Abstract
Objective: This study examines the International Classification of Functioning, Disability, and Health model (ICF) using a data set of 2,563 community-dwelling elderly with disease-independent measures of mobility, physical activity, and social networking, to represent ICF constructs. Method: The relationship between chronic disease and disability (independent and dependent variables) was examined using logistic regression. To demonstrate variability in activity performance with functional impairment, graphing was used. The relationship between functional impairment, activity performance, and social participation was examined graphically and using ANOVA. The impact of cognitive deficits was quantified through stratifying by dementia. Results: Disability is strongly related to chronic disease (Wald 25.5, p < .001), functional impairment with activity performance (F = 34.2, p < .001), and social participation (F= 43.6, p < .001). With good function, there is considerable variability in activity performance (inter-quartile range [IQR] = 2.00), but diminishes with high impairment (IQR = 0.00) especially with cognitive deficits. Discussion: Environment modification benefits those with moderate functional impairment, but not with higher grades of functional loss.
Keywords: chronic disease, disability evaluation, rehabilitation, Malaysia, social participation
Introduction
In the International Classification of Functioning, Disability, and Health model (ICF), pre-existing health conditions in an individual can give rise to functional impairments, which in turn cause activity limitation and restriction in social participation (Grimby & Smedby, 2001; World Health Organization, 2011). Disability in turn results from the interaction between these factors in the individual and the surrounding environment. In general, no attempt is made by the ICF to distinguish between cognitive and physical causes of disability, which often interact. The World Mental Health Survey Initiative has demonstrated that mental disorders were more likely to be associated with severe disability than physical ones (Scott et al., 2009).
The key improvement from the earlier disability model, the International Classification of Impairments, Disabilities, and Handicaps (ICIDH), was a shift from a purely medical to a bio-psycho-social framework reflecting the interaction between an individual’s health status and ambient factors, both personal and environmental. In addition, there was an emphasis on viewing disability as an expression of the aforementioned interaction, rather than as a characteristic of the individual (World Health Organization, 2011).
Previously, a person with paralysis after a stroke was deemed to be disabled due to loss of limb function. When viewed through the lens of the ICF model, the stroke is a chronic disease that gives rise to functional impairment (limb paralysis), which limits activity performance (driving), which in turn restricts social participation (going to play poker). The stroke itself may also limit activity performance and social participation directly by making it harder to retain a driving license or getting fewer invitations to play poker. Personal factors such as motivation (loves to play poker), and environmental factors (poker buddy who can drive) enable the affected person to still participate in his favorite social activity in spite of significant functional impairment or activity limitation. The interaction of all these elements thus gives rise to a certain level of disability in that person.
Although the above example was simplified for clarity, it can be observed that disability originates from the underlying chronic disease as it directly gives rise to functional impairment, and affects activity performance and social participation both directly and indirectly. In older people especially, multiple chronic diseases may co-exist contributing to disability, although not everyone with a high chronic disease burden is necessarily disabled. Hence, this relationship may not be as clear as one might assume.
The next observation is that for a certain degree of functional impairment, the level of restriction in activity and participation is variable depending on the personal and environmental factors surrounding the individual. The last observation is that functional impairment directly impacts on activity and participation, and although the correlation between them may be lessened by the aforementioned personal and environmental factors, it is still likely to be fairly strong.
Earlier studies on the ICF were restricted to examining disability in a particular disease-specific context or outcome, rather than looking at the general validity of the model as a whole (Dale et al., 2012; Faria-Fortini, Michaelsen, Cassiano, & Teixeira-Salmela, 2011; Jonsson, Ekholm, & Schult, 2008; Pollard, Johnston, & Dieppe, 2011). These disease-specific studies may also have limited generalizability to the elderly population, where there are often significant and concurrent co-morbidities contributing to disability. The use of a single large data set in examining the ICF model has the advantage of analyzing the connections between ICF constructs simultaneously, thus allowing a comparison of strengths between these inter-relationships. It would also be useful to determine how cognitive factors contribute to disability in the ICF model.
Thus, our study aimed to examine in a cohort of community-dwelling older adults some of the observations or hypotheses stemming from the ICF model, which are that (a) chronic disease burden is strongly related to disability as a whole; (b) for a given level of functional impairment, individuals vary significantly in performance of activities; and (c) functional impairment is strongly related to activity performance, which in turn correlates with social participation (World Health Organization, 2011). We also studied where relevant, the impact of dementia status on disability as well as the relationships between the ICF constructs.
Method
This study was based on the “Determinants of Wellness among Older Malaysians: A Health Promotion Perspective” survey conducted by trained interviewers from Universiti Putra Malaysia and the Malaysian Ministry of Health in 2008 to 2009. The questions were set in English and Malay, and translated into the primary language of the respondents by interviewers who were native speakers where necessary. It was not feasible to pre-translate into all the local languages due to the large number of tribes and ethnic groups especially in East Malaysia. The interviewers read out the individual questions and noted the responses in a set of printed study forms.
The study was approved by the Malaysian Ministry of Health Ethics Review Board (approval reference P08-219) in accordance with current guidelines on Good Clinical Practice and the Declaration of Helsinki. Specific informed consent was not required by the Ethics Review Board as the data were analyzed anonymously.
This study used a geographical cluster sampling design based on the World Health Organization STEPwise approach to Surveillance (STEPS), with clusters represented by enumeration blocks (EBs) determined by the Malaysian Department of Statistics (Department of Statistics Malaysia, 2010; World Health Organization, 2012). The inclusion criteria were above the age of 60 years, a Malaysian citizen, and willing to be interviewed. They were excluded from the study if mobility, mental functions, or hearing was sufficiently impaired to prevent them from completing the survey. Each EB had a target recruitment of eight people, but less could have been recruited if suitable residents were not found in the allocated houses.
The World Health Organization Disability Assessment Schedule II (WHODAS-II) was developed to assess disability based on the ICF (World Health Organization, 2001b). It has been validated across different cultures for use in subjects with chronic diseases, older people, and is literacy-independent (Garin et al., 2010; Sousa et al., 2010). In our study, we used simple percentile sum scoring. Severe disability was defined as a WHODAS-II score above the 90th percentile for the population (Scott et al., 2009).
Dementia status was defined using the Malaysian version of the 30-item Folstein mini-mental state examination (MMSE-M). The sample was stratified into two groups (with and without dementia) based on locally validated cutoffs, with good sensitivity (88%-97%) and specificity (75%-93%) in classifying dementia against Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association, 1994) criteria. The question testing attention and calculation was repeated using serial 7’s, serial 3’s, and spelling the word “WORLD” backwards, and was scored according to the best performance. The respective MMSE-M cutoffs for dementia classification were 21/22, 18/19, and 17/18, respectively, depending on the question used (Ibrahim et al., 2009).
Respondents were asked whether they had any of the chronic non-communicable disease groups, which may contribute to disability: cancer (all types), respiratory (asthma, obstructive airways disease, pulmonary tuberculosis), uncorrected visual or hearing impairment, renal, gastrointestinal-urinary (dyspepsia, constipation, incontinence), musculoskeletal (joint pain, gout, arthritis), and cardiovascular (hypertension, diabetes mellitus, heart disease, stroke). They were also questioned whether the diagnoses had been medically confirmed. Although pulmonary tuberculosis is not strictly a chronic non-communicable disease, it was included in the list as it can cause long-term impairment of lung function, and is commonly found among the elderly in Malaysia. Each of the seven chronic disease groups and dementia were marked as either present (1) or absent (0).
The Timed Get-Up and Go Test (GUGT), short International Physical Activity Questionnaire (IPAQ), and Lubben Social Network Scale (LSNS-6) are measures of basic functional mobility, physical activity, and social support (Bauman et al., 2009; Lubben, 2003; Podsiadlo & Richardson, 1991). They are feasible to perform, valid, and reliable in the elderly (Forsen et al., 2010; Hurtig-Wennlof, Hagstromer, & Olsson, 2010; Nordin, Lindelof, Rosendahl, Jensen, & Lundin-Olsson, 2008; Wall, Bell, Campbell, & Davis, 2000).
Unless specified in the original definition, missing values were replaced by the mean of the remaining values in the scale provided internal consistency was demonstrated to be high (Cronbach’s α > .7). Up to one missing value could be replaced in this way and further missing values invalidated the score for that respondent.
Education level, net monthly income, smoking, alcohol consumption, ethnicity, marital status, and religion were classified according to standard census criteria (Department of Statistics Malaysia, 2010). Fall status was determined as whether the respondent had any falls resulting in injury in the previous 6 months.
Body mass index (BMI) and waist circumference were obtained using a stadiometer (206CM Bodymeter Measuring Tape, SECA, Hamburg, Germany) and electronic weight scale (HD-314 Digital Weight Scale, Tanita, Arlington Heights, Illinois, USA). Dietary adequacy was assessed using a Dietary Diversity Score (DDS; Kant, Schatzkin, Harris, Ziegler, & Block, 1993).
Analysis
The overall contribution of chronic disease to severe disability was modeled using population attributable risk (PAR). PAR was calculated for each chronic disease group along with a total PAR for their combined effect (Sousa et al., 2009). The combined PAR for all (n) disease groups is shown below (Cole & MacMahon, 1971).
A composite chronic disease score (CDBS) for all (n) disease groups including dementia was derived for each respondent, weighted based on the PAR of each group as shown below. A composite chronic disease score without dementia (CDBS-ND) was also derived using the same method.
where CDBS is expressed as a percentage and Di represents the presence (1) or absence (0) of the chronic disease group “i.”
To test the first observation that chronic disease burden is strongly related to disability, a multivariate approach was used, including covariates that could potentially act as confounders. Subjects were divided into two groups based on the presence or absence of severe disability.
The initial list of covariates screened were age, gender, marital status, race, religion, years of formal education, net income, smoking and alcohol use, DDS, fall status, BMI, and waist circumference. To reduce the number of covariates, they were tested against the grouped WHODAS-II score using a Pearson’s chi-square test for categorical covariates, and either an unpaired T test or Mann–Whitney test for continuous covariates (Table 1).
Table 1.
Baseline Characteristics of Respondents, Selected Summary Data From the Malaysia 2010 Census, and Key Outcome Measures.
| Categorical covariates | Distribution |
|---|---|
| Gender | 45.0% males, 55.0% females |
| Ethnicity | 55.4% Malays, 13.9% Chinese, 5.3% Indians, |
| 25.4% Indigenous and Others | |
| Gender (Malaysia 2010 census) | 48.1% males, 51.9% females |
| Ethnicity (Malaysia 2010 census) | 65.9% Malays, 11.8% Chinese, 2.2% Indians, |
| 20.1% Indigenous and Others | |
| Marital status | 2.1% not married, 58.3% married, 2.2% divorced, 37.4% widowed |
| Religion | 71.1% Islam, 12.1% Christianity, 11.3% Buddhism, |
| 4.4% Hinduism, 1.1% no religion and others | |
| Smoking | 84% not current smoker, 16% current smoker |
| Alcohol use | 95.8% no current alcohol use, 4.2% current alcohol use |
| Fall status | 87% no falls, 13% fell with injury |
| Continuous covariates | M (SD)/distribution |
| Age | 63.2% (60-69 years), 29.9% (70-79 years), 7.0% (≥80 years) |
| Years of formal education | 3.4 years (3.7) |
| Net income | RM616 (1,329; approx. US$170, early 2015 conversion rates) |
| Dietary Diversity Score | 4.3 (0.8) (max 5.0) |
| Body mass index | 24.2 (4.8) |
| Waist circumference | 83.9 (15.0) cm |
| Key outcome measuresa | Median, IQR, non-response rate |
| WHODAS-II | Median 14.6, IQR 4.2-31.3, non-response 3.4% |
| CDBSb | Median 13.0, IQR 13.0-25.0, non-response <0.1% |
| CDBS-NDb | Median 14.0, IQR 14.0-29.0, non-response <0.1% |
| GUGT time (s) | Median 17.0, IQR 13.0-21.0, non-response 20.4% |
| IPAQ energy expenditure (MET-min/week) | Median 1233, IQR 0-3947, non-response <0.1% |
| LSNS-6 | Median 15.0, IQR 10.0-19.0, non-response 0.4% |
Note. IQR = inter-quartile range; WHODAS-II = World Health Organization Disability Assessment Schedule II; CDBS = composite chronic disease score; CDBS-ND = composite chronic disease score without dementia; GUGT = Get-Up and Go Test; IPAQ = International Physical Activity Questionnaire; LSNS-6 = Lubben Social Network Scale; MET = Metabolic Equivalent Task.
The key outcome measures were shown as medians and IQRs as most of them were non-normal in distribution.
Pearson’s correlation coefficient = .93 between CDBS and CDBS-ND.
Significant covariates were then entered into binary logistic regression analysis, with the CDBS as the main independent variable and the grouped WHODAS-II score as the binary dependent variable. The same analysis was also repeated excluding subjects with dementia on the MMSE-M, utilizing CDBS-ND as the main independent variable.
To test the second observation that individuals vary significantly in performance of activities for a given level of functional impairment, we plotted IPAQ activity levels (activity performance) for each GUGT functional status category (functional impairment) to illustrate the degree of variability in activity performance. As a comparison, LSNS-6 scores (social participation) were divided into quartiles and similarly charted against GUGT (functional impairment). The dispersion for IPAQ and LSNS-6 scores in each GUGT category was quantified using the inter-quartile range (IQR).
For the third observation, we tested the relationship between functional impairment and activity performance by comparing IPAQ energy expenditure (activity performance) with GUGT (functional impairment) using Spearman’s rank correlation coefficient, the Kruskal–Wallis non-parametric ANOVA, and also graphing the comparison. The relationship between activity performance and social participation was tested by comparing the LSNS-6 score (social participation) and IPAQ activity levels (activity performance) in a similar manner. This analysis was repeated stratifying by dementia status on the MMSE-M. As a comparison, we quantitatively tested the relationship between functional impairment and social participation in a similar manner.
Sample size was calculated using the STEPS Sample Size Calculator from the World Health Organization (2012). Based on a 95% confidence level, 5% margin of error, an estimated prevalence of risk factors of 50%, a design effect correction of 3, an expected response rate of 60%, the calculated sample size was 3,840 subjects, translating to 480 EBs of eight subjects each.
All computations were performed using SPSS for Windows version 20.0 (IBM Corp., Armonk, New York, USA). Statistical tests were two-tailed and conducted at 5% level of significance.
Results
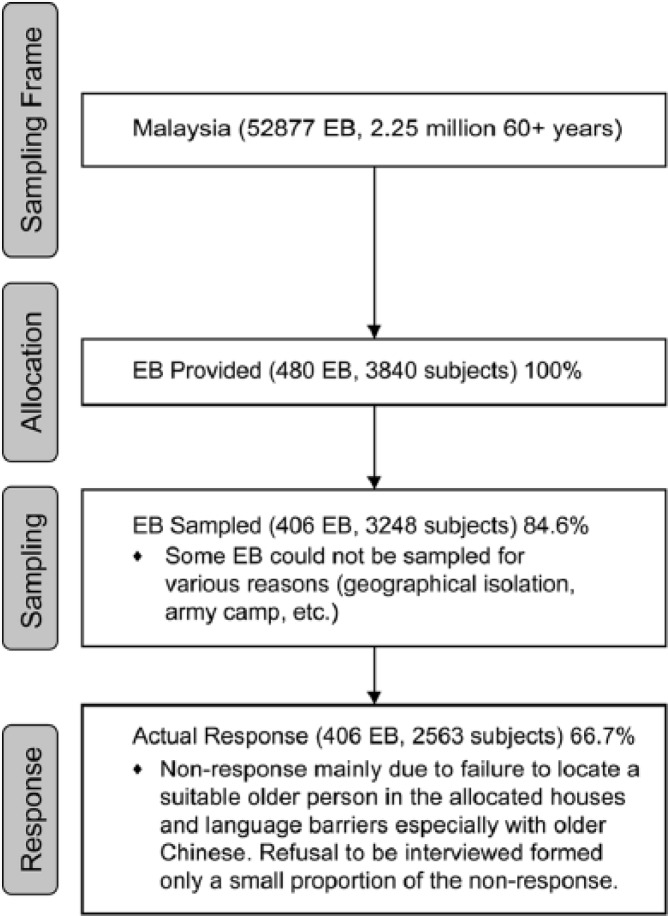
The sample included a total of 2,563 respondents with an overall response rate of 66.7%, which was more than the expected response rate of 60% (Figure 1). Baseline characteristics of respondents are detailed in Table 1, along with selected equivalent summary data from the Malaysia 2010 census (Department of Statistics Malaysia, 2010). The gender and ethnic distribution approximates that from the census data, with the exception of having less Malays and more Indigenous people.
Figure 1.
Flowchart showing recruitment process.
Note. EB = enumeration block.
The responses for medically confirmed diagnoses essentially followed the same pattern as self-reported conditions (Pearson’s correlation coefficient r = .68), and hence were not analyzed separately. Median values and non-response rates for the key outcome measures are summarized in Table 1; 21.5% were classified as having dementia using the locally validated cutoffs for MMSE-M. The non-response rate for GUGT testing (20.4%) was disproportionately higher than for other outcome measures, and mostly attributable to lack of proper equipment or space (7.7%) and refusal (5.5%). There was a significant difference in WHODAS-II scores between respondents who refused GUGT testing and those who agreed, with those who refused having higher scores (mean 4.4 points, 95% CI = [0.6, 8.1], p = .02). Cronbach’s alpha for WHODAS-II, DDS, LSNS-6, CDBS, and CDBS-ND were .92, .46, .83, .28, and .28, respectively. Replacement of missing values occurred in 8% of WHODAS-II scores but not for the DDS, LSNS-6, CDBS, or CDBS-ND.
For the first observation, the 90th percentile for WHODAS-II scores used to define severe disability was 50 points. Significant covariates obtained after initial testing against WHODAS-II were gender, marital status, smoking, fall status, age, years of formal education, and net income (Table 2). Binary logistic regression using these covariates gave a good model fit with a non-significant Hosmer and Lemeshow statistic (p = .26), −2 log-likelihood of 1,184, overall correct classification percentage of 92.4%, and Nagelkerke R-square of .78. The Wald statistic and significance level for the CDBS were 25.5 and p < .001. The other significant covariates from regression analysis were gender, age, education status, and social support (Table 3). When the analysis was repeated excluding subjects with dementia, the Wald statistic and significance level for the CDBS-ND were 28.5 and p < .001, with a Nagelkerke R-square of .78. The combined PAR for chronic disease was 53.8%, whereas the PAR for dementia alone was 15.3%.
Table 2.
Covariates Tested Against WHODAS-II Scores Using Pearson’s Chi-Square for Categorical Covariates and Unpaired T Test for Continuous Covariates.
| Categorical covariates | χ2 | Odds ratio (95% CI) | p |
|---|---|---|---|
| Gender (M/F) | 18.0 | 2.0 [1.4, 2.7] | <.001 |
| Marital status | 25.0 | n/aa | <.001 |
| Ethnicity | 2.9 | n/aa | .407 |
| Religion | 4.2 | n/aa | .515 |
| Smoking (Y/N) | 4.5 | 1.7 [1.0, 2.7] | .033 |
| Alcohol use (Y/N) | 1.2 | 1.6 [0.7, 4.1] | .281 |
| Fall status (Y/N) | 4.2 | 1.5 [1.0, 2.2] | .041 |
| Continuous covariates | T test/z score | p | |
| Age | −7.2b | <.001 | |
| Years of formal education | −6.3b | <.001 | |
| Net income | −5.1b | <.001 | |
| Dietary Diversity Score | −0.4b | .705 | |
| Body mass index | −0.6b | .527 | |
| Waist circumference | −0.6b | .581 |
Note. WHODAS-II = World Health Organization Disability Assessment Schedule II; CI = confidence interval. Boldfaced values have p<0.05.
Categorical covariate with more than two categories.
The z score for the Mann–Whitney Test was used where the variables were non-normal in distribution.
Table 3.
Relevant Statistics for the Logistic Regression Analysis From the First Observation (CDBS Only).
| Covariates | β | Wald | Significance | Exp(β) (95% CI) |
|---|---|---|---|---|
| Gender | 0.431 | 6.22 | .013 | 1.54 [1.01, 2.16] |
| Age | 0.024 | 58.06 | <.001 | 1.03 [1.02, 1.03] |
| Years of formal education | 0.118 | 18.27 | <.001 | 1.13 [1.07, 1.19] |
| CDBS | 1.874 | 25.48 | <.001 | 6.52 [3.15, 13.49] |
| LSNS-6 | 0.070 | 35.04 | <.001 | 1.07 [1.05, 1.10] |
Note. CDBS = composite chronic disease score; CI = confidence interval; LSNS-6 = Lubben Social Network Scale. Boldfaced values have p<0.05.
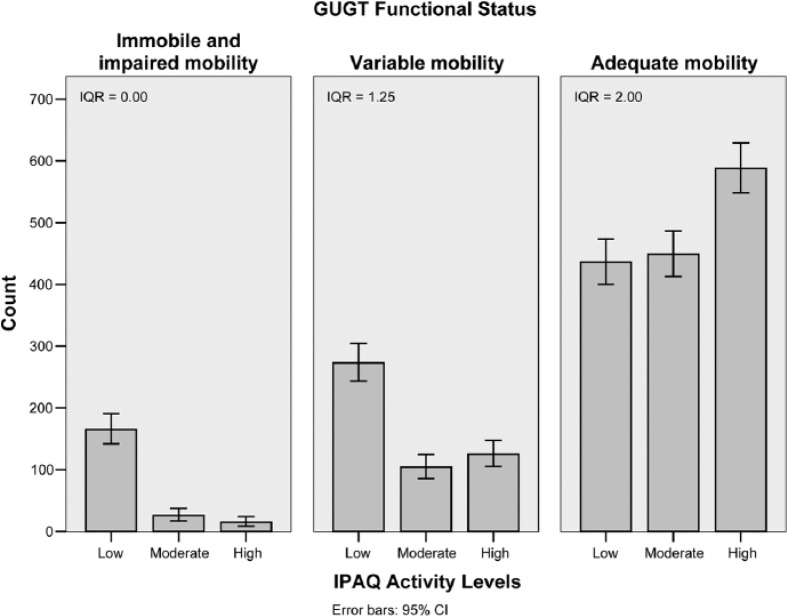
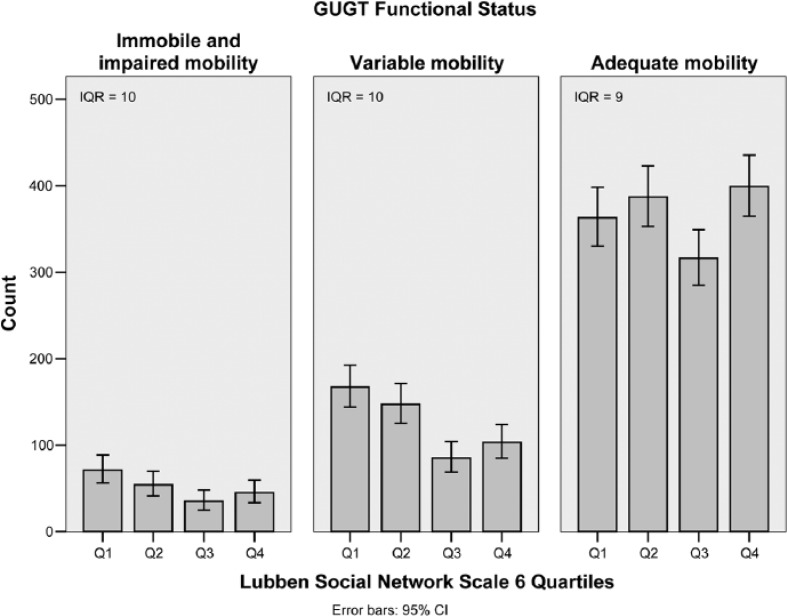
For the second observation, bar charts of IPAQ activity levels for each GUGT functional status category clearly showed that at low levels of functional impairment, activity performance varied considerably with a large IQR (2.00; Figure 2, right panel). However, at higher levels of functional impairment, activity performance was consistently low with a small IQR (0.00; Figure 2, left panel). In contrast, significant variability was present in social participation throughout all levels of functional impairment (IQR = 9-10; Figure 3).
Figure 2.
Bar charts of IPAQ activity levels for each GUGT functional status category.
Note. IPAQ = International Physical Activity Questionnaire; GUGT = Get-Up and Go Test; IQR = inter-quartile range; CI = confidence interval.
Figure 3.
Bar charts of LSNS-6 quartiles for each GUGT functional status category.
Note. LSNS = Lubben Social Network Scale; GUGT = Get-Up and Go Test; IQR = interquartile range; CI = confidence interval.
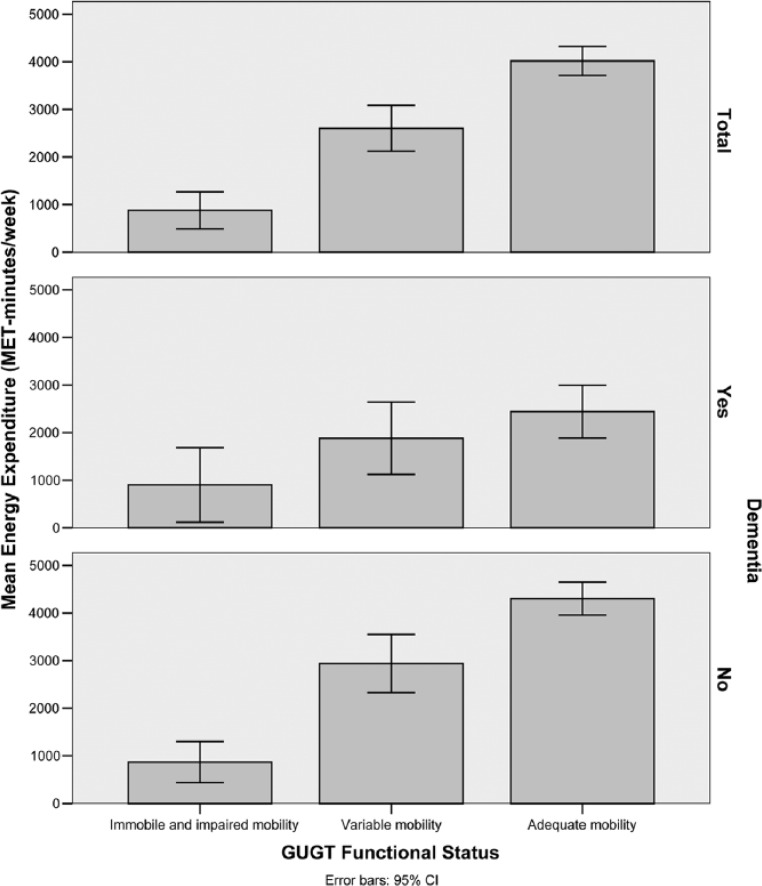
For the third observation, ANOVA showed a highly significant difference (χ2 = 253.3, p < .001) in IPAQ energy expenditure between GUGT functional status groups, and the bar chart showed a clear rise in energy expenditure with improving functional status (Spearman’s rho = 0.33, p < 0.001). When analyzed according to dementia status, the trends were generally similar although it could be seen that those without dementia were able to maintain a higher level of activity even with moderately reduced ambulatory function (Figure 4, bottom panel).
Figure 4.
Bar chart of mean energy expenditure against GUGT functional status category stratified according to dementia status (Total, Yes, No).
Note. GUGT = Get-Up and Go Test; CI = confidence interval; MET = Metabolic Equivalent Task.
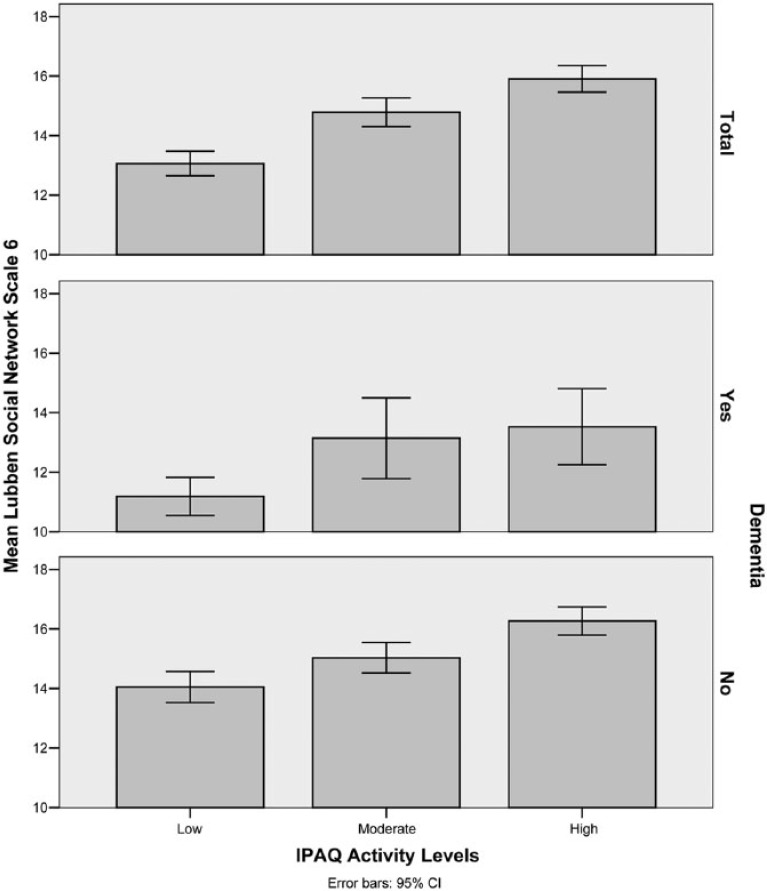
Similarly, ANOVA showed a highly significant difference (χ2 = 83.8, p < .001) in LSNS-6 scores between IPAQ activity levels, whereas the bar chart demonstrated improving social participation with better activity performance (Spearman’s rho = 0.18, p < .001). The presence of dementia was shown to substantially reduce social participation, and those with concurrent low physical activity and dementia had the lowest scores (median LSNS-6 = 11.0; Figure 5, middle panel).
Figure 5.
Bar chart of mean LSNS-6 scores against IPAQ activity levels stratified according to dementia status (Total, Yes, No).
Note. LSNS = Lubben Social Network Scale; IPAQ = International Physical Activity Questionnaire; CI = confidence interval.
The difference in LSNS-6 scores between GUGT groups was also significant (χ2 = 26.3, p < .001) showing improving social participation with better functional status (Spearman’s rho = 0.11, p < .001).
Discussion
For the first observation that examined the relationship between chronic disease burden and disability, the model used for regression analysis had a good fit and high correct classification percentage. Hence, the highly significant Wald statistic for CDBS indicates that chronic disease burden is strongly related to disability, even after accounting for significant confounding variables and dementia status. The large Nagelkerke R-square value shows that most of the variability in disability between individuals was accounted for by chronic disease and socio-demographic characteristics such as gender, age, education level, and social support. In addition, the PAR calculations indicate that chronic disease accounted for more than half of prevalent disability, with dementia causing a large proportion of it.
These findings are all consistent with results from the multi-national 10/66 Dementia Research Group, which reported that the significant covariates were chronic disease, age, gender, and education level. Similar to our study, PAR for chronic disease accounted for two thirds of disability with dementia also being the largest contributor (Sousa et al., 2009).
The second observation states that for a given level of functional impairment, activity performance varies significantly between individuals due to the effect of factors such as motivation, self-esteem, and a conducive environment. However, we found that this holds true only for individuals with relatively little functional impairment (Figure 2, right panel). With worsening function, the role of environmental factors diminishes as the ability to perform activities becomes limited (Figure 2, left panel). The reduction of variability in activity performance is evidenced by a corresponding diminution of the IQR. This means that once an individual’s physical function deteriorates beyond a certain point, modification of the environment cannot meaningfully improve that person’s disability.
In contrast, functional impairment does not seem to be a major limiting factor for social participation, and it is likely that individuals retain their pre-existing social networks even after their functional status worsens (Figure 3). This relates very well to Antonucci’s support bank theory, which postulates that social capital built up when a person is younger carries over into old age in the form of social support (Antonucci & Jackson, 1990).
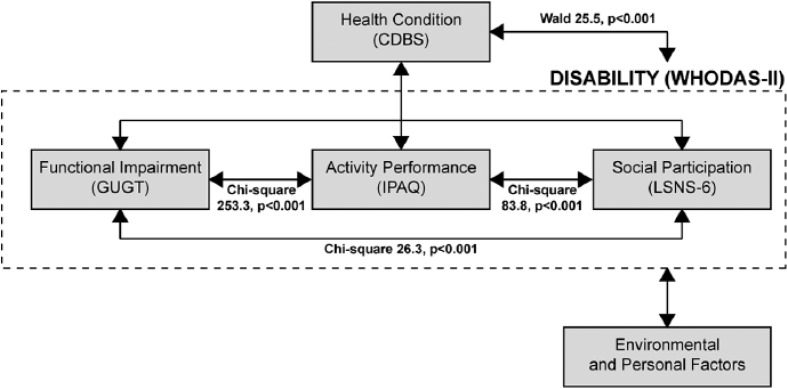
Figures 4 and 5 and the highly significant ANOVA tests all show a clear relationship between functional impairment, activity performance, and social participation, thus validating the third observation. The relative weakness of the relationship between functional impairment and social participation (χ2 = 26.3) compared with the other two relationships (χ2 = 253.3 and 83.8) suggests that activity performance is the intermediary construct in this model (Figure 6). A path analysis could provide more clarity although this is out of the scope of the present study.
Figure 6.
Diagram showing the International Classification of Functioning, Disability, and Health constructs, the instruments representing these constructs, and the relationships examined in this study.
Note. CDBS = composite chronic disease score; WHODAS-II = World Health Organization Disability Assessment Schedule II; GUGT = Get-Up and Go Test; IPAQ = International Physical Activity Questionnaire; LSNS = Lubben Social Network Scale.
Although the cognitive aspect of disability was not studied in depth, it could clearly be seen that the presence of dementia significantly worsened both physical activity and social participation. Furthermore, cognitively intact individuals with a moderate level of functional impairment retained a significant level of activity performance, implying that this group may have the potential for further improvement with appropriate rehabilitation (Figure 4 and 5). In contrast, concurrent low physical activity and poor cognitive function identify individuals who are at risk of social isolation (Lubben et al., 2006).
For the first observation, because chronic non-communicable disease is so strongly tied to disability, every effort should be made to identify the major conditions in each population, which contribute to it. These conditions should then be targeted with effective preventive care programs to reduce the eventual occurrence of end organ functional impairments in the community. Dementia is an especially good candidate to focus on due to its prevalence in community-dwelling elderly and the high PAR for severe disability (Hamid, Krishnaswamy, Abdullah, & Momtaz, 2010). In addition to these primary health conditions, attention should be paid to the secondary and co-morbid conditions that arise from them, the provision of adequate health care, and removing barriers to performance and participation (World Health Organization, 2011).
For the second observation, it can be seen that modification of the environment will be most effective in increasing activity performance in individuals with moderate functional impairment. In Japan, the Heart Building Law (1994), Barrier Free Transport Law (2000), and the Barrier Free Law (2006) provide a formal legal framework to enhance accessibility for the aged and disabled. Although these regulations have greatly improved facilities for the physically disabled, this study suggests that cognitive disablement is also an important issue that needs to be considered. For example, signage needs to be written in clear simple language with unambiguous universal symbols to aid understanding.
For those with high levels of functional impairment especially with concurrent cognition issues, this study shows that mobility is much harder to improve even with the best barrier-free environment. Resources would be better targeted in enhancing their support network, and hence social participation with interventions such as community social worker assistance and programs for organized home visits by school children and voluntary welfare organizations.
For the third observation, the strong linkages between functional impairment, activity performance, and social participation, mean that success in improving any one of these will have secondary benefits for the downstream components, hence reducing overall disability. Logically this means that resources would be better spent on areas that have the most potential to benefit each individual, rather than dealing with all aspects of disability simultaneously. From this study, the implication is that younger people are best targeted with prevention programs to reduce the risk of developing disability, whereas older people with milder disability should focus on removing barriers to activity performance, and those with more severe disability should be provided more support to improve their social participation (Loke, Abdullah, Chai, Hamid, & Yahaya, 2011).
It can be seen from Figures 4 and 5 that the presence of dementia has an important effect on the relationships between the ICF constructs. Moreover, data from this study and the 10/66 Dementia Research Group show that dementia contributes significantly to disability in the community-dwelling elderly (Sousa et al., 2009). Seen together, there would seem to be good reason to investigate the cognitive aspects of disability in greater detail that what was achieved in this study.
Study Considerations and Limitations
We opted to study disability in community-dwelling older people as very little work has been done locally in this population aside from the National Health and Morbidity Survey, which was based on the ICIDH (Institute for Public Health, 2008). Mindful of the need to establish the external validity of the model, we chose instruments that were developed separately from the ICF when testing the second and third observations. The relevant domains of the WHODAS-II (cognition, mobility, life activities, and participation) were unsuitable for this role as they were co-developed with the ICF. Moreover, the high level of internal consistency (Cronbach’s α = .94-.96 within domains, .98 across domains) virtually guarantees that the domains will cross-correlate with each other, thus defeating the intent of the study (World Health Organization, 2001b).
In addition, the instruments used focused on important aspects of an older person’s life, such as mobility (GUGT), overall physical activity and energy expenditure (IPAQ), and social networking (LSNS-6). There is very little overlap between these instruments, thus enabling the constructs to be clearly distinguished (Badley, 2008). The instruments are also not disease specific, thus allowing generalization across a variety of conditions.
The choice of instruments was made to reflect the conceptual framework behind the ICF model. For example, mobility (GUGT) was chosen to represent body functions and structures (ICF “b” and “s” codes, respectively) as the majority of impairments (mental, sensory, cardio-respiratory, metabolic, and neuro-musculoskeletal, corresponding to ICF chapters 1, 2, 4, 5, and 7, respectively) will result in reduced mobility. Similarly, physical activity (IPAQ) and social networking (LSNS-6) were chosen to represent activities and participation (ICF “d” codes) as these are consequent manifestations of an individual’s mobility in relation to the ICF model (World Health Organization, 2001a).
Although the WHODAS-II has been superseded by the more recent WHODAS 2.0, the 12-item instrument used is virtually identical in wording with the updated version (World Health Organization, 2015). This means that the findings and conclusions made in this article equally apply to the newer instrument and the ICF in general.
Conservative values were chosen for sample size calculation as a significant proportion of the survey was conducted in sparsely populated rural areas. A design effect correction of 3 was used rather than the recommended value of 1.5 as a study demonstrated that in such circumstances this value can range from 1 to 5 (Rowe, Lama, Onikpo, & Deming, 2002). The expected response rate was also reduced as certain allocated EBs were impossible to access due to geographical isolation. Considering this, the overall non-response rate for the study was within expectation. The individual item response rates were very good aside from GUGT, which was likely because some older people can be fairly averse to physical testing.
Whereas the complete ICF model depicts the interplay between disease states, human functioning, and environmental factors, this study is primarily scoped toward disease states and human functioning. A related study by the authors examined the contribution of environmental factors (ICF “e” codes) in greater detail (Hamid et al., 2010).
Some limitations of the study are worth highlighting. There is a systematic bias against severe disability due to the sampling method, which excluded people who were unable to complete the survey because of this. However, there is no good way of working around this issue as the alternative would be to rely on secondary information from caregivers, which would be vulnerable to confirmation bias. Although there may have been under-sampling of the Chinese population due to language barriers, the proportion of ethnic Chinese in the study is similar to that from the census data, suggesting that the magnitude of under-sampling was small (Table 1). Moreover, this should only give rise to minimal bias as WHODAS-II scores in this ethnic group are fairly similar to the other major races (Malays and Indians). Although there was possibly some minor element of bias from the GUGT non-response, this should not have affected the conclusions drawn as the results were all highly significant with large effect sizes. Most of the instruments used in this study were validated in English, and there were no validation studies either in Malay or the many languages and dialects used locally. Once again there is no realistic way around this issue, and this study should be interpreted with this in mind.
Although the validation article for MMSE-M recommended that serial 7’s or serial 3’s be tried as a first choice, followed by the spell backwards method, we found that most of the elderly in rural communities could not cope with this due to low levels of education. The MMSE-M was validated in city hospitals with subjects who had much better educational exposure, so the project team made a decision to use the best result of the three methods as a compromise (Ibrahim et al., 2009). Although this may potentially alter the diagnosis of dementia to a small degree, this risk was judged to be acceptable as cognition was not the main focus for the study.
Lastly, certain aspects of the study were either abbreviated or simplified to keep the questionnaire time to less than an hour. Although extending the time or using a multi-stage interview might have allowed collection of additional data, there was a strong likelihood that those with high levels of functional impairment would drop out or refuse in a developing country setting (Prince, 2000). This would introduce significant bias, particularly in a study focusing on disability. The cognitive aspect of disability was also not examined in depth, as other parallel studies by the authors will address this issue separately in greater detail (Hamid et al., 2010; Loke et al., 2011). It would have been desirable to examine the impact of each disease group individually, but the CDBS was used in this study as a compromise between survey time and greater detail. In any case, the relationship between CDBS and WHODAS-II was still found to be highly significant on logistic regression testing (p < .001), suggesting that this instrument was adequate for the limited purposes of this study.
Conclusion
Our study builds upon the body of evidence from earlier disease-specific studies by demonstrating the validity of the earlier observations based on the ICF model using empirical data from a large sample of community-dwelling older adults with a broad range of medical diagnoses. Consistent with earlier studies, our results confirm that disability is strongly related to chronic disease burden, and that functional impairment correlates with activity performance and consequently with social participation.
The novel finding from our study is that at low levels of functional impairment, there is considerable variability in the relationship between functional impairment and activity performance, but this diminishes at high impairment levels especially when complicated by cognitive deficits. This suggests that modification of the environment benefits those with moderate levels of functional impairment, but not those with higher grades of functional loss.
Acknowledgments
The authors would like to extend their gratitude to the director general of Health Malaysia for his kind support, funding of the research, and allowing the article to be published.
Footnotes
Author Contributions: Seng Cheong Loke conducted the data analysis, interpreted the results, and prepared the article. Wee Shiong Lim and Yoshiko Someya assisted with data interpretation and critically appraised the article. Tengku A. Hamid and Siti S. H. Nudin were involved in study design. Tengku A. Hamid directed the study implementation.
Authors’ Note: Siti S. H. Nudin was responsible for quality assurance and control. All authors reviewed the article and approved the final version.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was paid for using internal funding from the Ministry of Health Malaysia. The Ministry of Health through the Institute for Health Behavioural Research was fully involved in all aspects of the study as co-investigators.
References
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Antonucci T. C., Jackson J. S. (1990). The role of reciprocity in social support. In Sarason I. G., Sarason B. R., Pierce G. R. (Eds.), Social support: An interactional view (pp. 173–198). New York, NY: John Wiley. [Google Scholar]
- Badley E. M. (2008). Enhancing the conceptual clarity of the activity and participation components of the International Classification of Functioning, Disability, and Health. Social Science & Medicine, 66, 2335-2345. [DOI] [PubMed] [Google Scholar]
- Bauman A., Bull F., Chey T., Craig C. L., Ainsworth B. E., Sallis J. F. . . . IPS (International Prevalence Study) Group. (2009). The International Prevalence Study on Physical Activity: Results from 20 countries. International Journal of Behavioral Nutrition and Physical Activity, 6(1), Article 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P., MacMahon B. (1971). Attributable risk percent in case-control studies. British Journal of Preventive & Social Medicine, 25, 242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C., Prieto-Merino D., Kuper H., Adamson J., Bowling A., Ebrahim S., Casas J. P. (2012). Modelling the association of disability according to the WHO International Classification of Functioning, Disability and Health (ICF) with mortality in the British Women’s Heart and Health Study. Journal of Epidemiology & Community Health, 66(2), 170-175. [DOI] [PubMed] [Google Scholar]
- Department of Statistics Malaysia. (2010). Population distribution and basic demographic characteristics (1st ed.). Putrajaya: Author. [Google Scholar]
- Faria-Fortini I., Michaelsen S. M., Cassiano J. G., Teixeira-Salmela L. F. (2011). Upper extremity function in stroke subjects: Relationships between the International Classification of Functioning, Disability, and Health domains. Journal of Hand Therapy, 24, 257-264; quiz 265. [DOI] [PubMed] [Google Scholar]
- Forsen L., Loland N. W., Vuillemin A., Chinapaw M. J., van Poppel M. N., Mokkink L. B., . . . Terwee C. B. (2010). Self-administered physical activity questionnaires for the elderly: A systematic review of measurement properties. Sports Medicine, 40, 601-623. [DOI] [PubMed] [Google Scholar]
- Garin O., Ayuso-Mateos J. L., Almansa J., Nieto M., Chatterji S., Vilagut G. . . . MHADIE (Measuring Health and Disability in Europe) Consortium. (2010). Validation of the “World Health Organization Disability Assessment Schedule, WHODAS-2” in patients with chronic diseases. Health and Quality of Life Outcomes, 8, Article 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby G., Smedby B. (2001). ICF approved as the successor of ICIDH. Journal of Rehabilitation Medicine, 33, 193-194. [DOI] [PubMed] [Google Scholar]
- Hamid T. A., Krishnaswamy S., Abdullah S. S., Momtaz Y. A. (2010). Sociodemographic risk factors and correlates of dementia in older Malaysians. Dementia and Geriatric Cognitive Disorders, 30, 533-539. [DOI] [PubMed] [Google Scholar]
- Hurtig-Wennlof A., Hagstromer M., Olsson L. A. (2010). The International Physical Activity Questionnaire modified for the elderly: Aspects of validity and feasibility. Public Health Nutrition, 13(11), 1847-1854. [DOI] [PubMed] [Google Scholar]
- Ibrahim N. M., Shohaimi S., Chong H. T., Rahman A. H., Razali R., Esther E., Basri H. B. (2009). Validation study of the Mini-Mental State Examination in a Malay-speaking elderly population in Malaysia. Dementia and Geriatric Cognitive Disorders, 27, 247-253. [DOI] [PubMed] [Google Scholar]
- Institute for Public Health. (2008). The Third National Health and Morbidity Survey (NHMS III) 2006 (Vol. 1). Kuala Lumpur, Malaysia: Ministry of Health. [Google Scholar]
- Jonsson G., Ekholm J., Schult M. L. (2008). The International Classification of Functioning, Disability and Health environmental factors as facilitators or barriers used in describing personal and social networks: A pilot study of adults with cerebral palsy. International Journal of Rehabilitation Research, 31, 119-129. [DOI] [PubMed] [Google Scholar]
- Kant A. K., Schatzkin A., Harris T. B., Ziegler R. G., Block G. (1993). Dietary diversity and subsequent mortality in the First National Health and Nutrition Examination Survey Epidemiologic Follow-Up Study. American Journal of Clinical Nutrition, 57, 434-440. [DOI] [PubMed] [Google Scholar]
- Loke S. C., Abdullah S. S., Chai S. T., Hamid T. A., Yahaya N. (2011). Assessment of factors influencing morale in the elderly. PLoS ONE, 6(1), e16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubben J. (2003). Lubben Social Network Scale–6. Retrieved from https://www.bc.edu/schools/gssw/lubben/downloads/_jcr_content/content/download_0/file.res/LSNS6.pdf
- Lubben J., Blozik E., Gillmann G., Iliffe S., von Renteln Kruse W., Beck J. C., Stuck A. E. (2006). Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist, 46, 503-513. [DOI] [PubMed] [Google Scholar]
- Nordin E., Lindelof N., Rosendahl E., Jensen J., Lundin-Olsson L. (2008). Prognostic validity of the Timed Up-and-Go test, a modified Get-Up-and-Go test, staff’s global judgement and fall history in evaluating fall risk in residential care facilities. Age and Ageing, 37, 442-448. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D., Richardson S. (1991). The timed “up & go”: A test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39, 142-148. [DOI] [PubMed] [Google Scholar]
- Pollard B., Johnston M., Dieppe P. (2011). Exploring the relationships between International Classification of Functioning, Disability and Health (ICF) constructs of impairment, activity limitation and participation restriction in people with osteoarthritis prior to joint replacement. BMC Musculoskeletal Disorders, 12, Article 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M. (2000). Methodological issues for population-based research into dementia in developing countries. A position paper from the 10/66 Dementia Research Group. International Journal of Geriatric Psychiatry, 15, 21-30. [DOI] [PubMed] [Google Scholar]
- Rowe A. K., Lama M., Onikpo F., Deming M. S. (2002). Design effects and intraclass correlation coefficients from a health facility cluster survey in Benin. International Journal for Quality in Health Care, 14, 521-523. [DOI] [PubMed] [Google Scholar]
- Scott K. M., Von Korff M., Alonso J., Angermeyer M. C., Bromet E., Fayyad J., . . . Williams D. (2009). Mental-physical co-morbidity and its relationship with disability: Results from the World Mental Health Surveys. Psychological Medicine, 39, 33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa R. M., Dewey M. E., Acosta D., Jotheeswaran A. T., Castro-Costa E., Ferri C. P., . . . Prince M. J. (2010). Measuring disability across cultures—The psychometric properties of the WHODAS II in older people from seven low- and middle-income countries. The 10/66 Dementia Research Group population-based survey. International Journal of Methods in Psychiatric Research, 19, 1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa R. M., Ferri C. P., Acosta D., Albanese E., Guerra M., Huang Y., . . . Prince M. (2009). Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: A 10/66 Dementia Research Group population-based survey. The Lancet, 374, 1821-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. C., Bell C., Campbell S., Davis J. (2000). The Timed Get-Up-and-Go test revisited: Measurement of the component tasks. Journal of Rehabilitation Research & Development, 37, 109-113. [PubMed] [Google Scholar]
- World Health Organization. (2001a). International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland: Author. [Google Scholar]
- World Health Organization. (2001b). World Health Organization Disability Assessment Schedule II (WHODAS II). Retrieved from http://www.who.int/icidh/whodas/index.html
- World Health Organization. (2011). World report on disability. Geneva, Switzerland: Author. [Google Scholar]
- World Health Organization. (2012). STEPwise approach to surveillance (STEPS). Retrieved from http://www.who.int/chp/steps/en/
- World Health Organization. (2015). WHO Disability Assessment Schedule 2.0 (WHODAS 2.0), 12-item instrument scoring sheet. Retrieved from http://www.who.int/classifications/icf/whodasii/en/