We report herein that constitutive central nervous system (CNS) serotonin (5-HT) depletion via tryptophan hydroxylase 2 (Tph2) knockout in rats leads to postnatal mortality and ventilatory and thermoregulatory abnormalities at distinct postnatal ages. Furthermore, 5-HT precursor treatment in knockout rats can restore CNS 5-HT production and increases ventilation. This rat model offers an experimental system in which brain 5-HT levels can be manipulated in vivo and new insights into the role of 5-HT in breathing and temperature control.
Keywords: neonate, apnea, serotonin, chemoreflex, control of breathing
Abstract
Genetic deletion of brain serotonin (5-HT) neurons in mice leads to ventilatory deficits and increased neonatal mortality during development. However, it is unclear if the loss of the 5-HT neurons or the loss of the neurochemical 5-HT led to the observed physiologic deficits. Herein, we generated a mutant rat model with constitutive central nervous system (CNS) 5-HT depletion by mutation of the tryptophan hydroxylase 2 (Tph2) gene in dark agouti (DATph2−/−) rats. DATph2−/− rats lacked TPH immunoreactivity and brain 5-HT but retain dopa decarboxylase-expressing raphe neurons. Mutant rats were also smaller, had relatively high mortality (∼50%), and compared with controls had reduced room air ventilation and body temperatures at specific postnatal ages. In adult rats, breathing at rest and hypoxic and hypercapnic chemoreflexes were unaltered in adult male and female DATph2−/− rats. Body temperature was also maintained in adult DATph2−/− rats exposed to 4°C, indicating unaltered ventilatory and/or thermoregulatory control mechanisms. Finally, DATph2−/− rats treated with the 5-HT precursor 5-hydroxytryptophan (5-HTP) partially restored CNS 5-HT and showed increased ventilation (P < 0.05) at a developmental age when it was otherwise attenuated in the mutants. We conclude that constitutive CNS production of 5-HT is critically important to fundamental homeostatic control systems for breathing and temperature during postnatal development in the rat.
NEW & NOTEWORTHY
We report herein that constitutive central nervous system (CNS) serotonin (5-HT) depletion via tryptophan hydroxylase 2 (Tph2) knockout in rats leads to postnatal mortality and ventilatory and thermoregulatory abnormalities at distinct postnatal ages. Furthermore, 5-HT precursor treatment in knockout rats can restore CNS 5-HT production and increases ventilation. This rat model offers an experimental system in which brain 5-HT levels can be manipulated in vivo and new insights into the role of 5-HT in breathing and temperature control.
serotonergic (5-ht) neurons are embedded within, and project to, the neural networks that regulate breathing and body temperature and other homeostatic functions (18, 32, 39). The role of 5-HT neurons in cardiorespiratory control is thought to be particularly important during postnatal development, as evidenced by mouse knockout models in which most (Pet-1−/− mice) or all (Lmx1bf/f/p mice) central 5-HT neurons fail to develop (4, 9, 20). Lmx1bf/f/p mice have reduced growth rates and were apneic as much as 30-40% of their early life, which probably contributes to their relatively high neonatal mortality rates (20–25%) (20). Furthermore, genetic deletion (Pet-1−/− mice) or selective neurotoxin (5,7 DHT-treated rat pups) induced reductions in central 5-HT neurons (∼80%) result in reduced body size, high mortality, and age-dependent failures in cardiorespiratory/autonomic responses to episodic and severe hypoxia (5). However, both Pet-1−/− mice and Lmx1bf/f/p mice that survive the perinatal period regulate ventilation and body temperature relatively well as adults during unstressed conditions but fail to appropriately respond to increased CO2, reduced O2, or reduced environmental temperatures (19). Thus it appears that chronic loss of 5-HT-producing neurons significantly alters multiple homeostatic systems critical for survival during postnatal development and are important for normal adult chemoreflexes and body temperature control.
Are the above listed abnormalities in ventilatory and thermoregulatory control due to the loss of brain 5-HT per se or the 5-HT-producing neurons? Some investigators have begun to address this question by the generation and study of mice lacking brain 5-HT secondary to the knockout of the brain isoform of the rate-limiting enzyme in 5-HT biosynthesis tryptophan hydroxylase 2 (Tph2; Tph2−/− mice). These models have in common several phenotypes compared with mice with genetic deletion of 5-HT neurons, including reduced body size and increased mortality rates (1, 37). In addition, Tph2−/− mice also display a reduced capability to thermoregulate in the cold as adults, suggesting that central nervous system (CNS) 5-HT may be required for the maintenance of body temperature in mammals (1). Furthermore, increased CNS 5-HT in Lmx1bf/f/p mice restored the hypercapnic ventilatory response in adult mice (19). Thus it appears that constitutive loss of the neurochemical 5-HT can lead to reduced growth and mortality during development and alter the ventilatory CO2 chemoreflex and thermoregulation as adults. However, the effects of Tph2 knockout on ventilatory and thermoregulatory control during development, or ventilatory chemoreflexes in adult mammals, are not known.
We tested herein the hypothesis that truncation mutation of the Tph2 gene in dark agouti (DATph2−/−) rats would lead to reduced CNS TPH protein and constitutively reduced CNS levels of 5-HT. We further tested if these changes led to ventilatory abnormalities, postnatal mortality, or altered chemoreflexes and temperature control in adult rats. Finally, we tested the hypothesis that treatment of DATph2−/− rats with the 5-HT precursor 5-hydroxytryptophan (5-HTP) would restore CNS 5-HT production and improve ventilatory abnormalities during postnatal development.
METHODS
All rats were housed in the Biomedical Research Center at the Medical College of Wisconsin, allowed access to low salt chow (Dyets 0.4% NaCl) or standard Purina chow and water ad libitum, and maintained on a 12:12-h light-dark cycle. A total of 128 control (wild type and heterozygous) and 76 knockouts were used for this study. All experimental protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee before initiation of experimental protocols.
Generation of and Genotyping Procedures for Tph2 Knockout Rats
The Tph2 gene was targeted for mutation using custom-made zinc-finger nucleases (ZFNs; Sigma-Aldrich), which targeted exon 7 with the target sequence CATGGCTCCGAACCCCctctacACCCCGGAACCG where each ZFN monomer binds the target sequences underlined, on opposite strands. mRNAs encoding the Tph2 ZFNs were injected into one-cell DA rat embryos as described previously (13). Two mutant rat lines, DA-Tph2em2Mcwi (M2) and DA-Tph2em3Mcwi (M3) with overlapping 10-basepair (CTCTACACCC) or 11-basepair (CTCTACACCCC) ZFN-induced exonic deletions as identified by Sanger sequencing, were generated and studied. For each strain, heterozygous breeder pairs were set up, giving rise to litters containing wild-type (WT), heterozygous (Het), and homozygous mutant (DATph2−/−) rats for each mutation. A fragment analysis genotyping protocol was performed using M13-Tph2 forward (5′-TGTAAAACGACGGCCAGTGGCCTTTAGGTCCTGAGGTT-3′) and Tph2 reverse (5′-CCCTTCTCCACAGAAGTGCT-3′) primer sequences. Products were analyzed using an ABI 3730xl DNA analyzer (31).
Immunofluorescence and Tissue Monoamine Measurements
Immunofluorescence.
Adult rats (60 days of age) were anesthetized with isoflurane in propylene glycol (20% vol/vol) and transcardially perfused, and the brainstems removed, frozen (−80°C), and coronally sectioned (10 μm) and affixed to glass slides. Sections were subjected to an established immunostaining protocol (40) with minor modifications. Brainstem tissues were treated with a heat antigen retrieval solution (Dako) at 70°C overnight and washed in phosphate-buffered saline (PBS) before blocking (5% normal horse serum and 5% normal goat serum in PBS) for 1 h. Then, tissues were placed in 2.5% serum block and a primary antibody targeting TPH (1:1,000; mouse monoclonal, T0678; Sigma-Aldrich) overnight at 4°C, before PBS washes (3 × 10 min) and tagging with an Alexa-594 anti-mouse secondary antibody (1:500; 30 min; Vector Labs). The same tissues were then washed in PBS, placed in blocking solution again, and incubated with a primary antibody targeting dopa decarboxylase (DDC; 1:1,000; rabbit polyclonal, AB1569; Millipore) overnight at 4°C before additional PBS washes and tagging with an Alexa-488 anti-rabbit secondary antibody (1:500; 30 min; Vector Labs). Cover slipping followed final PBS washes, and the reagents contained DAPI (Vector Labs). Images of immunolabeled sections were obtained with a laser scanning confocal Nikon A1R system using Nikon Ti-E inverted microscope with a Plan Apo 20x/NA 0.75 objective. Laser excitation and emission filters for the labeled dyes were as follows: DAPI (405; 440/25 nm), Alexa-488 (488; 525/25 nm), and Alexa-594 (561; 600/25 nm). Fluorescence images were captured with Nikon NIS-Elements Advanced Research 3.2 64 bit software and were pseudocolored and merged with ImageJ.
High-performance liquid chromatography with electrochemical detection.
Adult WT (n = 5; 62.2 ± 2.3 days of age), Het (n = 7; 60.7 ± 1.7 days of age), and DATph2−/− (n = 7; 63.6 ± 1.3 days of age) rats were anesthetized with isoflurane in propylene glycol (20% vol/vol) and decapitated, and the brain tissues rapidly were removed; separated into cervical spinal cord, medulla, pons, hypothalamus, and forebrain; and frozen at −80°C for high-performance liquid chromatography (HPLC) measurements as previously described (15, 40). Samples were thawed [0.1 M perchloric acid (0.1 g/ml)], wet weights were taken, and tissues were sonicated and centrifuged (10,000 rpm) for 20 min (4°C), and the supernatant was removed for HPLC analysis of norepinephrine (NE), epinephrine, dopamine, dihydrophenylacetic acid (DOPAC), serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), and homovanillic acid (HVA). Epinephrine was not detected in all samples collected, and it was therefore not reported. Standards were injected with 3,4-dihydroxylbenzylamine [DHBA (internal standard); Sigma 858781] added and 0.1 M perchloric acid to establish the quantitative chemical profile. Samples were subjected to electrochemical detection (BAS LC4C; 0.65 V, 0.1 nA, 0.1-Hz filter with Ag/AgCl reference electrode) with a Waters uBondapak column (3.9X300) at ambient temperature.
Ventilation and Body Temperature Measurements
Ventilation measurements in neonatal rats.
Ventilatory measurements in young [postnatal day (P)1–22] male and female rats were made using a custom-built 200-ml cylindrical Plexiglas plethysmograph using methods similar to those described previously (16, 36). Briefly, gas inflow rate (150 ml/min) was balanced at the same or slightly lower flow rate as vacuum outflow rates to provide rapid gas exchange to avoid CO2 accumulation. Chamber temperature was warmed to ∼29–30°C (ages P1–P9) or ∼27°C (P10–P22) with a heated aluminum floor and controller (Dyna-sense; Scientific Instruments). Chamber pressure (Validyne differential pressure transducer), temperature (∼27–30°C; Warner Instruments), and relative humidity (HX15; Omega) were measured continuously. Volume calibrations (0.1 ml at 1 Hz) were performed to calibrate the ventilatory signal after each study similar to our previous studies (16, 36). All analog signals were connected to a 16-channel A/D converter and digitally recorded using data acquisition software (Windaq) sampled at 200 Hz. Animal temperature was obtained following each experimental period using a T-type rectal thermocouple probe and reader (Omega).
Ventilatory measurements in adult rats.
Ventilatory measurements in adult rats were made using a custom-built 10 l Plexiglas plethysmograph using methods similar to those described previously (16, 36). Briefly, gas inflow rate (10 l/min) was balanced at the same or slightly lower flow rate as vacuum outflow rates to provide rapid gas exchange to avoid CO2 accumulation and to maintain the absolute chamber pressure at or slightly above atmospheric pressure. Chamber O2 and CO2 levels (O2 Capnograph; 07-0193; Mountain View, CA), chamber pressure (Validyne differential pressure transducer), temperature (∼23°C), and relative humidity (∼0–30%; HX93A; Omega) were measured continuously. Volume calibrations (0.68 ml at 1.5–2 Hz) were performed to calibrate the ventilatory signal after each study similar to our previous studies (16, 36). All analog signals were connected to a 16-channel A/D converter and digitally recorded using data acquisition software (Windaq) sampled at 200 Hz. Animal temperature was obtained following each experimental period using a J-type rectal thermocouple probe and reader (BAT-12, Life Science Instruments).
5-HTP treatment studies in rat pups.
Ventilation and rectal temperatures were obtained from P11-16 WT and DATph2−/− rats and studied as described both before and after three intraperitoneal injections (4.5 μl/g body wt) of saline (0.9% NaCl) or 5-HTP (10 mg/kg in saline) separated by 12 h. This treatment regimen was based on unpublished observations from similar work in young DATph2−/− rats (K. J. Cummings, personal communication). Within 1 h of the final injection, ventilatory and rectal temperature measurements were again made while breathing room air. Immediately after the studies (within 1.5 h of final 5-HTP injection), the treated rats were anesthetized with isoflurane in propylene glycol (20% vol/vol) and decapitated and the whole brain tissues were rapidly removed, collected, and frozen at −80°C for HPLC measurements as described above.
Cooling studies in adult rats.
Nine- to fifteen-week-old adult WT, Het, or DATph2−/− rats were individually placed into standard rat cages fitted with wire tops. After >2 h of acclimation to room temperature, baseline body temperature (rectal) measurements (J-type thermocouple) were taken. The cages were then placed in a cooling chamber set to 4°C with rectal temperatures measured every 30 min for up to 4 h.
Data Analysis
All data collected were analyzed offline using a waveform browser (Windaq). Breathing frequency (f, breaths/min), tidal volume (VT; ml/breath), and their product minute ventilation (V̇e; ml/min) were calculated from multiple periods of raw data lasting more than 10–12 sequential breaths and no less than 400 total breaths per conditions (room air, hypoxia, CO2). The selected data were breathing segments devoid of animal movements, sniffing, or other behaviors and represented quiescent breathing in room air (selected from minutes 10–20 of a total of 20 min) or during the last 5 min of the hypoxic or hypercapnic ventilatory challenge (10 min in duration). Voltage deflections from peak to valley were calibrated to a known volume and corrected for animal and chamber temperature, relative humidity, and ambient barometric pressure to calculate the estimated VT per breath (7, 16, 40). Variability was determined by Poincare analyses as previously described (19). We were unable to reliably and unequivocally visually determine sex in the young rats and thus have combined the data from all rats studied grouped by genotype. However, adult rats could be distinguished by sex and all measurements were grouped accordingly.
Statistics
Statistical analyses were performed using the statistics package within SigmaPlot version 12.0. Data were compared using two-tailed t-tests and two-way ANOVAs or two-way repeated-measures (RM) ANOVAs to determine the main effects and all interactions, and appropriate post hoc tests (e.g., Bonferroni) were performed as needed. Significance thresholds were P < 0.05. Some animals were studied at all time points while others had missing data from a few time points. Thus, whenever possible we used two-way RM ANOVAs, but defaulted to using two-way ANOVAs when some of the time points were missing.
RESULTS
Tph2 Gene Mutation and Validation in the DA Rat
Multiple mutant rat lines (M1, M2, and M3) were generated using targeted deletion of exonic sequences in the Tph2 gene, where the M2 and M3 lines harbored 10- and 11-basepair deletions, respectively (Fig. 1A). Confocal imaging of immunolabeled brainstem tissues was indicative of a protein knockout in the M2 strain, as TPH immunofluorescence (Alexa-594, red pseudocolor) was negligible throughout the raphe nuclei including the raphe obscurus (Fig. 1B). Brainstem tissues from adult WT rats showed robust and specific immunolabeling of TPH-expressing raphe neurons throughout the classically described B nuclei (6, 10), with notably dense labeling along the medullary midline in raphe obscurus, pallidus, and magnus. In the same tissues there was an identical raphe distribution of immunolabeling of the biogenic amine biosynthetic enzyme DDC (Alexa-488, green pseudocolor, Fig. 1B). TPH and DDC expression within the medullary raphe was essentially identical, where an overlay of the Alexa-594 and Alexa-488 emission channels in the composite image showed complete coexpression (yellow color). In contrast, there was no detectible TPH immunoreactivity in DATph2−/− rats, despite the presence of DDC immunoreactive neurons throughout the rostro-caudal axis of the brainstem (Fig. 1B). Thus the presence of DDC and absence of TPH immunoreactivity are indicative of a TPH null mutation and sparing of many of the neurons that would otherwise produce 5-HT.
Fig. 1.
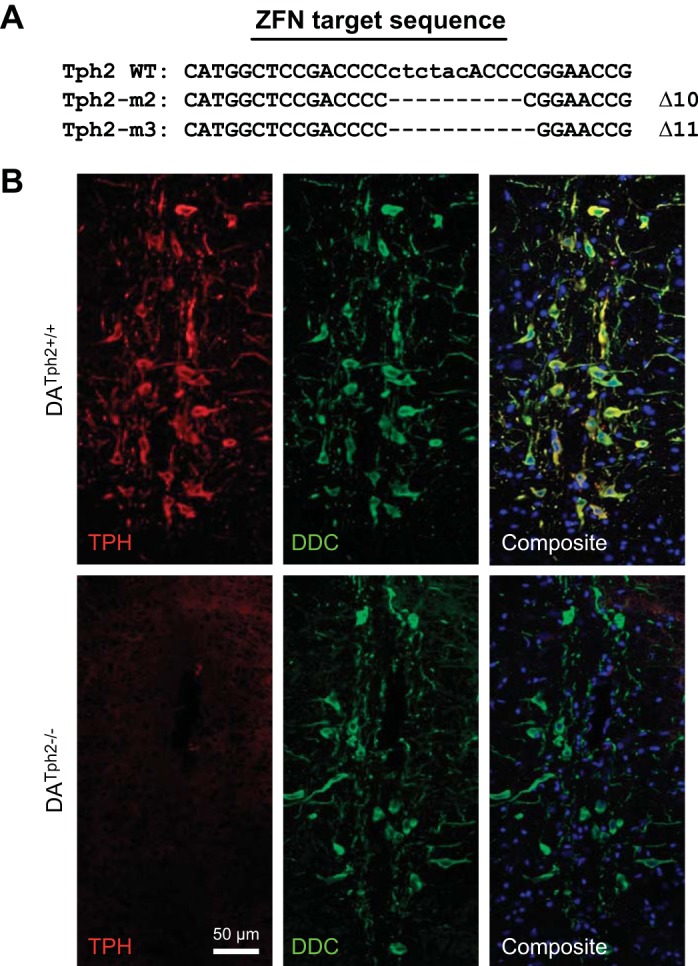
Zinc-finger nuclease (ZFN)-targeted mutation of the Tph2 gene in the rat eliminated tryptophan hydroxylase (TPH) immunoreactivity. A: basepair sequence of exon 7 of the native Tph2 allele [Tph2 wild type (WT)] and the 10- and 11-basepair deletions generated in two mutant lines of dark agouti (DA) rats (M2 and M3). B: tissue sections through the raphe obscurus nucleus of adult male rats that were stained to simultaneously detect TPH (red) and dopa decarboxylase (DDC; green). Compared with WT (DATph2+/+; top), DATph2−/− rats (bottom) showed a complete loss of TPH immunoreactivity but retained immunoreactivity for DDC that was similar to WT, indicating the presence of neurons in the DATph2−/− brainstem that would otherwise be serotonergic. The composite images show the coincidence of TPH and DDC staining in the WT rat (yellow) and emphasize the lack of TPH staining in the mutant. The composite images also show cell nuclei marked by DAPI staining (blue).
Measurements of monoamines in the forebrain of the M2 and M3 lines of DATph2−/− rats showed similar levels of NE and dopamine relative to WT and Het rats, but brain levels of 5-HT were undetectable in both the M2 and M3 mutant strains (Table 1). Given that both M2 and M3 mutations led to predicted truncation mutations and a total loss of CNS 5-HT, we combined monoamine data from both M2 and M3 lines collected from several CNS regions from adult WT, Het, and DATph2−/− rats (Table 2) and compared the data within male and female groups across genotypes to determine if there are sexual dimorphisms associated with the mutation and monoamine levels. HPLC analyses of samples from the forebrain, hypothalamus, pons, medulla, and spinal cord in adult male rats indicated that there were equal levels of NE (P = 0.101) and dopamine (P = 0.195) among WT, Het, and DATph2−/− rats, with the exception that DATph2−/− rats had lower dopamine (and DOPAC) levels in the forebrain (P < 0.05; Table 2). In addition, we found comparable levels of 5-HT and its metabolite 5-HIAA in most brain region tested in WT and Het rats. However, there was no detectible level of 5-HT or its metabolite 5-HIAA in all regions tested in the DATph2−/− rats (Table 2).
Table 1.
Forebrain levels of monoamines in two mutant lines of adult male control and DATph2−/− rats
| Mutation/Genotype | 5-HT | Norepinephrine | Dopamine |
|---|---|---|---|
| M2 | |||
| WT (n = 3) | 495.5 ± 24.1 | 382.2 ± 6.2 | 2424.7 ± 57.0 |
| Het (n = 3) | 401.9 ± 43.0 | 403.6 ± 28.9 | 2,047.9 ± 125.5 |
| DATph2−/− (n = 3) | Not detectable | 358.7 ± 22.1 | 1918.4 ± 51.5 |
| M3 | |||
| WT (n = 2) | 439.1 ± 4.9 | 368.6 ± 15.7 | 2,098.3 ± 63.0 |
| Het (n = 4) | 442.5 ± 20.7 | 411.3 ± 24.9 | 2,414.5 ± 168.7 |
| DATph2−/− (n = 4) | Not detectable | 362.7 ± 20.0 | 2,142.9 ± 60.7 |
Values are means ± SE in ng/g. 5-HT, serotonin; WT, wild-type; Het, heterozygous; DATph2−/−, homozygous mutant.
Table 2.
Regional levels of monoamines and metabolites in adult male WT, Het, and DATph2−/− rats
| Region/Genotype | 5-HT | 5-HIAA | NE | HVA | DA | DOPAC |
|---|---|---|---|---|---|---|
| Forebrain | ||||||
| WT | 473 ± 19 | 336 ± 19 | 377 ± 7 | 217 ± 17 | 2,294 ± 88 | 207 ± 4 |
| Het | 425 ± 21 | 218 ± 15* | 408 ± 17 | 201 ± 20 | 2,257 ± 126 | 201 ± 15 |
| DATph2−/− | ND† | ND† | 361 ± 14 | 184 ± 16 | 2,047 ± 59† | 173 ± 8† |
| Hypothalamus | ||||||
| WT | 537 ± 14 | 420 ± 29 | 961 ± 38 | ND | 247 ± 12 | 16 ± 3 |
| Het | 534 ± 20 | 414 ± 26 | 1,025 ± 46 | ND | 266 ± 21 | 10 ± 4 |
| DATph2−/− | ND† | ND† | 1,078 ± 19 | ND | 267 ± 16 | 8 ± 3 |
| Midbrain/pons | ||||||
| WT | 725 ± 46 | 516 ± 42 | 521 ± 20 | ND | 153 ± 8 | 19 ± 2 |
| Het | 643 ± 63 | 392 ± 28* | 501 ± 26 | ND | 149 ± 8 | 13 ± 2 |
| DATph2−/− | ND† | ND† | 534 ± 13 | ND | 160 ± 5 | 14 ± 5 |
| Medulla | ||||||
| WT | 358 ± 69 | 279 ± 39 | 615 ± 60 | ND | 54 ± 7 | 27 ± 3 |
| Het | 430 ± 27 | 225 ± 28 | 675 ± 51 | ND | 63 ± 9 | 26 ± 2 |
| DATph2−/− | ND† | ND† | 701 ± 57 | ND | 65 ± 3 | 26 ± 4 |
| Cervical Spinal Cord | ||||||
| WT | 1,599 ± 233 | 258 ± 71 | 294 ± 16 | ND | 36 ± 4 | 9 ± 5 |
| Het | 1,482 ± 79 | 177 ± 10 | 338 ± 18 | ND | 40 ± 5 | 2 ± 1 |
| DATph2−/− | ND† | ND† | 321 ± 24 | ND | 40 ± 5 | ND |
Values are means ± SE expressed in ng/g; WT: n = 5, Het: n = 7, DATph2−/−: n = 7. 5-HIAA, 5-hydroxyindoleacetic acid; NE, norepinephrine; HVA, homovanillic acid; DA, dopamine; DOPAC, dihydrophenylacetic acid.
Significant difference compared with WT group;
significantly different from WT and Het groups (P < 0.05, two-way repeated-measures ANOVA); ND, not detectable in ≥50% of samples/group.
We also measured 5-HT, 5-HIAA, NE, dopamine, and DOPAC (only in the medullas) obtained from adult female WT, Het, and DATph2−/− rats. We found no differences among WT and Het females in medullary 5-HT or 5-HIAA (P > 0.4), and there were no detectable levels of medullary 5-HT or 5-HIAA in adult female DATph2−/− rats (data not shown). In addition, medullary levels of dopamine, DOPAC, and NE were not different among adult female WT, Het, or DATph2−/− rats (P > 0.24). These data indicate that male and female rats homozygous for the Tph2 mutations failed to produce 5-HT (and 5-HIAA) in the CNS, with little or no effect on NE or dopamine levels.
Tph2 Knockout in the Rat Had Severe Effects on Growth, Mortality, and Eupneic Breathing During Development
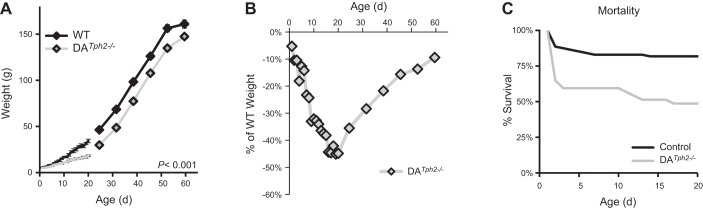
Rat pups were born to Het breeders giving rise to 20% WT, 51% Het, and 29% DATph2−/− rats with mean litter sizes of 7.9 ± 0.5 pups/litter. Shortly after birth the DATph2−/− pups appeared to have normal feeding behaviors and the appearance of milk bands in the abdomen. Body weights for DATph2−/− rats (male and females combined) were not different from control rats at birth until P6 but were significantly smaller beginning at P7 and remained less up to 60 days of age (P < 0.001; Fig. 2A). DATph2−/− rat weights, expressed as a percentage of the average WT and Het pups for a given age, lagged steadily up to weaning when their weights were nearly 46% less than control rats (Fig. 2B). Thereafter, body weights of DATph2−/− rats steadily increased and returned to within 10% of the controls by 60 days of age. We found little or no differences in weight among WT and Het rats at all ages studied.
Fig. 2.
DATph2−/− rats had reduced growth and increased mortality. A: DATph2−/− rats were smaller beginning at P7 and remained smaller thereafter up to 60 days of age (P < 0.001, two-way ANOVA). B: weights of DATph2−/− rats (green) ware expressed as a percentage of the WT weight at each age and plotted across age (days). C: survival (%pups born for each genotype) at each postnatal (P) age showed that after an initial high rate of mortality in DATph2−/− pups there was additional mortality on or after P10 that was rare in control [WT and heterozygous (Het)] pups.
Het dams appeared to exhibit normal maternal behaviors, building nests, and maintaining the pups in a group. However, survival of DATph2−/− rats after P1 rapidly decreased to ∼60% of those born by P3, during which time control (combined WT and Het) littermate survival rate was 88% (Fig. 2C). After a relative plateau in mortality from P3 up to P10, DATph2−/− pups began to have additional mortality up to P17 from 59% survival down to 49% (−10%), where percent survival over the same age range in control littermates went from 83 to 82% survival (−1%). Thus it appears that mortality was markedly greater in DATph2−/− rats compared with control littermates and that there could be two distinct phases of mortality, immediately after birth (P1-3) and from P10 to P17.
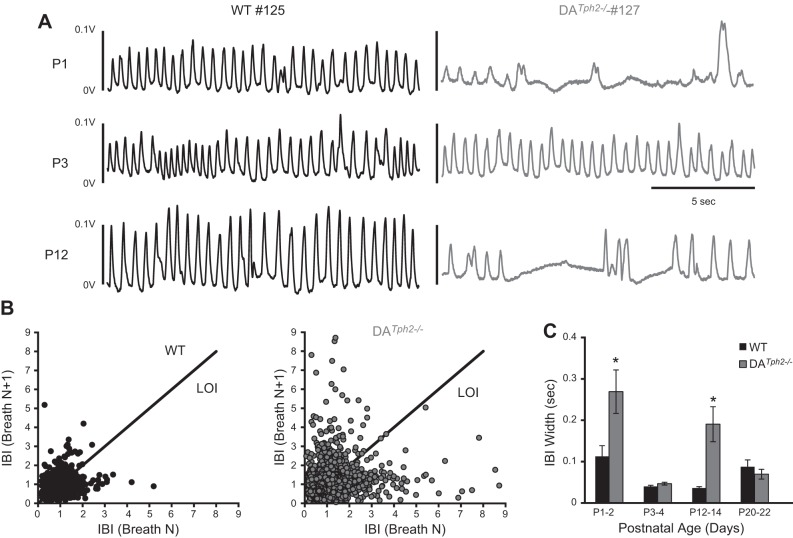
Room air (eupneic) ventilation (measured via whole body plethysmography) was disrupted in DATph2−/− rats (Fig. 3A). WT (and Het; not shown) pups had continuous and regular ventilation at all ages studied during postnatal development. In contrast, at P1 the DATph2−/− rats had highly irregular ventilatory patterns, having large and small breaths between long apneas mixed with some periods of regular breathing with reduced breathing frequency (Fig. 3A, top right). However, eupneic breathing patterns became more regular by P3 in most DATph2−/− rats and remained comparable to control littermates until approximately P10-12 when breathing frequency was reduced and apneas became apparent (Fig. 3A, bottom right). By approximately P21 however, there was no apparent disruption in ventilation during room air breathing in WT or DATph2−/− rats (not shown). We quantified variability in the interbreath interval (IBI) and VT of consecutive breaths during room air exposure at multiple ages using Poincare analyses as done previously (19). The IBI appeared more variable in the DATph2−/− compared with WT rats at P1-2 (Fig. 3B), with greater deviation away from the line of identity (LOI; IBI “width”) and from the mean IBI along the LOI (IBI “length”). The width of the IBI was significantly greater in DATph2−/− compared with WT rats at P1-2 and P12-14 (P < 0.05, two-way ANOVA; Fig. 3C) but similar at P3-4 and P20-22 (P > 0.05). Similarly the IBI length was significantly greater in DATph2−/− compared with WT rats at P12-14 (P = 0.007, two-way ANOVA; not shown). In contrast, we found no difference in the variability in VT at all ages studied (P > 0.05, two-way ANOVA; not shown), indicating that the increased variability in breathing in DATph2−/− rats was limited to the IBI and the measurement periods from P1-2 to P12-14.
Fig. 3.
Ventilation was disrupted at distinct postnatal ages in DATph2−/− rats. A: representative plethysmographic recordings of basal ventilation (measured as voltage deflections) in 1 WT (WT 125; blue) and a DATph2−/− littermate (DATph2−/− 127; green) rat at P1, P3, and P12 while breathing room air. Note that ventilation in the DATph2−/− rat alternated between regular and highly irregular with apneas and large, atypical breaths compared with the WT, but that there was improvement by P3. However, by P12 ventilation was reduced and irregular in the DATph2−/− rat. B: Poincare plots comparing the inter-breath interval (IBI) among consecutive breaths during room air exposure of WT (n = 9; 5,425 breaths) and DATph2−/− (n = 9; 3,594 breaths) rats from P1-2. C: variability in the IBI was calculated by distance from the line of identity (“width” from LOI) or distance from the mean IBI along the LOI (“length”; see results), and averaged for each genotype and age. Note that variability in the IBI was significantly greater (P < 0.003) in DATph2−/− rats at P1-2 and P11-12 but not at other ages tested (two-way ANOVA).
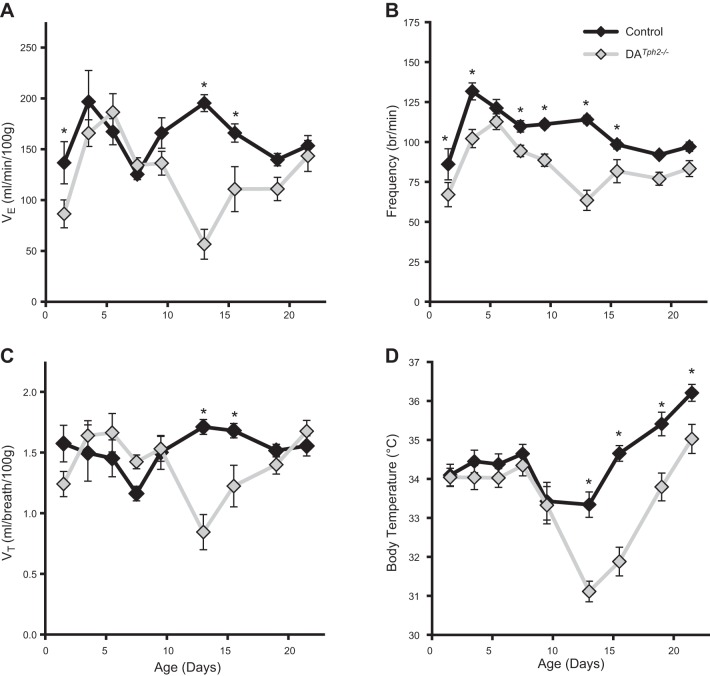
Consistent with these observations, minute ventilation (V̇e) in DATph2−/− rats was significantly reduced during two distinct phases of postnatal development, specifically during P1-2 and P12-16 (P < 0.05, two-way ANOVA; Fig. 4A). With one exception (P5-6), breathing frequency was lower in DATph2−/− rats while breathing room air from P1-16 (P < 0.05, two-way ANOVA; Fig. 4B), and VT was reduced from P12-16 (P < 0.05, two-way ANOVA; Fig. 4C). However, rectal temperatures measured after study periods were also lower in DATph2−/− rats beginning on P13 and remained lower up to P22, even with the ambient temperature warmed and clamped at ∼27°C for all studies (P < 0.05, two-way ANOVA; Fig. 4D). This suggests that the observed reductions in V̇e in DATph2−/− rats on and after P13 could be driven in part by reduced body temperatures and further suggests that body temperature control was impaired during postnatal development in the DATph2−/− rats.
Fig. 4.
Ventilation and body temperatures were reduced in DATph2−/− rats at distinct time points during development. Minute ventilation (V̇e; ml·min−1·100 g−1; A), breathing frequency (breaths/minute; B), tidal volume (VT; ml/100 g; C), and rectal temperatures (body temperature; °C; D) were plotted across postnatal age in 2- or 3-day bins. Note that resting V̇e is reduced in DATph2−/− rats (green; n = 7–15) compared with controls (WT and Het; blue; n = 6–15), due to a combination of reductions in frequency and VT. Note also that body temperatures in DATph2−/− rats were markedly lower compared with controls beginning on P13 despite the ambient temperature being warmed to ∼27°C. *Significant effect of genotype within age (P < 0.05, two-way ANOVA).
5-HT Precursor Treatment Partially Restored CNS 5-HT Biosynthesis and Augmented Eupneic Ventilation
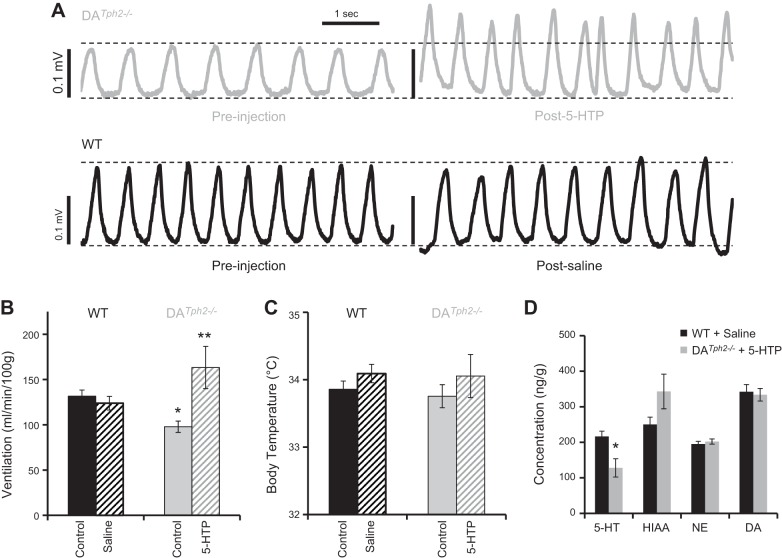
One advantage of the DATph2−/− rat model is the presence of the raphe neurons that express DDC, thus providing a pathway to circumvent Tph2 and produce endogenous 5-HT by supplying 5-HTP. We tested if three intraperitoneal 5-HTP injections (10 mg/kg) separated by 12 h was sufficient to restore CNS 5-HT in DATph2−/− rat pups and if the production of 5-HT was sufficient to restore breathing to WT levels at an age when DATph2−/− rats have reduced breathing (P12-16). Before 5-HTP treatment, DATph2−/− pups had significantly reduced eupneic V̇e (Fig. 5, A and B) and breathing frequency (P < 0.05; 2-way RM ANOVA). Saline injections had no effects on V̇e, breathing frequency or VT in WT littermates (Fig. 5, A and B; data not shown). However, 5-HTP treatment in DATph2−/− pups significantly increased V̇e, VT, and f (P < 0.05; 2-way RM ANOVA) from preinjection control, where V̇e (and VT) were greater in 5-HTP-treated DATph2−/− pups compared with saline-treated WT pups (P < 0.05, two-way RM ANOVA; Fig. 5, A and B). Whole brains collected from 5-HTP-treated DATph2−/− pups within 1.5 h after injection confirmed the presence of CNS 5-HT and its metabolite 5-HIAA, although the whole brain 5-HT levels were lower than saline-injected controls (P < 0.05, two-tailed t-test; Fig. 5D). HIAA levels in 5-HTP-treated DATph2−/− pups were slightly higher than WT controls (P = 0.063), and the 5-HT turnover ratio (5-HIAA/5-HT) was significantly greater in the treated DATph2−/− pups (3.4 ± 0.5) compared with controls (1.2 ± 0.1; P < 0.001, two-tailed t-test; not shown) similar to previous studies (28). In contrast, brain levels of dopamine and NE (Fig. 5D) and HVA (66.4 ± 9.2 in WT vs. 75.9 ± 15.5 ng/g; data not shown) were not different after saline or 5-HTP treatment in WT or DATph2−/− pups, respectively (P > 0.05, two-tailed t-test). DOPAC was not detectable in whole brain extracts from WT or DATph2−/− pups.
Fig. 5.
Resting ventilation is augmented in DATph2−/− rats treated with 5-HTP. A: representative plethysmographic recordings of basal ventilation (measured in voltage deflections) of individual DATph2−/− and WT rat pups from P12-16 breathing room air before (left) and after 3 injections of 5-HTP (10 mg/kg) or saline, respectively, administered over 24 h (right). Mean group data (±SE) for V̇e (B; expressed as ml·min−1·100 g−1) and body temperature (C; °C) in DATph2−/− rats (n = 9) and WT littermates (n = 12) while breathing room air. Saline injections in WT pups had no effect on V̇e, but 5-HTP injections increased V̇e compared with preinjection control and compared with saline-treated WT pups. *Significant effect of genotype in room air (RA); **significant differences from preinjection control and saline-injected WT pups in B and C (P < 0.05, two-tailed t-test). D: HPLC measurements of whole brains from the saline-injected WT and 5-HTP-treated DATph2−/− rats demonstrated the presence of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) after treatment, with little or no effects on norepinephrine (NE) or dopamine (DA) levels [P < 0.05; two-way repeated-measures (RM) ANOVA; *significant difference from saline-treated WT rats].
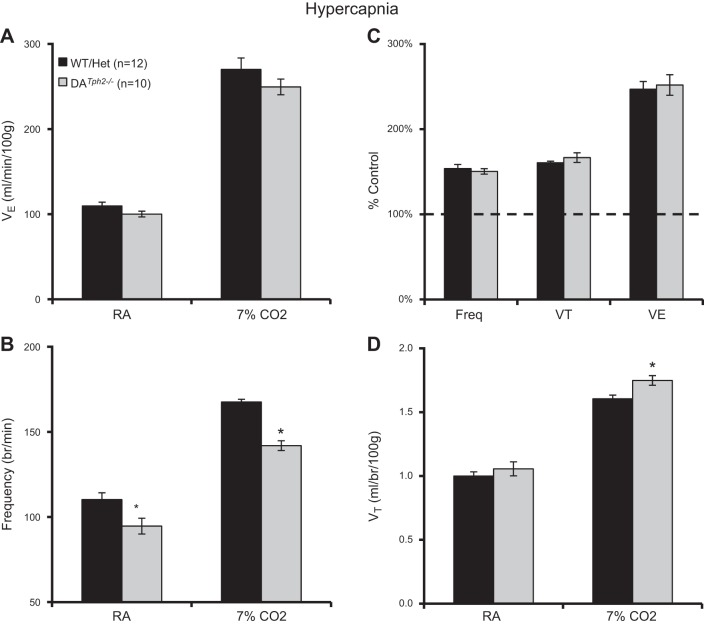
Tph2 Knockout Had Little Effects on Breathing, Chemoreflexes, or Temperature Control in Adult Rat
Based on the effects of 5-HT neuron (or 5-HT) depletion in mouse models, we tested the effects of Tph2 knockout in rats on eupneic breathing, ventilatory chemoreflexes, and temperature control in adult rats. Arterial blood gases obtained in a small number of male DATph2−/− rats (n = 3) showed values for resting PaCO2 (31.6 ± 0.2 mmHg), PaO2 (86.8 ± 0.8 mmHg), and arterial pH (7.474 ± 0.001 units) that were well within the normal range for adult rats shown previously (16, 36). Eupneic V̇e and VT were comparable among adult male DATph2−/− rats compared with control (WT and Het) rats (P > 0.05, two-way ANOVA; Fig. 6, A and D) but had a slightly reduced breathing frequency at rest (P < 0.05, two-way ANOVA; Fig. 6B). V̇e, f, and VT increased significantly during hypercapnia compared with baseline (7% CO2; P < 0.05). Breathing frequency was lower (P < 0.001) and VT was greater (P = 0.018) in DATph2−/− rats compared with control rats such that there was no overall difference among genotypes in V̇e during hypercapnia (P > 0.05, two-way ANOVA; Fig. 6A), consistent with the comparisons of breathing frequency, VT, and V̇e expressed as a percentage of control (room air) values (P > 0.05, two-way ANOVA; Fig. 6C). The same breathing measurements (in room air and 7% CO2) in adult female rats showed similar results when we compared absolute V̇e, VT, and breathing frequency among groups of control (WT and Het; n = 6) and DATph2−/− (n = 6), with no apparent differences in the hypercapnic ventilatory responses among genotypes when ventilation was expressed as a percentage of control (P > 0.05, two-way ANOVA; data not shown).
Fig. 6.
Minimal effects of Tph2 knockout on ventilation at rest or during hypercapnia in adult male rats. Ventilation (V̇e; ml·min−1·100 g−1; A), breathing frequency (breaths/min; B), V̇e [expressed as a percentage of room air breathing (%control); C], and tidal volume (VT; ml·breath−1·100 g−1; D) were compared among control (WT and Het) and DATph2−/− adult rats. Breathing frequency was significantly lower in room air and 7% CO2, and VT slightly greater in 7% CO2 in DATph2−/− rats compared with controls. *Significant effect of genotype (P < 0.05, two-way RM ANOVA).
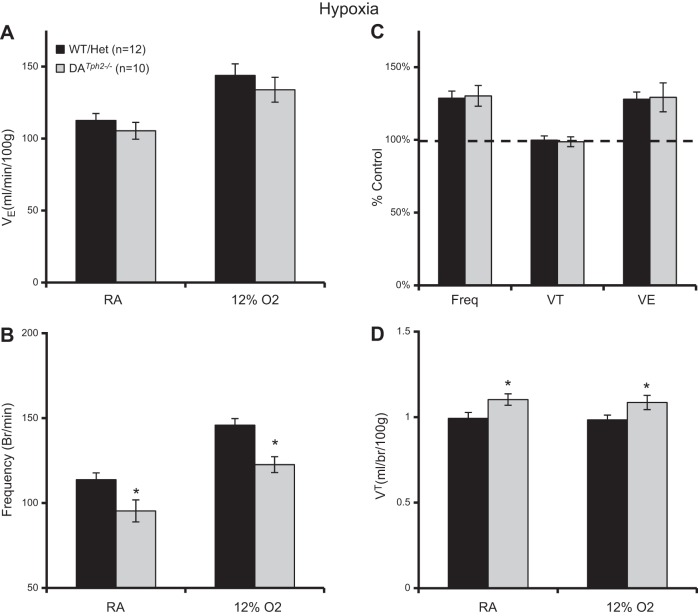
In separate experiments, we noted that V̇e was not different (P > 0.05; Fig. 7A) in adult male DATph2−/− rats compared with controls, despite having a significantly reduced frequency (P = 0.014) and increased VT (P = 0.031) while breathing room air at rest before hypoxia (Fig. 7, B and D; two-way ANOVA). Similarly, we found a significantly reduced breathing frequency (P = 0.002) and increased VT (P = 0.045) during hypoxia in DATph2−/− rats compared with controls, with no overall differences in weight normalized V̇e (Fig. 7A). In addition, none of these breathing parameters were different when expressed as a percentage of room air values (Fig. 7C). Overall, the data suggest that Tph2 knockout depressed breathing frequency under all conditions but also increased VT during hypoxia and CO2 breathing. Thus eupneic ventilation and blood gases, and hypercapnic or hypoxic ventilatory chemoreflexes, were comparable in DATph2−/− mutants and controls, indicating little or no effect of 5-HT depletion on these mechanisms in adult rats.
Fig. 7.
Minimal effects of Tph2 knockout on ventilation at rest or during hypoxia in adult rats. Ventilation (V̇e; ml·min−1·100 g−1; A), breathing frequency (breaths/min; B), V̇e [expressed as a percentage of room air breathing, (%control); C], and tidal volume (VT; ml·breath−1·100 g−1; D) were compared among control (WT and Het) and DATph2−/− adult rats. Breathing frequency was significantly lower and VT higher in DATph2−/− rats compared with controls when exposed to either RA or 12% O2. *Significant effect of genotype (P < 0.05; 2-way RM ANOVA).
Finally, given the major role for raphe 5-HT neurons in supporting thermogenic responses to cold exposures (14, 17, 19), we also measured body temperatures in adult control (WT and Het) and DATph2−/− rats while maintained at ambient temperatures of 23°C or 4°C for up to 4 h (Fig. 8). While maintained at a normal ambient (room) temperature, we did not detect any differences in basal body temperatures among the genotypes. In addition, control and DATph2−/− rats were able to maintain body temperatures over the course of 4 h while externally cooled to 4°C (P > 0.05, two-way ANOVA; Fig. 8). Thus, unlike mouse models of 5-HT neuron deletion or 5-HT depletion, Tph2 knockout in the adult rat appears to have no effects on thermoregulation at rest or during a cold challenge.
Fig. 8.
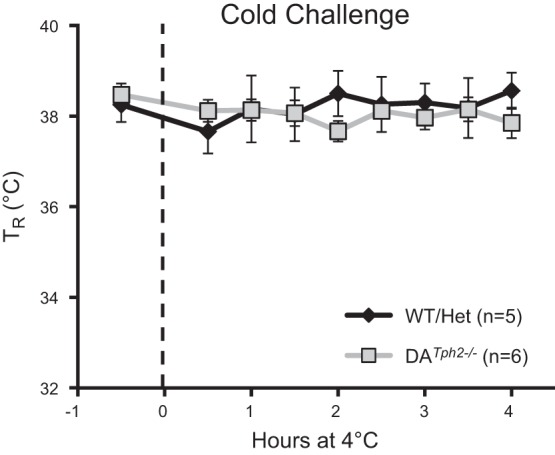
Tph2 knockout rats maintain body temperature during a cold challenge. Rectal temperature (TR; °C) was measured 30 min before (room temperature; 23°C) and at 30-min intervals for up to 4 h while exposed to an ambient temperature of 4°C. Note that control (WT and Het) and DATph2−/− rats both maintain body temperature during environmental cooling (P > 0.05, two-way ANOVA).
DISCUSSION
Understanding the fundamental roles 5-HT-producing neurons (and the neurochemical 5-HT) play in homeostatic control systems that regulate blood gases, heart rate, blood pressure, and body temperature in animal models has become a major focus of research due in part to the findings of multiple 5-HT system defects in human infants that have succumbed to sudden infant death syndrome (SIDS). A series of studies has shown that a majority of brainstems from SIDS cases exhibit one or more abnormalities, including decreased 5-HT receptor and transporter binding and increased TPH+ neuron counts (8, 22, 23, 38). Duncan et al. (8) showed that tissue levels of both TPH protein and 5-HT (via HPLC) were significantly reduced in SIDS cases compared with controls, indicating that despite the high numbers of 5-HT-producing neurons SIDS is largely defined by a TPH and 5-HT deficiency.
Thus an ideal animal model of SIDS would be one in which all of the identified 5-HT system abnormalities are present in the brainstem. However, there currently are no animal models of SIDS that recapitulate all of the 5-HT system abnormalities. Here, we created a Tph2 knockout rat positing that Tph2 mutations would lead to constitutive, selective, and complete loss of CNS 5-HT while retaining raphe neurons that normally synthesize 5-HT (1). In effect, this approach would account for two major deficits in SIDS, including the abrogation of TPH protein and tissue 5-HT levels (8), although the reductions in 5-HT and TPH protein levels in SIDS cases are relatively modest. Herein, we demonstrated that the DATph2−/− rat lacks TPH immunoreactivity throughout the brainstem but retains raphe neurons that express DDC that would otherwise synthesize 5-HT. Furthermore, these rats maintain normal levels of CNS NE and dopamine, suggestive of a selective CNS 5-HT depletion. Finally, our observations of reduced weights in the DATph2−/− rats are consistent with multiple mouse models of 5-HT neuron deletion and/or constitutive 5-HT deficiency (1, 9, 20, 37), although the mechanisms by which a CNS 5-HT depletion leads to reduced somatic growth remains unclear.
The generation of the DATph2−/− rat model allowed us to test the effects of selective and constitutive 5-HT loss, compared with multiple other mouse models of 5-HT neuron deletion like the Pet-1−/− and Lmx1bf/f/p mice. During postnatal development, the Pet-1−/− and Lmx1bf/f/p mice show severe and frequent apnea and altered breathing patterns, which were comparatively more severe in the Lmx1bf/f/p mouse. In both mouse models, the apnea and altered ventilatory patterns improve with increasing postnatal age. Similarly, the DATph2−/− rats had altered breathing patterns and apnea at early neonatal ages, which were reflected in significantly increased IBI variability and significant reductions in V̇e and breathing frequency 1–2 days after birth. In addition, these apneas and altered breathing patterns rapidly improved (within 2 days), effectively normalizing ventilation and variability in the DATph2−/− rats by approximately P3. Whereas the mouse models of 5-HT neuron deletion continue to breathe regularly thereafter into adulthood, the DATph2−/− rats by comparison showed additional periods of reduced ventilation and increased variability. Beginning at approximately P10-12 we found that DATph2−/− rats had large decreases in VT and breathing frequency, reducing minute ventilation to ∼30% of normal rats at P13-14. Thus there are similarities and differences among these various rodent models in their phenotypic ventilatory patterns across development.
The decrease in basal ventilation in the DATph2−/− rats coincided with increased breathing variability, reduced body temperatures, and additional mortality, which had been relatively stable from P3-P10. DATph2−/− rats exhibited an overall estimated mortality of ∼50%, which is nearly identical to that measured in Tph2−/− mice over a similar period of age (1). Although accurate measurements of mortality in mouse and rat pups is often problematic, the observations combined from Tph2−/− rats (∼50% mortality) and mice (∼50% mortality) suggest that constitutive 5-HT loss has a relatively greater effect on postnatal mortality compared with mouse models of moderate (Pet-1−/− mice; 25% mortality) and complete 5-HT neuron deletion (Lmx1bf/f/p mice; 20% mortality) (9, 20). The reduced body temperatures in DATph2−/− rats during mid-development were striking as they were obtained when they were exposed to elevated environmental temperatures (∼27–28°C). Similar measurements of ventilation or body temperature in the mouse models of 5-HT neuron deletion do not show these effects, even when measurements were obtained in nearly identical conditions of elevated (27°C) ambient temperature (20). Body temperatures in control and DATph2−/− rats were similar at P1-2, suggesting that the early mortality in rats devoid of CNS 5-HT may be linked to disrupted ventilation, but any later mortality (P10-17) may have resulted from a combination of reduced body temperatures and ventilation, neither of which have been shown in the 5-HT neuron deletion models in mice at this time in development.
Genetic manipulation in mice is currently far advanced from that available in the rat, but shifts in technologies are making transgenic (21) and genomic (12) and tissue specific knockout (41) rats increasingly available. Studying the physiology of genetically manipulated rat models has several advantages. Homeostatic control systems are better described and more widely studied in the laboratory rat (although this is rapidly changing). Perhaps more importantly, unlike mice there is a rich data set that describes neurochemical and receptor expression changes within the brainstem nuclei across postnatal development (11, 24–27, 29, 42). A major conclusion of these and supporting physiologic studies is that there appears to be a well-defined “critical window” in rat development at or around P12 where changes in expression of multiple neurotransmitters and receptors could give rise to major mechanistic shifts in homeostatic systems and could represent a period of inherent vulnerability. It is very difficult to equate relative age in rodents and humans, but one could speculate that P12 in rats represents a period of development following the neonatal period and before full neural maturation. While this is a gross comparison, we might gain additional insights into human diseases like SIDS, which has a relatively well-defined peak incidence (2–4 mo of age), through the study of neural control systems in rats that have a well-defined “critical window” of neurochemical activity. Furthermore, we propose that the DATph2−/− rat model could be of particular use in this regard, given that treatment with 5-HTP allows for a degree of control of CNS 5-HT production, although there are difficulties with controlling absolute 5-HT levels in the brain (34). Thus the DATph2−/− rat could be a useful model to study potential age-dependent contributions of 5-HT to homeostatic controls systems like breathing and thermoregulation.
While there are clear benefits in studying this novel rat model, there are also caveats and limitations. For example, while it was clear that there were DDC-expressing raphe neurons remaining in the DATph2−/− rat model, we have not quantified whether or not there is a measurable difference in the total number of DDC-expressing neurons among the genotypes. Furthermore, we did not probe the remaining DDC-expressing raphe neurons of changes in other 5-HT neuron-specific markers that could also have been altered in expression, such as Pet-1, SERT, or others. In addition, there are several neural mechanisms and/or structures that have been documented to change in mouse models with constitutive 5-HT loss, including delayed cortical maturation (37), altered adult neurogenesis (2), serotonergic circuit formation (30), and maternal (1) and social (33) behaviors and potential additional pathological behaviors (35), which could all be affected in the DATph2−/− rat model. Finally, exogenous 5-HTP administered to Tph2−/− rodents can be converted to 5-HT at sites beyond those intended, including many non-5-HT neurons that express DDC (dopaminergic and catecholaminergic neurons). In addition, excessive 5-HT production in the periphery could cause additional side effects, although we did not observe any obvious side effects. It is thus conceivable that some of the physiologic effects observed herein are due to a combination of constitutive 5-HT depletion, potential changes in DDC+ neuron counts, and/or other subtle alterations due to a lack of 5-HT present during development.
While there were a number of respiratory and thermoregulatory phenotypes during development, adult DATph2−/− rats displayed normal ventilation and arterial blood gases at rest while breathing room air, had no thermoregulatory deficits during cooling, and had essentially equivalent ventilatory responses to both hypoxia and hypercapnia compared with control rats. Moreover, these findings were identical in both male and female control and DATph2−/− rats. These findings are in contrast to the modest reductions in the hypoxic ventilatory chemoreflex in Pet-1−/− and Lmx1bf/f/p mice tested in equivalent ambient temperatures (14, 17), the large deficit in the CO2 chemoreflex in Lmx1bf/f/p mice at thermoneutral temperatures, and the complete failure of body temperature maintenance when cooled (19). The blunted CO2 chemoreflex in Pet-1−/− mice was specific to male mice, further contrasting the findings herein. Because in all of these mouse studies the major effects on breathing during development improve with age, and the major deficits observed in adult mice occurred during respiratory and thermoregulatory challenges, it was concluded that there was likely some form of time-dependent compensation or plasticity occurring in these models. The ventilatory deficits during development in DATph2−/− rats also improve with increasing age, which parallel those observed in the mouse models. However, the lack of effects in adult rats lacking CNS 5-HT suggests either that the compensatory mechanisms are more complete in this model or that losing the neurons has a larger impact on these homeostatic reflexes than losing the neurochemical. For example, when Lmx1bf/f/p mice (lacking nearly all 5-HT neurons) are treated with intracerebroventricular 5-HT, the major deficits in the ventilatory CO2 chemoreflex was eliminated but the thermoregulatory failure in the cold was not altered (19). We speculate that the differences in the ability of 5-HT to “rescue” these phenotypes may reflect differences in the nature of the role of the neurochemical 5-HT compared with serotonergic neurons in each neuronal circuit. It is possible that serotonergic neurons, some of which are CO2/pH sensitive, drive downstream respiratory neurons through increased 5-HT release specifically during hypercapnia. It is also possible that 5-HT production (or supplementation) is sufficient to activate other chemoreceptor cells important for this response through increased neuronal excitability, which can explain why intracerebroventricular 5-HT normalized the ventilatory response to hypercapnia. This may fundamentally differ from the role of serotonergic neurons and/or 5-HT in thermoregulatory control, whereby these neurons are activated by descending thermogenic efferents and may signal increased thermogenesis through the release of neurochemicals other than 5-HT, such as substance P, thyrotropin-releasing hormone, or others. In this case, the loss of 5-HT (or its restoration in models lacking serotonergic neurons) would fail to affect the thermogenic response to a cold environment. Thus we conclude that continued experimentation using animal models such as the DATph2−/− rat and Tph2−/− mice will provide additional clarity to these unresolved issues.
We believe that the data herein are unique in that they demonstrate the importance of constitutive CNS production of 5-HT in fundamental homeostatic control systems that are vital to supporting mammalian life during postnatal development, including breathing and body temperature control. In addition, the DATph2−/− rat represents a novel rat model with selective and complete CNS 5-HT depletion that has the advantage of temporal control of 5-HT synthesis through supplemental 5-HTP treatment. Finally, it appears that adult rats chronically lacking CNS 5-HT have little or no deficits in ventilatory or thermoregulatory control, indicative of potential compensatory mechanisms or yet to be determined roles for neurochemicals other than 5-HT released from serotonergic neurons in neural circuits that govern ventilatory chemoreflexes and/or thermogenic responses to cold temperatures.
GRANTS
This work was funded by the Children's Hospital of Wisconsin Research Institute (CRI) and National Institutes of Health Grants HL-097033 and HL-122358 (to M. R. Hodges) and DA-031561 (to A. M. Geurts and M. R. Hodges).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.K., A.E.E., B.M., M.M.P., O.P., A.M.G., and M.R.H. performed experiments; K.K., A.E.E., B.M., M.M.P., O.P., A.M.G., and M.R.H. analyzed data; K.K., A.E.E., B.M., M.M.P., A.M.G., and M.R.H. interpreted results of experiments; K.K., B.M., M.M.P., O.P., A.M.G., and M.R.H. prepared figures; K.K., O.P., A.M.G., and M.R.H. drafted manuscript; K.K., O.P., A.M.G., and M.R.H. edited and revised manuscript; K.K., A.E.E., B.M., M.M.P., O.P., A.M.G., and M.R.H. approved final version of manuscript; A.M.G. and M.R.H. conception and design of research.
ACKNOWLEDGMENTS
We thank Drs. Hubert V. Forster and Kevin J. Cummings for additional insights and comments on the manuscript and also thank Lisa Henderson, Camille Taylor, and Jenifer Phillips for their contributions to the HPLC analyses.
REFERENCES
- 1.Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106: 10332–10337, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alenina N, Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res 277: 49–57, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Cummings K, Commons K, Nattie EE. Destruction of serotonergic neurons in the neonatal rat brainstem decreases breathing and exacerbates apnea-induced bradycardia; evidence for a role for 5-HT signaling in Sudden Infant Death Syndrome (SIDS). Program No. 383.12. Neuroscience 2008 Abstracts. Washington, DC: Society for Neuroscience, 2008, online. [Google Scholar]
- 4.Cummings KJ, Commons KG, Hewitt JC, Daubenspeck JA, Li A, Kinney HC, Nattie EE. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: implications for SIDS. J Appl Physiol 111: 825–833, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol 589: 5247–5256, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia 20: 398–399, 1964. [DOI] [PubMed] [Google Scholar]
- 7.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- 8.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA 303: 430–437, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol 159: 85–101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. IV. distribution of monoamine nerve terminals in the central nervous system. Acta Physiol Scand Suppl 247: 37, 1965. [PubMed] [Google Scholar]
- 11.Gao XP, Liu QS, Liu Q, Wong-Riley MT. Excitatory-inhibitory imbalance in hypoglossal neurons during the critical period of postnatal development in the rat. J Physiol 589: 1991–2006, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325: 433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol 597: 211–225, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol 177: 133–140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodges MR, Echert AE, Puissant MM, Mouradian GC Jr. Fluoxetine augments ventilatory CO sensitivity in Brown Norway but not Sprague Dawley rats. Respir Physiol Neurobiol 186: 221–228, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol 93: 974–983, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol 164: 350–357, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol 173: 256–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodges MR, Tattersall G, Harris MB, McEvoy S, Richerson D, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katter K, Geurts AM, Hoffmann O, Mates L, Landa V, Hiripi L, Moreno C, Lazar J, Bashir S, Zidek V, Popova E, Jerchow B, Becker K, Devaraj A, Walter I, Grzybowksi M, Corbett M, Filho AR, Hodges MR, Bader M, Ivics Z, Jacob HJ, Pravenec M, Bosze Z, Rulicke T, Izsvak Z. Transposon-mediated transgenesis, transgenic rescue, and tissue-specific gene expression in rodents and rabbits. FASEB J 27: 930–941, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, Fingers ST, Youngman M, Habbe D, Fifer WP. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J Neuropathol Exp Neurol 64: 689–694, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. Serotonin and the brainstem in the sudden infant death syndrome: a review. Annu Rev Pathol 4: 517–550, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol 577: 957–970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Wong-Riley MT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol 95: 2285–2291, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Wong-Riley MT. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J Comp Neurol 518: 1082–1097, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Wong-Riley MT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Botzinger complex. J Appl Physiol 92: 923–934, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Jiang Y, Si Y, Kim JY, Chen ZF, Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature 472: 95–99, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YY, Wong-Riley MT. Developmental study of cytochrome oxidase activity in the brain stem respiratory nuclei of postnatal rats. J Appl Physiol 90: 685–694, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry 18: 1106–1118, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Moreno C, Kennedy K, Andrae JW, Jacob HJ. Genome-wide scanning with SSLPs in the rat. Methods Mol Med 108: 131–138, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 19: 67–74, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Mosienko V, Beis D, Alenina N, Wohr M. Reduced isolation-induced pup ultrasonic communication in mouse pups lacking brain serotonin. Mol Autism 6: 13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosienko V, Beis D, Pasqualetti M, Waider J, Matthes S, Qadri F, Bader M, Alenina N. Life without brain serotonin: reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav Brain Res 277: 78–88, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry 2: e122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation (CBD) on ventilation and chemoreflexes in three rat strains. J Physiol 590: 3335–3347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narboux-Neme N, Angenard G, Mosienko V, Klempin F, Pitychoutis PM, Deneris E, Bader M, Giros B, Alenina N, Gaspar P. Postnatal growth defects in mice with constitutive depletion of central serotonin. ACS Chem Neurosci 4: 171–181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puissant MM, Echert AE, Yang C, Mouradian GC Jr, Novotny T, Liu P, Liang M, Hodges MR. RNASeq-derived transcriptome comparisons reveal neuromodulatory deficiency in the CO2 insensitive brown Norway rat. J Physiol 593: 415–430, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber T, Schonig K, Tews B, Bartsch D. Inducible gene manipulations in brain serotonergic neurons of transgenic rats. PLoS One 6: e28283, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol 164: 28–37, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]