Abstract
Traditional Chinese medicine (TCM) has been used to treat tumors for years and has been demonstrated to be effective. However, the underlying molecular mechanisms of herbs remain unclear. This study aims to ascertain molecular targets of herbs prolonging survival time of patients with advanced hepatocellular carcinoma (HCC) based on network pharmacology, and to establish a research method for accurate treatment of TCM. The survival benefit of TCM treatment with Chinese herbal medicine (CHM) was proved by Kaplan–Meier method and Cox regression analysis among 288 patients. The correlation between herbs and survival time was performed by bivariate correlation analysis. Network pharmacology method was utilized to construct the active ingredient-target networks of herbs that were responsible for the beneficial effects against HCC. Cox regression analysis showed CHM was an independent favorable prognostic factor. The median survival time was 13 months and the 5-year overall survival rates were 2.61% in the TCM group, while there were 6 months, 0 in the non-TCM group. Correlation analysis demonstrated that 8 herbs closely associated with prognosis. Network pharmacology analysis revealed that the 8 herbs regulated multiple HCC relative genes, among which the genes affected proliferation (KRAS, AKT2, MAPK), metastasis (SRC, MMP), angiogenesis (PTGS2) and apoptosis (CASP3) etc.
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers worldwide1,2,3. More than 70% of new cases are diagnosed in Asia each year, and 50% are diagnosed in China alone4. The incidence of HCC is increasing and it is the second most common cause of cancer-related mortality2,3,5. The incidence and mortality rates are similar because most cases of HCC are diagnosed at an advanced stage6. Five-year survival rates had been improved but still less than 30%7. Previous studies reported that the median survival of patients with advanced HCC was less than 5 months8,9. But unfortunately, few therapies could be performed in advanced HCC (stage III–IV), only trans-arterial chemoembolization (TACE) and the drug Sorafenib have been shown to provide a survival benefit10,11.
Traditional Chinese medicine (TCM) has been widely used in China for thousands of years. Chinese herbal medicine (CHM) has become a treatment option in many cancer centers in Asia, Western countries and Africa12,13,14. Many clinical studies have shown that CHM is effective in the treatment of breast, gastric, lung and pancreatic cancers15,16,17. Our previous clinical and experimental studies indicated that CHM was effective on HCC18,19. However, whether patients with advanced HCC (stage III–IV) could obtain a benefit from CHM or not should be confirmed, and the mechanisms of CHM acting on HCC remain largely unsuspected.
Two famous Chinese herbal formulae, Carapacis Trionycis Bolus (Synopsis of Golden Chamber) and Cang Niu Fangji Decoction (Synopsis of Golden Chamber; Yao-zhong Fang, 1921–1995), are commonly used for cirrhosis, chronic liver disease and hepatocellular carcinoma. Chinese herbal formulae always contain many kinds of herbs and could have effect on many targets, so the anti-tumor mechanisms of herbal formulae are hard to understand. Encouragingly, the newly emerged network pharmacology approach, first proposed by Andrew L Hopkins20, brings the hope to study the effective constituents and targets of herbs in a traditional Chinese formula. As bioinformatics, systems biology and poly-pharmacology have rapidly progressed, network pharmacology method could clarify the mechanisms of drug action in a holistic way21,22,23,24. So, using the method, combined with the rich experience of TCM treatment, could hopefully shift the “one target, one drug” model to a “network targets, multi-component” strategy25. In recent years, there was an increasing concern about applying the network pharmacology to reveal the scientific basis and systematic features of CHM.
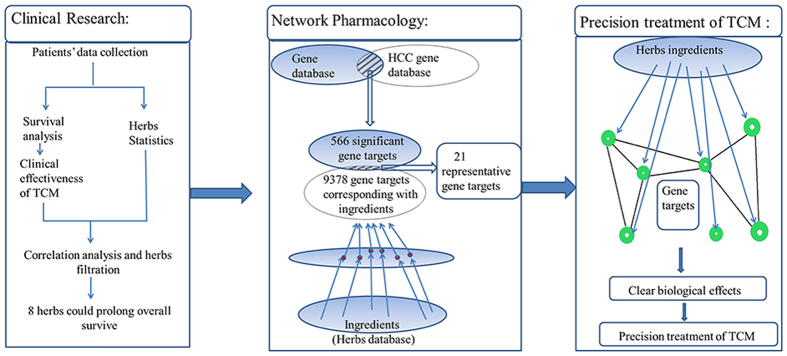
In the present work, the herbs are selected based on our clinical therapy; therefore it is much more reliable than those based on literature retrieval. The flowchart of the whole study design is illustrated in Fig. 1, and the following is a brief description: firstly, we proposed a method to screen out herbs that are effective for prolonging overall survival (OS) of patients with advanced HCC, and then predicted molecular mechanisms of these herbs by using the network pharmacology approach. This process would contribute to clarifying the molecular mechanisms of TCM and improving the effectiveness and specificity of TCM clinical treatment.
Figure 1. Process overview.
Results
Patient characteristics
In this study, a total of 288 patients with advanced HCC (stage III–IV) were included, and 227 (78.8%) patients were more than 50 years old when diagnosed. It has been demonstrated that age would not affect the survival time of patients with advanced HCC (P = 0.842) based on the univariate analysis. The baselines of patient demographics were summarized in Table 1. There were no significant differences in the patients’ age, gender, Child-Pugh score (A/B), etiology, serum AFP levels (<200 μg/L/>200 μg/L), palliative surgery (yes/no), TACE (yes/no), systemic chemotherapy (yes/no) and disease stage (III/IV) between the TCM and non-TCM groups.
Table 1. Patients’ baseline characteristics and treatments between TCM group and non-TCM group.
| |
TCM | non-TCM | ||
|---|---|---|---|---|
| t Variable | (n = 115) | (n = 173) | P- value | |
| Age | <30 | 1 | 0 | 0.176 |
| 30–40 | 9 | 5 | ||
| 40–50 | 17 | 29 | ||
| 50–60 | 40 | 89 | ||
| 60–70 | 31 | 36 | ||
| >70 | 17 | 14 | ||
| Gender (male/female) | 94/21 | 136/37 | 0.517 | |
| Child-Pugh stage (A/B) | 102/13 | 140/33 | 0.053 | |
| Etiology | Alcohol | 17 | 29 | 0.237 |
| HBV/HCV | 26 | 45 | ||
| HBV/HCV+Alcohol | 6 | 18 | ||
| Cryptogenic | 66 | 81 | ||
| Serum AFP levels (<200μg/L/>200μg/L) | 88/27 | 126/47 | 0.483 | |
| Palliative Surgery (yes/no) | 29/86 | 28/145 | 0.060 | |
| TACE (yes/no) | 46/69 | 67/106 | 0.829 | |
| Systemic Treatment (yes/no) | 8/107 | 22/151 | 0.117 | |
| Clinical Stage Composition (IIIB- IIIC /IV) | 96/19 | 142/31 | 0.759 | |
Abbreviations: AFP: Alpha-fetoprotein; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; TACE: Transcatheter arterial chemoembolization.
Univariate and multivariate analyses
The univariate analysis revealed that serum AFP (>200 μg/L) (P < 0.001) and clinical stage IV (P < 0.001) were significantly associated with the reduced median overall survival (Table 2). In contrast, TACE (P < 0.001), palliative surgery (P = 0.001) and TCM (P < 0.001) were protective factors.
Table 2. Univariate and Multivariate Analyses of Variables Influencing Survival of 288 Patients with HCC.
| Characteristics | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| N (%) | P-Value | β | Exp (β) | 95% CI for Exp (β) | P-Value | |
| Gender | 0.908 | – | – | – | – | |
| Male | 230 (79.9) | |||||
| Female | 58 (20.1) | |||||
| Age, years | 0.842 | – | – | – | – | |
| <50 | 61 (21.2) | |||||
| ≥50 | 227 (78.8) | |||||
| Child-Pugh stage | 0.126 | – | – | – | – | |
| A | 242 (84.0) | |||||
| B | 46 (16.0) | |||||
| Serum AFP | 214 (74.3) | <0.001 | −0.493 | 0.611 | 0.001 | |
| <200μg/L | 74 (25.7) | 0.457–0.817 | ||||
| >200μg/L | ||||||
| TACE | <0.001 | 0.597 | 1.816 | <0.001 | ||
| Yes | 113 (39.2) | 1.362–2.421 | ||||
| No | 175 (60.8) | |||||
| Palliative Surgery | 0.001 | 0.460 | 1.584 | 0.006 | ||
| Yes | 57 (19.8) | 1.142–2.198 | ||||
| No | 231 (80.2) | |||||
| Systemic Chemotherapy | 0.064 | – | – | – | – | |
| Yes | 28 (9.7) | |||||
| No | 260 (90.3) | |||||
| Clinical Stage Composition | <0.001 | −1.072 | 0.342 | <0.001 | ||
| III | 197 (68.4) | 0.247–0.474 | ||||
| IV | 91 (31.6) | |||||
| TCM | <0.001 | 0.845 | 2.328 | <0.001 | ||
| Yes | 115 (39.9) | 1.796–3.017 | ||||
| No | 173 (60.1) | |||||
P-values in bold font are statistically significant. TACE: Transcatheter arterial chemoembolization; AFP: Alpha-fetoprotein; CI: confidence interval; TCM: Traditional Chinese medicine.
Patient- and treatment-related variables were significantly associated with OS, and these results were then subsequently evaluated by Cox regression analysis to determine independent risk factors for the survival of patients with advanced HCC. Serum AFP > 200 μg/L and clinical stage IV were independent predictors of poor survival; TACE, palliative surgery and TCM were independent protective factors (Table 2).
Survival analysis
The OS curves for TCM and non-TCM groups are shown in Fig. 2. The median survival time was 13 months vs 6 months and the 1-, 2-, and 5-year overall survival rates was 54.78%, 21.74%, 2.61% vs 24.3%, 4.62%, 0 in the TCM and non-TCM group, respectively. The Log-rank test revealed significant differences between the two groups in terms of OS (P < 0.001).
Figure 2. Survival analysis between the TCM and non-TCM group.
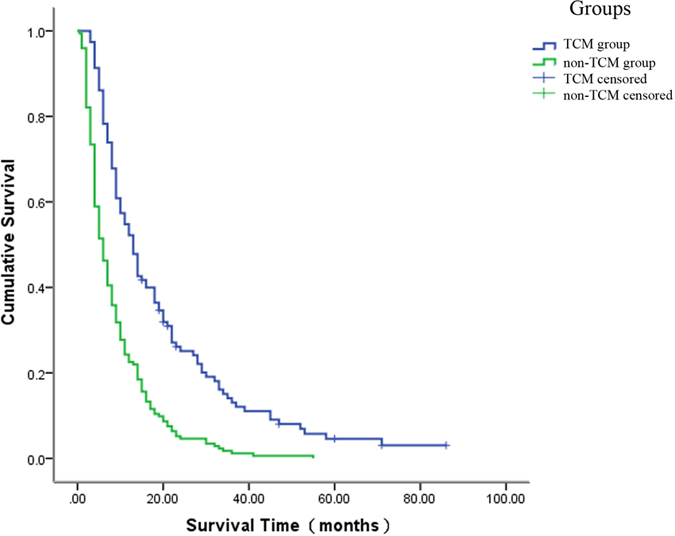
The median OS in the TCM group was longer than that in the on-TCM group (13 vs 6 months, respectively; P < 0.001). OS = overall survival, TCM = traditional Chinese medicine.
Candidate genes associated with HCC
A total of 566 significant genes were obtained from the OncoDB. HCC26 and Liverome27 databases after removing redundant entries (supplementary file 1, Table S1). These genes were differentially expressed or harbored genetic variations in HCC tissues compared to normal tissues.
Herbs, chemicals and targets information
All the Chinese herbal formulae used by 115 patients were collected. These formulae included a total of 240 types of herbs, and the highest using frequency was 425. There were 67 kinds of herbs, whose using frequency was more than 34 (425*8%) were selected to have a correlation analysis. Spearman bivariate correlate analysis showed 37 herbs had positive correlations with survival time, and the correlation coefficients of 20 herbs ≧0.25. Finally, 8 herbs (Radix Stephaniae Tetrandrae (RST), Flos Campsis (FC), Carapax Trionycis (CT), Radix Scutellariae (RS), Radix Achyranthis Bidentatae (RAB), Radix Bupleuri (RB), Semen Lepidii (SL), and Rhizoma Atractylodis Macrocephalae (RAM)) were found to have high correlation coefficients, and they were also components of the TCM formulae Carapacis Trionycis Bolus and Cang Niu Fangji Decoction.
For each of the 8 herbs, the ingredients with DL values ≥0.18 were listed in supplementary file 1, Table S2. As shown in Table 3, the 8 herbs were listed in descending order of their correlation coefficients. The detail target information for the ingredients of 8 herbs was shown in supplementary file 1, Table S3, and totally 9377 targets were found for the whole ingredients.
Table 3. The putative major ingredients and major targets of 8 herbs.
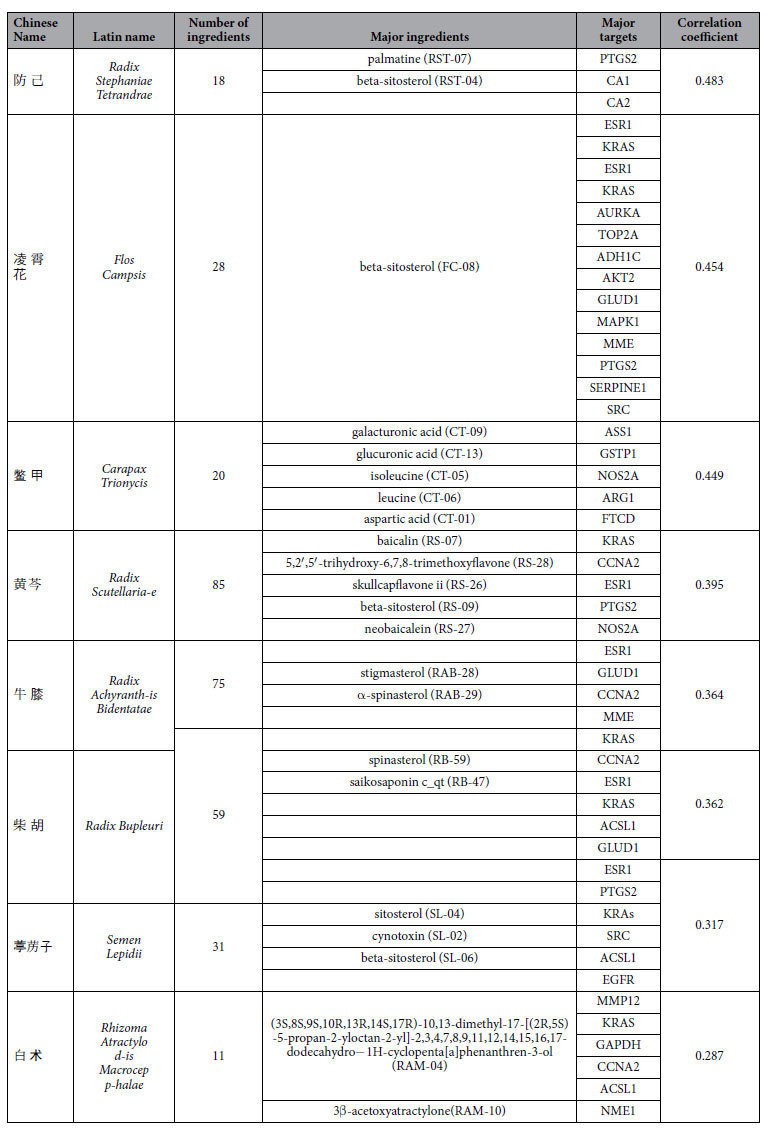
Putative major ingredients and major targets of the 8 herbs
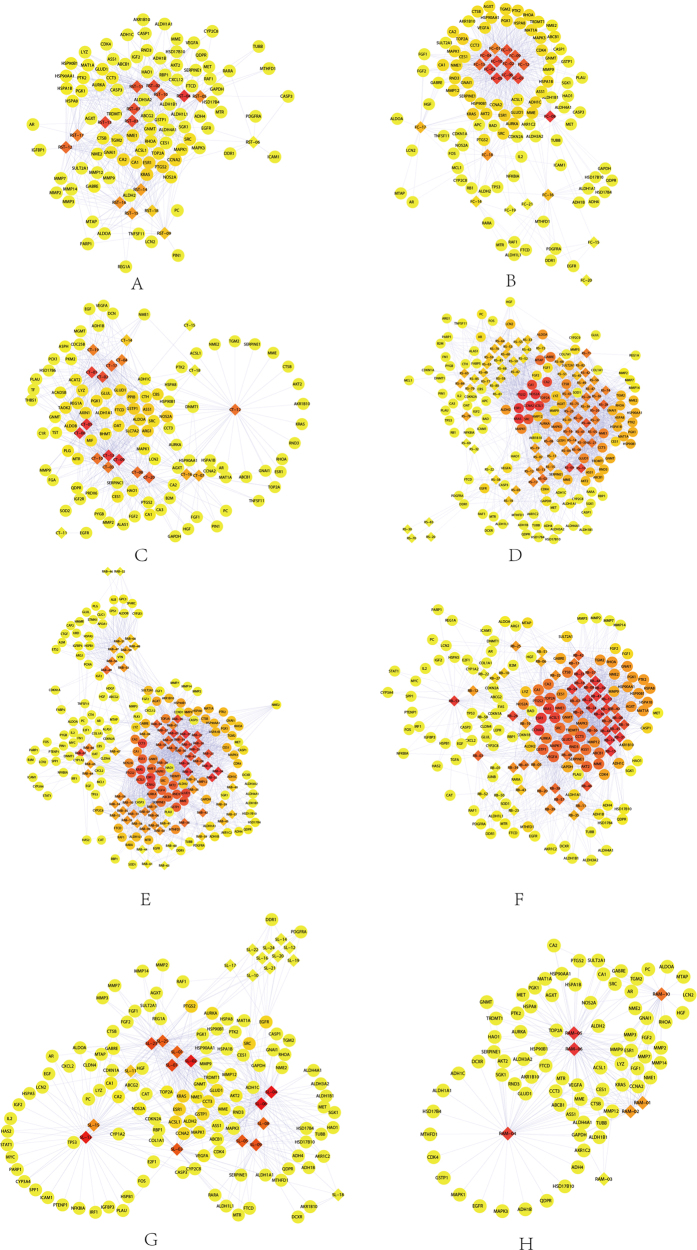
Ingredient-target networks were constructed for the 8 herbs (Fig. 3). The results revealed that 2 major ingredients in RST, 1 in FC, 5 in CT, 5 in RS, 2 in RAB, 2 in RB, 3 in SL and 2 in RAM may play important roles in anti-HCC. The major putative ingredients and targets of each herb were listed in Table 3.
Figure 3.
The ingredient-target networks of (A) RST, (B) FC, (C) CT, (D) RS, (E) RAB, (F) RB, (G) SL and (H) RAM. The diamond nodes represent ingredients, and the circular nodes represent targets. The colors of the nodes are illustrated from red to yellow in descending order of degree values.
As shown in Fig. 3(A), the RST network was consisted of 111 nodes and contains 12 validated targets and 83 predicted targets. The analysis of the network revealed that beta-sitosterol (1 validated target, 59 predicted targets) was predicted as the major ingredient in RST. Genes such as ESR1, KRAS and PTGS2 were predicted as the major targets of RST for the treatment of HCC. As shown in Fig. 3(B), the FC network was consisted of 116 nodes, including 22 validated targets and 73 predicted targets. Among the ingredients of FC, beta-sitosterol was also predicted as a major ingredient. Genes such as ESR1, KRAS and AURKA were predicted as major targets of FC for the treatment of HCC. The CT network consisted of 119 nodes and contained 1 validated target and 98 predicted targets (Fig. 3(C)). Galacturonic acid (0 validated targets, 52 predicted targets) was predicted as the major ingredient of CT for the treatment of HCC, and ASS1, GSTP1 and NOS2A were predicted as the major targets of CT. The RS network consisted of 199 nodes, including 32 validated targets and 91 predicted targets (Fig. 3(D)). Beta-sitosterol was also the major ingredient, and genes such as KRAS, CCNA2 and ESR1 were predicted as major targets of RS for the treatment of HCC.
As shown in Fig. 3(E), the RAB network consisted of 224 nodes and contained 49 validated targets and 107 predicted targets. Beta-sitosterol was predicted as the major ingredient, and genes such as ESR1, CCNA2 and CCT3 were predicted as major targets of RAB for the treatment of HCC. As illustrated in Fig. 3(F), the RB network consisted of 186 nodes, including 49 validated targets and 81 predicted targets. Spinasterol was predicted as the major ingredient, and genes such as CCNA2, ESR1 and KRAS were predicted as major targets of RB for the treatment of HCC. The SL network consisted of 144 nodes and contained 39 validated targets and 80 predicted targets (Fig. 3(G)). Beta-sitosterol was predicted as the major ingredient, and ESR1, PTGS2 and KRAS were predicted as the major targets of SL for the treatment of HCC. The RAM network consisted of 88 nodes and contained 0 validated targets and 77 predicted targets (Fig. 3(H)). 3β-acetoxyatractylone was predicted as the major ingredient, and genes such as MMP12, KRAS and GAPDH were predicted as the major targets of RAM for the treatment of HCC.
The major ingredients and underlying pharmacological mechanisms of TCM for the treatment of HCC
Although many ingredients may contribute to the HCC treatment effects of herbs, researching the major ingredients is an effective approach to elucidate the pharmacological mechanisms of action of herbs. In this study, network pharmacology was applied to explore the major ingredients. As shown in Table 3, beta-sitosterol was predicted as the major ingredient in 5 out of 8 herbs.
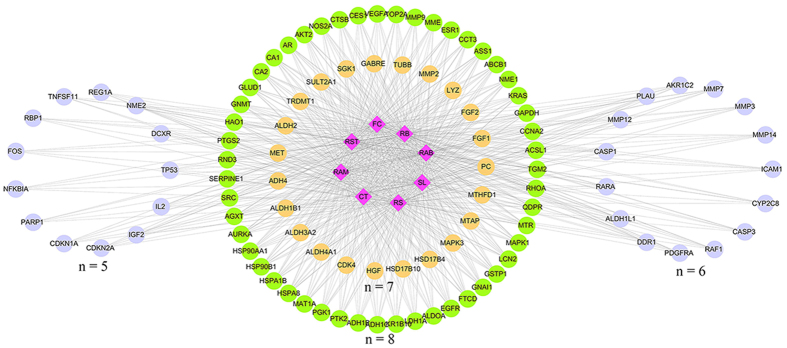
The common targets for at least 5 herbs were illustrated in Fig. 4. Among these genes, 61 were common targets of 8 herbs, 23 were common targets of 7 herbs, 15 were common targets of 6 herbs, and 13 were common targets of 5 herbs. The representative genes included ACSL1, ADH1C, ASS1, AURKA, CA1, CCNA2, CCT3, EGFR, ESR1, FTCD, GAPDH, GLUD1, GSTP1, KRAS, NME1, NOS2A, PTGS2, SRC, TOP2A and AKT2, MAPK. Gene enrichment analysis found that the phosphatidylinositol 3′ kinase/Akt (PI3K/Akt) signaling pathway, nuclear SMAD2/3 signaling, and E-cadherin might be influenced by these herbs. The anti-HCC roles may mainly play in four fields: anti-angiogenesis, induction of apoptosis, inhibition of proliferation and metastasis (Fig. 5).
Figure 4. The herb-target networks of the 8 herbs.
The diamond nodes represent herbs, and the circular nodes represent targets. The targets distributed in a circle represent they are acting by the same number of herbs, which illustrated as “n”.
Figure 5. Simplified pathways in HCC.
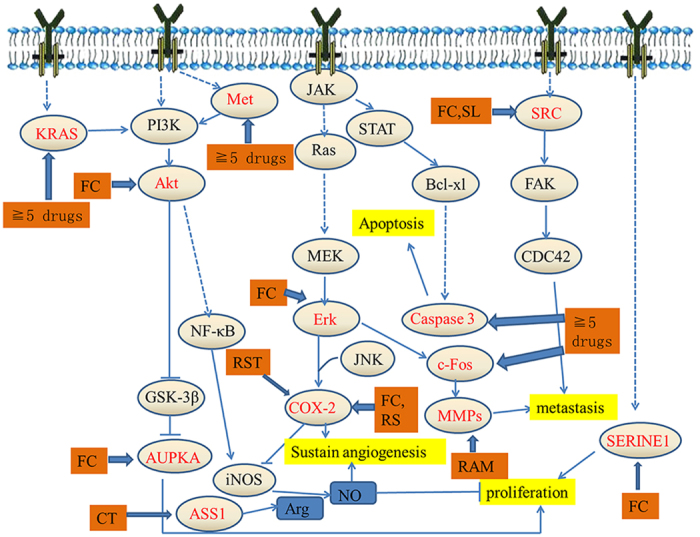
All of the targets are shown by the gene name.
Experimental validation
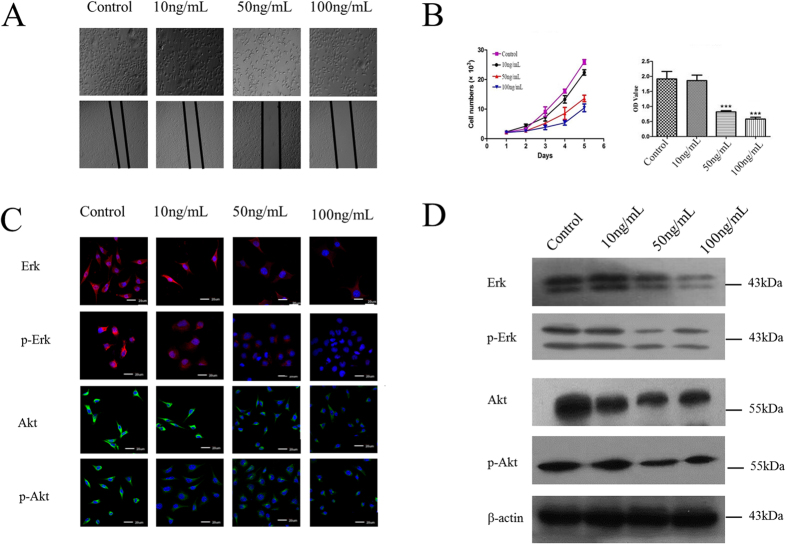
FC had high correlation coefficient with HCC (0.454), and multiple putative major targets (12) were selected for experimental validation. The effects of aqueous extract of FC on cell proliferation and wound healing assays were shown in Fig. 6(A); the statistical views of cell proliferation and cell migration after treating with FC were shown in Fig. 6(B). Our results revealed that aqueous of FC could obviously inhibit cell proliferation and cell migration (24 h) in dose of 50 ng/mL and 100 ng/mL in vitro.
Figure 6. Effect of the aqueous extract of FC on cell proliferation and cell migration.
Data are expressed as mean ± S.D. (A) The morphology of SMMC-7721 cells (upper) and the result of wound-scratch assay (lower). (B) The results of Typanblue staining assay (left) and CCK-8 assay (right), “***” represented for p < 0.001. (C) Immunocytochemical staining showed Erk, p-Erk, Akt and p-Akt of four groups. (D) Western blot assay analyzed Erk, p-Erk, Akt and p-Akt of four groups.
Aqueous of FC affected protein expression and also affected phosphorylation progress of Erk and Akt. As shown in Fig. 6(C,D), FC significantly inhibited the expression of Erk and Akt, and decreased p-Erk and p-Akt in dose of 50 ng/mL and 100 ng/mL.
Discussion
Many patients with HCC have a poor prognosis because most cases are diagnosed at the advanced stage6. In the field of western medicine (WM), many studies have focused on combination therapy for patients with unresectable HCC, but the prognostic improvement is limited. Studies on a variety of systemic cytotoxic chemotherapeutic agents have been reported in HCC28,29,30,31,32,33, however, most of these agents have not shown a survival benefit. TCM has been used for years to treat tumors worldwide especially in China and has been demonstrated effective in clinical practice. TCM seems appropriate for the treatment of cancer because the principle of TCM is to focus on systemic functional adjustments, which is in accordance with tumor treatment theories.
Our previous clinical study had proved TCM was effective in HCC patients from stage I to stage IV18. The present study included patients with advanced HCC (stage III–IV), of which the expected survival time was much shorter. Previous studies reported that the median survival of patients with advanced HCC without effective treatment was less than 5 months8,9. In the current study, the median survival times of the TCM group and the non-TCM group were 13 months and 6 months, and the survival time was significantly improved in the TCM group. These results indicated that TCM treatment was beneficial for improving survival time of patients with advanced HCC.
Carapacis Trionycis Bolus (Synopsis of Golden Chamber) and Cang Niu Fangji Decoction (Synopsis of Golden Chamber; Yao-zhong Fang, 1921–1995) are common used for cirrhosis, chronic liver disease and hepatocellular carcinoma in China. In the clinical treatment, herbs were used and adjusted according to different symptoms. At the end of the treatment, a total of 240 kinds of herbs were ever used. Correlation analysis showed 37 kinds of herbs had positive correlations with OS, and 20 kinds of herbs’ correlation coefficients were ≧0.25. We mainly concerned about 8 herbs, RST, FC, CT, RS, RAB, RB, SL and RAM, because FC, CT, RS, RB and SL were herbs of Carapacis Trionycis Bolus, RST, RAB, RAM were herbs of Cang Niu Fangji Decoction.
Network pharmacology approach could help us search for putative active ingredients and targets of herbs. 327 ingredients of these 8 herbs were found in total and 17 ingredients were screened out according to ingredients fishing rule for further research. Ingredients affected 95 targets in RST, 95 in FC, 99 in CT, 123 in RS, 156 in RAB, 130 in RB, 119 in SL and 77 in RAM. Furthermore, we found that 21 representative gene targets including ACSL1, ADH1C, ASS1, AURKA, CA1, CCNA2, CCT3, EGFR, ESR1, FTCD, GAPDH, GLUD1, GSTP1, KRAS, NME1, NOS2A, PTGS2, SRC, TOP2A, AKT2 and MAPK were regulated by these herbs. Particularly, it was found that AKT2 was the putative major target of FC. AKT2 is related to tumor growth and metastasis in HCC, and is activated by PI3K pathway. Overexpression of AKT2 is frequently observed in HCC and indicates poor prognosis34,35,36,37. Consistently, the experimental results showed that FC could inhibit the expression of Akt and phosphorylation of Akt, and could also obviously inhibit cell proliferation and cell migration in vitro. Furthermore, FC also had effects on MAPK1, whose activation promotes proliferation and metastasis in HCC36.
Repressing HCC progression and metastasis is important for prolonging the OS of patients with HCC. Other targets, such as CCNA2, involved in repressing the expression of cyclin E and tumor cell proliferation38. SRC2, NME1, GSTP1, FTCD3, COX-2, AUPKA, ASS1 and ADH1C associated with HCC. Beta-sitosterol, frequently predicted as the major ingredient of herbs, was found as a potential therapeutic agent for the hepatofibrosis in activating human hepatic stellate cell (HSC) model and dimethyl nitrosamine (DMN)-induced mouse hepatic fibrosis model39. Baicalin, a component of RS, had anticancer effects such as inducing autophagy and anti-proliferative function of tumor cells of HCC40. These facts support our research and confirm that TCM could affect HCC.
We conclude, from this retrospective study, that TCM treatment was associated with a survival benefit in patients with HCC. In addition, the use of network pharmacology method is beneficial for exploring the underlying anti-tumor mechanisms of herbs through prediction of active ingredients and molecular targets of herbs. The combination of clinical study and network pharmacology is a promising research method for “precise treatment of TCM”.
There are several limitations for the use of network pharmacology approach to predict active ingredients and potential targets. (i) the ingredients of herbs screened by DL values may be inconsistent with the ingredients actually existed in blood of the patients; (ii) the predicted targets were influenced by different target prediction tools; (iii) it is difficult to distinguish inhibitory effects from activated effects of the targets.
Materials and Methods
Statement
This retrospective study was supported by Medical Ethics Committee and the methods were carried out in accordance with the approved guidelines (The certificate no. 2016.4). Our study did not pose risk to patients, and did not have negative effects on patients’ rights and health. The subjects’ privacy and personal identity information were protected, and the majority of patients had died or lost to follow-up and unable to get in touch. According to guidelines of exempt from inform consent of Tianjin Medical University Cancer Institute and Hospital, our study meet the requirement of informed consent exemption.
Patient characteristics
From March 2009 to December 2014, 389 patients with HCC were followed up. Major inclusion criteria were as follows: aged 18 years or older; diagnosed by histology, cytology, or clinical and radiographic evidence. Moreover, patients in the TCM group should receive TCM treatment ≧2 months. The major exclusion criteria were as follows: serious complications (severe cardiac or pulmonary disease; acute upper gastrointestinal bleeding leading to death); Child-Pugh Class C; concurrent cancer; incomplete medical records; radical surgery or liver transplantation; or no accurate survival time. Diagnostic criteria included pathogenesis as well as clinical and radiographic evidence11. Baseline staging was performed based on age, gender, Child-Pugh score, serum alpha fetal protein (AFP) levels, palliative surgery, TACE, systemic chemotherapy and clinical stage. After screening, 288 patients were included in our study. Finally, 115 patients who received TCM together with WM were included in TCM group.
Treatment
TCM treatment was used according to different syndromes. In general, a formula contains 20–30 types of herbs in our study for patients with advanced HCC. The formula was taken orally 3 times a day, 30 minutes after meals, for 2 months or more in the TCM group. Every 2 weeks, some herbs were changed based on the patient’s symptoms. During TCM treatment, patients in the TCM group also received WM treatment. The patients in the non-TCM group only received WM treatment. The WM treatment included palliative surgery, TACE and systemic chemotherapy. Local radiation therapy and biotherapy were rarely used in patients.
Patients’ formulae were gathered every 2 weeks counted from the date received TCM treatment to the date the patients dead or the time of data closure. We analyzed the TCM formulae of 115 patients, who received 62940 days of TCM treatment with CHM and 240 types of herbs in total. The frequency of single-herb treatment was balanced by dividing the average treatment days and patient numbers. The frequency of single herb over 8% was selected out, and correlation coefficients between these herbs and survival time were calculated separately. The average of the correlation coefficient, which was 0.25, was set as a boundary for further selection. Herbs were included for further analysis if they satisfy the following criteria: using frequency/highest frequency >8%; correlation coefficient ≧0.25 and P-value < 0.05.
HCC significant genes collection
HCC significant genes were collected from 2 databases, OncoDB.HCC26 (http://oncodb.hcc.ibms.sinica.edu.tw) and Liverome27 (http://liverome.kobic.re.kr/index.php). A gene was selected if it satisfied at least one of the following criteria: (i) experimentally validated in OncoDB.HCC, or (ii) occurrence frequency >7 among the various collected gene signatures in Liverome. The repeated genes between the two databases were removed. To normalize the gene information, different ID types were converted to Swiss-Prot accession numbers.
Herb formulation ingredient collection and target fishing
The chemical ingredients were collected form TCM databases, including the Traditional Chinese Medicine Systems Pharmacology (TCMSP) Database41 (http://lsp.nwsuaf.edu.cn/tcmsp.php), the Traditional Chinese Medicine Integrated Database42 (TCMID, http://www.megabionet.org/tcmid/) and the TCM Potential Target Database (TCM-PTD, http://tcm.zju.edu.cn/ptd/). The ingredients were screened according to drug-likeness (DL) values, and the ingredients were retained if DL ≥ 0.18, a suggested criterion by TCMSP database. Specifically, the ingredients of CT were amino acids with low DL values; therefore they were not screened by these criteria.
Target fishing was performed to search for or predict the potential targets of small molecules. The validated targets were extracted from the Herbal Ingredients’ Targets (HIT) Database43 (http://lifecenter.sgst.cn/hit/). The predicted targets were obtained using ChemMapper44 (http://lilab.ecust.edu.cn/chemmapper/), an online tool for predicting targets based on 3D similarity. In this study, if the 3D similarity of a molecule was above 0.8545 compared with drugs in the DrugBank database, the targets of known drugs were predicted as the targets of the investigated molecules. The targets were retained if they were common targets between HCC and small molecules, and the prediction score was >0 as well.
Network construction and analysis
The ingredients-targets networks were constructed for these herbs using Cytoscape software46 (Version 3.2.1). The networks were analyzed using Cytoscape plugin CentiScaPe47 to calculate topological parameters, including the degree, betweenness, closeness and centroid. The significant nodes representing putative major ingredients and major targets of herbs were explored. Except for CT whose ingredients were amino acids, the putative major ingredients were remained if DL ≥ 0.18 and oral bioavailability values ≥30%.
Pathways analysis
All pathways associated with HCC were searched through the internet (http://cgap.nci.nih.gov/Pathways/BioCarta_Pathways, http://www.cellsignal.com and http://www.genome.ad.jp). The mechanisms of herbs were mapped to the pathway network according to their targets information.
Experimental validation
Hepatocarcinoma cell line SMMC-7721 was used to examine the effects of aqueous extract of FC.
Cell culture and morphologic observation
SMMC-7721 cells were obtained from the Tianjin cancer institute & hospital. Cells were cultured as described in literature. When SMMC-7721 cells reached 60% confluency, the cells were then continuously exposed to 10 ng/mL, 50 ng/mL and 100 ng/mL aqueous extract of FC. Photos were taken by inverted phase contrast microscope (Olympus).
Wound-scratch assay, Typanblue staining assay and CCK-8 assay
Wound-scratch assay was performed as previously described. CCK-8 assay was performed as manual. Typanblue staining was performed as described19.
Antibodies
The following antibodies were used: Erk (Cell Signaling Technology 4695, Danvers, MA, USA), Phospho-Erk1/2 (Cell Signaling Technology 4370, Danvers, MA, USA), β-actin (Cell Signaling Technology 3700), Akt (Cell Signaling Technology 4691, Danvers, MA, USA), Phospho-Akt (Cell Signaling 4060, Danvers, MA, USA).
Immunocytochemical staining
Immunocytochemical staining was performed according to the method published before19. The staining results were repeated at least three times. The representative pictures were shown in related figures captured by confocal microscope (Olympus Corporation, Beijing, China).
Western blot assay
Western blot analyses were operated as previously described48. The results were repeated at least three times.
Statistics
Baseline comparison was analyzed by the χ2 test. OS from the date of the definitive diagnosis of HCC was estimated by using the Kaplan–Meier method, and survival curves were established by the log-rank test. The continuous variables were divided into categories based on population quartiles, upper normal values, and published data to determine cutoff values. Multivariate Cox regression analysis was performed to determine survival trends adjusted for clinical and demographic factors. Spearman bivariate correlate analysis was used in searching for correlation between herbs and survival time. A P-value < 0.05 was considered to indicate statistical significance. Statistical analyses were performed by SPSS 19.0 for Windows.
Additional Information
How to cite this article: Gao, L. et al. Molecular targets of Chinese herbs: a clinical study of hepatoma based on network pharmacology. Sci. Rep. 6, 24944; doi: 10.1038/srep24944 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation of China (nos 81173376, 81473441 and 81503383).
Footnotes
Author Contributions L.G. and X.-d.W.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. Y.-y.N. acquisition of data; analysis and interpretation of data; added experiment results. D.-d.D., X.Y., J.H., C.-h.Z., K.-x.W. and X.-m.Q.: acquisition of data; analysis and interpretation of data. X.-z.W. and D.C.: study concept and design; critical revision of the manuscript for important intellectual content; obtained funding; administrative, technical, or material support; study supervision.
References
- Salhab M. & Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther 7, 463–475 (2011). [DOI] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2015. CA Cancer J Clin 65 (2015). [DOI] [PubMed] [Google Scholar]
- Ashtari S., Pourhoseingholi M. A., Sharifian A. & Zali M. R. Hepatocellular carcinoma in Asia: Prevention strategy and planning. World J Hepatol 7, 1708–1717 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerjomataram I. et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 380, 1840–1850 (2012). [DOI] [PubMed] [Google Scholar]
- Yang J. D. & Roberts L. R. Hepatocellular carcinoma: a global view. Nat Rev Gastro Hepat 7, 448–458 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard E. P., Ward E. M., Siegel R. & Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. Ca-Cancer J Clin 62, 118–128 (2012). [DOI] [PubMed] [Google Scholar]
- Lee J. O. et al. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann Oncol 20, 1402–1407 (2009). [DOI] [PubMed] [Google Scholar]
- Nakamura M. et al. Pilot study of combination chemotherapy of S-1, a novel oral DPD inhibitor, and interferon-alpha for advanced hepatocellular carcinoma with extrahepatic metastasis. Cancer 112, 1765–1771 (2008). [DOI] [PubMed] [Google Scholar]
- Lee Y. K. et al. Prognostic value of alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with hepatocellular carcinoma treated with transarterial chemoembolization. Bmc Cancer 13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. B. 3rd et al. Hepatobiliary cancers, version 2.2014. Journal of the National Comprehensive Cancer Network: JNCCN 12, 1152–1182 (2014). [DOI] [PubMed] [Google Scholar]
- Hyodo I. et al. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol 23, 2645–2654 (2005). [DOI] [PubMed] [Google Scholar]
- Evans M. A. et al. Men with cancer: is their use of complementary and alternative medicine a response to needs unmet by conventional care? Eur J Cancer Care 16, 517–525 (2007). [DOI] [PubMed] [Google Scholar]
- Swarup A. B., Barrett W. & Jazieh A. R. The use of complementary and alternative medicine by cancer patients undergoing radiation therapy. Am J Clin Oncol-Canc 29, 468–473 (2006). [DOI] [PubMed] [Google Scholar]
- Lee Y. W. et al. Adjunctive traditional Chinese medicine therapy improves survival in patients with advanced breast cancer: A population-based study. Cancer 120, 1338–1344 (2014). [DOI] [PubMed] [Google Scholar]
- Xu Y. et al. Survival Benefit of Traditional Chinese Herbal Medicine (a Herbal Formula for Invigorating Spleen) for Patients With Advanced Gastric Cancer. Integr Cancer Ther 12, 414–422 (2013). [DOI] [PubMed] [Google Scholar]
- Guo H. R., Liu J. X., Xu L., Madebo T. & Baak J. P. A. Traditional Chinese Medicine Herbal Treatment May Have a Relevant Impact on the Prognosis of Patients With Stage IV Adenocarcinoma of the Lung Treated With Platinum-Based Chemotherapy or Combined Targeted Therapy and Chemotherapy. Integr Cancer Ther 10, 127–137 (2011). [DOI] [PubMed] [Google Scholar]
- Man Y. N., Liu X. H. & Wu X. Z. Chinese medicine herbal treatment based on syndrome differentiation improves the overall survival of patients with unresectable hepatocellular carcinoma. Chin J Integr Med 21, 49–57 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang S. X. et al. Gekko-sulfated Glycopeptide Inhibits Tumor Angiogenesis by Targeting Basic Fibroblast Growth Factor. J Biol Chem 287, 13206–13215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. L. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4, 682–690 (2008). [DOI] [PubMed] [Google Scholar]
- Jia J. et al. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov 8, 111–128 (2009). [DOI] [PubMed] [Google Scholar]
- Keith C. T., Borisy A. A. & Stockwell B. R. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov 4, 71–U10 (2005). [DOI] [PubMed] [Google Scholar]
- Levinson A. D. Cancer Therapy Reform. Science 328, 137–137 (2010). [DOI] [PubMed] [Google Scholar]
- Schadt E. E., Friend S. H. & Shaywitz D. A. OPINION A network view of disease and compound screening. Nat Rev Drug Discov 8, 286–295 (2009). [DOI] [PubMed] [Google Scholar]
- Li S. & Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med 11, 110–120 (2013). [DOI] [PubMed] [Google Scholar]
- Su W. H. et al. OncoDB.HCC: an integrated oncogenomic database of hepatocellular carcinoma revealed aberrant cancer target genes and loci. Nucleic Acids Res 35, D727–D731 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. et al. Liverome: a curated database of liver cancer-related gene signatures with self-contained context information. Bmc Genomics 12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boige V. et al. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer 97, 862–867 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbio-Pallavicini E. et al. Epirubicin and etoposide combination chemotherapy to treat hepatocellular carcinoma patients: a phase II study. Eur J Cancer 33, 1784–1788 (1997). [DOI] [PubMed] [Google Scholar]
- Uka K. et al. Combination therapy of oral fluoropyrimidine anticancer drug S-1 and interferon alpha for HCC patients with extrahepatic metastases. Oncology-Basel 75, 8–16 (2008). [DOI] [PubMed] [Google Scholar]
- Ueshima K. et al. Combination therapy with S-1 and pegylated interferon alpha for advanced hepatocellular carcinoma. Oncology-Basel 75 Suppl 1, 106–113 (2008). [DOI] [PubMed] [Google Scholar]
- Okuda K. et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 56, 918–928 (1985). [DOI] [PubMed] [Google Scholar]
- Yeung Y. P. et al. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol 100, 1995–2004 (2005). [DOI] [PubMed] [Google Scholar]
- Manning B. D. & Cantley L. C. AKT/PKB signaling: Navigating downstream. Cell 129, 1261–1274 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. L. et al. FoxD3-regulated microRNA-137 suppresses tumour growth and metastasis in human hepatocellular carcinoma by targeting AKT2. Oncotarget 5, 5113–5124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L. K., Chiu Y. T., Sze K. M. & Ng I. O. Tensin4 is up-regulated by EGF-induced ERK1/2 activity and promotes cell proliferation and migration in hepatocellular carcinoma. Oncotarget 6, 20964–20976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. D. et al. Akt2 expression correlates with prognosis of human hepatocellular carcinoma. Oncol Rep 11, 25–32 (2004). [PubMed] [Google Scholar]
- Yue X. T. et al. Zinc Fingers and Homeoboxes 2 Inhibits Hepatocellular Carcinoma Cell Proliferation and Represses Expression of Cyclins A and E. Gastroenterology 142, 1559−+ (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S. et al. Effects of beta-sitosterol derived from Artemisia capillaris on the activated human hepatic stellate cells and dimethylnitrosamine-induced mouse liver fibrosis. BMC Complement Altern Med 14, 363, doi: 10.1186/1472-6882-14-363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. J. et al. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol Rep 27, 1128–1134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru J. et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 6, 13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R. C. et al. TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Res 41, D1089–D1095 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H. et al. HIT: linking herbal active ingredients to targets. Nucleic Acids Res 39, D1055–D1059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J. Y. et al. ChemMapper: a versatile web server for exploring pharmacology and chemical structure association based on molecular 3D similarity method. Bioinformatics 29, 1827–1829 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang Y. Q. et al. A systems biology-based investigation into the therapeutic effects of Gansui Banxia Tang on reversing the imbalanced network of hepatocellular carcinoma. Sci Rep-Uk 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoot M. E., Ono K., Ruscheinski J., Wang P. L. & Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardoni G., Petterlini M. & Laudanna C. Analyzing biological network parameters with CentiScaPe. Bioinformatics 25, 2857–2859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.