Abstract
Plant litter breakdown is a key ecological process in terrestrial and freshwater ecosystems. Streams and rivers, in particular, contribute substantially to global carbon fluxes. However, there is little information available on the relative roles of different drivers of plant litter breakdown in fresh waters, particularly at large scales. We present a global-scale study of litter breakdown in streams to compare the roles of biotic, climatic and other environmental factors on breakdown rates. We conducted an experiment in 24 streams encompassing latitudes from 47.8° N to 42.8° S, using litter mixtures of local species differing in quality and phylogenetic diversity (PD), and alder (Alnus glutinosa) to control for variation in litter traits. Our models revealed that breakdown of alder was driven by climate, with some influence of pH, whereas variation in breakdown of litter mixtures was explained mainly by litter quality and PD. Effects of litter quality and PD and stream pH were more positive at higher temperatures, indicating that different mechanisms may operate at different latitudes. These results reflect global variability caused by multiple factors, but unexplained variance points to the need for expanded global-scale comparisons.
Keywords: decomposition, latitudinal gradient, climate, litter quality, biodiversity, detritivore shredders
1. Introduction
Plant litter breakdown is a key process in organic matter recycling and supports food webs in terrestrial and aquatic ecosystems. Terrestrial plants produce ca 120 billion tonnes of organic carbon each year [1], 90% of which escapes herbivory and eventually enters the dead organic matter pool [2]. After death, the plant litter either can be decomposed, with its components recycled back into their inorganic forms, or the recalcitrant portion can be stored over long periods [3]. The rate at which plant litter is transformed to other forms of organic and inorganic carbon determines the rate of recycling of biologically essential nutrients, the capacity for carbon storage in ecosystems and the rate at which greenhouse gases such as carbon dioxide (CO2) are outgassed—all processes that influence regulation of the global climate [4].
Knowing which biotic and abiotic factors drive plant litter breakdown is crucial for understanding how ecosystems function and how vulnerable they are to environmental perturbations such as climate warming, biodiversity loss and biological invasions [5,6]. In terrestrial ecosystems, the relative roles of climate and plant litter quality vary among biomes [7,8]. A recent synthesis showed that climate had a greater role in cold or dry ecosystems and deciduous forests, whereas litter quality traits (i.e. the carbon : nitrogen (C : N) ratio and specific leaf area (SLA)) were more important in humid grasslands and agro-ecosystems, both factors being equally important in tropical wet forests [9]. Another study also highlighted the main role of climate on breakdown in cold areas of the Northern Hemisphere [10], whereas several terrestrial studies at the global scale found that effects of litter quality on breakdown were greater than effects of climate [11–13]. Detritivores can also be important contributors to breakdown rates in terrestrial ecosystems [9], particularly in temperate and wet tropical climates where biological activity is not constrained by temperature or moisture [14].
In contrast to terrestrial ecosystems, information on the relative roles of different drivers of plant litter breakdown in fresh waters is scarce. In freshwater ecosystems in general [15], and in streams and rivers in particular [16], large amounts of organic carbon are processed, contributing significantly to global carbon fluxes. For example, recent estimates show that mean CO2 evasion from fresh waters is 2.1 Pg C yr−1 (cf. ≈ 9 Pg C yr−1 from anthropogenic sources [15]), 86% of which comes from streams and rivers [17]. Breakdown of terrestrially derived plant litter is a pivotal component of stream ecosystem functioning but, despite numerous commonalities, the process is likely to differ in several respects between aquatic and terrestrial ecosystems, in part because of the contrast in water availability [18,19]. Specifically, water is not a limiting factor to the breakdown process within perennial streams, so climate is expected to affect breakdown rates mostly through the influence of temperature, and less so through precipitation (which can, however, cause floods that remove litter from stream reaches [20,21]).
Several local-scale stream studies (encompassing one or several streams within a single region) have demonstrated that litter quality traits (e.g. lignin or nutrient concentration) show tight relationships with litter breakdown rates [22–26]. The few comparable continental-scale and global-scale studies have shown that climate and detritivores can be key determinants of breakdown rates [27–30]. However, there have been few assessments of the relative roles of climate, litter quality and detritivores on litter breakdown in streams at continental or global scales, limiting our capacity to predict the effects of global change on the breakdown process. Furthermore, with one exception [28], global-scale breakdown studies have not considered the potential role of plant diversity on litter breakdown, although species loss is a major global concern [31] and can alter ecosystem processes [3]. The link between plant diversity and litter breakdown rate is still unclear and apparently weak [18,32,33], but virtually all studies examining plant diversity effects on litter breakdown have focused on species richness, neglecting other diversity components such as phylogenetic diversity (PD) [34].
Here, we present a global-scale study of litter breakdown in streams to assess the relative roles of biotic, climatic and other environmental factors on breakdown rates. We conducted an experiment in 24 streams on five continents encompassing a latitudinal range of 90° (47.8° N–42.8° S), using local litter mixtures differing in quality and PD (but not species richness), and an additional common litter type (black alder, Alnus glutinosa (L.) Gaertn.) to control to some extent for variation in litter quality and diversity across sites. Alder leaves were chosen because the genus is widely distributed across the North Temperate Zone, and because they are highly palatable to temperate and tropical stream detritivores [35]. We manipulated detritivore presence using coarse-mesh and fine-mesh bags, which allowed colonization of litter-consuming detritivores or excluded them, respectively.
We expected that the relative importance of litter quality and PD, detritivore presence, climate and other environmental factors would vary globally, based on the existing evidence from terrestrial ecosystems [9,11,13,14]. We hypothesized that (i) microbial breakdown would increase with temperature (through its effect on metabolic rate [36,37]), and hence decrease with latitude; (ii) detritivore-mediated breakdown would be greater at higher latitudes, where litter-consuming detritivores are more abundant and diverse [38]; (iii) litter of high quality would break down rapidly [24,26]; (iv) phylogenetically diverse plant litter would break down faster because of the potential presence of a wider range of species trait values, which increases the chance for niche partitioning [39]; (v) more basic water (higher pH) would enhance breakdown, as is typically the case [40]; (vi) breakdown would be faster in narrower streams where litter retentiveness is generally higher [41]; and (vii) climate would modulate the effects of other environmental factors and litter on breakdown rates; for example, higher temperatures could enhance any effects of litter quality or water chemistry [42].
2. Methods
(a). Field experiment
We conducted a litter breakdown experiment at 24 stream sites around the world with absolute latitudes ranging from 0.37° to 47.80° (electronic supplementary material, table S1 and figure S1); 14 sites were located within the tropics (less than or equal to 23.5° latitude) and 10 in temperate regions (more than 23.5°), comprising a large range of climatic patterns (electronic supplementary material, figure S2a,b). At each site, we chose a single stream reach draining a forested catchment that experienced little human influence. The experiment was run at a time of high litter inputs and low flood susceptibility at each site. We chose three native riparian tree species locally common in the riparian vegetation and well represented in stream leaf litter (electronic supplementary material, table S2). In total, we collected freshly abscised leaves of 70 species, as two species were shared between two sites each. Alder leaves were collected to serve as an approximate control for variation in litter quality across sites; however, the leaves were locally collected near the study sites or, when not locally available (tropical sites, Southern Hemisphere and some Northern Hemisphere temperate sites), shipped from either Portugal or Spain or collected in botanical gardens to avoid problems with import regulations. Although some intraspecific variation in alder litter quality across locations was likely to occur [25], we expected such variation to be much smaller than that among the 70 other species used in the experiment.
Leaves were air-dried, weighed, enclosed in coarse-mesh (10 mm) and fine-mesh (0.5 mm) bags (ca 1 g per species per bag in three-species mixtures, or ca 3 g for alder leaves) and secured in streams. Three coarse-mesh and three fine-mesh bags were retrieved on each of four dates: day 0 (to determine any mass loss due to handling), and approximately days 14, 28 and 56. Bags were collected with a net (0.5 mm mesh) and taken to the laboratory where leaves were cleaned, oven-dried and weighed. Ash-free dry mass was not estimated for logistical reasons; however, it is unlikely that the litter breakdown data were biased by mineral particles associated with the leaves as there was no indication of calcite precipitation in our streams (most of them being soft-water systems) or association of mineral particles with leaves (most streams having coarse substratum). In addition, all leaves were thoroughly cleaned under water before drying. At each site, we recorded pH as a measure of water chemistry, and wetted stream width as an estimate of stream size.
(b). Climatic and leaf quality data
Comprehensive water temperature data were not available for all sites, so mean annual air temperature data were extracted from the WorldClim database v. 1.3 [43] at the highest resolution (2.5 min of arc) using DIVA-GIS software, 7.5.0.0. (http://www.diva-gis.org). We assessed leaf quality by using the mean SLA, which is the ratio of leaf area (cm2) to leaf dry mass (g). SLA is a key ecological and physiological plant trait [44] that often correlates with leaf toughness, nutrient concentration and breakdown rate [45,46]. To measure SLA, we scanned 20 leaves of each plant species, estimated their areas with ImageJ 10.2, dried them to constant mass and weighed them to the nearest 0.1 mg. We then calculated the mean SLA at each site.
(c). Plant phylogenetic diversity
We used PD, defined as the total phylogenetic distance among species, as a measure of plant litter diversity [47]. To estimate PD among the three species selected at each site, we constructed a molecular phylogeny of the 70 species and 8935 bps of DNA (including five outgroups) using partial 18S ribosomal DNA, rbcl, matK, atpB, trnl, rpl16, rpoB and rpoC1 sequences available in GenBank (electronic supplementary material, table S3). We used these markers as they provided the most comprehensive sets of data for the target species. Data for several species were not available in GenBank, so we chose closely related congeners as replacements. We constructed alignments independently for each gene using Muscle v. 3.8.31 [48]. Nucleotide substitution models were selected for each gene using the Akaike information criterion (AIC) as implemented in jModelTest v. 0.1.1 [49]. We searched for the maximum-likelihood phylogeny using RAxML v. 7.2.8 [50], partitioning the dataset by gene. Random starting trees were used for each independent tree search, and topological robustness was investigated using 100 bootstrap replicates. We used a rate-smoothed Bayesian phylogeny, estimated using Beast v. 1.6.2 [51], assuming a relaxed uncorrelated lognormal clock with all other parameters set as default. The relaxed-clock analysis was used to estimate relative divergence times, thereby converting branch-length values from the substitutions per site to an estimate of time since divergence from a common ancestor. The Bayesian Markov chain Monte Carlo ran for 10 million generations sampled every 1000 generations, whereas stationarity and effective sample sizes (ESS > 200) were examined using Tracer v. 1.6 [52], discarding all trees under the asymptote as burn-in. Finally, we constructed a consensus tree (electronic supplementary material, figure S3) with mean node heights from the posterior distribution using TreeAnnotator v. 1.6.2 [51]. To calculate PD per community, we used the comdist function in the R package ‘picante’ [53].
(d). Data analysis
We used linear regression to detect latitudinal trends in litter variables, mean annual temperature and other environmental variables. Breakdown rates of local litter mixtures and alder were estimated for each site using the breakdown coefficient (k) resulting from the exponential decay model mf/mi = e−kt, where mf and mi are the final and initial litter mass (g), respectively, t is time in days (d) and k is the breakdown rate coefficient. We estimated the relative contributions of different factors to litter breakdown rates using general linear models, with key predictors selected based on the AIC following a stepwise search in backward and forward directions, using the stepAIC function in the R package ‘MASS’ [54]. The initial models for litter mixtures included litter variables (mean SLA and PD), mean annual temperature (hereafter temperature), other environmental variables (pH and stream width) and the interactions between temperature and the other variables. We included these interactions to explore the directional effects of climate on other variables. The models for alder included temperature and other environmental variables (and their interactions) but no litter traits, because SLA was expected to vary little within species (compared with variation in SLA across the total of 70 species) and PD = 1. We examined multicollinearity of the variables to be included in the models with the variance inflation factor (VIF) using the VIF function in the R package ‘fmsb’ [55].
We tested separate models for overall breakdown in coarse-mesh litter bags (kc), microbial breakdown in fine-mesh bags (kf) and detritivore-mediated breakdown (kd), which was calculated from the difference in the proportion of litter mass remaining between coarse-mesh and fine-mesh bags at each sampling date [29]. Normality of residuals was examined with Shapiro–Wilk's test; three variables (kc for alder and kf for litter mixtures and alder) showed lognormal distributions, which became normal after loge-transformation. Main and interaction effects were visually explored using the visreg and visreg2d functions in the R package ‘visreg’ [56]. We further examined the role of detritivores on breakdown of local litter mixtures and alder by comparing kc and kf for the global dataset and separately for tropical (n = 14) and temperate sites (n = 10), using Wilcoxon signed-rank tests.
3. Results
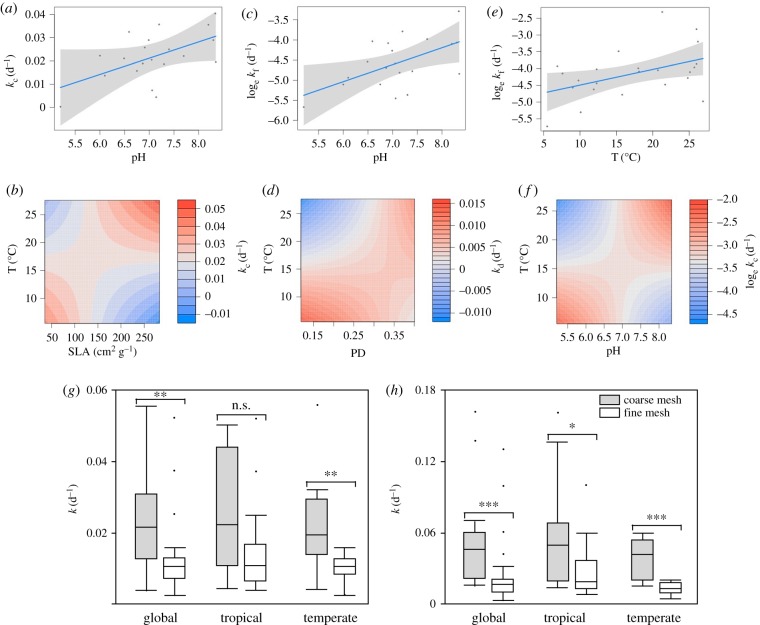
Mean annual temperature decreased with latitude (r = −0.73, p < 0.001), whereas the other environmental or litter variables showed no latitudinal trend (r < 0.26, p > 0.22 in all cases). VIFs were all less than 2, indicating the absence of multicollinearity (electronic supplementary material, table S4). Our final models included different factors and interactions (table 1). The overall model for total breakdown of the local litter mixtures was significant (p = 0.0499) and showed significant effects of litter SLA and pH and a significant interaction between temperature and SLA (figure 1a,b). The overall model for microbial breakdown of litter mixtures was significant (p = 0.042) and showed a significant effect of pH (figure 1c). For detritivore-mediated breakdown of litter mixtures, the overall model was not significant (p = 0.107) but it showed significant effects of litter PD and its interaction with temperature (figure 1d). For total alder breakdown, the overall model was significant (p = 0.046) and showed the effects of temperature and its interaction with pH (figure 1f). The overall model for microbial breakdown of alder was significant (p = 0.029) and showed an effect of temperature (figure 1e). Finally, detritivore-mediated breakdown of alder was not significantly affected by any factor (p = 0.90).
Table 1.
Results of general linear models (selected based on the Akaike information criterion, AIC, following a stepwise search in backward and forward directions) examining the influence of key predictors (mean annual temperature, T; mean specific leaf area, SLA, and litter phylogenetic diversity, PD, as litter variables; and pH and wetted width, SW, as stream environmental variables) on total litter breakdown rates of litter mixtures and alder in coarse-mesh bags (kc), microbial breakdown rates in fine-mesh bags (kf) and detritivore-mediated breakdown rates (kd). The AIC, adjusted r2, F-test and p-value are shown for each model, and the mean estimate and standard error, t-statistic and p-value for each factor (italic entries indicate significant results at the p < 0.05 level).
| litter breakdown | selected model | factor | estimate | s.e. | t | p |
|---|---|---|---|---|---|---|
| litter mixtures | ||||||
| total |
kc ∼ T + SLA + PD + pH + T × SLA + T × PD + T × pH |
|||||
| AIC =−175 | intercept | 0.081160 | −0.714000 | 0.49 | 0.489 | |
| adjusted r2 = 0.41 | T | 0.001409 | 0.004210 | 0.34 | 0.744 | |
| F7,12 = 2.92 | SLA | −0.000321 | 0.000110 | −2.92 | 0.013 | |
| p = 0.0499 | PD | −0.135900 | 0.088550 | −1.54 | 0.151 | |
| pH | 0.023480 | 0.010510 | 2.24 | 0.045 | ||
| T × SLA | 0.000019 | 0.000006 | 2.95 | 0.012 | ||
| T × PD | 0.009196 | 0.006253 | 1.47 | 0.167 | ||
| T × pH | −0.000975 | 0.000559 | −1.75 | 0.106 | ||
| microbial | kf ∼ T + SLA + pH + T × SLA | |||||
| AIC =−18 | intercept | −6.650175 | 1.632409 | −4.07 | 0.001 | |
| adjusted r2 = 0.32 | T | −0.058414 | 0.049330 | −1.18 | 0.255 | |
| F4,15 = 3.25 | SLA | −0.010108 | 0.005675 | −1.78 | 0.095 | |
| p = 0.042 | pH | 0.422662 | 0.166398 | 2.54 | 0.023 | |
| T × SLA | 0.000629 | 0.000325 | 1.94 | 0.072 | ||
| detritivore-mediated |
kd ∼ T + PD + pH + SW + T × PD + T × pH + T × SW |
|||||
| AIC =−208 | intercept | −0.009987 | 0.024860 | −0.40 | 0.693 | |
| adjusted r2 = 0.25 | T | 0.000562 | 0.001266 | 0.44 | 0.663 | |
| F7,16 = 2.08 | PD | −0.077460 | 0.028080 | −2.76 | 0.014 | |
| p = 0.107 | pH | 0.004855 | 0.003296 | 1.47 | 0.160 | |
| SW | 0.000021 | 0.000011 | 1.88 | 0.078 | ||
| T × PD | 0.005619 | 0.001892 | 2.97 | 0.009 | ||
| T × pH | −0.000305 | 0.000173 | −1.76 | 0.098 | ||
| T × SW | −0.000001 | 0.000001 | −1.60 | 0.129 | ||
| alder | ||||||
| total | kc ∼ T + pH + SW + T × pH | |||||
| AIC =−22 | intercept | 3.578300 | 3.945612 | 0.91 | 0.376 | |
| adjusted r2 = 0.27 | T | −0.475873 | 0.209678 | −2.27 | 0.036 | |
| F4,18 = 3.00 | pH | −1.029913 | 0.541762 | −1.90 | 0.073 | |
| p = 0.046 | SW | 0.000984 | 0.000525 | 1.87 | 0.077 | |
| T × pH | 0.068805 | 0.028986 | 2.37 | 0.029 | ||
| microbial | Kf ∼ T | |||||
| AIC =−16 | intercept | −4.963240 | 0.372650 | −13.32 | 0.000 | |
| adjusted r2 = 0.17 | T | 0.046910 | 0.019960 | 2.35 | 0.029 | |
| F1,21 = 5.52 | ||||||
| p = 0.029 | ||||||
| detritivore-mediated | kd ∼ T + pH + SW + T × pH + T × SW | |||||
| AIC =−223 | intercept | 0.029220 | 0.051040 | 0.572 | 0.574 | |
| adjusted r2=−0.19 | T | −0.001610 | 0.002725 | −0.591 | 0.562 | |
| F5,17 = 0.31 | pH | −0.003204 | 0.006897 | −0.465 | 0.648 | |
| p = 0.899 | SW | 0.000015 | 0.000022 | 0.665 | 0.515 | |
| T × pH | 0.000247 | 0.000369 | 0.667 | 0.514 | ||
| T × SW | −0.000001 | 0.000001 | −0.663 | 0.516 | ||
Figure 1.
(a–f) Significant main effects (top panels) and interaction effects (middle panels) of mean annual temperature (T), mean specific leaf area (SLA), litter phylogenetic diversity (PD) and stream pH on total breakdown rates in coarse-mesh bags (kc), microbial breakdown rates in fine-mesh bags (kf) and detritivore-mediated breakdown rates (kd) of litter mixtures (a–d) and alder (e,f). When a variable had a main effect and an interaction in the same model, only the interaction is shown. (g,h) Box plots show litter breakdown rates (k) in coarse-mesh bags (grey boxes) and fine-mesh bags (white boxes) for the global dataset and for tropical and temperate sites separately. Horizontal lines show medians, boxes show 25th and 75th percentiles, error bars show 10th and 90th percentiles, and points show outliers. Brackets and asterisks denote significant differences in breakdown rates of litter between coarse-mesh and fine-mesh bags (Wilcoxon signed-rank tests: *p < 0.05, **p < 0.01, ***p < 0.0001; n.s., not significant).
Litter breakdown rates were higher in coarse-mesh than in fine-mesh bags for litter mixtures and alder across all sites (litter mixtures: Z = −3.19, p = 0.0014; alder: Z = −3.87, p < 0.001) and at temperate sites (litter mixtures: Z = −2.76, p = 0.0058; alder: Z = −3.36, p < 0.001). In the tropics, breakdown was faster in coarse-mesh bags for alder, but the difference was weaker than in temperate sites (Z = −2.21, p = 0.027) and there was no difference for litter mixtures (Z = −1.81, p = 0.070; figure 1g,h).
4. Discussion
(a). Climatic effects on global patterns of litter breakdown in streams
The analysis of our global dataset revealed influences of several abiotic and biotic factors on litter breakdown rate in streams, paralleling findings for terrestrial ecosystems [9,11,14]. The only factor having no influence on breakdown was stream width, counter to our expectations [41], probably because we sampled only during stable base-flow periods. Mean annual temperature was a key influence on litter breakdown of alder, having a main effect on total and microbial breakdown. As expected, microbial breakdown rate increased with temperature and hence was higher towards lower latitudes. A companion study found that this rate increased with contemporaneous water temperature, and the relationship conformed to the metabolic theory of ecology [27]. Here, we found the relationship to hold with long-term air temperature data.
Although we did not detect any variation with temperature for detritivore-mediated breakdown when it was examined separately, total breakdown of alder increased towards cooler streams, and this pattern was most likely related to the higher abundance and diversity of litter-consuming detritivores at higher latitudes [38,57]. Accordingly, our comparison of coarse-mesh and fine-mesh bags revealed a greater role of detritivores on litter breakdown in temperate than in tropical streams, although this difference was most obvious for litter mixtures.
(b). Major influence of litter quality on breakdown of litter mixtures
While breakdown of a single substrate type (alder leaves) across latitudes was mostly influenced by environmental factors and especially temperature, litter variables (SLA and PD) were of major importance to explain variation in breakdown of litter mixtures. This agrees with the results of other global-scale studies [12] and a comprehensive meta-analysis [11] based on data from terrestrial ecosystems. Nevertheless, for litter mixtures, temperature was important as well in that it modulated litter effects on breakdown.
Counter to our prediction, litter SLA had a negative overall influence on total breakdown of litter mixtures. This result contrasts with those of previous research showing that breakdown is greater in leaves with lower lignin content [19,22], which tends to be correlated with SLA [45]. However, the positive interaction of SLA with temperature indicates that small SLAs enhanced breakdown only at low temperatures, whereas the opposite was true in streams of warmer regions, where breakdown of litter mixtures with a higher mean SLA was faster (figure 1b). This result is not easily explained but might reflect a relatively larger influence of detritivores at lower latitudes that use tough leaves for purposes other than nutrition, such as case construction by caddisflies [58].
(c). Detritivore-mediated breakdown driven by phylogenetic diversity in litter mixtures
Detritivore-mediated breakdown was mostly driven by PD of litter mixtures, but apparently in the direction opposite to our prediction. The negative effect of PD on breakdown suggests that phylogenetic proximity of litter mixtures enhances detritivore consumption. This finding could be due to lower phylogenetic distance between litter species resulting in a higher concentration of high-quality resources, whereas larger phylogenetic distance caused the dilution of such resources [59]. Similarly, one of the few studies to explore PD effects on breakdown found that a lower phylogenetic distance in litter mixtures promoted microbial biomass and litter nutrient concentration [60].
However, this pattern was inconsistent across climates, as occurred for SLA. Breakdown of litter mixtures composed of species showing higher phylogenetic proximity was faster only in cooler streams, whereas the opposite was true at higher temperatures (figure 1d). This discrepancy might be related to a higher specialization of litter-consuming detritivores at temperate latitudes [61], which would benefit from the concentration of their preferred resources, whereas more generalist detritivores in the tropics [62] could be relatively more efficient when more varied resources are available.
(d). Higher breakdown in more alkaline waters
Stream pH, which typically reflects basic catchment lithology [63,64], also influenced litter breakdown rates, especially for litter mixtures. Breakdown was faster at higher pH, which agrees with the well-known effect of greater breakdown in more basic waters [65]. The effect of pH on alder breakdown was, however, modulated by climate: while breakdown was faster in more alkaline waters at higher temperatures, the opposite was true in cooler streams (figure 1f). This interaction may reflect the facts that (i) microbial decomposition is most important in the tropics and is boosted by higher calcium concentration [66,67], and (ii) major litter consumers such as stoneflies are more important in circumneutral and acidic streams at higher latitudes [68,69], while they are rare in the tropics [57]. Consistent with this explanation, caddiflies, which dominate the guild of litter-consuming detritivores in tropical streams [57], tend to be more sensitive to low pH [70]. However, targeted experiments are required to test these possibilities.
5. Conclusion
Our study is one of very few to assess litter breakdown in streams at the global scale [27,28,30,71]. The number of samples was relatively small for such broad scope (24 study sites), limiting statistical power of the analyses; nevertheless, we were able to show that multiple biotic and abiotic factors influence rates of litter breakdown in streams at the global scale, with (i) a large positive influence of temperature on microbial breakdown of alder leaves; (ii) a greater role of litter-consuming detritivores on breakdown towards high latitudes, where these detritivores are typically more abundant and diverse; (iii) a notable influence of litter quality and PD on breakdown of litter mixtures that varies across climates, possibly through different effects on microbial and detritivore assemblages at different latitudes; and (iv) generally faster breakdown in more basic waters with some influence of temperature, possibly owing to interactions with microbial activity, which is the most important mechanism for litter breakdown in warmer waters. Our models explained up to 41% of the total variance in the datasets, indicating that our explanatory variables could be important drivers of litter breakdown. However, substantial unexplained variation indicates that further comparative research is required to develop a comprehensive picture of litter breakdown in streams at the global scale. Such understanding is vital to appropriate management of these ecosystems in the face of multiple anthropogenic stressors [72,73].
Supplementary Material
Acknowledgements
We thank the numerous people assisting with this research, including L. Buria, J. Castela, A. Cornejo, J. Davies, S. Lamothe, M. Schindler, V. Villanueva and D. West; and William Cornwell and two anonymous referees for their constructive comments on the manuscript.
Data accessibility
Data are available in the electronic supplementary material and the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.jg8r0.
Authors' contributions
L.B. and R.G.P. conceived the study; L.B. managed the overall project; L.B., C.H., J.P., M.A.A. and R.G.P. analysed the data; L.B. and R.G.P. wrote the manuscript with substantial contribution from M.O.G., M.A.S.G., J.P., B.J.C., C.H. and M.A.A.; and all other authors (listed alphabetically) locally coordinated or performed research and commented on the manuscript; all authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The study was supported by a grant from the National Geographic Society's Committee for Research and Exploration (grant no. 7980-06; PI, Luz Boyero) and various national funding sources.
References
- 1.Beer C, et al. 2010. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329, 834–838. ( 10.1126/science.1184984) [DOI] [PubMed] [Google Scholar]
- 2.Cebrian J. 1999. Patterns in the fate of production in plant communities. Am. Nat. 154, 449–468. ( 10.1086/303244) [DOI] [PubMed] [Google Scholar]
- 3.Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy E, Gamfeldt L, Balvanera P, O'Connor MI, Gonzalez A. 2011. The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 572–592. ( 10.3732/ajb.1000364) [DOI] [PubMed] [Google Scholar]
- 4.Heimann M, Reichstein M. 2008. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451, 289–292. ( 10.1038/nature06591) [DOI] [PubMed] [Google Scholar]
- 5.Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML. 2010. A review of allochthonous organic matter dynamics and metabolism in streams. J. N. Am. Benthol. Soc. 29, 118–146. ( 10.1899/08-170.1) [DOI] [Google Scholar]
- 6.Aerts R. 1997. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79, 439–449. ( 10.2307/3546886) [DOI] [Google Scholar]
- 7.Coûteaux MM, Bottner P, Berg B. 1995. Litter decomposition, climate and litter quality. Trends Ecol. Evol. 10, 63–66. ( 10.1016/S0169-5347(00)88978-8) [DOI] [PubMed] [Google Scholar]
- 8.Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ. 2000. Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob. Change Biol. 6, 751–765. ( 10.1046/j.1365-2486.2000.00349.x) [DOI] [Google Scholar]
- 9.García-Palacios P, Maestre FT, Kattge J, Wall DH. 2013. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 16, 1045–1053. ( 10.1111/ele.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelissen JH, et al. 2007. Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol. Lett. 10, 619–627. ( 10.1111/j.1461-0248.2007.01051.x) [DOI] [PubMed] [Google Scholar]
- 11.Cornwell WK, et al. 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071. ( 10.1111/j.1461-0248.2008.01219.x) [DOI] [PubMed] [Google Scholar]
- 12.Makkonen M, Berg MP, Handa IT, Hattenschwiler S, van Ruijven J, van Bodegom PM, Aerts R, Klironomos J. 2012. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 15, 1033–1041. ( 10.1111/j.1461-0248.2012.01826.x) [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Hui D, Luo Y, Zhou G. 2008. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J. Plant Ecol. 1, 85–93. ( 10.1093/jpe/rtn002) [DOI] [Google Scholar]
- 14.Wall DH, et al. 2008. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Change Biol. 14, 2661–2677. [Google Scholar]
- 15.Battin TJ, Luyssaert S, Kaplan LA, Aufdenkampe AK, Richter A, Tranvik LJ. 2009. The boundless carbon cycle. Nat. Geosci. 2, 598–600. ( 10.1038/ngeo618) [DOI] [Google Scholar]
- 16.Battin TJ, Kaplan LA, Findlay S, Hopkinson CS, Marti E, Packman AI, Newbold JD, Sabater F. 2008. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 1, 95–100. ( 10.1038/ngeo101) [DOI] [Google Scholar]
- 17.Raymond PA, et al. 2013. Global carbon dioxide emissions from inland waters. Nature 503, 355–359. ( 10.1038/nature12760) [DOI] [PubMed] [Google Scholar]
- 18.Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S. 2010. Diversity meets decomposition. Trends Ecol. Evol. 25, 372–380. ( 10.1016/j.tree.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 19.Frainer A, Moretti MS, Xu W, Gessner MO. 2015. No evidence for leaf-trait dissimilarity effects on litter decomposition, fungal decomposers, and nutrient dynamics. Ecology 96, 550–561. ( 10.1890/14-1151.1) [DOI] [PubMed] [Google Scholar]
- 20.Argerich A, Martí E, Sabater F, Ribot M, von Schiller D, Riera JL. 2008. Combined effects of leaf litter inputs and a flood on nutrient retention in a Mediterranean mountain stream during fall. Limnol. Oceanogr. 53, 631–641. ( 10.4319/lo.2008.53.2.0631) [DOI] [Google Scholar]
- 21.Pearson RG. 2005. Biodiversity of the freshwater invertebrates of the wet tropics region of north-eastern Australia: patterns and possible determinants. In Tropical rain forests: past, present and future (eds Bermingham E, Dick CW, Moritz C), pp. 470–485. Chicago, IL: University of Chicago Press. [Google Scholar]
- 22.Schindler MH, Gessner MO. 2009. Functional leaf traits and biodiversity effects on litter decomposition in a stream. Ecology 90, 1641–1649. ( 10.1890/08-1597.1) [DOI] [PubMed] [Google Scholar]
- 23.Graça MAS, Poquet JM. 2014. Do climate and soil influence phenotypic variability in leaf litter, microbial decomposition and shredder consumption? Oecologia 174, 1021–1032. ( 10.1007/s00442-013-2825-2) [DOI] [PubMed] [Google Scholar]
- 24.Gessner MO, Chauvet E. 1994. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75, 1807–1817. ( 10.2307/1939639) [DOI] [Google Scholar]
- 25.Lecerf A, Chauvet E. 2008. Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic Appl. Ecol. 9, 598–605. ( 10.1016/j.baae.2007.11.003) [DOI] [Google Scholar]
- 26.Hladyz A, Gessner MO, Giller PS, Pozo J, Woodward G. 2009. Resource quality and stoichiometric constraints on stream ecosystem functioning. Freshw. Biol. 54, 957–970. ( 10.1111/j.1365-2427.2008.02138.x) [DOI] [Google Scholar]
- 27.Boyero L, et al. 2011. A global experiment suggests climate warming will not accelerate litter decomposition in streams but may reduce carbon sequestration. Ecol. Lett. 14, 289–294. ( 10.1111/j.1461-0248.2010.01578.x) [DOI] [PubMed] [Google Scholar]
- 28.Handa IT, et al. 2014. Consequences of biodiversity loss for litter decomposition across biomes. Nature 509, 218–221. ( 10.1038/nature13247) [DOI] [PubMed] [Google Scholar]
- 29.Woodward G, et al. 2012. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336, 1438–1440. ( 10.1126/science.1219534) [DOI] [PubMed] [Google Scholar]
- 30.García-Palacios P, McKie BG, Handa IT, Frainer A, Hättenschwiler S. 2015. The importance of litter traits and decomposers for litter decomposition: a comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol. ( 10.1111/1365-2435.12589) [DOI] [Google Scholar]
- 31.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 32.Hooper DU et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. [DOI] [PubMed] [Google Scholar]
- 33.Spehn EM, et al. 2005. Ecosystem effects of biodiversity manipulations in European grasslands. Ecol. Monogr. 75, 37–63. ( 10.1890/03-4101) [DOI] [Google Scholar]
- 34.Srivastava DS, Cadotte MW, MacDonald AA, Marushia RG, Mirotchnick N. 2012. Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 15, 637–648. ( 10.1111/j.1461-0248.2012.01795.x) [DOI] [PubMed] [Google Scholar]
- 35.Graça MAS, Cressa C, Gessner MO, Feio MJ, Callies KA, Barrios C. 2001. Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshw. Biol. 46, 947–957. ( 10.1046/j.1365-2427.2001.00729.x) [DOI] [Google Scholar]
- 36.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 37.Yvon-Durocher G, et al. 2012. Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 487, 472–476. ( 10.1038/nature11205) [DOI] [PubMed] [Google Scholar]
- 38.Boyero L, et al. 2012. Global patterns of stream detritivore distribution: implications for biodiversity loss in changing climates. Glob. Ecol. Biogeogr. 21, 134–141. ( 10.1111/j.1466-8238.2011.00673.x) [DOI] [Google Scholar]
- 39.Flynn DFB, Mirotchnick N, Jain M, Palmer MI, Naeem S. 2011. Functional and phylogenetic diversity as predictors of biodiversity–ecosystem-function relationships. Ecology 92, 1573–1581. ( 10.1890/10-1245.1) [DOI] [PubMed] [Google Scholar]
- 40.Mulholland PJ, Palumbo AV, Elwood JW, Rosemond AD. 1987. Effects of acidification on leaf decomposition in streams. J. N. Am. Benthol. Soc. 6, 147–158. ( 10.2307/1467506) [DOI] [Google Scholar]
- 41.Larrañaga S, Diez JR, Elosegi A, Pozo J. 2003. Leaf retention in streams of the Agüera basin (northern Spain). Aquat. Sci. 65, 158–166. [Google Scholar]
- 42.Correa-Araneda F, Boyero L, Figueroa R, Sánchez C, Abdala R, Ruiz-García A, Graça MAS. 2015. Joint effects of climate warming and exotic litter (Eucalyptus globulus Labill.) on stream detritivore fitness and litter breakdown. Aquat. Sci. 77, 197–205. ( 10.1007/s00027-014-0379-y) [DOI] [Google Scholar]
- 43.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 44.Milla R, Reich PB. 2007. The scaling of leaf area and mass: the cost of light interception increases with leaf size. Proc. R. Soc. B 274, 2109–2114. ( 10.1098/rspb.2007.0417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fugère V, Andino P, Espinosa R, Anthelme F, Jacobsen D, Dangles O. 2012. Testing the stress-gradient hypothesis with aquatic detritivorous invertebrates: insights for biodiversity–ecosystem functioning research. J. Anim. Ecol. 81, 1259–1267. ( 10.1111/j.1365-2656.2012.01994.x) [DOI] [PubMed] [Google Scholar]
- 46.Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 182, 565–588. ( 10.1111/j.1469-8137.2009.02830.x) [DOI] [PubMed] [Google Scholar]
- 47.Faith DP. 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. ( 10.1016/0006-3207(92)91201-3) [DOI] [Google Scholar]
- 48.Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 1–19. ( 10.1186/1471-2105-5-113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Posada D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25, 1253–1256. ( 10.1093/molbev/msn083) [DOI] [PubMed] [Google Scholar]
- 50.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57, 758–771. ( 10.1080/10635150802429642) [DOI] [PubMed] [Google Scholar]
- 51.Drummond AJ, Rambaut A. 2007. Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 ( 10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rambaut A, Suchard MA, Xie D, Drummond AJ. 2014. Tracer v.1.6. Available at http://beast.bio.ed.ac.uk/Tracer.
- 53.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. ( 10.1093/bioinformatics/btq166) [DOI] [PubMed] [Google Scholar]
- 54.Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D. 2015. MASS. Support functions and datasets for Venables and Ripley's MASS. R package version 7.3–43. Available at http://cran.r-project.org/web/packages/MASS/MASS.pdf.
- 55.Nakazawa M. 2014. fmsb: Functions for medical statistics book with some demographic data. R package version 0.5.2. Available at http://minato.sip21c.org/msb/.
- 56.Breheny P, Burchett W. 2015. visreg: Visualization of regression models. R package version 2.2-2. Available at http://myweb.uiowa.edu/pbreheny/publications/visreg.pdf.
- 57.Boyero L, et al. 2011. Global distribution of a key trophic guild contrasts with common latitudinal diversity patterns. Ecology 92, 1839–1848. ( 10.1890/10-2244.1) [DOI] [PubMed] [Google Scholar]
- 58.Davies JN, Boulton AJ. 2009. Great house, poor food: effects of exotic leaf litter on shredder densities and caddisfly growth in 6 subtropical Australian streams. J. N. Am. Benthol. Soc. 28, 491–503. ( 10.1899/07-073.1) [DOI] [Google Scholar]
- 59.Duffy JE, Cardinale BJ, France KE, McIntyre PB, Thebault E, Loreau M. 2007. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–538. ( 10.1111/j.1461-0248.2007.01037.x) [DOI] [PubMed] [Google Scholar]
- 60.Pan X, Berg MP, Butenschoen O, Murray PJ, Bartish IV, Cornelissen JH, Dong M, Prinzing A. 2015. Larger phylogenetic distances in litter mixtures: lower microbial biomass and higher C/N ratios but equal mass loss. Proc. R. Soc. B 282, 20150103 ( 10.1098/rspb.2015.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friberg N, Jacobsen DJ. 1994. Feeding plasticity of two detritivore-shredders. Freshw. Biol. 32, 133–142. ( 10.1111/j.1365-2427.1994.tb00873.x) [DOI] [Google Scholar]
- 62.Bastian M, Boyero L, Jackes BR, Pearson RG. 2007. Leaf litter diversity and shredder preferences in an Australian tropical rain-forest stream. J. Trop. Ecol. 23, 219–229. ( 10.1017/S0266467406003920) [DOI] [Google Scholar]
- 63.Royer TV, Minshall GW. 2003. Controls on leaf processing in streams from spatial-scaling and hierarchical perspectives. J. N. Am. Benthol. Soc. 22, 352–358. ( 10.2307/1468266) [DOI] [Google Scholar]
- 64.Graça MAS, Ferreira V, Canhoto C, Encalada AC, Gerrero-Bolaño F, Wantzen KM, Boyero L. 2015. A conceptual model of litter breakdown in low order streams. Int. Rev. Hydrobiol. 100, 1–12. ( 10.1002/iroh.201401757) [DOI] [Google Scholar]
- 65.Dangles O, Gessner MO, Guerold F, Chauvet E. 2004. Impacts of stream acidification on litter breakdown: implications for assessing ecosystem functioning. J. Appl. Ecol. 41, 365–378. ( 10.1111/j.0021-8901.2004.00888.x) [DOI] [Google Scholar]
- 66.Suberkropp K, Klug MJ. 1980. The maceration of deciduous leaf litter by aquatic hyphomycetes. Can. J. Bot. 58, 1025–1031. ( 10.1139/b80-126) [DOI] [Google Scholar]
- 67.Jenkins CC, Suberkropp K. 2006. The influence of water chemistry on the enzymatic degradation of leaves in streams. Freshw. Biol. 33, 245–253. ( 10.1111/j.1365-2427.1995.tb01165.x) [DOI] [Google Scholar]
- 68.Jonsson M, Dangles O, Malmqvist B, Guerold F. 2002. Simulating species loss following perturbation: assessing the effects on process rates. Proc. R. Soc. Lond. B 269, 1047–1052. ( 10.1098/rspb.2002.1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dangles O, Guérold F. 1999. Impact of headwater stream acidification on the trophic structure of macroinvertebrate communities. Int. Rev. Hydrobiol. 84, 287–297. [Google Scholar]
- 70.Herrmann J, Degerman E, Almut G, Johansson C, Lingdell PE, Muniz IP. 1993. Acid-stress effects on stream biology. Ambio 22, 298–307. [Google Scholar]
- 71.Boyero L, et al. 2015. Leaf-litter breakdown in tropical streams: is variability the norm? Freshw. Sci. 34, 759–769. ( 10.1086/681093) [DOI] [Google Scholar]
- 72.Dudgeon D, et al. 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182. ( 10.1017/S1464793105006950) [DOI] [PubMed] [Google Scholar]
- 73.Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT. 2015. Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob. Change Biol. 22, 180–189. ( 10.1111/gcb.13028) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material and the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.jg8r0.