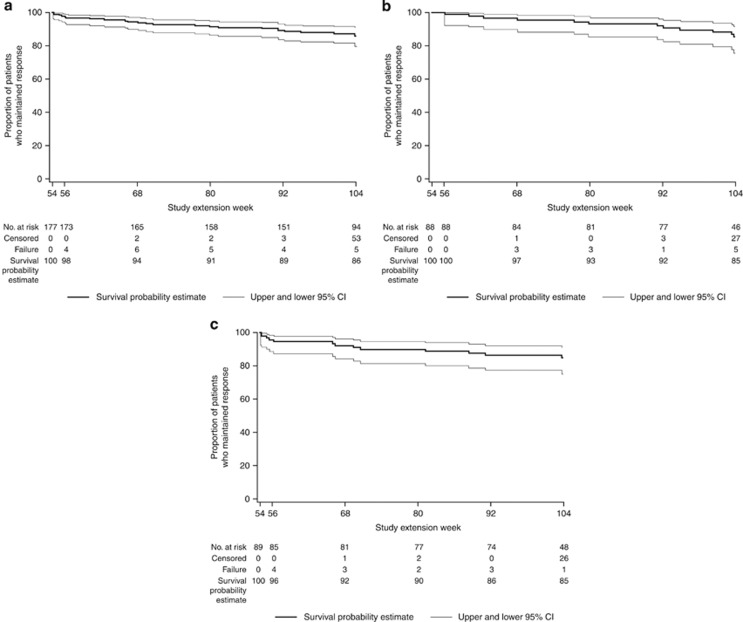
The durability of response through 1 year (week 104) of the LTE is shown in Figure 3a. In all, 177 patients in response (PGA=0/1) at week 54 were included in this analysis. Among them, 86% (Kaplan–Meier estimate) maintained inactive or mild disease activity through week 104. Similar results were obtained for the 50 and 100 mg golimumab groups (Figure 3b,c).
Figure 3.
Patients randomized to golimumab in the maintenance study who continued receiving golimumab in the long-term extension with PGA score of 0/1 at week 54 and maintained responsea: All golimumab induction responders who received golimumab (a), golimumab 50 mg (b), or golimumab 100 mg (c) on entry into the maintenance study. (a) Patients who entered the long-term extension and had a Physician's Global Assessment (PGA) subscore of 0/1 were evaluated for durability of response through week 104. Patients who met one of the treatment failure rules or who had a PGA score increase of ≥2 were considered to have lost response. Patients who discontinued before week 104 for reasons other than lack of efficacy were censored at their last follow-up visit. CI, confidence interval; PGA, Physician's Global Assessment.
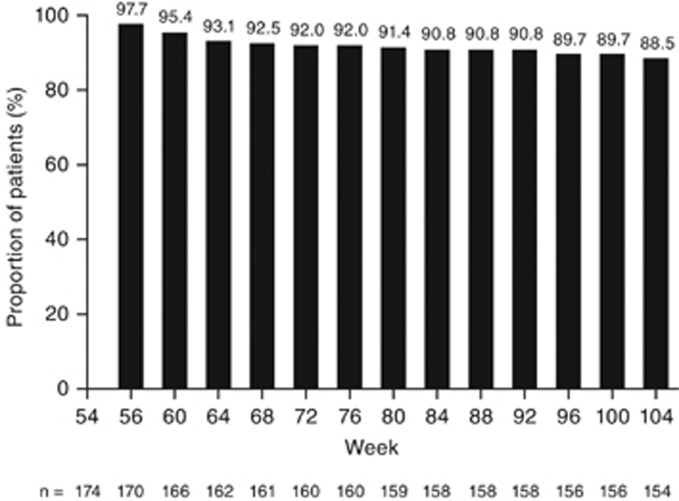
Of the 174 patients not receiving corticosteroids at week 54 (beginning of LTE), 88.5% (154/174) remained corticosteroid-free at week 104 (Figure 4).
Figure 4.
Patients not receiving corticosteroids during the long-term extension study; intention-to-treat analysisa. (a) These patients were not receiving corticosteroids at week 54. Patients with a missing corticosteroid value had their last value carried forward and patients who met one of the treatment failure rules were considered to be receiving concomitant corticosteroids.
Patient-reported outcomes (IBDQ)
As shown in Table 2, median and mean IBDQ scores remained similar from week 54 through week 104. Overall, 140 (72.5%) patients had an IBDQ score ≥170 at week 54. At week 104, a similar proportion (62.2%, ITT; 74.4%, observed) had an IBDQ score ≥170.
Table 2. Inflammatory bowel disease questionnaire results through week 104 of the maintenance study extension; patients randomized to golimumab in the maintenance study who continued receiving golimumab in the long-term extension.
| Time point | Index | Intent-to-treat analysis (N=195) | Observed data analysis (N=195) | |
|---|---|---|---|---|
| Week 54 | IBDQ scorea,b | n | 193 | 193 |
| Mean±s.d. | 182.6±32.04 | 182.6±32.04 | ||
| Median (IQR) | 191.0 (165.0; 209.0) | 191.0 (165.0; 209.0) | ||
| IBDQ score ≥170c,d | % (n/N) | 72.5% (140/193) | 72.5% (140/193) | |
| Week 80 | IBDQ scorea,b | n | 193 | 185 |
| Mean±s.d. | 179.5±35.23 | 183.3±31.02 | ||
| Median (IQR) | 188.0 (156.0; 208.0) | 192.0 (163.0; 208.0) | ||
| IBDQ score ≥170c,d | % (n/N) | 64.2% (124/193) | 70.8% (131/185) | |
| Week 104 | IBDQ scorea,b | n | 193 | 176 |
| Mean±s.d. | 177.0±38.80 | 185.1±31.59 | ||
| Median (IQR) | 189.0 (151.0; 206.0) | 193.5 (168.5; 208.5) | ||
| IBDQ score ≥170c,d | % (n/N) | 62.2% (120/193) | 74.4% (131/176) | |
IBDQ, inflammatory bowel disease questionnaire; IQR, interquartile range.
For the intent-to-treat analysis, patients who initiated oral or parenteral corticosteroids (including budesonide), had an ostomy or colectomy, or who discontinued study agent due to lack of therapeutic effect before the week 104 visit had their week 0 value of an induction study carried forward from the time of the event onward.
For the intent-to-treat analysis, patients who had a missing IBDQ score will have their last available value carried forward.
For the intent-to-treat analysis, patients who initiated oral or parenteral corticosteroids (including budesonide), had an ostomy or colectomy, or who discontinued study agent due to lack of therapeutic effect visit before week 80 (or week 104) are considered not to have an IBDQ score ≥170.
For the intent-to-treat analysis, patients who had a missing IBDQ score at week 80 (week 104) are considered not to have an IBDQ score ≥170.
Absolute mean and median scores are presented for each time point.
Safety
With adjustment for length of follow-up, the safety profile per 100 patient-years of therapy for all patients treated with at least one golimumab dose at any time from week 0 of induction through week 104 of LTE (N=1240) was similar to that observed through week 54 (Table 3). Patients were followed for an average of 1.3 years and 15.5 golimumab administrations through week 104. Duration of follow-up through week 104 among 1,240 golimumab-treated patients totaled 1664.0 patient-years (Table 3). Rates of AEs and serious AEs per 100 patient-years of exposure through weeks 54 and 104 were similar (Table 3). Rates of serious AEs (19.65 vs. 11.10) and AEs leading to study agent discontinuation (12.72 vs. 5.98) were higher for golimumab 100 mg compared with 50 mg (Table 4). It is worth noting that patients receiving golimumab 100 mg in this study were either randomized golimumab responders, nonrandomized golimumab induction nonresponders, or patients whose disease activity worsened and increased their dose to golimumab 100 mg.
Table 3. Safety findings per 100 patient-years of follow-up through week 104.
| Adverse events | Randomized golimumab-treated |
All golimumab-treated |
|
|---|---|---|---|
| Week 54 through 104 (N=200) | Through week 54a (N=1,233) | Through week 104a (N=1,240) | |
| Average duration of follow-up (years) | 0.9 | 0.9 | 1.3 |
| Average exposure (no. of administrations) | 12.4 | 11.0 | 16.9 |
| Total patient-years of follow-up | 185.2 | 1,080.1 | 1,664 |
| Patients who died | 0.5 | 0.4 | 0.6 |
| Patients who discontinued due to ≥1 adverse event | 4.3 | 16.1 | 12.4 |
| Patients with ≥1 of the following: | |||
| Adverse events | 251.1 | 406.3 | 349.9 |
| Infectionsb | 72.4 | 95.7 | 89.1 |
| Infections requiring antimicrobial therapyb | 33.5 | 48.0 | 44.5 |
| Injection site reaction | 17.3 | 17.2 | 14.6 |
| Serious adverse events | 8.6 | 24.3 | 19.4 |
| Serious infectionsb | 3.2 | 5.2 | 4.5 |
| Infections of special interest | |||
| Sepsis | 0.00 | 0.56 | 0.42 |
| Pneumonia | 4.32 | 1.76 | 2.04 |
| Tuberculosis | 0.00 | 0.37 | 0.24 |
| Opportunistic infectionsb | 0.00 | 0.28 | 0.18 |
| Cellulitis | 1.62 | 1.48 | 1.38 |
| Other adverse events of special interest | |||
| Malignancies | 1.08 | 0.46 | 0.6 |
| Demyelination | 0.00 | 0.09 | 0.06 |
| Congestive heart failure | 0.00 | 0.19 | 0.12 |
| Hypersensitivity reactions | 2.70 | 2.13 | 2.46 |
| Serum sickness and anaphylactic reactions | 0.00 | 0.09 | 0.00 |
Includes data from the time of the first golimumab dose onward.
Infection as assessed by the investigator.
Table 4. Selected safety findings through week 104 of the maintenance study extension from week 0 of an induction study by treatment group.
|
Placebo induction |
Golimumab induction |
Any Golimumaba | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Maintenance |
Maintenance |
No Maintenance |
Maintenance |
||||||
| Placebob | Golimumabc | Placebod | 50 mgd | 100 mgc,d | 200 mgc,e | ||||
| Treated patients | 49 | 358 | 293 | 77 | 157 | 155 | 681 | 22 | 1,240 |
| Mean weeks of follow-up | 12.3 | 24.0 | 63.3 | 11.9 | 51.1 | 78.6 | 67.2 | 50.4 | 69.8 |
| Mean exposure (number of administrations) | 1.6 | 6.1 | 15.1 | 1.6 | 12.9 | 19.9 | 16.2 | 13.0 | 16.9 |
| Total patient-years of follow-up | 12 | 165 | 356 | 18 | 154 | 234 | 880 | 21 | 1,664 |
| Number of specified events per hundred patient-years of follow-up (95% confidence interval)f | |||||||||
| Adverse events | 1,024.99 (849.12, 1,226.55) | 397.18 (367.34, 428.80) | 348.11 (329.01, 368.04) | 828.56 (699.62, 974.39) | 376.89 (346.86, 408.82) | 323.15 (300.53, 347.01) | 340.71 (328.62, 353.12) | 459.93 (373.39, 560.50) | 349.87 (340.94, 358.97) |
| Serious adverse events | 223.95 (146.29, 328.13) | 13.34 (8.36, 20.20) | 16.27 (12.35, 21.03) | 221.33 (157.39, 302.56) | 15.57 (9.98, 23.16) | 11.10 (7.25, 16.26) | 19.65 (16.83, 22.81) | 9.39 (1.14, 33.91) | 19.35 (17.29, 21.58) |
| Infectionsg | 232.56 (153.26, 338.36) | 69.13 (57.02, 83.04) | 100.42 (90.29, 111.38) | 255.38 (186.28, 341.72) | 83.03 (69.27, 98.73) | 89.22 (77.53, 102.17) | 80.77 (74.95, 86.94) | 145.49 (98.85, 206.51) | 89.06 (84.58, 93.71) |
| Serious infectionsg | 68.91 (29.75, 135.77) | 1.21 (0.15, 4.38) | 2.81 (1.35, 5.16) | 73.78 (39.28, 126.16) | 5.19 (2.24, 10.23) | 4.70 (2.34, 8.40) | 3.64 (2.49, 5.13) | 0.00 (0.00, 14.06) | 4.45 (3.49, 5.58) |
| Neoplasms (malignant) | 0.00 (0.00, 25.80) | 0.61 (0.02, 3.38) | 0.56 (0.07, 2.03) | 11.35 (1.37, 41.00) | 0.00 (0.00, 1.94) | 0.43 (0.01, 2.38) | 0.57 (0.18, 1.33) | 0.00 (0.00, 14.06) | 0.60 (0.29, 1.11) |
| Injection-site reaction | 0.00 (0.00, 25.80) | 9.10 (5.09, 15.00) | 2.24 (0.97, 4.42) | 0.00 (0.00, 17.00) | 24.00 (16.90, 33.08) | 16.22 (11.48, 22.27) | 16.81 (14.21, 19.75) | 56.32 (29.10, 98.38) | 14.60 (12.82, 16.56) |
| Adverse events leading to discontinuation of study agent | 43.07 (13.98, 100.50) | 5.46 (2.50, 10.36) | 16.83 (12.84, 21.66) | 34.05 (12.50, 74.11) | 9.08 (4.97, 15.24) | 5.98 (3.27, 10.03) | 12.72 (10.48, 15.31) | 4.69 (0.12, 26.15) | 12.44 (10.80, 14.25) |
| Death | 0.00 (0.00, 25.80) | 0.61 (0.02, 3.38) | 0.56 (0.07, 2.03) | 5.68 (0.14, 31.62) | 0.00 (0.00, 1.94) | 0.43 (0.01, 2.38) | 0.68 (0.25, 1.48) | 0.00 (0.00, 14.06) | 0.60 (0.29, 1.11) |
Includes data from the time of the first golimumab dose onward.
Includes data up to the time of the first golimumab dose.
Includes data from the time of dose adjustment onward for those who increased to the indicated dose.
Includes data up to the time of dose adjustment for those who increased dose from the indicated dose.
Includes data from the time of dose increase for patients who were dose adjusted to golimumab 200 mg from golimumab 100 mg and subsequently had their dose decreased from 200 mg to 100 mg.
Confidence intervals based on exact method.
Infection as assessed by the investigator.
Overall rates of infections, infections requiring antimicrobial therapy, and serious infections per 100 patient-years of therapy did not increase with continued exposure to golimumab (Table 3). Rates of infections of special interest (sepsis, pneumonia, tuberculosis, opportunistic infections, and cellulitis) remained low and comparable to week 54. Although tuberculosis was reported in four patients through week 54, no additional cases occurred over the next 50 weeks. In addition, the rates of other AEs of special interest, including demyelination, congestive heart failure, and hypersensitivity reactions, and serum sickness and anaphylactic reactions, remained low and comparable to those through week 54 (Table 3). For the population of randomized golimumab-treated patients in the LTE, the rates of AEs and serious AEs per 100 patient-years of therapy are summarized in Table 3.
The incidence of malignancy through week 104 of golimumab therapy was similar to that observed through week 54; four malignancies were reported through week 541 and three additional malignancies (two non-melanoma skin cancers and one metastatic colon cancer) were observed between weeks 54 and 104.
Six (0.5%) deaths were reported through 104 weeks of therapy, with an incidence of 0.4 per 100 patient-years of follow-up. All deaths were reported previously: peritonitis and sepsis following ischorectal abscess surgery complications in a patient receiving concomitant prednisolone 20 mg (golimumab 400/200 mg, SC induction; no maintenance) during PURSUIT-SC;2 malnutrition and sepsis (golimumab 2 mg/kg, IV induction; golimumab 100 mg; maintenance); cardiac failure in a patient with a history of thrombosis (golimumab 400/200 mg, SC induction; golimumab 100 mg; maintenance); disseminated tuberculosis in a patient who tested positive for latent tuberculosis on induction study entry despite prophylactic therapy (golimumab 200/100 mg, SC induction; golimumab 100 mg; maintenance) through week 54 of PURSUIT-M;1 and biventricular heart dysfunction in a patient (golimumab 100/50 mg, SC induction; golimumab 50 mg; maintenance) with pronounced atherosclerosis and stenosis of the aorta, large arteries, and coronary arteries; and sepsis in a 47-year-old woman (golimumab 2 mg/kg, IV induction; golimumab 100 mg; maintenance) following consumption of raw goat's milk, both after week 54 of PURSUIT-M.1
Discussion
After achieving a short-term clinical response in patients with UC, longer-term treatment goals include the maintenance of clinical response and discontinuation of corticosteroids. The current LTE study demonstrated maintenance of inactive or mild disease without corticosteroid use among patients who initially had moderate-to-severe disease activity, and who responded to golimumab induction therapy followed by SC maintenance therapy through week 54. The majority of patients entered the LTE study with inactive or mild disease and maintained this disease activity through week 104 without corticosteroids.
Patients assessed for efficacy in the LTE study were those who responded to golimumab induction therapy, were randomized to golimumab in the maintenance study, and, in the opinion of the investigator, were expected to benefit from continued golimumab therapy. Their demographics and disease characteristics at the time of randomization into an induction study were similar to those of all patients enrolled and generally reflect the characteristics of patients with moderate-to-severe active disease who participated in trials of other biologic agents.4, 7, 8, 9 At the time of entry into the LTE, the patient population had improved considerably since beginning induction therapy according to all criteria, including the Mayo score (median 2.0 from 8.0), serum C-reactive protein concentration (1.6 from 3.6 mg/l) and IBDQ score (191.0 from 130.0). Results showed that golimumab therapy through week 104 in the LTE maintained the early clinical benefit that was established in the main study, as measured by consistent proportions of patients with PGA scores of 0 (inactive disease), or PGA 0/1 (inactive or mild disease). A concomitant reduction in corticosteroid use was also noted. These findings were similar both quantitatively and qualitatively to those reported for patients who participated in the ACT-1 and ACT-2 studies of infliximab, data from which were reported through extension studies lasting 3 years.4 Golimumab therapy SC q4w through week 104 also resulted in IBDQ remission for approximately 71 and 74% of patients at weeks 80 and 104, respectively.
There were no new safety signals observed with continued golimumab treatment through week 104 and the AE profile was similar to that reported through week 54.1 This applied to serious AEs and infections, including serious infections. Rates of AEs of special interest (i.e., sepsis, pneumonia, tuberculosis, opportunistic infections, or cellulitis) through week 104 remained similar to those previously observed through week 54 of the main study. Malignancies that were diagnosed during the second year of therapy may have been related to environmental exposures (non-melanoma skin cancers with concomitant thiopurine exposure) and the underlying disease (metastatic colon cancer). Two patients died during the second 12 months of therapy; one had at-risk behavior with subsequent sepsis (consuming raw goat's milk) and the other had comorbidities (pronounced atherosclerosis and stenosis of the aorta, large arteries, and coronary arteries).1 Such events underline the importance of recognizing and where possible modifying potential risk factors for patients with moderate-to-severe ulcerative colitis who are receiving immunosuppressive agents.
The current study has limitations. Selection bias may have influenced the results; patients who participated in the LTE were highly selected since they had initially responded to and had tolerated induction therapy with golimumab and had completed the 52-week maintenance trial, although this generally reflects usual clinical practice where physicians determine whether a patient will benefit from continued drug therapy. Observer bias also may have influenced the results, as the opinion of the investigator determined which patients would benefit from continued treatment. The intent of this manuscript was to assess the sustained effect of golimumab beyond 1 year of maintenance therapy for those who responded to golimumab induction therapy and were randomized to golimumab q4w at baseline of the 52-week maintenance trial. No efficacy subanalyses were done for nonrandomized patients who were eligible to receive at least three golimumab-100 mg doses q4w on entry into the maintenance study; thus, the overall results presented here may benefit the therapeutic profile of golimumab. Any patient receiving placebo at the time of study unblinding was discontinued from the LTE; thus, there was no control group for comparison of efficacy or safety. Furthermore, efficacy was summarized by using the PGA subscore of the Mayo score, not clinical response or remission using the total Mayo score, as was reported for the main studies. Thus, endoscopic confirmation of patients' disease activity and biomarker results are lacking.
In conclusion, in patients with moderate-to-severe active UC who respond to golimumab induction therapy and maintain this response through 52 weeks are highly likely to maintain well-controlled disease activity over the ensuing year while continuing golimumab q4w. No new safety issues were identified, and the risks of AEs noted for golimumab over 52 weeks continue at a similar rate during the second year of drug exposure.
Study Highlights

Acknowledgments
Writing and editorial support was provided by James P. Barrett, BS and Mary Whitman, PhD of Janssen Scientific Affairs, LLC.
Footnotes
Guarantor of the article: Peter R. Gibson, MD.
Specific author contributions: P.R. Gibson, B.G. Feagan, W.J. Sandborn, J. Collins, D. Tarabar, Z. Hebzda, P. Rutgeerts, and W. Reinisch collected the data; L. Padgett and J. Johanns performed statistical analysis; all the authors contributed to the design of the study. All the authors contributed to the interpretation of data and critical review and revision of each draft of the manuscript. All the authors had access to the data and approved the final version for submission.
Financial support: Janssen Research & Development, LLC (Spring House, PA, USA) funded the study and analyses, and reviewed and approved the publication. Janssen Scientific Affairs, LLC (Horsham, PA, USA) provided writing support.
Potential competing interests: P.R. Gibson reports having received consulting fees from Ferring Pharmaceuticals, Janssen Research & Development, LLC, Shering-Plough, AbbVie, having received speaker fees from Ferring Pharmaceuticals, Janssen Research & Development, LLC, Abbott/AbbVie, Shering-Plough, and Fresenius Kabi, having received research support from Ferring Pharmaceuticals, Janssen Research & Development, LLC, Schering-Plough, AbbVie, Fresenius Kabi, and Norgine. B.G. Feagan reports having received consulting fees from Abbott/AbbVie, Actogenix, Albireo Pharma, Amgen, Astra Zeneca, Avaxia Biologics, Axcan, Baxter Healthcare, Boehringer-Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging, GSK, Ironwood Pharma, Janssen Biotech (Centocor), Johnson & Johnson/Janssen Research & Development, LLC, Kyowa Kakko Kirin, Lexicon, Lilly, Merck, Millennium, Nektar, Novonordisk, Prometheus Therapeutics and Diagnostics, Pfizer, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma, Takeda, Teva Pharma, Tillotts, UCB Pharma, Vertex Pharma, Warner-Chilcott, Wyeth, Zealand, Zyngenia, having received speaker fees from Abbott/AbbVie, Johnson & Johnson/Janssen Research & Development, LLC, Takeda, Warner-Chilcott, UCB Pharma, having been a scientific advisory board member for Abbott/AbbVie, Amgen, Astra Zeneca, Avaxia Biologics, Bristol-Myers Squibb, Celgene, Centocor, Elan/Biogen, Ferring, Johnson & Johnson/Janssen Research & Development, LLC, Merck, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Salix Pharma, Takeda, Teva, Tillotts Pharma AG, UCB Pharma, and having received research support from Abbott/AbbVie, Amgen, Astra Zeneca, Bristol-Myers Squibb (BMS), Johnson & Johnson/Janssen Research & Development, LLC, Roche/Genentech, Millennium, Pfizer, Receptos, Santarus, Sanofi, Tillotts, UCB Pharma. W.J. Sandborn reports having received received consulting fees from Abbott, ActoGeniX NV, AGI Therapeutics, Alba Therapeutics, Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Astellas, Athersys, Atlantic Healthcare, Aptalis, BioBalance, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Celek Pharmaceuticals, CellerixSL, Cerimon Pharmaceuticals, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle Pharmaceuticals, EnGene, Eli Lilly, Enteromedics, Exagen Diagnostics, Ferring Pharmaceuticals, Flexio Therapeutics, Funxional Therapeutics, Genzyme, Gilead Sciences, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood Pharmaceuticals, Janssen Research & Development, LLC, KaloBios Pharmaceuticals, Lexicon Pharmaceuticals, Lycera Corp, Meda Pharmaceuticals, Merck Research Laboratories, Merck Serono, Millenium Pharmaceuticals, Nisshin Kyorin Pharmaceuticals, Novo Nordisk, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen Therapeutics, PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb, Purgenesis Technologies, Relypsa, Roche, Salient Pharmaceuticals, Salix Pharmaceuticals, Santarus, Schering Plough, Shire Pharmaceuticals, Sigmoid Pharma, Sirtris Pharmaceuticals, SLA Pharma UK, Targacept, Teva Pharmaceuticals, Therakos, Tilliotts Pharma AG, TxCell SA, UCB Pharma, Viamet Pharmaceuticals, Vascular Biogenics, Warner Chilcott UK, and Wyeth; has received research grants from Abbott, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Janssen Research & Development, LLC, Milennium Pharmaceuticals, Novartis, Pfizer, Procter and Gamble, Shire Pharmaceuticals, and UCB Pharma; has received payments for lectures/speakers bureau from Abbott, Bristol-Myers Squibb, and Janssen Research & Development, LLC; and holds stock/stock options in Enteromedics. J. Collins reports having received research funding from Janssen Research & Development, LLC, and UCB Pharma, and has served on the Speakers bureau for Salix. D. Tarabar reports no conflicts of interest. Z. Hebzda, reports no conflicts of interest. P. Rutgeerts reports having received research funding and/or served as a speaker, consultant, and/or advisory board member for Abbott Laboratories, Janssen Research & Development, LLC, Merck Research Laboratories, Merck Serono, UCB Pharma, Millenium/Takeda, Genentech/Hoffman LaRoche, Neovacs, Bristol Myers Squibb, Robarts, Tillotts, Pfizer, and Falk Pharma. W. Reinisch reports having served as a speaker, consultant, and/or advisory board member for Abbott Laboratories, Aesca, Amgen, Astellas, Astra Zeneca, Biogen IDEC, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Janssen Research & Development, LLC, Danone Austria, Elan, Ferring, Genentech, Grünenthal, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Schering-Plough, Setpointmedical, Shire, Takeda, Therakos, Tigenix, UCB Pharma, Vifor, Yakult Austria, and 4SC. R. Strauss, C. Marano, J. Johanns, L. Padgett are employees of Janssen Research & Development, LLC. and may own Johnson & Johnson stock and/or options.
References
- Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 96–109. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Feagan BG, Marano CW et al. Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther 2015; 42: 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch W, Sandborn WJ, Rutgeerts P et al. Long-term infliximab maintenance therapy for ulcerative colitis: the act-1 and -2 extension studies. Inflamm Bowel Dis 2012; 18: 201–211. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Feagan B, Rochon J et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology 1994; 106: 287–296. [DOI] [PubMed] [Google Scholar]
- Hlavaty T, Persoons P, Vermeire S et al. Evaluation of short-term responsiveness and cutoff values of inflammatory bowel disease questionnaire in Crohn's disease. Inflamm Bowel Dis 2006; 12: 199–204. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Van Assche G, Reinisch W et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012; 142: 257–265. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Rutgeerts P, Sands BE et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]