Abstract
Dynamic, epigenetic mechanisms can regulate macrophage phenotypes following exposure to different stimulating conditions and environments. However, temporal patterns of microRNAs (miRNAs or miRs) across multiple macrophage polarization phenotypes have not been defined. We determined miRNA expression in bone marrow-derived murine macrophages over multiple time points (0.5, 1, 3, 24 h) following exposure to cytokines and/or LPS. We hypothesized that dynamic changes in miRNAs regulate macrophage phenotypes. Changes in macrophage polarization markers were detected as early as 0.5 and as late as 24 h; however, robust responses for most markers occurred within 3 h. In parallel, many polarization-specific miRNAs were also changed by 3 h and expressed divergent patterns between M1 and M2a conditions, with increased expression in M1 (miR-155, 199a-3p, 214-3p, 455-3p, and 125a) or M2a (miR-511 and 449a). Specifically, miR-125a-5p exhibited divergent patterns: increased at 12–24 h in M1 macrophages and decreasing trend in M2a. VEGF in the culture media of macrophages was dependent upon the polarization state, with greatly diminished VEGF in M2a compared with M1 macrophage culture media despite similar VEGF in cell lysates. Inhibition of miR-125a-5p in media-only controls (MO) and M1 macrophages greatly increased expression and secretion of soluble VEGF receptor-1 (sVEGFR1) leading to diminished VEGF in the culture media, partially converting MO and M1 into an M2a phenotype. Thus, the divergent expression patterns of polarization-specific miRNAs led to the identification and demonstrated the regulation of a specific macrophage polarization phenotype, sVEGFR1 by inhibition of miR-125a-5p.
Keywords: microRNAs, temporal, M1, M2a, M2c, VEGF
macrophages are essential in pathogen defense (48), inflammatory diseases (42), tissue repair/regeneration (45, 59, 76, 80), metabolic disorders (8), allergy (14), atherosclerosis (51), fibrosis (63), and cancer (60). The prevailing model of macrophages in injury and disease is based upon the concept of macrophage polarization, a plastic, agonist-dependent activation resulting in distinct phenotypes. Defined in vitro, macrophage polarization classifies the activation of mature macrophages into two divergent subsets: classically activated (M1) proinflammatory macrophages and alternatively activated (M2) anti-inflammatory macrophages, with further subdivision of M2 into three subsets M2a, M2b, and M2c. M1 macrophages are characterized by increased production of proinflammatory mediators, such as TNF-α, IL-1, and IL-6, increased reactive NO (nitric oxide), and increased killing of phagocytized pathogens (16, 18, 52, 54). M2a macrophages are characterized by increased arginase expression (16, 80), growth factor production such as IGF-1 (82), and mannose receptor expression (75). M2b macrophages exhibit a mixture of inflammatory (TNF-α and IL-6) (37, 85) and anti-inflammatory (IL-10) (21) phenotypes. M2c macrophages achieve a “deactivated” phenotype marked by silencing of cytokine production (53), and there are some reports of anti-inflammatory mediator production (17, 85). The regulatory mechanisms responsible for these distinct phenotypes and means of manipulating polarization states could be used to treat a variety of diseases.
Molecular mechanisms regulating macrophage polarization have revealed multiple transcriptional networks (79) and distinct transcriptome signatures (46). However, epigenetic regulation of macrophage polarization by microRNAs (miRNA or miR) is poorly understood. miRNAs are small, noncoding RNAs ∼22 nucleotides long that regulate gene expression at the mRNA stability/translational level. miRNA regulation of macrophage polarization has been characterized as primarily feedback or fine-tuning based upon miRNA targeting mRNAs that are downstream of proinflammatory pathways (12, 58). However, given the antagonist behavior of transcription factor families (79) and that miRNAs directly target transcription factors and negative regulators controlling major inflammatory pathways, such as miR-9 targets NF-κB1, while miR-155 targets C/EBPβ and SOCS1 (40, 71), miRNAs may actually drive macrophage polarization. Studies of polarization-specific miRNA expression in human (9, 11, 23) and mouse (7, 50, 87) monocytes/macrophages in vitro have mostly focused on a single time point, typically many hours (≥8–18 h) (7, 23, 50, 87) to days (11) following perturbation with polarizing agonists. The implied assumption in these studies is that miRNA expression was perpetually altered following macrophage polarization without consideration of the dynamic temporal patterns of miRNAs during polarization. Limited reports exploring temporal patterns of miRNA in macrophage polarization have studied only a single polarizing agonist (9) or multiple agonists with a limited selection of miRNAs (50). We have defined the temporal expression of multiple macrophage markers during polarization: cytokines, NO, etc. Dramatic changes in polarization markers were evident as early as 0.5 h following stimulation (49). Therefore, the present study has defined miRNA polarization signatures at early time points in macrophage phenotype development.
We recently reported the divergent levels of VEGF in the culture media of M1 and M2a cultures as a novel polarization marker. Extracellular VEGF was drastically decreased from 12 to 24 h in M2a and increased by 24 h in M1 macrophage culture media. Interestingly, VEGF in the cellular lysates was present at low levels in all conditions except for an increase at 24 h in M1 macrophages, corresponding to the elevated VEGF in the M1 culture media (49). Given the comparably low cellular VEGF, the decreased VEGF in M2a culture media was not likely due to cellular uptake, but possibly sequestration or inactivation. A potential candidate for sequestering VEGF is soluble VEGF receptor-1 (sVEGFR1) (81). VEGFR1 is a cell membrane receptor that binds VEGF-A and placental growth factor (81). A secreted, soluble form of VEGFR1 was identified from a truncated transcript derived primarily from alternative splicing of the full-length Vegfr1 (29). sVEGFR1 was capable of sequestering VEGF (81) and functioned as an inhibitory, mimic receptor antagonizing the activation of the membrane forms of VEGFR1. However, sVEGFR1 can also antagonize VEGF signal transduction by forming dominant negative heterodimers with the membrane form of VEGFR1 (30). A better understanding of the mechanisms of polarization-specific VEGF regulation could lead to macrophage-based therapies.
The goal of this study was to determine the early changes and dynamic patterns of polarization-specific miRNA expression across multiple macrophage polarization states. We hypothesized that the dynamic expression patterns of polarization-specific miRNAs will suggest regulatory roles of miRNA in macrophage polarization to guide future studies. This approach led to the identification of divergent temporal expression patterns for miR-125a-5p across polarization conditions and a regulatory role for miR-125a-5p in VEGFR1 expression in macrophages.
MATERIALS AND METHODS
Bone marrow-derived macrophage cultures.
We created L929-conditioned medium by plating 4 × 105 L929 cells (ATCC, Manassas, VA) in 50 ml of media [DMEM/F12 + 10% FBS + 1% penicillin-streptomycin (Life Technologies, Grand Island, NY)] in a 175 mm culture flask (Corning, Corning, NY) for 10 days. L929-conditioned media were filtered (0.22 μm) and stored at −30°C. Bone marrow was harvested bilaterally from the hindlimbs of C57BL/6J 3–4 mo old male mice (Jackson Labs, Bar Harbor, ME). All procedures complied with the National Institutes of Health Animal Care and Use Guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and at the South Texas Veterans Health Care Systems, San Antonio, TX. Bone marrow-derived macrophages (BMDM) were obtained by plating bone marrow at 5 × 106 cells/plate in 10 ml of bone marrow macrophage media #1 (BMM#1: 30% L929-conditioned media + DMEM/F12 + 10% FBS + 1% penicillin-streptomycin; Life Technologies) on 100 mm nontissue culture-treated polystyrene petri dishes (BD Bioscience, San Jose, CA); after day 3, an additional 5 ml of BMM#1 was added to each plate and incubated for an another 3 days.
Cultures for miRNA RT-quantitative PCR array.
BMDM were subcultured (Fig. 1) into 60 mm nontissue culture-treated polystyrene petri dishes (BD Bioscience) at 5 × 106 cells/plate in 3 ml of bone marrow macrophage media #2 (BMM#2: 10% L929-conditioned media + DMEM/F12 + 10% FBS + 1% penicillin-streptomycin; Life Technologies) and allowed to adhere and proliferate with a medium change every 24 h. BMDMs were stimulated with IFN-γ (20 ng/ml, Miltenyi Biotech, San Diego, CA; prepared in PBS + 1% FBS) + LPS (1,000 ng/ml, Invivogen, San Diego, CA; prepared in PBS + 1% FBS) for M1, IL-4 (20 ng/ml, Miltenyi Biotech; prepared in PBS + 1% FBS) for M2a, or IL-10 (20 ng/ml, Miltenyi Biotech; prepared in PBS + 1% FBS) for M2c, or maintained in BMM#2 as a media-only control. Cells were either cultured for 0.5, 1, 3, 6, 12, or 24 h following this media change or collected as a 0 h time point.
Fig. 1.
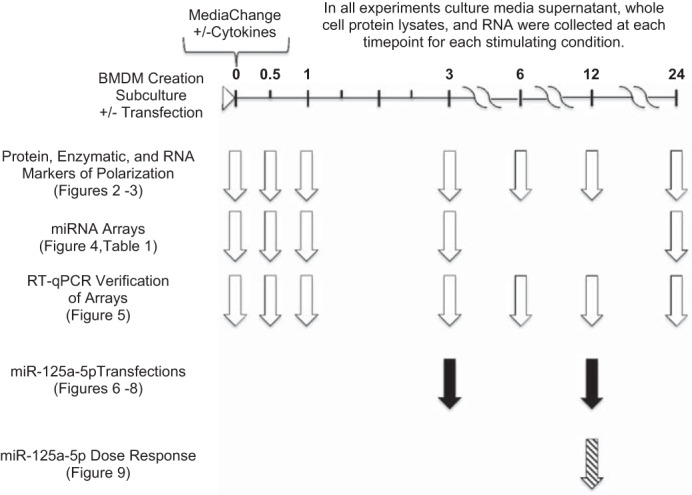
Experimental design and timeline. All bone marrow-derived macrophage (BMDM) cultures were created similarly, subcultured, ± transfected, media change with media only or media with cytokines, and collected at specified times (0, 0.5, 1, 3, 6, 12, or 24 h) after media change. Study design was separated into 3 experimental series shown by the white, black, or hashed arrows. Assays, measurements, and treatments conducted in these experiments are noted on the left with associated figures and tables included. All experiments were n = 3–4 biological replicates per condition and time point.
Collection of samples.
Cell culture media (CM) were collected at the indicated time points (Fig. 1) for n = 4, centrifuged (1,000 g) at 4°C for 10 min, and supernatants were aliquoted and stored at −80°C. Cells were lysed for RNA with QIAzol (Qiagen, Valencia, CA). In parallel plates, cells were collected and counted, and cellular lysates were prepared at 107 cells/ml in lysate buffer (69), aliquoted, and stored at −80°C.
Cellular and CM cytokine quantification.
Cytokines (TNF-α, IL-1α, and IL-6) in cellular protein lysates (Fig. 1, n = 4) and in CM were determined by Myriad Rules Based Medicine (Austin, TX) using microsphere-based immuno-multiplexing technology to quantify analyte concentrations. In addition, samples of BMM#2 were analyzed to detect cytokines present in the L929-conditioned media prior to contact with cells.
Supernatant NO quantification.
NO in the CM (Fig. 1, n = 4) was quantified by use of Griess reagent per manufacturer's protocol (Promega, Madison, WI).
Arginase enzyme assay.
Arginase activity was determined by use of a well-established assay (10). In brief, cellular protein lysates (Fig. 1, n = 4) in the presence of 100 mM MnCl2 (Sigma-Aldrich) were incubated at 56°C for 6 min to activate arginase. The substrate, 0.5 M arginine (Sigma-Aldrich), was added and incubated at 37°C for 2 h to allow for the enzymatic conversion of arginine to urea. An acid mixture consisting of 1:3:7 H3PO4/H2SO4/H2O (EMD) was added to urea standards (Sigma-Aldrich) and to samples to stop the reaction. An indicator, 6% α-isonitrosopropiophenone (Sigma-Aldrich), was added to standards and samples and incubated at 95°C for 30 min followed by incubation at 4°C for an additional 30 min. Samples and standards were read at 540 nm on a microplate spectrophotometer. Arginase activity was calculated: [(μg urea/molecular weight of urea) × (dilution factor/time in minutes of arginine incubation)]. Data were reported as units of arginase activity: specifically, 1 unit of arginase activity = the amount of enzyme required to hydrolyze 1 μM of arginine per minute.
RNA extraction.
RNA was extracted using miRNeasy kit (Qiagen) per manufacturer's protocol without DNase treatment. A NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE) was used to measure RNA concentration and adjusted to 150 ± 1 ng/μl. The RNA purity was confirmed by an A260/A280 ratio of ∼2. RNA quality in each sample was determined by gel electrophoresis and analysis of the 28S and 18S rRNA peaks (Agilent 2100 Bioanalyzer; Santa Clara, CA). All RNA samples used in array studies had RNA integrity numbers ≥ 9.
Quantitative PCR miRNA array.
A total of 300 ng of RNA per 7.5 μl reaction for a selection of samples including time points (0.5, 1, 3, 24 h) for n = 3/time point/polarization state (Fig. 1) were converted to cDNA using TaqMan Megaplex RT Primer Rodent Pools (both A and B) v3.0 and TaqMan MicroRNA Reverse Transcription Kit (Life Technologies). cDNA (1.25 μl per 12.5 μl reaction) was preamplified using TaqMan Megaplex PreAmp Primer Rodent Pools (both A and B) v3.0 (Life Technologies). Preamplified cDNA was diluted to 100 μl by addition of 88.5 μl 1× Tris-EDTA Buffer (Promega). miRNA quantification was determined in TaqMan Low Density miRNA Array Rodent Cards A+B Set v3.0 with TaqMan Universal PCR Master Mix, no AmpErase UNG (cat. #4324018, Life Technologies) on a 7900HT Fast Real Time PCR machine (Life Technologies). Primers/probes for a plant miRNA (ath-miR-159a) included on both cards were used as a spike-in control to detect aberrations in RNA extraction, aliquoting, and amplification. All samples used in this study yielded consistent cycle threshold (Ct) values for the spike-in control miRNA resulting in 3.7 and 4.2% coefficient of variation (CV) on the A card and B card, respectively, indicating consistency in sample preparation and handling. Additionally, a nontemplate control (water only) indicated low (>35) to absent signal (undefined) for all miRNAs of interest. Raw Ct values were obtained by use of ExpressionSuite Software v1.0.3. The ΔΔCt relative expression analysis method was performed as per the manufacturer. Ct values of each group were averaged, normalized to the most stable (lowest % CV) endogenous controls [snoRNA135 (A card), snoRNA202 (B card)] to obtain the ΔCt values, compared with the ΔCt value of the 0 h time point to obtain the ΔΔCt values, and transformed to reflect the fold change relative to 0 h time point. Fold change was presented as an average linkage heat map.
Verification of miRNA array: quantitative real-time PCR.
RNA was converted to cDNA for all time points for n = 3 (Fig. 1) and preamplified as described above. Each 10 μl PCR reaction included 0.1 μl of preamplified/diluted cDNA and a 9.9 μl mixture of TaqMan (Life Technologies) primer/probe miRNA expression assays for mmu-miRs-125a-3p (assay ID #2199), -125a-5p (assay ID #2198), -155-5p (assay ID #2571), or -511-5p (assay ID #2549), and TaqMan Universal PCR Master Mix, no AmpErase UNG (cat. #4324018, Life Technologies) per the manufacturer and run on a 7900HT Fast Real Time PCR machine. Raw Ct values were obtained by use of SDS Software v2.4. Range of raw Cts were mmu-miR-125a-3p (26.6–31.7), -125a-5p (19.8–23.5), -155-5p (16.9–24.0), -511-5p (25.6–28.8), and snoRNA55 (19.7–23.1). Results were normalized to snoRNA55 (assay ID #1228; %CV = 2.5) and analyzed with the ΔΔCt method as described by the manufacturer. Data were reported as −ΔΔCt ± SE relative to the 0 h time point. Reporting adheres to MIQE (minimum information for publication of quantitative real-time PCR experiments) recommendations (6).
BMDM cultures for miR-125a-5p manipulation.
BMDM were subcultured (Fig. 1, n = 4) into 12-well nontissue culture-treated polystyrene plates (BD Bioscience) at 0.9 × 106 cells/well in 0.5 ml of BMM#2 and allowed to adhere and proliferate with a media change every 24 h. Transfection of 20 nM of Scramble #2 Mimic (cat. #CN-0020000-01-05; GE Dharmacon, Lafayette, CO), miR-125a-5p Mimic (cat. #C-310392-07-005, GE Dharmacon), Scramble A Inhibitor (cat. #199006-001 Exiqon, Woburn, MA), or miR-125a-5p Inhibitor (cat. #4104499-001, Exiqon) was conducted with Lipofectamine RNAiMax (Life Technologies) per manufacturer forward transfection protocol in 0.5 ml of BMM#2 without antibiotics. Six hours after transfection, the media were replaced with either BMM#2 without additional cytokines (media only, MO) or BMM#2 containing IFN-γ + LPS (for M1) or IL-4 (for M2a) and incubated for 3 or 12 h. A sample of each medium (Media) was aliquoted, snap-frozen, and stored at −80°C. CM and RNA were harvested at 3 or 12 h from parallel cultures following the media change per the collection of sample protocol previously detailed.
Quantitative real-time PCR.
The same RNA input, reaction volumes, and RT kit reagents used for the array miRNA RT- quantitative (q)PCR were used; except individual (not pooled) primer/probe miRNA expression assays for mmu-miR-125a-5p (assay ID #2198) and snoRNA55 (assay ID #1228) were used with TaqMan Universal PCR Master Mix, no AmpErase UNG (cat. #4324018, Life Technologies) per the manufacturer and run on a 7900HT Fast Real Time PCR machine. Raw Ct values were obtained by use of SDS Software v2.4. Ranges of raw Cts were mmu-miR-125a-5p (10.4–31.6) and snoRNA55 (20.4–23.4). Results were normalized to snoRNA55 (%CV = 1.8) and analyzed with the ΔΔCt method as described by the manufacturer. Data are reported as normalized values (−ΔCt), means ± SE. Reporting adheres to MIQE recommendations (6).
For gene expression, a total of 1,350 ng of RNA input per 20 μl were converted to cDNA via TaqMan High Capacity cDNA Reverse Transcriptase Kit (Life Technologies). Each 10 μl PCR reaction included 1 μl of cDNA and a 9 μl mixture of TaqMan (Life Technologies) primer/probe gene expression assay for Irf4 (assay ID #Mm00516431_m1), Vegfr1 (also known as Flt1, assay ID #Mm00438980_m1), or Vegfa (assay ID #Mm01281449_m1), and TaqMan Universal PCR Master Mix (Life Technologies) per the manufacturer and run on a 7900HT Fast Real Time PCR machine. Raw Ct values were obtained by use of SDS software v2.4. Ranges of raw Cts were Irf4 (22.4–33.2), Vegfr1 (21.4–29.3), Vegfa (18.2–24.6), and Gapdh (14.8–17.9). Results were normalized to Gapdh (assay ID #Mm99999915_g1; CV% < 4) and analyzed with the ΔΔCt method as described by the manufacturer. Data were reported as −ΔΔCt relative to 0 h time point for Irf4 to better display diminished expression in the M1 condition, while Vegfr1 and Vegfa were presented as normalized and transformed values (not relative expression), 2−ΔCt due to the lack of twofold diminished expression relative to any appropriate control. Reporting adheres to MIQE recommendations (6).
Enzyme-linked immunosorbent assays.
CM from each culture condition and time point and media (BMM#2) were assayed for sVEGFR1 (cat. #MVR100) and VEGF (cat. #MMV00) (R&D Systems, Minneapolis, MN) per manufacturer's instructions. All samples had detectable levels of sVEGFR and VEGF.
miRNA gene target discovery.
Experimentally observed and highly predicted gene targets of the miR-125a-5p/125b-5p/351-5p cluster (with seed sequence of CCCUGAG) were obtained using the miRNA target filter in Qiagen's Ingenuity Pathway Analysis (IPA, Qiagen Redwood City, http://www.qiagen.com/ingenuity) software. Target genes were further filtered based upon their membership in specific pathways reported to be regulate Vegfr1 [ERK/MAPK (13), PI3K/Akt (61), HIF-1α (56), IL-4 signaling (83), GM-CSF signaling (19, 83), p38 MAPK (83, 84), and Wnt/Ca+ (73, 74)] and verified in TargetScanMouse release 6.2 (38).
Statistical analysis.
miRNA array data were preprocessed to mitigate the effect of technical variation and noise related to large Ct values. For values Ct >35, outlying values were systematically removed for each (n = 3) time point/stimulation combination according to a majority-rule so that if one out of three had a Ct value >35 and the other two were <35, the Ct value >35 was set to missing. Likewise, if one out of the three values were <35 and the other two were >35, the value <35 was set to missing. We applied quantile normalization to the Ct values from all arrays to minimize the effects of technical variation; the normalized Ct values were used in downstream analyses. miRNAs that were ≥2-fold changed in only one stimulating condition (M1, M2a, or M2c) at any time point were extracted from the whole dataset for statistical analysis (48 miRNAs total).
For the filtered miRNA array dataset (48 miRNAs) we used a two-way ANOVA to identify changes due to time and stimulation factors. We tested the interaction between time and stimulation to determine whether trajectories over time were different between at least two stimulations. To identify the differences at each time point between stimulations, we performed one-way ANOVA with the stimulation factor and applied Tukey's honest significant difference (HSD) test for all pairwise comparisons between stimulations at that time point. To identify changes over time for each stimulation, we performed one-way ANOVA with time as the factor and applied Tukey's HSD for all pairwise comparisons between time points. The Benjamini-Hochberg correction was applied to compute the false discovery rate (FDR) to account for multiple hypotheses for each type of comparison. Those miRNAs that exhibited P ≤ 0.05 and an FDR ≤ 0.05 comprised our final dataset (12 miRNAs; Fig. 4). The array analysis was performed with R v3+ (Vienna, Austria) software.
Fig. 4.
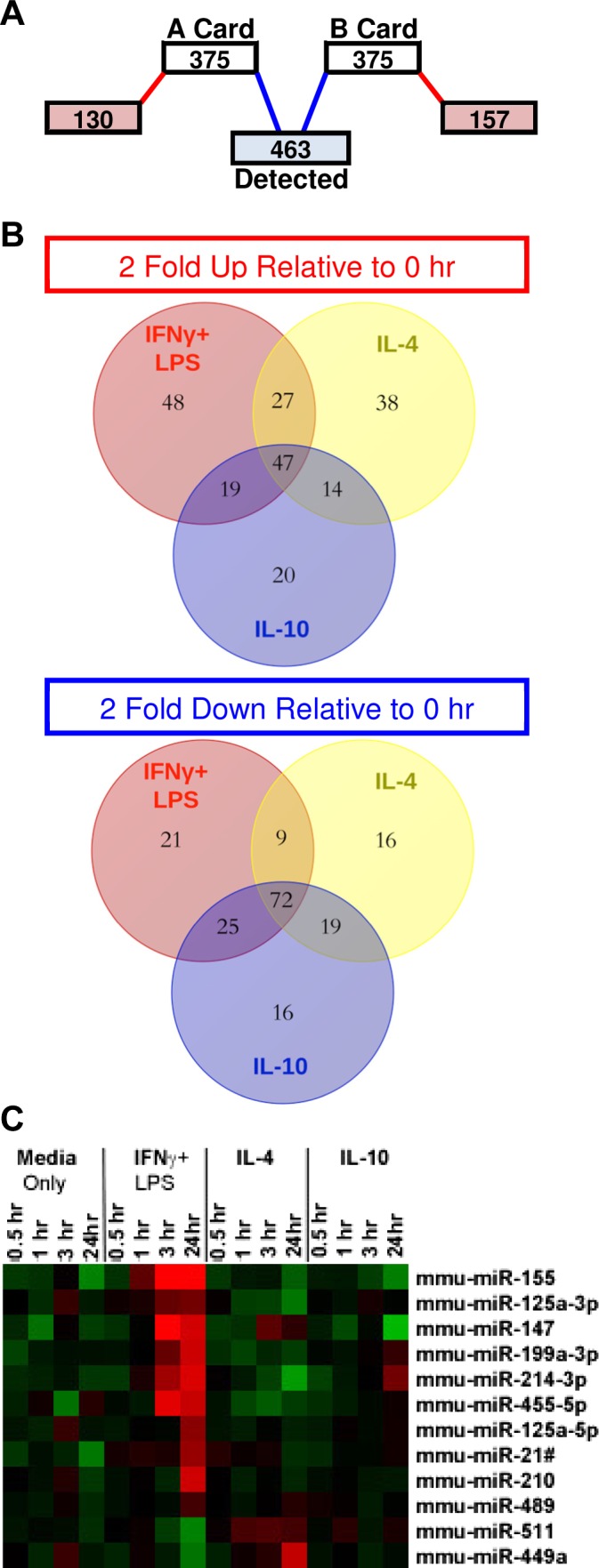
Temporal expression of polarization-specific microRNAs (miRNAs). Control (Media Only), IFN-γ + LPS (M1), IL-4 (M2a), or IL-10 (M2c) conditions were used to achieve macrophage polarization. miRNA expression was measured at various time points after stimulation including a 0 h time point prior to stimulation. A: miRNAs that were not detected in any samples were eliminated; 463 miRNAs were analyzed. B: distribution of miRNAs that exhibited either ≥2-fold increase or decrease relative to the miRNA levels in the 0 h sample in each stimulating condition. C: average linkage heat map demonstrating the temporal expression patterns of miRNAs that exhibited significant (P ≤ 0.05, FDR ≤ 0.05), polarization-specific 2-fold upregulation; n = 3 separate primary cultures/stimulating condition/time point.
Verification of miRNA array (Fig. 5) was analyzed with a two-way ANOVA. All data were log-transformed before statistical analysis. All data that failed a Kolmogorov-Smirnov (KS) normality test were log-transformed before statistical analysis. A Dunnett's test adjustment for multiple comparisons was performed to compare result from each stimulating condition (M1, M2a, M2c) to the MO control at the corresponding time point with a two-sided significance threshold of P ≤ 0.05.
Fig. 5.
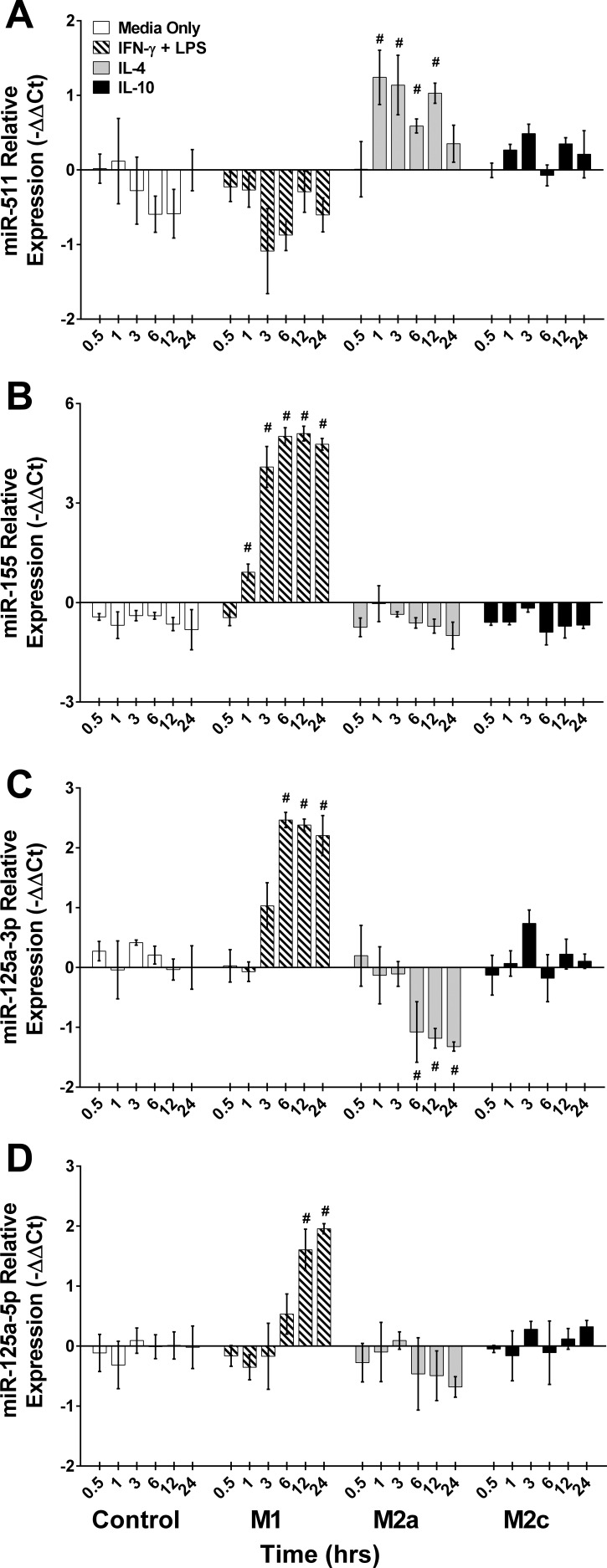
Verification of divergently expressed miRNAs from miRNA array. Single tube RT-qPCR assay performed on samples used in the miRNA arrays to verify miRNA expression. Control (Media Only), IFN-γ + LPS (M1), IL-4 (M2a), or IL-10 (M2c) conditions were used to achieve macrophage polarization. miRNA expression were measured at various time points after stimulation including a 0 h time point prior to stimulation. A: miR-511, B: miR-155, C: miR-125a-3p, and D: miR-125a-5p. Data presented as −ΔΔCt, means ± SE; #P ≤ 0.03 compared with media-only control at the corresponding time point; n = 3 biological replicates/stimulating condition/time point in all experiments.
All other data were analyzed with a two-way ANOVA to assess the effects of time and stimulation. Results below the level of detection were assigned a value equivalent to the square root of the lowest detectable limit (27). All data that failed a KS normality test were log-transformed before statistical analysis. A Dunnett's test adjustment for multiple comparisons was performed to compare result from each stimulating condition (M1, M2a, M2c) to the MO control at the corresponding time point (Figs. 2, 3, and 5) and to compare results from each relevant Scramble (Mimic or Inhibitor) to the miR-125a-5p Mimic/Inhibitor result at the same time point in each condition (MO, M1, or M2a) (Figs. 6–8) with a two-sided significance threshold of P ≤ 0.01. A Bonferroni test adjustment for multiple comparisons was performed to compare result from each Scramble to the same dose of miR-125a-5p Inhibitor result (Table 1 and Fig. 9) with a two-sided significance threshold of P ≤ 0.01. Unless otherwise noted, all statistical analysis was performed with GraphPad Prism 6 v.6.03 (La Jolla, CA); data are presented as means ± SE.
Fig. 2.
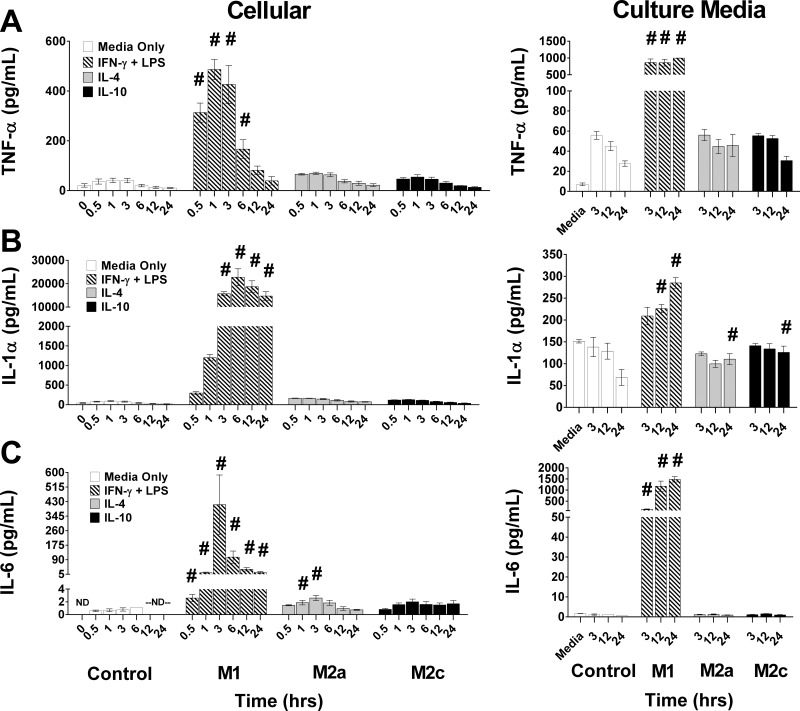
Temporal changes in cellular and culture medium (CM) proinflammatory cytokines in polarized macrophages. Control (Media Only), IFN-γ + LPS (M1), IL-4 (M2a), or IL-10 (M2c) conditions were used to achieve macrophage polarization. Cytokines were measured at various time points after stimulation in cellular protein lysates (Cellular, left column), including a 0 h time point prior to stimulation and in the culture media (right column), including the conditioned media [bone marrow macrophage medium #2 (BMM#2), Media] not exposed to cells. Protein levels of TNF-α (A), IL-1α (B), and IL-6 (C), data are reported as means ± SE; #P ≤ 0.01 compared with media-only control at the corresponding time point; n = 3 biological replicates/stimulating condition/time point in all experiments.
Fig. 3.
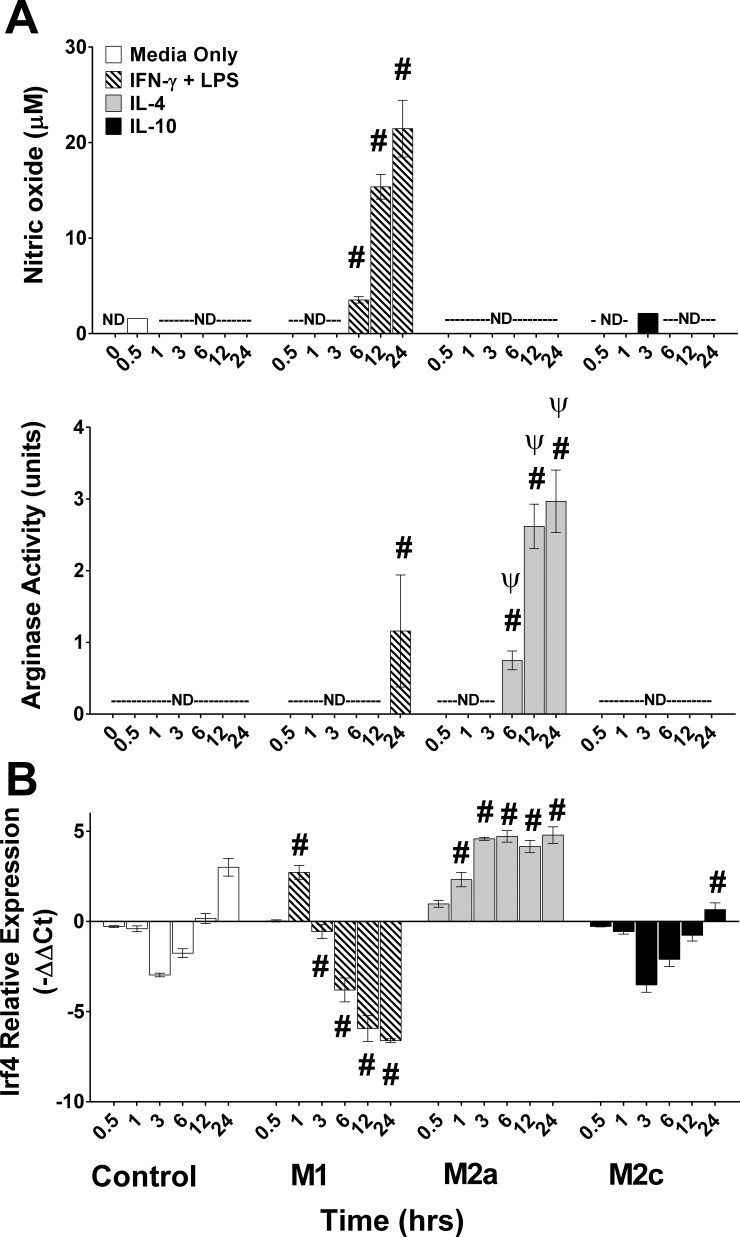
Temporal changes in enzyme activities and Irf4 mRNA levels in polarized macrophages. Control (Media Only), IFN-γ + LPS (M1), IL-4 (M2a), or IL-10 (M2c) conditions were used to achieve macrophage polarization. Nitric oxide (NO) in culture media, arginase activity in cellular protein lysates, and Irf4 mRNA expression were measured prior to (0 h) or at various time points after stimulation. A: NO in the CM and cellular arginase activity. B: Irf4 mRNA. Data reported as means ± SE; #P ≤ 0.002 compared with media only control at corresponding time point; ΨP < 0.001 compared with IFN-γ + LPS condition at corresponding time point; n = 3 biological replicates/stimulating condition/time point in all experiments.
Fig. 6.
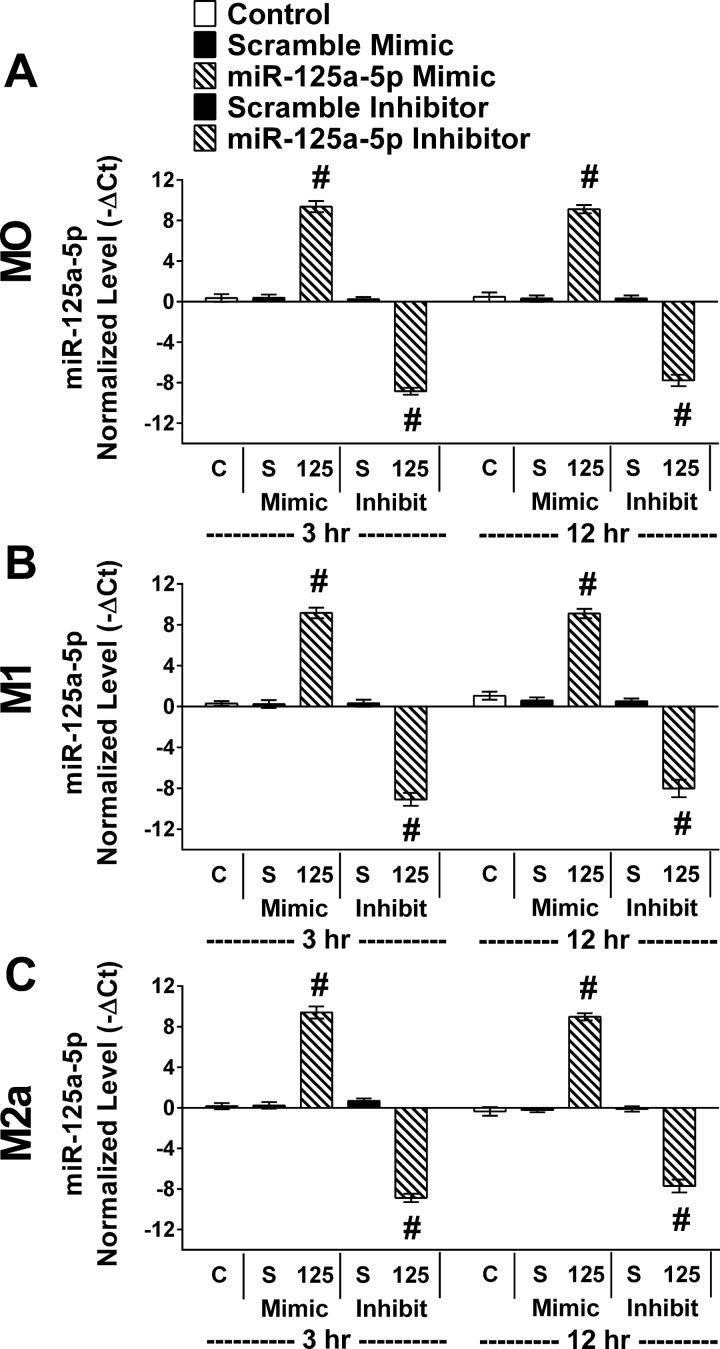
miR-125a-5p levels following miR-125a-5p manipulation. In parallel, control cultures without transfection (C), transfected with relevant scramble (S) or miR-125a-5p inhibitor/mimic (125). Six hours after transfection the media were changed and BMDMs were either maintained in BMM#2 without added cytokines [media only (MO), top row] or stimulated with IFN-γ + LPS (M1, middle row) or IL-4 (M2a, bottom row) for 3 or 12 h before RNA collection. RT-qPCR was performed using primers for mmu-miR-125a-5p and snoRNA55. Raw Ct values were mmu-miR-125a-5p (10.4–31.2) and snoRNA55 (20.4–22.7). Results were normalized to snoRNA55. Data reported as normalized values (−ΔCt), means ± SE; #P ≤ 0.01 compared with relevant scramble; n = 4 biological replicates/stimulating condition/time point in all experiments.
Fig. 8.
miR-125a-5p inhibition elevated both Vegfr1 and Vegf mRNAs in BMDMs. In parallel, control cultures without transfection (C), transfected with relevant scramble (S) or miR-125a-5p inhibitor/mimic (125). Six hours after transfection the media were changed and BMDMs were either maintained in media only (MO, top row) or stimulated with IFN-γ + LPS (M1, middle row) or IL-4 (M2a, bottom row) for 3 or 12 h before RNA collection. RT-qPCR was performed using primers for (A, C, E) Vegfr1 and (B, D, F) Vegfa to measure gene expression in all experimental groups. Raw Ct values were Vegfr1 (21.4–29.3), Vegfa (18.2–24.6), and Gapdh (14.8–17.9). Results were normalized to Gapdh. Data reported as normalized and transformed values (2−ΔCt), means ± SE; #P ≤ 0.006 compared with relevant scramble; n = 4 biological replicates/stimulating condition/time point in all experiments.
Table 1.
microRNA specifically and significantly altered temporally during macrophage polarization
| All Time Points and Conditions |
Across Conditions at Relevant Time Point |
|||||
|---|---|---|---|---|---|---|
| Condition | microRNA | Overall P Value | FDR | Time Point, h | P Value | FDR |
| M1 related | mmu-miR-155 | 8.3E-09 | 7.2E-08 | 1 | 0.08 | 0.53 |
| 3 | 1.8E-04 | 2.5E-03 | ||||
| 24 | 7.4E-09 | 3.9E-07 | ||||
| mmu-miR-125a-3p | 1.3E-04 | 6.2E-04 | 3 | 2.9E-04 | 3.6E-03 | |
| 24 | 2.1E-04 | 2.4E-03 | ||||
| mmu-miR-147 | 2.4E-04 | 0.01 | 3 | 2.0E-03 | 0.02 | |
| 24 | 2.7E-05 | 4.4E-04 | ||||
| mmu-miR-199a-3p | 0.01 | 0.05 | 3 | 0.08 | 0.44 | |
| 24 | 5.3E-03 | 0.03 | ||||
| mmu-miR-214-3p | 6.4E-03 | 0.02 | 3 | 0.03 | 0.18 | |
| 24 | 9.9E-04 | 7.3E-03 | ||||
| mmu-miR-455-5p | 0.01 | 0.04 | 3 | 3.5E-03 | 0.03 | |
| 24 | 0.05 | 0.18 | ||||
| mmu-miR-125a-5p | 2.5E-03 | 0.01 | 24 | 4.6E-04 | 3.9E-03 | |
| mmu-miR-21# | 8.8E-05 | 4.4E-04 | 3.5E-04 | 3.5E-03 | ||
| mmu-miR-210 | 4.5E-07 | 3.0E-06 | 4.0E-04 | 3.9E-03 | ||
| mmu-miR-489 | 4.8E-12 | 5.8E-11 | 1.5E-05 | 2.9E-04 | ||
| M2a related | mmu-miR-511 | 8.0E-05 | 4.0E-04 | 1 | 0.02 | 0.16 |
| 3 | 4.1E-04 | 4.8E-03 | ||||
| mmu-miR-449a | 3.6E-03 | 0.01 | 24 | 3.4E-04 | 3.5E-03 | |
microRNAs (miRNA or miR) that were specifically up- or downregulated (≥2-fold) relative to the 0 h time point in only 1 stimulating condition [M1 (IFN-γ + LPS) or M2a (IL-4)] and were statistically significant across 3 biological replicates/time point/condition. The miRNAs chosen for this dataset must have an overall P value ≤0.05 and false discovery rate (FDR) of ≤0.05. Additionally, miRNAs in this dataset must have exhibited a P value ≤0.05 and FDR ≤0.05 when compared with the other stimulating conditons in at least 1 time point.
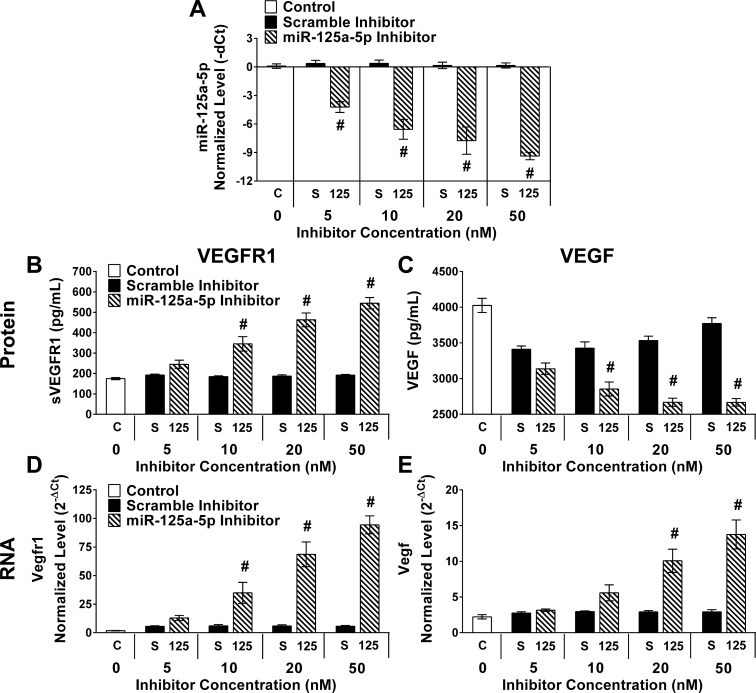
Fig. 9.
Titration of miR-125a-5p inhibitor elevated VEGFR1 and depressed VEGF in a dose-dependent manner. Control cultures without transfection (C, 0 nM), transfected with scramble (S) or miR-125a-5p inhibitor (125) at various doses (5, 10, 20, 50 nM). Six hours after transfection the media were changed and BMDMs were maintained in media only for 12 h before culture media and RNA collection. RT-qPCR was performed using primers for mmu-miR-125a-5p and snoRNA55 (A). Raw Ct values were mmu-miR-125a-5p (20.2–31.6) and snoRNA55 (20.5–23.4). Results were normalized to snoRNA55. ELISA assays for sVEGFR1 (B) and VEGF (C) were performed on culture media from experimental groups. RT-qPCR was performed using primers for Vegfr1 (D) and Vegfa (E) to measure gene expression in all experimental groups. Raw Ct values were Vegfr1 (24.0–28.9), Vegfa (19.9–21.8), and Gapdh (15.9–17.6). Results were normalized to Gapdh and reported as normalized and transformed values (2−ΔCt). Data reported as means ± SE; #P ≤ 0.007 compared with scramble at the same dose; n = 4 biological replicates/stimulating condition/time point in all experiments.
RESULTS
Temporally measured markers revealed the time course for macrophage polarization.
To validate our in vitro model, we measured cytokines, enzyme activities, and gene expression induced with polarization states in classically activated M1, wound-healing M2a, and M2c macrophages (44, 52) at multiple time points following stimulation with polarizing cytokines and/or LPS. Though many studies have measured cytokines in cell CM of stimulated macrophages, we measured cytokines in both the CM and in whole cell protein lysates (Cellular) to define the earliest changes in cytokines (Fig. 2). Established proinflammatory cytokines, TNF-α, IL-6, and IL-1α, rapidly increased in the cellular compartment following stimulation with IFN-γ + LPS. Cellular IL-1α and IL-6 increased (P < 0.001) and reached peak levels by 3 h, while TNF-α (P < 0.001) was maximum after only 0.5–3 h of stimulation compared with the MO controls at the same time point. Cellular IL-1α remained elevated throughout 24 h, whereas cellular TNF-α and IL-6 rapidly decreased 3 h after induction of polarization. In contrast to the dynamic changes in cellular cytokines, TNF-α in the CM was increased (P < 0.001) and stable 3–24 h. IL-1α and IL-6 progressively increased in the CM. Cellular and CM levels of these cytokines were similar or only slightly increased relative to MO controls in IL-4- and IL-10-stimulated macrophages.
Arginase and iNOS are enzyme markers of IL-4- and IFN-γ + LPS-stimulated macrophages, respectively (16), and this observation was confirmed in our macrophage culture system. Increased (P < 0.001) cellular arginase activity and NO in the CM were both evident by 6 h, further increased at 12 h, and remaining elevated through 24 h after stimulation in IL-4 and IFN-γ + LPS conditions, respectively (Fig. 3A). Importantly, NO was not detectable in the media of control, IL-4-, or IL-10-stimulated conditions. Unexpectedly, we detected increased (P < 0.001) arginase activity in the IFN-γ + LPS-stimulated macrophages at only the 24 h time point, although this activity was much lower (P < 0.001) compared with IL-4-stimulated macrophages.
Lastly, an mRNA associated with IL-4-stimulated macrophages, Irf4 (66), was temporally measured. Irf4 expression was elevated (P ≤ 0.002) and was maximally expressed by 3–24 h of IL-4 stimulation (Fig. 3B). In contrast, Irf4 was decreased (P < 0.001) by 6–24 h in IFN-γ + LPS-stimulated macrophages. This divergent expression pattern clearly distinguished M1 and M2a polarization conditions. In contrast to the well-defined phenotypic markers of IL-4 and IFN-γ + LPS-stimulated macrophages, we did not observe expression of any M2c specific markers, consistent with previous reports that IL-10-stimulated cells resulted in a “silencing” phenotype (47, 53).
With few exceptions (iNOS and arginase), most macrophage markers were significantly changed within 3 h of stimulation, suggesting that 3 h may represent an important time point for studying the regulation of macrophage polarization. However, the diversity of temporal patterns indicated that earlier time points (0.5–3 h) present the best window to study regulatory mechanisms in macrophage polarization, including miRNA expression.
Temporal polarization-specific miRNA levels in cytokine/LPS-stimulated BMDMs.
Given that most macrophage polarization markers increased by 3 h of stimulation, miRNA expression was examined at early (0.5, 1, and 3 h) or late (24 h) time points after stimulation. Of the 750 total miRNAs measured (Fig. 4A), 287 were not detected in any samples and were eliminated. The remaining 463 miRNAs were detected in at least one replicate of one condition at one time point. A distribution of miRNAs with a twofold increase or decrease (relative to the 0 h time point) at any time point within each stimulating condition was used to identify polarization-specific miRNAs. Some miRNAs were uniquely upregulated (2-fold) only after IFN-γ + LPS (48 miRs), IL-4 (38 miRs), or IL-10 (20 miRs) stimulation (Fig. 4B). Similarly, some miRNAs were uniquely downregulated (2-fold) only after IFN-γ + LPS (21 miRs), IL-4 (16 miRs), and IL-10 (16 miRs) stimulation (Fig. 4B). Filtering based upon statistical significance culminated in the final dataset of 12 polarization-specific miRNAs (Fig. 4C); statistical analyses are provided in Table 1. This approach to discover miRNAs was supported by the inclusion of many previously reported, polarization-specific miRNA in the final dataset, such as miR-155 (2, 4, 26, 57, 65, 77), miR-147 (41), and miR-125a-5p (5, 9). miRNAs specifically upregulated by IL-4 stimulation included miR-511 and -449a, while IFN-γ + LPS induced the expression of miRs-155, -125a-3p, -147, -199a-3p, -214-3p, -455-5p, -125a-5p, -21#, -210, and -489. miRNAs associated with IL-10 stimulation were eliminated due to lack of statistical significance.
Several miRNAs were divergently expressed in IL-4 vs. the IFN-γ + LPS conditions (miR-449a, -155, -125a-3p, -214-3p, -455-5p, -125a-5p, and -210). We verified a selection of these divergently expressed miRNA using a pooled single tube assay RT-qPCR (Fig. 5, A–D). As anticipated, divergently expressed miR-511-5p (Fig. 5A), miR-155-5p (Fig. 5B), miR-125a-3p (Fig. 5C), and miR-125a-5p (Fig. 5D) demonstrated in the miRNA array were confirmed using the same RNA extracted in the miRNA array experiments in single RT-qPCR assays with additional time points included. miR-511-5p and -155-5p increased (P ≤ 0.03) by 1 h of stimulation in IL-4 and IFN-γ + LPS-stimulated conditions, respectively. In particular, we became interested in miR-125a-5p as our analysis revealed a divergent polarizing trend in the -3p strand and moderately in the -5p strand (Fig. 5, C and D). While both strands similarly increased in the magnitude of upregulation with IFN-γ + LPS stimulation, the -3p strand increased (P < 0.001) by 6 h, while the increase (P = 0.003) in the -5p strand was evident at a later time point (12 h).
miR-125a-5p inhibition increased sVEGFR1 in BMDMs.
Our previous studies characterized polarization-specific VEGF changes in CM of IL-4 and IFN-γ + LPS-stimulated cultures. VEGF is present in L929-conditioned media (22) and was also high in the media (BMM#2) used in all our conditions (Table 2). Consistent with our previous study (49), VEGF in the CM precipitously decreased (P < 0.001) compared with Media in IL-4-stimulated BMDM cultures by 12 h (Table 2). As previous work demonstrated low and stable cellular VEGF over time, suggesting that decreased VEGF was not secondary to cellular uptake from the CM (49), we hypothesized decreased VEGF in the CM may be due to sequestration by a soluble receptor for VEGF, sVEGFR1. This was confirmed by a large sVEGFR1 increase (P < 0.001) compared with Media in the CM of IL-4-stimulated cultures at 12 h (Table 1). Previous work revealed increased VEGF in CM following IFN-γ + LPS stimulation and concomitant decrease in Vegfr1 expression after 24 h exposure but not at earlier time points of 3 or 12 h (49). The late pattern of increased expression (12–24 h) of miR-125a-5p immediately preceded and corresponded to increased VEGF in the IFN-γ + LPS condition, suggesting a possible mechanism. We hypothesized that inhibition of miR-125a-5p would result in increased sVEGFR1 with resultant decreases in VEGF in CM.
Table 2.
VEGF and sVEGFR1 in media or in culture media after 12 h of macrophages exposed to MO, M1, or M2a conditions
| VEGF, pg/ml |
sVEGFR1, pg/ml |
|||
|---|---|---|---|---|
| Condition | Media | Culture Media | Media | Culture Media |
| MO | 4,105 ± 57 | 3,806 ± 80 | 191 ± 6 | 208 ± 5 |
| M1 | 4,143 ± 97 | 3,536 ± 137 | 182 ± 7 | 248 ± 11 |
| M2a | 3,829 ± 191 | 493 ± 41# | 186 ± 9 | 2,793 ± 323# |
“Media” is the bone marrow macrophage media (BMM#2) used in experiments containing 10% FBS and 10% conditioned media from L929 cells (media only “MO”) plus the addition of IFN-γ + LPS (M1) or IL-4 (M2a). Vascular endothelial growth factor (VEGF) and soluble vascular endothelial growth factor receptor 1 (sVEGFR1) were measured in the Media (BMM#2 with or without polarizing agonists) prior to contact with cells and in the “Culture Media” of macrophages 12 h after exposure in MO, M1, or M2a conditions. Data presented as means ± SE, n = 4 biologic replicates/stimulating condition,
P < 0.001 compared with Media.
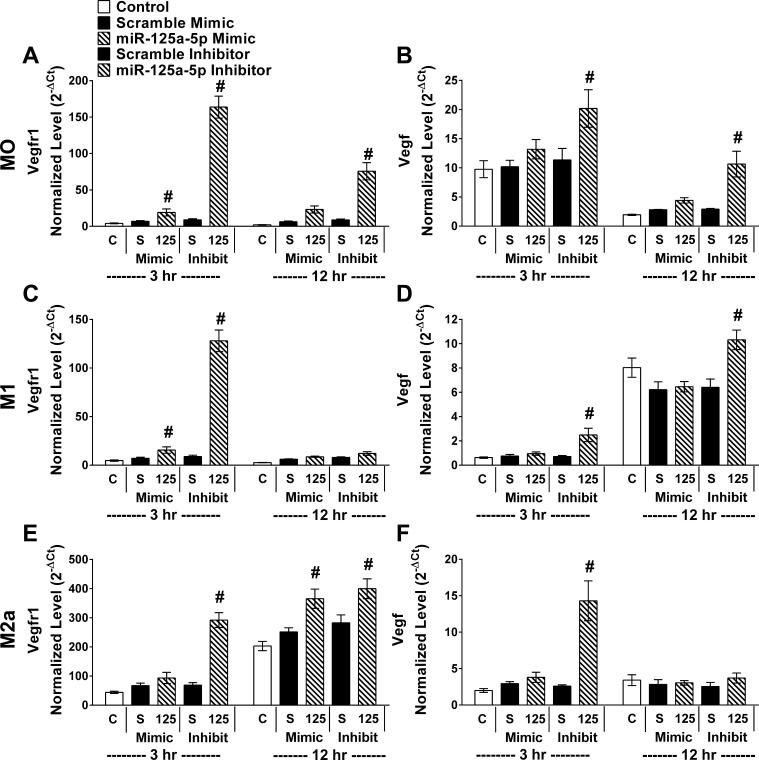
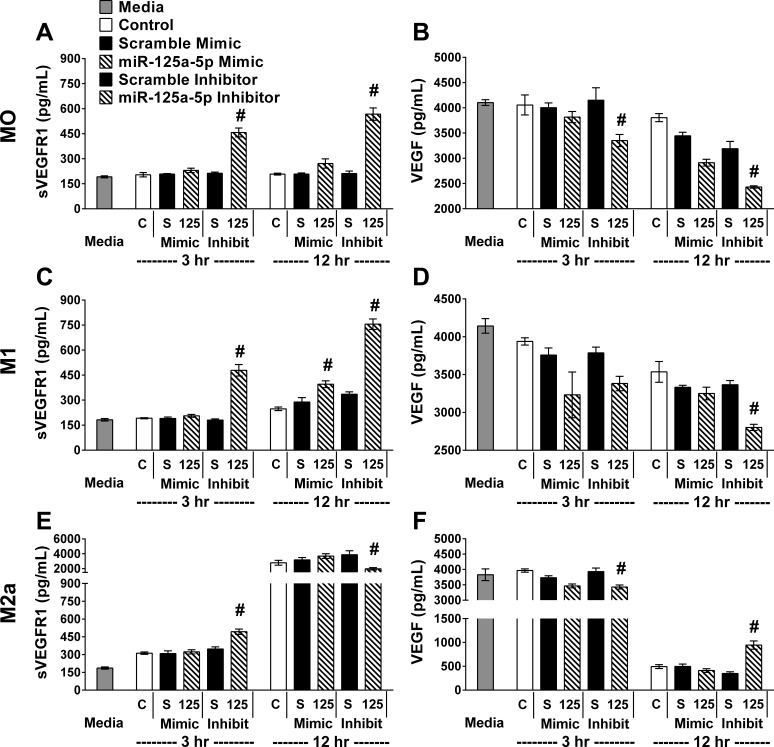
Following transfection of miR-125a-5p mimics or inhibitors in each condition (MO, M1, or M2a), the expected changes in miR-125a-5p levels were confirmed to be over eightfold increased or decreased (P < 0.001) compared with scramble mimic or inhibitor, respectively, for each condition (Fig. 6). Interestingly, transfection with the scramble mimic or inhibitor resulted in comparable miR-125a-5p and was remarkable similar to controls that did not receive any transfecting reagents in each stimulating condition. Thus, transfection did not alter miR-125a-5p levels. sVEGFR1 (Fig. 7, left column) and VEGF (Fig. 7, right column) were measured in the BMM#2 prior to exposure to cells (Media), in CM of BMDMs that were never transfected (Control), or in CM of BMDMs that were transfected with scrambles or miR-125a-5p mimics or inhibitors. These experimental groups were repeated in each condition and cultured for 3 or 12 h (Fig. 7). Regardless of stimulating condition (MO, M1, or M2a), the sVEGFR1 in the CM was elevated (P ≤ 0.01) in miR-125a-5p-inhibited cultures at 3 h (Fig. 7, A, C, E) relative to the scramble inhibitor with corresponding decreases (P < 0.001) in MO and M2a in VEGF in the CM of miR-125a-5p-inhibited cultures at 3 h (Fig. 7, B and F). After 12 h, sVEGFR1 progressively increased (P < 0.001) with miR-125a-5p inhibition relative to scramble inhibitor in MO and M1 conditions. sVEGFR1 was dramatically elevated (P < 0.001) and VEGF drastically diminished (P < 0.001) in every experimental group in the 12 h IL-4 condition (Fig. 7, E and F). While still highly elevated compared with MO and M1 conditions, a small decrease (P < 0.001) in sVEGFR1 (Fig. 7E) with miR-125a-5p inhibition in the IL-4 condition relative to the scramble inhibitor was observed with a concomitant small increase (P < 0.001) in VEGF (Fig. 7F). Interestingly, increasing miR-125a-5p levels with the mimic did not alter sVEGFR1 or VEGF in any condition or time point.
Fig. 7.
miR-125a-5p inhibition elevated sVEGFR1 and decreased VEGF in BMDMs. In parallel, control cultures without transfection (C), transfected with relevant scramble (S) or miR-125a-5p inhibitor/mimic (125). Six hours after transfection the media were changed and BMDMs were either maintained in media only (MO, top row) or stimulated with IFN-γ + LPS (M1, middle row) or IL-4 (M2a, bottom row) for 3 or 12 h before culture media collection. ELISA assays for sVEGFR1 (A, C, E) and VEGF (B, D, F) were performed on BMM#2 media without additional cytokines (in MO) or with cytokines added (M1 or M2a) before exposure of the media to cells (Media) or on culture media from experimental groups. Data reported as means ± SE; #P ≤ 0.01 compared with relevant scramble; n = 4 biological replicates/stimulating condition/time point in all experiments.
miR-125a-5p inhibition increased expression of Vegfr1 in BMDMs.
To determine if the increased sVEGFR1 was regulated at the transcriptional level, we measured expression of Vegfr1 (Fig. 8, left column) and Vegfa (Fig. 8, right column) in each experimental group using RNA extracted from the same macrophage cultures used to measure sVEGFR1 and VEGF in CM (Fig. 7). Consistent with the elevation of sVEGFR1 protein with miR-125a-5p inhibition, Vegfr1 mRNA was elevated (P < 0.001) at 3 h in all conditions (Fig. 8, A, C, E). However, expression of Vegfr1 by 12 h, while still increased compared with scramble inhibitor (P < 0.001), was decreased in the MO conditions relative to the 3 h time point and was comparable to the scramble inhibitor in the M1 condition. In M2a macrophages, Vegfr1 remained elevated (Fig. 8E, P ≤ 0.02) at 12 h. Notably, the relatively small decrease (P < 0.001) in sVEGFR1 protein observed in the M2a condition at 12 h (Fig. 7E) was not explained by decreased expression of Vegfr1 (Fig. 8E). Unexpectedly, inhibition of miR-125a-5p increased (P ≤ 0.01) expression of Vegfa in MO and M1 conditions at both 3 and 12 h (Fig. 8, B and D), or only at 3 h (M2a, Fig. 8C, P < 0.001) compared with scramble inhibitor. Interestingly, Vegfa elevation was observed with miR-125a-5p inhibition when VEGF in the CM was significantly diminished (comparing Fig. 7 and 8, B, D, F). Additionally, the relatively small increase (P < 0.001) in VEGF present at 12 h in the miR-125a-5p inhibited M2a condition (Fig. 7F) did not correspond to increased expression of Vegfa in the same samples (Fig. 8F). Consistent with the protein data, convincing changes in Vegfr1 and Vegfa were not observed in miR-125a-5p mimic samples under any condition.
Dose response in sVEGFR1 and VEGF with miR-125a-5p inhibition.
Given the lack of an opposing phenotype with the miR-125a-5p mimic, a possible explanation could be a nonspecific effect of the inhibitor to produce changes in sVEGFR1. Therefore, a titration of the inhibitor and scramble were conducted with the hypothesis that a dose response in the inhibitor and absence of the same response in the scramble control would provide evidence of a specific effect. Increasing doses of miR-125a-5p inhibitor progressively diminished (P < 0.001) miR-125a-5p without any effect in scramble transfected conditions (Fig. 9A). Both culture media protein (Fig. 9, B and C) and RNA (Fig. 9, D and E) were measured for VEGFR1 (Fig. 9, B and D) and VEGF (Fig. 9, C and E) in cultures maintained in MO for 12 h following transfection of 5, 10, 20, or 50 nM of miR-125a-5p inhibitor/scramble or no transfection (0 nM). A clear dose response was present with progressively increased (P < 0.001) sVEGFR1 (Fig. 9A) and decreased (P < 0.001) VEGF (Fig. 9B) with increasing amounts of miR-125a-5p inhibitor contrasting with comparable sVEGFR1 and VEGF regardless of the dose of scramble inhibitor used. Expression of Vegfr1 mirrored the dose-dependent changes in sVEGFR1 (Fig. 9C) with increased (P ≤ 0.007) in Vegfr1 expression relative to scramble inhibitor detectable at the 10 nM dose. The increased (P < 0.001) expression of Vegfa was most readily observed at a higher dose (20 nM) than the most precipitous change of VEGF protein at the lower dose (10 nM), suggesting that increased Vegfa expression may be in response to the diminished VEGF protein levels.
Predicted gene targets of miR-125a-5p in pathways associated with Vegfr1 regulation.
To predict gene targets, the miRNA target filter in the Ingenuity Pathway Analysis software was used and revealed 985 experimentally observed and highly predicted gene targets of miR-125a-5p. Unfortunately, the regulation of Vegfr1 transcription and production of VEGFR1 and sVEGFR1 are not fully understood. However, several studies have minimally explored the effect of perturbing specific pathways upon Vegfr1 expression and VEGFR1/sVEGFR1 production. This literature supports a regulatory role for the ERK/MAPK (13), PI3K/Akt (61), HIF-1α (56), IL-4 signaling (83), GM-CSF signaling (19, 83), p38 MAPK (83, 84), and Wnt/Ca+ (73, 74) pathways in Vegfr1 expression. Therefore, the 985 gene targets were further filtered to include those involved in these high-yield pathways, resulting in 32 individual genes (Table 3), 29 of which are highly predicted targets and three that are experimentally observed (20, 39, 86).
Table 3.
Potential miR-125a-5p targets in pathways implicated in Vegfr1 regulation
| ERK/MAPK (13) | PI3K/Akt (61) | HIF-1α (56) | IL-4 (83) | GM-CSF (19, 83) | p38 MAPK (83, 84) | Wnt/Ca+ (73, 74) | |
|---|---|---|---|---|---|---|---|
| BclII | # | ||||||
| Ccr5 (39) | Ψ | ||||||
| Creb1 | # | # | # | ||||
| Dvl3 | # | ||||||
| Eif4ebp1 [4e-bp1] | # | # | |||||
| Epo | # | ||||||
| Ets1 | # | # | |||||
| Gab2 | # | ||||||
| Il1rn [Il1ra] | # | ||||||
| Irf4 | # | ||||||
| Mapk12 [p38γ] | # | # | |||||
| Mapk14 [p38α] | # | # | |||||
| Mapkapk2 | # | ||||||
| Mcl1 | # | ||||||
| Mef2d | # | ||||||
| Mknk2 [Mnk2] | # | # | |||||
| Mmp11 | # | ||||||
| Subunits of PI3K | |||||||
| Pik3c2b | # | # | # | # | # | ||
| Pik3cd | # | # | # | # | # | ||
| Pik3r5 | # | # | # | # | # | ||
| Ppp1ca | # | ||||||
| Subunits of PP2 | |||||||
| Ppp2ca | # | # | |||||
| Ppp2r4 | # | # | |||||
| Ppp2r5c | # | # | |||||
| Rps6ka1 [Rsk1] | # | # | |||||
| Smo (20) | Ψ | ||||||
| Srf | # | # | |||||
| Stat3 | # | # | |||||
| Tnfrsf1b [CD120b, p75, Tnfr2] | # | ||||||
| Trp53 [p53] (86) | Ψ | Ψ | Ψ | ||||
| Traf6 | # | ||||||
| Vegfa | # |
Highly predicted (#) and experimentally observed (Ψ) targets (rows) of the miR-125a-5p/125b-5p/351-5p cluster (with seed sequence of CCCUGAG) that are also members of pathways (columns) associated with changes in Vegfr1 expression were obtained using the miRNA target filter in Ingenuity Pathway Analysis and verified with TargetScanMouse. Better known synonyms for each gene are included in brackets. BclII: B cell leukemia/lymphoma 2, Ccr5: chemokine (C-C motif) receptor 5, Creb1: cAMP responsive element binding protein 1, Dvl3: dishevelled segment polarity protein 3, Eif43bp1: eukaryotic translation initiation factor 4E binding protein 1, Epo: erythropoietin, Ets1: E26 avian leukemia oncogene 1, 5′ domain, Gab2: growth factor receptor bound protein 2-associated protein 2, Il1rn: interleukin 1 receptor antagonist, Irf4: interferon regulatory factor 4, Mapk12: mitogen-activated protein kinase 12, Mapk14:mitogen-activated protein kinase 14, Mapkapk2: MAP kinase-activated protein kinase 2, Mcl1: myeloid cell leukemia sequence 1, Mef2d: myocyte enhancer factor 2D, Mknk2: MAP kinase-interacting serine/threonine kinase 2, Mmp11: matrix metallopeptidase 11, Pik3c2b: phosphoinositide-3-kinase, class 2, beta polypeptide, Pik3cd: phosphatidylinositol 3-kinase catalytic delta polypeptide, Pik3r5: phosphoinositide-3-kinase, regulatory subunit 5, p101, Ppp1ca: protein phosphatase 1, catalytic subunit, alpha isoform, Ppp2ca: protein phosphatase 2 (formerly 2A), catalytic subunit, alpha isoform, Ppp2r4: protein phosphatase 2A activator, regulatory subunit B, Ppp2r5c: protein phosphatase 2, regulatory subunit B', gamma, Rps5ka1: ribosomal protein S6 kinase polypeptide 1, Smo: smoothened, frizzled class receptor, Srf: serum response factor, Stat3: signal transducer and activator of transcription 3, Tnfrsf1b: tumor necrosis factor receptor superfamily, member 1b, Trp53: transformation related protein 53, Traf6: TNF receptor-associated factor 6, Vegfa: vascular endothelial growth factor A.
DISCUSSION
The sophistication of macrophage polarization and the plasticity exhibited between states suggest complex regulatory mechanisms. One potential mechanism is posttranscriptional/translational control by miRNA. While some work has been conducted to define polarization-specific miRNAs (7, 9, 11, 23, 50, 87), the current study has extended the understanding of miRNA regulation of macrophage polarization. Our work has revealed early temporal changes in miRNA expression in macrophages following exposure to polarizing cytokines/agonists. This approach identified miRNAs with divergent patterns across polarization states and time providing insights into the role of miRNAs in macrophage polarization. Of the identified polarization-specific miRNAs, miR-125a-5p exhibited a later temporal pattern (elevation 12–24 h in M1 condition) coinciding with VEGF elevations (24 h) in the CM of M1 macrophages (49). Our results suggested that macrophages, regardless of stimulating condition, increased production and secretion of sVEGFR1 with miR125a-5p inhibition with a resultant and consistent decrease in VEGF in the CM.
It was vital to define the earliest evidence of macrophage polarization to determine the optimal time points to study miRNA regulation. Phenotypic markers including cytokines, growth factors, enzyme activities, and transcription factor mRNAs were used to temporally characterize macrophage polarization. Proinflammatory cytokines TNF-α, IL-1α, and IL-6 were prominently elevated following IFN-γ + LPS (M1) stimulation in the cellular lysates very early (by 0.5 h) followed by secretion into the CM as early as 3 h, and possibly even earlier, indicating rapid mechanisms of activation. Secretion of another proinflammatory macrophage marker, NO, exhibited a more delayed pattern (by 6 h), suggesting macrophage polarization was temporally coordinated. As previously reported, markers of IL-4 (M2a) stimulated macrophage phenotypes occurred later relative to that of M1 stimulated macrophage phenotypes (49), as exhibited by arginase (by 6 h) and Irf4 (plateau by 3 h). The divergent expression of the M2a-related transcription factor, Irf4, provided evidence of macrophage polarization M1 vs. M2a; down- vs. upregulation, respectively. Another M2a-related mRNA previously identified by our group was Vegfr1, whose expression reached a plateau by 3 h of stimulation that was maintained through 24 h. However, the divergent nature of this mRNA marker was not detectable in IFN-γ + LPS-stimulated macrophages until 24 h of stimulation (49). Rapid macrophage polarization detected by early changes in polarization marker expression/production argued the need to study earlier time points (0.5–3 h) than previously used (8 h–5 days) (7, 11, 23, 87). Additionally, autocrine/paracrine effects of secreted agonists may dramatically alter the regulatory landscape of miRNA expression causing secondary, compensatory changes (e.g., feedback inhibition) and therefore may not reflect initial changes contributing to macrophage polarization.
The early temporal approach to miRNA expression during macrophage polarization in the current study provided patterns consistent with previous work while also revealing novel miRNAs. Consistent with previous studies, miRs-155, -125a, and -147 exhibited increased expression in IFN-γ + LPS-stimulated macrophages (2–5, 7, 11, 23, 41, 50, 55, 57, 65). With regard to temporal changes, miR-155 (23, 65), -147 (41), and -125a (5, 23) expression was consistent with the patterns identified in this study. More specifically, miR-155 was highly expressed as early as 1–3 h and remained elevated. In contrast, miR-125a-5p was most reliably elevated by 12–24 h with the -3p strand increasing earlier (6–24 h). Interestingly, miR-155 promoted (2–4, 15, 34, 55) and miR-125a-5p inhibited proinflammatory macrophage polarization (5). Two other miRNAs identified in the current study also have some evidence for promoting proinflammatory macrophage polarization: miR-214-3p and miR-210. Increasing expression of miR-214-3p using a mimic resulted in increased Nos2 and decreased Arg1, promoting a proinflammatory state in tumor-associated macrophages (43). Similarly, increasing miR-210 expression resulted in decreased while decreasing miR-210 resulted in increased IL-6, TNF-α, and Nos2 in LPS-stimulated peritoneal macrophages, suggesting a feedback inhibition mechanism (62). Little is known about early-expressed miRs-199a-3p and -455-5p and late-expressed miRs-21# and -489 in macrophage polarization; future studies may help to reveal any functional role in proinflammatory macrophage polarization.
Two miRNAs previously reported to be increased in proinflammatory macrophage polarization, miR-125b and miR-146a (58), failed to reach our threshold of twofold expression change in IFN-γ + LPS-stimulated macrophages at any time point. However, these miRNAs have not consistently demonstrated change in inflammatory macrophages (7, 11, 50). In fact, miR-146a was upregulated by IL-10 stimulation as well as LPS in BMDMs (50). This possibly was another reason miR-146a was eliminated in our analysis; due to elevations in more than one stimulating condition (M1 and M2c) miR-146a therefore lacked polarization specificity. Timing of experiments (surveying miRNAs >24 h) may be responsible for differences. Finally, sex differences may be another possible reason for inconsistencies in miRNA expression across studies. Admittedly, this study focused only on male murine macrophages; future studies will explore sex-based differences.
miRNA regulation of proinflammatory macrophage polarization has been more extensively studied than in alternatively activated macrophages. Only a handful of miRNA changes has been discovered in IL-4-stimulated macrophages, such as miR-378-3p (64) and miR-511 (11, 28, 72). In the current study, miR-511-5p and -449a were identified as ≥2-fold changed and specific to IL-4-stimulated macrophages. Recently, increased miR-511-5p expression in macrophages following IL-4 treatment was observed both in vitro and in vivo (28). Our study revealed that miR-511-5p exhibited an early temporal expression pattern in IL-4-stimulated BMDMs (elevated 1–12 h but not 24 h) and a trend toward a decrease in IFN-γ + LPS-stimulated macrophages. Therefore, miR-511-5p could be important in IL-4 macrophage polarization.
Our initial strategy for identifying miRNAs regulating polarization was to compare polarization marker production patterns with miRNA expression. Elevated expression of miR-125a-5p preceded (12 h) and coincided (24 h) with a polarization phenotype we had previously characterized (49), the late production and secretion (24 h) of VEGF from IFN-γ + LPS-stimulated macrophages. We hypothesized that miR-125a-5p regulated VEGF in the CM of M1 macrophage cultures. A possible contributing mechanism for this observation may be the diminished Vegfr1 expression also observed in IFN-γ + LPS-stimulated macrophages at 24 h (49). Presumably, decreased Vegfr1 expression would diminish extracellular sVEGFR1, preventing sequestration of VEGF. While coupled with increased production of VEGF, the final result would be elevated VEGF in the CM of IFN-γ + LPS-stimulated macrophages. Consistent with this hypothesis, inhibition of miR-125a-5p in IFN-γ + LPS-stimulated macrophages diminished VEGF, correlating with elevated sVEGFR1 in the CM. Interestingly, this observation was seen in the MO and the IL-4-stimulated (at 3 h) conditions as well. The expression of Vegfr1 in the MO and IFN-γ + LPS-stimulated conditions predominated at 3 h with miR-125a-5p inhibition. However, by 12 h the expression was similar to controls (IFN-γ + LPS stimulated) or moderately elevated (MO), suggesting a possible feedback mechanism.
The most obvious possible mechanism of increased sVEGFR1 in response to miR-125a-5p inhibition is the direct targeting of Vegfr1 by miR-125a-5p. The presence of a predicted binding site (7-mer) for miR-125a-5p in the Vegfr1 3′-untranslated region (TargetScanMouse 6.2) suggests that miR-125a-5p could target Vegfr1. However, the lack of diminished Vegfr1/sVEGFR1 with mimic transfection in any condition supports an indirect mechanism. Given the high abundance of miR-125a-5p in all conditions and time points (range of raw Ct values of 20.2–21.4), further increasing miR-125a-5p did not result in an opposite effect, possibly due to endogenous repression of mRNA targets. By extension, this also suggests that the increase in miR-125a-5p may not be directly responsible for diminished Vegfr1 in the IFN-γ + LPS-stimulated condition as hypothesized. Additionally, the elevated endogenous expression of Vegfr1 in the IL-4-stimulated condition compared with MO (average raw Cts: 3 h, 24.0 vs. 27.5; 12 h, 21.0 vs. 28.5) with a consistently high expression of miR-125a-5p does not suggest a direct targeting of Vegfr1 by miR-125a-5p. The lack of any definable phenotypic change with mimic transfection compelled us to confirm that the effects with the miR-125a-5p inhibitor were indeed specific by demonstrating a dose response in the MO condition. A dose-dependent increase in sVEGFR1 and Vegfr1 with concomitant depression in VEGF with miR-125a-5p inhibition and absence of any trend in the scramble transfected cultures supported this observation as specific to miR-125a-5p.
IL-4-stimulated macrophages presented unique findings in regards to miR-125a-5p, VEGFR1, and VEGF. While miR-125a-5p expression decreased late (12–24 h) in the IL-4-stimulated condition, our previous work revealed an earlier (3 h) increased expression of Vegfr1 mRNA and rapid (20-fold) drop in VEGF-A protein (49). The increased sVEGFR1 in the CM of IL-4-stimulated macrophages was confirmed in the current study. These dynamic patterns did not suggest a direct regulatory link. However, inhibiting miR-125a-5p early (3 h) did elevate sVEGFR1 and depress VEGF in a magnitude consistent with the MO and IFN-γ + LPS-stimulated conditions. Even more intriguing was the moderate depression of sVEGFR1 and elevation of VEGF in the CM of IL-4-stimulated cultures at 12 h that was not observed in either MO or IFN-γ + LPS-stimulated conditions. The expression of Vegfr1 at 12 h did not follow this observed depression in sVEGFR1 in the CM. Vegfr1 mRNA remained elevated at the 12 h time point, suggesting that the small decrease in sVEGFR1 was not due to expression changes. The primers used to quantify Vegfr1 do not discriminate between the alternatively spliced and truncated transcript that produces the soluble form and the full-length transcript. Therefore, the sustained expression of Vegfr1 may be shunted into an increased membrane form of VEGFR1 rather than the soluble form. Functionally, VEGFR1 on the cell surface and sVEGFR1 are antagonistic in nature and with regards to macrophages may reflect a polarization-specific means of regulating the biological availability and effect of extracellular VEGF. The polarization-specific shift between these two antagonistic forms of VEGFR1 and the effects of miR-125a-5p may reveal novel regulatory processes for miRNAs in macrophage polarization.
VEGF and its receptors VEGFR1/sVEGFR1 have been implicated in multiple human diseases, such as septic shock (36), pre-eclampsia (35, 68), cancer (31, 67), diabetic retinopathy (25), and tissue repair/regeneration (59). Functionally, by virtue of its anti-VEGF capabilities (81), sVEGFR1 has been characterized as antiangiogenic (1, 32), antiedema (33), and anti-inflammatory (78). Macrophages also play important roles in these pathologies and diseases (25, 35, 70). Whether macrophage polarization contributes to disease through secretion of sVEGFR1 and/or rebalancing of VEGFR1/sVEGFR1 remains to be determined. The ubiquitous distribution of macrophages both in various tissues and in diseases in which VEGF may play a central role to the pathology (cancer, retinopathy, and tissue inflammation) makes macrophage-derived sVEGFR1 a potential therapeutic strategy.
Determining the targets of miR-125a-5p responsible for the changes in Vegfr1 will require more extensive exploration as the regulation of Vegfr1 and, more specifically, production of sVEGFR1 are poorly understood. Studies that have manipulated specific pathways resulting in changes in VEGFR1/sVEGFR1 have demonstrated that multiple regulatory avenues are utilized: ERK/MAPK (13), PI3K/Akt (61), HIF-1α (56), IL-4 signaling (83), GM-CSF signaling (19, 83), p38 MAPK (83, 84), and Wnt/Ca+ (73, 74) pathways. Our survey of miR-125a-5p-predicted gene targets within these pathways revealed some promising candidates. Notably, Pik3c2b, Pik3cd, and Pik3r5 are all subunits of PI3K and are central in most of the probed pathways. Additionally, Mapk12 (p38γ) and Mapk14 (p38α) are important components of the p38/MAPK and HIF-1α pathways. Interestingly, many transcription factors with predicted binding sites in the Vegfr1 gene were direct targets (Creb1 and Stat3) of miR-125a-5p or regulated in pathways associated with Vegfr1 containing miR-125a-5p targets such as Egr1 (24) and Hif1a (13) as determined by SABiosciences' DECODE database (65a).
In conclusion, we studied temporal miRNA regulation of macrophage polarization identifying early (by 3 h) and late (by 24 h) temporal patterns. Inhibition of a late-expressed miRNA, miR-125a-5p, in the IFN-γ + LPS-stimulated and the MO control condition resulted in increased production/secretion of sVEGFR1 with a resultant reduction in VEGF in the CM. Elevation of sVEGFR1 and reduction in VEGF in the CM had been previously characterized as a distinct IL-4 condition phenotype. Therefore, inhibition of miR-125a-5p in the MO and IFN-γ + LPS-stimulated conditions shifted this particular polarization phenotype toward an IL-4 phenotype. The capability of modulating extracellular VEGF by manipulating macrophage sVEGFR1 production is of immense therapeutic potential in many diseases.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-074236 and HL-110743, the Veterans Administration Merit Review (1I01BX001186), and the Institute for the Integration of Medicine and Science, UL1 TR001120.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.W.M. and P.K.S. conception and design of research; D.W.M. performed experiments; D.W.M., X.L., and J.A.L.G. analyzed data; D.W.M., X.L., and P.K.S. interpreted results of experiments; D.W.M. prepared figures; D.W.M. drafted manuscript; D.W.M., X.L., J.A.L.G., and P.K.S. edited and revised manuscript; D.W.M., X.L., J.A.L.G., and P.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the assistance of the University of Texas Health Science Center at San Antonio Genomics Core Shared Resource, which is supported by National Cancer Institute Grant P30CA-054174 (CTRC).
REFERENCES
- 1.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 31: 220–231, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED, Ieronymaki E, Androulidaki A, Venihaki M, Margioris AN, Stathopoulos EN, Tsichlis PN, Tsatsanis C. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA 109: 9517–9522, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem 286: 1436–1444, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee S, Cui H, Xie N, Tan Z, Yang S, Icyuz M, Thannickal VJ, Abraham E, Liu G. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem 288: 35428–35436, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang CY, Zen K. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol 4: 341–343, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Chawla A, Nguyen KD, Goh YPS. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 11: 738–749, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, Wang C. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res 83: 131–139, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Classen A, Lloberas J, Celada A. Macrophage activation: classical vs alternative. Meth Mol Biol 531: 29–43, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Cobos Jiménez V, Bradley EJ, Willemsen AM, van Kampen AHC, Baas F, Kootstra NA. Next-generation sequencing of microRNAs uncovers expression signatures in polarized macrophages. Physiol Genomics 46: 91–103, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Contreras J, Rao DS. MicroRNAs in inflammation and immune responses. Leukemia 26: 404–413, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Das B, Yeger H, Tsuchida R, Torkin R, Gee MFW, Thorner PS, Shibuya M, Malkin D, Baruchel S. A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1 through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma. Cancer Res 65: 7267–7275, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta P, Keegan AD. Contribution of alternatively activated macrophages to allergic lung inflammation: a tale of mice and men. J Innate Immun 4: 478–488, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du F, Yu F, Wang Y, Hui Y, Carnevale K, Fu M, Lu H, Fan D. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 34: 759–767, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80: 1298–1307, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrchen J, Steinmüller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, Nordhues U, Sorg C, Sunderkötter C, Roth J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 109: 1265–1274, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Engström A, Erlandsson A, Delbro D, Wijkander J. Conditioned media from macrophages of M1, but not M2 phenotype, inhibit the proliferation of the colon cancer cell lines HT-29 and CACO-2. Int J Oncol 44: 385–392, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eubank TD, Roberts R, Galloway M, Wang Y, Cohn DE, Marsh CB. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity 21: 831–842, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferretti E, De Smaele E, Miele E, Laneve P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E, Screpanti I, Bozzoni I, Gulino A. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J 27: 2616–2627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc receptors. J Immunol 166: 6861–6868, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Gersuk GM, Razai LW, Marr KA. Methods of in vitro macrophage maturation confer variable inflammatory responses in association with altered expression of cell surface dectin-1. J Immunol Methods 329: 157–166, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem 287: 21816–21825, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregg J, Fraizer G. Transcriptional regulation of EGR1 by EGF and the ERK signaling pathway in prostate cancer cells. Genes Cancer 2: 900–909, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Wang H, Liu Y, Li W, Kim D, Huang H. Blockade of vascular endothelial growth factor receptor 1 prevents inflammation and vascular leakage in diabetic retinopathy. J Ophthalmol 2015: 1–11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He M, Xu Z, Ding T, Kuang DM, Zheng L. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPβ. Cell Mol Immunol 6: 343–352, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5: 46–51, 1990. [Google Scholar]
- 28.Karo-Atar D, Itan M, Pasmanik-Chor M, Munitz A. MicroRNA profiling reveals opposing expression patterns for miR-511 in alternatively and classically activated macrophages. J Asthma 52: 545–553, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 90: 10705–10709, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun 226: 324–328, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Kerber M, Reiss Y, Wickersheim A, Jugold M, Kiessling F, Heil M, Tchaikovski V, Waltenberger J, Shibuya M, Plate KH, Machein MR. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res 68: 7342–7351, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Kommareddy S, Amiji M. Antiangiogenic gene therapy with systemically administered sFlt-1 plasmid DNA in engineered gelatin-based nanovectors. Cancer Gene Ther 14: 488–498, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Kumai Y, Ooboshi H, Ibayashi S, Ishikawa E, Sugimori H, Kamouchi M, Kitazono T, Egashira K, Iida M. Postischemic gene transfer of soluble Flt-1 protects against brain ischemia with marked attenuation of blood-brain barrier permeability. J Cereb Blood Flow Metab 27: 1152–1160, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA 108: 11193–11198, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol 94: 247–257, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Lee MC, Wei SC, Tsai-Wu JJ, Wu CHH, Tsao PN. Novel PKC signaling is required for LPS-induced soluble Flt-1 expression in macrophages. J Leukoc Biol 84: 835–841, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Lefèvre L, Galès A, Olagnier D, Bernad J, Perez L, Burcelin R, Valentin A, Auwerx J, Pipy B, Coste A. PPARγ ligands switched high fat diet-induced macrophage m2b polarization toward m2a thereby improving intestinal candida elimination. PLoS One 5: 1–12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Lin KY, Zhang XJ, Feng DD, Zhang H, Zeng CW, Han BW, Zhou AD, Qu LH, Xu L, Chen YQ. miR-125b, a target of CDX2, regulates cell differentiation through repression of the core binding factor in hematopoietic malignancies. J Biol Chem 286: 38253–38263, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol 33: 170–177, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci USA 106: 15819–15824, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci 10: 520–529, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu S, Gao Y, Huang X, Wang X. Cantharidin exerts anti-Hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int J Biol Sci 10: 415–425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Martinez CO, McHale MJ, Wells JT, Ochoa O, Michalek JE, McManus LM, Shireman PK. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am J Physiol Regul Integr Comp Physiol 299: R832–R842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez F, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177: 7303–7311, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mège JL, Mehraj V, Capo C. Macrophage polarization and bacterial infections. Curr Opin Infect Dis 24: 230–234, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Melton DW, McManus LM, Gelfond JAL, Shireman PK. Temporal phenotypic features distinguish polarized macrophages in vitro. Autoimmunity 48: 161–176, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monk CE, Hutvagner G, Arthur JSC. Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS One 5: e13669, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13: 709–721, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray P. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA 102: 8686–8691, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158: 670–689, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest 122: 4190–4202, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nevo O. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol 291: R1085–R1093, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA 104: 1604–1609, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 11: 163–175, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Ohkubo H, Ito Y, Minamino T, Eshima K, Kojo K, Okizaki S, Hirata M, Shibuya M, Watanabe M, Majima M. VEGFR1-positive macrophages facilitate liver repair and sinusoidal reconstruction after hepatic ischemia/reperfusion injury. PLoS One 9: e105533, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA, Sarukhan A. Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Front Immunol 5: 127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park JK, Jeong JW, Kang MY, Baek JC, Shin JK, Lee SA, Choi WS, Lee JH, Paik WY. Inhibition of the PI3K-Akt pathway suppresses sflt1 expression in human placental hypoxia models in vitro. Placenta 31: 621–629, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C. MicroRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-κB1 in murine macrophages. FEBS Lett 586: 1201–1207, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Ramachandran P, Pellicoro A, Vernon M, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield J, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA 109: E3186–E395, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rückerl D, Jenkins SJ, Laqtom NN, Gallagher IJ, Sutherland TE, Duncan S, Buck AH, Allen JE. Induction of IL-4Rα-dependent microRNAs identifies PI3K/Akt signaling as essential for IL-4-driven murine macrophage proliferation in vivo. Blood 120: 2307–2316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruggiero T, Trabucchi M, De Santa F, Zupo S, Harfe BD, McManus MT, Rosenfeld MG, Briata P, Gherzi R. LPS induces KH-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. FASEB J 23: 2898–2908, 2009. [DOI] [PubMed] [Google Scholar]
- 65a.SABiosciences. DECODE: DECipherment Of Elements [Online] DNA. Qiagen, http://www.sabiosciences.com/chipqpcrsearch.php?species_id=0&factor=Over+200+TF&gene=MUC13&nfactor=n&ninfo=n&ngene=n&B2=Search.
- 66.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley D, Nakanishi K, Nakai K, Akira S. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 11: 936–944, 2010. [DOI] [PubMed] [Google Scholar]
- 67.Shibuya M. Involvement of Flt-1 (VEGF receptor-1) in cancer and preeclampsia. Proc Jpn Acad Ser B Phys Biol Sci 87: 167–178, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibuya M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153: 13–19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shireman PK, Contreras-Shannon V, Reyes-Reyna SM, Robinson SC, McManus LM. MCP-1 parallels inflammatory and regenerative responses in ischemic muscle. J Surg Res 134: 145–157, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Squadrito ML, Etzrodt M, De Palma M, Pittet MJ. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol 34: 350–359, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L, De Palma M. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell Rep 1: 141–154, 2012. [DOI] [PubMed] [Google Scholar]
- 73.Stefater JA 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474: 511–515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stefater JA, Rao S, Bezold K, Aplin AC, Nicosia RF, Pollard JW, Ferrara N, Lang RA. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood 121: 2574–2578, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun D, Martinez CO, Ochoa O, Ruiz-Willhite L, Bonilla JR, Centonze VE, Waite LL, Michalek JE, McManus LM, Shireman PK. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J 23: 382–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tili E, Michaille J, Cimino A, Costinean S, Dumitru CD, Adair B, Alder H, Liu CG, Adrian G, Croce CM, Fabbri M, Calin GA. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179: 5082–5089, 2010. [DOI] [PubMed] [Google Scholar]
- 78.Tsao PN, Chan FT, Wei SC, Hsieh WS, Chou HC, Su YN, Chen CY, Hsu WM, Hsieh FJ, Hsu SM. Soluble vascular endothelial growth factor receptor-1 protects mice in sepsis. Crit Care Med 35: 1955–1960, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol 33: 1135–1144, 2013. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 14: 678–688, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu FTH, Stefanini MO, Gabhann Mac F, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: Its biological function, pathogenic role and therapeutic use. J Cell Mol Med 14: 528–552, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wynes MW, Riches DWH. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. J Immunol 171: 3550–3559, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Xia L, Dong Z, Zhang Y, Zhang X, Song X, Sun M, Hu Y, Liu S, Wang K, Qu X, Wei F. Interleukin-4 and granulocyte-macrophage colony-stimulating factor mediates the upregulation of soluble vascular endothelial growth factor receptor-1 in RAW264.7 cells-a process in which p38 mitogen-activated protein kinase signaling has an important role. J Microbiol Immunol Infect doi: 10.1016/jjmii.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Xiong Y, Liebermann DA, Tront JS, Holtzman EJ, Huang Y, Hoffman B, Geifman-Holtzman O. Gadd45a stress signaling regulates sFlt-1 expression in preeclampsia. J Cell Physiol 220: 632–639, 2009. [DOI] [PubMed] [Google Scholar]
- 85.Zhang W, Xu W, Xiong S. Macrophage differentiation and polarization via phosphatidylinositol 3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P component. J Immunol 187: 1764–1777, 2011. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Gao JS, Tang X, Tucker LD, Quesenberry P, Rigoutsos I, Ramratnam B. MicroRNA 125a and its regulation of the p53 tumor suppressor gene. FEBS Lett 583: 3725–3730, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med 31: 797–802, 2013. [DOI] [PubMed] [Google Scholar]