Abstract
HIV-infected drug users have increased age-matched morbidity and mortality compared with HIV-infected people who do not use drugs. Substance-use disorders negatively affect the health of HIV-infected drug users, who also have frequent medical and psychiatric comorbidities that complicate HIV treatment and prevention. Evidence-based treatments are available for the management of substance-use disorders, mental illness, HIV and other infectious complications such as viral hepatitis and tuberculosis, and many non-HIV-associated comorbidities. Tuberculosis co-infection in HIV-infected drug users, including disease caused by drug-resistant strains, is acquired and transmitted as a consequence of inadequate prescription of antiretroviral therapy, poor adherence, and repeated interfaces with congregate settings such as prisons. Medication-assisted therapies provide the strongest evidence for HIV treatment and prevention efforts, yet are often not available where they are needed most. Antiretroviral therapy, when prescribed and adherence is at an optimum, improves health-related outcomes for HIV infection and many of its comorbidities, including tuberculosis, viral hepatitis, and renal and cardiovascular disease. Simultaneous clinical management of multiple comorbidities in HIV-infected drug users might result in complex pharmacokinetic drug interactions that must be adequately addressed. Moreover, interventions to improve adherence to treatment, including integration of health services delivery, are needed. Multifaceted, interdisciplinary approaches are urgently needed to achieve parity in health outcomes in HIV-infected drug users.
Introduction
Drug use, especially the injection of drugs, has been associated with some of the most severe HIV epidemics worldwide. HIV-infected drug users have increased prevalence and frequency of medical, psychiatric, and substance-use disorders that result in increased age-matched morbidity and mortality compared with HIV-infected people who do not use drugs. The number and range of these comorbid disorders complicates diagnosis and treatment, resulting in several challenges in the provision of comprehensive care. HIV-infected drug users accessing antiretroviral therapy (ART) have worse clinical outcomes than do matched people living with HIV/AIDS who do not use drugs.1 Medical, psychiatric, and substance-use disorder comorbidities complicate care and must be simultaneously addressed to achieve health outcome parity. We describe some of the comorbidities affecting HIV-infected drug users and discuss adherence interventions and continuity of care issues that can be used to achieve the best possible care for this group.
Substance-use disorders
Drug and alcohol dependence, comorbidities that are highly prevalent in HIV-infected drug users, can each contribute to poor health outcomes. They are associated with decreased access to and use of health care, reduced likelihood of being prescribed ART, and once prescribed it, reduced adherence.2 Table 1 lists commonly used illicit drugs and their evidence-based treatments.
Table 1.
Common legal and illegal drugs and their effect on HIV
| Common adverse clinical consequence of ingestion | Modes of use | Effect on HIV | Evidence-based medication-assisted therapies | |
|---|---|---|---|---|
| Opioids (heroin, morphine, hydromorphone, codeine, poppy straw) | Respiratory depression, coma, overdose; physical and psychological dependence | Injection; inhalation (smoked or snified); oral (synthetic only) | Decreases access to and use of care, decreased prescription of ART, decreased adherence to ART | Methadone (oral); buprenorphine (sublingual); naltrexone (oral, injectable) |
|
| ||||
| Cocaine (white powder, crack) | Agitation, hyperthermia, tachycardia, arrhythmia, hypertension, convulsions, cardiac and CNS disturbances, hallucinations, death, psychological dependence | Injection; inhalation (smoked or snified) | Decreases access to and use of care; decreased prescription of ART; decreased adherence to ART; increased sexual and drug risk behaviours | None |
|
| ||||
| Benzodiazepines | CNS depression, sedation, ataxia, amnesia and coma; deaths are rare when benzodiazepines are taken alone; physical and psychological dependence are rapid and profound | Injection; oral | Associated with increased sexual and drug risk behaviours; decreased adherence to ART; increased STIs | Slow supervised taper and withdrawal needed |
|
| ||||
| Club drugs | ||||
| Methamphetamine (rINN metamfetamine) and amphetamine-group substances | CNS stimulation; increased alertness and energy; high doses induce euphoria, enhance self-esteem, and increase sexual pleasure; physiologically causes increased heart rate and blood pressure, vasoconstriction (including cerobrovascular events), bronchodilation, and hyperglycaemia; neurotoxic resulting in permanent brain damage | Injection; inhalation; per rectum | Decreases access to and use of care, decreased prescription of ART, decreased adherence to ART | None |
| MDMA | With overdose: serotonin syndrome, stimulant psychosis, and/or hypertensive crisis, cognitive and memory impairment, acute delirium, cardiac arrhythmias or infarction, coma; profound depression several days after use | Oral (tablet) | Decreased adherence to ART on days of MDMA use | None |
| Ketamine | Hypertension, cardiac arrhythmias, cognitive impairment | Injection; inhalation (snified or smoked) | Not known | None |
| Gamma-hydroxybutyrate | Oversedation, coma, death, seizures, hypotension and shock, psychosis and agitation | Oral (liquid) | Not known, but likely similar to alcohol | None |
| Nitrates/nitrates (poppers) | Methaemoglobinaemia, haemolytic anaemia (especially in those with G6PD deficiency), hypotension, cardiac arrhythmias | Inhalation (liquid) | Assoicated with increased HIV risk behaviours | None |
|
| ||||
| Alcohol | CNS sedation, some malignant diseases, hepatic injury, dietary deficiencies, pancreatitis, gastritis, neurocognitive deficits | Oral | Increases hepatotoxicity; increases peripheral neuropathy; decreases access to and use of care, decreased prescription of ART, decreased adherence to ART | Naltrexone (oral, depot injection); acamprosate; disulfiram |
ART=antiretroviral therapy. STIs=sexually transmitted infections. MDMA=methylenedioxymethamphetamine (rINN methylenedioxymethamfetamine). G6PD=glucose-6-phosphate dehydrogenase.
Key messages.
HIV-infected drug users are at increased risk for several medical and psychiatric comorbidities including viral hepatitis, tuberculosis, bacterial infections, and mental illness.
Evidence-based treatment for substance-use disorders improves the psychological and physiological disruptions that perpetuate the often unstable life of HIV-infected drug-dependent individuals.
Treatment of HIV infection, substance-use disorders, and comorbidities in HIV-infected drug users is improved by comprehensive and multidisciplinary management of these disorders.
Medication-assisted therapy, when appropriately dosed, enhances adherence to antiretroviral therapy, treatment for comorbidities, and retention on antiretroviral therapy and in HIV care, and decreases HIV risk behaviours.
As antiretroviral therapy is successfully made universally available to HIV-infected drug users, non-AIDS comorbidities and tuberculosis will emerge as leading problems that will complicate care.
Tuberculosis diagnosis and treatment poses complex clinical challenges in HIV co-infected drug users.
Adherence to treatment for HIV infection, substance use, and comorbidities can be enhanced through a range of interventions, such as counselling, contingency management, supervised therapy (eg, directly observed therapy), medication-assisted therapy, and integrated health services delivery.
Incarceration is detrimental to disease control programmes for HIV-infected drug users, particularly through increased transmission of drug-susceptible and drug-resistant tuberculosis. It can also serve as a point of entry to care. Once diagnosis and treatment are initiated, successful transitional programmes and continuity of care after release to the community are needed.
The drugs most closely associated with HIV infection worldwide are heroin and cocaine, but amphetamine-group substance use is an evolving problem. Injection with shared contaminated needles and syringes or other injection equipment carries the greatest risk for transmission of HIV and other bloodborne infections. Non-injection drug and alcohol use, however, increasingly facilitates HIV transmission, particularly among women, through its association with the exchange of sex or money for drugs. Recognition of local drug availability and routes of use is important for provision of treatment and care.
Heroin, the most widely used opioid, results in both physical and psychological dependence with chronic use. Apart from overdose, there are few direct medical consequences of heroin use. Its unsterile method of injection, unpredictable concentrations in street-derived samples, acute euphoria resulting in disinhibitory HIV risk behaviours, adulterants in the injection mixture, and the lifestyle necessary to procure drugs, however, are responsible for many heroin-associated medical complications.2
Among the stimulants, cocaine is most commonly used and rapidly leads to psychological dependence. Cocaine induces feelings of elation, omnipotence, and invincibility that often disrupt clinical care. The most severe medical consequence of cocaine use is vasospasm, an idiosyncratic response that can result in myocardial infarction and cerebrovascular accidents in young people without any evidence of vascular disease.2
Benzodiazepines, a class of sedative hypnotics, are sometimes injected after crushing the tablet formulation, often resulting in soft-tissue and vascular complications.3 When combined with other drugs, benzodiazepine use is associated with decreased retention in drug treatment, increased HIV risk behaviours, fatal and non-fatal overdose, and increased mortality.4,5 Like opioids, benzodiazepines cause both physical and psychological dependence and can result in dangerous complications associated with withdrawal.6 Prolonged supervised withdrawal is therefore needed for safe discontinuation of benzodiazepines.
Evidence-based treatment for chemical dependence
Chemical dependence is a chronic, relapsing, and treatable disease, characterised by compulsive drug-seeking behaviour and drug use. Although exposure to addictive substances is widespread in society, high vulnerability to addiction is more limited and is the product of biological, psychological, and environmental factors. Thus, identification of addictive disease and provision of and referral to appropriate treatment services are essential parts of the clinical management of HIV-infected drug users. Indeed, successful treatment of HIV-infected drug users is greatly improved by treatment of substance-use disorders. There are many mind-altering substances that are clinically encountered (table 1) as well as a range of treatment modalities to manage these substance-use disorders (table 2). Selection of appropriate treatment is an individual decision based in part upon the drug used, the length and pattern of the patient’s drug use, personal psychosocial characteristics, and availability of local resources. Options are often limited, however, resulting in substantially reduced referral to or provision of integrated treatment.
Table 2.
Available pharmacological medication-assisted therapies used for treatment of substance-use disorders
| Type of dependence | Mechanism of action | Pharmacological properties | Side-effects | Substance-use treatment outcomes | Effect on HIV | Other issues | |
|---|---|---|---|---|---|---|---|
| Methadone | Opioid | Pure opioid μ-receptor agonist | Half-life 24–36 h; ingested orally as tablet or liquid; achieves steady state within 5 days | Tolerance to side-effects usually develops; diaphoresis, constipation, and amenorrhoea (menses usually return after 12–18 months); excessive dosing or when combined with alcohol might cause overdoses or death | Decreases relapse to illicit opioid use; decreases number of days using illlicit opioids; decreases opioid and cocaine use after release from prison; decreases criminal activity; increases employment; cost effective | Decreases injection and HIV transmission; increases retention in HIV care; effective supporter of DAART; increases effectiveness of ART | No euphoria felt after being on stable methadone dose; doses of 30–60 mg per day will block opioid withdrawal symptoms, but this dose seldom produces abstinence. Instead, higher doses in the 80–120 mg per day range are needed to decrease opioid craving and decrease illicit drug use. These higher doses are also associated with greater retention in treatment |
| Buprenorphine | Opioid | Partial opioid μ-receptor agonist and partial κ–receptor antagonist | Half-life 24–36 h; administered sublingually; slow dissociation from the μ-receptor allowing alternate-day dosing | Improved safety profile compared with methadone; unlikely to cause overdose or respiratory depression; higher binding affinity for the μ receptor than heroin or methadone, therefore precipitates withdrawal in person still with opioids in their system | Decreases relapse to illicit opioid use; decreases number of days using illlicit opioids; decreases opioid and cocaine use after release from prison; decreases criminal activity; increases employment; cost effective | Decreases injection and HIV transmission; increases retention in HIV care; effective supporter of DAART; increases effectiveness of ART; increases retention on ART after release from prison | Decreased likelihood for medication diversion; injection of buprenorphine in opioid-dependent individuals precipitates withdrawal symptoms |
| Buprenorphine-naloxone | Opioid | Partial opioid μ-receptor agonist and partial κ–receptor antagonist; naloxone is a shortacting μ-receptor but not orally bioavailable antagonist | Half-life 24–36 h; administered sublingually; slow dissociation from the μ receptor allowing alternate-day dosing | Same as for buprenorphine; naloxone used to reduce likelihood for diversion and injection | Compared with high dose methadone, retention in treatment is lower | Same as for buprenorphine; when injected, probably results in more frequent injecting and increased risk for HIV transmission | Used to decrease likelihood of illicit injection and injection frequency; however, might increase the frequency of injections |
| Naltrexone | Opioid | Pure μ-receptor opioid antagonist | Oral formulation dosing is daily or alternate-day dosing; injectable formulation given intramuscularly monthly (improves adherence) | Hepatoxicity possible; has been administered safely in HCV-infected patients | Retention in treatment is lower than for methadone or buprenorphine, but might be considered in highly motivated patients | ·· | Discourages opioid use by diminishing the pleasurable effect of and craving for opioids, and has shown efficacy in highly motivated populations |
| Naltrexone | Alcohol | Blocks the pleasant and reinforcing effects of alcohol by preventing the stimulation of opioid receptors and the reduction of dopamine release in the ventral tegmental area | Oral formulation dosing is daily or alternate-day dosing; injectable formulation given intramuscularly monthly (improves adherence7,8) | Hepatoxicity possible; has been administered safely in HCV-infected patients | Superior to behavioural counselling and acamprosate for treatment of alcohol-use diseases in HIV-uninfected individuals; increases time to relapse, decreases number of days of heavy drinking7,8 | ·· | ·· |
| Acamprosate | Alcohol | Structural analogue of the γ-aminobutyric acid neurotransmitter; normalises glutamatergic neurotransmission; slow acting, can attenuate relapse in some | Orally dosed with two tablets three times per day; adherence might be problematic | Few side-effects | Increased abstinence confirmed in placebo-controlled trials, but no benefit in preventing relapse by itself or in combination with counselling | Not studied in HIV-infected patients | ·· |
| Disulfiram | Alcohol | Inhibits acetaldehyde dehydrogenase and causes accumulation of acetaldehyde when alcohol is consumed; leads to painful symptoms such as facial flushing, dypsnoea, nausea, vomiting, and headache, thereby discouraging relapse to alcohol consumption | Orally dosed, half-life 24 h; should not be combined with amprenavir (probable) or metronidazole (probable) | Nausea and vomiting if alcohol is ingested; hepatotoxicity | Not studied in HIV-infected individuals | Hepatotoxicity; causes profound nausea and vomiting when alcohol is ingested | Might be useful when combined with other interventions, including methadone when a patient is opioid-dependent |
| Varenicline | Tobacco | Binds with high affinity and selectivity at α4β2-neuronal nicotinic acetylcholine receptors and exerts its effect by producing agonist activity at a subtype of the α4β2-nicotinic receptor while also preventing nicotine binding to α4β2 receptors | Orally ingested, half-life 24 h and 92% renally cleared; no hepatic metabolism | Rare but serious neuropsychiatric events (eg, depression, suicidal ideation, suicide attempt, and completed suicide) have been reported | In meta-analysis, associated with the highest rates of abstinence and greatest reduction in smoking | Has not been systematically studied in HIV-infected individuals | ·· |
| Nicotine-replacement therapy | Tobacco | Competitive binding of α4β2-neuronal nicotinic acetylcholine receptors | Route of administration includes transdermal, orally ingested, sublingual or inhaled | Can rarely cause cardiac dysrhythmia, hypertension, or tachyarrhythmia; more commonly, causes nicotine withdrawal, dizziness, headache, insomnia | Is more effective than placebo or counselling, but results in greatest abstinence rates and reductions in tobacco smoking when combined with bupropion or with two forms of nicotine replacement9,10 | Has not been systematically studied in HIV-infected individuals | ·· |
| Buproprion | Tobacco | Acts as a norepinephrine and dopamine reuptake inhibitor for treating depression, as well as α3β4-nicotinic receptor antagonist | Orally administered; half-life 24 h, efavirenz and lopinavir moderately decrease bupropion concentrations | Rare but serious neuropsychiatric events, including depression, suicidal ideation, suicide attempt, and completed suicide have been reported in patients with and without pre-existing psychiatric disease who were taking bupropion for smoking cessation; some had worsening of their psychiatric illnesses | Superior to placebo but most optimum outcomes associated with combination with nicotine replacement therapy in RCTs | Has not been systematically studied in HIV-infected individuals | ·· |
DAART=directly administered antiretroviral therapy. ART=antiretroviral therapy. HCV=hepatitis C virus. RCTs=randomised controlled trials.
As with management of most diseases, a comprehensive, multidisciplinary approach is ideal for treatment of substance-use disorders. Medication-assisted therapies use clinician-prescribed agents to reduce the negative consequences of drug use. These treatments alone, or when combined with brief counselling or education, can lead to impressive outcomes.11 These results are encouraging in view of the dearth of manpower and financial resources to provide health care in many low-income and middle-income countries. Barriers to providing medication-assisted therapies in these settings have been identified as cost, medication availability, lack of coordination between governmental agencies, name-based registries, and governmental restrictions that limit eligibility, supply, distribution, or licensing.12,13
Table 2 provides a list of evidence-based medication-assisted therapies for opioid dependence, alcohol-use disorders, and nicotine dependence. Medication-assisted therapies constitute the most effective treatment for opioid dependence, but they are not currently available for treatment of cocaine or amphetamine-group substance use.14,15 Generally, medication-assisted therapy is reserved for injecting drug users (IDUs) and chronic opioid users (ie, those who have used opioids for more than 2 years).15 Since the availability of medication-assisted therapy is often limited and dosing is usually inadequate, improvement of access to and prescription of effective doses should be the first priority. Relapse rates in chronic opioid users exceed 85% when behavioural therapy alone is used.16 Methadone and buprenorphine are the most effective medication-assisted therapies for opioid dependence,17 although naltrexone has shown some success in highly motivated patients.18 Patients with polysubstance use or psychiatric comorbidity have increased benefit from comprehensive and integrated drug treatment programmes that enhance rehabilitation. Medication-assisted therapy, when appropriately dosed, is particularly important for HIV-infected drug users because it enhances access and adherence to ART and HIV treatment, improves retention in HIV care, and decreases HIV risk behaviours (table 2).19 Moreover, evidence-based treatment for substance-use disorders improves the psychological and physiological disruptions that perpetuate the often unstable life of a drug-dependent person.
Methadone is the medication-assisted therapy that has been available for the longest time (more than 40 years), whereas buprenorphine has been extensively available in France since 1996. Naltrexone has been available since the mid-1980s, but its longacting depot formulation only became available in 2006. Compared with tablets, the newer depot formulation has fewer adverse effects and improved adherence, but is more costly. Naltrexone therefore holds some promise as a treatment of opioid dependence in HIV-infected drug users, but has yet to be empirically tested in them.
In most settings, methadone remains the most highly regulated medication-assisted therapy because of its narrow therapeutic index, problems with drug diversion, and the possibility of overdose. When highly regulated, integration of HIV and primary care within medication-assisted therapy settings can be successful.20 Conversely, when medication-assisted therapy is not highly regulated, both HIV treatment and prescription of medication-assisted therapy can be provided within HIV primary care settings, correctional settings, syringe exchange programmes, and safe injecting facilities, resulting in effective integration of services.21–27
Buprenorphine, when prescribed with flexible dosing, is 20% less effective than is high-dose methadone (≥80 mg per day) for treatment retention, but similar to methadone for reducing opioid use. Medium-dose buprenorphine, however, is more likely than low-dose methadone (<60 mg per day) to suppress opioid use.28 Buprenorphine has fewer pharmacokinetic drug interactions with ART than does methadone.29 Oral naltrexone has been shown to be inferior to methadone and buprenorphine, mainly because of poor retention.18,30,31 Longacting depot naltrexone administration, however, might result in improved treatment adherence and retention.
Alcohol-use disorders are common in people living with HIV/AIDS and in IDUs. Heavy alcohol use negatively affects people living with HIV/AIDS in several ways, including increased risk of HIV transmission to others,32 decreased retention in care,33 poor adherence to treatment,34,35 increased HIV risk behaviours,36–38 decreased likelihood of suppression of HIV,39,40 and acceleration of hepatic fibrosis, particularly in the setting of co-infection with hepatitis C virus (HCV) and prescribed ART.41 Treatment of alcohol-use disorders, therefore, has the potential to positively affect HIV treatment outcomes and reduce HIV transmission.
Behavioural interventions, the mainstay of treatment for alcohol-use disorders for decades, have typically shown a small to modest benefit. A review of 16 trials of behavioural interventions showed that theory-based behavioural treatment was better than standard supportive 12-step treatment at reducing alcohol relapse.42,43 A review of acupuncture trials for treatment of alcohol dependence did not support its use.44
Although pharmacotherapy for treatment of alcohol-use disorders has proved more effective than have behavioural interventions, its use in people living with HIV/AIDS has not been systematically assessed. Naltrexone is the most effective treatment for relapse prevention and treatment of alcohol-use disorders.18,43 Although less effective than naltrexone and equivalent to counselling alone,45,46 acamprosate is superior to placebo.47 Disulfiram is seldom used to treat alcohol-use disorders because of its poor efficacy and hepatotoxicity.42,48
Smoking is prevalent in HIV-infected drug users. Although AIDS-related mortality has decreased with expanded prescription of ART, cardiovascular disease, which is greatly accelerated in smokers, is emerging as one the leading causes of morbidity and mortality.49 The effects of smoking in HIV-infected drug users are often compounded by the added contribution of HCV and HIV infections, and some classes of ART that lead to accelerated atherosclerosis and increased morbidity and mortality. Use of medication-assisted therapy to reduce the harm from smoking is crucial to improve health outcomes in HIV-infected drug users. Although medication-assisted therapy for nicotine dependence is better than behavioural treatments, its use in people living with HIV/AIDS has not yet been fully examined. Hence, there is an urgent need to use available therapies to reduce or stop tobacco consumption.
Comorbidities and their treatments
Viral hepatitis
Chronic hepatitis B and C are the most prevalent viral infections in IDUs, especially in those with HIV infection. Hepatitis B virus (HBV) and HCV share common routes of transmission with HIV, and therefore co-infection is frequent. Chronic HCV infection is the most common comorbidity in HIV-infected drug users, occurring in approximately 20% of all people living with HIV/AIDS and 60–90% of HIV-infected drug users. Transmission of HCV, however, is mainly parenteral because of its less efficient sexual transmission compared with HBV and HIV. Increased sexual transmission of HCV has been associated with sex with a male IDU in women50 and traumatic sex practices and concomitant ulcerative sexually transmitted infections (eg, syphilis, herpes simplex virus) in men who have sex with men.51 Chronic hepatitis B affects 10% of HIV-infected individuals worldwide, ranging from 5% in developed countries to 20% in some Asian and African regions where HBV infection is endemic. Multiple hepatic viral infections complicate care in a subset of people living with HIV/AIDS, greatly contributing to poor outcomes.
Co-infection with HIV accelerates HCV infection to end-stage liver disease. A meta-analysis of patients with HIV/HCV co-infection suggested that cirrhosis develops in around 21% of patients after 20 years of HCV infection and in around 49% after 30 years.52 With a mean injection initiation age of 17 years and HCV transmission typically occurring within the first 2 years of injection,53 morbidity and mortality related to end-stage liver disease has increased as HIV-infected drug users live longer.54 Factors contributing to accelerated progression of fibrosis in people with HIV/HCV co-infection include low CD4-cell counts, detectable HIV-1 RNA concentrations, use of hepatotoxic agents, and frequent alcohol use. HIV-infected drug users, compared with other people living with HIV/AIDS, are more likely to present with advanced disease, poorly control their HIV infection, and drink alcohol, thereby accelerating progression to end-stage liver disease.49 Effective provision of ART, despite its potential for hepatotoxity, reduces HCV progression to end-stage liver disease.55 Moreover, co-infection with HCV contributes to development or acceleration of cardiovascular disease,56 neurocognitive impairment,57 insulin resistance,58 and renal insufficiency,59 emerging comorbidities that complicate care in people living with HIV/AIDS.
Few HIV-infected drug users receive treatment for HCV infection for many reasons, including cost, physician reluctance, unsubstantiated concerns about poor treatment adherence, misperception that HCV infection is not harmful, and pessimism about tolerability and effectiveness of HCV treatment in HIV-infected drug users.60 Despite these concerns, a growing number of studies in methadone clinics,61 primary care settings,62 and prisons63 provide support for HCV treatment in HIV-infected drug users.
Therapeutic options for treatment of HCV infection are scarce and tolerance is a major obstacle, thereby creating substantial challenges (table 3). Pegylated interferon plus weight-based ribavirin treatment results in successful eradication of HCV in around 40% of co-infected patients (30% with HCV genotypes 1 or 4 and 70% with genotypes 2 or 3).64 When treatment is successful, survival in these patients is substantially improved.65 New antiviral treatments for HCV infection that offer hope of decreased treatment duration and improved efficacy are eagerly awaited, but this optimism is balanced by concerns about added toxicity, drug interactions with antiretroviral agents, and risk for selection of drug resistance to HCV therapeutics.
Table 3.
Complications related to drug use in HIV-infected injecting drug users2
| Organisms or cause | Treatment | Comments | |
|---|---|---|---|
|
Skin and soft-tissue disorders
| |||
| Cellulitis | Group A and other streptococci, Staphylococcus aureus | Antistaphylococcal and antistreptococcal agents | Requires hospital admission; consider MRSA depending on local epidemiology |
| Abscess | Same as for cellulitis | Same as for cellulitis | Incision and drainage |
| Necrotising fasciitis | Polymycrobial, clostridial infections | Parenteral antibiotics to cover both gram-positive and gram-negative organisms | Consider if crepitus noted; immediate surgical consultation needed |
| Septic thrombophlebitis | S aureus | Antistaphylococcal agents | Surgical exploration and vein ligation |
|
| |||
|
Cardiovascular disorders
| |||
| Endocarditis | S aureus, streptococci, enteric gram-negative rods | Antistaphylococcal agents until cultures grow; treat for 4–6 weeks | Consider biopsy if: (1) regurgitant murmur; (2) presence of peripheral or pulmonary emboli; (3) blood culture positive; (4) echocardiogram evidence of vegetation |
| Myocardial infarction | Substance induced; associated with vascular spasm and cocaine and amphetamine-group substance use; increased pro-inflammatory response from HIV and HCV; potential small increases from protease inhibitor-based regimens | Fibrinolytic agents and supportive care; lipid-lowering agents in those with hyperlipidaemia and smoking cessation | Drug-induced myocardial infarction associated with no evidence of endovascular stenosis on angiogram |
|
| |||
|
Pulmonary disorders
| |||
| Community-acquired pneumonia | Streptococcus pneumoniae, Haemophilus influenzae, atypical organisms | Penicillin, cephalosporin, macrolide, or tetracyclines | Treatment is typically empiric based upon local epidemiology |
| Pneumonia | Pneumocystis jirovecii | Co-trimoxazole, trimethoprim-dapsone, atovaquone, pentamidine, primaquine-clindamycin | Most common when CD4-cell count <200 cells per μL or CD4 percentage <14%; consider even with normal chest radiograph |
| Pneumonia | Mycobacterium tuberculosis | Isoniazid, rifampicin, pryzinamide, ethambutol | Treat with rifabutin due to protease-inhibitor interactions; rifampicin markedly decreases methadone and buprenorphne concentrations; start ART as soon as possible |
| Pneumonia | Atypical mycobacteria (Mycobacterium kansasii, Mycobacterium fortuitum, Mycobacterium xenopi, etc) | Antimicrobial agents dependent on specific organism | ·· |
| Pneumonia | Influenza A | Oseltamavir, zanamivir, amantadine, rimantidine | Influenza symptoms prolonged and influenza-related complications higher in HIV-infected people; yearly influenza vaccination recommended |
| Pneumonia | Influenza A H1N1 | Oseltamavir, zanamivir | ART and increased CD4-cell count associated with decreased hospital admission and mortality; H1N1 influenza vaccination recommended |
| Septic emboli | S aureus, streptococci, enteric gram-negative rods | Antistaphylococcal agents until cultures grow | Common complication, consider with pleuritic chest pain; treatment similar to endocarditis |
|
| |||
|
Liver disorders
| |||
| Hepatitis B | Hepatitis B virus | ART should be used in all co-infected patients and should include tenofovir plus lamivudine or emtricitibine; interferon occasionally for certain HBV genotypes; entecavir and telbivudine used only if on fully suppressive ART regimen | HBsAg positive; if HBV is to be treated, ART regimen should be used; exclude hepatitis D superinfection in all HBsAg-positive patients |
| Hepatitis C | Hepatitis C virus | Pegylated interferon plus weight-based ribavirin | HCV-antibody positive with detectable RNA |
|
| |||
|
CNS disorders
| |||
| Altered mental status | Substance-induced psychosis | Observation and removal of the inciting agent | Includes opioids, cocaine, amphetamine-group substances, phencyclidine, psilocibin, ketamine, MDMA, others |
| Focal brain lesions | Brain abscess and embolism | Same as for endocarditis | CNS imaging (CT or MRI) used to monitor response to therapy |
| Opportunistic infection | Toxoplasma encephalitis | Trimethoprim-sulfadiazine or trimethoprim-clindamycin | 85–95% have positive anti-toxoplasma antibody; presentation typically with focal findings (eg, seizure, altered mental status, cerebrovascular accident, etc); CNS imaging (CT or MRI) used to diagnose and monitor response to therapy |
| Opportunistic infection | Tuberculosis meningitis or encephalitis | Same as for tuberculosis except isoniazid dose increased to 600 mg per day | ART may be started cautiously, but might need serial lumbar punctures to decrease intracranial pressure associated with immune reconstitution |
| Opportunistic infection | Cryptococcal meningitis | Amphotericin B induction followed by fluconazole maintenance | ART should be initiated as soon as possible (see tuberculosis meningitis) |
| Altered mental status | Dementia | ART is recommended | Might be exacerbated by chronic drug use; must rule out all other causes; diagnosis of exclusion |
| Altered mental status | Head trauma | Can cause neurocognitive impairment or seizures | ·· |
| Neuropathy | HIV and HCV | Treatment aimed at HIV and HCV | ·· |
| Cerebrovascular accident | Substance-induced (cocaine, amphetamine-group substances) | Supportive care | Associated with 70% likelihood of developing depression |
| Cerebrovascular accident | Haemorrhage due to septic emboli | Same as for endocarditis | ·· |
|
| |||
|
Renal disorders
| |||
| Heroin or HIV nephropathy | Both present with nephrotic syndrome | Renal biopsy to establish diagnosis; electron microscopy distinguishes diagnosis; ART should be started immediately for HIV-associated nephropathy | Focal and segmental glomerular sclerosis with progression to renal failure in weeks to months |
| Glomerulonephritis | HBV, HCV | Treat underlying viral infection | ·· |
| Glomerulonephritis | Systemic bacterial infection | Same as for endocarditis | ·· |
MRSA=meticillin-resistant S aureus. HCV=hepatitis C virus. ART=antiretroviral therapy. HBV=hepatitis B virus. HbsAg=hepatitis B surface antigen. MDMA=methylenedioxymethamphetamine (rINN methylenedioxymethamfetamine).
All HIV-infected drug users needing treatment for HBV infection should be simultaneously treated with suppressive ART regimens. At the least, these regimens should include two antiviral agents that effectively treat both infections. Single anti-HBV agents such as lamivudine incompletely suppress HBV and HIV replication, thereby promoting treatment resistance. The combination of tenofovir with either lamivudine or emtricitabine is now recommended to avoid selection and transmission of lamivudine-resistant HBV.64 Few patients with HBV infection meet criteria for 1 year of interferon monotherapy, including those with highly controlled HIV infection. Entecavir, and potentially telbivudine, have modest anti-HIV activity, and must only be prescribed along with effective antiretroviral agents.66 Their use is further discouraged by the paucity of information about the pharmacodynamic interaction between the guanosine analogues entecavir and abacavir and between the thymidine analogues telbivudine and zidovudine or stavudine.64
Tuberculosis
Tuberculosis and HIV infection have been tightly linked since the early years of the HIV/AIDS epidemic. Tuberculosis has emerged as a leading cause of morbidity and mortality in HIV-infected drug users. Incidence of tuberculosis has fallen or stabilised in IDUs in many developed countries during the past three decades—possibly as a result of earlier case detection, attention to infection control, and isoniazid preventive treatment—but not in eastern Europe or countries of the former Soviet Union.67 Moreover, in many of the most populous countries of Asia, injecting drug use contributes greatly to expanding HIV epidemics and high rates of tuberculosis.
Tuberculosis-associated morbidity and mortality in HIV-infected drug users results from latent tuberculosis infection, with increasing reactivation from HIV-induced immunosuppression, and from increased transmission in crowded and poorly ventilated congregate settings (eg, prisons, drug treatment programmes, and health-care facilities). Tuberculosis reactivation in HIV-infected drug users with latent tuberculosis infection is 9% per year, by contrast with a similar lifelong risk in HIV-uninfected populations with latent tuberculosis infection. Although most cases of tuberculosis worldwide are susceptible to antituberculosis treatment, drug-resistant tuberculosis has now emerged as a growing threat.68 This problem is compounded in HIV-infected drug users because of decreased rates of completion of tuberculosis treatment, resulting in selection of drug-resistant mutants, and increased exposure to congregate settings that facilitate their transmission. WHO estimates that worldwide there are more than 500 000 incident cases of multidrug-resistant (MDR) tuberculosis (resistance to isoniazid and rifampicin) per year and 1 000 000 prevalent cases per year.69 China, India, and Russia, countries in which injecting drug use contributes greatly to HIV transmission, account for 62% of the global burden of tuberculosis drug resistance. Increasing numbers of cases of extensively drug-resistant tuberculosis (MDR tuberculosis plus resistance to second-line tuberculosis drugs) worldwide have renewed awareness of drug-resistant tuberculosis and its individual and public health impact.68 This awareness has unveiled the daunting challenges confronting successful treatment of tuberculosis (panel 1), HIV/AIDS, and substance-use disorders.70
Panel 1. Crucial issues for drug users with tuberculosis and HIV co-infection.
Intensive case finding and enhanced screening for identification of tuberculosis and HIV infection early in the course of disease
Screening for latent tuberculosis infection and provision of isoniazid prophylaxis therapy
Enhanced control of airborne infection in clinical care and other congregate settings (eg, prisons, detention centres, drug treatment programmes)
Screening of all tuberculosis patients for HIV infection
Screening of all HIV patients for tuberculosis
Training and experience with special tuberculosis diagnostic challenges in HIV co-infected patients
Recognition of the need for and implementation of adequate adherence support, including the use of directly observed therapy and linkage to supervised medication-assisted therapy (eg, methadone or buprenorphine maintenance)
Awareness of increasing rates of drug-resistant tuberculosis
Appreciation of pharmacokinetic drug interactions between treatments to effectively treat substance use, HIV infection, and tuberculosis
Promotion and development of comprehensive, collaborative, and integrated services for substance use, tuberculosis, and HIV prevention and treatment
Tuberculosis co-infection in HIV-infected drug users presents special diagnostic challenges. HIV-induced immunosuppression confounds diagnosis, reducing the usefulness of tuberculin skin testing and interferon-γ release assays; both techniques are increasingly insensitive as immunosuppression progresses. Advanced HIV co-infection confounds diagnosis of pulmonary tuberculosis because of negative sputum smears (40–60% of cases), atypical chest radiographs, and high rates of extra-pulmonary tuberculosis, which is difficult to diagnose. This difficulty is further exaggerated by poor access to care and advanced clinical presentation in HIV-infected drug users. The diagnostic capabilities of tuberculosis laboratories are deficient worldwide, adversely affecting vulnerable populations such as HIV-infected drug users. When available, tuberculosis culture and drug-sensitivity testing takes weeks to months to complete. Diagnosis of tuberculosis in HIV-infected drug users is therefore delayed, often unconfirmed, affects treatment decisions, and results in increased mortality. These challenges thereby increase opportunities for tuberculosis transmission in congregate and public settings.
Isoniazid preventive treatment of latent tuberculosis infection has proved effective in HIV-infected drug users.71 The period of prophylaxis is long, however, and is associated with increased hepatoxicity in HCV-infected patients72 and poor adherence, both concerning for HIV-infected drug users. ART also substantially reduces incidence and recurrence of tuberculosis in susceptible populations.73 Long-term use of isoniazid preventive treatment (36 months) combined with ART in people living with HIV/AIDS who do not use drugs is associated with a more than 90% reduction in incident cases of tuberculosis in individuals with positive tuberculin skin tests; however, isoniazid preventive treatment is less beneficial for patients with negative tuberculin skin tests, and might even be detrimental.74 Enhanced control of airborne infection in congregate settings prevents transmission of drug-susceptible and drug-resistant tuberculosis to HIV-infected drug users.75 Despite confirmed effectiveness of known strategies, their widespread application for HIV-infected drug users who frequent congregate settings remains limited.76
Treatment of drug-susceptible tuberculosis in HIV-infected drug users can be very successful. Early diagnosis, adherence to combination antituberculosis agents, and co-administration of ART are essential. Coordination and integration of services are crucial to ensure therapeutic success for HIV-infected drug users.77 Standard first-line tuberculosis treatment for HIV-infected drug users is the same as for the general population. Treatment of drug-resistant tuberculosis is more complicated because available agents have less potency, increased toxicities, longer treatment duration, greater cost, and limited availability. Several observational studies have shown that ART reduces tuberculosis transmission and mortality from HIV/tuberculosis co-infection. A randomised clinical trial of integrated versus sequential tuberculosis and antiretroviral treatment showed a 55% reduction in all-cause mortality in patients who were assigned to integrated treatment, supporting both early ART initiation and integration of HIV and tuberculosis care.78 Current WHO guidelines recommend initiation of ART in all people living with HIV/AIDS who have tuberculosis, irrespective of CD4-cell count, as soon as possible within the initial phase of tuberculosis treatment.79
Several challenges are associated with treatment of tuberculosis in HIV-infected drug users. These include additive toxicities, pharmacokinetic interactions, and special strategies needed for ensuring treatment success. HIV-infected drug users show increased frequencies of side-effects and toxicities from tuberculosis and antiretroviral treatments, probably resulting from the high prevalence of hepatic, renal, neurological, psychiatric, gastrointestinal and haematological comorbidities in IDUs.2 Table 4 shows pharmacological interactions between treatments for tuberculosis, HIV infection, and substance-use disorders. Rifampicin’s effect on some medication-assisted therapy and antiretroviral agents poses particular problems.
Table 4.
Common interactions between methadone and buprenorphine with treatment for HIV infection and other comorbidities7,29
| Effect on methadone | Effect on buprenorphine | Antiretroviral medication | Comments | |
|---|---|---|---|---|
|
Nucleoside reverse transcriptase inhibitor
| ||||
| Abacavir | Increased clearance of methadone | Not studied | No effect | Unclear if increase in methadone clearance is caused by abacavir; monitor for symptoms of withdrawal |
| Didanosine | No effect | No effect | Methadone decreases didanosine AUC by 57% for buffered tablet, partly corrected by enteric-coated capsule to within range in historical controls | Enteric-coated capsule recommended for patients on methadone |
| Emtricitabine | Not studied | Not studied | Not studied | No expected pharmacokinetic interactions |
| Lamivudine | No effect | No effect | Not studied | Zidovudine-lamivudine co-formulation studied only; no effect on methadone |
| Stavudine | No effect | Not studied | Decrease in stavudine AUC12 h by 23% and Cmax by 44% | Changes unlikely to be clinically significant |
| Tenofovir | No effect | No effect | Not studied | ·· |
| Zidovudine | No effect | No effect | Increase in zidovudine AUC by 40% | Watch for zidovudine-related toxicity (symptoms and laboratory) when on methadone |
|
| ||||
|
Non-nucleoside reverse transcriptase inhibitors
| ||||
| Delavirdine | Increases methadone AUC by 19%; increases Cmax by 10% | Not studied | No effect with methadone | Possibly not clinically relevant, but should be used with caution since long-term effects (greater than 7 days) unknown |
| Efavirenz | Significant effect: decrease in mean methadone AUC by 57% | Significant effect: mean decrease in buprenorphine AUC by around 50%; no clinical symptoms of withdrawal | Not studied | Opiate withdrawal common with methadone; increase in methadone dose necessary; no change in buprenorphine dose |
| Nevirapine | Significant effect: decrease in mean methadone AUC by 46% | No effect | No effect on nevirapine with methadone or buprenorphine | Opioid withdrawal symptoms common with methadone; increase in methadone dose necessary |
| Etravirine | Studied with low dose (100 mg twice a day); no effect on methadone | Not studied in human beings | No effect when combined with methadone | No dose adjustments necessary |
|
| ||||
|
Protease inhibitors
| ||||
| Amprenavir | Decreases AUC of R-methadone by 13% | Not studied in human beings | Decrease in AUC by 30% | Decrease in AUC does not seem to be clinically significant |
| Atazanavir | No effect | When atazanavir combined with ritonavir, increased buprenorphine concentrations | No effect with methadone or buprenorphine | Oversedation possible with atazanavir-ritonavir; titrate buprenorphine dose slowly |
| Darunavir | AUC, Cmax, and Cmin decrease by 24–40% | Norbuprenorphine, but not buprenorphine AUC increases by 46%; no clinical symptoms | No effect of methadone on darunavir | Darunavir might precipitate opioid withdrawal symptoms in patients on methadone |
| Fosamprenavir | S-methadone but not R-methadone concentrations decreased | Not studied in human beings | Not studied | No clinically significant interactions reported |
| Indinavir | No effect | Not studied | Decreased Cmax between 16% and 28% and increased Cmin between 50–100% | Differences do not seem to be clinically significant |
| Lopinavir-ritonavir | Decreases methadone AUC by 26–36% | No effect | No effect by methadone or buprenorphine on antiretroviral drugs | Decrease in AUC of methadone caused by lopinavir; one study reported opioid withdrawal symptoms in 27% of patients; increase in methadone dose might be necessary in some patients |
| Nelfinavir | Decreases methadone AUC by 40% | Not studied | Decrease in AUC of active M8 metabolite by 48% but not on nelfinavir itself when combined with methadone | Despite decrease in methadone AUC, clinical withdrawal is usually absent and a priori dose adjustments are not needed; decrease in AUC of M8 unlikely to be clinically significant; TDM might be useful in patients with good adherence and virological failure |
| Ritonavir | Decreases methadone AUC by 37% in one study and no effect in another (see text) | Not studied | Not studied | No dose adjustment necessary |
| Saquinavir | Decreases methadone AUC by 20–32% | Not studied | Not studied | Saquinavir boosted with ritonavir studied; despite decrease in methadone AUC, clinical withdrawal was not reported |
| Tipranavir | Decreases methadone by 50%* | No effect | Buprenorphine decreases tipranavir concentrations by 19–25% | Methadone might need to be increased; no change in buprenorphine dose needed; TDM of tipranavir possibly needed when given with buprenorphine |
|
| ||||
|
Integrase inhibitors
| ||||
| Raltegravir | Methadone AUC unchanged when co-administered with raltegravir | Not studied; common metabolic pathway with UGT1A1 | No significant interactions with ART drugs | Titrate buprenorphine dose slowly |
|
| ||||
|
Entry inhibitors
| ||||
| Enfuvirtide | Not studied | Not studied | Not studied | No interactions anticipated; enfuvirtide given intramuscularly |
| Maraviroc | Not studied | Not studied | Not studied | ·· |
|
| ||||
|
Other common treatment drugs for HIV-related comorbidities
| ||||
| Rifampicin | Decreases methadone AUC by 30–65%; 70% of patients on methadone developed withdrawal symptoms 1–33 days after receiving rifampicin | Not studied in human beings | Substantial reduction in concentrations of all protease inhibitors, raltegravir, enfuvirtide, and nevirapine | Should not be combined with protease inhibitors or nevirapine; clinical pharmacodynamic studies support efavirenz given at 600 mg or 800 mg per day; raltegravir dose should be increased to 800 mg twice a day |
| Rifabutin | No significant interaction | Not studied in human beings | Protease inhibitors significantly increase rifabutin concentrations | In patients requiring a protease inhibitor, the rifabutin dose should be decreased to 150 mg thrice weekly |
| Ciprofloxacin | Not studied in human beings | Not studied in human beings | No significant change with enteric-coated didanosine formulation | No expected interaction |
| Ofloxacin | Not studied in human beings | Not studied in human beings | No significant interactions | No expected interaction |
| Clarithromycin | Not studied in human beings | Not studied in human beings | Atazanavir increases clarithromycin concentrations by 50% and can cause QT prolongation; darunavir, tipranavir, and lopinavir increase clarithromycin concentrations and increase side-effects; efavirenz and nevirapine decreases clarithromycin AUC by 39%; efavirenz associated with increased rash; fluconazole increases clarithromycin concentrations and is associated with QTc prolongation; clarithromycin increases maraviroc and saquinavir concentrations | Adjust clarithromycin with lopinavir or darunavir only if renal insufficiency; use azithromycin instead of clarithromycin when given with efavirenz, etravirine; decrease clarithromycin dose by 50% with atazanavir |
| Azithromycin | Not studied in human beings | Not studied in human beings | No change in azithromycin AUC; Cmax increased by 22% | Preferred over clarithromycin |
| Fluconazole | Increases methadone AUC levels by 35%; no signs or symptoms of opioid excess | Not studied in human beings | Several interactions, but not of clinical significance | No clinical need for dose adjustment |
| Pegylated interferon alfa | No interactions | Not studied in human beings | No interactions | No interaction with methadone; no expected interactions with buprenorphine or ART |
| Ribavirin | Not studied in human beings | Not studied in human beings | ·· | No interaction with methadone |
| Telbivudine | Not studied in human beings | Not studied in human beings | Not studied, might need caution with other thymidine analogues | Renally cleared; no expected interactions with methadone or buprenorphine |
| Entecavir | Not studied in human beings | Not studied in human beings | Not studied, might need caution with other guanosine analogues; use with ART that is virologically suppressive | Renally cleared; no expected interactions with methadone or buprenorphine |
|
| ||||
|
Common psychiatric medications
| ||||
| Amitriptyline | Increases methadone concentrations (via decreased clearance) | Not studied in human beings | Increased amitriptyline concentrations (dry mouth, hypotension, confusion) | Dry mouth, hypotension, confusion; monitor and adjust amitriptyline as indicated |
| Citalopram | No clinically signficant interaction | No clinically signficant interaction | Not studied in human beings | ·· |
| Desipramine | Associated with increased desipramine concentrations | Not studied in human beings | Desipramine levels decreased by 59% | Start with lower desipramine and monitor and adjust desipramine clinically |
| Duloxetine | May lead to increased duloxetine concentrations, not studied in human beings | Not studied in human beings | Not studied in human beings | ·· |
| Sertraline | No clinically signficant interaction | No clinically signficant interaction | Darunavir decreases sertraline AUC by 50% | Might need to titrate sertraline dose upwards |
| Mirtazapine | Not studied in human beings | Not studied in human beings | Not studied in human beings | No expected interactions |
| Fluvoxamine | Increases methadone concentrations; discontinuation associated with precipitation of opioid withdrawal symptoms | Not studied in human beings | Not studied in human beings | Monitor for symptoms of opioid excess and withdrawal depending on initiation and stopping of duloxetine, respectively |
| Fluoxetine | Might increase methadone concentration | Not studied in human beings | Increase in ritonavir AUC by 19% | No dose adjustment necessary |
| Haloperidol | Not studied in human beings | Not studied in human beings | Not studied in human beings | ·· |
| Risperidone | Decreases methadone concentrations | Not studied in human beings | Not studied in human beings | Monitor for symptoms of opioid withdrawal |
| Aripiprazole | Not studied in human beings | Not studied in human beings | Not studied in human beings | No anticipated interactions |
| Olanzapine | Not studied in human beings | Not studied in human beings | Ritonavir decreases olanzapine AUC by 50% | Increase olanzapine dose to symptoms |
| Quetiapine | Results in increased methadone concentrations | Not studied in human beings | Not studied in human beings | Monitor for symptoms of opioid excess |
| Carbamazepine | Decreases methadone concentrations; precipitates opioid withdrawal symptoms | Not studied in human beings | Decreases concentrations of many antiretroviral drugs and should be avoided when possible | Symptoms of withdrawal reported; monitor for symptoms of opioid withdrawal |
| Lamotrigine | No clinically signficant interaction | Not studied in human beings | Lopinavir and ritonavir decrease lamotrigine concentrations | ·· |
| Topiramate | Not studied in human beings | Not studied in human beings | Not studied in human beings | None expected; not hepatically metabolised |
| Valproic acid | No clinically signficant interaction | Not studied in human beings | Lopinavir, tipranavir, and ritonavir decrease valproic acid concentrations and valproic acid increases lopinavir concentrations | ·· |
| Diazepam or midazolam | Increase methadone concentrations; associated with increased sedation | No effect | Should avoid or use carefully with most boosted protease inhibitors | Monitor for symptoms of opioid excess |
See text for references. AUC=area under curve. AUC12 h=area under curve from 0 h to 12 h. Cmax=maximum concentration. Cmin=minimum concentration. TDM=therapeutic drug monitoring. UGT1A1=UDP-glucuronosyltransferase 1A1. ART=antiretroviral therapy.
Decrease in methadone not specified as AUC or Cmax.
Outcomes of tuberculosis treatment are less favourable in HIV-infected drug users than in other populations. Apart from the previously noted challenges, problems in health-care structure and patient behaviour can confound treatment success. Treatment of substance-use disorders is often a prerequisite for successful HIV and tuberculosis treatment, but lack of access to medication-assisted therapy is common in settings where these comorbidities occur. Poor adherence to treatment, decreased retention in care, and poor continuity of care between community and prison settings frequently occurs, with increasing risk for tuberculosis and HIV drug resistance. This, in turn, reduces treatment success for both diseases in individual patients and results in transmission of resistant organisms to other IDUs and the general population.
Increased stigma associated with multiple comorbidities and reluctance of IDUs to use traditional health-care settings often result in delayed entry into care and treatment. As a result, WHO advocates intensified tuberculosis case-finding in IDUs in non-medical settings. In addition to medication-assisted therapy, adherence support, directly observed therapy (DOT), and integrated health-care delivery are strongly recommended and necessary to ensure long-term adherence and reduced morbidity and mortality.70,77
Bacterial and other infections
Bacterial infections cause substantial morbidity and mortality in HIV-infected drug users, accounting for up to 25% of deaths.80 These infections are frequently associated with syringe reuse, non-sterilisation of the injection site, injection of crack cocaine, or injection into sites other than the arm.81 Injection breaches the natural integument defences, increasing risk for vascular and soft-tissue injuries by exposure to pathogens that can cause localised or systemic infections. Commensal staphylococcal and streptococcal species are predominantly involved. High rates of staphyloccus colonisation in HIV-infected drug users, including meticillin-resistant strains, contribute to the high prevalence of bacterial infections in IDUs.82 Meticillin-resistant Staphylococcus aureus bacteraemia has increased substantially in HIV-infected patients, and is associated with injecting drug use, end-stage renal disease, and low CD4-cell counts.83 Poor hygiene, injection of non-sterile preparations, and poor technique predispose HIV-infected drug users to infections with other bacterial pathogens such as Pseudomonas spp, Clostridium spp, and Candida spp.84 Fatal outbreaks of botulism, tetanus, and fungal infections caused by injection of contaminated heroin have also been reported.85,86
Bacterial infections range from the more prevalent localised skin and soft-tissue infections to less common deep-seated infections including pyomyositis, septic arthritis, osteomyelitis, and endocarditis.87 IDUs who develop endocarditis are more likely to have tricuspid valve involvement, but aortic or mitral valve involvement and HIV infection are associated with increased rates of hospital admission,88 morbidity, and mortality.89 Clonal outbreaks of invasive group A streptococcal infection in Europe resulted in life-threatening infections in IDUs.90 A Spanish surveillance study associated these outbreaks with specific environmental factors (drug-purchase site or dealer, homelessness) and injection-related factors (injection frequency, sharing equipment).91 The cost of treating and managing injection-related skin and soft-tissue infections and their complications is substantial.92 IDUs in the USA who sought care for skin and soft-tissue infections had an increased risk for subsequent hospital admission and death.93 Visits for skin and soft-tissue infections therefore represent missed opportunities for preventive care for IDUs.
Concomitant drug use can lead to additional complications to many organ systems, resulting in increased morbidity and mortality and complicating treatment (table 2). ART combined with co-trimoxazole for pneumocystis prophylaxis reduces the rate of bacterial infections, particularly pneumonia, but not injection-related infections,94 which are reduced only by alcohol disinfection of skin. Injection-related infections can result in endocarditis (and other endovascular infections), resulting in cardiac decompensation and peripheral embolisation, which can in turn damage kidneys, brain, and limbs.
Non-HIV-related complications
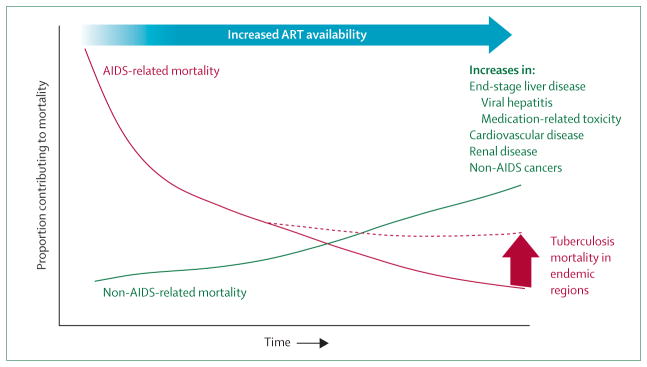
Chronic kidney disease is increased in HIV-infected drug users and has infectious (eg, HBV, HCV) and non-infectious causes, including direct medication-associated kidney damage and heroin-related nephropathy. Ultimately, chronic kidney disease complicates treatment for HIV infection, HCV infection, and tuberculosis because laboratory monitoring is limited and some medications must be renally adjusted or precluded altogether. Cocaine and amphetamine-group substances increase blood pressure and vascular spasm of the coronary and cerebral arteries, resulting in myocardial infarction and cerobrovascular accidents. Atherosclerosis that results in increased cardiovascular disease is reduced by ART, but is accelerated by chronic inflammation from HIV and HCV infections and by some protease inhibitors.56 Non-AIDS malignant diseases are emerging as ART successfully averts development of opportunistic infections. Infection-related cancers (anal and liver cancer, Hodgkin’s lymphoma) and non-infection-related cancers (lung and skin cancers) are increasingly reported.95 Cohort studies undertaken in Europe and North America, where ART is readily available, have highlighted that non-AIDS complications are emerging as leading causes of mortality in people living with HIV/AIDS.96 These cohorts insufficiently represent HIV-infected drug users or other groups associated with substantial tuberculosis-related mortality. Therefore, they markedly under-represent the expected burden of these complications where the HIV epidemic is driven by IDUs, especially in eastern Europe and Asia (figure). These non-AIDS comorbidities are already straining health-care delivery in these regions and are likely to worsen with time.
Figure. Emerging medical comorbidity and mortality in HIV-infected drug users.
This graph depicts the relative proportion of comorbidities over time if antiretroviral therapy (ART) is widely accessed. ART results in marked reductions in AIDS-related opportunistic infections and malignant diseases. Although ART also decreases several non-AIDS-related morbidities through a reduction in cytokine production and inflammatory responses, over time these comorbidities will increasingly contribute to mortality, but not as profoundly as mortality before availability of ART.
Mental illness
Mental illness and substance-use disorders are closely inter-related with HIV infection and concentrated further among prisoners.97 Individuals with all three diagnoses are likely to engage in high-risk behaviours,98 and when untreated, continue to fuel the HIV epidemic. Untreated mental illness results in worse outcomes for treatment of HIV infection and substance-use disorders. Mental illness should be distinguished from neurocognitive impairment that can masquerade as mental illness, result in similar adverse outcomes, and complicate treatment.99 Successful therapy requires screening, diagnosis, and treatment of all comorbidities. For this reason, it is essential to ensure a comprehensive and integrated approach.
In addition to routine screening and best possible treatment of mental illness, it is essential to recognise any potential drug interaction that can arise as a consequence of shared metabolism with medication-assisted therapy (table 4). Some pharmacokinetic and pharmacodynamic evidence exists to guide practitioners. Most selective serotonin-reuptake inhibitors and tricyclic antidepressants can be safely given with medication-assisted therapy, although many are associated with decreased metabolism of methadone without any identified clinical sequelae. Abrupt discontinuation of fluvoxamine, however, has been associated with precipitation of opioid withdrawal symptoms in methadone-maintained patients. Carbemazepine, a mood stabiliser, reduces serum concentrations of methadone (and many antiretroviral drugs) by 60% and might precipitate opioid withdrawal symptoms.
Pharmacokinetic drug interactions between treatments for HIV infection, HIV-related comorbidities, and substance-use disorders
As the number of HIV-infected drug users enrolled in medication-assisted therapy expands, so too must the knowledge of drug interactions that occur between medication-assisted therapy, ART, and medications to treat comorbidities (table 4).11,29 This knowledge is crucial because medication-assisted therapy might alter metabolism of antiretroviral drugs and other medications, resulting in increased toxicity or reduced effectiveness. Alternatively, these other medications might alter the concentrations of medication-assisted therapy, resulting in clinical opioid withdrawal or overdose. When combining medications with known or potential pharmacological interactions, the monitoring of signs of opioid withdrawal or opioid excess and appropriate adjustment of methadone dosing is central for competent care. Several common medications, including rifampicin, nevirapine, and efavirenz, are potent inducers of cytochrome P450 isoenzymes. They can therefore precipitate opioid withdrawal symptoms. In such situations, dosing for medication-assisted therapy must be rapidly escalated to retain patients in treatment. Alternatively, medications such as rifabutin should be substituted for rifampicin to avoid precipitation of opioid withdrawal symptoms; when co-administered with protease inhibitors, the rifabutin dose should be reduced to 150 mg thrice weekly.29
Improving adherence to treatment in HIV-infected drug users
Table 5 provides an overview of 13 interventions that examine adherence to treatment in IDUs. The range of interventions is diverse and includes cues and reminders, adherence counselling, contingency management, supervised therapy, medication-assisted therapy, and integrated health services delivery.
Table 5.
Studies of interventions for adherence to treatment for HIV infection, tuberculosis, and substance use in people who use drugs
| Medical intervention | Study design | Treatment setting | Population | Type of intervention | Description of intervention group | Description of comparison group | Primary outcomes | |
|---|---|---|---|---|---|---|---|---|
| Chaisson et al (2001)100 | Isoniazid prophylaxis for latent tuberculosis | RCT, 3×2 factorial design | Community setting | 300 IDUs in Baltimore, MD, USA, positive TST, no active tuberculosis; 150 patients in intervention group | Contingency management | US$10 stipend for maintaining adherence (in three groups—peer, supervised therapy, self-administered therapy) | No stipend | Completion rates: contingency management, 83%; control, 75%; NS. Proportion taking 100% of treatment: 77% (DOT) |
| Chaisson et al (2001)100 | Isoniazid prophylaxis for latent tuberculosis | RCT, 3×2 factorial design | Community setting | 300 IDUs in Baltimore, MD, USA, positive TST, without active tuberculosis; 99 patients in DOT intervention group and 101 patients in peer intervention group | Supervised therapy and peer group intervention | Twice-weekly DOT by professional outreach workers or peer counselling and education | SAT as standard of care | Completion rates: DOT, 80%; peer, 78%; control, 79%; NS. Proportion taking 100% of treatment: DOT, 77%; peer, 6%; control, 10%; p<0·001 |
| Batki et al (2002)101 | Isoniazid prophylaxis for latent tuberculosis | Three-group RCT | Drug treatment programme | 111 opioid-dependent IDUs in San Francisco, CA, USA | Supervised therapy plus counselling | 6 months of DOT within MMT 7 days per week plus twice-monthly substance-use counselling, social work referrals, and urine toxicology screens | MMT without counselling; routine referral without MMT to tuberculosis programme | Isoniazid completion rates: MMT plus counselling, 77%; MMT alone, 60%; routine care, 13% |
| Kinlock et al (2009)25 | MMT | Three-group RCT | Correctional facility | 211 opioid-dependent prisoners with history of heroin use in Baltimore, MD, USA | Supervised therapy (methadone) plus counselling | MMT in prison with continued care upon release | Counselling only; counselling plus transfer to MMT upon release | Opioid-free urine at 1 month post-release: counselling, 37%; counselling plus MMT on transfer, 59%; counselling plus MMT in prison and upon transfer, 72% |
| Macalino et al (2007)102 | ART | RCT | Community setting | 87 active substance users in Providence, RI, USA; alcohol, cocaine, or heroin; 44 in intervention group | Supervised therapy (DAART) | DAART by an outreach worker for 3 months | SAT as standard of care | End-treatment HIV-1 <400 copies per mL: DAART, 64%; control, 41%; p<0·05 |
| Altice et al (2007)103 and Maru et al (2009)104 | ART | RCT | Community setting | 141 active drug users in New Haven, CT, USA; cocaine or heroin users, on no more than twice-daily ART; 88 in intervention group | Supervised therapy (DAART) | DAART 5 days per week by an outreach worker via a mobile health unit for 6 months | SAT as standard of care | End-treatment virological success: DAART, 71%; control, 54%; p<0·05. 6 months after intervention stopped: DAART, 58%; control, 56%; p=0·64 |
| Arnsten et al (2009)105 | ART | RCT | MMT programme | 77 patients in Bronx, NY, USA; all on stable methadone dose; 39 in intervention group and 38 in control group | Supervised therapy (DAART) | DAART provided within 12 outpatient methadone clinics; received supervised therapy once a day with methadone for 6 months | SAT as standard of care | DAART vs control had significantly higher adherence (86% vs 56%) and higher viral suppression defined as HIV-1 RNA <75 copies per mL (72% vs 45%); p=0·001 for both |
| Lucas et al (2006)106 | ART | Longitudinal cohort | MMT programme | 82 IDUs in Baltimore, MD, USA; compared with groups of 75 methadone IDUs, 244 non-methadone IDUs, 490 non-IDUs | Supervised therapy (DAART) | DAART provided within an outpatient methadone programme; received supervised therapy once a day with methadone for 12 months | SAT as standard of care | End-treatment viral load <400 copies per mL: DAART, 56%; control, 32%; p<0·01 |
| Lucas et al (2004)107 | ART | Longitudinal cohort | MMT programme | 40 drug users receiving methadone for >30 days in Baltimore, MD, USA; compared with 33 DAART recipients | Adherence counselling | Adherence support group that included case management, nursing education and medication tailoring, group education, and peer advocacy | DAART through an outpatient MMT programme | Viral load <400 copies per mL at 6 months on therapy: DAART, 56%; control, 32%; p<0·01 |
| Sorensen et al (2007)108 | ART | RCT | MMT programme | 66 drug users in an outpatient methadone programme on ART for at least 1 month | Contingency management | Medication counselling plus financial incentive for each MEMS-verified appropriate pill ingestion for 12 weeks, starting at US$1 per day and ramping up; total possible compensation $1172 | Medication counselling alone | On-treatment mean adherence: contingency management, 78%; control, 56%; p<0·0001 |
| Moatti et al (2000)109 | ART | Case control study within a longitudinal cohort | Primary care setting | 54 opioid dependent patients on buprenorphine in France; 19 active IDUs not on buprenorphine | Buprenorphine treatment | Outpatient buprenorphine maintenance therapy | Not receiving OST | Odds ratio of 4·91 (95% CI 1·2–20·8) of active IDU not on OST compared with those on OST |
| Purcell et al (2007)110 | ART | RCT | Community setting | 966 HIV-infected IDUs in four US cities | Peer-group intervention | Ten sessions of a peer mentoring programme over 12 months | Eight sessions of an adherence video | No differences in adherence measures at 3, 6, or 12 months; control group was an active intervention |
| Avants et al (2004)111 and Margolin et al (2003)112 | ART | RCT | MMT programme | 90 HIV-infected drug users in New Haven, CT, USA | Risk reduction and adherence counselling | 12-session HHRP intervention over 6 months | Harm-reduction counselling | Both groups reduced HIV risk behaviours; HHRP improved ART adherence and abstinence from illegal drugs |
IDUs=injecting drug users. TST=tuberculin skin test. NS=not significant. DOT=directly observed therapy. SAT=self-administered therapy. RCT=randomised controlled trial. MMT=methadone maintenance therapy. DAART=directly administered antiretroviral therapy. ART=antiretroviral therapy. MEMS=medication electronic monitoring system. OST=opioid substitution therapy. HHRP=Holistic Health Recovery Program.
Several aids and reminders have been developed to improve adherence to pharmacotherapeutics: beepers and alarms, blister packs, pill boxes, and calendars.113,114 Although they provide only a modest effect on adherence, they can be useful for patients for whom a major reason for missed doses is “forgetting” because of their lifestyle, comorbid mental illness, or HIV-associated neurocognitive impairment. For active drug users, use of injection cues linked to pill-taking has improved adherence and HIV treatment outcomes.115 These methods are inexpensive, making it feasible to integrate them with other adherence interventions.
Adherence counselling can modestly improve adherence by changing patients’ attitudes and beliefs about medical treatment and thus altering behaviour. Counselling strategy interventions, based on distinct theoretical frameworks, including social action theory, social cognitive theory, the information-motivation-behavioural skills model, and the health belief model, show equivocal efficacy in HIV-infected drug users, and few randomised clinical trials have been done in this population. Two trials that did not target drug users reported a benefit from strategy interventions,116 while four additional ones that either included HIV-infected drug users as the entire sample107,117,118 or as a subsample119 did not. In one study that compared a peer mentoring intervention consisting of ten sessions with a video discussion intervention consisting of eight sessions, adherence improved in both groups.110 In the only study in HIV-infected drug users, 12 sessions of the Holistic Health Recovery Program reduced HIV risk behaviours and improved adherence.111,112 Therefore, although data from the general HIV-infected population suggest usefulness of educational counselling, the benefits specifically to HIV-infected drug users are less well supported. Moreover, continuation of counselling interventions beyond the intervention remains an important challenge and data do not support sustained benefits.120,121
Contingency management has its roots in mental health treatment, where it has been used for the management of several substance-use disorders.122 Participants are rewarded for positive health behaviours and a series of sanctions are imposed for negative ones. Such interventions can take the form of financial compensation,100 vouchers,108 and positive and negative reinforcing medications (eg, methadone).108
Primary financial incentives for isoniazid preventive therapy failed to improve isoniazid adherence and retention,100 but these were improved by combining isoniazid with methadone administration.101 Similarly, combining disulfiram for treatment of alcohol-use disorders with methadone has also shown promise. Contingency management has also been applied to adherence to HIV treatment. In a randomised controlled trial in HIV-infected drug users, adherence to ART increased by 20% when patients receiving methadone earned money vouchers; however, the benefit was not sustained after the intervention ended.108 This strategy has raised concerns from policy makers that patients should not receive payments for engaging in health-promoting behaviours, despite the cost-effectiveness of these approaches at the societal level.123
Supervised treatment has been shown to be effective in several longitudinal cohort studies and randomised controlled trials and is a major component of the global strategy to control tuberculosis.124 Despite one review suggesting no benefit from supervised treatment (there was remarkable heterogeneity of DOT interventions) in 5609 patients from 11 studies,125 DOT remains central to tuberculosis control efforts by WHO. The ancillary and social support services that often accompany DOT are believed to contribute most to the success of the approach rather than the observation of treatment itself.125
To address concerns about poor adherence, DOT has been adapted for use with HIV treatment as directly administered antiretroviral therapy (DAART).126 One meta-analysis of randomised DAART trials, undertaken mainly in people who do not use drugs, did not substantiate the benefit of DAART.127 A more recent meta-analysis that included longitudinal studies, however, confirmed that DAART substantially improved virological, immunological, and adherence outcomes, especially when targeting drug users and others with increased risk for non-adherence.128 Data from feasibility pilot studies115,129–132 case-control studies,106,107 and randomised controlled trials,102,103,105 however, lend support to implementation of DAART in HIV-infected drug users. The success of DAART, as with DOT for tuberculosis, relies on sufficiently trained and motivated staff to deliver the services and develop trust.26 Ancillary services provided by DAART staff, such as social support and case management services, improve HIV treatment outcomes.26 Although DAART has not proven sustainable after fixed time periods,103,104 the opportunities for implementing DAART in the long term within other venues of supervised therapy (eg, DOT for tuberculosis or medication-assisted therapy) could prove to be more durable.
Medication-assisted therapy—mainly the prescription of buprenorphine or methadone for opioid dependence—is essential for accessing care and improving adherence in HIV-infected drug users.70 WHO, UNAIDS, and the UN Office on Drugs and Crime each support expansion of medication-assisted therapy133 for its health-promoting medical and social benefits, including cost-effectiveness and increased ART uptake, retention on and adherence to ART, and the resulting reductions in HIV-1 RNA levels that are associated with reduced morbidity in HIV-infected drug users.70,106,134
Similarly, buprenorphine has a direct effect of improving adherence to ART and other HIV treatment outcomes. In HIV-infected drug users in France,109 long-term retention on buprenorphine or methadone was associated with increased suppression of HIV-1 RNA levels compared with no medication-assisted therapy. Although methadone seemed to be more effective, the non-randomised study design did not allow for direct comparison between these prescribed medications.135 In a larger study of HIV-infected drug users, prescription of buprenorphine was associated with increased receipt of ART and improvements in CD4-cell count during a 12-month period.136
Additional medical and social services not directly related to adherence can substantially affect the effectiveness, cost-effectiveness, and scalability of adherence interventions. This association is related to underlying social instability, homelessness, and poverty that can affect adherence.137 Ancillary services, such as case management, can enhance adherence through coordination of primary health care, social services, and social support.138,139 One randomised controlled trial in a population consisting mainly of HIV-infected people who did not use drugs, however, did not lend support to the benefits of case management on HIV treatment outcomes.140 Such studies need confirmation in HIV-infected drug users and others with known poor adherence.141,142 Alternatively, ancillary services can take the form of integrated services within a general primary care or supervised therapy programme, although cost-effectiveness has yet to be confirmed.143
WHO guidelines for the management of HIV infection and tuberculosis in HIV-infected drug users support integration of services (panel 2).70 Colocation of medical care and drug treatment services for HIV-infected drug users144 and integrated mobile health-care services138 result in improved health outcomes, which are particularly evident when social support is included as part of DOT programmes for treatment of tuberculosis145 and HIV infection.26
Panel 2. Case study from Mykolaiv, Ukraine.
Dima* is in his 40s and is a welder at one of the local shipbuilding yards. He was diagnosed with HIV infection after leaving prison 8 years ago when he had pneumonia and has been using “shirka” (liquid opioid derived from poppy straw) for more than 20 years.
“The only reason I’m not in the cemetery like all of my friends is that I’m tough like the steel I weld. Honest, I like my doctors. I go crazy though when they all tell me different things. I’ve been in and out of the hospitals at both the TB [tuberculosis] and AIDS centres for the past 3 years and no one seems to agree with each other. The HIV doctor hospitalises me and treats me for TB and then they transfer me to the TB hospital where they then tell me they can’t find TB and stop all my medications. All I know is that most of the time when they give me the TB medications, my fevers go away and I gain weight.
The last time I came to the hospital with the fevers, the medications didn’t work. I’m sure part of it is my fault. For the past 2 years, I was lucky enough to get methadone after they allowed me to get my driver’s licence back. Every time I would be hospitalised, I would go ‘cold turkey’ and get really sick because they said they could not bring the methadone from the narcology centre to the hospital. I finally got fed up with getting sick and just left. Just a few months ago, they also started giving me my methadone in the TB and AIDS centres. The only problem is that when I took the TB medications, they made me feel really dope sick, worse than I did when I came off the shirka. My doctors are afraid to raise my methadone dose because they tell me I take too many pills already and that it will hurt my liver.
I’m finally doing better these days. I think it’s because I’m working really hard and making enough money to pay for my TB medications. The ones I take now are new and I have to pay for them. They don’t eat up my methadone either. Thankfully, I have a good-paying job that lets me buy them. I decided to pay for them myself rather than go back to the TB hospital where all they do is stop them. I was tired of going back and forth and I just want to get treated and feel better. I didn’t have a choice. All my friends who I knew who went back and forth to the two hospitals are now in heaven. I’m not ready for that yet. I think my job and my family is the only thing that saves me.
The HIV doctors tell me I have AIDS now and that my T cells are low. I want to take the HIV cocktail that other guys get, but I’m told that I have to wait because the HIV medications will make me sick, be too much for my liver and eat up my methadone like my other TB medications did. Maybe if I offer to buy the HIV medications like I did the TB medications, they will give them to me. I just don’t want to die like all my friends did. I have a wife and kids to take care of. If I get sick or die, there is no one to help them.”
*Name changed for confidentiality.
The criminal justice system and HIV-related comorbidity
The high rate of HIV infection in prisoners worldwide is directly associated with society’s approach to controlling illicit drug use. Mass incarceration of drug users has resulted in high rates of HIV/AIDS, tuberculosis, and other comorbidities in prisons compared with surrounding communities.146 Although the single most important strategy in controlling HIV in prison is to reduce the rate of incarceration,147 ample evidence suggests that the criminal justice system can be an effective place to identify and treat people with HIV infection when appropriate services are available.148
Incarceration increases risk of HIV infection through several factors, including disruption of social networks,149,150 multiple partners, and high-risk sexual behaviour;151 the reduced likelihood of gaining meaningful employment or entrance into social service and rehabilitation programmes is also a factor. Inadequate treatment of mental illness and lack of social support put prisoners at a greater risk of substance-use disorders and reincarceration.152 Most incarcerated HIV-infected drug users have restricted or no access to ART or medication-assisted therapy within these settings. HIV-infected drug users who have access to ART show clinical improvement while incarcerated, but recidivism and poor continuity of care upon release result in deteriorating clinical outcomes.153
When widescale ART is prescribed in prison settings, prison-related mortality from HIV infection achieves parity with the community.154 This finding is partly because of reduced drug use and supervised therapy, substantiated by higher rates of HIV suppression in prisoners who receive DAART. HIV-infected drug users in Italian prisons who received DAART were more likely to achieve HIV suppression (62% vs 34%) and increase their CD4-cell count above 200 cells per μL (95% vs 68%) than were those who self-administered their antiretroviral medications.155 US prisoners who received DAART had a two-fold greater reduction in mean HIV-1 RNA levels compared with those who did not.153 In Spain, ART adherence levels in HIV-infected prisoners improved while they were incarcerated.156 DAART in prisons, however, is not effective when supervised by non-medical professionals such as guards.140 Also, antiretroviral treatment benefits have not been confirmed to persist beyond the period of incarceration. Three studies, two from the USA153,157 and one from Canada158 have shown that adherence rates and virological outcomes in HIV-infected individuals improve while in prison, but these benefits are lost upon release.153,157
Medication-assisted therapy, provided as methadone or buprenorphine, has been implemented in prisons in at least 29 countries or territories; however, as few as 1–14% of eligible prisoners were prescribed it because of the pilot nature of these programmes.159 The largest randomised trial of 204 HIV-undifferentiated opioid-dependent prisoners compared pre-release methadone with community linkages with pre-release counselling with or without active referral to methadone. Prisoners receiving pre-release methadone were substantially more likely to be retained in drug treatment and to remain abstinent from opioids and cocaine than were those in the other two groups.25 The only study in HIV-infected drug users receiving ART in prison who initiated buprenorphine upon prison-release confirmed retention in care and sustained CD4-cell counts and HIV-1 RNA concentrations 3 months after release, suggesting the potential for medication-assisted therapy in HIV-infected drug users.160 Despite a large body of evidence, very few medication-assisted therapy programmes that help to reintegrate incarcerated HIV-infected drug users into the community have been developed.161
Incarceration itself is detrimental to disease control programmes for HIV-infected drug users, especially the increased transmission of tuberculosis and drug-resistant tuberculosis.67,147,162 Nevertheless, since HIV-infected drug users have such a high risk of imprisonment, these congregate settings are important for diagnosis, treatment, and implementation of adherence interventions. When appropriately organised, time-restricted treatment for tuberculosis163 and HCV infection164 has been effectively implemented within prisons. Unfortunately, most treatments for infectious diseases in HIV-infected drug users need effective transitional care to the community.
Important public health funds diverted towards prisons that house largely non-violent off enders are wasted on cost-ineffective programmes. Annual costs of treating addiction are five times less than costs of incarceration,159 and research suggests that rehabilitation programmes are more effective than incarceration for non-violent off enders.165,166 This finding is particularly true for non-violent off enders with several treatable comorbidities such as HIV infection, mental illness, and substance-use disorders.97 Approaches needed to abrogate the role of prisoners in the HIV epidemic are provided in panel 3. Until these problems are solved, there will remain crucial health disparities among HIV-infected drug users who move in and out of the criminal justice system and either do not receive effective therapy for their comorbidities or have treatment interrupted, potentially resulting in the development of resistance to HIV and tuberculosis treatments.
Panel 3. Essential strategies for the criminal justice system to improve the health of HIV-infected drug users.
Initiate and expand access to medication-assisted therapies for alcohol and drug dependence within communities, but also introduce and coordinate them with the criminal justice system
Engage the criminal justice system and police to work on solutions to the drug problem that effectively balance public safety and public health
Advocate for legislative changes to decriminalise drug use, promote alternatives to incarceration for non-violent off enders, and remove mandatory minimum sentences for drug possession
Encourage activism by communities affected by the incarceration epidemic, as has been achieved in drug users in southeast Asia and northern Europe
For HIV-infected drug users who are imprisoned, ensure timely provision of evidence-based treatments for HIV infection, tuberculosis, viral hepatitis, mental illness, and substance-use disorders
Develop effective transitional programmes within the criminal justice system that promote continuity of care for treatment of HIV infection, tuberculosis, viral hepatitis, mental illness, and substance-use disorders
Conclusions
HIV-infected drug users have substantial HIV-related and non-HIV-related medical and psychiatric comorbidities. As a result, care is often complicated for the individual and for the health-care system. Several evidence-based interventions are available to improve treatment outcomes for this vulnerable population, but parity in treatment outcomes to reduce morbidity and mortality in HIV-infected drug users will be achieved only with further resources, expertise, political will, and commitment by the health-care establishment.
Supplementary Material
Acknowledgments
We acknowledge funding from the National Institutes on Drug Abuse for career development (K24 DA017072, FLA) and research support for FLA (R01 DA013805, R01 DA017059, and R01 DA025943) and GHF (R01 DA025932) that contributed substantially to the creation of this report.
Footnotes
Contributors
FLA was responsible for the first draft and integration of all sections of the report. All authors contributed to the scientific content and final review.
Steering committee
This article is part of The Lancet Series on HIV in people who use drugs, which was developed and coordinated by Chris Beyrer (Center for Public Health and Human Rights, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA); Steffanie Strathdee (University of California, San Diego, CA, USA); Adeeba Kamarulzaman (University of Malaya, Kuala Lumpur, Malaysia); and Kasia Malinowska-Sempruch (Open Society Institute, Drug Policy Program, Warsaw, Poland).
Conflicts of interest
We declare that we have no conflicts of interest.
Search strategy and selection criteria
We searched PubMed, Google Scholar, PsychInfo, Ovid, and Scopus databases for publications on substance-use disorders using MeSH terms “HIV” or “AIDS” combined with “substance abuse”, “drug dependence”, “drug user”, “addiction”, “opioids/opiates”, “stimulants”, “cocaine”, “amphetamine”, “alcohol or injection drug use (IDU)” or “intravenous drug use/abuse (IVDU/IVDA)”. Additional details of the literature search and supplemental references are available in the webappendix.
References
- 1.Lewden C Mortality Working Group of COHERE. Time with CD4 cell count above 500 cells/mm3 allows HIV-infected men, but not women, to reach similar mortality rates to those of the general population: a seven-year analysis. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, USA. Feb 16–19, 2010; Abstract 527. [Google Scholar]
- 2.Bruce RD, Altice FL. Clinical care of the HIV-infected drug user. Infect Dis Clin North Am. 2007;21:149–79. doi: 10.1016/j.idc.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partanen TA, Vikatmaa P, Tukiainen E, Lepantalo M, Vuola J. Outcome after injections of crushed tablets in intravenous drug abusers in the Helsinki University Central Hospital. Eur J Vasc Endovasc Surg. 2009;37:704–11. doi: 10.1016/j.ejvs.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Brands B, Blake J, Marsh DC, Sproule B, Jeyapalan R, Li S. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis. 2008;27:37–48. doi: 10.1080/10550880802122620. [DOI] [PubMed] [Google Scholar]
- 5.Kerr T, Fairbairn N, Tyndall M, et al. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007;87:39–45. doi: 10.1016/j.drugalcdep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Ashton H. The diagnosis and management of benzodiazepine dependence. Curr Opin Psychiatry. 2005;18:249–55. doi: 10.1097/01.yco.0000165594.60434.84. [DOI] [PubMed] [Google Scholar]
- 7.Lobmaier P, Kornor H, Kunoe N, Bjorndal A. Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev. 2008;2:CD006140. doi: 10.1002/14651858.CD006140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005;1:CD001867. doi: 10.1002/14651858.CD001867.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–62. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–55. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruce RD, Kresina TF, McCance-Katz EF. Medication-assisted treatment and HIV/AIDS: aspects in treating HIV-infected drug users. AIDS. 2010;24:331–40. doi: 10.1097/QAD.0b013e32833407d3. [DOI] [PubMed] [Google Scholar]
- 12.Thirthalli J, Chand PK. The implications of medication development in the treatment of substance use disorders in developing countries. Curr Opin Psychiatry. 2009;22:274–80. doi: 10.1097/YCO.0b013e32832a1dc0. [DOI] [PubMed] [Google Scholar]
- 13.Izenberg J, Altice FL. Next steps for Ukraine—abolition of HIV registries, implementation of routine HIV testing and expansion of services. Addiction. 2010;105:569–70. doi: 10.1111/j.1360-0443.2009.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amato L, Minozzi S, Davoli M, Vecchi S, Ferri MM, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2008;4:CD005031. doi: 10.1002/14651858.CD005031. [DOI] [PubMed] [Google Scholar]
- 15.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray JB. Effectiveness of methadone maintenance for heroin addiction. Psychol Rep. 1998;83:295–302. doi: 10.2466/pr0.1998.83.1.295. [DOI] [PubMed] [Google Scholar]
- 17.Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28:321–29. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Lobmaier P, Kornor H, Kunoe N, Bjorndal A. Sustained-release naltrexone for opioid dependence. Cochrane Database Syst Rev. 2008;2:CD006140. doi: 10.1002/14651858.CD006140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18:262–70. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Lintzeris N, Ritter A, Panjari M, Clark N, Kutin J, Bammer G. Implementing buprenorphine treatment in community settings in Australia: experiences from the Buprenorphine Implementation Trial. Am J Addict. 2004;13(suppl 1):S29–41. doi: 10.1080/10550490490440799. [DOI] [PubMed] [Google Scholar]
- 21.Altice FL, Sullivan LE, Smith-Rohrberg D, Basu S, Stancliff S, Eldred L. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clin Infect Dis. 2006;43(suppl 4):S178–83. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin Infect Dis. 2006;42:716–21. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- 23.Carrieri MP, Leport C, Protopopescu C, et al. Factors associated with nonadherence to highly active antiretroviral therapy: a 5-year follow-up analysis with correction for the bias induced by missing data in the treatment maintenance phase. J Acquir Immune Defic Syndr. 2006;41:477–85. doi: 10.1097/01.qai.0000186364.27587.0e. [DOI] [PubMed] [Google Scholar]
- 24.Durand E. Changes in high-dose buprenorphine maintenance therapy at the Fleury-Merogis (France) prison since 1996. Ann Med Interne (Paris) 2001;152(suppl 7):9–14. in French. [PubMed] [Google Scholar]
- 25.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37:277–85. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altice FL, Mezger JA, Hodges J, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38(suppl 5):S376–87. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 27.Broadhead RS, Kerr TH, Grund JPC, Altice FL. Safer injection facilities in North America: their place in public policy and health initiatives. J Drug Issues. 2002;32:329–55. [Google Scholar]
- 28.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;2:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006;41:563–72. doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- 30.Greenstein RA, Evans BD, McLellan AT, O’Brien CP. Predictors of favorable outcome following naltrexone treatment. Drug Alcohol Depend. 1983;12:173–80. doi: 10.1016/0376-8716(83)90042-x. [DOI] [PubMed] [Google Scholar]
- 31.Roth A, Hogan I, Farren C. Naltrexone plus group therapy for the treatment of opiate-abusing health-care professionals. J Subst Abuse Treat. 1997;14:19–22. doi: 10.1016/s0740-5472(96)00164-x. [DOI] [PubMed] [Google Scholar]
- 32.Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta-analysis. Int J Public Health. 2009;55:159–66. doi: 10.1007/s00038-009-0095-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim TW, Kertesz SG, Horton NJ, Tibbetts N, Samet JH. Episodic homelessness and health care utilization in a prospective cohort of HIV-infected persons with alcohol problems. BMC Health Serv Res. 2006;6:19. doi: 10.1186/1472-6963-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim TW, Palepu A, Cheng DM, Libman H, Saitz R, Samet JH. Factors associated with discontinuation of antiretroviral therapy in HIV-infected patients with alcohol problems. AIDS Care. 2007;19:1039–47. doi: 10.1080/09540120701294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons JT, Rosof E, Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. J Health Psychol. 2007;12:357–70. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher JC, Bang H, Kapiga SH. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34:856–63. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 37.Palepu A, Raj A, Horton NJ, Tibbetts N, Meli S, Samet JH. Substance abuse treatment and risk behaviors among HIV-infected persons with alcohol problems. J Subst Abuse Treat. 2005;28:3–9. doi: 10.1016/j.jsat.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Samet JH, Walley AY, Bridden C. Illicit drugs, alcohol, and addiction in human immunodeficiency virus. Panminerva Med. 2007;49:67–77. [PubMed] [Google Scholar]
- 39.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 40.Palepu A, Tyndall MW, Li K, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. J Urban Health. 2003;80:667–75. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiavini M, Angeli E, Mainini A, et al. Risk factors for fibrosis progression in HIV/HCV coinfected patients from a retrospective analysis of liver biopsies in 1985–2002. HIV Med. 2006;7:331–37. doi: 10.1111/j.1468-1293.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 42.Mattick RP, Ali R, Auriacombe M, et al. Cochrane Drugs and Alcohol Group: the development of systematic reviews of treatment outcome. Alcohol Alcohol. 2001;36:109–11. doi: 10.1093/alcalc/36.2.109. [DOI] [PubMed] [Google Scholar]
- 43.Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005;1:CD001867. doi: 10.1002/14651858.CD001867.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Cho SH, Whang WW. Acupuncture for alcohol dependence: a systematic review. Alcohol Clin Exp Res. 2009;33:1305–13. doi: 10.1111/j.1530-0277.2009.00959.x. [DOI] [PubMed] [Google Scholar]
- 45.Mason BJ, Ownby RL. Acamprosate for the treatment of alcohol dependence: a review of double-blind, placebo-controlled trials. CNS Spectr. 2000;5:58–69. doi: 10.1017/s1092852900012827. [DOI] [PubMed] [Google Scholar]
- 46.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 47.Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:1105–10. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 48.Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36:S15–23. [PubMed] [Google Scholar]
- 49.Ferreros I, Lumbreras B, Hurtado I, Perez-Hoyos S, Hernandez-Aguado I. The shifting pattern of cause-specific mortality in a cohort of human immunodeficiency virus-infected and non-infected injecting drug users. Addiction. 2008;103:651–59. doi: 10.1111/j.1360-0443.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- 50.Frederick T, Burian P, Terrault N, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women’s Interagency HIV Study (WIHS) AIDS Patient Care STDS. 2009;23:915–23. doi: 10.1089/apc.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS. 2009;23:F1–7. doi: 10.1097/QAD.0b013e32832e5631. [DOI] [PubMed] [Google Scholar]
- 52.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 53.De P, Roy E, Boivin JF, Cox J, Morissette C. Risk of hepatitis C virus transmission through drug preparation equipment: a systematic and methodological review. J Viral Hepat. 2008;15:279–92. doi: 10.1111/j.1365-2893.2007.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 55.Macias J, Mira JA, Lopez-Cortes LF, et al. Antiretroviral therapy based on protease inhibitors as a protective factor against liver fibrosis progression in patients with chronic hepatitis C. Antivir Ther. 2006;11:839–46. [PubMed] [Google Scholar]
- 56.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010 doi: 10.1111/j.1468-1293.2009.00815.x. published online Feb 16. [DOI] [PubMed] [Google Scholar]
- 57.Peixoto B, Lopez L, Areias J, Cerqueira R, Arias JL. Executive functions in chronic hepatitis C virus-infected patients. Adv Clin Exp Med. 2008;17:53–60. [Google Scholar]
- 58.Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29(suppl 2):13–25. doi: 10.1111/j.1478-3231.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 59.Izzedine H, Sene D, Cacoub P, et al. Kidney diseases in HIV/HCV-co-infected patients. AIDS. 2009;23:1219–26. doi: 10.1097/QAD.0b013e32832ac36a. [DOI] [PubMed] [Google Scholar]
- 60.Scott JD, Wald A, Kitahata M, et al. Hepatitis C virus is infrequently evaluated and treated in an urban HIV clinic population. AIDS Patient Care STDS. 2009;23:925–29. doi: 10.1089/apc.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litwin AH, Harris KA, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37:32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grebely J, Petoumenos K, Matthews GV, et al. Factors associated with uptake of treatment for recent hepatitis C virus infection in a predominantly injecting drug user cohort: The ATAHC Study. Drug Alcohol Depend. 2009;107:244–49. doi: 10.1016/j.drugalcdep.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maru DS, Bruce RD, Basu S, Altice FL. Clinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populations. Clin Infect Dis. 2008;47:952–61. doi: 10.1086/591707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010;85:303–15. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 67.Podlekareva DN, Mocroft A, Post FA, et al. Mortality from HIV and TB coinfections is higher in eastern Europe than in western Europe and Argentina. AIDS. 2009;23:2485–95. doi: 10.1097/QAD.0b013e3283326879. [DOI] [PubMed] [Google Scholar]
- 68.WHO. [accessed Feb 24, 2010];Guidelines for surveillance of drug resistance in tuberculosis. 2009 http://www.who.int/tb/publications/mdr_surveillance/en/index.html.
- 69.WHO. [accessed Feb 24, 2010];Anti-tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance, fourth global report. 2008 http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf.
- 70.WHO. [accessed Jan 15, 2010];Policy guidelines for collaborative TB and HIV services for injecting and other drug users: an integrated approach. 2008 http://whqlibdoc.who.int/publications/2008/9789241596930_eng.pdf. [PubMed]
- 71.Golub JE, Astemborski J, Ahmed M, et al. Long-term effectiveness of diagnosing and treating latent tuberculosis infection in a cohort of HIV-infected and at-risk injection drug users. J Acquir Immune Defic Syndr. 2008;49:532–37. doi: 10.1097/QAI.0b013e31818d5c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadaphal P, Astemborski J, Graham NMH, et al. Isoniazid preventive therapy, hepatitis C virus infection, and hepatotoxicity among injection drug users infected with Mycobacterium tuberculosis. Clin Infect Dis. 2001;33:1687–91. doi: 10.1086/323896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawn SD, Harries AD, Wood R. Strategies to reduce early morbidity and mortality in adults receiving antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2009;5:18–26. doi: 10.1097/COH.0b013e328333850f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samandari T, Mosimaneotsile B, Agizew T, et al. Randomized, placebo-controlled trial of 6 vs 36 months isoniazid TB preventive therapy for HIV-infected adults in Botswana. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, USA. Feb 16–19, 2010; Abstract 104LB. [Google Scholar]
- 75.WHO. Tuberculosis infection control in the era of expanding HIV care and treatment. [accessed Jan 15, 2009];Addendum to WHO guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. 2007 http://www.who.int/tb/publications/who_tb_99_269/en/index.html.
- 76.WHO. [accessed Feb 24, 2010];WHO policy on TB infection control in health-care facilities, congregate settings and households. 2009 http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf. [PubMed]
- 77.Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18:306–12. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdool-Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO. [accessed Feb 24, 2010];Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. 2009 http://www.who.int/hiv/pub/arv/advice/en/
- 80.Kohli R, Lo Y, Howard AA, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:864–72. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 81.Hope V, Kimber J, Vickerman P, Hickman M, Ncube F. Frequency, factors and costs associated with injection site infections: findings from a national multi-site survey of injecting drug users in England. BMC Infect Dis. 2008;8:120. doi: 10.1186/1471-2334-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crum-Cianflone N, Weekes J, Bavaro M. Recurrent community-associated methicillin-resistant Staphylococcus aureus infections among HIV-infected persons: incidence and risk factors. AIDS Patient Care STDS. 2009;23:499–502. doi: 10.1089/apc.2008.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burkey MD, Wilson LE, Moore RD, Lucas GM, Francis J, Gebo KA. The incidence of and risk factors for MRSA bacteraemia in an HIV-infected cohort in the HAART era. HIV Med. 2008;9:858–62. doi: 10.1111/j.1468-1293.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reyes MP, Ali A, Mendes RE, Biedenbach DJ. Resurgence of Pseudomonas endocarditis in Detroit, 2006–2008. Medicine (Baltimore) 2009;88:294–301. doi: 10.1097/MD.0b013e3181b8bedc. [DOI] [PubMed] [Google Scholar]
- 85.Barry J, Ward M, Cotter S, et al. Botulism in injecting drug users, Dublin, Ireland, November–December 2008. Euro Surveill. 2009;14 pii=19082. [PubMed] [Google Scholar]
- 86.Murray-Lillibridge K, Barry J, Reagan S, et al. Epidemiological findings and medical, legal, and public health challenges of an investigation of severe soft tissue infections and deaths among injecting drug users—Ireland, 2000. Epidemiol Infect. 2006;134:894–901. doi: 10.1017/S0950268805005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frazee BW, Fee C, Lynn J, et al. Community-acquired necrotizing soft tissue infections: a review of 122 cases presenting to a single emergency department over 12 years. J Emerg Med. 2008;34:139–46. doi: 10.1016/j.jemermed.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 88.Hsieh YH, Rothman RE, Bartlett JG, Yang S, Kelen GD. HIV seropositivity predicts longer duration of stay and rehospitalization among nonbacteremic febrile injection drug users with skin and soft tissue infections. J Acquir Immune Defic Syndr. 2008;49:398–405. doi: 10.1097/qai.0b013e318183ac84. [DOI] [PubMed] [Google Scholar]
- 89.Fernandez Guerrero ML, Gonzalez Lopez JJ, Goyenechea A, Fraile J, de Gorgolas M. Endocarditis caused by Staphylococcus aureus: a reappraisal of the epidemiologic, clinical, and pathologic manifestations with analysis of factors determining outcome. Medicine (Baltimore) 2009;88:1–22. doi: 10.1097/MD.0b013e318194da65. [DOI] [PubMed] [Google Scholar]
- 90.Curtis SJ, Tanna A, Russell HH, et al. Invasive group A streptococcal infection in injecting drug users and non-drug users in a single UK city. J Infect. 2007;54:422–26. doi: 10.1016/j.jinf.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Sierra JM, Sanchez F, Castro P, et al. Group A streptococcal infections in injection drug users in Barcelona, Spain: epidemiologic, clinical, and microbiologic analysis of 3 clusters of cases from 2000 to 2003. Medicine (Baltimore) 2006;85:139–46. doi: 10.1097/01.md.0000224707.24392.52. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi TA, Maciejewski ML, Bradley K. US hospitalizations and costs for illicit drug users with soft tissue infections. J Behav Health Serv Res. 2009 doi: 10.1007/s11414-009-9177-z. published online April 21. [DOI] [PubMed] [Google Scholar]
- 93.Binswanger IA, Takahashi TA, Bradley K, Dellit TH, Benton KL, Merrill JO. Drug users seeking emergency care for soft tissue infection at high risk for subsequent hospitalization and death. J Stud Alcohol Drugs. 2008;69:924–32. doi: 10.15288/jsad.2008.69.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohli R, Lo Y, Homel P, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis. 2006;43:90–98. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen ML, Farrell KJ, Gunthel CJ. Non-AIDS-defining malignancies in patients with HIV in the HAART era. Current Infect Dis Rep. 2009;12:46–55. doi: 10.1007/s11908-009-0075-6. [DOI] [PubMed] [Google Scholar]
- 96.D:A:D Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–26. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zahari MM, Hwan Bae W, Zainal NZ, Habil H, Kamarulzaman A, Altice FL. Psychiatric and substance abuse comorbidity among HIV seropositive and HIV seronegative prisoners in Malaysia. Am J Drug Alcohol Abuse. 2010;36:31–38. doi: 10.3109/00952990903544828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalichman SC, Rompa D. HIV treatment adherence and unprotected sex practices in people receiving antiretroviral therapy. Sex Transm Infect. 2003;79:59–61. doi: 10.1136/sti.79.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS Behav. 2010 doi: 10.1007/s10461-010-9684-1. published online March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaisson RE, Barnes GL, Hackman J, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med. 2001;110:610–15. doi: 10.1016/s0002-9343(01)00695-7. [DOI] [PubMed] [Google Scholar]
- 101.Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug Alcohol Depend. 2002;66:283–93. doi: 10.1016/s0376-8716(01)00208-3. [DOI] [PubMed] [Google Scholar]
- 102.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–77. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 103.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–78. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maru DS, Bruce RD, Walton M, Springer SA, Altice FL. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;50:176–81. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arnsten JH, Berg KM, Mouriz J, Li X, Goldberg U, Goldstein H. Final results from a 6-month, randomized controlled trial of directly observed therapy delivered in methadone clinics. Fourth International Conference on HIV Treatment Adherence; Miami, FL, USA. April 5–7, 2009; Abstract 030. [Google Scholar]
- 106.Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42:1628–35. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- 107.Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38(suppl 5):S409–13. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- 108.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88:54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moatti JP, Carrieri MP, Spire B, Gastaut JA, Cassuto JP, Moreau J. Adherence to HAART in French HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance treatment. The Manif 2000 study group. AIDS. 2000;14:151–55. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- 110.Purcell DW, Latka MH, Metsch LR, et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46(suppl 2):S35–47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- 111.Avants SK, Margolin A, Copenhaver M, Usubiaga MH, Doebrick C. Targeting HIV-related outcomes with intravenous drug users maintained on methadone: a randomized clinical trial of a harm reduction group therapy. J Subst Abuse Treat. 2004;26:67–78. doi: 10.1016/S0740-5472(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 112.Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychol. 2003;22:223–28. [PubMed] [Google Scholar]
- 113.Kalichman SC, Cherry J, Cain D. Nurse-delivered antiretroviral treatment adherence intervention for people with low literacy skills and living with HIV/AIDS. J Assoc Nurses AIDS Care. 2005;16:3–15. doi: 10.1016/j.jana.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 114.Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45:908–15. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Altice FL, Springer S, Buitrago M, Hunt DP, Friedland GH. Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. J Urban Health. 2003;80:416–27. doi: 10.1093/jurban/jtg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goujard C, Bernard N, Sohier N, et al. Impact of a patient education program on adherence to HIV medication: a randomized clinical trial. J Acquir Immune Defic Syndr. 2003;34:191–94. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 117.Broadhead RS, Heckathorn DD, Altice FL, et al. Increasing drug users’ adherence to HIV treatment: results of a peer-driven intervention feasibility study. Soc Sci Med. 2002;55:235–46. doi: 10.1016/s0277-9536(01)00167-8. [DOI] [PubMed] [Google Scholar]
- 118.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26:488–95. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rawlings MK, Thompson MA, Farthing CF, et al. Impact of an educational program on efficacy and adherence with a twice-daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34:174–83. doi: 10.1097/00126334-200310010-00007. [DOI] [PubMed] [Google Scholar]
- 120.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:443–50. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morin SF, Chesney MA, Ehrhardt AA, et al. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: The Healthy Living Project randomized controlled study. J Acquir Immune Defic Syndr. 2007;44:213–21. doi: 10.1097/QAI.0b013e31802c0cae. [DOI] [PubMed] [Google Scholar]
- 122.Rogers RE, Higgins ST, Silverman K, et al. Abstinence-contingent reinforcement and engagement in non-drug-related activities among illicit drug abusers. Psychol Addict Behav. 2008;22:544–50. doi: 10.1037/0893-164X.22.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sindelar JL, Olmstead TA, Peirce JM. Cost-effectiveness of prize-based contingency management in methadone maintenance treatment programs. Addiction. 2007;102:1463–71. doi: 10.1111/j.1360-0443.2007.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: Consensus Statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279:943–48. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 125.Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2007;4:CD003343. doi: 10.1002/14651858.CD003343.pub3. [DOI] [PubMed] [Google Scholar]
- 126.Lucas GM, Flexner CW, Moore RD. Directly administered antiretroviral therapy in the treatment of HIV infection: benefit or burden? AIDS Patient Care STDS. 2002;16:527–35. doi: 10.1089/108729102761041083. [DOI] [PubMed] [Google Scholar]
- 127.Ford N, Nachega JB, Engel ME, Mills EJ. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374:2064–71. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- 128.Hart JE, Jeon CY, Ivers LC, et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr. 2010;54:167–79. doi: 10.1097/QAI.0b013e3181d9a330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Behforouz HL, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J Acquir Immune Defic Syndr. 2004;36:642–45. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 130.Conway B, Prasad J, Reynolds R, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38(suppl 5):S402–08. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- 131.Mitchell CG, Freels S, Creticos CM, Oltean A, Douglas R. Preliminary findings of an intervention integrating modified directly observed therapy and risk reduction counseling. AIDS Care. 2007;19:561–64. doi: 10.1080/09540120601040813. [DOI] [PubMed] [Google Scholar]
- 132.Mitty JA, Macalino GE, Bazerman LB, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J Acquir Immune Defic Syndr. 2005;39:545–50. [PubMed] [Google Scholar]
- 133.WHO, United Nations Office on Drug and Crime, UNAIDS. [accessed Jan 15, 2009];Substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention: position paper. 2004 http://www.unodc.org/docs/treatment/Brochure_E.pdf.
- 134.Palepu A, Tyndall MW, Joy R, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84:188–94. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 135.Roux P, Carrieri MP, Cohen J, et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clin Infect Dis. 2009;49:1433–40. doi: 10.1086/630209. [DOI] [PubMed] [Google Scholar]
- 136.Lucas GM, Chaudhry A, Hsu J, et al. Clinic-based treatment of opioid dependent HIV-infected patients versus referral to an opioid treatment program: a randomized trial. Ann Intern Med. 2010;152:704–11. doi: 10.1059/0003-4819-152-11-201006010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kidder DP, Wolitski RJ, Royal S, et al. Access to housing as a structural intervention for homeless and unstably housed people living with HIV: rationale, methods, and implementation of the housing and health study. AIDS Behav. 2007;11(suppl 6):149–61. doi: 10.1007/s10461-007-9249-0. [DOI] [PubMed] [Google Scholar]
- 138.Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S48–53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- 139.Kushel MB, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clin Infect Dis. 2006;43:234–42. doi: 10.1086/505212. [DOI] [PubMed] [Google Scholar]
- 140.Wohl AR, Garland WH, Valencia R, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42:1619–27. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 141.Smith-Rohrberg D, Altice FL. Randomized, controlled trials of directly administered antiretroviral therapy for HIV-infected patients: questions about study population and analytical approach. Clin Infect Dis. 2006;43:1221–22. doi: 10.1086/508357. [DOI] [PubMed] [Google Scholar]
- 142.Altice FL, Springer SA. DAART for human immunodeficiency virus-infected patients: studying subjects not at risk for nonadherence and use of untested interventions. Arch Intern Med. 2010;170:109–10. doi: 10.1001/archinternmed.2009.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weaver MR, Conover CJ, Proescholdbell RJ, et al. Cost-effectiveness analysis of integrated care for people with HIV, chronic mental illness and substance abuse disorders. J Ment Health Policy Econ. 2009;12:33–46. [PubMed] [Google Scholar]
- 144.Gourevitch MN, Chatterji P, Deb N, Schoenbaum EE, Turner BJ. On-site medical care in methadone maintenance: associations with health care use and expenditures. J Subst Abuse Treat. 2007;32:143–51. doi: 10.1016/j.jsat.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 145.Lertmaharit S, Kamol-Ratankul P, Sawert H, Jittimanee S, Wangmanee S. Factors associated with compliance among tuberculosis patients in Thailand. J Med Assoc Thai. 2005;88(suppl 4):S149–56. [PubMed] [Google Scholar]
- 146.Hammett TM. HIV/AIDS and other infectious diseases among correctional inmates: transmission, burden, and an appropriate response. Am J Public Health. 2006;96:974–78. doi: 10.2105/AJPH.2005.066993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maru DS-R, Basu S, Altice FL. HIV control efforts should directly address incarceration. Lancet Infect Dis. 2007;7:568–69. doi: 10.1016/S1473-3099(07)70190-1. [DOI] [PubMed] [Google Scholar]
- 148.Springer SA, Altice FL. Managing HIV/AIDS in correctional settings. Curr HIV/AIDS Rep. 2005;2:165–70. doi: 10.1007/s11904-005-0011-9. [DOI] [PubMed] [Google Scholar]
- 149.Wallace R. Social disintegration and the spread of AIDS—II. Meltdown of sociogeographic structure in urban minority neighborhoods. Soc Sci Med. 1993;37:887–96. doi: 10.1016/0277-9536(93)90143-r. [DOI] [PubMed] [Google Scholar]
- 150.Thomas JC. From slavery to incarceration: social forces affecting the epidemiology of sexually transmitted diseases in the rural South. Sex Transm Dis. 2006;33(suppl 7):S6–10. doi: 10.1097/01.olq.0000221025.17158.26. [DOI] [PubMed] [Google Scholar]
- 151.Adimora AA, Schoenbach VJ, Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(suppl 1):S115–22. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 152.Harrison LD. The revolving prison door for drug-involved off enders: challenges and opportunities. Crime Delinq. 2001;47:462–85. [Google Scholar]
- 153.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38:1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 154.Maruschak LM, Beaver R. HIV in prisons, 2008. Washington, DC: US Department of Justice; Dec, 2009. Report no. NCJ 228307. [Google Scholar]
- 155.Babudieri S, Aceti A, D’Offizi GP, Carbonara S, Starnini G. Directly observed therapy to treat HIV infection in prisoners. JAMA. 2000;284:179–80. doi: 10.1001/jama.284.2.179. [DOI] [PubMed] [Google Scholar]
- 156.Soto Blanco JM, Perez IR, March JC. Adherence to antiretroviral therapy among HIV-infected prison inmates (Spain) Int J STD AIDS. 2005;16:133–38. doi: 10.1258/0956462053057503. [DOI] [PubMed] [Google Scholar]
- 157.Stephenson BL, Wohl DA, Golin CE, Tien HC, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Rep. 2005;120:84–88. doi: 10.1177/003335490512000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Palepu A, Tyndall MW, Chan K, Wood E, Montaner JS, Hogg RS. Initiating highly active antiretroviral therapy and continuity of HIV care: the impact of incarceration and prison release on adherence and HIV treatment outcomes. Antivir Ther. 2004;9:713–19. [PubMed] [Google Scholar]
- 159.Larney S, Dolan K. A literature review of international implementation of opioid substitution treatment in prisons: equivalence of care? Eur Addict Res. 2009;15:107–12. doi: 10.1159/000199046. [DOI] [PubMed] [Google Scholar]
- 160.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health. 2010 doi: 10.1007/s11524-010-9438-4. published online Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Smith-Rohrberg D, Bruce RD, Altice FL. Review of corrections-based therapy for opiate-dependent patients: implications for buprenorphine treatment among correctional populations. J Drug Issues. 2004;34:451–80. [Google Scholar]
- 162.Kerr T, Marshall A, Walsh J, et al. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17:539–49. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 163.Rodrigo T, Cayla JA, Garcia de Olalla P, et al. Effectiveness of tuberculosis control programmes in prisons, Barcelona 1987–2000. Int J Tuberc Lung Dis. 2002;6:1091–97. [PubMed] [Google Scholar]
- 164.McVay D, Schiraldi V, Ziedenberg J. Treatment or incarceration? National and state findings on the efficacy and cost savings of drug treatment versus imprisonment. Washington, DC: Justice Policy Institute; 2004. [Google Scholar]
- 165.Chandler C, Patton G, Job J. Community-based alternative sentencing for HIV-positive women in the Criminal Justice System. Berkeley Womens Law J. 1999;14:66–69. [Google Scholar]
- 166.Drucker E. Population impact of mass incarceration under New York’s Rockefeller drug laws: an analysis of years of life lost. J Urban Health. 2002;79:434–35. doi: 10.1093/jurban/79.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.