Abstract
The nuclear receptors for heme, REV-ERBα and REV-ERBβ, play important roles in the regulation of metabolism and inflammation. Recently it was demonstrated that reduced REV-ERBα expression in hematopoetic cells in LDL receptor null mice led to increased atherosclerosis. We sought to determine if synthetic REV-ERB agonists that we have developed might have the ability to suppress atherosclerosis in this model. A previously characterized synthetic REV-ERB agonist, SR9009, was used to determine if activation of REV-ERB activity would affect atherosclerosis in LDL receptor deficient mice. Atherosclerotic plaque size was significantly reduced (p < 0.05) in mice administered SR9009 (100 mg/kg) for seven weeks compared to control mice (n = 10 per group). SR9009 treatment of bone marrow-derived mouse macrophages (BMDM) reduced the polarization of BMDMs to proinflammatory M1 macrophage while increasing the polarization of BMDMs to anti-inflammatory M2 macrophages. Our results suggest that pharmacological targeting of REV-ERBs may be a viable therapeutic option for treatment of atherosclerosis.
Keywords: Atherosclerosis, REV-ERB, Nuclear receptor, Macrophages
1. Introduction
Formation of atherosclerotic plaque is an intricate process involving lipid deposition, macrophage recruitment, smooth muscle cell proliferation and vascular wall remodeling. Both hyperlipidemia and inflammation are shown to have causal roles in the development of atherosclerosis [1–3]. Macrophages play key roles in various stages of plaque development by influencing the interplay between lipid metabolism and the inflammatory responses [3,4]. Macrophages can be broadly classified into pro-inflammatory (M1) or anti-inflammatory (M2) subtypes [5,6]. Macrophages are known for their plasticity and polarization into various subtypes, which depends on the cytokine signals they receive and the tissue environment they occupy [7]. M1 macrophages promote a pro-inflammatory immune response and cytotoxicity and are characterized by the expression of high levels of interleukins IL-18, IL-6, iNos and TNF alpha. These macrophages also display increased expression of chemokines, including MIP-18, MIP-18, MCP-1, and MIF, and cell surface markers such as CD80 and CD86 [7]. On the other hand, M2 macrophages play regulatory roles in tissue repair and remodeling and express mannose receptor and proteins such Arg1, Ym1, Ym2 [8]. Macrophages within atherosclerotic plaques are heterogeneous and the balance between these macrophage populations is crucial in the progression of atherosclerosis [4,9].
REV-ERBs (REV-ERBα and REV-ERBβ [NR1D1 and NR1D2]) are members of the nuclear receptor (NR) superfamily and play important roles in the regulation of metabolism and inflammation [10–12]. REV-ERBs have been shown to regulate the expression of inflammatory cytokines interleukin-6 and Ccl2 [13,14]. REV-ERBs are widely expressed and it has been demonstrated that REVERBα is expressed in macrophages found in human atherosclerotic lesions [15]. This study also revealed that suppression of REV-ERBα expression in hematopoetic cells in LDL receptor-deficient mice resulted in increased atherosclerotic lesions in these mice without affecting plasma lipid levels. Additional knockdown and over-expression experiments in macrophages indicated that REV-ERB affects macrophage differentiation into M1/M2 states. These findings suggest that REV-ERBα has anti-atherogenic function and may represent a novel target for prevention and treatment of atherosclerosis. While this study employed genetic manipulation to identify important roles for REV-ERBα and possible underlying mechanisms in the pathogenesis of atherosclerosis, further studies are needed to assess the roles of these NRs in atherosclerosis. Like most nuclear receptors, the REV-ERBs are ligand-dependent transcription factors and we have made considerable progress identifying both endogenous and synthetic ligands specific for these NRs [16]. The identification of heme as a physiological ligand for REV-ERB suggested that synthetic ligands could be designed to modulate the activity of these receptors [17]. Since heme is not an ideal compound to investigate REV-ERB function due to its rapid metabolism and lack of specificity, other small molecule ligands with high specificity and efficacy have been developed and characterized. REV-ERB is a ligand-regulated repressor of transcription and an agonist increases the transcriptional repressive activity of REV-ERB [18]. Use of REV-ERB-specific synthetic ligands that activate the receptor, in an atherosclerosis model would be beneficial in determining whether pharmacological modulation of these receptors holds potential utility in the treatment or prevention of atherosclerosis. In this study, we examined the utility of pharmacological modulation of REV-ERB activity on atherosclerosis and in altering macrophage phenotype.
2. Materials and methods
2.1. Animals and treatment
All procedures were approved and conducted in accordance to the Scripps Florida Institutional Animal Care and Use Committee and Saint Louis University Institutional Animal Care and Use Committee. Twenty homozygous LDL receptor deficient (ldlr−/−) male mice were obtained from Jackson Laboratory (Bar Harbor, ME) at the age of 7 weeks and were housed individually in 12 h light/dark cycles. The mice were allowed to acclimate for a week and were maintained on standard chow diet. During the experiment the mice were fed Western diet containing 1.25% (w/w) cholesterol and 42% calories from fat (w/w) (Research diets, NJ). The REV-ERB agonist SR9009 was formulated in 15% cremophor. 100 mg/kg SR9009 or vehicle was administered once a day intraperitoneally for seven weeks. Body weight and food intake was monitored daily.
2.2. Atherosclerotic lesion analysis
At the end of seven weeks, mice from both groups were sacrificed and the left ventricle was perfused with PBS. The aorta sections from aortic root to iliac artery were collected and fixed in 4% paraformaldehyde overnight and washed with PBS the next day. To carry out en face analysis of aorta, the connective tissues were removed and aorta was cut open longitudinally under dissecting microscope. Plaques in aorta sections were visualized by staining with Oil Red O (Sigma) and subsequent washing with 80% isopropanol (Fischer). Aortas were imaged with Leica S4E microscope and plaque area was quantified as percentage of total aortic surface area.
2.3. Plasma lipid and liver enzyme analysis
Mice were euthanized after 4–5 h fasting and blood was collected via cardiac puncture. Concentration of plasma total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride, glucose and liver enzymes were assessed using COBAS clinical chemisrty analyzer (Roche).
2.4. Isolation and culture of bone marrow-derived macrophages (BMDM)
Eight to twelve week old C57BL/6 mice were euthanized according to the animal protocol approved by Scripps Research Institute and Saint Louis University. Tibia and femur were flushed with PBS and mononuclear phagocyte progenitor cells from bone marrow were collected and differentiated in Dulbecco’s minimum essential medium (DMEM, Life Technologies Inc., MD) supplemented with L929 cell-conditioned medium. Culture media was added on day 3 and day 6 and cells were collected and plated at the density of 4 × 105/ml in twelve-well culture plates on day 7. Cells were treated with 100 ng/ml IFNg (Millipore) and 10 ng/ml LPS(Millipore) for M1 polarization. M2 differentiation was induced by incubating macrophages with 15 ng/ml IL-4 (Millipore).
2.5. Cell viability assay
The Nuclear-ID® Blue/Red cell viability reagent (Enzo life sciences) was used to determine the cell viability of BMDM during polarization and drug treatment. The live and dead cell populations were examined using fluorescent microscope. Additionally, Cell-Titer 96® Aqueous One Solution Reagent (Promega) was used according to the manufacturer’s directions to determine viability and metabolic activity of cells via absorbance reading in plate reader.
2.6. Quantitative real-time PCR
Total RNA was isolated from BMDM using PureLink RNA mini kit (Ambion). RNA was reverse-transcribed to make cDNA using qScript™ cDNA Synthesis Kit (Quanta biosciences) according to the manufacturer’s instructions. Real-time PCR was performed using an SYBR-green PCR master mix kit (Roche). Primers purchased from Integrated DNA Technologies.
Relative mRNA levels were calculated using the 2−ΔΔCt method and normalized against m36B4. Each experiment was performed three times.
| Primer sequences |
|---|
| mIL1R11 Forward – ACAGTGGGATTGTGGTTGTC |
| mIL1R1 Reverse – TTCCACTGTGGGGTATCAGT |
| m iNOS Forward – CACCTTGGAGTTCACCCAGT |
| m iNOS Reverse – ACCACTCGTACTTGGGATGC |
| mMR Forward – ATGCCAAGTGGGAAAATCTG |
| mMR Reverse – TGTAGCAGTGGCCTGCATAG |
| mCD86 Forward – TACGAGCACTATTTGGGCAC |
| mCD86 Reverse – GAGCCCATGTCCTTGATCTG |
| mCD80 Forward – AGAGGACTTCACCTGGGAAA |
| mCD80 Reverse – TATTACTGCGCCGAATCCTG |
| mMCP-1 Forward – ATCCCAATGAGTAGGCTGGA |
| mMCP-1 Reverse – CCATTCCTTCTTGGGGTCAG |
| mMIP1a Forward – CATGAAGGTCTCCACCACTG |
| mMIP1a Reverse – GTCAACGATGAATTGGCGTG |
| mMIP1b Forward – CCACTTCCTGCTGTTTCTCTT |
| mMIP1b Reverse – TCTGTCTGCCTCTTTTGGTC |
| mMIF Forward – GCTCCACGTAGTGTTCTGTG |
| mMIF Reverse – ACCATTTATTTCTCCCGGCT |
| mTNFa Forward – TCAGCCGATTTGCTATCTCAT |
| mTNFa Reverse – TGGAAGACTCCTCCCAGGTAT |
| mIL-1b Forward – TGACGGACCCCAAAAGATGA |
| mIL-1b Reverse – TCTCCACAGCCACAATGAGT |
| mIL-6 Forward – CTGCAAGAGACTTCCATCCAG |
| mIL-6 REVERSE – AGTGGTATAGACAGGTCTGTTGG |
| mYm1 Forward – AGAAGCAATCCTGAAGACACCA |
| mYm1 REVERSE – CTATTGGCCTGTCCTTAGCCC |
| mYm2 Forward – AGCCCTCCTAAGGACAAACAT |
| mYm2 Reverse – TTGGAATGTGGTTCAAAGTGGA |
| mBmal1 Forward – CTCCAGGAGGCAAGAAGATTC |
| mBmal1 Reverse – ATAGTCCAGTGGAAGGAATG |
| m36B4 Forward – ACCTCCTTCTTCCAGGCTT |
| m36B4 Reverse – CCCACCTTGTCTCCAGTCTTT |
2.7. Statistical analysis
Data are expressed as mean ± SEM. A student’s two-tailed t-test was used to calculate statistical significance. P < 0.05 was considered significant.
3. Results
3.1. Reduced atherosclerotic lesions in LDL receptor deficient mice treated with a REV-ERB agonist
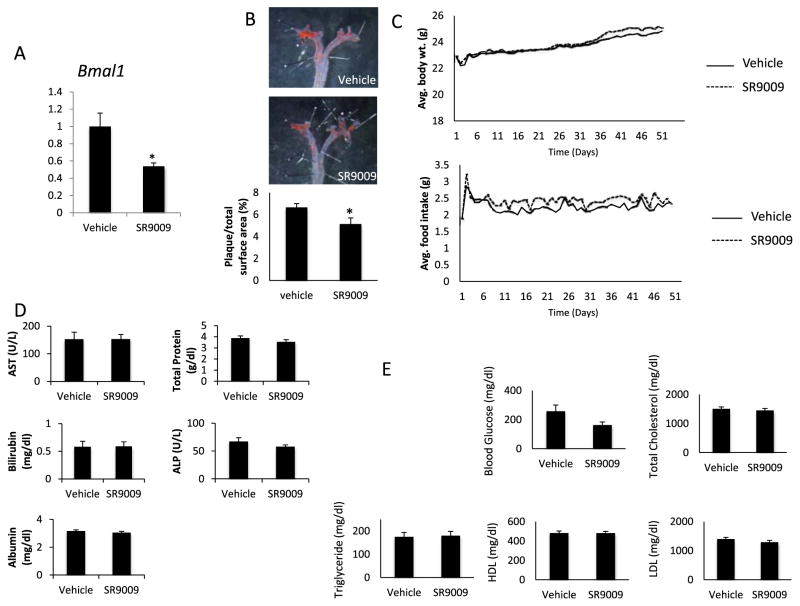
Given the results from a recent study demonstrating that genetic REV-ERB deficiency in macrophages exacerbates atherosclerosis [15], the effect of pharmacologic activation of REV-ERB activity on atherosclerosis was examined in LDL receptor deficient mice. These mice were fed a western diet throughout the experiment and were treated with the REV-ERB agonist, SR9009 (100 mg/kg, q.d.), or vehicle for seven weeks. We previously identified SR9009 as a REV-ERB agonist with in vivo activity [18]. The expression of the REV-ERB target gene, Bmal1, was suppressed indicating effectiveness of the treatment (Fig. 1A). Plaque formation was assessed in the aorta at the end of the experiment using oil red o staining. As shown in Fig. 1B, SR9009 significantly lowered atherosclerotic plaque by 23% compared to vehicle-treated group. The treatment did not affect food intake or body weight in these mice (Fig. 1C). Elevated liver enzymes and protein in plasma are indicative of liver damage and were used in the study as parameters to assess any adverse effects of the treatment regimen on these mice [19]. The levels of liver transaminases, total albumin and protein in plasma were within the normal range in both groups demonstrating that the drug was well tolerated by the mice (Fig. 1D). We also assessed plasma lipids in these mice. Lipids were highly elevated in LDLR null mice on western diet. However, no significant changes were observed in total plasma cholesterol, HDL, LDL or triglycerides between the vehicle and SR9009-treated groups (Fig. 1E). Thus, our data indicating that gain of function (REV-ERB agonists treatment in LDLR null mice) decreases atherosclerotic plaque burden is consistent with that of Ma et al. [15] demonstrating that loss of function (hematopoetic REV-ERB knock down in LDLR null mice) increases atherosclerotic plaque burden. In both cases, no effect on plasma lipids was noted.
Fig. 1.
The REV-ERB ligand SR9009 reduces atherosclerotic plaque size in ldlr−/− mice. A) mRNA expression of Bmal1 in mouse liver. mRNA was normalized to cyclophilin. B) Atherosclerotic plaque stained with Oil Red O in ldlr−/− mice on western diet treated with vehicle or SR9009 for seven weeks. The data represents mean ± SEM (n = 10 per group) p < 0.05 °C) Average body weight and food intake of ldlr−/− mice during the period of the experiment. D) Hepatic enzymes and proteins in ldlr−/− mice at the end of the seven week dosing. E) Plasma glucose and lipid levels of ldlr−/− mice on western diet at the end of the experiment. *, indicates p < 0.05.
3.2. REV-ERB agonist impairs the appearance of pro-inflammatory M1 phenotype and enhances anti-inflammatory M2 phenotype
Macrophages are important components of atherosclerotic lesions. They play a key role in formation of plaque, inflammation and progression of atherosclerosis. Given the important role of macrophages during atherosclerosis as well as previous findings demonstrating the presence of REV-ERB in macrophages in atherosclerotic plaques [5,15] and our observation that SR9009-treatment reduced plaque size without alteration in plasma lipid levels, we sought to evaluate the effect of pharmacological REV-ERB activation on macrophages.
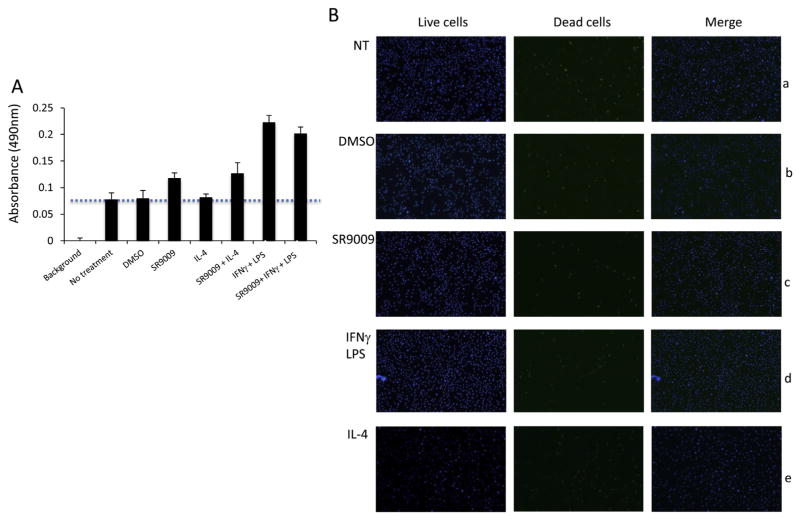
First we examined the effect of M1 or M2 polarizing signals and SR9009 treatment on cell viability. Bone marrow cells were isolated from mice and differentiated in L929 cell-conditioned medium for seven days. On day 7 these mouse bone marrow – derived macrophages (BMDMs) were plated and assays were carried out to determine metabolic activity and cell viability when treated with SR9009 and polarizing cytokines. The metabolic activity of BMDMs was increased in the presence of polarizing cytokines and SR9009 suggesting no adverse effect of treatment on the cells (Fig. 2A). The number of dead cells was not altered in the treatment group compared to non-treatment group demonstrating that cell viability was not affected by cytokines or SR9009 treatment (Fig. 2B).
Fig. 2.
Cell viability and metabolic activity of bone marrow derived macrophages were assessed using A) CellTiter 96® Aqueous One Solution assay and B) Blue/Green cell viability assay in non-treated bone marrow derived macrophages (BMDMs) (panel a) or BMDMs treated with DMSO (panel b), 10 uM SR9009 (panel c), 100 ng/ml IFNg, 10 ng/ml LPS and DMSO (panel d and 15 ng/ml IL-4 and DMSO (panel e) for 24 h.
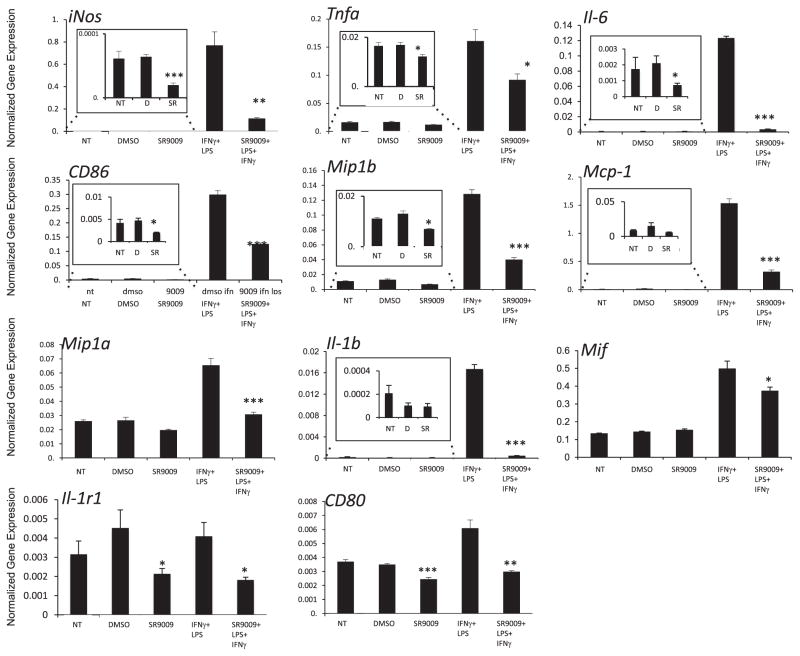
Polarization of macrophages into two subtypes, M1 and M2, are known to be associated with atherogenicity and the inflammatory state [5]. Using bone marrow - derived mouse macrophages (BMDM) we next investigated the role of REV-ERB during macrophage polarization into M1 or M2 subtypes. BMDMs were stimulated with IFNγ and LPS to induce M1 polarization and were co-treated with SR9009 for 24 h. Our data revealed that mRNA expression of markers of M1 macrophages such as iNos, Tnfa, CD80, CD86, inflammatory cytokines Il-6, Il-1b and chemokines Mif, Mip1a, Mip1b, Mcp-1 were significantly reduced in SR9009 treated BMDM cells (Fig. 3). These results indicate that REV-ERB impairs classical activation of macrophages. Many of these markers were also reduced when cells were treated with SR9009 alone without stimulation (Fig. 3 insets). These results suggest that the agonist alone, in the absence of LPS and IFNγ, suppresses the ability of macrophages to become M1 macrophages. These data also suggest that REV-ERB activation leads to stimulation of pathways that suppress macrophage M1 polarization and are consistent with studies indicating that the endogenous REV-ERB agonist, heme, behaves in a similar manner with regard to suppressing M1 macrophage polarization [15].
Fig. 3.
Mouse bone marrow derived macrophages (BMDMs) stimulated with IFNγ and LPS to obtain M1 polarization were co-treated with SR9009 for 24 h mRNA expression was normalized to 36B4. The insets represent magnified portions of the larger graphs as indicated. *p < 0.05; **p < 0.001, ***p < 0.0001 NT – No treatment, D – DMSO, and SR – SR9009.
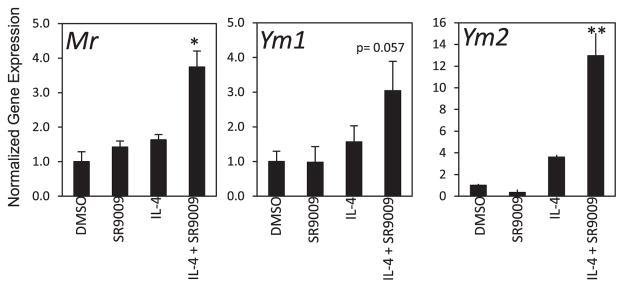
M2 macrophages are a more heterogeneous population [5]. Previously, it was demonstrated that knock-down of REV-ERBα led to decreased M2 polarization whereas overexpression of REV-ERBα resulted in increased M2 polarization [15]. To explore the effects of REV-ERB agonist (SR9009) treatment on M2 macrophage activation we stimulated BMDM with IL-4. Seven-day differentiated BMDMs were treated with DMSO, SR9009, IL-4 alone or with IL-4 in the presence of DMSO or SR9009 for 24 h. Our results show that a range of M2 macrophage markers such as Ym1, Ym2 and Mr increase with cotreatment with SR9009 (Fig. 4). One marker that we examined, Arg1, was unaffected by SR9009 cotreatment (data not shown). In summary, our data indicate that activation of REV-ERB by SR9009 results in reduced proinflammatory M1 macrophage polarization and increased anti-inflammatory M2 polarization.
Fig. 4.
Mouse bone marrow derived macrophages (BMDMs) were either left unstimulated and treated with DMSO or 10 μM SR9009 only or to polarize to M2 macrophages BMDMs were stimulated with IL-4 alone or co-treated with SR9009 for 24 h mRNA expression was normalized to 36B4. *p < 0.05; **p < 0.001.
4. Discussion
REV-ERBs have been demonstrated to play important roles in regulation of metabolism and immune function [11,18,20–22]. We were particularly intrigued by a recent study demonstrating that hematopoetic knock down of REV-ERBα increases atherosclerosis in LDLR null mice providing clear evidence that REV-ERB plays a role in atherosclerosis [15]. In this study, plaque burden increased and was attributed to an increase in proinflammatory M1 macrophages and a decrease in anti-inflammatory M2 macrophages [15]. Given our development of synthetic REV-ERB agonists [16,18], we were particularly interested in examining whether pharmacological activation of REV-ERB could inhibit or delay the development of atherosclerosis.
The focus of the current study was to use a previously characterized synthetic REV-ERB agonist, SR9009, in an identical mouse model of atherosclerosis as previously described. Our results demonstrate that pharmacological gain of function of REV-ERB results in reduced atherosclerosis, which is consistent with the genetic loss of function experiments indicating that hematopoetic knock-down of REV-ERB results in increased atherosclerosis [15]. In both of these cases, no alterations in plasma lipid levels were observed. However, we were surprised by these results since in our previous studies, significant effects on lipid levels were seen when mice were treated with SR9009 twice a day [18]. Thus, lack of change in plasma lipid levels in our present study could be due to once a day dosing with our compound vs. twice a day. However, given this recent study with REV-ERBα knock down, we believe that it was very important to determine if pharmacological activation of REV-ERB would have an anti-atherogenic effect as predicted, and indeed it does. Our results examining alterations in macrophage polarization using pharmacological activation of REV-ERB are also consistent with genetic gain- and loss-of-function studies by Ma et al. [15]. Regardless, we suspect that pharmacological modulation of REV-ERB activity may lead to a number of beneficial effects that may be anti-atherosclerotic and alteration of the M1/M2 macrophage balance may only be one important effect. Given these effects, future studies investigating the use of this compound in immune function and inflammation would be valuable.
It has become increasingly evident that REV-ERB has important functions in metabolism, inflammation and cardiovascular disease. Developing synthetic ligands has allowed us to study the role of these nuclear receptors to a greater extent and in the context of therapeutic development. This study further demonstrates the utility of these compounds and potential therapeutic potential in the treatment of metabolic diseases such as atherosclerosis and other inflammatory conditions. Our findings demonstrate that targeting the REV-ERBs might be a viable therapeutic option for metabolic and inflammatory diseases such as atherosclerosis and the development of improved and optimized synthetic ligands may increase the effects observed with these compounds. In conclusion, our data suggests an important role of REV-ERB in macrophage biology and atherosclerosis and indicates therapeutic potential of modulating this receptor in the treatment of metabolic diseases.
Acknowledgments
Sources of funding
This research was supported by AHA Predoctoral Fellowship and NIH R01MH093429 grants.
Footnotes
Conflict of interest
None.
Disclosures
TPB and TMK have patents pending on REV-ERB synthetic ligands.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2015.03.070.
References
- 1.Tabas I. Biochem Lipids, Lipoproteins Membr. 2008:579–605. http://dx.doi.org/10.1016/B978-044453219-0.50023-4.
- 2.Kerenyi L, Mihalka L, Csiba L, Bacso H, Bereczki D. Role of hyperlipidemia in atherosclerotic plaque formation in the internal carotid artery. J Clin Ultrasound. 2006;34:283–288. doi: 10.1002/jcu.20233. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 6.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramakrishnan SN, Muscat GEO. The orphan rev-erb nuclear receptors: a link between metabolism. circadian rhythm and inflammation? Nucl Recept Signal. 2006;4:e009. doi: 10.1621/nrs.04009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs JE, et al. The nuclear receptor REV-ERB alpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato S, et al. Direct and indirect suppression of Interleukin-6 gene expression in Murine macrophages by nuclear orphan receptor REV-erbα. Sci World J. 2014;2014:685854. doi: 10.1155/2014/685854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato S, et al. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 15.Ma H, et al. Increased atherosclerotic lesions in LDL receptor deficient mice with hematopoietic nuclear receptor Rev-erbα knock- down. J Am Heart Assoc. 2013;2:e000235. doi: 10.1161/JAHA.113.000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee S, et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat Commun. 2014;5 doi: 10.1038/ncomms6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raghuram S, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solt LA, et al. Regulation of circadian behavior and metabolism by synthetic REV-erb agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol. 1998;27:119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- 20.Delezie J, et al. The nuclear receptor REV-ERB is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012;26:3321–3335. doi: 10.1096/fj.12-208751. [DOI] [PubMed] [Google Scholar]
- 21.Ripperger JA, Albrecht U. REV-ERB-erating nuclear receptor functions in circadian metabolism and physiology. Cell Res. 2012;22:1319–1321. doi: 10.1038/cr.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bugge A, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]