Breastfeeding is protective against hormone receptor-negative breast cancers, which are more common in younger women, African-American women, and BRCA1 carriers and generally have a poorer prognosis than other breast cancers. More prospective research on the optimal duration of breastfeeding and the association between breastfeeding and receptor-positive breast cancers is required.
Keywords: breast cancer, breastfeeding, estrogen receptor, HER2 receptor, meta-analysis, progesterone receptor
Abstract
Background
Breastfeeding is inversely associated with overall risk of breast cancer. This association may differ in breast cancer subtypes defined by receptor status, as they may reflect different mechanisms of carcinogenesis. We conducted a systematic review and meta-analysis of case–control and prospective cohort studies to investigate the association between breastfeeding and breast cancer by estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status.
Design
We searched the PubMed and Scopus databases and bibliographies of pertinent articles to identify relevant articles and used random-effects models to calculate summary odds ratios (ORs) and 95% confidence intervals (CIs).
Results
This meta-analysis represents 27 distinct studies (8 cohort and 19 case–control), with a total of 36 881 breast cancer cases. Among parous women, the risk estimates for the association between ever (versus never) breastfeeding and the breast cancers negative for both ER and PR were similar in three cohort and three case–control studies when results were adjusted for several factors, including the number of full-term pregnancies (combined OR 0.90; 95% CI 0.82–0.99), with little heterogeneity and no indication of publication bias. In a subset of three adjusted studies that included ER, PR, and HER2 status, ever breastfeeding showed a stronger inverse association with triple-negative breast cancer (OR 0.78; 95% CI 0.66–0.91) among parous women. Overall, cohort studies showed no significant association between breastfeeding and ER+/PR+ or ER+ and/or PR+ breast cancers, although one and two studies (out of four and seven studies, respectively) showed an inverse association.
Conclusions
This meta-analysis showed a protective effect of ever breastfeeding against hormone receptor-negative breast cancers, which are more common in younger women and generally have a poorer prognosis than other subtypes of breast cancer. The association between breastfeeding and receptor-positive breast cancers needs more investigation.
introduction
Breast cancer is the most common cancer in women [1]. In the United States, one in eight women will develop breast cancer over the course of their lifetime [2]. Breast cancer is a heterogeneous disease with various subtypes. Only <5%–10% of breast cancers can be primarily attributed to an inherited genetic mutation, such as breast cancer 1 or 2, early onset (BRCA1 or 2) genes [3, 4]. More commonly, breast cancer is associated with lifestyle, reproductive, and other environmental factors, including aging, nulliparity, early age at menarche, late menopause and first full-term pregnancy, the use of exogenous hormones (oral contraceptives and combined postmenopausal hormone replacement therapy), alcohol consumption, excess weight, insulin resistance, and possibly smoking [5], many of which are potentially modifiable.
Breast cancer subtypes defined by receptor status may reflect different mechanisms of carcinogenesis. Many studies have reported an inverse association between breastfeeding and breast cancer [6, 7], including those suggesting a stronger protective effect on hormone receptor-negative breast cancers [8–14], which are cancers lacking both estrogen receptor (ER) and progesterone receptor (PR). This subtype constitutes 19%–22% of breast cancers in large-scale population-based studies in the United States and Europe [15, 16], but they are more common in younger women [17], patients with advanced disease [18], women in Sub-Saharan Africa [19], African-American women [2], and BRCA1 carriers [18]. Over two-third of hormone receptor-negative cancers are additionally negative for human epidermal growth factor receptor 2 (HER2) [15, 16]; this subtype is defined as triple-negative breast cancer (TNBC). Given the high incidence of breast cancer, any action that could significantly lower its risk would have a major public health impact. Additionally, identifying effective strategies to reduce the risk of receptor-negative breast cancers might be even more impactful, because they are more common in younger women and generally have a poorer prognosis than receptor-positive breast cancers [18].
The only previous meta-analysis of the association between breastfeeding and breast cancer risk by receptor status was based on seven case–control studies published up to December 2005. It showed inverse associations between breastfeeding and the breast cancers that were positive or negative for both hormone receptors (ER+ and PR+; or ER− and PR−) [20], but it did not take HER2 into consideration. Since then, results of several cohort and other case–control studies on this association have been published. We conducted this updated systematic review and meta-analysis of epidemiological studies to investigate the impact of breastfeeding on the incidence of breast cancer by hormone receptor and HER2 status, with further focus on receptor-negative subtypes.
methods
study selection
We searched the PubMed and Scopus databases to identify articles in English from case–control and prospective cohort studies on the association between breastfeeding and breast cancer risk, when these associations were reported by ER, PR, or HER2 status. We followed the Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [21]. The following terms were used to search the PubMed database: (‘Breast feeding’[Mesh] OR ‘breast-feeding’ OR breastfeeding OR reproductive OR lactation) AND (‘Breast Neoplasms’[Mesh] OR (breast AND (neoplasm OR cancer OR tumor OR tumors))) AND (‘Receptors, Estrogen’[Mesh] OR ‘Receptors, Progesterone’[Mesh] OR ((estrogen OR progesterone OR HER2) AND (receptor OR receptors))) AND ‘English’[Language]; and the Scopus database: ALL((‘breast feeding’) OR (breastfeeding) OR (lactation)) AND (ALL(Breast) AND ALL(Neoplasms OR neoplasm OR cancer OR tumor OR tumors)) AND ALL(Receptor OR Receptors) AND ALL(Estrogen OR Progesterone OR HER2) AND (LIMIT-TO(DOCTYPE, ‘ar’)) AND (LIMIT-TO(SUBJAREA, ‘MEDI’)) AND LANGUAGE (ENGLISH). Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. There was no limitation with regard to publication year. All results were updated on 27 August 2014. We identified a total of 1301 articles from PubMed and 1718 articles from Scopus. A total of 158 articles were present in both databases; so the combined set comprised 2861 distinct articles.
We examined article abstracts, retrieved and reviewed full texts of potentially eligible articles, and searched bibliographies of relevant articles to identify other publications not retrieved in our electronic search. Six additional potentially eligible publications were identified in bibliography search. We included publications reporting (i) original research, (ii) human studies, (iii) case–control or prospective cohort studies, and providing (iv) information about the association between breastfeeding and breast cancer by ER, PR, or HER2 status (risk estimates or enough information to calculate risk estimates, such as distribution of cases and controls by breastfeeding and receptor status). Of 2867 articles in our list (Figure 1), we excluded 2826 articles that did not meet the inclusion criteria. Five other articles, including one cohort and four case–control studies, were excluded for other reasons (supplementary Table S1, available at Annals of Oncology online): four articles were excluded because results were only reported by increments of breastfeeding duration (e.g. per 3 months of breastfeeding; three studies) [9, 22, 23] or by average number of children breastfed (one study) [24]; another study was excluded because the reference group in that study included those with the longest duration of breastfeeding, and not enough information was available to change the reference group (e.g. distribution of participants by categories of breastfeeding and receptor status) [25]. Six additional articles were excluded because similar or more complete data from the same study were available in another included publication. Of remaining 30 articles [10, 11, 13, 16, 26–51], 27 were from distinct studies; the other 3 articles [49–51] provided additional information for 3 [26, 35, 38] of the above 27 studies. It should be noted that a pooled analysis of four studies of African-American women was published since the completion of our analysis [52]. We did not include this article because all of three original studies with data on breastfeeding in this pooled analysis were included in our analysis [28, 39, 47], and the fourth study had no data on breastfeeding.
Figure 1.
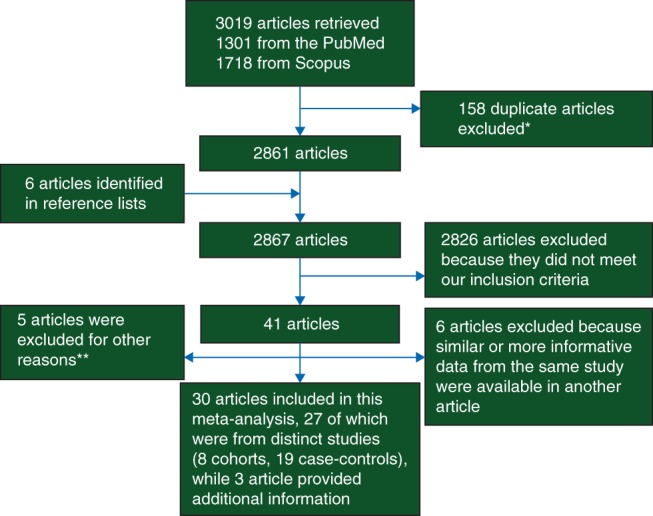
Flowchart of selection of studies. *When articles were indexed in both databases, only one was considered for further review. **Four studies were excluded because results were only reported by increments of breastfeeding duration or by average number of children breastfed; another study was excluded because the reference group was not those with the lowest duration of breastfeeding.
Three authors (FI, YL, JZ) independently performed the search and evaluated the articles. Two authors (FI, YL) independently abstracted the data. Any inconsistency was solved by consensus. We did not contact the authors of original studies.
data abstraction
We abstracted data on first author, publication year, study design, the number of cancer cases, the number of controls (case–control studies) or study sizes at baseline (cohort studies), the odds ratios (ORs) or relative risks (RRs) and 95% confidence intervals (CIs), and the variables for which the results were controlled by standardization or statistical adjustments. We used the maximally adjusted results when several risk estimates with various adjustments were reported. As sometimes only a subset of study subjects (e.g. only parous women) had information on receptor status, the numbers of total cases and controls shown in this meta-analysis could be smaller than the total numbers in the original studies.
Only a modest number of prospective cohort studies reported the results by single receptor status, in particular for PR status alone (supplementary Table S2, available at Annals of Oncology online). Therefore, we only show the results of individual studies and do not present pooled estimates by single receptor status. Also, the results by combination of receptors might be more relevant to the development of breast cancer than those by single receptor status. The definitions commonly used in original articles for combinations of receptors included: luminal (positive for either ER or PR or both: ER+ and/or PR+); luminal A (ER+ and/or PR+ and HER2−); luminal B (ER+ and/or PR+ and HER2+); nonluminal (negative for both ER and PR: ER−/PR−); and triple negative (ER−, PR−, and HER2−). Two cohort studies used additional biomarkers to define basal-like breast cancers [ER−, PR−, HER2−, and positive for cytokeratins (CK) 5/6 and/or epidermal growth factor receptor (EGFR)] and breast cancers that lacked expression of all of ER, PR, HER2, CK 5/6, and EGFR [13, 31]. We combined the results for these two latter groups and included them in analysis of TNBC. The distribution of cases, controls/noncases or person-years at risk, ORs, RRs, and 95% CIs were abstracted separately by receptor status and for breastfeeding (ever versus never) and for various categories of breastfeeding duration, when data were available. Ever breastfeeding in this analysis includes any duration of breastfeeding. However, the never breastfeeding group additionally included women with some duration of breastfeeding in one cohort (breastfeeding <4 months) [30] and three case–control studies (breastfeeding ≤11 months [37], <36 months [41], and ≤13 months [46]); the cohort study reported the results by single receptor status only (i.e. ER, PR, or HER2 status individually) [30]. As the results of our sensitivity analysis with inclusion and exclusion of these studies were similar, we reported the results with inclusion of these studies.
statistical analysis
We calculated the summary risk estimates and 95% CIs and plotted Forest plots using random-effects models (DerSimonian-Laird method) [53] for the association between ever breastfeeding and breast cancer by receptor status. We presented the summary estimates for cohort and case–control studies separately. As results of these two settings might not be comparable, we did not combine the overall results from cohort and case–control studies. However, when we did our preplanned subgroup analysis for adjusted studies, results of cohort and case–control studies appeared to be comparable in several receptor categories. Therefore, we showed combined results for adjusted results from both study designs. Adjustment variables differed across studies; we considered a study as adjusted when the results were adjusted at least for age, body mass index (BMI), parity (the number of births/full-term pregnancies when only parous women were included), and family history of breast cancer, as some of major potential confounding factors. We did not include other potential confounders because the number of studies with results adjusted for those factors was much more limited. We also combined results from cohort and case–control studies of parous women only. We had planned to do a subgroup analysis by outcome (incident cases; mortality), but the outcome in all included studies was the incident cases of breast cancer.
Only few cohort studies provided information on the association between duration of breastfeeding and breast cancer risk for each receptor status. We did not apply meta-regression models to cohort studies to examine a dose–response analysis, because models based on small number of studies could be unstable and might provide misleading results. We did not examine dose–response associations in case–control studies either because we were not able to verify these associations in cohort studies. We only show dose–response associations reported in individual cohort and case–control studies.
Heterogeneity among articles was estimated using the I2 statistic and P values associated with Q statistics. I2 statistic indicates the percentage of total variability explained by heterogeneity, and values of 25%, 50%, and 75% are arbitrarily considered as indicative of low, moderate, and high heterogeneity, respectively [54]. We plotted funnel plots and used Egger's weighted regression method and the Begg and Mazumdar's adjusted rank correlation test to examine publication bias for all and adjusted studies by receptor status when there was at least five cohort or five adjusted studies [55, 56]. All statistical analyses were conducted with Stata (Stata Corp. LP, version 13) statistical software. Throughout the article, associations with 95% CIs that do not include unity or two-sided P values of <0.05 were considered as statistically significant. An inverse association refers to a statistically significant association with an RR or OR of <1.
results
Thirty articles were included in this systematic review representing 27 distinct studies: 8 prospective cohort (supplementary Table S2, available at Annals of Oncology online) and 19 case–control studies (supplementary Table S3, available at Annals of Oncology online), with a total of 23 658 cases and 31 304 controls from case–control and 13 223 cases from cohort studies; the total number of participants in cohorts at baseline was 736 308 persons. The publication year varied from 2007 to 2014 for cohort studies and from 1983 to 2014 for case–control studies. The cohort studies were conducted in the United States (N = 5) and Europe (N = 3). The case–control studies were conducted in North America (N = 11), Asia (N = 6), and Australia (N = 1), and one pooled study was conducted in the United States, Canada, and Australia. Three and two studies exclusively included premenopausal [10, 29, 37] and postmenopausal [27, 32] women, respectively.
ER−/PR− and TBNC
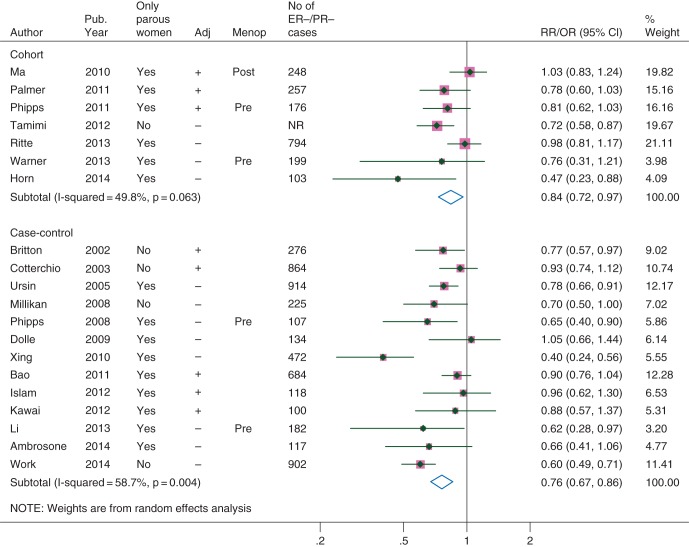
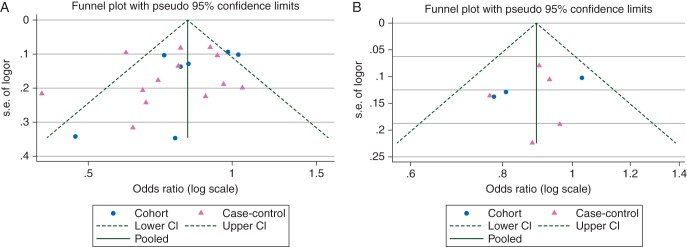
Risk estimates from individual studies on the association between breastfeeding and the breast cancers that were negative for both ER and PR (ER−/PR−) are shown in Figure 2. The ORs were similar in the cohort and case–control studies that were adjusted at least for age, BMI, parity, and family history of breast cancer (Table 1). The combined OR (95% CI) for three cohort and three case–control studies of parous women with adjusted results was 0.90 (0.82–0.99) with little heterogeneity (I2 = 0%, P for heterogeneity = 0.61). The funnel plot shows more studies in the lower left than lower right side of the plot when all studies were considered, suggesting some bias toward publication of smaller studies (with greater standard errors of OR) reporting inverse associations (Figure 3). However, for adjusted studies, neither the funnel plot nor formal statistical tests did show any indication of publication bias. Similarly, the combined results suggest an inverse association between breastfeeding and TNBC, but there were only three adjusted cohort or case–control studies among parous women (OR 0.78; 95% CI 0.66–0.91) in this category (Table 1, supplementary Figure S1, available at Annals of Oncology online).
Figure 2.
Association between ever breastfeeding and the breast cancers that are negative for both estrogen and progesterone receptors. Adj, adjusted for at least age, body mass index, parity, and family history of breast cancer; Menop, menopausal status of study participants (‘Pre’ and ‘Post’ indicate that participants were premenopausal or postmenopausal women, respectively); NR, not-reported; Pub. year, publication year.
Table 1.
Association between ever breastfeeding and breast cancer risk by receptor status
| Receptor status | No. of studies | Relative risk/odds ratio (95% CI) | I2 statistics (%) | P for heterogeneity |
|---|---|---|---|---|
| ER−/PR− | ||||
| Cohort | 7 | 0.84 (0.72–0.97) | 50 | 0.06 |
| Adjusteda | 3 | 0.88 (0.74–1.06) | 42 | 0.18 |
| Only parous women | 6 | 0.88 (0.76–1.02) | 39 | 0.15 |
| Adjusteda, only parous | 3 | 0.88 (0.74–1.06) | 42 | 0.18 |
| Case–control | 13 | 0.76 (0.67–0.86) | 59 | 0.004 |
| Adjusteda | 5 | 0.89 (0.80–0.99) | 0 | 0.83 |
| Only parous women | 9 | 0.77 (0.65–0.91) | 55 | 0.02 |
| Adjusteda, only parous | 3 | 0.91 (0.79–1.04) | 0 | 0.94 |
| All, adjusteda | 8 | 0.89 (0.82–0.97) | 0 | 0.66 |
| All, only parous women | 15 | 0.81 (0.73–0.91) | 51 | 0.01 |
| All, adjusteda, only parous | 6 | 0.90 (0.82–0.99) | 0 | 0.61 |
| Triple negative | ||||
| Cohort | 3 | 0.73 (0.62–0.87) | 0 | 0.43 |
| Adjusteda | 1 | 0.81 (0.62–1.04) | – | – |
| Only parous women | 2 | 0.76 (0.53–1.08) | 15 | 0.28 |
| Adjusteda, only parous | 1 | 0.81 (0.62–1.04) | – | – |
| Case–control | 9 | 0.73 (0.64–0.84) | 12 | 0.34 |
| Adjusteda | 2 | 0.75 (0.61–0.93) | 0 | 0.67 |
| Only parous women | 7 | 0.71 (0.59–0.85) | 26 | 0.23 |
| Adjusteda, only parous | 2 | 0.75 (0.61–0.93) | 0 | 0.67 |
| All, adjusteda | 3 | 0.78 (0.66–0.91) | 0 | 0.83 |
| All, only parous women | 9 | 0.72 (0.63–0.84) | 17 | 0.29 |
| All, adjusteda, only parous | 3 | 0.78 (0.66–0.91) | 0 | 0.83 |
| ER+/PR+ | ||||
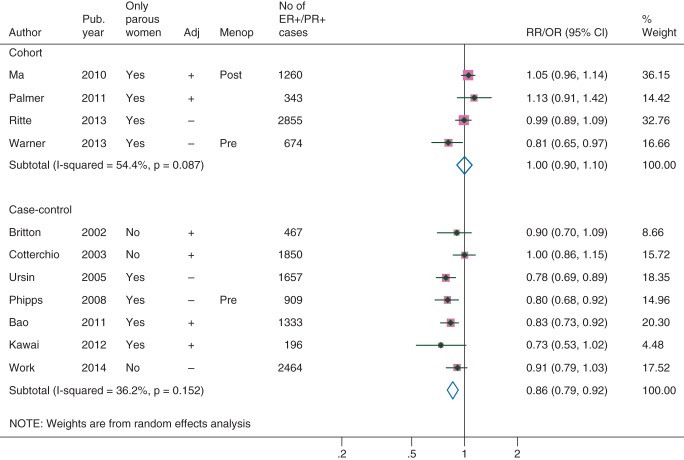
| Cohort | 4 | 1.00 (0.90–1.10) | 54 | 0.09 |
| Adjusteda | 2 | 1.06 (0.98–1.15) | 0 | 0.55 |
| Only parous women | 4 | 1.00 (0.90–1.10) | 54 | 0.09 |
| Adjusteda, only parous | 2 | 1.06 (0.98–1.15) | 0 | 0.55 |
| Case–control | 7 | 0.86 (0.79–0.92) | 36 | 0.15 |
| Adjusteda | 4 | 0.88 (0.78–0.99) | 43 | 0.16 |
| Only parous women | 4 | 0.80 (0.75–0.86) | 0 | 0.84 |
| Adjusteda, only parous | 2 | 0.82 (0.73–0.91) | 0 | 0.47 |
| All, adjusteda | 6 | 0.95 (0.85–1.06) | 68 | 0.008 |
| All, only parous women | 8 | 0.89 (0.80–0.99) | 77 | <0.001 |
| All, adjusteda, only parous | 4 | 0.94 (0.79–1.12) | 80 | 0.002 |
| ER+ and/or PR+ | ||||
| Cohort | 7 | 0.97 (0.88–1.07) | 78 | <0.001 |
| Adjusteda | 3 | 1.04 (0.98–1.10) | 0 | 0.56 |
| Only parous women | 5 | 1.00 (0.92–1.09) | 43 | 0.14 |
| Adjusteda, only parous | 2 | 1.04 (0.95–1.14) | 15 | 0.28 |
| Case–control | 18 | 0.82 (0.76–0.89) | 69 | <0.001 |
| Adjusteda | 5 | 0.88 (0.76–1.02) | 72 | 0.006 |
| Only parous women | 12 | 0.83 (0.74–0.92) | 70 | <0.001 |
| Adjusteda, only parous | 3 | 0.89 (0.71–1.11) | 75 | 0.02 |
| All, adjusteda | 8 | 0.95 (0.87–1.05) | 75 | <0.001 |
| All, only parous women | 17 | 0.88 (0.81–0.97) | 78 | <0.001 |
| All, adjusteda, only parous | 5 | 0.97 (0.85–1.11) | 77 | 0.002 |
aResults adjusted at least for age, body mass index, parity, and family history of breast cancer.
ER, estrogen receptor; ER−/PR−, negative for both ER and PR; ER+/PR+, positive for both ER and PR; ER+ and/or PR+, positive for either estrogen or progesterone receptor or both; PR, progesterone receptor.
Figure 3.
Funnel plot for the association between breastfeeding and the breast cancers that are negative for both estrogen and progesterone receptors. (A) All studies. P value for publication bias—Egger's method: 0.16; Begg's method: 0.12. (B) Studies adjusted for at least age, body mass index, parity, and family history of breast cancer. P value for publication bias—Egger's method: 0.47; Begg's method: 0.32.
ER+ and/or PR+ cancers
In cohort studies, breastfeeding did not show any associations with the breast cancers that were positive for both ER and PR (ER+/PR+; Table 1, Figure 4, Funnel plot in supplementary Figure S2, available at Annals of Oncology online) or ER+ and/or PR+ cancers (supplementary Figures S3–S4, available at Annals of Oncology online). There was a high heterogeneity, but the only cohort studies that showed inverse associations [13, 29] were from different, not overlapping phases of a cohort of registered nurses with relatively younger participants (supplementary Table S2, available at Annals of Oncology online). The RRs in other cohort studies were close to or above unity. Case–control studies generally showed an inverse association between breastfeeding and these two subtypes (Table 1).
Figure 4.
Association between ever breastfeeding and the breast cancers that were positive for both estrogen and progesterone receptors. Adj, adjusted for at least age, body mass index, parity, and family history of breast cancer; Menop, menopausal status of study participants (‘Pre’ and ‘Post’ indicate that participants were premenopausal or postmenopausal women, respectively); Pub. year, publication year.
In a sensitivity analysis, we included only the studies with results on both ER−/PR− and ER+ and/or PR+ breast cancers. The combined estimates were comparable with the overall results: in cohort studies, the OR (95% CI) was 0.84 (0.72–0.97) for ER−/PR− and 0.96 (0.85–1.08) for ER+ and/or PR+ cancers.
dose–response association
Of eight cohort studies included in this analysis, six provided risk estimates for the association between duration of breastfeeding and breast cancer risk by receptor status (supplementary Table S2, available at Annals of Oncology online). In five [16, 26–28, 30] of six studies reporting the association between duration of breastfeeding and ER+ and/or PR+ breast cancers [13, 16, 26–28, 30], risk estimates for any durations were generally close to or above unity. There were five studies reporting results for ER−/PR− or TNBC [13, 16, 26–28], of which three showed some indication of inverse association [13, 26, 28] (two of which were adjusted for parity) [13, 28]. Several case–control studies showed inverse dose–response associations between breastfeeding and hormone receptor-negative breast cancers or TNBC [10, 11, 35, 38, 39, 44, 46, 48], as well as ER+ and/or PR+ cancers [11, 33, 35, 38, 41, 44, 46, 48].
discussion
In this meta-analysis of cohort and case–control studies in women with diverse ethno-cultural backgrounds, ever breastfeeding was associated with a 10% decrease in the risk of the breast cancers that were negative for both ER and PR in parous women when results were adjusted for age, BMI, number of full-term pregnancies, and family history of breast cancer, with minimal heterogeneity and little evidence for publication bias. This inverse association was even stronger for TNBC—∼20% reduction in risk—but this was based on a modest number of studies. Several case–control studies showed inverse dose–response associations between breastfeeding and hormone receptor-negative breast cancers and TNBC, and there was some indication of such an association in a few cohort studies; however, the number of cohort studies reporting on dose–response association was limited. Breastfeeding was not significantly associated with the risk of ER+ and/or PR+ breast cancer in cohort studies. Although several case–control studies reported a dose–response inverse association for ER+ and/or PR+ cancers, cohort studies did not identify such an association.
It is long known that parity has an inverse association with breast cancer overall [57]. Although breastfeeding and parity are highly correlated, a large pooled analysis showed a 4% reduction in breast cancer risk associated with every 12 months of breastfeeding, which was independent and in addition to a 7% reduction in the risk with each live birth [6]. The pattern of associations between parity/breastfeeding and breast cancer by receptor status provide another piece of evidence for an independent protective effect of breastfeeding. Although parity reduces the risk of breast cancer overall, it has a paradoxical effect by receptor status: early age at first full-term pregnancy and multiparity are generally associated with a lower risk of ER+ and/or PR+ breast cancers but with an increased risk of [28, 39] or no association [13, 16, 29, 49, 51] with ER−/PR− and TNBC. Therefore, the inverse association of breastfeeding with ER−/PR− and TNBC in our analysis cannot be explained by parity. The magnitude of the inverse association between breastfeeding and ER−/PR− and TNBC in parous women remained strong in our analysis (∼10%–20%) even when the results were adjusted for the number of full-term pregnancies and a few other potential confounding factors, indicating that the inverse association in the above pooled analysis (4%) [6], might have been diluted by receptor-positive breast cancers, which are much more common than receptor-negative subtypes and showed little association with breastfeeding in our analysis. Although the 4% reduction in risk in the pooled study was for 12 months of breastfeeding, ever breastfeeding in our analysis is unlikely to represent much longer durations of breastfeeding (if not shorter), especially in Western countries [6], so a longer duration of breastfeeding is unlikely to be the reason for a stronger inverse association in our analysis.
Consistent with results of this meta-analysis, studies on breast cancer subtypes in specific racial or ethnic groups who are at higher risk for hormone receptor-negative breast cancers and TNBC are more likely to demonstrate the greatest benefit to breastfeeding. Two systematic reviews of risk factors of breast cancer in BRCA1 and BRCA2 carriers showed an inverse association between breastfeeding and breast cancer in BRCA1 carriers but not in BRCA2 carriers [58, 59]. BRCA1 carriers are at higher risk for developing ER−/PR− and TNBC, whereas BRCA2 carriers are more likely to develop ER+ and/or PR+ breast cancer [60]. A pooled analysis of several studies of African-American women (24% ER− and 15% TNBC) demonstrated an ∼10% reduction in the risk of all breast cancers with ever breastfeeding (19% reduction in ER− or TNBC risk but without any effect on ER+ and/or PR+ subtypes) [52].
The mechanisms of breastfeeding's protective effect on ER−/PR− and TNBC subtypes are unclear and need further investigations. This protective effect may be partly due to alterations in hormones other than estrogen and progesterone, such as androgens, which can suppress cell proliferation in ER+ tumors but can promote tumorigenesis in ER− tumors [61], as well as nonhormonal mechanisms, including changes in immune responses, alterations in proteins involved in tight junctions and cell-to-cell adhesion, and apoptosis [62, 63]. The mechanisms of action of parity and breastfeeding's separate and additive protective effects are likely to work through their effects on the molecular maturation and the complete involution of the terminal ductal units, the milk-making cells, which confer resistance to carcinogenesis [64]. It has been suggested that higher mammographic breast density is associated with an increased risk of breast cancer [65–68]. However, it is unclear whether the inverse association between breastfeeding and breast cancer is through an effect of breastfeeding on breast density. Although some studies have suggested that breastfeeding could eventually reduce the risk of having high breast density [69, 70], especially among younger women [69], some other studies do not support this notion [71–74].
Although this meta-analysis showed no significant overall association between breastfeeding and the risk of ER+ and/or PR+ cancers, there were a number of studies (mainly case–control studies) that did demonstrate a benefit against this subtype [11, 13, 33, 35, 38, 39, 41, 43, 44, 46, 48]. This suggests why the earlier meta-analysis, which included only case–control studies, showed an inverse association between breastfeeding and ER+/PR+ breast cancers [20]. More prospective research is required before making any definite conclusion about the nature of the association between breastfeeding and receptor-positive breast cancer, including on whether or not there is a more modest inverse association, especially in younger women [13, 29].
Results of this study have important public health implications, because breastfeeding can be a practical way to potentially reduce the risk of receptor-negative breast cancers, which are more common in younger women. These cancers are unlikely to respond to antiestrogen therapies, which are important strategies for treatment and prevention of recurrence of more common hormone receptor-positive breast cancers [75], and they generally are more aggressive and have a poorer prognosis than receptor-positive cancers [18]. Results of our analysis could be especially important to individuals at a higher risk of receptor-negative cancers, including BRCA1 carriers who want to utilize all potential protective measures as they accelerate child-bearing before recommended prophylactic surgeries. African-American women not only are at a higher risk of receptor-negative breast cancer, but also have a much lower rate of breastfeeding than White or Hispanic women in the United States; for example, the respective rates for 6 and 12 months of breastfeeding after childbirth in 2007 was 27.5% and 12.5% among African-Americans, 45.1% and 23.6% among Whites, and 46.0% and 24.7% among Hispanics [76]. In addition, breastfeeding could represent an important complement to other risk reduction strategies in women [77], such as sustained weight management [78], as well as several well-established benefits to babies [79]. The duration of breastfeeding necessary to protect against breast cancer, however, is yet to be accurately ascertained. The modest number of prospective cohort studies with several categories of exposure did not allow us to perform a dose–response meta-analysis. Short durations of breastfeeding in western countries, where all cohort studies included in this analysis came from, might have been one of the factors that interfered with proper examination of dose–response associations in original reports.
Our analysis has several strengths, including thorough and exhaustive search of two databases and reference lists of relevant articles by three researchers and abstraction of data independently by two researchers. On the other hand, we were unable to include studies in other languages than English. However, the results of major prospective cohort studies on this topic are most likely to be published in English. One of the limitations of all meta-analyses of observational studies is heterogeneity in the study design and variations in definition of exposures and outcomes across studies. Nevertheless, in almost all studies, the outcome (breast cancer) was histopathologically confirmed. Errors or inconsistencies in measurement of breastfeeding could be more likely [80]. However, any exposure misclassifications in prospective cohort studies are likely to be nondifferential and less likely to cause spurious associations. The moderate heterogeneity between results of some cohort studies could be related to differences in the usual duration of breastfeeding in populations and changes in this duration over time [81]. In addition to differences between high-income and low- and middle-income countries, e.g. a lifetime average duration of breastfeeding of 8.7 months for parous controls in high-income countries versus a median duration of breastfeeding of 24 months per child in rural areas of Asia and Africa in the 1990s, duration of breastfeeding varies across high-income populations [6]. These variations and differences in definition of breastfeeding may explain part of the heterogeneity between cohort and case–control studies, but methodological issues in case–control studies, including recall bias, may play a major role. Finally, although this analysis provides a better insight into the association between breastfeeding and breast cancer, the results of subgroup analyses are based on modest numbers of studies, so this association and its detailed aspects, including optimal duration of breastfeeding, need to be examined in further prospective studies. In addition, interventional trials are required to identify effective ways to promote breastfeeding among women, in particular those at high risk of receptor-negative breast cancers.
conclusions
This meta-analysis showed a protective effect of ever breastfeeding against hormone receptor-negative breast cancers, and this effect seems to be several times stronger than what had been suggested by studies of all breast cancers without stratification by receptor status. Our finding has clear public health implications, because hormone receptor-negative cancers constitute at least one-fifth of breast cancers in the general population, they are more common in younger women, and they generally have a poorer prognosis. Women with the highest risk of receptor-negative breast cancers, such as African-American women and BRCA1 carriers, can potentially benefit more from breastfeeding. Although our results showed little association between breastfeeding and receptor-positive breast cancers in cohort studies, more prospective research is required before making any definite conclusion about the nature of this association.
Supplementary Material
acknowledgements
We thank Lauren Hanley (Cambridge Health Alliance and Harvard Medical School, Cambridge, USA) and Melissa Bartick (Massachusetts General Hospital, Boston, USA) for reviewing this manuscript and providing thoughtful comments.
disclosure
The authors have declared no conflicts of interest.
references
- 1.Jemal A, Bray F, Center MM et al. Global cancer statistics. CA Cancer J Clin. 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin 2014; 64: 52–62. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury AR, Olopade OI. Genetic susceptibility to breast cancer. Rev Endocr Metab Disord 2007; 8: 255–267. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Choi DH. Distribution of BRCA1 and BRCA2 mutations in Asian patients with breast cancer. J Breast Cancer 2013; 16: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colditz GA, Bohlke K. Priorities for the primary prevention of breast cancer. CA Cancer J Clin 2014; 64: 186–194. [DOI] [PubMed] [Google Scholar]
- 6.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002; 360: 187–195. [DOI] [PubMed] [Google Scholar]
- 7.Anothaisintawee T, Wiratkapun C, Lerdsitthichai P et al. Risk factors of breast cancer: a systematic review and meta-analysis. Asia Pac J Public Health 2013; 25: 368–387. [DOI] [PubMed] [Google Scholar]
- 8.Shinde SS, Forman MR, Kuerer HM et al. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer 2010; 116: 4933–4943. [DOI] [PubMed] [Google Scholar]
- 9.Gaudet MM, Press MF, Haile RW et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat 2011; 130: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CI, Beaber EF, Tang MT et al. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat 2013; 137: 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phipps AI, Malone KE, Porter PL et al. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer 2008; 113: 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redondo CM, Gago-Dominguez M, Ponte SM et al. Breast feeding, parity and breast cancer subtypes in a Spanish cohort. PLoS One 2012; 7: e40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamimi RM, Colditz GA, Hazra A et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat 2012; 131: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 2014; 144: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlader N, Altekruse SF, Li CI et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014; 106: pii: dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritte R, Tikk K, Lukanova A et al. Reproductive factors and risk of hormone receptor positive and negative breast cancer: a cohort study. BMC Cancer 2013; 13: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheridan W, Scott T, Caroline S et al. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat 2014; 147: 617–629. [DOI] [PubMed] [Google Scholar]
- 18.Gluz O, Liedtke C, Gottschalk N et al. Triple-negative breast cancer—current status and future directions. Ann Oncol 2009; 20: 1913–1927. [DOI] [PubMed] [Google Scholar]
- 19.Brinton LA, Figueroa JD, Awuah B et al. Breast cancer in Sub-Saharan Africa: opportunities for prevention. Breast Cancer Res Treat 2014; 144: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res 2006; 8: R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 22.Yoo KY, Tajima K, Miura S et al. Breast cancer risk factors according to combined estrogen and progesterone receptor status: a case-control analysis. Am J Epidemiol 1997; 146: 307–314. [DOI] [PubMed] [Google Scholar]
- 23.Chung S, Park SK, Sung H et al. Association between chronological change of reproductive factors and breast cancer risk defined by hormone receptor status: results from the Seoul Breast Cancer Study. Breast Cancer Res Treat 2013; 140: 557–565. [DOI] [PubMed] [Google Scholar]
- 24.Cummings SR, Lee JS, Lui LY et al. Sex hormones, risk factors, and risk of estrogen receptor-positive breast cancer in older women: a long-term prospective study. Cancer Epidemiol Biomarkers Prev 2005; 14: 1047–1051. [DOI] [PubMed] [Google Scholar]
- 25.Rusiecki JA, Holford TR, Zahm SH, Zheng T. Breast cancer risk factors according to joint estrogen receptor and progesterone receptor status. Cancer Detect Prev 2005; 29: 419–426. [DOI] [PubMed] [Google Scholar]
- 26.Chlebowski RT, Anderson GL, Lane DS et al. Predicting risk of breast cancer in postmenopausal women by hormone receptor status. J Natl Cancer Inst 2007; 99: 1695–1705. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Henderson KD, Sullivan-Halley J et al. Pregnancy-related factors and the risk of breast carcinoma in situ and invasive breast cancer among postmenopausal women in the California Teachers Study cohort. Breast Cancer Res 2010; 12: R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer JR, Boggs DA, Wise LA et al. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev 2011; 20: 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warner ET, Colditz GA, Palmer JR et al. Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: are there differences before and after age 40? Breast Cancer Res Treat 2013; 142: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butt S, Borgquist S, Anagnostaki L et al. Breastfeeding in relation to risk of different breast cancer characteristics. BMC Res Notes 2014; 7: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn J, Opdahl S, Engstrom MJ et al. Reproductive history and the risk of molecular breast cancer subtypes in a prospective study of Norwegian women. Cancer Causes Control 2014; 25: 881–889. [DOI] [PubMed] [Google Scholar]
- 32.Hildreth NG, Kelsey JL, Eisenfeld AJ et al. Differences in breast cancer risk factors according to the estrogen receptor level of the tumor. J Natl Cancer Inst 1983; 70: 1027–1031. [PubMed] [Google Scholar]
- 33.McTiernan A, Thomas DB, Johnson LK, Roseman D. Risk factors for estrogen receptor-rich and estrogen receptor-poor breast cancers. J Natl Cancer Inst 1986; 77: 849–854. [PubMed] [Google Scholar]
- 34.Cooper JA, Rohan TE, Cant EL et al. Risk factors for breast cancer by oestrogen receptor status: a population-based case-control study. Br J Cancer 1989; 59: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Britton JA, Gammon MD, Schoenberg JB et al. Risk of breast cancer classified by joint estrogen receptor and progesterone receptor status among women 20–44 years of age. Am J Epidemiol 2002; 156: 507–516. [DOI] [PubMed] [Google Scholar]
- 36.Cotterchio M, Kreiger N, Theis B et al. Hormonal factors and the risk of breast cancer according to estrogen- and progesterone-receptor subgroup. Cancer Epidemiol Biomarkers Prev 2003; 12: 1053–1060. [PubMed] [Google Scholar]
- 37.Nichols HB, Trentham-Dietz A, Love RR et al. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomarkers Prev 2005; 14: 41–47. [PubMed] [Google Scholar]
- 38.Ursin G, Bernstein L, Lord SJ et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer 2005; 93: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millikan RC, Newman B, Tse CK et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 2008; 109: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweeney C, Baumgartner KB, Byers T et al. Reproductive history in relation to breast cancer risk among Hispanic and non-Hispanic white women. Cancer Causes Control 2008; 19: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dey S, Boffetta P, Mathews A et al. Risk factors according to estrogen receptor status of breast cancer patients in Trivandrum, South India. Int J Cancer 2009; 125: 1663–1670. [DOI] [PubMed] [Google Scholar]
- 42.Dolle JM, Daling JR, White E et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev 2009; 18: 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing P, Li J, Jin F. A case-control study of reproductive factors associated with subtypes of breast cancer in Northeast China. Med Oncol 2010; 27: 926–931. [DOI] [PubMed] [Google Scholar]
- 44.Bao PP, Shu XO, Gao YT et al. Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the shanghai breast cancer study. Am J Epidemiol 2011; 174: 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Islam T, Matsuo K, Ito H et al. Reproductive and hormonal risk factors for luminal, HER2-overexpressing, and triple-negative breast cancer in Japanese women. Ann Oncol 2012; 23: 2435–2441. [DOI] [PubMed] [Google Scholar]
- 46.Kawai M, Kakugawa Y, Nishino Y et al. Reproductive factors and breast cancer risk in relation to hormone receptor and menopausal status in Japanese women. Cancer Sci 2012; 103: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambrosone CB, Zirpoli G, Ruszczyk M et al. Parity and breastfeeding among African-American women: differential effects on breast cancer risk by estrogen receptor status in the Women's Circle of Health Study. Cancer Causes Control 2014; 25: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Work ME, John EM, Andrulis IL et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer 2014; 110: 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phipps AI, Chlebowski RT, Prentice R et al. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst 2011; 103: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trivers KF, Lund MJ, Porter PL et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control 2009; 20: 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma H, Wang Y, Sullivan-Halley J et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women's contraceptive and reproductive experiences study. Cancer Res 2010; 70: 575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer JR, Viscidi E, Troester MA et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst 2014; 106: pii: dju237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin.Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 54.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst 1989; 81: 107–115. [DOI] [PubMed] [Google Scholar]
- 56.Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998; 316: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Press DJ, Pharoah P. Risk factors for breast cancer: a reanalysis of two case-control studies from 1926 and 1931. Epidemiology 2010; 21: 566–572. [DOI] [PubMed] [Google Scholar]
- 58.Pan H, He Z, Ling L et al. Reproductive factors and breast cancer risk among BRCA1 or BRCA2 mutation carriers: results from ten studies. Cancer Epidemiol 2014; 38: 1–8. [DOI] [PubMed] [Google Scholar]
- 59.Friebel TM, Domchek SM, Rebbeck TR. Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst 2014; 106: dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lakhani SR, Van De Vijver MJ, Jacquemier J et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol 2002; 20: 2310–2318. [DOI] [PubMed] [Google Scholar]
- 61.McNamara KM, Yoda T, Takagi K et al. Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol 2013; 133: 66–76. [DOI] [PubMed] [Google Scholar]
- 62.Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol 2014; 141: 462–477. [DOI] [PubMed] [Google Scholar]
- 63.Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. New strategies for triple-negative breast cancer—deciphering the heterogeneity. Clin Cancer Res 2014; 20: 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi S, Sugiura H, Ando Y et al. Reproductive history and breast cancer risk. Breast Cancer 2012; 19: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2007; 99: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 66.Ramon YCT, Chirivella I, Miranda J et al. Mammographic density and breast cancer in women from high risk families. Breast Cancer Res 2015; 17: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maskarinec G, Nakamura KL, Woolcott CG et al. Mammographic density and breast cancer risk by family history in women of white and Asian ancestry. Cancer Causes Control 2015; 26: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vachon CM, Pankratz VS, Scott CG et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst 2015; 107: pii: dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riza E, dos Santos Silva I, De Stavola B et al. Correlates of high-density mammographic parenchymal patterns by menopausal status in a rural population in Northern Greece. Eur J Cancer 2005; 41: 590–600. [DOI] [PubMed] [Google Scholar]
- 70.Ursin G, Sun CL, Koh WP et al. Associations between soy, diet, reproductive factors, and mammographic density in Singapore Chinese women. Nutr Cancer 2006; 56: 128–135. [DOI] [PubMed] [Google Scholar]
- 71.Sung J, Song YM, Stone J et al. Reproductive factors associated with mammographic density: a Korean co-twin control study. Breast Cancer Res Treat 2011; 128: 567–572. [DOI] [PubMed] [Google Scholar]
- 72.Ahmadinejad N, Movahedinia S, Movahedinia S et al. Distribution of breast density in Iranian women and its association with breast cancer risk factors. Iran Red Crescent Med J 2013; 15: e16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crew KD, Campbell J, Reynolds D et al. Mammographic density and serum 25-hydroxyvitamin D levels. Nutr Metab (Lond) 2014; 11: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y, Liu J, Gu R et al. Influence of factors on mammographic density in premenopausal Chinese women. Eur J Cancer Prev 2015. June 11 [epub ahead of print], doi: 10.1097/CEJ.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 75.den Hollander P, Savage MI, Brown PH. Targeted therapy for breast cancer prevention. Front Oncol 2013; 3: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spencer BS, Grassley JS. African American women and breastfeeding: an integrative literature review. Health Care Women Int 2013; 34: 607–625. [DOI] [PubMed] [Google Scholar]
- 77.Bartick MC, Stuebe AM, Schwarz EB et al. Cost analysis of maternal disease associated with suboptimal breastfeeding. Obstet Gynecol 2013; 122: 111–119. [DOI] [PubMed] [Google Scholar]
- 78.Sharma AJ, Dee DL, Harden SM. Adherence to breastfeeding guidelines and maternal weight 6 years after delivery. Pediatrics 2014; 134(Suppl 1): S42–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ip S, Chung M, Raman G et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007; 153: 1–186. [PMC free article] [PubMed] [Google Scholar]
- 80.Huttly SR, Barros FC, Victora CG et al. Do mothers overestimate breast feeding duration? An example of recall bias from a study in southern Brazil. Am J Epidemiol 1990; 132: 572–575. [DOI] [PubMed] [Google Scholar]
- 81.Lee H, Li JY, Fan JH et al. Risk factors for breast cancer among Chinese women: a 10-year nationwide multicenter cross-sectional study. J Epidemiol 2014; 24: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.