‘Allogeneic Stem Cell Transplantation in Patients with Cutaneous Lymphoma: Updated Results from a Single Institution’ for publication in Annals of Oncology. This is the largest single-center prospective analysis of stem cell transplantation in patients with cutaneous T-cell lymphoma.
Keywords: allogeneic stem-cell transplantation, cutaneous T-cell lymphoma, mycosis fungoides, Sézary syndrome
Abstract
Background
Cutaneous T-cell lymphomas (CTCLs) and its common variants mycosis fungoides (MF) and leukemic Sézary syndrome (SS) are rare extranodal non-Hodgkin's lymphomas. Patients who present with advanced disease and large-cell transformation (LCT) are incurable with standard treatments. In this article, we report the largest single-center experience with allogeneic stem-cell transplantation (SCT) for advanced CTCL.
Patients and methods
This is a prospective case series of 47 CTCL patients who underwent allogeneic SCT after failure of standard therapy between July 2001 and September 2013. The Kaplan–Meier method was used to estimate overall survival (OS) and progression-free survival (PFS) curves. The method of Fine and Gray was used to fit regression models to the same covariates for these cumulative incidence data.
Results
The Kaplan–Meier estimates of OS and PFS at 4 years were 51% and 26%, respectively. There was no statistical difference in the OS in patients who had MF alone, SS, MF with LCT, or SS with LCT. PFS at 4 years was superior in patients who had SS versus those who did not (52.4% versus 9.9%; P = 0.02). The cumulative incidences of grade 2–4 acute graft-versus-host disease (GVHD) and chronic GVHD were 40% and 28%, respectively. The cumulative nonrelapse mortality rate was 16.7% at 2 years.
Conclusion
Allogeneic SCT may result in long-term remissions in a subset of patients with advanced CTCL. Although post-SCT relapse rates are high, many patients respond to immunomodulation and achieve durable remissions.
ClinicalTrials.gov
introduction
Cutaneous T-cell lymphomas (CTCLs) and its common variants mycosis fungoides (MF) and leukemic Sézary syndrome (SS) are rare extranodal non-Hodgkin lymphomas arising from the clonal proliferation of skin-homing lymphocytes and have an incidence of 7.7 cases per 1 000 000 person years in the United States [1, 2]. Patients with early-stage MF (stage IA–IIA) generally have a good prognosis; with median overall survival (OS) duration of more than 25 years [3]. However, patients with advanced MF or SS (stage IIB–IVB), which may include tumors, nodal, blood, or visceral involvement, and patients with folliculotropic MF or large-cell transformation (LCT) have a poor prognosis with reported median OS of 5 years or less [3]. Unlike other T-cell lymphomas, patients with CTCL generally have dismal responses to autologous stem-cell transplantation (SCT), with median progression-free survival (PFS) durations of <6 months reported in most cases [4–6]. Although more promising results have been reported with the use of allogeneic SCT in this patient population, most such reports are limited by small patient numbers, heterogeneous preparative regimens, and/or diverse T-cell histologies [7–13]. In a previous study, we reported encouraging results with allogeneic SCT in 19 patients with advanced CTCL. In this article, we provide updated data on 47 patients, including those included in our previous study, who underwent SCT for advanced CTCL [10, 14].
patients and methods
All consecutive CTCL patients who underwent allogeneic SCT at The University of Texas MD Anderson Cancer Center between July 2001 and September 2013 were included. All provided written informed consent. The study was approved by the Institutional Review Board. To be eligible for SCT, patients had to have advanced MF (folliculotropic variant or stage IIB or higher) or SS, a Karnofsky performance status score of more than 70%, and adequate pulmonary, cardiac, hepatic, and renal function. Eligible patients also had to have failed prior standard therapy and have no active infections or skin ulcers. Visceral sites of disease had to be adequately treated before SCT. Patients were required to have an 8/8 or 7/8 human leukocyte antigen (HLA)-compatible related or unrelated donor (class I HLA-A, HLA-B, and HLA-C loci by intermediate/high-resolution typing and class II HLA-DRB1 by molecular typing). Patients underwent SCT on any available transplant protocol for hematologic malignancies or as standard of care. Patients with >10% body surface area (BSA) skin involvement received tumor debulking with total body skin electron beam radiation (TBSEB) therapy completed 2–3 weeks before the planned transplant with healing of all areas of skin breakdown before initiation of transplant conditioning regimen [15]. All patients underwent a complete physical examination, including assessment of BSA involved with patches, plaques, and/or tumors and pruritus scoring before SCT. Baseline evaluations and imaging studies included bone marrow aspiration and biopsy, flow cytometric analysis of peripheral blood for T-cell lymphoma panel, and positron emission tomography–computed tomography (PET-CT) scans if clinically indicated.
conditioning regimen
Forty-two patients (89%) received a conditioning regimen consisting of fludarabine and melphalan with or without antithymocyte globulin (ATG). Two patients received the nonmyeloablative conditioning regimen of fludarabine, cyclophosphamide, and rituximab (FCR) and three patients received fludarabine and busulfan. Only patients who received unrelated or mismatched grafts received ATG as a part of their conditioning regimen. Graft-versus-host-disease (GVHD) prophylaxis included minimethotrexate and tacrolimus (adjusted to a blood concentration of 5–15 ng/ml starting 2 days before SCT and maintained for ≥6 months). Supportive care was administered per institutional guidelines as previously described [10].
Forty-two patients (89%) received TBSEB radiation therapy before SCT, of whom 25 (60%) achieved a complete remission (CR) in the skin. Of the five patients who did not receive TBSEB radiation therapy, three had no skin disease at the time of SCT, one had limited facial erythema, and one had scalp tumors that were treated with local radiation therapy. TBSEB was delivered using the modified Stanford technique for a median dose of 32 cGy (range, 12–36) within 8–9 weeks [16]. Two days per week, 2 Gy were delivered to all 12 fields; starting at week 4, supplemental radiation was delivered to the underdosed areas on the remaining 5 days per week according to the thermoluminescent dosimetry measurements obtained at week 1 [17]. One patient had to discontinue TBSEB radiation therapy after receiving 12 cGy owing to a hypersensitivity reaction.
follow-up
All patients had complete physical examination for assessment of pruritus scores, GVHD grading, and BSA involved with patches, plaques, and tumors 1 month, 3 months, 6 months, and 1 year after transplant and yearly thereafter. Bone marrow aspiration and biopsy, chimerism studies, PET-CT scans (if clinically indicated), and peripheral blood T-cell lymphoma panel by flow cytometry were also performed.
treatment of disease progression/relapse
Patients with disease progression or relapse after SCT were tapered off immunosuppressive therapies, depending on disease status and the presence/absence of GVHD. Additional treatment for disease management included topical steroids and/or nitrogen mustard, extracorporeal photopheresis (ECP), local radiation therapy, and standard chemotherapy as indicated. Approximately 2–4 weeks after successful tapering of tacrolimus, patients underwent restaging. Patients with progressive disease with no evidence of GVHD were considered for donor lymphocyte infusion (DLI).
study end points
The primary aim of this study was to report the outcomes of patients with CTCL following allogeneic SCT. The analysis included patient, disease, and transplant characteristics, time to neutrophil and platelet engraftment, incidence of acute and chronic GVHD, pruritus scores, nonrelapse mortality (NRM), disease progression/relapse, PFS, and OS. Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) >0.5 × 109/l. Platelet engraftment was defined as first of the 7 consecutive days with platelet counts of >20 × 109/l without platelet transfusions. Acute GVHD was graded according to the Modified Consensus Criteria while chronic GVHD was classified according to the National Institute of Health's guidelines [18, 19]. The incidence of chronic GVHD was calculated in patients surviving beyond 100 days and classified as none, limited, or extensive. NRM was defined as death from any cause without evidence of progression/relapse of lymphoma. Relapse was defined as disease progression after a CR. Disease progression was defined as 25% increase in skin scores, increase in size of 25% of the sum of the products of the pretreatment measurements, or appearance of new lesions. PFS was measured from the time of transplantation to relapse (patients in CR), progression [patients in partial remission (PR)], and death from any cause or last follow-up. OS was defined as the time from transplantation to the date of death from any cause or last follow-up.
statistical analysis
The Kaplan–Meier method was used to estimate the distribution of OS and PFS from the transplant date. Cox proportional hazards regression analysis was used to assess the association between survival end points and disease and demographic covariates of interest. Acute GVHD and NRM were assessed in a competing risks framework, and the method of Fine and Gray was used to fit regression models to the same covariates for these cumulative incidence data. The cumulative incidence of relapse and NRM was assessed in a separate analysis. All statistical analyses were carried out using R (http://www.r-project.org) version 3.1.0. All statistical tests used a significance level of 0.05, and no adjustment for multiplicity was made. The follow-up for all patients was updated, and the database was closed for analysis on 31 August 2014.
results
patient characteristics
Patient characteristics are summarized in Table 1. A total of 47 patients with biopsy-proven CTCL staged by tumor, node, blood, metastasis) [2] underwent allogeneic SCT during this period. The patients' median age at SCT was 51.5 years (range, 19–72 years); their median follow-up time after SCT was 2 years (range, 15 days to 12.5 years). The median number of prior systemic therapeutic regimens received was 6 (range 2–11). The most commonly used regimens used were combined immunomodulatory therapy composed of interferon (50%) bexarotene (65%), and ECP (42%) for patients with SS or multiagent chemotherapy or targeted agents (32%) for patient with lymph node or visceral involvement. All had failed skin-directed therapies (i.e. steroids, retinoids, or nitrogen mustard) pretransplant [10].
Table 1.
Characteristics of 47 patients who underwent allogeneic stem-cell transplantation for cutaneous T-cell lymphoma
| Characteristic | No. of patients (%) |
|---|---|
| Median age at transplant, years (range) | 51.5 years (19–72) |
| Sex | |
| Male | 20 (43) |
| Female | 27 (57) |
| Ethnicity | |
| Caucasian | 33 (69) |
| African American | 11 (23) |
| Hispanic | 4 (8) |
| Median no. of prior chemotherapy regimens (range) | 6 (2–11) |
| Time from diagnosis to transplant, median (range) | 2.25 years (126 days–22 years) |
| Mycosis fungoides | 1.3 years (258 days–22 years) |
| Mycosis fungoides with LCT | 3.77 years (283 days–11.3 years) |
| Mycosis fungoides with SS | 2.2 years (126 days–6.31 years) |
| Mycosis fungoides with SS and LCT | 2.45 years (376 days–9.3 years) |
| Response to pretransplant therapy (%) | |
| CR/CRu | 7 (15) |
| PR | 28 (60) |
| SD/PD | 12 (26) |
| Median pruritus score (range) | |
| At diagnosis (n = 44) | 8 (1–10) |
| Before transplant (n = 25) | 3 (0–8) |
| One year after transplant (n = 22) | 0 (0–8) |
| Stem cell source | |
| HPC-A | 35 (74) |
| HPC-M | 12 (26) |
| Donor type | |
| Matched sibling | 21 (45) |
| Matched unrelated | 24 (51) |
| One antigen mismatched related | 2 (4) |
| Median CD34+ cell dose (106 cells/kg) | 5.3 (0.99–33.56) |
| Recipient → donor | |
| Male → male | 14 |
| Male → female | 6 |
| Female → female | 6 |
| Female → male | 21 |
| Conditioning regimen | |
| Fludarabine/melphalan ± thymoglobulin | 42 (89) |
| Fludarabine/busulfan | 3 (6) |
| Fludarabine/cyclophosphamide ± rituximab | 2 (4) |
| Graft-versus-host-disease prophylaxis (%) | |
| Tacrolimus/methotrexate | 47 (100) |
| Total body skin electron beam radiation (%) | 42 (89) |
All data are the number of patients (%) unless otherwise noted.
CR, complete remission; CRu, complete remission unconfirmed; PR, partial remission; SD, stable disease; PD, progressive disease; HPC-A, hematopoietic progenitor cells-apheresis; HPC-M, hematopoietic progenitor cells-marrow; LCT, large-cell transformation; SS, Sézary syndrome.
platelet and neutrophil engraftment
Two patients died before platelet and neutrophil engraftment due to infections (bacterial −1; fungal −1). For the remaining 45 patients, the median time to ANC recovery was 12 days (range, 9–23), and the median time to platelet count recovery was 12 days (range, 0–88). The median number of platelet transfusions received was 4 (range 0–47) and the median number of packed red blood cell transfusion received was 2 (range, 0–20).
chimerism analysis
Chimerism analysis was not carried out in three patients (including two who died before engraftment). Of the 44 patients for whom chimerism analysis was carried out, 36 (82%) achieved full-donor chimerism in the myeloid and T-cell compartments and 6 (14%) had mixed chimerism at last assessment. Two patients experienced graft rejection (one received FCR conditioning regimen followed by a HLA-matched sibling graft and the second patient received fludarabine, melphalan, ATG followed by a 9/10-matched unrelated donor transplant) and had autologous reconstitution; of these two patients, one underwent a successful second allogeneic SCT and has had an ongoing CR since 2001.
graft-versus-host disease
The cumulative incidences of grade 2–4 and grade 3 or 4 acute GVHD were 40% and 10%, respectively. The most common organ involved was the skin. Of the 17 patients who developed acute cutaneous GVHD, 3 patients had grade 2 involvements, 8 patients had grade 3 involvements, and 1 patient had grade 4 involvement. Eight of these 17 patients developed GVHD after undergoing immunomodulation for disease progression or relapse. One patient died from acute GVHD.
The cumulative incidence of chronic GVHD was 28%. Of the 42 patients who survived more than 100 days after SCT, 15 developed chronic GVHD; 12 had chronic extensive GVHD. All patients with chronic GVHD had cutaneous involvement. The development of chronic GVHD was not significantly associated with disease relapse (P = 0.75). Three patients died from chronic GVHD.
nonrelapse mortality
The cumulative NRM rate was 10.4% and 16.7% at 1 and 2 years, respectively. Other causes of death included infections in four patients (bacterial 2; viral 1; fungal 1), nonsmall-cell lung cancer in 2 patients, and pulmonary failure in 1 patient. The most common cause of death was progressive disease in 9 of 20 patients.
disease progression/relapse
The incidence of disease progression/relapse following SCT was 50%. A majority of patients progressed within 6 months of SCT. At the time of this report, 27 of the 47 patients who underwent SCT are alive; of these patients, 20 are in CR, 1 in PR, 3 have stable disease, and 3 have progressive disease. A total of 22 patients received additional therapy, 8 of 22 achieved a second CR. Most patients who relapsed or progressed post-transplant received a combination of immunosuppression withdrawal, skin-directed therapies, ECP, and/or chemotherapy. Two patients received DLI. One patient underwent a second allogeneic SCT after disease progression but died due to complications of transplant. The eight patients who achieved a CR are alive and in remission 2–11 years after achieving a second CR. Three patients have stable disease and two have progressive disease after the withdrawal of immunosuppression and additional therapy.
pruritus scores, OS, and PFS
The median pruritus score at diagnosis was 8 (range 1–10), pretransplant after completion of TBSEB was 3 (range, 0–8) and, at 1-year post-transplant, it was 0 (range 0–8). Patients with SS who responded to allogeneic SCT experienced complete clearing of their pruritus symptoms.
The results of the univariate Cox regression analysis for OS and PFS are provided in Table 2. The Kaplan–Meier estimate of OS at 4 years was 51% (Figure 1A). There was no difference in the OS in patients who had SS versus all others (P = 0.33).
Table 2.
Disease stage at diagnosis
| Disease stage | No. of patients (N = 47) |
|---|---|
| Clinicopathologic stage at diagnosis | |
| Mycosis fungoides | 12 |
| MF with large-cell transformation | 18 |
| Mycosis fungoides with Sézary syndrome | 9 |
| Mycosis fungoides with Sézary syndrome and | 8 |
| Large-cell transformation | 4 nodal; 1 tumor |
| Folliculotropic mycosis fungoides | 4 |
| TNMB stage at diagnosis | |
| IB–IIA Refractory IB | 2 |
| IIB Tumors (includes tumors with large-cell transformation) | 12 |
| IIIA Erythrodermic mycosis fungoides (<B2) | 1 |
| IVA Sézary syndrome (B2) and/or nodes | 18 |
| IVB Bone marrow positive, liver (n = 2) | 15 |
| TNMB maximum stage pretransplant | |
| IB–IIA Refractory IB | 4 |
| IIB Tumors (including large-cell transformation) | 8 |
| IIIA Erythrodermic mycosis fungoides (<B2) | 3 |
| IVA Sézary syndrome (B2) and/or nodes | 11 |
| IVB bone marrow positive, liver (n = 2) | 21 |
TNMB, tumor, node, blood, metastasis; MF, mycosis fungoides.
Figure 1.
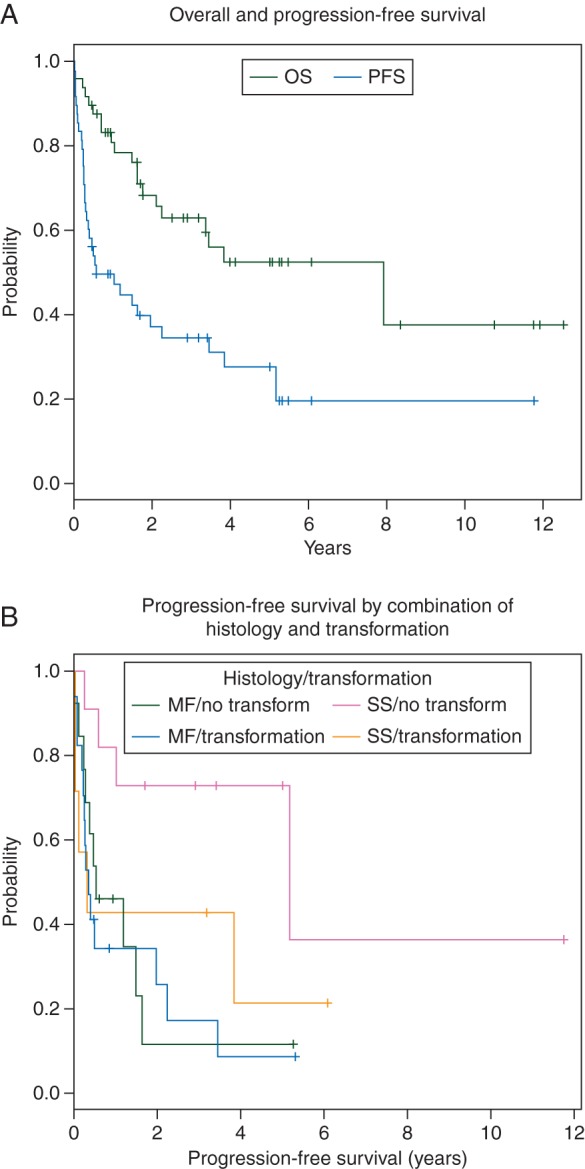
(A) Kaplan–Meier estimates of the overall survival rate and progression-free survival (PFS) rate of 47 CTCL patients who underwent stem cell transplantation. (B) Kaplan–Meier estimates of PFS rates after stem cell transplantation in patients with mycosis fungoides (MF)/no transformation; mycosis fungoides/Sézary syndrome (MF/SS), mycosis fungoides/large-cell transformation (MF/LCT), and mycosis fungoides/Sézary syndrome/large-cell transformation (MF/SS/LCT).
The Kaplan–Meier estimate of the 4-year PFS for the entire cohort was 26% (Figure 1A). The PFS rate at 4 years was superior in patients who had SS versus those with MF (72.7% versus 11.5%; P = 0.04). The Kaplan–Meier estimate of PFS at 4 years was 8.6% in patients with MF with LCT (Figure 1B).
Multivariate analysis was not carried out because of the small patient numbers.
discussion
Advanced CTCL and its variants MF and SS have a poor prognosis with median survival of <5 years [3]. Since curative therapy for advanced CTCL does not exist at this time, allogeneic SCT may produce acceptable results and may cure a subset of patients [9, 10, 14, 20].
We describe here the largest single-center cohort of patients who underwent allogeneic SCT for MF/SS. Our study provides important information about the survival of patients with advanced CTCL who undergo SCT. In the present study, the 4-year OS and PFS rates were 51% and 26%, respectively, which are similar to those described previously [7, 11]. An analysis from the Center for International Blood and Marrow Transplant Research, which likely included several patients from this single-center analysis; the 5-year PFS rate of 129 CTCL patients' was 17% (95% confidence interval 9% to 26%) [11]. Our results suggest a graft-versus-lymphoma effect in CTCL given that only 15% of patients were in CR at the time of transplant and the long-term remissions observed in 8 of 22 patients with immunomodulation post-transplant. We also found in the present study that the 4-year PFS rate of SS alone (without concomitant LCT) patients was 73%, which was higher than that of patients with MF (P = 0.04) or LCT. The 4-year PFS in patients with MF and LCT was only 8.6%. The ideal conditioning regimen for transplant is unknown; however, most reports in literature have used reduced intensity conditioning regimens. At least two prior studies have shown an increased risk of disease relapse with T-cell depletion [8, 12]. In the study by de Masson et al. in multivariate analysis, the use of ATG was the only factor significantly associated with reduced PFS (P = 0.04) [12]. We were not able to show an increased risk of relapse or disease progression with the use of in vivo T-cell depletion with ATG. We found no significant differences in survival outcomes between patients who received SCT from a matched sibling and those who received SCT from a matched unrelated donor. All patients who received SCT from an unrelated donor received ATG in the preparative regimen, whereas those who received SCT from a matched sibling donor did not.
A substantial number of patients in the present study experienced disease progression after SCT. In many patients, progressive disease responded to a combination of immunomodulation plus additional ECP, topical therapy, local radiation therapy, DLI, and/or chemotherapy. Unfortunately, because most of these patients received multiple simultaneous treatments, assessing responses to individual therapies was not possible. However, of the 22 patients who had disease progression after SCT, 8 are alive and continue to be in a CR 2–11 years after receiving additional therapy.
We also included TBSEB as part of the treatment regimen. Although the primary goal of TBSEB radiation therapy was to debulk the skin disease, we hypothesized that such treatment would also reduce the numbers of resident skin-homing lymphocytes and antigen-presenting Langerhans cells in the skin, thereby ameliorating the risk of cutaneous GVHD [21]. However, of the 25 patients who developed acute GVHD after SCT, 17 had cutaneous GVHD, 8 of who developed GVHD owing to early tacrolimus withdrawal following disease progression.
A 2012 Cochrane review [22] concluded that allogeneic SCT induces durable successes with acceptable side-effects and thus should be considered as a promising treatment option for patients with advanced CTCL. However, the authors also recommended that prospective, genetically randomized, multicenter controlled trials should be conducted to identify the precise role of allogeneic SCT in treating patients with advanced CTCL [22]. Given the rarity of CTCL, it is unlikely that a randomized, controlled trial will be conducted; however, a prospective, multicenter, national or international collaborative trial of transplant versus no transplant assigned on the basis of donor availability should be considered.
In the absence of randomized data, the findings of our prospective study suggest that allogeneic SCT has an important role in treating patients with advanced CTCL patients particularly those with SS without LCT. In all other subgroups, rates of disease progression/relapse following SCT are high; indicating that novel conditioning regimens and maintenance therapy to prevent progression/relapse should be explored.
funding
MD received funding from the Sherry Anderson CTCL Research Fund. No grant numbers apply.
disclosure
The authors have declared no conflicts of interest.
references
- 1.Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood 2009; 113: 5064–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen E, Vonderheid E, Pimpinelli N et al. . Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 3.Talpur R, Singh L, Daulat S et al. . Long-term outcomes of 1,263 patients with mycosis fungoides and Sezary syndrome from 1982 to 2009. Clin Cancer Res 2012; 18: 5051–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell-Jones R, Child F, Olavarria E et al. . Autologous peripheral blood stem cell transplantation in tumor-stage mycosis fungoides: predictors of disease-free survival. Ann N Y Acad Sci 2001; 941: 147–154. [DOI] [PubMed] [Google Scholar]
- 5.Olavarria E, Child F, Woolford A et al. . T-cell depletion and autologous stem cell transplantation in the management of tumour stage mycosis fungoides with peripheral blood involvement. Br J Haematol 2001; 114: 624–631. [DOI] [PubMed] [Google Scholar]
- 6.Bigler RD, Crilley P, Micaily B et al. . Autologous bone marrow transplantation for advanced stage mycosis fungoides. Bone Marrow Transplant 1991; 7: 133–137. [PubMed] [Google Scholar]
- 7.Duarte RF, Boumendil A, Onida F et al. . Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a European society for blood and marrow transplantation lymphoma working party extended analysis. J Clin Oncol 2014; 32: 3347–3348. [DOI] [PubMed] [Google Scholar]
- 8.Duarte RF, Canals C, Onida F et al. . Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2010; 28: 4492–4499. [DOI] [PubMed] [Google Scholar]
- 9.Molina A, Zain J, Arber DA et al. . Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol 2005; 23: 6163–6171. [DOI] [PubMed] [Google Scholar]
- 10.Duvic M, Donato M, Dabaja B et al. . Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol 2010; 28: 2365–2372. [DOI] [PubMed] [Google Scholar]
- 11.Lechowicz MJ, Lazarus HM, Carreras J et al. . Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant 2014; 49: 1360–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Masson A, Beylot-Barry M, Bouaziz JD et al. . Allogeneic stem cell transplantation for advanced cutaneous T-cell lymphomas: a study from the French Society of Bone Marrow Transplantation and French Study Group on Cutaneous Lymphomas. Haematologica 2014; 99: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paralkar VR, Nasta SD, Morrissey K et al. . Allogeneic hematopoietic SCT for primary cutaneous T cell lymphomas. Bone Marrow Transplant 2012; 47: 940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polansky M, Talpur R, Daulat S et al. . Long-term complete responses to combination therapies and allogeneic stem cell transplants in patients with Sezary syndrome. Clin Lymphoma Myeloma Leuk 2015; 15: e83–e93. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe RT, Harrison C, Tavallaee M et al. . Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol 2015; 72: 286–292. [DOI] [PubMed] [Google Scholar]
- 16.Navi D, Riaz N, Levin YS et al. . The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol 2011; 147: 561–567. [DOI] [PubMed] [Google Scholar]
- 17.Karzmark CJ, Loevinger R, Steele RE, Weissbluth M. A technique for large-field, superficial electron therapy. Radiology 1960; 74: 633–644. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P et al. . 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828. [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S et al. . National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956. [DOI] [PubMed] [Google Scholar]
- 20.Olsen EA, Rook AH, Zic J et al. . Sezary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol 2011; 64: 352–404. [DOI] [PubMed] [Google Scholar]
- 21.Kreutz M, Karrer S, Hoffmann P et al. . Whole-body UVB irradiation during allogeneic hematopoietic cell transplantation is safe and decreases acute graft-versus-host disease. J Invest Dermatol 2012; 132: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaak M, Pickenhain J, Theurich S et al. . Allogeneic stem cell transplantation versus conventional therapy for advanced primary cutaneous T-cell lymphoma. Cochrane Database Syst Rev 2012; 1: CD008908. [DOI] [PubMed] [Google Scholar]


