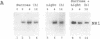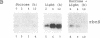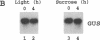Abstract
Nitrate reductase, the first enzyme in nitrate assimilation, is located at the crossroad of two energy-consuming pathways: nitrate assimilation and carbon fixation. Light, which regulates the expression of many higher-plant carbon fixation genes, also regulates nitrate reductase gene expression. Located in the cytosol, nitrate reductase obtains its reductant not from photosynthesis but from carbohydrate catabolism. This relationship prompted us to investigate the indirect role that light might play, via photosynthesis, in the regulation of nitrate reductase gene expression. We show that sucrose can replace light in eliciting an increase of nitrate reductase mRNA accumulation in dark-adapted green Arabidopsis plants. We show further that sucrose alone is sufficient for the full expression of nitrate reductase genes in etiolated Arabidopsis plants. Finally, using a reporter gene, we show that a 2.7-kilobase region of 5' flanking sequence of the nitrate reductase gene is sufficient to confer the light or the sucrose response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Borthwick H. A., Hendricks S. B., Parker M. W., Toole E. H., Toole V. K. A Reversible Photoreaction Controlling Seed Germination. Proc Natl Acad Sci U S A. 1952 Aug;38(8):662–666. doi: 10.1073/pnas.38.8.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. L., Acedo G. N., Dewdney J., Goodman H. M., Conkling M. A. Differential expression of the two Arabidopsis nitrate reductase genes. Plant Physiol. 1991 May;96(1):275–279. doi: 10.1104/pp.96.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. L., Dewdney J., Nam H. G., den Boer B. G., Goodman H. M. A new locus (NIA 1) in Arabidopsis thaliana encoding nitrate reductase. EMBO J. 1988 Nov;7(11):3309–3314. doi: 10.1002/j.1460-2075.1988.tb03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowri G., Campbell W. H. cDNA Clones for Corn Leaf NADH:Nitrate Reductase and Chloroplast NAD(P):Glyceraldehyde-3-Phosphate Dehydrogenase : Characterization of the Clones and Analysis of the Expression of the Genes in Leaves as Influenced by Nitrate in the Light and Dark. Plant Physiol. 1989 Jul;90(3):792–798. doi: 10.1104/pp.90.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Brendle-Behnisch E. Rapid Modulation of Spinach Leaf Nitrate Reductase Activity by Photosynthesis : I. Modulation in Vivo by CO(2) Availability. Plant Physiol. 1991 Jun;96(2):363–367. doi: 10.1104/pp.96.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C., Schaal S., Werr W. A feedback control element near the transcription start site of the maize Shrunken gene determines promoter activity. EMBO J. 1990 Nov;9(11):3447–3452. doi: 10.1002/j.1460-2075.1990.tb07552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace G. M., Volk R. J., Jackson W. A. Nitrate Reduction in Response to CO(2)-Limited Photosynthesis : Relationship to Carbohydrate Supply and Nitrate Reductase Activity in Maize Seedlings. Plant Physiol. 1990 Feb;92(2):286–292. doi: 10.1104/pp.92.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekhar V. K., Gowri G., Campbell W. H. Phytochrome-mediated light regulation of nitrate reductase expression in squash cotyledons. Plant Physiol. 1988 Oct;88(2):242–244. doi: 10.1104/pp.88.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffner A. R., Sheen J. Maize rbcS promoter activity depends on sequence elements not found in dicot rbcS promoters. Plant Cell. 1991 Sep;3(9):997–1012. doi: 10.1105/tpc.3.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990 Oct;2(10):1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]