Abstract
Cirrhosis is a common, complex, chronic condition requiring care by multiple specialists in different locations. Emerging data demonstrates limitations in the quality of care these patients receive—in large part due to the problems with care coordination rather than failures of individual providers. This article will discuss approaches for measuring quality, and provide a step-by-step guide for developing quality improvement programs for this patient population.
EPIDEMIOLOGY OF CIRRHOSIS IN THE UNITED STATES
Cirrhosis is the final common pathway for most chronic liver diseases, afflicting ~0.27% of the adult population and accounting for more than 60,000 deaths in the United States each year.1, 2 Although the general public perceives liver disease to be rare, possibly because of the associated stigma, attributable mortality surpasses that from diabetes or kidney disease.2, 3 Cirrhosis is also a resource intensive and costly condition. The number of emergency department visits for complications of cirrhosis increased from 411,869 in 2006 to 548,092 in the United States in 2011, and the number of hospitalizations increased in parallel; from 436,901 in 2006 to 576,573 in 2011. These numbers confirm what anyone caring for these patients has experienced—cirrhotic patients are rarely discharged from the emergency department without an admission!4 Nearly 70% of cirrhosis patients who survive their hospitalization experience readmission, at a cost of >$20,000 each time.5 Finally, quality of life is abysmal, which can further worsen survival.6
THE QUALITY GAP IN MANAGEMENT OF CIRRHOSIS
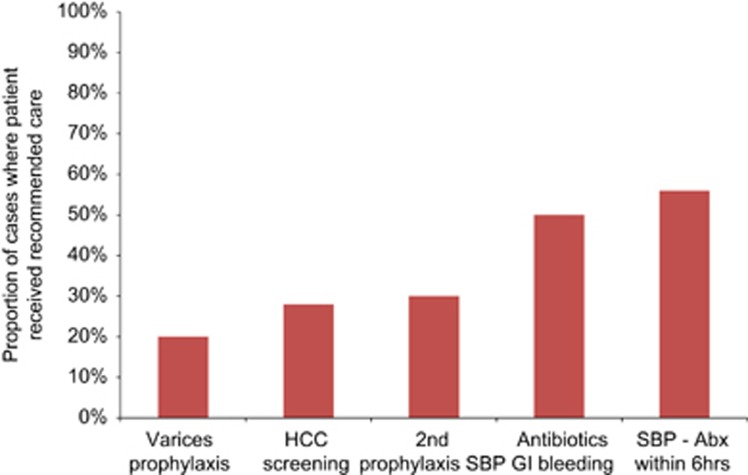
The past three decades have seen tremendous advances in treatment and prevention of cirrhosis. Data from numerous randomized trials now exist to guide management in cirrhosis, as shown in Table 1. Adherence to these guidelines may delay complications, improve quality of life, and prolong survival among cirrhotic patients. For example, nonselective B-blockers or variceal ligation reduce the risk of variceal bleeding and mortality.7 Similarly, enrollment in a hepatocellular carcinoma surveillance program may be associated with increased detection of early stage cancer and increased utilization of potentially curative therapies.8 However, numerous studies have found that evidence-based guidelines are frequently not followed (Figure 1). Furthermore, clinical experience suggests that even when guidelines are followed, patients and their caregivers are often not provided adequate education on how to follow through with often complex plans of care.9 Therefore, a quality gap clearly exists.
Table 1. Guidelines for cirrhosis care supported by strong evidencea 17, 32, 33 .
| Cirrhosis care category | Recommendation |
|---|---|
| TIPS | In patients with good liver function, either a TIPS or a surgical shunt is an appropriate choice for the prevention of rebleeding in patients who have failed medical therapy. |
| TIPS will decrease the need for repeated large-volume paracentesis in patients with refractory cirrhotic ascites. | |
| Prophylactic use of nonabsorbable disaccharides or antibiotics does not appear to lower the risk of encephalopathy after TIPS creation. | |
| ePTFE-covered stents are preferred to bare stents to lower the risk of shunt dysfunction. | |
| Varices | In patients with medium/large varices who have not bled but have a high risk of hemorrhage, nonselective β-blockers or EVL may be recommended for prevention of first variceal hemorrhage. |
| In patients with medium/large varices who have not bled and are not at highest risk for hemorrhage, nonselective β-blockers are preferred and EVL should be considered in patients with contraindications, intolerance, or noncompliance to β-blockers. | |
| Short-term (maximum, 7 d) antibiotic prophylaxis should be instituted within 24 h in any patient with cirrhosis and gastrointestinal hemorrhage: oral norfloxacin or intravenous ciprofloxacin are the recommended antibiotics. | |
| Therapy with somatostatin or its analogues, octreotide and vapreotide, or terlipressin should be initiated as soon as variceal hemorrhage is suspected and continued for 3–5 days after diagnosis is confirmed. | |
| Esophagogastroduodenoscopy, performed within 12 h, should be used to make the diagnosis and to treat variceal hemorrhage, either with EVL or sclerotherapy. | |
| If patients with cirrhosis are found to have bleeding esophageal varices, they should receive EVL or sclerotherapy at time of index endoscopy. | |
| Patients with cirrhosis who survive an episode of active variceal hemorrhage should receive therapy to prevent recurrence of variceal hemorrhage. | |
| Combination of nonselective β-blockers plus EVL is the best option for secondary prophylaxis of variceal hemorrhage. | |
| Ascites | If patients have clinically apparent moderate to severe ascites, they should be managed with a combination of sodium-restricted diet and diuretics (including a combination of both spironolactone and loop diuretics). |
| If hospitalized patients with ascites have ascitic fluid PMN count ≥250 cells/mm3, they should receive empiric antibiotics within 6 h of their test result. | |
| If ambulatory patients with ascites have an ascites fluid PMN count ≥250 cells/mm3, they should receive empiric antibiotics within 24 h of their test result. | |
| If patients have ascites fluid total protein <1.1 g/dl and serum bilirubin >2.5 mg/dl, they should receive prophylactic antibiotics. | |
| Patients who have survived an episode of spontaneous bacterial peritonitis should receive long-term outpatient prophylaxis with daily norfloxacin (or similar medication). | |
| Hepatic encephalopathy | Patients with cirrhosis who have persistent hepatic encephalopathy should receive oral disaccharides or rifaximin |
| Hepatocellular carcinoma | If patients have cirrhosis, they should receive surveillance for HCC by using imaging with or without α-fetoprotein every 6–12 mo. |
EVL, endoscopic variceal ligation; HCC, hepatocellular carcinoma; PMN, polymorphonuclear; TIPS, transjugular intrahepatic portosystemic shunt.
This table is far from an exhaustive list of all randomized trials in cirrhosis management, but rather reflects the most widely accepted guidelines supported by the strongest evidence.
Figure 1.
Many patients with cirrhosis fail to receive evidence-based treatments.13, 29, 30, 31 Abx, antibiotics; HCC, hepatocellular carcinoma; SBP, spontaneous bacterial peritonitis.
In the following section, we will discuss (1) the underlying reasons that might partially explain this gap, and (2) the steps that individual providers can take to improve the quality of care for their population of cirrhotic patients.
SOCIETAL AND DELIVERY SYSTEM BARRIERS TO QUALITY CARE
The natural inclination upon viewing Figure 1 is to assume that improved provider education is needed. Indeed, the ever-enlarging body of medical literature does make it difficult to keep up, particularly for generalist physicians who must stay on top of multiple fields. However, education alone is not the sole answer. A number of systems-based barriers exist that likely explain many of the observed gaps in care. The first barrier is access to timely care—cirrhotic patients are more likely than the general population to be poor and uninsured. Among US patients, approximately one quarter of the insured patients are covered by Medicaid, which lapses easily and has a limited provider network owing to low reimbursement rates. Even among those with premium insurance, access to specialty care may be difficult. There are only 560 board certified transplant hepatologists in the United States, and perhaps another several hundred physicians without that certification who focus their practice on liver disease.10 Most of these hepatologists tend to be clustered at liver transplant centers, and as a result, most of the care for patients with liver disease is provided by gastroenterologists and primary care physicians. In an analysis of the Nationwide Inpatient Sample, 82% of hospitalizations occurred at non-transplant hospitals.11 In another study, only 45% of elderly cirrhotic hospitalized patients had an encounter with a gastroenterologist during their hospitalization or in the ensuring year after discharge.12
A related barrier is the difficulty in coordinating care among multiple different providers working in different offices and healthcare systems. The associated confusion and diffusion of responsibility can lead to under-testing, over-testing, and even conflicting interventions. One real-life example we have encountered more than once is a patient who is prescribed diuretics for ascites by one provider, and salt tablets for hyponatremia by another provider. This lack of coordination can also occur between the provider and other members of the care team. For example, in a study on predictors of timely antibiotics for spontaneous bacterial peritonitis, delays occurred because providers were not notified when laboratory results were posted, and then nurses were not notified when antibiotic orders were placed.13 Finally, lack of coordination frequently exists in transitions between different sites of care, such as when a patient is discharged from the hospital.5 These examples highlight the “Swiss cheese” model of medical errors: both errors and quality gaps tend to be caused by multiple small holes in a system rather than one large gaping hole in care by one individual.
Even when providers and their systems of care function perfectly, optimal management still relies on the patient to follow through on medical recommendations. Medical encounters last for a small fraction of a patient's course of illness; much of the “management” (taking medications, proper diet, exercise and travel to appointments) is done outside the presence of a healthcare provider. Standard approaches to patient education are inadequate. A study of 150 cirrhotic patients followed in a specialty clinic found woefully poor knowledge about basic topics: 54% thought that nonsteroidal anti-inflammatories were safer than acetaminophen, and 58% thought that sea salt is low in sodium.9 Patients with cirrhosis also depend heavily on their caregivers (friends and family) to manage their care between visits.14 Unfortunately, education and involvement of these individuals is often neglected in current practice.
MEASURING QUALITY
A standard maxim in quality improvement is “you can't improve what you can't measure.” If something cannot be measured, it cannot be improved. A usable measurement taxonomy of healthcare quality was first developed by Avedis Donabedian, who divided it into structure, process, and outcome of care.15 Structural elements include, for example, whether all physicians in a practice are board certified, or whether a hospital offers advanced treatments such as transjugular intrahepatic portosystemic shunt (TIPS). Processes reflect evidence-based medical decision-making and health care-related activity, such as prescribing antibiotics for secondary prophylaxis of spontaneous bacterial peritonitis. Outcomes can be intermediate endpoints such as re-bleeding rate after endoscopic hemostasis of varices, or more distal measures such as mortality or health related quality of life. Although the ultimate goal of medicine is to improve quality and/or quantity of life, outcome measurement is susceptible to confounding variables, statistical error, provider manipulation, and may depend on many factors—many of which are not under the control of healthcare providers.16 In addition, with distal outcomes such as mortality, it is often difficult to determine what changes should be implemented to create improvement. Therefore, most quality measurement focuses on either processes or intermediate outcomes. Kanwal et al.17 have proposed a set of 41 process measures for cirrhosis. These measures, which were developed from literature review and input from a multidisciplinary expert panel, provide a useful starting point for QI efforts—some examples are provided in Table 1.
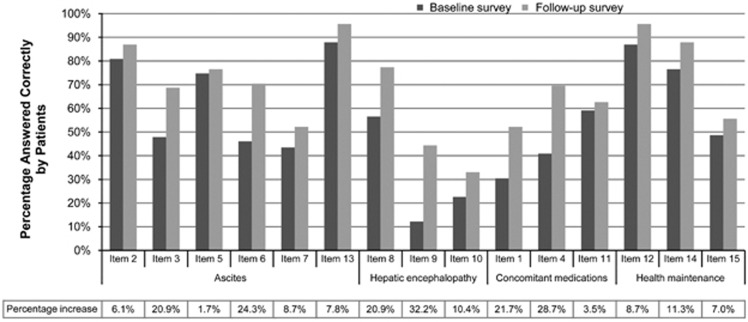
Additional quality measures of importance include patient-centered measures, such as knowledge, self-efficacy, and satisfaction with care. Most medical interventions require an engaged patient to carry them out, and the extent to which a clinician educates and involves the patient in the decision making process (i.e., shared decision-making) is an important feature of quality care. For example, in our practice, patients' knowledge about their condition and its management improved significantly after implementing a structured education program (Figure 2).18
Figure 2.
Patient knowledge about disease self-management, before and after a structured educational intervention. Knowledge improved significantly across all domains (P<0.001). Reprinted with permission from Volk et al.18
EXAMPLES OF SUCCESSFUL QUALITY IMPROVEMENT PROGRAMS
The field of quality improvement in Hepatology is in its infancy, but several early successes are worth noting. Wigg et al.19 used a chronic disease management paradigm and found improved patient adherence and attendance to clinic visits compared with usual care. Morando et al.20 applied the concept of a specialty “day hospital” and found an improvement in mortality among patients with ascites. More recently, Tapper et al.21 used checklists to improve care among hospitalized patients and found lower rates of re-admissions compared with historical controls. Several other institutions have published their experiences—it is notable that most have been unfunded endeavors, driven primarily by a passion for excellence.
A PRACTICAL GUIDE TO IMPROVING QUALITY IN CLINICAL PRACTICE
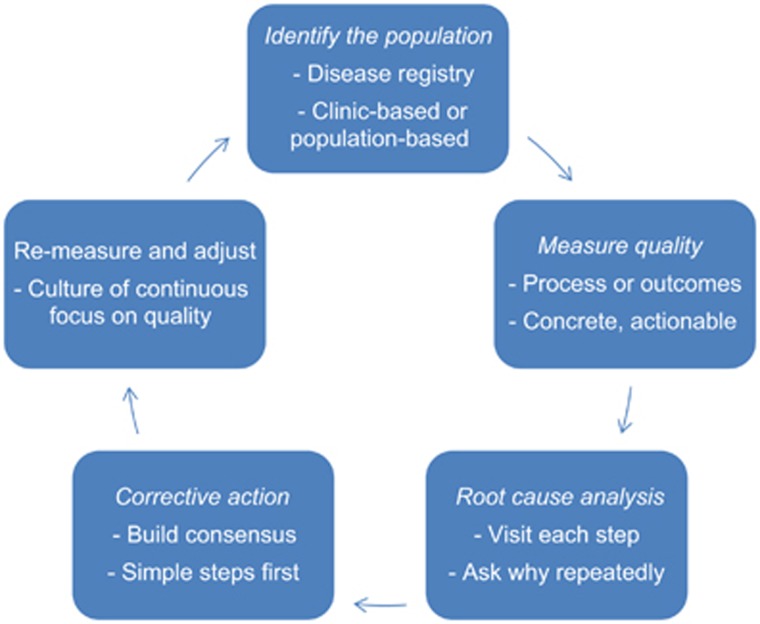
There are numerous approaches to quality improvement that can be adopted, such as Six Sigma or Lean.22 Most of these philosophies come from the manufacturing sector, and consultants can be hired to come in and provide advice. However, not only can this be costly, but hiring external consultants also goes against the principles that QI needs to be continuous and driven by those doing the everyday work. Therefore, in many instances a provider group may be better served to develop their own system. The following steps outline a practical approach (Figure 3):
Figure 3.
A practical guide to improving quality in clinical practice.
Identify the population
The first step is to be able to identify patients with cirrhosis on an ongoing basis, for inclusion in a continuously updated clinical registry. One method is to develop an automated feed from the billing database, using ICD-9 codes 571.5 and 571.2. The advantage of this method is the automation; it also allows for population-based case identification. Although these codes have fairly good positive predictive values at 80% and 87%, respectively, they are limited by less robust negative predictive values at 52% and 46%, respectively.23 Combing these codes or using data from both inpatient and outpatient encounters can increase the positive and negative predictive values.2 The group at Yale has developed a more sophisticated method based on billing codes, which categorizes patients according to the complications and location of service.24 Another method is prospective clinician-driven identification. In one of our prior practices, when a clinician sees a cirrhosis patient in clinic (diagnosed by liver biopsy or imaging/laboratory evidence), he or she notifies the support staff to enroll the patient in the registry. This method is slightly more time consuming, but more precise. A decision will then need to be made about how to store the registry data. The options range from simple (e.g., Excel), to more complex programs—we have used a disease management program called Avitracks, which links to our electronic medical record and provides reminders when labs or imaging are due. Epic, an electronic medical record used by many healthcare systems, can also support disease registries in some versions of the software.
Measure quality
The next step is to decide on quality measures. For reasons discussed above, a combination of process and intermediate outcome measures is recommended. Each measure will need a clearly defined denominator (e.g., patients with prior SBP), numerator (those prescribed antibiotic prophylaxis with norfloxacin, ciprofloxacin, or perhaps Bactrim), and denominator exclusions (e.g., those who no longer have ascites). In addition to the evidence base supporting the measures, several other factors are important to consider. One is the reliability and ease of data collection. For example, a measure focusing on management of variceal bleed will suffer from poor inter-rater reliability of the denominator with variceal bleeding; many patients have stopped bleeding by the time the endoscopy is done, and no “nipple sign” is present. Descriptive data that require skilled chart review to collect will pose greater measurement burden than discrete data, which can be gathered in an automated fashion. Another important consideration is to generate data that are actionable. The data should be current, and permit clinicians to drill down to individual patients to remedy any deficits. It is also important to measure areas that affect a large proportion of patients, and areas where less-than-optimal quality is suspected (if performance is already 100% then no improvement is needed). Finally, it may sometimes be useful to measure practice variation in the absence of an explicit quality measure.25 An example would be the utilization of TIPS for patients with ascites—although it may be difficult to discern appropriateness of TIPS for this indication, a finding of large practice variation could lead to efforts at developing consensus and standardization. In the manufacturing world this consensus is called a “shared baseline.” Conversely, processes with very little variation are probably constrained by non-remediable factors, and thus may not be readily amenable to improvement.
Identify root causes for inadequate quality
Root cause analysis involves developing an understanding of the sequence of actions that led to an event. Like other areas in medicine, this means developing hypotheses and gathering data to test them. A critical component of this process involves going to see where the work is done, called a “gemba walk” in Lean. The investigator should talk to all people involved, and ask why repeatedly (often as many as five times). It is important to maintain a nonjudgmental attitude and take the position that all medical errors are systems errors—humans will inevitably make mistakes, so backup systems should be in place to prevent patient harm. In addition, in most clinical scenarios, quality measures lack sufficient statistical power to accurately discriminate between individual clinicians. For this reason, QI experts distinguish between “measurement for selection” vs. “measurement for improvement.”26 Measurement for improvement focuses less on individuals and more on processes of care. Common process failures include lack of duplicative systems in place to act on test results, confusion about who is supposed to be responsible for each step of the process, and breakdown of communication (between providers, providers and support staff, and/or with patients). For example, we found that many of our patients were not receiving timely screening for hepatocellular carcinoma because the test was ordered when they were seen in clinic, to be done at a local hospital. Oftentimes, the patient would not understand they needed to call and schedule, or the test was actually done but no results made it back to our clinic—and nobody realized this until their return visit 6 months later.
Implement corrective action
Once the root causes are identified, the lead individual on the project will typically present findings to the rest of the group. This is where a consensus should be developed about the appropriate steps—if many people in the group disagree on the action to be taken, it will not happen. It is also important to make the changes as simple as possible. For example, in partnership with our nursing staff we improved our hepatocellular carcinoma screening rates from 74 to 93%, by (a) encouraging clinicians to schedule ultrasounds in conjunction with clinic visits, so they occur at our institution, (b) including the importance of HCC screening in our educational booklet, (c) establishing a reminder system, and (d) writing out a clinical protocol, and empowering the nurses to order screening when due without the need for a physician order (while still allowing opt-out for certain patients).27
Re-measure and adjust
Quality improvement is a continuous process, for several reasons. First, QI interventions are often mini-experiments: it is not practical to conduct a randomized trial for each change, so follow-up is needed to determine whether the change worked as intended. This is the basis behind the “Plan-Do-Study-Adjust” cycle popularized by W. Edwards Deming.28 Second, clinical medicine changes: what was appropriate care 1 year ago may be outdated the next year. Third, the individuals in an organization change, and “institutional memory” about process changes can wane over time. Finally, a continuous focus on quality makes it an appropriate focus of emphasis in the organizational culture.
SUMMARY
Patients with cirrhosis represent an ideal population for quality improvement efforts, and every clinician is facing increasing incentives to participate in such efforts. A downside to QI is that it does take time, a commodity that few clinicians possess in surplus. However, most of the work is up front, with minimal time required once the infrastructure is in place. These efforts can also sometimes provide a return on investment by improving efficiency in a practice. Finally, clinicians will benefit from the satisfaction that their efforts will result in an immediate positive impact on patient care. Delivering quality care, after all, is why we went into medicine in the first place.
Guarantor of the article: Michael L. Volk, MD, MSc, AGAF.
Specific author contributions: Both authors had an equal role in reviewing the literature and writing the article.
Financial support: None.
Potential competing interests: None.
References
- Scaglione S, Kliethermes S, Cao G et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol 2015; 49: 690–696. [DOI] [PubMed] [Google Scholar]
- Asrani SK, Larson JJ, Yawn B et al. Underestimation of liver-related mortality in the United States. Gastroenterology 2013; 145: 375–382. e371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn-Sandler V, Sherman C, Aronsohn A et al. Consequences of perceived stigma among patients with cirrhosis. Dig Dis Sci 2014; 59: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HCUP. Available at http://hcupnet.ahrq.gov/.
- Volk ML, Tocco RS, Bazick J et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal F, Gralnek IM, Hays RD et al. Health-related quality of life predicts mortality in patients with advanced chronic liver disease. Clin Gastroenterol Hepatol 2009; 7: 793–799. [DOI] [PubMed] [Google Scholar]
- Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev 2012; 8: CD004544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014; 11: e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk ML, Fisher N, Fontana RJ. Patient knowledge about disease self-management in cirrhosis. Am J Gastroenterol 2013; 108: 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABIM. Available at http://www.abim.org/pdf/data-candidates-certified/all-candidates.pdf.
- Mellinger JL, Richardson CR, Mathur AK et al. Variation among United States hospitals in inpatient mortality for cirrhosis. Clin Gastroenterol Hepatol 2015; 13: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clin Gastroenterol Hepatol 2013; 11: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JT, Volk ML. Can quality of care for patients with cirrhosis be measured? Dig Dis Sci 2011; 56: 3488–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Wade JB, Gibson DP et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011; 106: 1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian A. The quality of medical care. Science 1978; 200: 856–864. [DOI] [PubMed] [Google Scholar]
- Hofer TP, Hayward RA, Greenfield S et al. The unreliability of individual physician "report cards" for assessing the costs and quality of care of a chronic disease. JAMA 1999; 281: 2098–2105. [DOI] [PubMed] [Google Scholar]
- Kanwal F, Kramer J, Asch SM et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol 2010; 8: 709–717. [DOI] [PubMed] [Google Scholar]
- Volk ML, Roney M, Merion RM. Systematic bias in surgeons' predictions of the donor-specific risk of liver transplant graft failure. Liver Transpl 2013; 19: 987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg AJ, McCormick R, Wundke R et al. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol 2013; 11: 850–858. e851–854. [DOI] [PubMed] [Google Scholar]
- Morando F, Maresio G, Piano S et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol 2013; 59: 257–264. [DOI] [PubMed] [Google Scholar]
- Tapper EB, Finkelstein D, Mittleman MA et al. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clin Gastroenterol Hepatol 2016. (in press). [DOI] [PMC free article] [PubMed]
- Wellman J, Hagan P, Jeffries H. Leading the Lean Healthcare Journey: Driving Culture Change to Increase Value. Productivity Press, Taylor & Francis Group: New York, 2011. [Google Scholar]
- Nehra MS, Ma Y, Clark C et al. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol 2013; 47: e50–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune BE, Golus A, Barsky CL et al. Linking a hepatology clinical service line to quality improvement. Clin Gastroenterol Hepatol 2015; 13: 1391–1395. [DOI] [PubMed] [Google Scholar]
- James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Affairs 2011; 30: 1185–1191. [DOI] [PubMed] [Google Scholar]
- Berwick DM, James B, Coye MJ. Connections between quality measurement and improvement. Med Care 2003; 41: I30–I38. [DOI] [PubMed] [Google Scholar]
- Aberra FB, Essenmacher M, Fisher N et al. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci 2013; 58: 1157–1160. [DOI] [PubMed] [Google Scholar]
- Deming WE. Quality, Productivity, and Competitive Position. Massachusetts Institute of Technology, Center for Advanced Engineering Study: Cambridge, MA, 1982. [Google Scholar]
- Davila JA, Henderson L, Kramer JR et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med 2011; 154: 85–93. [DOI] [PubMed] [Google Scholar]
- Kanwal F, Kramer JR, Buchanan P et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology 2012; 143: 70–77. [DOI] [PubMed] [Google Scholar]
- Wilbur K, Sidhu K. Beta blocker prophylaxis for patients with variceal hemorrhage. J Clin Gastroenterol 2005; 39: 435–440. [DOI] [PubMed] [Google Scholar]
- AASLD. Practice Guidelines. Available at http://www.aasld.org/publications/practice-guidelines-0.
- EASL. Clinical Practice Guidelines. Available at http://www.easl.eu/research/our-contributions/clinical-practice-guidelines.