Abstract
The comorbidity of substance- and alcohol-use disorders (AUD) with other psychiatric conditions, especially those related to stress such as post-traumatic stress disorder (PTSD), is well-established. Binge-like intoxication is thought to be a crucial stage in the development of the chronic relapsing nature of the addictions, and self-medication through binge-like drinking is commonly seen in PTSD patients. We have selectively bred two separate High Drinking in the Dark (HDID-1 and HDID-2) mouse lines to reach high blood ethanol concentrations (BECs) after a 4-h period of access to 20% ethanol starting shortly after the onset of circadian dark. As an initial step toward the eventual goal of employing binge-prone HDID mice to study PTSD-like behavior including alcohol binge drinking, we sought first to determine their ability to acquire conditioned fear. We asked whether these mice acquired, generalized, or extinguished conditioned freezing to a greater or lesser extent than unselected control HS/Npt mice. In two experiments, we trained groups of 16 adult male mice in a standard conditioned fear protocol. Mice were tested for context-elicited freezing, and then, in a novel context, for cue-induced freezing. After extinction tests, renewal of conditioned fear was tested in the original context. Mice of all three genotypes showed typical fear responding Context paired with shock elicited freezing behavior in a control experiment, but cue unpaired with shock did not. These studies indicate that fear learning per se does not appear to be influenced by genes causing predisposition to binge drinking, suggesting distinct neural mechanisms. However, HDID mice are shown to be a suitable model for studying the role of conditioned fear specifically in binge-like drinking.
Keywords: Ethanol binge drinking, Conditioned fear, Selective breeding, Drinking in the dark, Mouse, Genetics
Introduction
Alcoholism and the alcohol-use disorder (AUD) spectrum afflict more than 18 million people in the USA aged 18 or older (Warren, 2009), and the annual cost to the US economy was estimated already to exceed $223 billion in 2009 (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011). Binge-like intoxication is thought to be a crucial stage in the development of the chronic relapsing nature of the addictions (Mandyam & Koob, 2012). Risk for AUDs is substantially heritable (Goldman, Oroszi, & Ducci, 2005). There are many rodent models for high levels of alcohol drinking. These models have generally been developed by selectively breeding for high preferential intake for 10% ethanol versus water when both fluids are offered continuously. Many such selected lines have been created and studied (for review, see Crabbe, 2014). One curious feature of these selected lines is that they generally do not drink in patterns that lead to behaviorally intoxicating blood ethanol concentrations (BECs). Unlike many humans diagnosed with alcohol dependence, they do not achieve the BEC (80 mg%) established by the NIAAA (2004) to define a binge.
Considering this to be a limitation of current models, we set out 10 years ago to develop a better model of focused, binge-like drinking in mice. Since rodents ingest most of their food and fluids early during their circadian dark period, we replaced the water bottle with a single bottle of 20% ethanol (a relatively high concentration for rodents) and found that C57BL/6J mice drank enough alcohol in a 4-h session of drinking in the dark (DID) to exceed 80 mg% BECs (Rhodes, Best, Belknap, Finn, & Crabbe, 2005). To develop a model enriched for genetic contributions to such drinking, we bred mice from a genetically heterogeneous population to produce a High Drinking in the Dark selected line (HDID-1). The basis for selection was BEC (not g/kg intake), and these animals showed increased BECs across generations and drank ethanol to the point of becoming behaviorally intoxicated (Crabbe et al., 2009). With continued selection, the current (32nd selected) generation of HDID-1 mice reach BECs averaging 180 mg%; they also have very high ethanol intake (6.9 g/kg in 4 h). A second, genetically independent replicate of this selection was initiated later. The HDID-2 (generation S26) reaches an average BEC of 155 mg% and drinks somewhat more ethanol. During the most recent test of the entire population to choose breeders, 58% of HDID-1 and 44% of HDID-2 mice reached BECs ≥160 mg%, twice the NIAAA standard for a binge. BECs ≥240 mg% (3 times the threshold) were reached in 25% of HDID-1 and 6% of HDID-2 mice. This is a unidirectional selection. Thus, no lines were bred for low BECs. Details of this selection’s recent progress have been published (Crabbe et al., 2014).
A useful feature of selected lines is that if the lines differ (in this case, from their unselected control line, HS/Npt mice) on another trait, it is demonstrated that some of the genes leading to high DID-BEC also affect the correlated trait. Given the practical constraints of selection, this inference is greatly strengthened if one sees a parallel response in a genetically independent replicate of the selected line (Crabbe, Phillips, Kosobud, & Belknap, 1990).
The extensive comorbidity of AUD, other substance-use disorders, and other psychiatric disorders is well established (Kendler, Prescott, Myers, & Neale, 2003), notably including disorders clearly related to stress such as post-traumatic stress disorder (PTSD). Prevalence of PTSD among US military veterans is especially high (Davis, Bush, Kivlahan, Dobie, & Bradley, 2003) and was estimated to be between 6 and 24% depending on the definition of PTSD; comorbidity of AUD with either PTSD or depression was approximately 50% (Thomas et al., 2010). Currently, little is known about the biological mechanisms common to A/SUDs and PTSD (Norman et al., 2012). Combat experience leads young veterans to express a highly prevalent and disabling form of AUD, binge-like alcoholic drinking, leading to especially high BECs (Cucciare, Darrow, & Weingardt, 2011). The long-term goal of the studies we report here is to implement the HDID lines as a model of PTSD-like exacerbated binge drinking. Although the lines already achieve binge-like BECs and drink excessively, the BEC ranges reported above make it clear that these lines have not yet reached any biological limits to their intakes/BECs. Many rodent models of PTSD are based on classically conditioned fear, on the theory that PTSD resembles inappropriate generalization of a panic-like, anxious response to cues in the post-traumatic environment (Maren & Holmes, 2015). Thus, a predominant therapeutic approach is to decrease cue-induced fear responses by exposing patients to cues associated with trauma or anxiety, allowing the fearful response to diminish with repeated exposure through a process known as extinction. A common murine model for fear conditioning builds upon the unconditioned behavioral arrest (freezing) response displayed by mice to cues previously associated with foot shock (Fanselow, 1980; Kaouane et al., 2012). Robust freezing responses occur after a single pairing between a neutral conditioned stimulus (CS) and a biologically significant unconditioned stimulus (US). This learned response can persist across the lifespan of rodents (e.g., Gale et al., 2004) and, although it decreases over the course of repeated exposures to the CS in the absence of the US, conditioned freezing often returns even after successful extinction treatments (e.g., Lattal & Maughan, 2012).
As an initial step toward the eventual goal of employing our alcohol binge-prone HDID mice to study PTSD-related behavior, particularly including alcohol binge drinking, we sought first to determine their ability to acquire conditioned fear. We asked whether these mice acquired, generalized, or extinguished shock-induced freezing to a greater or lesser extent than unselected control HS/Npt mice. We used a protocol for assessing conditioned fear routinely used in the Lattal laboratory (Raybuck & Lattal, 2011). HDID-1 and HDID-2 mice have previously been shown to acquire a preference for a location associated with ethanol injections, and they do not differ from HS/Npt mice in that conditioned place preference (Barkley-Levenson, Cunningham, Smitasin, & Crabbe, 2015). In contrast, when ethanol injections were used to establish a conditioned aversion to a novel taste (saline solution), both HDID-1 and HDID-2 mice showed reduced sensitivity to ethanol compared to HS/Npt. While a high dose of ethanol (4 g/kg, administered intraperitoneally [i.p.]) conditioned a strong aversion in all mice, a stronger conditioned taste aversion was seen in HS/Npt mice to an intermediate dose (2 g/kg) than in either HDID replicate line. All three genotypes showed equivalent taste conditioning induced by injections of lithium chloride (Barkley-Levenson et al., 2015). Although HDID mice do not seem to differ in ethanol reward sensitivity, they are less sensitive to the aversive properties of ethanol. If this is a general insensitivity to aversive outcomes, then one might expect that they would show deficits in fear conditioning. Alternatively, given that they show normal associative learning in associating reward with a context in a CPP procedure, they may show normal fear conditioning, which also involves associating a contextual stimulus with an outcome.
Experimental procedures
Animals and husbandry
Male mice from the HDID-1, HDID-2, and non-selected HS/Npt lines were bred in our colonies in the VA Portland Health Care System Veterinary Medical Unit. All mice were naïve at the beginning of each experiment and were between 70- and 135-days-old at the start of testing. HDID-1 mice were from the 29th selected generation and HDID-2 mice were from generation S22 and S23. HS/Npt mice were from filial generation G78. For the third experiment, HDID-1 mice of the 31st selection generation, HDID-2 mice of generation S25, and HS/Npt of filial generation G81 were used. The HS animals were the genetically heterogeneous population from which both HDID-1 and HDID-2 lines were selected, starting about 2 years apart. The HS/Npt animals were created by systematically intercrossing eight inbred mouse strains (Hitzemann, Dains, Kanes, & Hitzemann, 1994) and are maintained as 48 rotationally mated breeding pairs (Crabbe, Spence, Brown, & Metten, 2011).
Mice were maintained in standard plastic cages on Bed-o’Cobs® bedding (Andersons, Maumee, OH, USA) with stainless steel wire bar tops with a recess for chow. Rodent chow 5001 (PMI Nutrition International, Brentwood, MO, USA) and tap water were available ad libitum, and colonies and testing rooms were maintained on a 12-h:12-h reversed light:dark schedule (lights on at 9:30 PM, lights out at 9:30 AM) at a temperature of 21 ± 1 °C. Two weeks before the start of an experiment, mice were transferred to a procedure room with the same environmental conditions other than the light:dark schedule. During this time they acclimated to a forward light:dark schedule (lights on at 6:00 AM, lights off at 6:00 PM) in order to test mice in the light, as per standard protocols, during the human daytime. All procedures were approved by the VA Portland Health Care System Institutional Animal Care and Use Committee and were performed according to NIH Guidelines for the Care and Use of Laboratory Animals.
Apparatus
The Freeze Monitors (San Diego Instruments, San Diego, CA) were 10″ × 10″ × 7″ (height) clear acrylic enclosures with 1/8-in stainless steel grid floors space 1/2 in apart. Movement was detected with a 16 × 16 array of infrared photobeams spaced 1/2 in apart. A novel context was effected with a black acrylic triangular insert splitting each chamber in two – on alternate context trials, the subject was confined to the inside of the triangular compartment. Tone cues (3 kHz, 85 dB) were emitted from a small speaker in the lid covering the chambers. House lights illuminated the chamber to approximately 333 lux. Shock (0.4 mA) was scrambled and delivered through the grid floor. Four freeze monitors were run in parallel under the control of a dedicated computer, which acquired data (beam interruptions) each 0.1 s.
Statistical analyses
Occurrence of an incident of freezing was defined as initiated by a beam break followed by a period of >3 s without an additional beam break (Lattal & Maughan, 2012). The dependent variable for analyses was percent time freezing within a defined interval (see individual experiments). Data were analyzed using Systat (version 13). We first assessed the significance of genotype and treatment (Shock vs. No Shock) and their interaction using factorial ANOVAs for data within a time period. For further analyses, we used simpler ANOVAs and/or the Tukey HSD post hoc test. Differences were considered significant if p < 0.05.
Experiment 1: fear conditioning – 5-day protocol
A total of 96 male mice, 73–133-days-old at the start of the experiment, were used. Sixteen mice of each genotype (HDID-1, HDID-2, HS/Npt) were randomly assigned to either the Shock or Control group. All testing commenced between 8:00 and 9:00 AM, when all animals were transported in their home cages down two floors to the procedure room. They rested there for at least 60 min near a white noise generator (60–65 dB) that ran throughout the experiment to mask the sounds of the tone for animals before and after testing. Testing all 96 mice required 5.5 h or less. To initiate a trial, four mice were moved to the other end of the room and each mouse was placed in its designated monitor. All four lids were placed over the monitors, and each monitor was placed into a separate sound-attenuating enclosure. At the end of each day’s test, all mice in a squad were moved back into their home cages to await the end of all testing, at which point all mice were moved back upstairs to the colony room. To help distinguish contextual cues, the experimenter wore a paper gown, used nitrile gloves, and cleaned the chambers between mice with Dermachlor (Henry Schein, Dublin, OH) before and after the first, second, and last day of testing (all of which occurred in the conditioning context). On the other days of testing (all of which occurred in the novel context), the experimenter wore a cloth gown, used latex gloves, and cleaned the apparatus with Simple Green (Sunshine Makers, Huntington Beach, CA).
Day 1 – acquisition
After 2 min with no events, four, 30-s CS (tone) trials were administered. On each trial, the last 2 s were accompanied by a 0.4-mA foot shock US, which co-terminated with the CS. A variable intertrial interval (ITI) followed (90, 60, 90, and 120 s). After the final ITI, the trial ended and animals were removed. The entire test session was 10 min.
Day 2 – context testing
Twenty-four hours later, animals were simply replaced in the same box for 6 min. Neither tones nor shocks were delivered.
Day 3 – cued testing
Box configuration was first changed to a novel context by inserting triangular black partitions. The experimenter wore different clothes and gloves, and used the alternate cleaner between mice. Each mouse remained in the chamber for five, 3-min periods, and during minutes 3–6 and 9–12, the 85-dB tone was sounded. At 15 min, the session was terminated.
Day 4 – extinction testing
On this day, the same procedures described for Day 3 were followed.
Day 5 – renewal testing
Chambers (and experimenter garb) were reconfigured to their condition on Days 1 and 2. Each mouse was tested as described for Day 3. No shocks were administered.
Experiment 2: fear conditioning – 7-day protocol
We considered the possibility that the two extinction sessions in Experiment 1 (Days 3–4) might not have led to complete extinction of conditioned freezing. We therefore repeated Experiment 1 and included two additional extinction trials. A total of 96 male mice, 59–79-days-old at the start of the experiment, were used. Sixteen mice of each genotype (HDID-1, HDID-2, HS/Npt) were randomly assigned to either the Shock or Control group. During Days 1–4, all procedures and testing were as described for Experiment 1. Days 5 and 6 were conducted as described for Day 4. The final day (Day 7) of the experiment comprised the renewal test, as described for Experiment 1, Day 5.
Experiment 3: shock control conditions
One complication in examining fear conditioning in mice is that freezing sometimes occurs in response to cues even when there is a degraded contingency between the cue and the shock, such as when the cue and the shock are presented in an unpaired fashion (e.g., Tipps, Raybuck, Buck, & Lattal, 2014). To evaluate the effects of shock exposure on subsequent freezing to the tone CS, we used a pseudorandom procedure in which the time between CS and US presentation was varied in each trial.
A total of 96 male mice, 65–98-days-old at the start of the experiment, were used. Sixteen mice of each genotype (HDID-1, HDID-2, HS/Npt) were randomly assigned to either the Shock or Control group. All handling and testing procedures were as described for Experiment 1; however, the temporal placement of the occurrence of tones and shocks during the Acquisition session on Day 1 differed for the groups. The No Shock group was given the temporal placement of tones identically to the Shock group, but were administered no shocks. The testing room for this experiment was also different (now on the same floor as the colony room) but the handling procedures were very nearly identical to Experiments 1 and 2. We administered the shocks at exactly the same times as they occurred in Experiments 1 and 2. Two-second shocks were given between minutes 2:28–2:30, 4:28–4:30, 5:58–6:00, and 7:58–8:00. Thirty-second tone CS exposures were presented between minutes 0:30–1.00, 3:30–4:00, 6:00–6:30, and 7:00–7:30. The total duration on Day 1 was 10.5 min. Days 2–5 were conducted as described for Experiment 1.
Results
Experiment 1: 5-day protocol
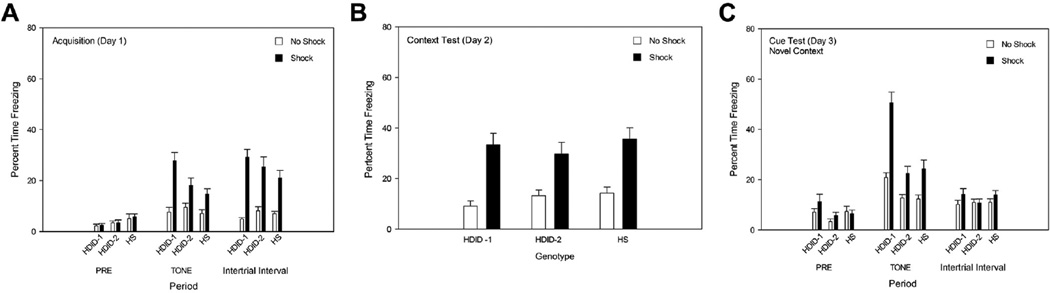
For acquisition (Fig. 1A), we combined the data across the four, 30-s CS-US intervals (“Tone” in Fig. 1A) and across the three inter-trial intervals plus the last 2 min (“ITI” in Fig. 1A). Responding during the 2 min at the start of the session prior to any stimulus is also shown (“Pre”). During the pre-stimulus period, there was only a significant main effect of genotype [F(2,116) = 3.80, p < 0.05]; HS mice showed greater freezing than HDID-1 mice [p < 0.05]. HDID-2 mice did not differ from either HDID-1 or HS, and the interaction of genotype and treatment group was not significant [F values < 1].
Fig. 1.
Freezing in HDID-1, HDID-2, and HS mice in Experiment 1 (5-day protocol) before stimulus presentations (Pre), during Tone (with or without shock), and during intertrial intervals (ITI). For conditioning and testing parameters, temporal data binning strategy, and statistical outcomes, see the text. Percent time spent freezing ± SE is shown. Panel A. Acquisition (Day 1). Shock group data are shown in filled bars, and No Shock group data in open bars. Periods of tone CS presentation culminated with 2-s shocks on this day for the Shock groups. Panel B. Context testing (Day 2). No tones or shocks were presented during this test period. Panel C. Cued testing in the novel context (Day 3). Two, 180-s tone CS periods occurred, with no shocks presented.
The genotypes differed during tone presentations [Fig. 1A: F(2,116) = 4.43, p < 0.05], with HDID-1 mice showing greater freezing than HS mice [p ≤ 0.01], while HDID-2 mice did not differ from HDID-1 or HS. The Shock groups showed significantly greater freezing than the No Shock groups [F(1,116) = 41.34, p < 0.0001], and treatment group also interacted with genotype [F(2,116) = 4.50, p < 0.05]. All shocked groups showed greater percent freezing during the tone than their respective non-shocked groups [HDID-1: F(1,38) = 28.46, p < 0.0001; HDID-2: F(1,39) = 6.88, p < 0.05; HS: F(1,39) = 8.14, p < 0.01]. In the Shock groups, HDID-1 mice showed greater freezing scores than either HDID-2 or HS [p values < 0.05, Tukey’s HSD], while the No Shock groups did not differ among the three genotypes [F < 1].
During the ITI (Fig. 1A), the Shock groups showed significantly greater freezing than the No Shock groups [F(1,116) = 78.10, p < 0.0001]. Neither genotype nor the genotype × treatment group interactions were significant [F values < 1].
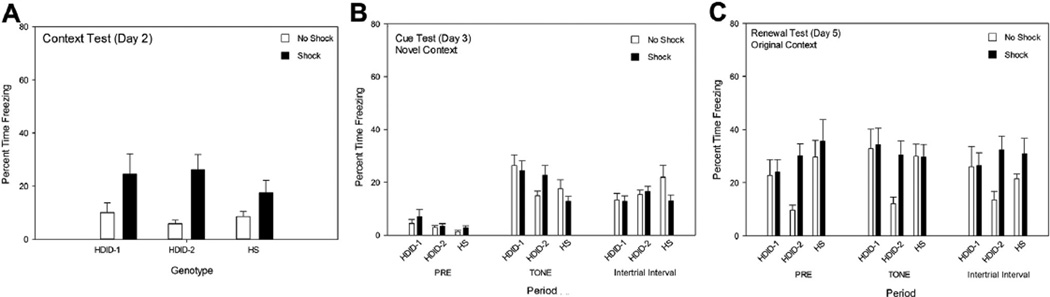
Testing on Day 2 revealed that the context alone had acquired efficacy to induce conditioned freezing in all three genotypes (Fig. 1B). There was a significant effect of treatment group [F(1,116) = 48.46, p < 0.0001], with all animals shocked the previous day freezing more compared with the non-shocked animals, but genotypes did not differ or interact significantly with treatment group [F values < 1].
Results in the novel context on Day 3 are shown in Fig. 1C. For the Pre period, a main effect of genotype was significant [F(2,116) = 3.14, p < 0.05], but not other effects. Tukey’s HSD Test showed that HDID-1 mice showed more freezing than HDID-2 [p < 0.05], but not HS, mice. During tone presentations, genotype, treatment, and their interaction were significant [F values ≥ 7.40, p values ≤ 0.001]. Post hoc tests revealed that mice of each genotype in the Shock group froze significantly more than those in their respective No Shock group [p values ≤ 0.005]. Furthermore, HDID-1 mice in the Shock group froze significantly more than either of the other genotypes’ Shock group mice [p values ≤ 0.01; Tukey’s HSD]. There were no significant differences among groups of mice during the ITI period.
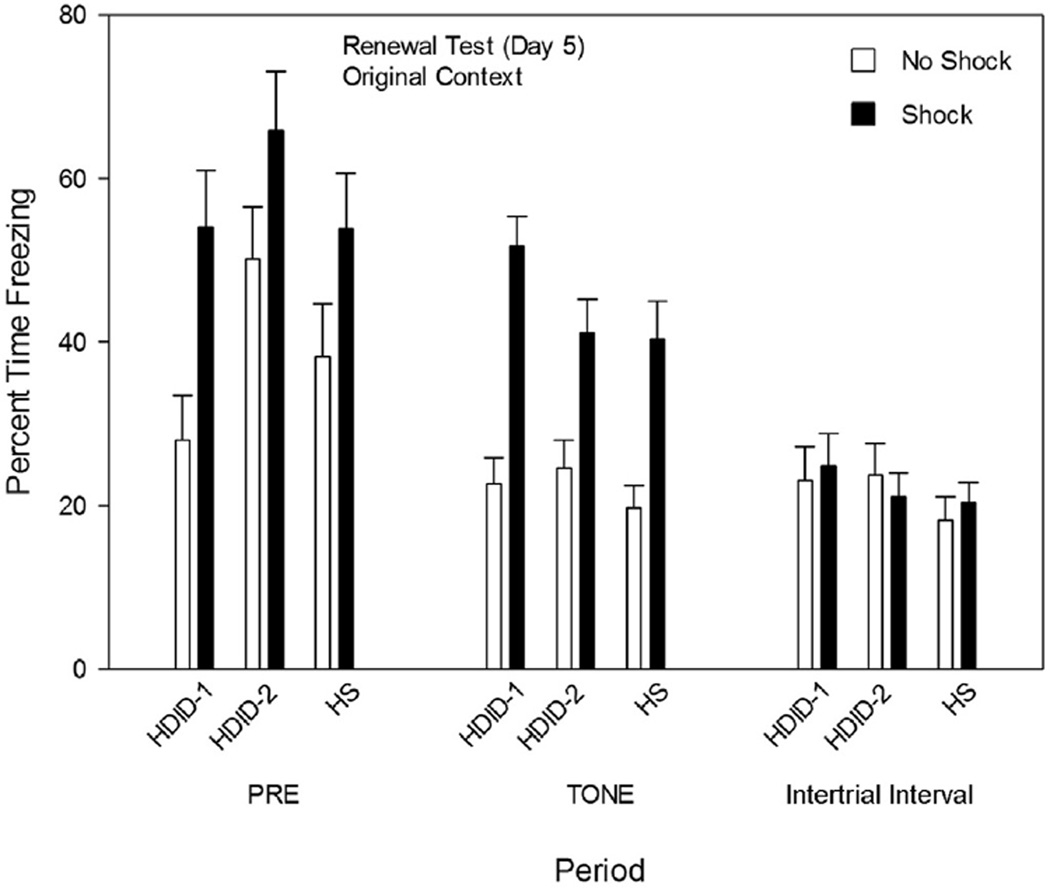
Fig. 2 shows that when mice were retested on Day 5 in the original context, all genotypes retained contextual conditioning, showing high levels of freezing in the context without further increase when the tones were activated. During the Pre-stimulus period, there were significant main effects of genotype (HDID-2 > HDID-1, but HS was not different from either [p < 0.05, Tukey’s HSD]) and treatment group (Shock > No Shock) [F values ≥ 3.5, p values < 0.05], but no significant interaction. During the tone period, there was only a significant main effect of treatment group [F(1,116) = 54.53, p < 0.0001], with Shock groups showing greater freezing than No Shock groups. Fig. 2 shows that, although there is an ordinal increase in freezing in the No Shock groups during the context test, the Shock vs. No Shock difference continues to persist. Finally, there were no significant differences among groups of mice during the ITI periods [all F values ≤ 1].
Fig. 2.
Renewal testing (Day 5). See caption to Fig. 1, Panel C.
Experiment 2: 7-day protocol
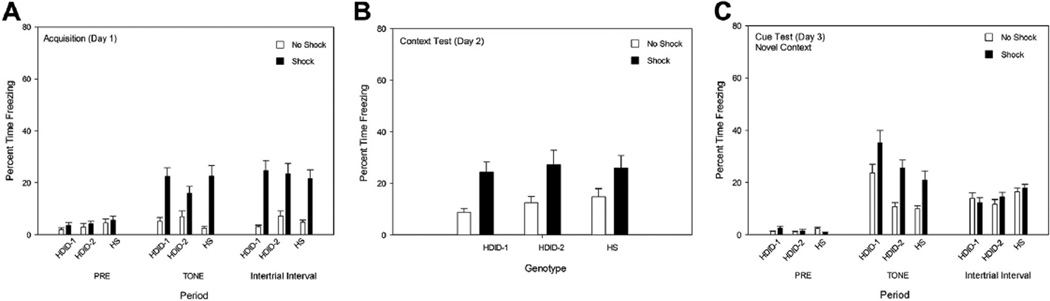
Results from the acquisition day are shown in Fig. 3A. There were no significant effects during the Pre period (all F values < 1.9, p values ≥ 0.16). During the tone period, the Shock groups froze more than the No Shock groups [F(1,88) = 44.42, p < 0.0001], but the main effect of genotype was not significant, nor did it interact significantly with treatment [F values ≤ 2.1, p values ≥ 0.13]. The same pattern was seen during the ITI periods.
Fig. 3.
Freezing in HDID-1, HDID-2, and HS mice in Experiment 2 (7-day protocol). See caption to Fig. 1.
Testing on Day 2 revealed that the context alone had acquired efficacy to induce conditioned freezing in all three genotypes (Fig. 3B). There was a significant effect of treatment [F(2,88) = 16.70, p < 0.0005], but genotypes did not differ or interact significantly with treatment [F values < 1].
In the novel context on Day 3, there was very little freezing during the Pre period (Fig. 3C). A significant genotype by treatment interaction [F(2,88) = 3.75, p ≤ 0.05] was due to HS mice in the No Shock group showing greater freezing than the HS Shocked group [p < 0.05]. No significant differences were seen between treatments in the other genotypes [F values < 1]. Similar to results from Experiment 1, tone presentation was sufficient to elicit conditioned freezing [F(1,88) = 20.15, p ≤ 0.0001] and genotypes differed significantly [F(2,88) = 9.69, p < 0.0005]; HDID-1 mice showed significantly greater percent freezing than either of the other genotypes [p values < 0.05]. HS mice of either treatment group froze more than the other genotypes during the ITIs [F(2,88) = 3.46, p < 0.05] (Fig. 3C).
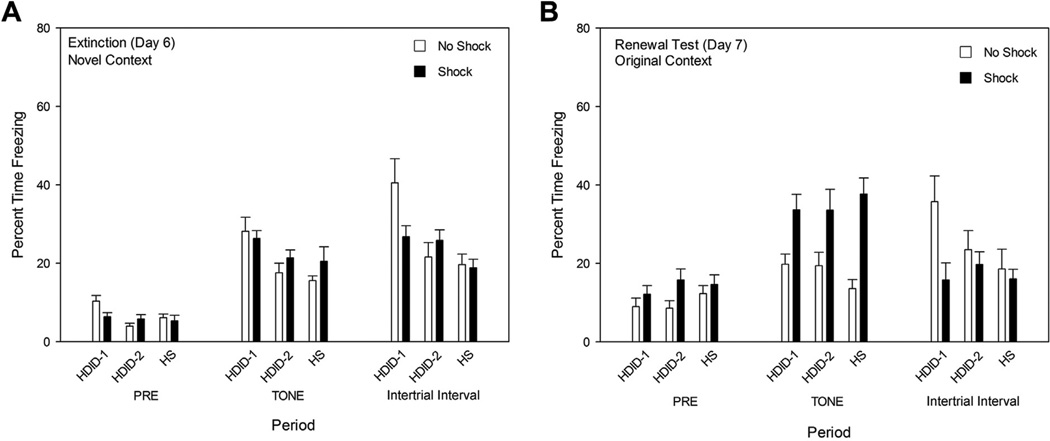
Differences between the Shock and No Shock groups tended to dissipate across extinction Days 4 and 5 (data not shown). Analysis of Day 6 tone period data revealed more freezing in HDID-1 mice [F(2,88) = 6.87, p < 0.01] but no main effect of treatment or genotype by treatment interactions [F values < 1.2, p values ≥ 0.29] (Fig. 4A), suggesting that extinction had occurred in all genotypes. Finally, when mice were retested in the original context and exposed to the tone CS on Day 7, all mice in the Shock groups showed greater freezing compared to the No Shock groups, consistent with renewal of conditioned freezing (Fig. 4B). Fig. 4B also shows the main effects of treatment group in all three periods [all F values ≥ 4.70, p values < 0.05], but not genotype or its interaction with treatment group [all F values ≤ 2.40, p values > 0.09]. During the Pre and Tone periods, the Shock groups showed greater freezing than the No Shock groups; however, during the ITI period, this effect was reversed. A trend toward a treatment by genotype interaction [p = 0.097] appeared to be primarily driven by the HDID-1 line showing greater freezing by the No Shock group.
Fig. 4.
Panel A, Extinction (Day 6) and Panel B, Renewal testing (Day 7) in Experiment 2. See caption to Figs. 1 and 2.
Experiment 3: pseudorandom presentations of CS and US
Due to the irregular (hence, unassociated) progression of events, Acquisition-day freezing data for the 12 sequential events are summarized in Table 1. Freezing scores across time (event) bins were analyzed using repeated-measures ANOVA. There were significant main effects of treatment group [F(1,57) = 35.49, p < 0.0001] but not genotype or their interaction [F values < 2.12, p values > 0.12]. Freezing increased over time [F(11,627) = 8.77, p < 0.001], and time interacted significantly with treatment group [F(11,627) = 4.09, p < 0.0001] but not with genotype or their interaction [F values < 1.3, p values > 0.16]. Examination of the pattern of responses (see Table 1) revealed that the Shock groups froze more than the No Shock groups, regardless of genotype, and HDID-1 mice froze more than either HDID-2 or HS mice. Greater freezing in the Shock group emerged later during the session for HDID-2 mice than HDID-1 or HS.
Table 1.
Experiment 3: Percent time freezing during unpaired tone and shock presentations on Day 1.
| Presentation | Duration(s) | HDID-1 | HDID-2 | HS | |||
|---|---|---|---|---|---|---|---|
| Shock | No Shock | Shock | No Shock | Shock | No Shock | ||
| Pre | 30 | 1.4 ± 1.4 | 0.0 ± 0.0 | 1.0 ± 1.0 | 3.4 ± 1.9 | 4.9 ± 2.0 | 1.0 ± 1.0 |
| Tone 1 | 30 | 20.6 ± 6.6 | 4.1 ± 2.9 | 6.1 ± 3.4 | 2.7 ± 1.8 | 1.4 ± 1.4 | 3.5 ± 2.4 |
| ITI, then + Shock | (90) 88 + 2 | 9.0 ± 3.1 | 2.4 ± 0.8 | 3.9 ± 1.2 | 3.4 ± 1.2 | 6.4 ± 1.7 | 2.8 ± 1.6 |
| ITI | 60 | 4.8 ± 2.0 | 1.4 ± 1.0 | 1.9 ± 1.1 | 2.2 ± 1.0 | 6.8 ± 2.4 | 1.8 ± 0.9 |
| Tone 2 | 30 | 9.4 ± 3.3 | 2.4 ± 2.4 | 7.9 ± 6.0 | 5.3 ± 2.6 | 6.8 ± 2.3 | 3.6 ± 2.4 |
| ITI, then + Shock | (30) 28 + 2 | 8.2 ± 5.1 | 1.4 ± 1.4 | 9.6 ± 2.1 | 4.6 ± 2.0 | 14.1 ± 3.3 | 3.3 ± 1.7 |
| ITI, then + Shock | (90) 88 + 2 | 16.2 ± 6.6 | 4.3 ± 1.3 | 8.1 ± 2.6 | 4.1 ± 1.4 | 7.0 ± 2.8 | 5.5 ± 2.1 |
| Tone 3 | 30 | 17.0 ± 4.6 | 3.8 ± 1.9 | 15.9 ± 3.8 | 5.1 ± 2.3 | 5.7 ± 3.3 | 6.8 ± 2.9 |
| ITI | 30 | 25.8 ± 5.2 | 4.7 ± 3.2 | 15.8 ± 4.9 | 3.7 ± 2.0 | 7.7 ± 2.9 | 1.2 ± 1.2 |
| Tone 4 | 30 | 19.0 ± 8.2 | 8.6 ± 5.5 | 24.5 ± 6.4 | 3.4 ± 1.9 | 16.7 ± 5.6 | 3.8 ± 2.0 |
| ITI, then + Shock | (60) 58 + 2 | 30.4 ± 8.2 | 2.7 ± 1.8 | 18.6 ± 4.6 | 10.4 ± 2.7 | 11.6 ± 5.3 | 8.3 ± 2.3 |
| ITI | 120 | 25.6 ± 7.8 | 5.8 ± 2.1 | 23.4 ± 4.9 | 4.6 ± 1.5 | 20.7 ± 7.9 | 4.3 ± 1.3 |
ITI = intertrial interval.
Context alone clearly elicited freezing increases in the Shock groups [F(1,57) = 14.40, p < 0.0005] on Day 2 (Fig. 5A), but this was not influenced by genotype [F values < 1]. Day 3 results in the novel context (Fig. 5B) demonstrated that the unpaired tone presentations had not acquired the efficacy to enhance freezing in Shock groups, relative to the No Shock groups (for treatment and genotype by treatment, F values < 2.40, p values > 0.10). HDID-1 mice, regardless of treatment group, showed more freezing than HS mice [p < 0.01]. During the Pre period, a main effect of genotype [F(2,57) = 3.44, p < 0.05] was found that was due to greater freezing overall by HDID-1 mice than HS mice [p < 0.05, Tukey’s HSD]. No other effects were significant during the Pre period [F values < 1.8, p values > 0.18]. There were no significant differences during the ITI period [F values < 2.17, p values > 0.12]. When tones were presented in the original context on Day 5 during the Renewal test (Fig. 5C), genotypes tended to differ [F(2,57) = 3.10, p = 0.052], but there were no other significant differences [F values < 2.38, p values > 0.12]. During the Pre period, the Shock groups showed greater freezing than the No Shock groups [F(1,57) = 4.38, p < 0.05] and a trend for a genotype difference was found [F(2,57) = 3.03, p = 0.56], but there was no interaction [F < 1.81, p > 0.17]. There were similar effects of treatment during the ITI period [F(1,57) = 5.67, p < 0.05], but no other significant effects [F values < 1.8, p values > 0.17].
Fig. 5.
Panel A. Context testing (Day 2). No tones or shocks were presented during this test period. Panel B. Cued testing in the novel context (Day 3). Two, 180-s tone CS periods occurred, with no shocks presented. Panel C. Renewal testing (Day 5). See caption to Fig. 1, Panel C.
Discussion
The outcomes reported here offer strong evidence that the genetic contributions to high DID-BEC are substantially independent of those contributing to differences in fear conditioning. Furthermore, these experiments establish that HDID mice of both genetic replicates, and the heterogeneous stock from which they were derived, display intact abilities to acquire a conditioned fear response. In two experiments, re-exposure to the context in which 4 CS-US tone-shock pairings were administered was sufficient to elicit enhanced behavioral freezing 24 h later. Exposure to the tone CS in a novel context on Day 3 also elicited freezing in mice that had previously received pairings of CS and shock. After either 1 or 3 additional post-test extinction trials, mice were re-exposed to the original context and the CS was later presented. In a renewal test in both experiments, the overall level of freezing appeared to be greater in Experiment 1 than in Experiment 2 (Figs. 2 and 4B), although our designs did not permit formal statistical comparison of this difference. All three genotypes responded with more freezing if they had been previously shocked. The overall level of freezing to the context only (“Pre” period in), as well as the relative increases in the Shock groups, also appeared to be greater in Experiment 1. We attribute this to the greater number of extinction trials in Experiment 2. In both experiments, the strength of renewed responding to the tone CS also appeared to be similar across genotypes. Furthermore, renewal of responding to the tone was greater than that only to the context (“Pre” period in both figures).
Murine genotypes are known to differ in fear-conditioned responding (Camp et al., 2012; Tipps et al., 2014), even when extensive experimental procedures are followed to eliminate the role of non-genetic sources for those differences (e.g., equating initial levels of acquisition across strains before comparing patterns of extinction; see Lattal & Maughan, 2012). While Experiment 1 suggested that contextual and tone conditioning may have been stronger in HDID-1 mice than in the other two genotypes, the three genotypes responded similarly to identical procedures in Experiment 2. We conclude that there is no apparent difference among genotypes. Because HDID-1 and HDID-2 mice differ markedly from the HS Control line in their BEC attained after binge-like alcohol consumption in the drinking in the dark assay (DID-BEC), the trait for which they were selectively bred, there must be different genes contributing to fear-conditioning processes, presumably through different neural mechanisms. For us to conclude that there is genetic overlap in influences, we would expect to see both replicates of the HDID lines differing systematically from HS (Crabbe et al., 1990). The HDID lines differ in a number of behavioral responses both from HS and from each other. Few of these responses, however, appear to be correlated responses to selection (for review, see Barkley-Levenson & Crabbe, 2014). Rather, the practical constraints of maintaining selected lines with relatively few breeding pairs leads inevitably to genetic divergence among the three genotypes for genes unrelated to high DID-BEC, which then lead to behavioral differences. It does appear to us, however, that the HDID-1 line displays greater levels of freezing overall than either HDID-2 or HS. While this outcome was not evident on every test day or during every test condition, examinations of the figures, and several statistical outcomes reported above, support this generalization.
Experiment 3 assuaged the potential concern that the genotypic differences, where seen, might have been adventitious due to nonassociative differences in response to the tone stimulus even if it were not predictive of shock. Fig. 5A shows that all three genotypes displayed equivalent conditioned freezing to the contextual cues, which include housing, transportation, handling, and experimenter-related cues. And, as seen in Fig. 5B, isolation of the tone as the relevant cue through the use of a novel context, experimenter garb, and olfactory cues shows that the tone itself acquired no efficacy to increase freezing.
Fear conditioning serves as the structural scaffold for many rodent models of post-traumatic stress disorder (PTSD) (Clay et al., 2011; Cohen, Kozlovsky, Alona, Matar, & Joseph, 2012; Fanselow, 1980; Johnson, McGuire, Lazarus, & Palmer, 2012; Maren & Holmes, 2015; Norman et al., 2012; Olson et al., 2011; Pynoos, Ritzmann, Steinberg, Goenjian, & Prisecaru, 1996; Siegmund & Wotjak, 2007). Animal model evidence shows a variety of effects of acute and chronic alcohol, and particularly withdrawal from alcohol dependence, on fear-related responses, whether conditioned or unconditioned (for review, see Tipps et al., 2014). It was recently shown that murine genotypes differed in their response to acute alcohol withdrawal on fear-conditioned responses. Furthermore, context-elicited and cue-elicited responses were differentially affected: the former were blunted, and the latter enhanced (Tipps, Raybuck, Buck, & Lattal, 2015). Because of the high prevalence of both binge-like drinking and PTSD in combat veterans (Cucciare et al., 2011; Davis et al., 2003), we plan to adapt existing PTSD models for use in HDID mice. The studies reported here show that these mice have the capacity to acquire and extinguish conditioned fear, and they are genetically predisposed to binge intoxication, which will allow us to pursue the relationship between drinking and conditioned fear in these genotypes.
Acknowledgments
These studies were supported by Grants AA010760, AA013519, and AA020245 from the NIH-NIAAA; by the Department of Veterans Affairs; and by the Department of the Army/DoD-TATRC grant 10245005.05. We thank Stephanie E. Spence and Lawrence C. Huang for maintaining the colonies and supplying the mice for these experiments.
References
- Barkley-Levenson AM, Crabbe JC. High drinking in the dark mice: a genetic model of drinking to intoxication. Alcohol. 2014;48:217–223. doi: 10.1016/j.alcohol.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Cunningham CL, Smitasin PJ, Crabbe JC. Rewarding and aversive effects of ethanol in high drinking in the dark selectively bred mice. Addiction Biology. 2015;20:80–90. doi: 10.1111/adb.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. American Journal of Preventive Medicine. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, et al. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology. 2012;37:1534–1547. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay R, Hebert M, Gill G, Stapleton LA, Pridham A, Coady M, et al. Glucocorticoids are required for extinction of predator stress-induced hyperarousal. Neurobiology of Learning and Memory. 2011;96:367–377. doi: 10.1016/j.nlm.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z. Animal model for PTSD: from clinical concept to translational research. Neuropharmacology. 2012;62:715–724. doi: 10.1016/j.neuropharm.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. The genetic complexity of alcohol drinking in rodents. In: Noronha AB, Cui C, Harris RA, Crabbe JC, editors. Neurobiology of alcohol dependence. San Diego: Elsevier/Academic Press; 2014. pp. 359–375. [Google Scholar]
- Crabbe JC, Metten P, Belknap JK, Spence SE, Cameron AJ, Schlumbohm JP, et al. Progress in a replicated selection for elevated blood ethanol concentrations in HDID mice. Genes, Brain, and Behavior. 2014;13:236–246. doi: 10.1111/gbb.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu C-H, Brown LL, Phillips TJ, et al. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biological Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcoholism: Clinical and Experimental Research. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011;45:427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucciare MA, Darrow M, Weingardt KR. Characterizing binge drinking among U.S. military Veterans receiving a brief alcohol intervention. Addictive Behaviors. 2011;36:362–367. doi: 10.1016/j.addbeh.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Davis TM, Bush KR, Kivlahan DR, Dobie DJ, Bradley KA. Screening for substance abuse and psychiatric disorders among women patients in a VA Health Care System. Psychiatric Services. 2003;54:214–218. doi: 10.1176/appi.ps.54.2.214. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. The Pavlovian Journal of Biological Science. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. The Journal of Neuroscience. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nature Reviews. Genetics. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Hitzemann B, Dains K, Kanes S, Hitzemann R. Further studies on the relationship between dopamine cell density and haloperidol-induced catalepsy. The Journal of Pharmacology and Experimental Therapeutics. 1994;271:969–976. [PubMed] [Google Scholar]
- Johnson LR, McGuire J, Lazarus R, Palmer AA. Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology. 2012;62:638–646. doi: 10.1016/j.neuropharm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Kaouane N, Porte Y, Vallée M, Brayda-Bruno L, Mons N, Calandreau L, et al. Glucocorticoids can induce PTSD-like memory impairments in mice. Science. 2012;335:1510–1513. doi: 10.1126/science.1207615. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Maughan DK. A parametric analysis of factors affecting acquisition and extinction of contextual fear in C57BL/6 and DBA/2 mice. Behavioural Processes. 2012;90:49–57. doi: 10.1016/j.beproc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends in Neurosciences. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2015;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. Vol. 3. Bethesda, MD: DHHS-NIH; 2004. [Google Scholar]
- Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, Huang Z, et al. Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology. 2012;62:542–551. doi: 10.1016/j.neuropharm.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VG, Rockett HR, Reh RK, Redila VA, Tran PM, Venkov HA, et al. The role of norepinephrine in differential response to stress in an animal model of posttraumatic stress disorder. Biological Psychiatry. 2011;70:441–448. doi: 10.1016/j.biopsych.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biological Psychiatry. 1996;39:129–134. doi: 10.1016/0006-3223(95)00088-7. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One. 2011;6:e15982. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. Journal of Psychiatric Research. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Archives of General Psychiatry. 2010;67:614–623. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, Lattal KM. Delay and trace fear conditioning in C57BL/6 and DBA/2 mice: issues of measurement and performance. Learning & Memory. 2014;21:380–393. doi: 10.1101/lm.035261.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, Lattal KM. Acute ethanol withdrawal impairs contextual learning and enhances cued learning. Alcoholism: Clinical and Experimental Research. 2015;39:282–290. doi: 10.1111/acer.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KR. NIAAA Director’s Statement. Appropriations: Labor, HHS, education, and related agencies. 2009 [Google Scholar]