Abstract
This review updates the conceptual basis for the association of alcohol abuse with an insidious adaptation that facilitates negative affect during withdrawal from chronic intermittent alcohol (CIA) exposure – a change that later supports sensitization of stress-induced anxiety following alcohol abstinence. The finding that a CRF1-receptor antagonist (CRF1RA) minimized CIA withdrawal-induced negative affect supported an association of alcohol withdrawal with a stress mechanism. The finding that repeated stresses or multiple CRF injections into selected brain sites prior to a single 5-day chronic alcohol (CA) exposure induced anxiety during withdrawal provided critical support for a linkage of CIA withdrawal with stress. The determination that CRF1RA injection into positive CRF-sensitive brain sites prevented CIA withdrawal-induced anxiety provided support that neural path integration maintains the persistent CIA adaptation. Based upon reports that stress increases neuroimmune function, an effort was undertaken to test whether cytokines would support the adaptation induced by stress/CA exposure. Twenty-four hours after withdrawal from CIA, cytokine mRNAs were found to be increased in cortex as well as other sites in brain. Further, repeated cytokine injections into previously identified brain sites substituted for stress and CRF induction of anxiety during CA withdrawal. Discovery that a CRF1RA prevented the brain cytokine mRNA increase induced by CA withdrawal provided critical evidence for CRF involvement in this neuroimmune induction after CA withdrawal. However, the CRF1RA did not block the stress increase in cytokine mRNA increases in controls. The latter data supported the hypothesis that distinct mechanisms linked to stress and CA withdrawal can support common neuroimmune functions within a brain site. As evidence evolves concerning neural involvement in brain neuroimmune function, a better understanding of the progressive adaptation associated with CIA exposure will advance new knowledge that could possibly lead to strategies to combat alcohol abuse.
Keywords: adaptation, kindling, chronic intermittent alcohol withdrawal, anxiety, stress, CRF, neuroimmune function, circuitry, signaling
Introduction
Ballenger and Post (1978) hypothesized that chronic intermittent alcohol (CIA) exposure and accompanying withdrawals over an extended period were responsible for seizures during withdrawal in alcoholics – a process proposed to depend upon “kindling”. These investigators further speculated that if the “kindling hypothesis” accounted for the incidence of seizures, this persistent adaptation induced by the CIA exposure also contributed to the maintenance of alcohol abuse (alcoholism) (Ballenger & Post, 1978). Somewhat later, McCown and Breese (1990) demonstrated that exposure of rats to a CIA protocol for 10 weeks facilitated kindling of seizures from the inferior colliculus – a change not duplicated in rats continuously exposed to this 10-week period of alcohol diet. This latter observation indicated that CIA cycling was critical for inducing the adaptation that supported the kindling of seizures (McCown & Breese, 1990). Subsequently, repeated CIA exposures were reduced to three to test the possibility that early stages of adaptation induced by repeated CIA exposures and withdrawals would worsen negative withdrawal symptoms by a “kindling” process (Overstreet, Knapp, & Breese, 2002). Whereas withdrawal from three cycles of CIA exposure facilitated anxiety-like behavior, continuous exposure to alcohol did not (Overstreet et al., 2002). This facilitation of anxiety during a shorter repeated exposure to CIA (Overstreet et al., 2002; Overstreet, Knapp, & Breese, 2004a, 2004b) but not following withdrawal from continuous alcohol provided a further conceptual foundation for an accumulated adaptation being induced by repeated CIA exposures that could contribute to the etiology of alcoholism (Ballenger & Post, 1978; Breese, Overstreet, & Knapp, 2005). Based upon this framework, the present review describes our current understanding of how such CIA exposures interact with CRF and neuroimmune neural mediation of stress to sustain this persistent adaptation that follows chronic repeated alcohol exposures.
CRF-receptor (CRFR) antagonism of CIA withdrawal-induced anxiety implicates a contribution of “stress”
The neural basis of the hypothesized kindling-like neuroadaptation that sensitized seizures (Ballenger & Post, 1978; McCown & Breese, 1990) and withdrawal-induced anxiety (Koob, 2003; Overstreet et al., 2002) first began to be elucidated more than a decade after the kindling hypothesis was advanced (Ballenger & Post, 1978). The finding that a corticotropin releasing-factor 1 receptor antagonist (CRFR1A) prevented the anxiety that occurs following withdrawal from continuous alcohol exposure (Knapp, Overstreet, Moy, & Breese, 2004; Fig. 1A) provided part of the impetus to test drugs against sensitization arising during the repeated withdrawals (Overstreet et al., 2004a). In this latter testing, the CRF1RA given during each withdrawal in the repeated-withdrawal paradigm blocked the appearance of anxiety-like behavior during an untreated final (third) withdrawal (Fig. 1B) – a result that was particularly important in suggesting a basis for the neuroadaptation that accompanied alcohol-withdrawal anxiety. This finding was also consistent with the earlier finding by Baldwin et al. (1991) that CRF release during a final withdrawal contributes to the persistent adaptation that contributes to the anxiety linked to withdrawal from CA exposure (Baldwin, Rassnick, Rivier, Koob, & Britton, 1991; Rassnick, Heinrichs, Britton, & Koob, 1993). Additional studies confirmed and expanded on this pharmacological approach to manipulate the CIA effect on anxiety-like behavior (Breese, Knapp, & Overstreet, 2004; Knapp, Overstreet, Angel, Navarro, & Breese, 2007; Knapp et al., 2004; Overstreet et al., 2004a; Overstreet, Knapp, & Breese, 2005;Overstreet, Knapp, Moy, & Breese, 2003). These efforts provide further evidence that repeated CRF release during multiple withdrawals supports the developing adaptation associated with the withdrawal-induced anxiety following CIA exposure. A recent report that CIA exposure up-regulates CRF/CRF1R signaling supports this conclusion (Eisenhardt, Hansson, Spanagel, & Bilbao, 2014).
Fig. 1.
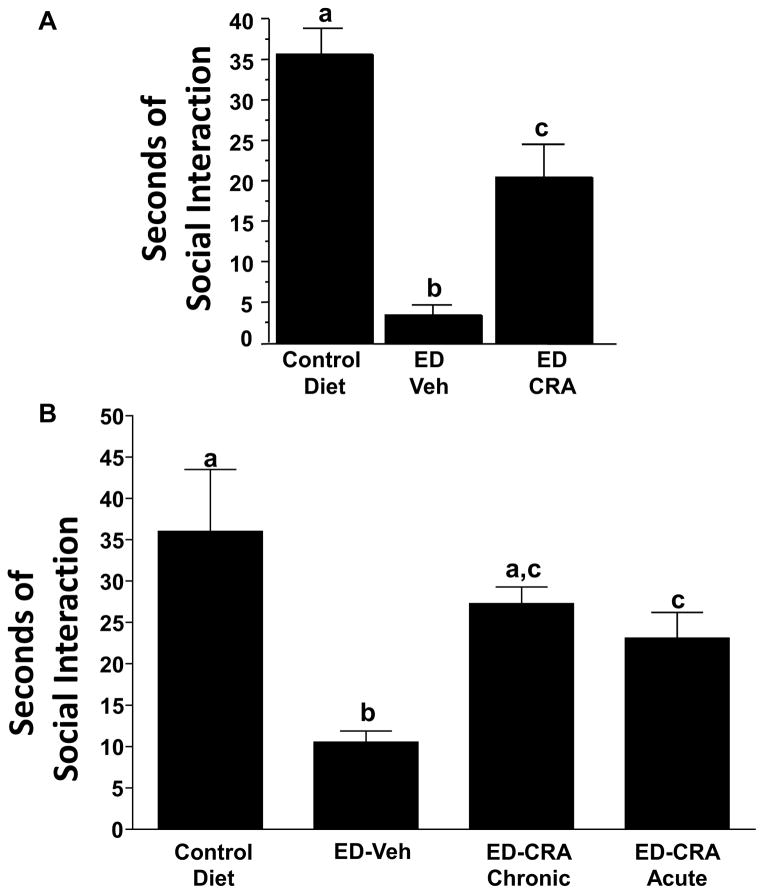
Fig. 1A. Mean social interaction time for control and alcohol-withdrawn (ED) rats treated with CRA1000. Rats were treated for 17 days with 7% (w/v) ethanol (alcohol) diet and then tested between 5 and 6 h into withdrawal. CRA1000 was given intra-peritoneally (i.p.) to one ED group 30 min prior to behavioral testing (1 mg/kg). Reduced SI behavior is interpreted as an elevated anxiety-like state. The data represent the mean seconds ± S.E.M. of time spent in SI. A one-way ANOVA showed significant group differences (p < .01). The letter(s) above each bar, if different from those appearing above other bars, reflect statistically significant post hoc comparisons between those respective bars according to Tukey’s test (p < .01). CMC = 0.5% solution of carboxymethylcellulose vehicle. Adapted from Knapp et al., 2004.
Fig. 1B. Effects of the CRF1 (corticotrophin releasing factor-1) receptor antagonist CRA1000 (CRA) on social interaction (SI) behavior of rats subjected to repeated withdrawals from alcohol. Rats were exposed to control liquid diets throughout (n = 8) or to three cycles of 5 days’ exposure to an ethanol- (alcohol) containing liquid diet (ED, 7% w/v). The rats were maintained on control diet during the 2 days of withdrawal between the first and second, and the second and third cycles, and between alcohol withdrawal and behavior testing after the third cycle. One group was injected with CMC vehicle 4 h into the first and second withdrawal (ED–Veh), another was pretreated with 3 mg/kg CRA1000 at the same times (ED–CRA Chronic), and the final group was injected acutely with 1 mg/kg CRA1000 30 min before the SI test during the third withdrawal, 4.5 h after alcohol removal (ED–CRA Acute). The other groups exposed to ED were also tested in the SI arena 5 h after removal of alcohol. Error bars, letters above bars, and CMC are described in Fig. 1A above. Adapted from Overstreet et al., 2004.
Subsequently, antagonists and agonists associated with a variety of neural systems were given during the initial withdrawals of the CIA protocol and found to prevent the sensitization of anxiety associated with the final withdrawal from CIA (Knapp, Overstreet, & Breese, 2005, 2007; Knapp et al., 2004; Overstreet et al., 2003, 2004a, 2005). In these extended evaluations, antagonists of receptors for 5-HT2C and benzodiazepine receptors as well as a GABAB receptor agonist attenuated the CIA and chronic alcohol-withdrawal anxiety (Breese, Overstreet, Knapp, & Navarro, 2005; Knapp et al., 2004). Equally important was the finding that antagonists of NMDA, 5-HT3, and CRF2 receptors did not affect the anxiety following withdrawal from chronic alcohol (CA) (Knapp et al., 2004; Overstreet et al., 2004a). Currently, it is not yet resolved how these other neural systems are implicated in the withdrawal anxiety that involves CRF. Regardless, these outcomes support the view that CRF as well as the other neural systems can contribute to the persistent adaptation that follows withdrawal from CA and CIA exposures.
Finding that a CRF1RA mitigated the sensitization of withdrawal anxiety induced by the CIA exposure (Knapp, Overstreet, Angel, et al., 2007; Knapp et al., 2004; Overstreet et al., 2003, 2004a, 2004b, 2005) and that a BZD inverse agonist (DMCM) substituted for the earlier withdrawals of CIA exposure (Knapp, Overstreet, Angel, et al., 2007) raised the possibility that the initial withdrawals from the CIA protocol induced a “stress-like” response that initiated CRF release (Bale & Vale, 2004; Dunn & Berridge, 1990a, 1990b; Imaki, Shibasaki, Hotta, & Demura, 1993; Vale, Spiess, Rivier, & Rivier, 1981). To determine whether activation of a neural “stress” pathway was a critical component in the CIA-induced anxiety during withdrawal (Overstreet et al., 2002), single bi-weekly stresses were substituted for the two initial weekly repeated withdrawals of CIA exposure. This strategy tested whether sensitization of negative symptoms (anxiety) would occur upon withdrawal from the single 5 days of alcohol exposure that alone induced no behavioral change during withdrawal (Breese et al., 2004, 2008). In accord with the hypothesized stress induction of adaptation occurring during repeated alcohol withdrawals, the repeated weekly stresses prior to the single 5-day cycle of alcohol exposure sensitized anxiety during withdrawal (Breese et al., 2004; Fig. 2A). Further, this repeated stress sensitization of alcohol withdrawal anxiety was prevented by a CRF1RA (Huang et al., 2010; Knapp et al., 2011). Moreover, in related studies where repeated withdrawal preceded a stress challenge 3 days following the withdrawal, the CRF1RA applied either during the first two of the three repeated withdrawals or prior to the stress challenge were effective in limiting the magnitude of anxiety-like behavior (Breese, Overstreet, Knapp, et al., 2005; Fig. 2B, C). Together these findings suggested the possibility that stress in the recovering alcoholic could contribute to craving and relapse – an outcome that provides evidence for the idea that the brain’s “neural stress system” is a critical component of the kindling process that supports the CIA persistent adaptation and the stress facilitation of anxiety following withdrawal (Overstreet et al., 2004a, 2005).
Fig. 2.
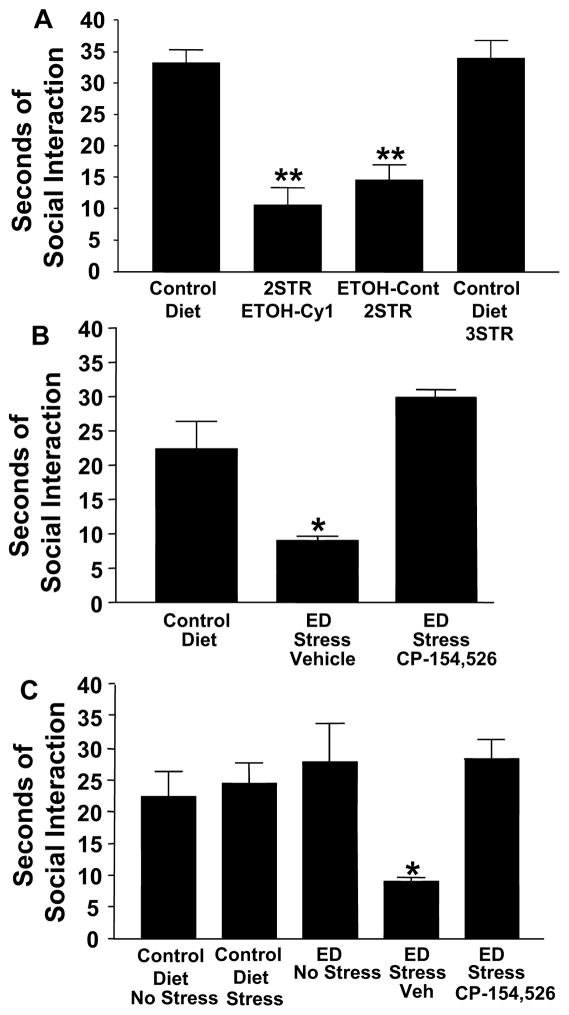
Fig. 2A (top). Sensitization of alcohol withdrawal-induced anxiety (reduced social interaction) with repeated stresses. Male rats were exposed to either continuous control diet or to stress applied at 6 and 11 days while on CD followed by a single 5-day cycle of 4.5% ethanol liquid diet (2STR-ETOH-Cy1). Social interaction testing commenced between 5 and 6-h after the ethanol exposure ended.
2STR-ETOH-Cy1 = two stresses + ethanol + withdrawal. Control Diet-3STR = stressed at 6, 11, and 16 days. ETOH-Cont-2STR = stress at 6 and 11 days during continuous (Cont) ethanol diet + withdrawal. **p < 0.01 compared to CD or CD-3STR. Adapted from Breese et al., 2004.
Fig. 2B (middle). Effect of a CRF-1R antagonist administered prior to testing for stress-induced reductions in social interaction behavior at three days into abstinence from repeated alcohol withdrawals. The CRF-1R antagonist CP-154,526 (10 mg/kg) was administered 30 min prior to restraining of rats for 45 min on day 3 after the last of three withdrawals. Vehicle (Veh) was given to rats exposed to ethanol (alcohol) diet and stress (ED Stress Veh). CP-154,526 significantly blocked the deficit induced by stress in repeatedly withdrawn rats (*p < 0.01 compared to Control Diet group with no stress). Adapted from Breese et al., 2005.
Fig. 2C (bottom). Effect of a CRF-1R antagonist administered during repeated withdrawals on subsequent behavioral responses to stress three days into abstinence from repeated alcohol withdrawals. The CRF1-receptor antagonist, CP-154,526 (10 mg/kg) was administered during the first two withdrawal periods, but not the final withdrawal, of the multiple withdrawal protocol. Vehicle was given to the group that received alcohol diet and stress (ED Stress Veh). CP-154,526 significantly prevented the reduction in social interaction induced by stress applied three days following the repeated withdrawals. *p < 0.01 compared to ED No Stress, Control Diet Stress, and Control Diet No Stress groups and CP-154,526 group. Adapted from Breese et al., 2005.
Humans have been observed to consume excessive amounts of alcohol under stressful conditions, and alcoholic patients have an increased susceptibility to stress – a response not observed in social drinkers (Breese, Chu, et al., 2005; Breese, Sinha, & Heilig, 2011; Gilman & Hommer, 2008; Gilman, Ramchandani, Davis, Bjork, & Hommer, 2008; Sinha, 2001). The Marlatt laboratory (Higgins & Marlatt, 1973, 1975; Marlatt & Gordon, 1985; Vujanovic, Bonn-Miller, & Marlatt, 2011) found that stressful circumstances consistently resulted in greater alcohol drinking. Subsequently, other research linked excessive alcohol use with stressful environments (Breese, Chu, et al., 2005; Breese et al., 2004; Brown et al., 1990; Brown, Vik, Patterson, Grant, & Schuckit, 1995; Kofeod, Friedman, & Peck, 1993; Koob, 2008; Sillaber & Henniger, 2004; Tate, McQuaid, & Brown, 2005; Uhart & Wand, 2009). Additionally, psychiatric disorders commonly susceptible to stress such as depression, generalized anxiety disorder, social phobia, and posttraumatic stress disorder (PTSD) have also been commonly identified with alcohol abuse (Anisman & Merali, 2003; Dunn, Swiergiel, & de Beaurepaire, 2005; Hammen, 2005; Hayley & Anisman, 2005; Kofoed et al., 1993; Miller, Maletic, & Raison, 2009; Pucak & Kaplin, 2005; Raison, Capuron, & Miller, 2006; Uddin et al., 2011). Because alcohol pharmacologically can be viewed as exerting an “anti-tension” action (Gilman et al., 2008), the alcohol tension-reduction hypothesis was proposed to explain the excessive use of alcohol associated with stress (Cappell & Herman, 1972; Conger, 1951, 1956). This latter concept presumably could also explain the behavior of drinking to reduce the negative symptoms accompanying alcohol withdrawal that have been linked to “stress” (Breese et al., 2008), and the consistent finding that previous CIA exposures enhance stress-induced anxiety during alcohol withdrawal (Breese, Overstreet, Knapp, & et al., 2005; Valdez, Zorrilla, Roberts, & Koob, 2003). It has not been determined whether stress-associated elevation in drinking in alcoholics is dependent on the developing adaptation associated with CIA exposure. The demonstration of a contribution of “stress” to the CIA cumulative adaptation (Breese et al., 2011; Heilig & Koob, 2007; Koob & Heinrichs, 1999) may provide a means to implicate neural systems that support “stress” in excessive alcohol ingestion.
Clinically, the stress that accompanies withdrawal from alcohol results in a susceptibility for craving – a reflection of the persistent neural dysregulation in alcoholics (Breese, Overstreet, & Knapp, 2005; Breese et al., 2011; Brown et al., 1990; Cooney, Litt, Morse, Bauer, & Gaupp, 1997; Fox, Bergquist, Hong, & Sinha, 2007; Fox, Bergquist, Peihua, & Rajita, 2010; Fox, Hong, Siedlarz, & Sinha, 2008; Fox, Hong, & Sinha, 2008; Monti et al., 1987; Payne et al., 1992; Sinha, 2007; Sinha & O’Malley, 1999). Over time, the association of negative affect and craving that occurs during acute abstinence is accompanied by altered basal HPA axis function and a suppressed HPA cortisol response to stress in alcoholics compared with non-addicted counterparts (al’Absi, Hatsukami, & Davis, 2005; Badrick et al., 2008; Junghanns et al., 2003; Lovallo, Dickensheets, Myers, Thomas, & Nixon, 2000; Munro, Oswald, Weerts, McCaul, & Wand, 2005). The altered pattern of HPA and autonomic dysregulation in the alcoholic is similar to that documented in chronic high distress states such as PTSD (Ehlert, Gaab, & Heinrichs, 2001; Yehuda, Giller, Southwick, Lowy, & Mason, 1991). Furthermore, the negative emotional state that accompanies withdrawal from alcohol is accompanied by physiological dysregulation, including elevated blood pressure and heart rate. These reflections of adaptation can be accompanied by negative affect, sleep disturbances, and cognitive and behavioral changes – alterations which persist in the alcoholic for an extended period (Brower, 2001; Drummond, Gillin, Smith, & DeModena, 1998; Sinha et al., 2009). Distress and craving in response to stress cues are also observed in abstinent alcoholics but not in controls (Sinha et al., 2009, 2011; Seo et al., 2011). The Sinha Laboratory (Fox, Hong, & Sinha, 2008; Sinha, 2007; Sinha et al., 2009, 2011; see Breese et al., 2011) has pursued the possibility that the persistent maladapted neural changes associated with alcoholism contribute to high relapse rates. In spite of the strong evidence that a persistent adaptation develops over time in the alcoholic (Ballenger & Post, 1978), duration and timing of alcohol abuse required to have a persistent consequence is presently not known.
Multiple brain site involvement of CRF in stress substitution for CIA withdrawal sensitization of anxiety-like behavior
Merlo Pich et al. (1995) used microdialysis to demonstrate that extracellular CRF release increased in the amygdala of rats not only following restraint stress, but also during alcohol withdrawal. Consistent with this report, sheep exposed acutely to a predator (i.e., a stress) also exhibited an increase in CRF release from the amygdala (Cook, 2004). These observations support the possibility that CRF in the amygdala contributes to the link between stress and alcohol withdrawal. Microinjection of a CRF1R antagonist (CRF1RA) into the central amygdala (CeA) prior to each stress application given prior to the final alcohol diet exposure prevented withdrawal anxiety (Fig. 3; Huang et al., 2010). This inhibitory effect of the CRF1RA antagonist in the CeA suggested that this site was a primary contributor to the stress induction of adaptation associated with the negative affect in alcohol abuse (Gilpin, Herman, & Roberto, 2015). CRF1RA microinjection into the dorsal raphe nucleus (DRN) or the bed nucleus of the stria terminalis (BNST) prior to each weekly stress exposure also prevented the sensitization of anxiety observed during alcohol withdrawal (Fig. 3; Huang et al., 2010; Knapp et al., 2011). Recently, Daniel and Rainnie (2015) reviewed evidence that the BNST has an opposing circuitry that can minimize stress modulation of anxiety-like behavior. From these findings, differing brain sites in an integrated CRF (stress) circuit appear to contribute to the repeated stress-induced cumulative adaptation responsible for the anxiety induced during the single withdrawal from a CA exposure.
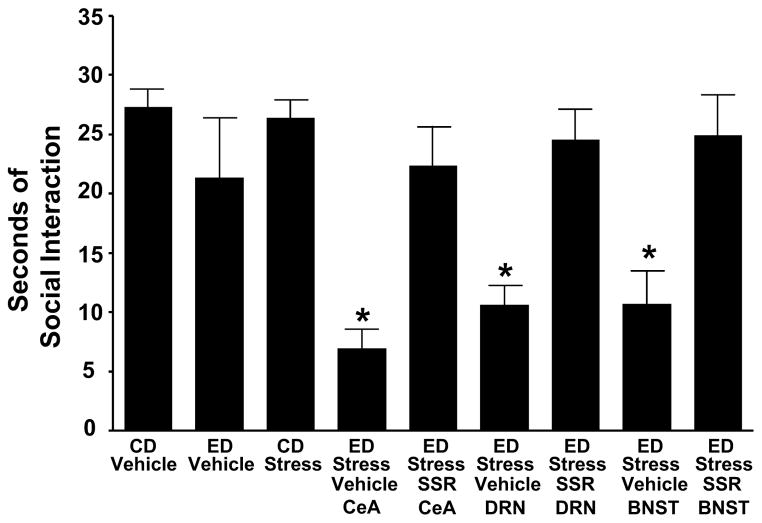
Fig. 3. Microinjection of corticotrophin releasing factor 1 receptor (CRF1R) SSR125543 (SSR) into the CeA, DRN, or d-BNST before restraint stress limits sensitization of alcohol withdrawal-induced anxiety-like behavior in rats.
SSR125543 (SSR; 10 μg/0.5 μL) was microinjected into the CeA, DRN, or the d-BNST 15 min before the two weekly 60-min restraint stresses before exposure to 5 days of 4.5% alcohol (ethanol) liquid diet (ED). Social interaction (SI) was measured 5 to 6 h after the alcohol diet removal. The anxiogenic effect of stress on alcohol withdrawal-induced anxiety was blocked by the SSR microinjected in the selected brain sites. For the control diet- (CD) vehicle and ED-vehicle groups, vehicle was administered into each of the brain sites (n = 3–6 for each site). The vehicle data for each of these controls were combined because a significant change across sites was not observed. No significant effect on SI (p > 0.05) was observed during alcohol withdrawal when the CD-vehicle group was compared with the ED-vehicle group. *p < 0.001 compared with the CD-vehicle, ED-vehicle, and CD stress groups as well as the groups that received the CRF-1 receptor antagonist before the repeated stresses. [F(8,75) = 7.385, *p < 0.001]. CeA = central amygdala; DRN = dorsal raphe nucleus;
d-BNST = dorsolateral bed nucleus of the stria terminalis. Adapted from Huang et al., 2010.
To further test the hypothesis that CRF acts on CRF1Rs in differing brain sites to contribute to the stress facilitation of alcohol-withdrawal anxiety, CRF was microinjected prior to a single 5-day cycle of alcohol exposure at bi-weekly intervals into the CeA, DRN, or BNST to substitute for weekly stress applications (Huang et al., 2010; Knapp et al., 2011). This repeated administration of CRF into these brain sites prior to the single CA exposure also sensitized anxiety during alcohol withdrawal (Fig. 4A). On the other hand, repeated microinjections of CRF into the hypothalamic PVN, the nucleus accumbens, and the CA1 region of the hippocampus prior to the withdrawal from alcohol did not support sensitization of anxiety during alcohol withdrawal (Huang et al., 2010). Furthermore, peripheral injection of the CRF1RA prior to each CRF injection into the CeA, DRN, or BNST prevented the anxiety arising from the subsequent withdrawal from the single 5-day alcohol exposure (Fig. 4B). These observations with CRF1RA injection constituted additional evidence that an integration of CRF action within specific brain sites is likely involved in the stress sensitization of anxiety during withdrawal from the subsequent single CA exposure (Huang et al., 2010). Importantly, microinjection of a specific CRF2R agonist, urocortin-3, into the positive CRF sites produced no sensitization of anxiety during alcohol withdrawal (Deussing et al., 2010). Collectively, this work that demonstrated brain site selectivity for CRF on CRF1Rs (Huang et al., 2010) supported involvement of an integrated neural circuit in the stress sensitization of anxiety during withdrawal from CA (Breese et al., 2011; Huang et al., 2010).
Fig. 4.
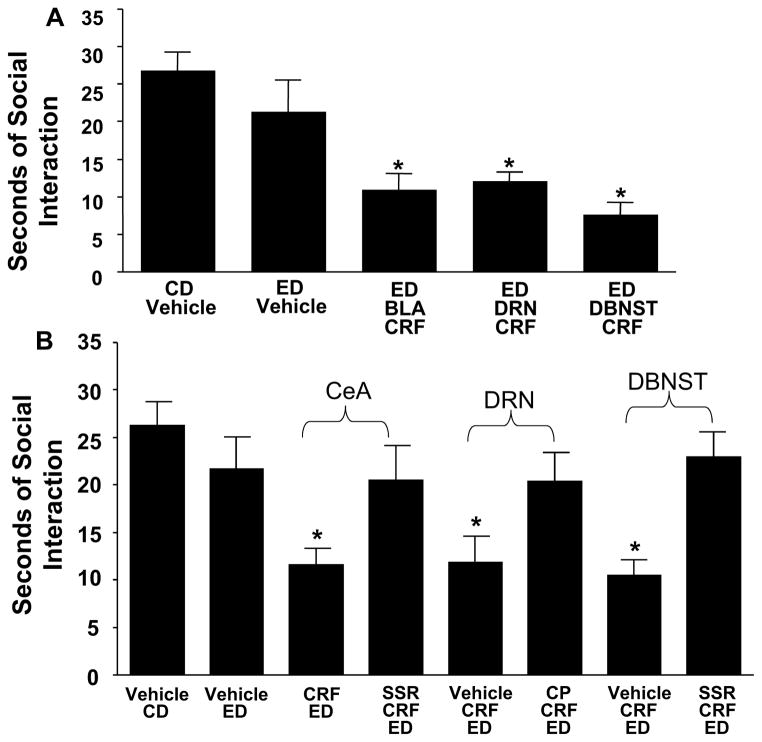
Fig. 4A. Effects of repeated corticotrophin releasing factor (CRF) into the basolateral amygdala (BLA), dorsal raphe nucleus (DRN), and dorsolateral bed nucleus of the stria terminalis (d-BNST) before exposure to chronic alcohol liquid diet reduces social interaction (SI) behaviors during withdrawal. CRF (0.5 μg/0.5 μL) was microinjected twice, once per week, into the BLA, DRN, or d-BNST before exposure to 5 days of 4.5% ethanol (alcohol) liquid diet (ED). In the Control Diet (CD)-vehicle and ED-vehicle groups, vehicle was administered into each of the brain sites (n = 3–4 for each site), and data for these vehicle injections were combined because a significant difference in SI across sites was not observed across these groups. No significant difference was found for the SI between the CD-vehicle and ED-vehicle groups (p > 0.05). A group that received CRF and was on CD only was not included for each of the present sites because previous data demonstrated that intracerebroventricular administration of CRF to rats that received CD sensitize SI deficits (Overstreet et al., 2004), and the repeated CRF in the CeA of control diet-treated animals likewise did not sensitize withdrawal-induced anxiety. SI was measured 5 to 6 h after the ED removal. *Significantly different from CD-vehicle and ED-vehicle [F(4,42) = 9.227, p < 0.001].
Fig. 4B. CRF-1 receptor antagonist blockade of CRF-sensitized alcohol withdrawal-induced anxiety-like behavior when CRF is injected into the central amygdala, dorsal raphe, or dorsolateral bed nucleus of the stria terminalis. A 10 mg/kg dose of the CRF-1 receptor antagonist, SSR125543 (SSR), was administered i.p. 15 min before the microinjection of CRF (0.5 μg) into either the central amygdala or the d-BNST followed by the 5 days of ethanol (alcohol) diet. Another CRF-1 receptor antagonist CP154526 (CP; 10 mg/kg i.p.) was administered 15 min before each of the CRF (0.5 μg) microinjections into the dorsal raphe. CRF in these brain sites sensitized social interaction deficits (reduced SI), and this effect was prevented by the CRF-1 receptor antagonists. SI was measured 5 to 6 h after the removal of ED. In the CD-vehicle group and the ED-vehicle group, vehicle was administered into each of the brain sites (n = 4–6 for each site), and data were combined because a significant change across sites was not observed. No significant difference in SI (p > 0.05) was observed when the CD-vehicle group was compared with the ED-vehicle group. *p < 0.01 compared with vehicle CD- and vehicle ED-treated groups and the groups that received the SSR125543 systemically [F(7,76) = 3.005, p < 0.01]. Adapted from Huang et al., 2010.
Neurocircuitry related to stress supports the negative affect (anxiety) induced by withdrawal from CIA exposure
Sensitization of anxiety is a major outcome of the repeated withdrawals from CIA exposure (Baldwin et al., 1991; Breese, Overstreet, Knapp, et al., 2005; Rassnick et al., 1993). An early review by Davis (2002) provided evidence for specific brain site involvement in anxiety and stress. More recent reviews have focused on defining specific neurocircuitry involvement in the chronic alcohol withdrawal associated with anxiety and stress (Herman & Roberto, 2015; Koob, 2014, 2015; Tovote, Fadok, & Lüthi, 2015). The evidence reviewed herein concerning the specific neurocircuitry associated with CIA withdrawal anxiety seems to indicate that circuitry associated with CRF involvement in stress and alcohol withdrawal is critical for negative affect (Breese et al., 2004, 2008; Huang et al., 2010). In accord with this concept, data from several sources have clearly implicated the CeA as a critical site for supporting CRF involvement in this withdrawal anxiety (Huang et al., 2010; Knapp, Overstreet, Angel, et al., 2007). Namburi et al. (2015) and Silberman and Winder (2015) have also suggested the involvement of a circuit involving the amygdala. Specific brain sites that must interact within a circuit involving CRF include the CeA, BNST, and BLA because CRF placed within these sites substituted for stress enhancement of withdrawal anxiety (Huang et al., 2010; Knapp et al., 2011; Koob, 2014). Supporting the association of CRF in the BNST with anxiety, Daniel and Rainnie’s review (2015) documents the importance of CRF involvement in the BNST in stress-induced anxiety-like behavior. This latter review also provides evidence that norepinephrine as well as serotonin action in the BNST can contribute to stress-induced anxiety – a clue that brain sites linked to these transmitter systems are associated with stress (Daniel & Rainnie, 2015).
The negative emotional state associated with the BNST following chronic stress and CA exposure was antagonized by a CRF1RA in support of CRF involvement (Daniel & Rainnie, 2015; Francesconi et al., 2009). A recent overview documents a role for the BNST in the anxiety and addiction associated with chronic stress in humans (Avery, Clauss, & Blackford, 2015). However, an issue to be clarified is whether the circuitry that is related to the CRF association with anxiety is identical to that activated by stress and CIA exposure (Breese et al., 2011; Shin & Liberzon, 2010; Sinha, 2001; Sinha et al., 2011; Tovote et al., 2015). Findings to date suggest that a neural circuit associated with CRF involvement in stress and alcohol withdrawal is critical for the negative affect noted during withdrawal (Silberman & Winder, 2015). In accord with this concept, Namburi et al. (2015) described a circuit mechanism that may be responsible for functional involvement of the amygdala. As these reports emphasize, the neurocircuitry associated with the role of stress and alcohol with alcohol and other abused drugs remains an active area of interest (Mantsch, Baker, Funk, Lê, & Shaham, 2015; Tovote et al., 2015).
One can conclude from the various reports on stress and CA consequences that the associated neurocircuitry supporting these changes related to alcohol withdrawal is complex. Even though documentation of the involvement of differing brain sites in the CRF and stress contribution to CA adaptation is informative, the means by which CRF action on CRF1Rs in these differing brain regions individually support the cumulative adaptation that sensitizes alcohol withdrawal to induce anxiety has not been defined. Consequently, efforts are warranted to define how CRF released by stress in these differing brain sites interacts within a neural circuit to sensitize anxiety during the withdrawal from CA intake (Breese et al., 2008). One focus could be on determining whether these brain sites form a circuit that supports the regional effects of CRF on sensitization of withdrawal anxiety. Future work should define how CRF released by stress in differing brain sites interacts within a proposed neural circuit to sensitize anxiety during alcohol withdrawal (Breese et al., 2008). A key experiment could test whether induction of anxiety by CRF placement into one of these sensitive sites would be prevented by simultaneous placement of a CRF1RA into the CeA or one of the other CRF sites supporting ethanol withdrawal-induced anxiety (Huang et al., 2010). Such research would also shed light on the degree to which these specific brain sites involved in CRF sensitization of withdrawal anxiety form an interactive circuit that is the basis of the regional effects of CRF action observed during stress and withdrawal.
The Roth laboratory has recently published several reviews related to Designer Receptors Exclusively Activated by Designer Drugs (DREADD) technology (Sternson & Roth, 2014; Urban & Roth, 2015). Utilizing a DREADD protocol, our laboratory demonstrated that inhibition of glutamate output from the BLA prevented the anxiety that follows withdrawal from CA exposure (unpublished observation) – a finding confirming that the BLA is involved in the adaptation that induces anxiety during withdrawal from CIA exposure. This latter observation is consistent with the finding that chronic early stress that activates the BLA facilitates anxiety (Rau, Chappell, Butler, Ariwodola, & Weiner, 2015). Whether this aspect of BLA involvement in the withdrawal anxiety is associated with a selective BLA link to the CeA has yet to be confirmed. The degree to which GABA neural output from the medial portion of the CeA to distant sites is involved in the CA withdrawal and stress-induced anxiety also warrants further elucidation. Approaches to further identify the specific circuitry involved will be essential for fully understanding the neural basis of the persistent adaptation that supports alcohol-induced withdrawal anxiety. Future strategies may require new approaches to characterize fully the specific circuitry and sites involved in the withdrawal anxiety and sensitization of stress following withdrawal from CIA exposure. This new effort must also document how specific brain sites identified within a given circuit interact. The approach by Richiardi et al. (2015), who have explored how gene expression correlates with brain networks, could possibly be a means to explore interactions of differing brain regions associated with molecular and behavioral outcomes during withdrawal from chronic alcohol. Fig. 5 provides a summary of the possible brain sites that may interact to form circuits that support involvement of CRF and stress in withdrawal-induced anxiety associated with CA exposure, and illustrates how these sites may be linked to involvement of neuroimmune function (details to follow).
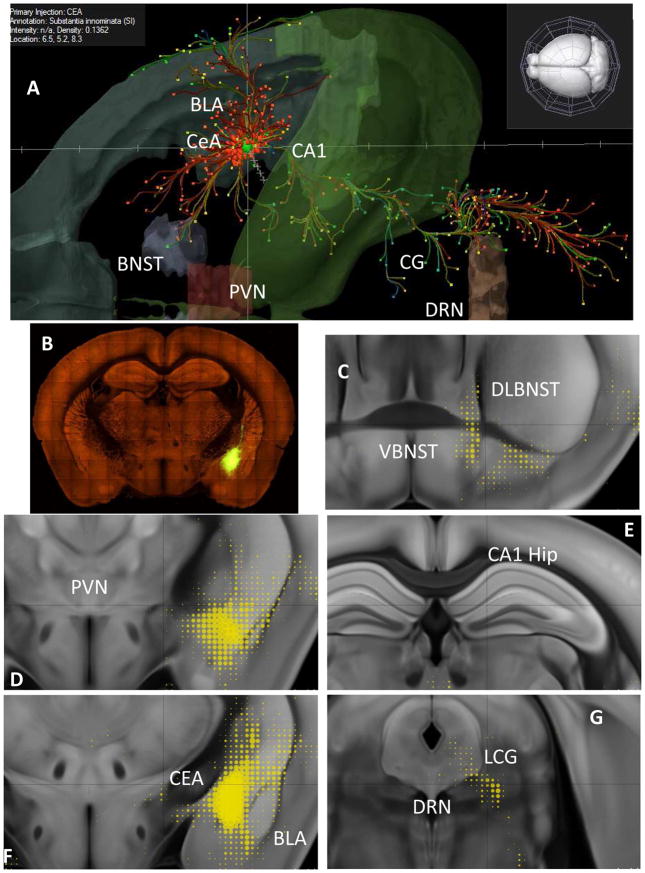
Fig. 5. Anatomical depictions of brain sites where CRF actions influence alcohol withdrawal-induced anxiety.
Positive sites (central nucleus of the amygdala, CeA; basolateral nucleus of the amygdala, BLA; dorsolateral bed nucleus of the stria terminalis, DLBNST; and dorsal raphe nucleus, DRN) and negative sites (VBNST, PVN, and hippocampus) identified in Huang et al. (2010) are overlaid on mouse images derived from the Allen Mouse Brain Connectivity Atlas (2015, Allen Institute for Brain Science; http://connectivity.brain-map.org) and Oh et al., 2014. Above, the reference animal was an erbb4-2A-creERT2 mouse (Madisen et al., 2010; see also Bi et al., 2015) injected with virus (89-nanoliters) that led to expression in GABAergic neurons of the CeA (Bregma: −0.94, 2.43, 4.47) (Bi et al., 2015). A. CeA projection patterns customized from the Mouse Connectivity Atlas and extending anteriorly in the extended amygdala, laterally toward nearby cortex, and posteriorly into the brain stem. B. Serial two-photon tomography of the CeA injection site. C. Ventral and dorsal BNST projections (yellow; dot sizes reflect relative density of innervation of that region as per the Composite Projection Viewer). D. Absence of PVN projections. E. Absence of hippocampal projections. F. CeA injection site and local/regional projections. G. DRN and lateral central gray (LCG) projections.
Withdrawal from chronic alcohol (CA) exposure facilitates an increase in cytokine mRNA in brain – relationship to stress
During the period when investigations implicated CRF involvement in the contribution of stress in alcohol withdrawal-induced anxiety, literature was accumulating that stress alone increased cytokines, cytokine mRNAs, and other neuroimmune factors in brain (Blandino, Barnum, & Deak, 2006; Blandino et al., 2009; Deak et al., 2005; Girotti, Donegan, & Morilak, 2011; Hueston et al., 2011; Johnson, O’Connor, Watkins, & Maier, 2004; Johnson et al., 2005; Minami et al., 1991; Nguyen et al., 1998, 2000; O’Connor et al., 2003; Porterfield et al., 2011; Shintani, Nakaki, Kanba, Kato, & Asai, 1995; Shintani, Nakaki, Kanba, Sato, et al., 1995; Shizuya et al., 1997; Suzuki, Shintani, Kanba, Asai, & Nakaki, 1997). The latter findings considered in the context of the increased susceptibility of alcoholics to stress (Gilman & Hommer, 2008; Gilman et al., 2008; Sinha, 2001) suggested the possibility that the adaptation induced by CIA exposure and stress might be associated with a facilitation of neuroimmune (cytokine) function (Breese et al., 2004; 2008, 2011). Blednov et al. (2005) had earlier provided evidence for this possibility by finding that deleting genes of chemokines altered alcohol reinforcement – a conclusion later confirmed (Blednov et al., 2012). Measurement of cortical cytokine mRNA levels prior to withdrawal from CA exposure revealed no increase in these mRNAs (Whitman, 2012; Whitman, Knapp, Werner, Crews, & Breese, 2013), even though stress alone activated neuroimmune function (Blandino et al., 2009; Deak et al., 2005; Johnson et al., 2004, 2005; Minami et al., 1991). Nonetheless, cytokine levels have been found to be elevated in plasma of alcoholics (Achur, Freeman, & Vrana, 2010; Kiefer, Jahn, Schick, & Wiedemann, 2002) as well as in their brains at autopsy (Crews, Qin, Sheedy, Vetreno, & Zou, 2013; He & Crews, 2008). Additionally, a cytokine-gene polymorphism was found to be associated with alcohol dependence (Liu, Hutchinson, White, Somogyi, & Coller, 2009; Saiz et al., 2009). The implications of these findings concerning neuroimmune function in alcoholism have not as yet been fully explored.
As noted earlier (Overstreet et al., 2002), three cycles of withdrawal from a 4.5% CIA exposure facilitated anxiety-like behavior whereas continuous alcohol exposure did not. A subsequent investigation assessed whether an increase in neuroimmune function would be altered in a similar fashion by these CA exposures (Whitman, 2012; Whitman et al., 2013). However, the cycled and continuous introduction of the lower 4.5% alcohol diet, which had previously been demonstrated to sensitize anxiety during withdrawal (Overstreet et al., 2002), did not increase cortical cytokine mRNAs or cytokines 24 h after withdrawal (Harper, Knapp, & Breese, 2015; Whitman, 2012; Whitman et al., 2013). In response to a 24-h alcohol withdrawal from 7% alcohol CIA diet, CCL2 (MCP-1), IL1β, and TNFα mRNAs were increased in cortex (Whitman, 2012; Whitman et al., 2013; Fig. 6). Unexpectedly, a comparable increase in neuroimmune mRNAs was also found in cortex following withdrawal from the 7% continuous alcohol diet exposure (Fig. 6), just as seen with withdrawal from the CIA 7% diet exposure (Whitman, 2012; Whitman et al., 2013). These comparable cytokine mRNA increases obtained during the CIA and continuous 7% alcohol exposures implicate a requirement for higher alcohol doses to increase cytokine mRNAs during alcohol withdrawal. Doremus-Fitzwater et al. (2014) have since reported that withdrawal from CA exposure also increases expression of both central and peripheral cytokine mRNAs. Additional investigations have found similar brain cytokine mRNA effects in adolescents exposed to a 5.3% alcohol diet compared with adults that received the 7% alcohol diet (Harper et al., 2015; Kane et al., 2014). These latter age-dependent findings could be relevant to reports that adolescent exposure to alcohol is associated with the appearance of alcoholism in adulthood (Hingson, Heeren, & Winter, 2006). Whether the increase in neuroimmune function associated with stress and withdrawal from chronic alcohol is associated with proliferation of microglia (Zou, Vetreno, & Crews, 2012) is not known. Likewise unknown is the degree to which lipopolysaccharide- (LPS) associated activation of neuroimmune function after CA exposure (Qin et al., 2007; 2008) is related to the increase in cytokine mRNAs associated with withdrawal from CA exposure. Additionally, given the extensive literature that females respond differently to chronic stress than males (see review by McEwen, Nasca, & Gray, 2015), further investigation of sex differences to withdrawal from CA exposure should be considered.
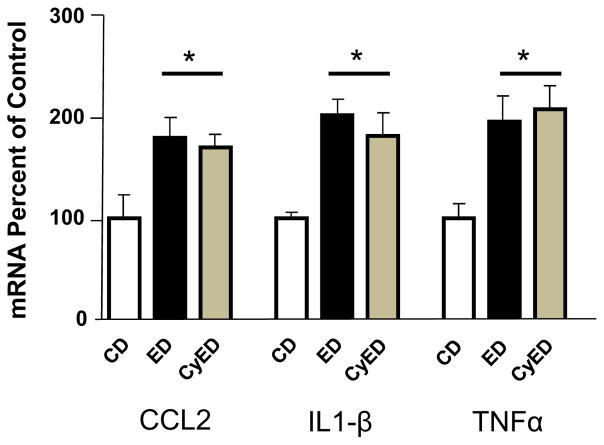
Fig. 6. Comparison of chronic 7% dietary ethanol (alcohol) (ED, 15 days continuous diet) and chronic intermittent/cycled alcohol diet (ED, three 5-day cycles with 2 days of control liquid diet (CD) between cycles) on cytokine mRNAs in brain cortex.
Twenty-four hours into withdrawal from ED, both the chronic continuous ED and cycled ED treatment groups had elevated CCL2, IL1β, and TNFa mRNA, F(2,18) = 5.211, p = 0.018; F(2,24) = 9.718, p = 0.0009; F(2,25) = 6.475, p = 0.006, respectively. No significant difference was found between the cytokine mRNAs for the two chronic ethanol-treated groups relative to each other (p > 0.05). *Significantly different from CD (p < 0.05). CCL2, chemokine(C-Cmotif) ligand 2; IL1β, interleukin-1β, TNFα, tumor necrosis factor-α; CyED = chronic intermittent/cycled alcohol. Diet: ED = chronic continuous alcohol diet. Adapted from Whitman et al., 2013.
To follow up on the possibility that the stress increase in brain neuroimmune function (Johnson et al., 2004, 2005) could relate to CA withdrawal anxiety, Breese et al. (2008) found that individual cytokines (i.e., TNFα, IL1β, and CCL2 [MCP-1]) injected into brain prior to a single exposure to CA sensitized withdrawal anxiety (Fig. 7), just as multiple stresses and microinjections of CRF did when given prior to the single alcohol exposure (Breese et al., 2004, 2008; Huang et al., 2010). In accord with this latter work, Arakawa, Blandino, and Deak (2009) found that central infusion of an IL1β antagonist prevented the social interaction change produced by a subsequent stress exposure. Additionally, Knapp et al. (2011) determined that repeated administration of TNFα into the CeA prior to 5 days of alcohol exposure sensitized anxiety during withdrawal from a single alcohol exposure – a finding further implicating the CeA in the adaptation associated with sensitization of withdrawal-induced anxiety. Knapp, Whitman, Harper, and Breese (2015) subsequently demonstrated that withdrawal from exposure to continuous CA selectively induced a different pattern of CCL2-, IL1β-, and TNFα-mRNA levels in amygdala, hippocampus, and hypothalamus than observed in cortex, a finding consistent with the idea that not all brain sites contribute equally to the neuroimmune response that occurs during withdrawal from CA exposure.
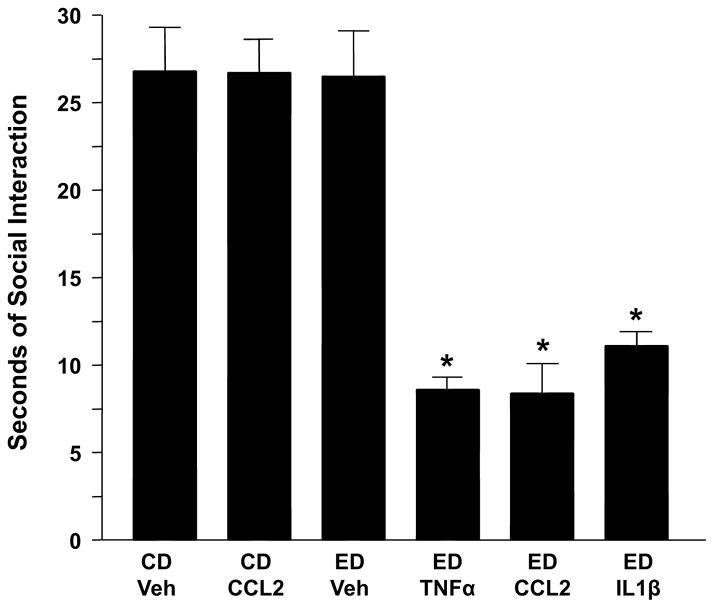
Fig. 7. Repeated IL1β, TNFα, or CCL2 sensitize alcohol withdrawal-induced anxiety-like behavior.
Rats were injected twice at weekly intervals with either vehicle or cytokine (IL1β, TNFα, or CCL2; 100 ng/5 μL, intracerebroventricularly [i.c.v.]) while drinking control diet (CD) and then continued on CD or switched to a 4.5% alcohol (ethanol) diet (ED) for 5 days. MCP1 was also given twice in a group exposed only to CD to test for an effect on social interaction (SI) deficits (the operational definition of anxiety-like behavior) without prior ED exposure. SI for all groups was measured 5–6 h into withdrawal. CCL2 = CC chemokine ligand 2 (formerly MCP-1 = monocyte chemo-attractant protein-1); IL1β = interleukin-1β; TNFα = tumor necrosis factor-α;
CD = control diet; Veh = artificial cerebrospinal fluid. *p < 0.001 compared to CD-Veh or ED-Veh groups. Adapted from Breese et al., 2008.
The prevention of brain cytokine mRNAs by a CRF1RA prior to initial withdrawals from CA in rats suggests a specific CRF mechanism for the withdrawal increase in cytokine mRNAs (Whitman et al., 2013). The observation that cytokine microinjection into the CeA substituted for CRF sensitization of withdrawal-induced anxiety supported the conclusion that cytokine mRNAs increase in sites where CRF acts to support adaptive changes associated with stress (Knapp et al., 2011). Given that CRF and cytokines in the CeA likely mediate stress influences on chronic alcohol-associated neuroimmune changes, a future investigation should determine whether microinjection of CCL2 and IL1β into the DRN or the BNST will sensitize anxiety during a withdrawal from a subsequent single alcohol exposure (Breese et al., 2008; Huang et al., 2010). This future investigation would determine whether all brain sites previously related to CRF sensitization of withdrawal anxiety also support cytokine sensitization of alcohol withdrawal-induced anxiety. Given the recent observation by Gray et al. (2015) that CRF drives anandamide hydrolysis to promote stress-induced anxiety, it may be warranted to test whether antagonism of anandamide action in the CeA, the DRN, or the BNST (Huang et al., 2010; Knapp et al., 2011) will prevent the anxiety and the accompanying increase in cytokine mRNAs that follow withdrawal from chronic alcohol (Knapp et al., 2011; Overstreet et al., 2002; Whitman et al., 2013). Such new information could be critical for understanding the persistent adaptation associated with the neuroimmune activation that follows CIA exposure.
Brain cytokine mRNAs after stress alone in controls and after CA withdrawal may be supported by distinct mechanisms
In a recent study from our laboratory (Knapp et al., 2015), restraint stress was confirmed to increase cytokine mRNAs in the cortex of control rats (Deak et al., 2005; Girotti et al., 2011; Johnson et al., 2004, 2005; Porterfield et al., 2011) to approximately the same degree observed following withdrawal from the CA treatment (Whitman, 2012; Whitman et al., 2013). Given that a CRF1RA prevents the increase in CCL2, IL1β, and TNFα mRNAs induced by withdrawal from continuous alcohol exposure (Whitman et al., 2013), it was presumed that the CRF1RA would prevent the stress increase in neuroimmune function in controls. However, the CRF1RA did not block the restraint stress increase in cytokine mRNAs in control rats (Knapp et al., 2015, & unpublished). These findings suggest that the mechanism of stress induction of cytokine mRNAs in cortex of controls is distinct from that responsible for the cortical increase in cytokine mRNAs induced during withdrawal from CA exposure (Knapp et al., 2015; Whitman et al., 2013).
A possible clue to the distinct mechanisms induced by these challenges comes from finding that a β-adrenergic receptor antagonist blocks the increase in neuroimmune function by stress in controls (Johnson et al., 2005; Porterfield et al., 2012) – an outcome that may be related to stress priming of microglia (Johnson, Zimomra, & Stewart, 2013). β-adrenergic input has also been shown to activate an inflammatory response in controls (Madrigal et al., 2010), just as stress increases IL1β and other cytokine mRNAs (Porterfield et al., 2012). Accordingly, β-adrenergic antagonism also prevents microglial activation and anxiety-like behavior induced by stress (Wohleb et al., 2011). Whether β-adrenergic antagonism will prevent the increase in cortical cytokine mRNAs induced by withdrawal from CA exposure has yet to be defined. The later experiment should include an assessment of whether a β-adrenergic antagonist will alter brain cytokine mRNA increases in the CeA, the DRN, or the BNST following withdrawal from CA exposure. Another future experiment should also define whether a β-adrenergic antagonist during CIA exposure will influence sensitization of anxiety induced during withdrawal (Huang et al., 2010).
When stress is applied 24 h after withdrawal, the anxiety induced is enhanced (Breese et al., 2004; 2008, 2011); therefore, it was presumed that stress 24 h after the withdrawal from CA exposure would cause a further increase in selected cytokine mRNAs in brain. The extended absence from CA exposure was believed to be necessary for the stress to cause a greater response, because previous work had demonstrated that stress during withdrawal from an acute alcohol exposure did not increase neuroimmune function (Buck, Hueston, Bishop, & Deak, 2011). In an initial test of this hypothesis, stress increased CCL2 and IL1β mRNAs above that induced by withdrawal from CA intake alone. As this initial elevation of neuroimmune function by stress after CA exposure was not confirmed in a subsequent investigation, future studies will be required to resolve whether stress after CA exposure indeed causes a further increase in cytokine mRNAs after withdrawal from CA exposure. If an elevated level in cytokine mRNAs is confirmed to occur with stress after withdrawal from chronic alcohol, we will seek to define if a CRF1RA or a β-adrenergic receptor antagonist given prior to this stress challenge after CA withdrawal will affect the degree to which cytokine mRNAs are increased.
Cytokine influences on neural function
The close relationship of cytokine function to stress and CRF led many to posit that cytokines may act as neuromodulators (Adler, Geller, Chen, & Rogers, 2006; Bauer, Kerr, & Patterson, 2007; Lukàts, Egyed, & Karádi, 2005; Rostène, Kitabgi, & Parsadaniantz, 2007; Shintani, Nakaki, Kanba, Kato, et al., 1995). Given that a CRF1RA prevented the effect of cytokine microinjection into brain from substituting for stress (Knapp et al., 2011), a question that arose was whether this CRF1R antagonism provided indirect evidence for an association of cytokine actions in the CeA with neural function. Such efforts to understand cytokine influences on neural function have been proceeding on a number of fronts. A subsequent electrophysiological study demonstrated that TNFα decreased the threshold for triggering an action potential from CeA neurons without altering membrane properties during current-clamp recording (Ming, Criswell, & Breese, 2013). Further, TNFα increased amplitude but not frequency of mEPSCs from some but not all CeA neurons (Ming et al., 2013). Similarly, CCL2 was found to increase firing rates in the striatum (Guyon et al., 2009). In the hippocampus, CCL2 increased synaptic transmission via presynaptic influences on transmitter release (Zhou, Tang, Liu, Dong, & Xiong, 2011).
Electrophysiological studies also demonstrated that TNFα influences GABA function in the CeA (Ming et al., 2013). TNFα increased the frequency but not the amplitude of mIPSCs from some but not all CeA neurons – an outcome consistent with a selective neural influence of TNFα on GABA function in this brain site. Bajo et al. (Bajo et al., 2014; Bajo, Herman, et al., 2015; Bajo, Varodayan, et al, 2015) have also reported that immune factors modulated alcohol influences on GABAergic transmission in the CeA. In unpublished work, our laboratory also found that CCL2 can enhance GABA release from some CeA neurons. In accord with these latter findings, Bajo and colleagues (Bajo, Herman, et al., 2015) found that IL1β influences mIPSCs of some CeA neurons but not others, further evidence for a similar influence of this cytokine on GABAergic function in the CeA as observed with TNFα (Ming et al., 2013). Bajo, Varodayan, et al. (2015) reported that an IL1β receptor antagonist prevented the IL1β increase in mIPSC frequency. Consistent with a relationship between alcohol and cytokine action, alcohol-like cytokines significantly increased eIPSP amplitude and increased mIPSC frequencies (Bajo, Varodayan, et al., 2015). While co-application of IL1β and alcohol did not alter the increase in eIPSP amplitude by alcohol, the alcohol-induced increase in mIPSCs was prevented by IL1β co-application (Bajo, Varodayan, et al., 2015). Liu et al. (2011) reported that the influence of binge alcohol drinking on GABAA receptor function was related to TLR4 expression in the amygdala. It is not clear whether this latter result directly relates to cytokine involvement in neural function.
Based upon work showing that CRF1RA into the CeA prevented CIA withdrawal-induced anxiety (Huang et al., 2010) and that CRF release is induced by IL1β (Turnbull & Rivier, 1995), Ming et al. (2013) explored whether a CRF1RA would alter the TNFα-induced increase in mIPSC frequency. The CRF1RA prevented the TNFα-induced increase in mIPSC frequency without altering the TNFα-induced amplitude increase in mEPSCs or the TNFα-reduced threshold for action potentials. (Ming et al., 2013). This finding suggested the possibility that at least some cytokine actions are mediated via indirect actions through CRF receptor signaling – a conclusion consistent with the observation that the CRF1RA blocked the action of TNFα to support alcohol withdrawal anxiety (Breese et al., 2004; Knapp et al., 2004; Knapp, Overstreet, Angel, et al., 2007; Overstreet et al., 2003, 2004a, 2005). Bale & Vale (2004) hypothesized that stress release of CRF causes a release of cytokines that in turn further facilitates CRF release (del Cerro & Borrell, 1990). To clarify how TNFα might involve CRF release in the presence of tetrodotoxin, the possibility that preventing glial activation with minocycline (Giuliani, Hader, & Yong, 2005; Gong, Zou, Fuchs, & Lin, 2015) would alter the elevated mIPSC frequency by TNFα was tested (Ming et al., 2013). The minocycline action prevented the TNFα-induced increase in mIPSC frequency – a finding consistent with a glial contribution in this CRF neural involvement (Ming et al., 2013). Cytokine effects on calcium and sodium channels have also been documented. For example, actions of CCL2 were found to depend upon T-type Ca2+ channels or Na+ channels (Banisadr, Gosselin, Mechigel, Rostène et al., 2005; Belkouch et al., 2011; Gosselin et al., 2005; van Gassen, Netzeband, de Graan, & Gruol, 2005). In the striatum, CCL2 was found to increase firing rates via CCL2 effects on membrane leak channels (Guyon et al., 2009). In other neuronal preparations, actions of CCL2 via T-type Ca2+ channels or Na+ channels have been identified (Banisadr, Gosselin, Mechighel, Rostène, et al., 2005; Belkouch et al., 2011; Gosselin et al., 2005; van Gassen et al., 2005). The possible relationship of these channels to CRF and CA influences on cells has not been identified.
These basic electrophysiological observations of cytokine action provide renewed support for the view that neural and immune functions in brain may interact to support withdrawal anxiety and the increases in brain cytokine mRNAs associated with CA adaptation. However, the observation that not all neurons in the CeA responded to TNFα also needs further clarification (Bajo, Herman, et al., 2015; Bajo, Varodayan, et al., 2015; Ming et al., 2013). Likewise, as only TNFα and IL1β have been tested in the CeA, the preliminary observation that CCL2 enhances mIPSC frequency will need confirmation, and a possible linkage of this CCL2 action to CRF function will need to be explored. Whether all cytokines linked to stress and CRF induction of withdrawal anxiety (Huang et al., 2010; Whitman et al., 2013) will be associated with neural actions in all brain regions like those observed in the CeA (Ming et al., 2013) also remains to be assessed.
CRF and neuroimmune signaling links to CA adaptation
Phenotypes related to anxiety and CA drinking are responsive to manipulations by stress, CRF, and cytokines (e.g., Blednov et al., 2005; Breese et al., 2008; Knapp et al., 2011). Further, demonstration of cytokines within neurons and glia (Banisadr, Gosselin, Mechighel, Kitabgi, et al., 2005; June et al., 2015) and evidence for cytokine receptors on both neurons and glia (Banisadr et al., 2002; Banisadr, Gosselin, Mechighel, Rostène, et al., 2005) suggest the potential for cytokine signaling systems across cell types and circuits that will influence neural function. The underlying signaling mechanisms that drive actions of cytokines and CRF are not well understood, although physiological studies described above (“Cytokine Influences on Neuronal Function”) provide clues that can guide future efforts to isolate relevant mechanisms. Involvement of the amygdala in anxiety has been clearly outlined for CRF and cytokines. In addition, classic neurotransmitters like GABA, glutamate, norepinephrine, and serotonin are all well known to play a role in amygdala contribution to anxiety-related behavior. These neural systems have long been known to directly impact both conditioned and unconditioned negative emotional responding via the amygdala. Less well understood is how cytokine and CRF systems may interact across these behavioral domains via common or independent signaling mechanisms to regulate behaviors influenced by the amygdala, including alcohol consumption and alcohol withdrawal-related behaviors. It is also possible that individuals at risk for alcohol abuse have differential engagement of CRF or cytokines in brain. The recent observation that CRF and alcohol action on glutamate transmission in the CeA is selectively altered in Marchigian Sardinian alcohol-preferring rats but not in Wistar rats is an example of such differential involvement in animals (Herman, Varodayan, et al., 2015). Assuming that alcoholism has a genetic basis, perhaps such distinction in CRF involvement observed in differing rat strains occurs between non-alcoholics and alcoholics. Such differential vulnerability across species and strains could be a fruitful area for additional research to elucidate mechanisms of the persistent adaptation that supports alcohol abuse.
Several laboratories have proposed that TLR4 signaling is a key link between alcohol and activation of neuroimmune function (Akira & Takeda, 2004; Alfonso-Loeches & Guerri, 2011; Alfonso-Loeches, Pascual-Lucas, Blanco, Sanchez-Vera, & Guerri, 2010; Alfonso-Loeches et al., 2012; Blanco, Vallés, Pascual, & Guerri, 2005; Fernandez-Lizarbe, Pascual, Gascon, Blanco, & Guerri, 2008; Fernandez-Lizarbe, Pascual, & Guerri, 2009; Gárate et al., 2013; Pandey, 2012; Pascual, Baliño, Alfonso-Loeches, Aragón, & Guerri, 2011; Pascual, Baliño, Aragón, & Guerri, 2015; Weber, Frank, Sobesky, Watkins, & Maier, 2013; Wu et al., 2012; Yamada & Maruyama, 2007; Yang, Wang, Czura, & Tracey, 2005; Yu et al., 2006; Zou & Crews, 2010). In support of this view, TLR4 was increased in mouse brain following CA intake as well as in cortex of post mortem alcoholics (Crews et al., 2013; He & Crews, 2008). Likewise, the increase in cytokine production after withdrawal from prolonged alcohol was prevented in TLR4 knockout mice (Pascual, Baliño, Aragón, & Guerri, 2015; Pla, Pascual, Renau-Piqueras, & Guerri, 2014). Further, TLR4 mRNA was increased in cortex after withdrawal from both the CIA and continuous alcohol protocols (Whitman, 2012; Whitman et al., 2013). The increase in TLR4 mRNA induced by the CA exposures could be an important factor in the reported involvement of TLR4 signaling in alcohol action (Crews et al., 2013; Pascual et al., 2015). Montesinos et al. (2015) found that TLR4 elimination prevented synaptic and cognitive dysfunctions in adolescent mice that received CIA exposure. Additionally, Weber et al. (2013) found that blockade of TLR4 during stress prevented a subsequent LPS-induced neuroimmune response.
Other factors that may contribute to the potential involvement of TLR4 in CA adaptation are MyD88 and TRIF – adaptor proteins that influence the transcription factor NF-kB (Cheng, Taylor, Ourthiague, & Hoffman, 2015). Another endogenous factor linked to TLR4 is the high-mobility group box 1 protein (HMGB1) (Andersson & Tracey, 2011), which initiates cytokine release (Frank, Weber, Watkins, & Maier, 2015). Like TLR4 mRNA, HMGB1 mRNA was elevated in cortex after both CIA and continuous CA exposure (Whitman, 2012; Whitman et al., 2013). Functional antagonists of HMGB1 block the cumulative increase in cytokine mRNAs that follows withdrawal from CA exposure (Davé et al., 2009; Kim et al., 2006; Lin et al., 2011; Park et al., 2004, 2006; Su, Wang, Zhao, Pan, & Mao, 2011; Ulloa et al., 2002; Whitman et al., 2013). Collectively, these findings support an interactive link between TLR4 activity and the increase in neuroimmune function that follows withdrawal from CA exposure. Further investigation of the effects of TLR4 and factors linked to repeated CA withdrawals and stress activation of cytokine mRNAs in various brain regions is warranted.
Relatedly, CRF-amplified neuronal TLR4/MCP-1 (CCL2) signaling was shown to regulate alcohol drinking (June et al., 2015); therefore, it is apparent that regions of brain known to regulate drinking (e.g., accumbens, amygdala, and others) are attractive brain sites for further investigation of these cytokine interactions with CRF. Recent reports from Herman, Roberto, and colleagues (Bajo, Herman, et al., 2015; Bajo, Varodayan, et al., 2015; Herman & Roberto, 2014, 2015) provide a classic neurotransmitter and local circuit framework through which cytokine effects may be manifested. Moreover, these investigators (Bajo, Herman, et al., 2015; Bajo, Varodayan, et al., 2015; Herman & Roberto, 2014, 2015) have shown that different populations of CeA neurons defined via physiological phenotyping respond differentially to alcohol. Consequently, interleukin-1β (IL1β) receptors on these CeA-GABA neurons may regulate their effects via signaling that ultimately regulates amygdala outputs that control emotion and other behaviors. Such effects are even more notable in the context of data showing that IL1β induces anxiety through interactions with the endocannabinoid system in the brain (Rossi et al., 2012) – a finding consistent with an association of dysregulation of endocannabinoid function with anxiety (Lutz, Marsicano, Maldonado, & Hillard, 2015). Additionally, Hodge and colleagues (Agoglia, Holstein, Reid, & Hodge, 2015) have provided evidence that cytokines acting through glutamate could be involved. These investigators (Agoglia et al., 2015) showed that binge drinking linked to glutamate function decreased CaMKIIα T286- and CaMKIIα GluA1S831-dependent phosphorylation (pGluA1S831) in adolescent rat amygdala. This observation (Agoglia et al., 2015) and others (Ming et al., 2013) that relate phosphatidyl inositol-3 kinase antagonist action to glutamate suggest that glutamate signaling may be another avenue through which cytokines could act to influence the persistent outcome of CA exposure.
The fact that kinases generally are worthy of further assessment in connection with cytokine/CRF interaction in brain is suggested by work showing that p38-mediated protein kinase is induced by CCL2 in cultured hippocampal cells (Cho & Gruol, 2008). The second messenger protein kinase C (PKC) epsilon, which supports CRF action in the amygdala, is likely engaged within GABA neurons (Bajo, Cruz, Siggins, Messing, & Roberto, 2008), by CRF1R action linked to Gs- and Gq-mediated signaling (Hanoune & Defer, 2001; Papadopoulou et al., 2004). Another established signaling mechanism involving CRF1-receptor signaling is PKA activation (Papadopoulou et al., 2004) – an engagement confirmed in the amygdala (Cruz, Herman, Kallupi, & Roberto, 2012). The CRF1R activation of PKA via the Gs and Gq proteins (Hanoune & Defer, 2001; Papadopoulou et al., 2004) likely regulates amygdala-related anxiety-like behavior via this mechanism. Thus, one might speculate that the action of CRF on CRF1Rs in other behaviorally significant regions such as the BNST and dorsal raphe may also be linked to these same kinase signaling mechanisms. Additionally, the CCL2 cytokine action on CCR2 receptors activates MAPK (Jiménez-Sainz, Fast, Mayor, & Aragay, 2003). The fact that a phosphatidyl inositol-3 kinase antagonist prevents the TNFα-increased amplitude of mEPSCs suggests TNFα has an intracellular influence on this neural function (Ming et al., 2013). Such findings imply that the potential routes through which CRF and cytokines interact to influence function are diverse. More generally, in areas where classic neurotransmitter receptors engage signaling systems important to behavior, particularly those behaviors regulated by amygdala activities following chronic alcohol exposure, cytokine modulation via their respective receptors may alter the effects associated with classic transmitters such as CRF. It would seem essential that future studies focus on cells in behaviorally relevant brain regions that have cytokine/chemokine receptors and their specific signaling systems in order to fully characterize the effects cytokines and CRF have on behavior during and after CA exposure.
Defining the signaling links will provide support for a number of avenues and mechanisms that could be explored to further define CRF and cytokine actions. This information will contribute to our understanding of the functional basis of the persistent adaptation responsible for the behavioral effects associated with CIA withdrawal and sensitization of stress after CIA exposure. For the future, an area that could be of interest would be defining whether second messengers are common to the varied types of neural systems that appear to be involved in CIA-induced withdrawal anxiety. Likewise, further exploration of stress having a differing basis for increasing cytokine mRNAs in the cortex than withdrawal from CA exposure could assist in resolving other unexpected outcomes. Defining the potential involvement of cytokines and other peptides in neural systems implicated in circuits associated with stress and withdrawal from CA would also seem worthy of future investigation. Chief among these future explorations may be additional delineation of cytokine action on classic neurotransmitter effects in the amygdala and associated brain regions that relate to the anxiety that follows withdrawal from CA and CIA exposure. An understanding of the functional significance of cytokines interacting with classic neural types may be important to understanding the complex involvement of cytokines in the adaptation induced by chronic alcohol exposure. Through such studies, novel targets of therapeutic intervention could emerge.
Do cytokines act through neurocircuits to support negative affect (anxiety) induced by stress and withdrawal from CIA exposure?
The demonstration that cytokines can be linked to stress supports the examination of specific brain sites and circuits that may contribute to the anxiety associated with cytokine influences on withdrawal-induced anxiety. The possible involvement of cytokines in neurocircuitry associated with stress and CA exposure has been raised by Banisadr, Gosselin, Mechighel, Kitabgi, et al. (2005), who showed that CCL2 was highly localized with neurotransmitters and neuropeptides systems in brain. Other data suggest that the previously described increase in cortical cytokine mRNAs induced by CA withdrawal (Whitman et al., 2013) may also occur in the amygdala (Knapp et al., 2015). Szcytkowski and Lysle (2010) implicated DA1Rs in the BLA in the mediation of heroin-induced conditioned immunomodulation – another example of a drug of abuse influencing cytokine changes within a specific brain site. Additionally, the role of inflammasomes in the cytokine mRNA increases in differing brain regions associated with stress and withdrawal from CA is yet another area worthy of future investigation (Fleshner, 2013; Iwata, Ota, & Duman, 2013).
The recent research of Marshall et al. (2015) implicating IL-1 receptor signaling in the BLA in alcohol binge drinking in mice complements the finding that CRF amplifies neuronal TLR4/CCL2 signaling by alcohol self-administration (June et al., 2015). Muscatell et al. (2015) have also reported that stress enhanced inflammatory activity by way of cortical-amygdala coupling. Recently, Knapp et al. (2015) demonstrated that withdrawal from continuous CA exposure differentially affected levels of CCL2, IL1β, and TNFα mRNAs across the cortex, amygdala, hippocampus, and hypothalamus. These distinct regional differences in cytokine mRNA expression are plausibly mediated by distinct neurocircuitry. Whether selected cytokines placed into those specific brain sites with differing cytokine mRNA responses would all sensitize anxiety during a single alcohol-withdrawal exposure, just as is found when TNFα is placed in the CeA prior to alcohol exposure (Huang et al., 2010), would be of interest. Investigations of differences between adolescents and adults in immune responses across brain regions following stress and CIA withdrawal are also warranted (Kane et al., 2014), given the association of adolescent alcohol exposure with later alcoholism (Hingson et al., 2006). These strategies for studying immune involvement in alcohol action across brain sites and neural circuits may help point the way to mechanisms that support the association of cytokines with the adaptation induced by stress and withdrawal from chronic alcohol. Such new information about cytokines and brain sites could advance an understanding of the association of neuroimmune influences with the withdrawal anxiety linked to alcohol abuse.
Is stress activation of CRF and neuroimmune function linked to the neurobiology of alcoholism?
As outlined earlier, alcoholics have an increased susceptibility to stress (Gilman & Hommer, 2008; Gilman et al., 2008; Sinha, 2001) and neuroimmune function is related to stress (Breese et al., 2004, 2008, 2011). Further, the stress link to CRF and neuroimmune activation is associated with a “kindling”-like neural adaptation that supports the symptoms associated with chronic alcohol exposure (Breese et al., 2011). CRF acting through the CRF1R has been implicated in both stress sensitization of the anxiety during CIA exposure (Breese et al, 2011; Huang et al., 2010; Knapp et al., 2011) and the sensitization induced by repeated cytokine injection into brain prior to a single alcohol withdrawal (Knapp et al., 2011). Mantsch et al. (2015) have recently reviewed the various brain sites and transmitters involved in stress involvement in alcohol and other drugs of abuse. The Crews laboratory, citing evidence that alcohol can influence measures of neuroimmune function in alcoholics (Crews et al., 2013; He & Crews, 2008), has contended that induction of immune-related genes in brain contribute integrally to the neurobiology of addiction (Crews & Vetreno, 2011; Crews, Zou, & Qin, 2011) – a view shared by others (Achur et al., 2010; Mayfield, Ferguson, & Harris, 2013). The finding that repeated microinjections of cytokines into brain prior to a single alcohol exposure sensitized withdrawal anxiety is consistent with this view (Breese et al., 2008).
A CRF1RA does not block a stress increase in cytokine mRNAs in controls, but does prevent the CA withdrawal-induced increase in neuroimmune function (Whitman, 2012; Whitman et al., 2013) – findings that suggest distinct mechanisms support these neuroimmune changes induced by the different “stressful” challenges. Thus, released cytokines might not always be involved in the sensitization of anxiety that follows alcohol withdrawal. Nonetheless, the stress-induced return to alcohol seeking was prevented by a CRF1RA in animals (reviewed by Heilig & Koob, 2007). Overall, it seems reasonable to suggest that the CIA exposures and stress engage CRF and neuroimmune function to contribute to the persistent adaptation and the symptomatic consequences observed in animals (Breese et al., 2011; Cui et al., 2015; Koob, 2015).
The present overview clearly associates stress and alcohol withdrawal with CRF and neuroimmune involvement (Breese et al., 2008; Huang et al., 2010; Knapp et al., 2011). It is not known whether altered CRF and neuroimmune function associated with the kindling-like adaptation associated with repeated stresses and withdrawals from CA exposure contributes to the varied symptoms observed in abstinent alcoholics. In this context, Kwako et al. (2015) recently reported that CRF1RA administration to alcohol-dependent individuals after withdrawal did not affect stress or alcohol cues on craving, anxiety, or neuroendocrine responses. Such apparent inconsistencies between the animal and human findings point to the need for further work to elucidate the neural basis of the stress and CIA consequences responsible for the persistent adaptation that supports alcohol abuse.
Highlights.
Withdrawals from chronic intermittent alcohol exposure involve stress release of CRF.
CRF involvement in stress substitution for chronic intermittent alcohol withdrawals involves multiple brain sites.
Withdrawal from chronic alcohol exposure facilitates an increase in cytokine mRNAs that is dependent upon CRF release.
Brain regions are capable of increasing common cytokine mRNAs by distinct mechanisms.
Neurocircuitry related to stress is involved in the anxiety and induction of cytokine mRNAs that follow withdrawal from chronic alcohol exposure.
Acknowledgments
This work was supported by grants AA17462, AA-22234, AA-21275, and AA-11605 from the NIAAA. We are grateful for the editorial assistance of Dr. Jane Saiers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. Journal of Neuroimmune Pharmacology. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. The AAPS Journal. 2006;7:E865–870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoglia AE, Holstein SE, Reid G, Hodge CW. CaMKIIα-GluA1 activity underlies vulnerability to adolescent binge alcohol drinking. Alcoholism: Clinical and Experimental Research. 2015;39:1680–1690. doi: 10.1111/acer.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Critical Reviews in Clinical Laboratory Sciences. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Gómez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia. 2012;60:948–964. doi: 10.1002/glia.22327. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of Neuroscience. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Mouse Brain Connectivity Atlas. Allen Institute for Brain Science. 2015 Available from: http://connectivity.brain-map.org.
- Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annual Review of Immunology. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Annals of Medicine. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blandino P, Jr, Deak T. Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiology & Behavior. 2009;98:139–146. doi: 10.1016/j.physbeh.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU. The Human BNST: Functional role in anxiety and addiction. Neuropsychopharmacology. 2015;41:126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrick E, Bobak M, Britton A, Kirschbaum C, Marmot M, Kumari M. The relationship between alcohol consumption and cortisol secretion in an aging cohort. The Journal of Clinical Endocrinology and Metabolism. 2008;93:750–757. doi: 10.1210/jc.2007-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Herman MA, Varodayan FP, Oleata CS, Madamba SG, Harris RA, et al. Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain, Behavior, and Immunity. 2015;45:189–197. doi: 10.1016/j.bbi.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Madamba SG, Roberto M, Blednov YA, Sagi VN, Roberts E, et al. Innate immune factors modulate alcohol interaction with GABAergic transmission in mouse central amygdala. Brain, Behavior, and Immunity. 2014;40:191–202. doi: 10.1016/j.bbi.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Varodayan FP, Madamba SG, Roberts AJ, Casal LM, Oleata CS, et al. IL-1 interacts with alcohol effects on GABAergic transmission in the mouse central amygdala. Frontiers in Pharmacology. 2015;6:49. doi: 10.3389/fphar.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual Review of Pharmacology and Toxicology. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. The British Journal of Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Kitabgi P, Rostène W, Parsadaniantz SM. Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. The Journal of Comparative Neurology. 2005;489:275–292. doi: 10.1002/cne.20598. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostène W, Kitabgi P, Mélik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. The Journal of Comparative Neurology. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Quéraud-Lesaux F, Boutterin MC, Pélaprat D, Zalc B, Rostène W, et al. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. Journal of Neurochemistry. 2002;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nature Reviews Neuroscience. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Belkouch M, Dansereau MA, Réaux-Le Goazigo A, Van Steenwinckel J, Beaudet N, Chraibi A, et al. The chemokine CCL2 increases Nav1.8 sodium channel activity in primary sensory neurons through a GBγ-dependent mechanism. The Journal of Neuroscience. 2011;31:18381–18390. doi: 10.1523/JNEUROSCI.3386-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi LL, Sun XD, Zhang J, Lu YS, Chen YH, Wang J, et al. Amygdala NRG1-ErbB4 is critical for the modulation of anxiety-like behaviors. Neuropsychopharmacology. 2015;40:974–986. doi: 10.1038/npp.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Vallés SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by alcohol in cultured astrocytes. Journal of Immunology. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. Journal of Neuroimmunology. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain, Behavior, and Immunity. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of alcohol. Behavioural Brain Research. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction Biology. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcoholism: Clinical and Experimental Research. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2008;33:867–876. doi: 10.1038/sj.npp.1301468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology. 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & Therapeutics. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research & Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. Journal of Abnormal Psychology. 1990;99:344–348. doi: 10.1037//0021-843x.99.4.344. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. Journal of Studies on Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Buck HM, Hueston CM, Bishop C, Deak T. Enhancement of the hypothalamic-pituitary-adrenal axis but not cytokine responses to stress challenges imposed during withdrawal from acute alcohol exposure in Sprague-Dawley rats. Psychopharmacology. 2011;218:203–215. doi: 10.1007/s00213-011-2388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, Herman CP. Alcohol and tension reduction: A review. Quarterly Journal of Studies on Alcohol. 1972;33:33–64. [PubMed] [Google Scholar]
- Cheng Z, Taylor B, Ourthiague DR, Hoffman A. Distinct single-cell signaling characteristics are conferred by the MyD88 and TRIF pathways during TLR4 activation. Science Signaling. 2015;8:ra69. doi: 10.1126/scisignal.aaa5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Gruol DL. The chemokine CCL2 activates p38 mitogen-activated protein kinase pathway in cultured rat hippocampal cells. Journal of Neuroimmunology. 2008;199:94–103. doi: 10.1016/j.jneuroim.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JJ. The effects of alcohol on conflict behavior in the albino rat. Quarterly Journal of Studies on Alcohol. 1951;12:1–29. [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Quarterly Journal of Studies on Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiology & Behavior. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. Journal of Abnormal Psychology. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Addiction, adolescence, and innate immune gene induction. Frontiers in Psychiatry. 2011;2:19. doi: 10.3389/fpsyt.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain, Behavior, and Immunity. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic alcohol exposure. Biological Psychiatry. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Noronha A, Warren KR, Koob GF, Sinha R, Thakkar M, et al. Brain pathways to recovery from alcohol dependence. Alcohol. 2015;49:435–452. doi: 10.1016/j.alcohol.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG. Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2015;41:103–125. doi: 10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davé SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, et al. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. Journal of Leukocyte Biology. 2009;86:633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neurocircuitry of anxiety and stress disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: Fifth Generation of Progress. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2002. pp. 931–951. [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, et al. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Research Bulletin. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- del Cerro S, Borrell J. Interleukin-1 affects the behavioral despair response in rats by an indirect mechanism which requires endogenous CRF. Brain Research. 1990;528:162–164. doi: 10.1016/0006-8993(90)90212-t. [DOI] [PubMed] [Google Scholar]
- Deussing JM, Breu J, Kühne C, Kallnik M, Bunck M, Glasl L, et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. The Journal of Neuroscience. 2010;30:9103–9116. doi: 10.1523/JNEUROSCI.1049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial alcohol exposure. Alcoholism: Clinical and Experimental Research. 2014;38:2186–2198. doi: 10.1111/acer.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcoholism: Clinical and Experimental Research. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Annals of the New York Academy of Sciences. 1990a;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Research Brain Research Reviews. 1990b;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neuroscience and Biobehavioral Reviews. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biological Psychiatry. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Eisenhardt M, Hansson AC, Spanagel R, Bilbao A. Chronic intermittent ethanol exposure in mice leads to an up-regulation of CRH/CRHR1 signaling. Alcoholism: Clinical and Experimental Research. 2014;39:752–762. doi: 10.1111/acer.12686. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Molecular Immunology. 2008;45:2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by alcohol. Journal of Immunology. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain, Behavior, and Immunity. 2013;27:1–7. doi: 10.1016/j.bbi.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Peihua G, Rajita S. Interactive effects of cumulative stress and impulsivity on alcohol consumption. Alcoholism: Clinical and Experimental Research. 2010;34:1376–1385. doi: 10.1111/j.1530-0277.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive Behaviors. 2008;33:388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. The Journal of Neuroscience. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain, Behavior, and Immunity. 2015;48:1–7. doi: 10.1016/j.bbi.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gárate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, et al. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biological Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addiction Biology. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. The Journal of Neuroscience. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biological Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Donegan JJ, Morilak DA. Chronic intermittent cold stress sensitizes neuro-immune reactivity in the rat brain. Psychoneuroendocrinology. 2011;36:1164–1174. doi: 10.1016/j.psyneuen.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani F, Hader W, Yong VW. Minocycline attenuates T cell and microglia activity to impair cytokine production in T cell-microglia interaction. Journal of Leukocyte Biology. 2005;78:135–143. doi: 10.1189/jlb.0804477. [DOI] [PubMed] [Google Scholar]
- Gong K, Zou X, Fuchs PN, Lin Q. Minocycline inhibits neurogenic inflammation by blocking the effects of tumor necrosis factor-α. Clinical and Experimental Pharmacology & Physiology. 2015 doi: 10.1111/1440-1681.12444. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, et al. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. Journal of Neurochemistry. 2005;95:1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. The Journal of Neuroscience. 2015;35:3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovère C, Conductier G, Trocello JM, et al. Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience. 2009;162:1072–1080. doi: 10.1016/j.neuroscience.2009.05.048. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annual Review of Pharmacology and Toxicology. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- Harper KM, Knapp DJ, Breese GR. Withdrawal from chronic alcohol induces a unique CCL2 mRNA increase in adolescent but not adult brain – relationship to blood alcohol levels and seizures. Alcoholism: Clinical and Experimental Research. 2015;39:2375–2385. doi: 10.1111/acer.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Anisman H. Multiple mechanisms of cytokine action in neurodegenerative and psychiatric states: neurochemical and molecular substrates. Current Pharmaceutical Design. 2005;11:947–962. doi: 10.2174/1381612053381611. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic alcohol. Addiction Biology. 2014;21:72–86. doi: 10.1111/adb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Roberto M. The addicted brain: understanding the neurophysiological mechanisms of addictive disorders. Frontiers in Integrative Neuroscience. 2015;9:18. doi: 10.3389/fnint.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Varodayan FP, Oleata CS, Luu G, Kirson D, Heilig M, et al. Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects. Neuropharmacology. 2015;102:21–31. doi: 10.1016/j.neuropharm.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA. Effects of anxiety arousal on the consumption of alcohol by alcoholics and social drinkers. Journal of Consulting and Clinical Psychology. 1973;41:426–433. doi: 10.1037/h0035366. [DOI] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA. Fear of interpersonal evaluation as a determinant of alcohol consumption in male social drinkers. Journal of Abnormal Psychology. 1975;84:644–651. doi: 10.1037//0021-843x.84.6.644. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Archives of Pediatrics & Adolescent Medicine. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, et al. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. The Journal of Pharmacology and Experimental Therapeutics. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiology & Behavior. 2011;104:187–198. doi: 10.1016/j.physbeh.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Hotta M, Demura H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Research. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain, Behavior, and Immunity. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Sainz MC, Fast B, Mayor F, Jr, Aragay AM. Signaling pathways for monocyte chemoattractant protein 1-mediated extracellular signal-regulated kinase activation. Molecular Pharmacology. 2003;64:773–782. doi: 10.1124/mol.64.3.773. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Zimomra ZR, Stewart LT. Beta-adrenergic receptor activation primes microglia cytokine production. Journal of Neuroimmunology. 2013;254:161–164. doi: 10.1016/j.jneuroim.2012.08.007. [DOI] [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, et al. CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 2015;40:1549–1559. doi: 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol and Alcoholism. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, et al. Effects of alcohol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcoholism: Clinical and Experimental Research. 2014;38:384–391. doi: 10.1111/acer.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Schick M, Wiedemann K. Alcohol intake, tumour necrosis factor-alpha, leptin and craving: factors of a possibly vicious circle? Alcohol and Alcoholism. 2002;37:401–404. doi: 10.1093/alcalc/37.4.401. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. The Journal of Neuroscience. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Angel RA, Navarro M, Breese GR. The amygdala regulates the antianxiety sensitization effect of flumazenil during repeated chronic ethanol or repeated stress. Alcoholism: Clinical and Experimental Research. 2007;31:1872–1882. doi: 10.1111/j.1530-0277.2007.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcoholism: Clinical and Experimental Research. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcoholism: Clinical and Experimental Research. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block alcohol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Whitman BA, Harper K, Breese GR. Stress and chronic alcohol differentially induce neuroimmune mRNAs in subregions of the rat brain. Alcoholism: Clinical and Experimental Research. 2015;39:127a. doi: 10.1111/acer.12898. supplement S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Whitman BA, Wills TA, Angel RA, Overstreet DH, Criswell HE, et al. Cytokine involvement in stress may depend on corticotrophin releasing factor to sensitize ethanol withdrawal anxiety. Brain, Behavior, and Immunity. 2011;25:S146–154. doi: 10.1016/j.bbi.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofoed L, Friedman MJ, Peck R. Alcoholism and drug abuse in patients with PTSD. The Psychiatric Quarterly. 1993;64:151–171. doi: 10.1007/BF01065867. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. European Neuropsychopharmacology. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2008;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handbook of Clinical Neurology. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. The dark side of emotion: the addiction perspective. European Journal of Pharmacology. 2015;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Research. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Yang XP, Fang D, Ren X, Zhou H, Fang J, et al. High-mobility group box-1 mediates toll-like receptor 4-dependent angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:1024–1032. doi: 10.1161/ATVBAHA.111.224048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hutchinson MR, White JM, Somogyi AA, Coller JK. Association of IL-1B genetic polymorphisms with an increased risk of opioid and alcohol dependence. Pharmacogenetics and Genomics. 2009;19:869–876. doi: 10.1097/FPC.0b013e328331e68f. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism: Clinical and Experimental Research. 2000;24:651–658. [PubMed] [Google Scholar]
- Lukáts B, Egyed R, Karádi Z. Single neuron activity changes to interleukin-1beta in the orbitofrontal cortex of the rat. Brain Research. 2005;1038:243–246. doi: 10.1016/j.brainres.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nature Reviews Neuroscience. 2015;16:705–718. doi: 10.1038/nrn4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Garcia-Bueno B, Hinojosa AE, Polak P, Feinstein DL, Leza JC. Regulation of MCP-1 production in brain by stress and noradrenaline-modulating drugs. Journal of Neurochemistry. 2010;113:543–551. doi: 10.1111/j.1471-4159.2010.06623.x. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2015;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York, NY: The Guilford Press; 1985. [Google Scholar]
- Marshall SA, Casachahua JD, Rinker JA, Blose AK, Lyse DT, Thiele TE. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain, Behavior, and Immunity. 2015;51:258–267. doi: 10.1016/j.bbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Current Opinion in Neurobiology. 2013;23:513–520. doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic alcohol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcoholism: Clinical and Experimental Research. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2015;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and alcohol withdrawal as measured by microdialysis. The Journal of Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Kuraishi Y, Yamaguchi T, Nakai S, Hirai Y, Satoh M. Immobilization stress induces interleukin-1 beta mRNA in the rat hypothalamus. Neuroscience Letters. 1991;123:254–256. doi: 10.1016/0304-3940(91)90944-o. [DOI] [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Breese GR. Evidence for TNFα action on excitatory and inhibitory neurotransmission in the central amygdala: a brain site influenced by stress. Brain, Behavior, and Immunity. 2013;33:102–111. doi: 10.1016/j.bbi.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, Maldonado C, Rodríguez-Arias M, Miñarro J, et al. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent alcohol treatment. Brain, Behavior, and Immunity. 2015;45:233–244. doi: 10.1016/j.bbi.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of Abnormal Psychology. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Munro CA, Oswald LM, Weerts EM, McCaul ME, Wand GS. Hormone responses to social stress in abstinent alcohol-dependent subjects and social drinkers with no history of alcohol dependence. Alcoholism: Clinical and Experimental Research. 2005;29:1133–1138. doi: 10.1097/01.alc.0000172459.71517.05. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, et al. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, et al. Exposure to acute stress induces brain interleukin-1beta protein in the rat. The Journal of Neuroscience. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, et al. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Research. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, et al. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Research. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated alcohol withdrawals. Alcoholism: Clinical and Experimental Research. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacology, Biochemistry, and Behavior. 2004a;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from alcohol. Pharmacology, Biochemistry, and Behavior. 2004b;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Pharmacological modulation of repeated ethanol withdrawal-induced anxiety-like behavior differs in alcohol-preferring P and Sprague-Dawley rats. Pharmacology, Biochemistry, and Behavior. 2005;81:122–130. doi: 10.1016/j.pbb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC. TLR4-MyD88 signalling: a molecular target for alcohol actions. British Journal of Pharmacology. 2012;165:1316–1318. doi: 10.1111/j.1476-5381.2011.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1alpha receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Molecular Endocrinology. 2004;18:624–639. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. American Journal of Physiology Cell Physiology. 2006;290:C917–924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. The Journal of Biological Chemistry. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Alfonso-Loeches S, Aragón CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain, Behavior, and Immunity. 2011;25(Suppl 1):S80–91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Aragón CM, Guerri C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology. 2015;89:352–359. doi: 10.1016/j.neuropharm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Rychtarik RG, Rappaport NB, Smith PO, Etscheidt M, Brown TA, et al. Reactivity to alcohol-relevant beverage and imaginal cues in alcoholics. Addictive Behaviors. 1992;17:209–217. doi: 10.1016/0306-4603(92)90026-r. [DOI] [PubMed] [Google Scholar]
- Pla A, Pascual M, Renau-Piqueras J, Guerri C. TLR4 mediates the impairment of ubiquitin-proteasome and autophagy-lysosome pathways induced by ethanol treatment in brain. Cell Death & Disease. 2014;5:e1066. doi: 10.1038/cddis.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield VM, Gabella KM, Simmons MA, Johnson JD. Repeated stressor exposure regionally enhances beta-adrenergic receptor-mediated brain IL-1β production. Brain, Behavior, and Immunity. 2012;26:1249–1255. doi: 10.1016/j.bbi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Porterfield VM, Zimomra ZR, Caldwell EA, Camp RM, Gabella KM, Johnson JD. Rat strain differences in restraint stress-induced brain cytokines. Neuroscience. 2011;188:48–54. doi: 10.1016/j.neuroscience.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucak ML, Kaplin AI. Unkind cytokines: current evidence for the potential role of cytokines in immune-mediated depression. International Review of Psychiatry. 2005;17:477–483. doi: 10.1080/02646830500381757. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Research. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. The Journal of Neuroscience. 2015;35:9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, et al. Brain networks. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–1244. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, et al. Interleukin-1β causes anxiety by interacting with the endocannabinoid system. The Journal of Neuroscience. 2012;32:13896–13905. doi: 10.1523/JNEUROSCI.1515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostène W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nature Reviews Neuroscience. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- Saiz PA, Garcia-Portilla MP, Florez G, Corcoran P, Arango C, Morales B, et al. Polymorphisms of the IL-1 gene complex are associated with alcohol dependence in Spanish Caucasians: data from an association study. Alcoholism: Clinical and Experimental Research. 2009;33:2147–2153. doi: 10.1111/j.1530-0277.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Kato R, Asai M. Role of interleukin-1 in stress responses. A putative neurotransmitter. Molecular Neurobiology. 1995;10:47–71. doi: 10.1007/BF02740837. [DOI] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, et al. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. The Journal of Neuroscience. 1995;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuya K, Komori T, Fujiwara R, Miyahara S, Ohmori M, Nomura J. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sciences. 1997;61:PL 135–140. doi: 10.1016/s0024-3205(97)00608-5. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. Ethanol and corticotropin releasing factor receptor modulation of central amygdala neurocircuitry: An update and future directions. Alcohol. 2015;49:179–184. doi: 10.1016/j.alcohol.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Henniger MS. Stress and alcohol drinking. Annals of Medicine. 2004;36:596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current Psychiatry Reports. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, O’Malley SS. Craving for alcohol: findings from the clinic and the laboratory. Alcohol and Alcoholism. 1999;34:223–230. doi: 10.1093/alcalc/34.2.223. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain function. Annual Review of Neuroscience. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Su X, Wang H, Zhao J, Pan H, Mao L. Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-κB pathway after traumatic brain injury in the rat. Mediators of Inflammation. 2011:ARTICLE ID/807142. doi: 10.1155/2011/807142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Shintani F, Kanba S, Asai M, Nakaki T. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cellular and Molecular Neurobiology. 1997;17:557–562. doi: 10.1023/a:1026319107528. [DOI] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT. Dopamine D1 receptors within the basolateral amygdala mediate heroin-induced conditioned immunomodulation. Journal of Neuroimmunology. 2010;226:38–47. doi: 10.1016/j.jneuroim.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SR, McQuaid JR, Brown SA. Characteristics of life stressors predictive of substance treatment outcomes. Journal of Substance Abuse Treatment. 2005;29:107–115. doi: 10.1016/j.jsat.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain, Behavior, and Immunity. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- Uddin M, Koenen KC, Aiello AE, Wildman DE, de los Santos R, Galea S. Epigenetic and inflammatory marker profiles associated with depression in a community-based epidemiologic sample. Psychological Medicine. 2011;41:997–1007. doi: 10.1017/S0033291710001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addiction Biology. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annual Review of Pharmacology and Toxicology. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted alcohol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL. The chemokine CCL2 modulates Ca2+ dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. The European Journal of Neuroscience. 2005;21:2949–2957. doi: 10.1111/j.1460-9568.2005.04113.x. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Bonn-Miller MO, Marlatt GA. Posttraumatic stress and alcohol use coping motives among a trauma-exposed community sample: the mediating role of non-judgmental acceptance. Addictive Behaviors. 2011;36:707–712. doi: 10.1016/j.addbeh.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Sobesky JL, Watkins LR, Maier SF. Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain, Behavior, and Immunity. 2013;32:112–121. doi: 10.1016/j.bbi.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman B. Dissertation in partial fulfillment of the requirements for degree of Doctor of Philosophy in the Curriculum of Neurobiology. UNC; Chapel Hill: 2012. Neuroimmune consequences of chronic alcohol exposure: Relationship to stress. [Google Scholar]
- Whitman B, Knapp DJ, Werner DF, Crews FT, Breese GR. The cytokine mRNA increase induced by withdrawal from chronic alcohol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcoholism: Clinical and Experimental Research. 2013;37:2086–2097. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of Neuroscience. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, et al. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. British Journal of Pharmacology. 2012;165:1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Maruyama I. HMGB1, a novel inflammatory cytokine. Clinica Chimica Acta. 2007;375:36–42. doi: 10.1016/j.cca.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. Journal of Leukocyte Biology. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW. Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biological Psychiatry. 1991;30:1031–1048. doi: 10.1016/0006-3223(91)90123-4. [DOI] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. Journal of Neurochemistry. 2011;116:406–414. doi: 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcoholism: Clinical and Experimental Research. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- Zou J, Vetreno RP, Crews FT. ATP-P2X7 receptor signaling controls basal and TNFα-stimulated glial cell proliferation. Glia. 2012;60:661–673. doi: 10.1002/glia.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]