Anemia is a cardinal manifestation of primary myelofibrosis (PMF) and constitutes a negative prognostic factor, being an independent risk factor for survival both in the international prognostic score system (IPSS)1 and dynamic IPSS (DIPSS)-plus.2 Anemia in myelofibrosis is also inversely correlated with patient-reported quality of life. Between 35 and 54% of patients with PMF are anemic at diagnosis with a hemoglobin level of <10 g/dl1 with the proportion increasing to 47–64% after about 1 year from the time of diagnosis.3
Pathogenesis of PMF-associated anemia is poorly understood and is multifactorial, involving hypersplenism, chronic low-grade hemolysis and ineffective hematopoiesis, the latter influenced by both abnormal expression of proinflammatory cytokines and other growth factors and, intrinsic erythroid cell defects.3, 4, 5
Current drugs, including JAK inhibitors, are suboptimal in the treatment of PMF-associated anemia and better information on its pathogenesis is critical for the development of more effective drugs. In the current study of JAK2/CALR/MPL-annotated patients with PMF, we examined the correlation between anemia with both driver and non-driver mutations,6, 7 as well as cytogenetic abnormalities, in order to gain better insight into its pathogenesis.
This study was approved by the institutional review board of Mayo Clinic (Rochester, MN, USA). Study patients were selected from our institutional database of myeloproliferative neoplasms based on the 2008 World Health Organization criteria diagnosis of PMF.8 Risk stratification was according to DIPSS-plus system.2 Screening for JAK2, CALR, MPL, ASXL1, TET2, EZH2, IDH1, IDH2 and spliceosome (SF3B1, U2AF1, SRSF2, ZRSR2) mutations was performed according to previously described methods.6, 7 Statistical analyses considered clinical and laboratory data collected at the time of diagnosis or referral to the Mayo Clinic. Differences in the distribution of continuous variables between categories were analyzed by either Mann–Whitney or Kruskal–Wallis test. Patient groups with nominal variables were compared by X2 test. Survival analysis was considered from the date of diagnosis or referral to date of death (uncensored) or, last contact (censored). Survival curves were prepared by the Kaplan–Meier method and compared by the log-rank test. The Cox proportional hazard regression model was used for multivariate survival analysis. P<0.05 was considered significant. The Stat View (SAS Institute, Minneapolis, MN, USA) statistical package was used for all calculations.
A total of 722 PMF patients (median age was 64, and 64% were males) were studied. Presenting clinical and laboratory details are outlined in Table 1. Cytogenetic studies were available in 703 patients and included normal karyotype in 61% and normal or favorable abnormalities in 88%.9 Other mutations that were concomitantly analyzed included ASXL1 (n=480), SF3B1 (n=415), U2AF1 (n=457), SRSF2 (n=474), TET2 (n=180), EZH2 (n=374), ZRSR2 (n=180), IDH1 (n=187) and IDH2 (n=187); their respective frequencies were 38, 8, 16, 15, 18, 4, 11, 5 and 7%.
Table 1. Clinical and laboratory features of 722 patients with primary myelofibrosis patients stratified by grades of anemia.
| Variables | All (n=722) | No anemia (n=94) | Mild anemia (n=295) | Moderate anemia (n=98) | Severe anemia (n=235) | P-value univariate | P-value multivariate |
|---|---|---|---|---|---|---|---|
| Age at referral in years median (range) | 64 (22–90) | 60.5 (30–87) | 61 (22–88) | 64.5 (28–89) | 69 (37–90) | <0.0001 | 0.0002 |
| Male (%) | 464 (64%) | 55 (59%) | 192 (65%) | 61 (62%) | 156 (66%) | 0.55 | |
| Leukocytes, × 109/l median (range) | 9 (0.8–236.1) | 13.950 (2.8–4.1) | 9.0 (1.1–176.0) | 6.450 (1.7–236.1) | 7.9 (0.8–218.5) | <0.0001 | 0.061 |
| Platelets, × 109/l median (range) | 212.0 (10.0–466.0) | 309.0 (15.0–288.0) | 243.0 (13.0–255.0) | 202.0 (14.0–282.0) | 140.0 (10.0–2466.0) | <0.0001 | 0.03 |
| Circulating blasts % median (range) | 1 (0–15) | 0 (0–5) | 0 (0–15) | 1 (0–8) | 1 (0–15) | 0.0014 | 0.36 |
| Presence of constitutional symptoms n (%) | 231 (32%) | 20 (21%) | 79 (27%) | 31 (32%) | 101 (43%) | <0.0001 | 0.04 |
| Presence of palpable splenomegaly N evaluable=706 n (%) | 514 (73%) | 63 (68%) | 208 (72%) | 68 (72%) | 175 (75%) | 0.62 | |
| DIPSS-plusa risk N evaluable =703 | <0.0001 | ||||||
| Low | 94 (13%) | 31 (34%) | 61 (21%) | 2 (2%) | 0 (0%) | ||
| Intermediate-1 | 119 (17%) | 27 (29%) | 85 (30%) | 7 (7%) | 0 (0%) | ||
| Intermediate-2 | 259 (37%) | 27 (29%) | 109 (38%) | 62 (64%) | 61 (27%) | ||
| High | 231 (33%) | 7 (8%) | 29 (10%) | 26 (27%) | 169 (73%) | ||
| Driver mutations | <0.0001 | ||||||
| JAK2 mutated n (%) | 476 (66%) | 73 (78%) | 175 (59%) | 66 (67%) | 162 (69%) | 0.03 | |
| CALR type 1/type 1-like n (%) | 115 (16%) | 12 (13%) | 70 (24%) | 14 (14%) | 19 (8%) | 0.01 | |
| CALR type 2/type 2-like n (%) | 24 (3%) | 1 (1%) | 14 (5%) | 5 (5%) | 4(2%) | ||
| MPL mutated n (%) | 38 (5%) | 0 (0%) | 18 (6%) | 6 (6%) | 14 (6%) | ||
| Triple negative n (%) | 69(10%) | 8 (8%) | 18 (6%) | 7 (7%) | 36 (15%) | ||
| Cytogenetic categories N evaluable =703 (97%) | |||||||
| Normal | 426 (61%) | 64 (70%) | 173 (61%) | 56 (58%) | 133 (58%) | ||
| Normal vs abnormal | 0.24 | ||||||
| Favorable | 620 (88%) | 82 (89%) | 255 (90%) | 87 (90%) | 196 (85%) | ||
| Favorable vs unfavorable | 0.40 | ||||||
| ASXL1 N evaluable=480 | 181(38%) | 21 (31%) | 70 (36%) | 28 (42%) | 62 (41%) | 0.50 | |
| SF3B1 N evaluable=415 | 35 (8%) | 4 (7%) | 11 (7%) | 6 (10%) | 14 (10%) | 0.64 | |
| U2AF1 N evaluable=457 | 72 (16%) | 2 (3%) | 15 (8%) | 11 (18%) | 44 (30%) | <0.0001 | <0.0001 |
| SRSF2 N evaluable=474 | 70 (15%) | 9 (13%) | 24 (13%) | 6 (9%) | 31 (20%) | 0.09 | |
| TET2 N evaluable=180 | 32 (18%) | 2 (7%) | 13 (19%) | 7 (30%) | 10 (16%) | 0.21 | |
| EZH2 N evaluable=374 | 16 (4%) | 2 (4%) | 8 (5%) | 2 (4%) | 4 (3%) | 0.85 | |
| ZRSR2 N evaluable=180 | 19 (11%) | 1 (4%) | 11 (16%) | 0 (0%) | 7 (11%) | 0.11 | |
| IDH1 N evaluable=187 | 9 (5%) | 1 (4%) | 3 (4%) | 2 (8%) | 3 (5%) | 0.87 | |
| IDH2 N evaluable=187 | 14 (7%) | 1 (4%) | 5 (7%) | 1 (4%) | 7 (11%) | 0.58 | |
Abbreviation: DIPSS-plus, dynamic international prognostic scoring system-plus.
DIPSS-plus:5 DIPSS-plus uses eight independent predictors of inferior survival: age >65 years, hemoglobin <10 g/dl, leukocytes >25 × 109/l, circulating blasts ⩾1%, constitutional symptoms, red cell transfusion dependency, platelet count <100x109/l and unfavorable karyotype (that is, complex karyotype or sole or two abnormalities that include þ8, −7/7q−, i(17q), inv,3 −5/5q−, 12p− or 11q23 rearrangement). The presence of 0, 1, ‘2 or 3' and 4 adverse factors defines low, intermediate-1, intermediate-2 and high-risk disease. Statistically significant P-values are in bold.
Patients were stratified according to the grade of anemia as mild (for hemoglobin level ⩾10 g/dl but below sex-adjusted normal value), moderate (for hemoglobin level between 8 g/dl and <10 g/dl) and severe (hemoglobin <8 g/dl or transfusion-dependent). At time of referral, 628 (87%) patients had anemia, of which 295 (47%) were mild, 98 (16%) moderate and 235 (37%) severe.
In univariate analysis, anemia was significantly associated with advanced age (P<0.0001), lower leukocyte count (P<0.0001), lower platelet count (P<0.0001), presence of circulating blasts (P=0.0014), presence of constitutional symptoms (P<0.0001), driver mutational status (P<0.0001) and U2AF1 mutation (P<0.0001). On multivariate analysis, advanced age (P=0.0002), lower platelet count (P=0.03), the presence of constitutional symptoms (P=0.04), absence of JAK2 mutation (P=0.03), absence of CALR type 1/type 1-like mutation (P= 0.01) and presence of U2AF1 (P<0.0001) retained significance.
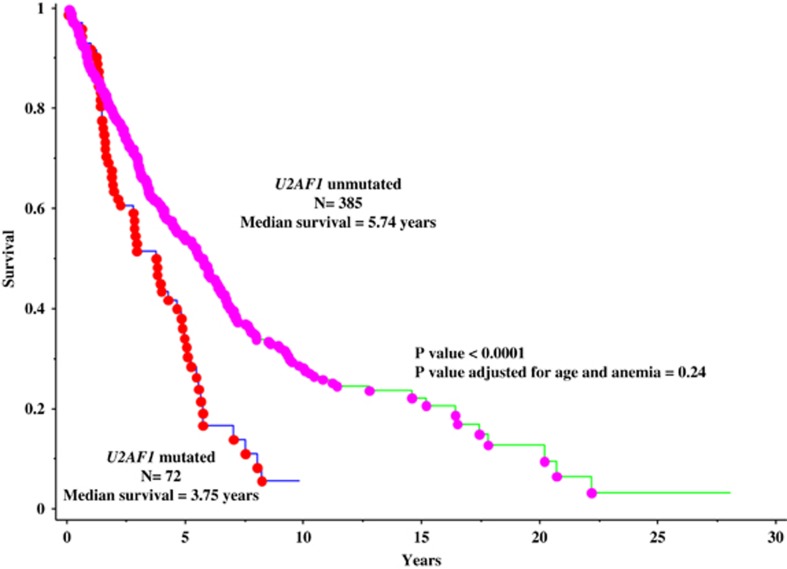
At a median follow-up of 3 years (range 0–28.1 years), 439 (61%) deaths were recorded. In univariate analysis, the presence of U2AF1 mutation is associated with inferior survival (P<0.0001; Figure 1). However, significance was lost when analysis was adjusted for age and anemia (P=0.24)
Figure 1.
Overall survival of 457 patients with PML stratified by the presence or absence of U2AF1 mutations.
The current study confirms our previous observation regarding the association of mutant U2AF1 with anemia,10 supporting its role in hematopoiesis11 and in the pathogenesis of PMF-associated anemia. Our observation also supports the need to screen patients with PMF for U2AF1 mutations, in order to identify those who are at risk for the development of anemia, as well as those who might benefit from accordingly targeted therapy.12
The authors declare no conflict of interest.
References
- Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009; 113: 2895–2901. [DOI] [PubMed] [Google Scholar]
- Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 2011; 29: 392–397. [DOI] [PubMed] [Google Scholar]
- Guglielmelli P, Vannucchi AM. Struggling with myelofibrosis-associated anemia. Leuk Res 2013; 37: 1429–1431. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2 R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol 2011; 29: 1356–1363. [DOI] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013; 369: 2379–2390. [DOI] [PubMed] [Google Scholar]
- Lasho TL, Jimma T, Finke CM, Patnaik M, Hanson CA, Ketterling Rp et al. SRSF2 mutations in primary myelofibrosis: significant clustering with IDH mutations and independent association with inferior overall and leukemia-free survival. Blood 2012; 120: 4168–4171. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013; 27: 1861–1869. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- Caramazza D, Begna KH, Gangat N, Vaidya R, Siragusa S, Van Dyke DL et al. Refined cytogenetic-risk categorization for overall and leukemia-free survival in primary myelofibrosis: a single center study of 433 patients. Leukemia 2011; 25: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Finke CM, Lasho TL, Wassie EA, Knudson R, Ketterling RP et al. U2AF1 mutations in primary myelofibrosis are strongly associated with anemia and thrombocytopenia despite clustering with JAK2V617F and normal karyotype. Leukemia 2014; 28: 431–433. [DOI] [PubMed] [Google Scholar]
- Shirai CL, Ley JN, White BS, Kim S, Tibbitts J, Shao J et al. Mutant U2AF1 expression alters hematopoiesis and pre-mRNA splicing in vivo. Cancer cell 2015; 27: 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Lasho TL, Begna KH, Patnaik MM, Zblewski DL, Finke CM et al. A pilot study of the telomerase inhibitor lmetelstat for myelofibrosis. N Engl J Med 2015; 373: 908–919. [DOI] [PubMed] [Google Scholar]