Abstract
In many primates, including humans, the vocalizations of males and females differ dramatically, with male vocalizations and vocal anatomy often seeming to exaggerate apparent body size. These traits may be favoured by sexual selection because low-frequency male vocalizations intimidate rivals and/or attract females, but this hypothesis has not been systematically tested across primates, nor is it clear why competitors and potential mates should attend to vocalization frequencies. Here we show across anthropoids that sexual dimorphism in fundamental frequency (F0) increased during evolutionary transitions towards polygyny, and decreased during transitions towards monogamy. Surprisingly, humans exhibit greater F0 sexual dimorphism than any other ape. We also show that low-F0 vocalizations predict perceptions of men's dominance and attractiveness, and predict hormone profiles (low cortisol and high testosterone) related to immune function. These results suggest that low male F0 signals condition to competitors and mates, and evolved in male anthropoids in response to the intensity of mating competition.
Keywords: sexual selection, anthropoid primates, mating system, vocal fundamental frequency, dominance, attractiveness
1. Introduction
Explaining why sexual dimorphisms evolve is central to understanding the evolution of primate mating systems and social organization. In many primate species, the vocalizations of males and females differ dramatically, with male vocalizations and vocal anatomy often seeming to exaggerate the appearance of body size [1–7]. Among humans, men's approximately 60% longer vocal folds [8,9] contribute to an average rate of vocal fold vibration during phonation (fundamental frequency, F0) that is about 5 standard deviations below women's [5]. To human listeners, utterances lower in F0 are perceived as being deeper in pitch and as emanating from larger individuals [10,11]. The evolutionary reasons for such apparent size exaggeration have been the subject of speculation since Darwin noted the pubertal enlargement of male vocal structures and their intensified employment during the breeding season in many mammals [12].
Some have suggested that male vocalizations evolve to intimidate male competitors and/or attract mates [6,13]. For example, among orang-utans, lower-ranking males avoid long calls given by higher-ranking males [14], suggesting that acoustic cues convey threat potential to conspecifics. Several studies in humans suggest that F0 has relevance under both inter- and intrasexual competition: experimentally lowering F0 increases perceptions of men's dominance and attractiveness [11,15], and raising F0 increases women's vocal attractiveness [16,17]. However, little is known about whether these effects persist in unmanipulated speech when F0 and other acoustic parameters vary naturally and simultaneously.
Moreover, it is unclear why F0 should signal formidability to same-sex competitors or mate value to potential mates; F0 is only weakly associated with body size [5,7,18–20] and perhaps strength [5,21] in humans, although F0 may be modulated according to relative formidability [22] and mate quality [23,24]. Steroid hormones may provide a link between F0 and condition. Growing evidence indicates that glucocorticoids such as the stress hormone cortisol negatively interact with testosterone in predicting immune function and influencing the expression of secondary sex traits [25–27]. Infection stimulates cortisol production [28], which downregulates androgen receptors and inhibits the action of testosterone on target tissues [29–33]. Hence, testosterone should be more potent in individuals in good condition with low immune system activation. In humans, positive relationships between testosterone and immune response to a vaccine [34], and between testosterone and both facial attractiveness [34] and dominance [35], were stronger in males with low cortisol. Furthermore, the interactive effect of testosterone and cortisol on attractiveness was mediated by immune function, supporting the stress-linked immunocompetence handicap hypothesis (SL-ICHH) that testosterone-related traits that interact with cortisol are linked to immunocompetence [34]. Although previous studies have found negative relationships between men's testosterone and F0 [5,20], it is unknown whether testosterone and cortisol negatively interact in predicting F0, as the SL-ICHH would suggest if F0 reflects underlying condition.
More generally, scant evidence exists to support a role for sexual selection in shaping F0 and other vocal sexual dimorphisms across primates [6], and there are plausible alternative hypotheses: F0 dimorphism may represent a by-product of selection for greater male size or long-distance transmission of male calls [36], or reflect selection for sex identification. The latter two hypotheses predict relationships between habitat and F0 dimorphism. In general, open, terrestrial habitats are poor acoustic environments in which sounds greatly attenuate over distance compared with arboreal habitats. This is particularly true for the heights at which each habitat's primate residents tend to communicate and for lower-frequency sounds [37]. Thus, all else equal, selection for long-distance transmission of male calls should tend to produce relatively lower-frequency male calls in arboreal environments than in terrestrial ones (for caveats, see Discussion). Likewise, because primates are both more visible and more sexually dimorphic in terrestrial species than in arboreal ones [38], selection for sex identification should favour greater F0 dimorphism in arboreal species.
Here, we report the results of three studies designed to clarify the evolution of sexual dimorphism in F0. In study 1, we examined the evolution of F0 dimorphism as a function of mating system, as well as body mass dimorphism and habitat, across anthropoid primates. Studies 2 and 3 focused on humans. Not only are humans of special interest, but they are also highly useful as a model organism: there is strong evidence implicating sexual selection in the evolution of human F0 [39,40], as well as a unique richness to the data available for addressing the questions outlined earlier, as we detail in the following. In study 2, we tested the stimulus–response properties of F0 on intrasexual competitiveness in humans by examining the independent contributions of F0 and other acoustic parameters related to assessments of attractiveness and dominance. In study 3, we explored the indexical value of F0 by testing the SL-ICHH prediction that F0 will be more strongly linked to testosterone in individuals with low cortisol.
2. Study 1: F0 across anthropoid primates
(a). Methods
Please refer to electronic supplementary material, Materials and methods for additional details.
We obtained the recordings of non-human primate calls from our own fieldwork and by contacting other primatologists. From these, we selected 1721 files, such that each was without substantial background noise and was produced by a single individual of known species, sex and adult status. The acoustic properties of primate calls vary across call types and contexts [13]. We chose to use measurements across all available call types (but see also electronic supplementary material, Results) rather than, for example, selecting only calls believed to be analogous across species, or only calls shared between males and females. Our reasoning was that averaging across call types should maximize our ability to capture information about the physical properties of the sound source (e.g. vocal fold length and thickness), especially if some call types may provide more information than others. If F0 is driven by underlying anatomy—as it is fundamentally—then any influence on F0 should be manifest similarly across call types, even if not specifically adapted to be, because the same anatomy supports the production of all calls. Although it is possible that some other special mechanism of vocal fold action is in play for some calls (e.g. loud calls compared with close calls), this is not a given and certainly cannot be assumed a priori. Moreover, it is unclear whether call types are truly analogous across species, which complicates comparisons of only a particular call type or set of types. In addition, the repertoire shared between males and females can be very limited in some primate species, occasionally down to one call type, as in the orang-utan. Finally, we do not know of any reason that our sampling procedures would have systematically biased our sample, and the size of our sample—the largest ever compiled for this type of research—should reduce any bias owing to random sampling.
Files were measured as uncompressed .wav or .aiff files using the acoustic analysis software Praat v. 5.3. F0 was measured from each file by identifying in the raw waveform a segment in which cycles were clearly discernible. Cycles were counted along this segment up to 20 cycles, and then divided by the duration of the interval to calculate F0. This procedure was repeated for a second segment, if possible (78% of files, n = 1343). Mean F0 values from each recording were averaged with all other mean F0 values per sex to arrive at separate male and female F0 averages for each species (electronic supplementary material, table S2). Between-segment reliability was high for files with two measurable segments (Cronbach's α = 0.973). First segments of a randomly chosen 11% of files (n = 184) were re-measured to determine intrameasurer reliability, which was very high (Cronbach's α = 1.000). Body size, habitat and mating system were obtained from the literature (electronic supplementary material, table S2).
Mating system was used as a proxy for the intensity of sexual selection [41,42]. We categorized mating system as monogamous, promiscuous or polygynous [43] rather than using an interval-level measure such as socionomic sex ratio [44], as such measures often vary widely within species and hold uncertain relationships to the intensity of intermale competition [41,44,45]. Promiscuity differs from monogamy and polygyny in that females far more frequently copulate with multiple males in promiscuous species. Although evidence suggests intermediate levels of male contest competition in promiscuous species, the ability of males to monopolize females varies widely [46]; other mechanisms of sexual selection (such as sperm competition) are more salient [47,48], and the degree of sexual dimorphism relative to monogamous or polygynous species varies widely by trait [47]. Such apparent diversity in the mechanisms and intensity of sexual selection, as well as their influence on sexual dimorphism, precludes straightforward predictions regarding F0 dimorphism in promiscuous species. By contrast, predictions regarding monogamous and polygynous species are clearer: in polygynous species, some males are able to monopolize multiple mates, whereas in monogamous species, males do not, leaving fewer males unmated. Hence, sexual selection—particularly intrasexual selection—tends to be more intense in polygynous than in monogamous primates, which are less dimorphic in size and weaponry [47]. Dimorphism in F0 was therefore predicted to increase during evolutionary transitions towards polygyny and decease during transitions towards monogamy.
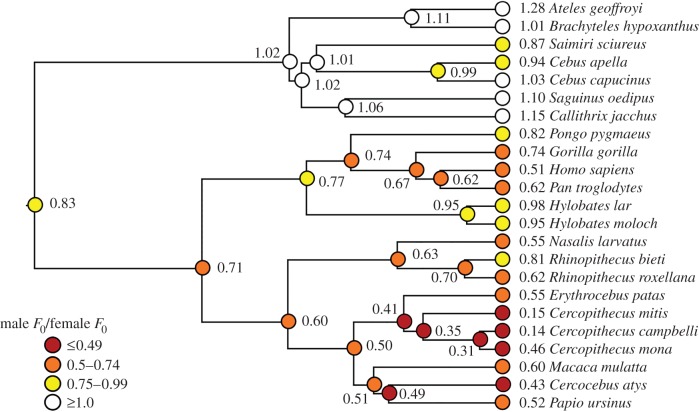
Habitat was categorized as arboreal, terrestrial or arboreal/terrestrial. We conducted phylogenetically informed analyses using a consensus phylogeny for all species represented in our sample [49] (figure 1) and assessed correlated evolution among our variables with phylogenetic generalized least-squares regression using the caper package, v. 0.5.2, in R [50].
Figure 1.
Phylogenetic tree of anthropoid primates included in study 1 for which data were available on at least two vocalizations from each sex (mean number of vocalizations: females = 38.6, males = 22.1; max: females = 181, males = 155; electronic supplementary material, table S1). Tree was constructed using a consensus phylogeny for all species in our sample from the 10kTrees website (http://10ktrees.fas.harvard.edu/). Sexual dimorphism (male/female) in F0 is shown in the column to the left of species names. Inferred ancestral states were determined using squared change parsimony and are shown at nodes on the tree (coloured to highlight evolutionary trends). (Online version in colour.)
(b). Results
Across analyses, F0 and F0 dimorphism exhibited strong phylogenetic signals (λ > 0.8). In general, New World primates showed little sexual dimorphism in F0, averaging a mean F0 dimorphism (male F0/female F0) of 1.05 across seven species, whereas male cercopithecines averaged half of the F0 of females (mean F0 dimorphism = 0.48 across 10 species). With a similar F0 dimorphism of 0.51, humans surprisingly exhibited the greatest dimorphism that we measured in any ape.
We tested whether increases in body size predict decreases in F0 across species for each sex. Previous studies relied on published acoustic data measured using varying methodologies, and either averaged male and female measurements [51] or included only males [36]. In our data, body mass negatively predicted F0 (both variables natural log-transformed) in males and females (table 1). These results suggest that body size constrains the evolution of primate call frequencies in both sexes [51].
Table 1.
Phylogenetic generalized least-squares regression models predicting evolutionary changes in F0 (body mass models) or F0 dimorphism (mating system and habitat models). In both sexes, evolutionary increases in body mass predicted decreases in F0 (body mass models). Consequently, changes in body mass dimorphism were statistically controlled in models showing that F0 dimorphism increases during transitions from monogamy to polygyny (mating system models), and from arboreality to terrestriality (habitat model).
| F | d.f. | R2 | λ | est. | t | p | ||
|---|---|---|---|---|---|---|---|---|
| body mass | model (males) | 14.01 | 2.27 | 0.32 | 1.00 | <0.0001 | ||
| ln(mass) | −0.74 | −3.74 | <0.001 | |||||
| model (females) | 6.88 | 2.26 | 0.21 | 0.98 | ||||
| ln(mass) | −0.56 | −2.62 | 0.014 | |||||
| mating system | modela | 6.42 | 3.13 | 0.50 | 0.82 | 0.007 | ||
| polygyny versus monogamy | 0.55 | 3.51 | 0.004 | |||||
| male/female mass | 0.16 | 2.62 | 0.021 | |||||
| modelb | 6.31 | 3.13 | 0.49 | 1.00 | 0.007 | |||
| polygyny versus monogamy | 0.58 | 2.89 | 0.013 | |||||
| male/female mass | 0.30 | 3.55 | 0.004 | |||||
| modelc | 6.03 | 3.12 | 0.50 | 0.85 | <0.01 | |||
| polygyny versus monogamy | 0.56 | 3.40 | 0.005 | |||||
| male/female mass | 0.17 | 2.50 | 0.028 | |||||
| habitat | model | 3.33 | 4.19 | 0.34 | 1.00 | 0.032 | ||
| terrestrial versus arboreal | −0.18 | −2.58 | 0.018 | |||||
| terrestrial versus arb./terr. | −0.16 | −1.37 | 0.188 | |||||
| male/female mass | 0.06 | 1.30 | 0.209 |
aHumans treated as polygynous.
bHumans treated as monogamous.
cHumans excluded.
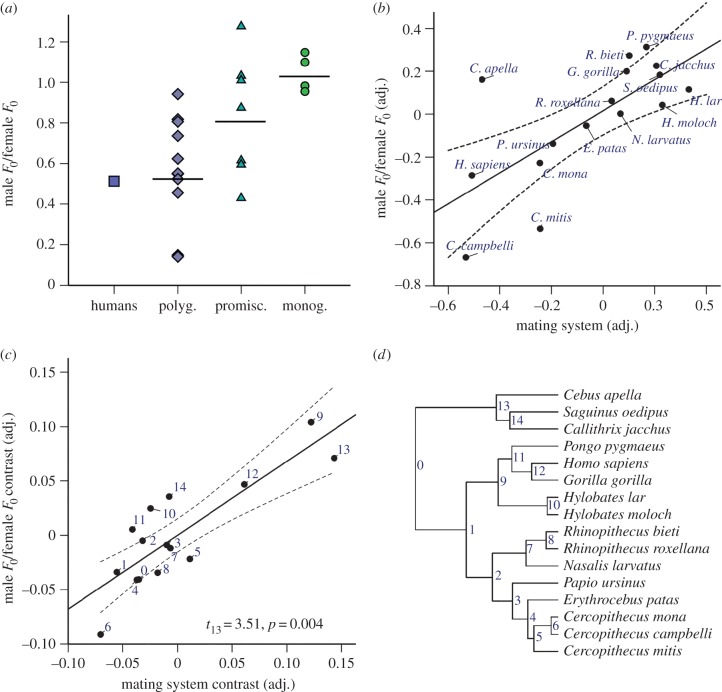
We then regressed F0 dimorphism on mating system, controlling for body size dimorphism (male mass/female mass). Because diversity in the mechanisms and intensity of sexual selection precludes straightforward predictions regarding F0 dimorphism in promiscuous species (see above), we excluded promiscuous species from this analysis; however, promiscuous species appear intermediate in F0 dimorphism (figure 2a). We found that greater F0 dimorphism evolves in transitions to polygyny than in transitions to monogamy (table 1 and figure 2c). In this model, changes towards greater F0 dimorphism also tended to be accompanied by decreases in body size dimorphism. Humans were treated as polygynous [52] and exhibited F0 dimorphism that was outside the range of monogamous species (figure 2a); however, we obtained similar results when humans were treated as monogamous, or excluded from the analysis (table 1).
Figure 2.
Sexual dimorphism in vocal F0 as a function of mating system. In panel (a), data points represent individual species, and horizontal bars represent mean F0 dimorphism for each mating system. Sexual dimorphism in F0 is most extreme in polygynous anthropoid primates and lowest in monogamous species. This remains true after adjusting for body mass dimorphism (b), and after adjusting for both body mass dimorphism and phylogenetic non-independence (c). Panel (c) shows results from phylogenetic generalized least-squares regression with statistics for the effect of mating system on F0 dimorphism. In panels (b) and (c), least-squares regression lines with 95% CI are plotted, mating system refers to polygyny (coded as −1) versus monogamy (coded as 1), and adjusted values are residuals after each variable was regressed on body mass dimorphism. The key for independent contrasts in panel (c) is shown in panel (d). (Online version in colour.)
Finally, we tested the relationship between F0 dimorphism and habitat. If male vocalizations are selected primarily to propagate over distance, or if F0 dimorphism evolves primarily for sex discrimination, then arboreal species should tend to exhibit relatively lower male F0 than terrestrial species. We found the reverse: arboreal primates showed less F0 dimorphism than terrestrial primates when F0 dimorphism was regressed on habitat and mass dimorphism (table 1).
3. Study 2: F0, dominance and attractiveness in humans
(a). Methods
Please refer to electronic supplementary material, Materials and methods for additional details.
Two hundred and fifty-eight female (mean age ± s.d. = 20.0 ± 1.6 years) and 175 male (20.1 ± 1.7 years) students from Michigan State University provided written consent to participate in this study approved by the university's institutional review board. Participants were recorded reading a standard voice passage [53] in an anechoic, soundproof booth using a Shure SM58 vocal cardioid dynamic microphone (frequency response: 50–15 000 Hz) positioned at approximately 30° and 9.5 cm from the speaker's mouth, and connected to a desktop computer via a Sound Devices USBPre 2 preamplifier. Voices were recorded in mono at a sampling rate of 44 100 Hz and 16-bit quantization, and saved as uncompressed .wav files. Recordings were rated by 558 female (19.1 ± 2.4 years) and 568 male (19.4 ± 1.8 years) students from Pennsylvania State University. Each female recording was rated by 15 men for attractiveness for short- and long-term romantic relationships using seven-point Likert scales. Each male recording was rated by 15 men for dominance (seven-point scale) and 15 women for short- and long-term attractiveness. Ratings were averaged to produce composite ratings of short- and long-term attractiveness for each recording, and dominance for each male recording.
Recordings were analysed using Praat v. 5.3 for mean F0, standard deviation in F0 across the utterance (F0-SD), duration, number of voice breaks, harmonics, four measures of jitter (cycle-to-cycle variation in F0) and five measures of shimmer (cycle-to-cycle variation in amplitude) using the ‘voice report’ function in Praat (electronic supplementary material, table S3). Pitch floors were set to 75 and 100 Hz, and pitch ceilings were 300 and 500 Hz, for men and women, respectively. Otherwise, default settings were used. We also measured the first four formant frequencies (F1−F4, electronic supplementary material, table S3). Formants were measured at each glottal pulse, averaged across measurements and then used to compute formant position (Pf), the average standardized formant value for the first four formants [5].
Using SPSS v. 22, we performed separate male and female principal components analyses to reduce the number of acoustic control variables (electronic supplementary material, Materials and methods and table S4), and we used multiple regression to examine the effects of acoustic parameters on perceptual variables.
(b). Results
F0 predicted men's perceived dominance to heterosexual male listeners (β = −0.43, p < 0.0001) and attractiveness to heterosexual female listeners for both prospective short-term (β = −0.36, p < 0.001) and long-term (β = −0.33, p = 0.001) romantic relationships (electronic supplementary material, table S5). When perceived dominance and short-term attractiveness were entered into a multiple regression to predict men's F0 (model F2,171 = 12.36, p < 0.0001, R2 = 0.13), dominance negatively predicted F0 (β = −0.28, p = 0.002), but short-term attractiveness did not (β = −0.10, p = 0.259), suggesting a stronger role for male contests than female choice in shaping men's F0. F0 did not predict women's attractiveness to men for either short- (β = −0.03, p = 0.650) or long-term (β = −0.03, p = 0.652) relationships when other acoustic parameters were statistically controlled (electronic supplementary material, table S5). These results are thus more consistent with sexual selection (primarily intrasexual selection) on males, rather than intersexual selection on females, influencing the evolution of human F0 dimorphism.
4. Study 3: F0 and hormonal profiles in humans
(a). Methods
Please refer to electronic supplementary material, Materials and methods for additional details.
Participants from Pennsylvania State University provided written consent to participate in this study approved by the university's institutional review board. Fifty-three normally cycling women (mean age ± s.d. = 19.4 ± 1.6 years) and 62 men (19.9 ± 2.0 years) were recorded in an anechoic recording booth in a quiet room (sample 1), and 58 men (19.9 ± 1.2 years) were recorded in a quiet room (sample 2) using the equipment and methods in study 2 above.
Participants rinsed their mouths with water before providing two saliva samples of 1–2 ml each via passive drool approximately 30 (sample 1) or 20 (sample 2) min apart. From each sample, 0.5 ml of saliva was aliquotted into a third tube, which was shaken and then frozen at −20°C until analysis by the Johns Hopkins Center for Interdisciplinary Salivary Bioscience Research (Baltimore, MD) using Salimetrics kits. Samples were analysed in duplicate via enzyme immunoassay. Duplicates correlated highly for both cortisol and testosterone (all r ≥ 0.97, p < 0.0001), and were consequently averaged. For cortisol assays, sensitivity is less than 0.003 µg dl−1, and average intra-assay coefficient of variation is 3.5%. For testosterone assays, sensitivity is less than 1.0 pg ml−1, and average intra-assay coefficient of variation is 4.6%.
We used multiple regression to examine the effects of cortisol and testosterone (natural log-transformed to correct skew) on F0, and statistically controlled for diurnal decreases in cortisol and testosterone [54], but results were similar without controlling for these effects (electronic supplementary material, table S6, figures S1 and S2).
(b). Results
In women, F0 was unrelated to cortisol, testosterone, and their interaction (electronic supplementary material, table S6). However, in both male samples, cortisol and testosterone interacted in predicting F0 (sample 1: β = 0.36, p = 0.007; sample 2: β = 0.28, p = 0.033; electronic supplementary material, table S6) such that testosterone was negatively related to F0 only in low-cortisol men (median split for cortisol; sample 1: partial r = −0.44, p = 0.018; sample 2: partial r = −0.40, p = 0.034; see also electronic supplementary material, figure S2). This pattern of relationships between hormones and a putative sexually selected trait has been found to indicate men's immune function [34], as well as attractiveness [34] and dominance [35], and is consistent with the SL-ICHH [34].
5. Discussion
Our data supported the sexual selection hypothesis: F0 dimorphism increased with evolutionary changes towards polygyny and decreased with transitions towards monogamy across anthropoid primates. Although our primary analyses averaged across call types in order to maximize data, we also examined the patterns of F0 dimorphism across mating systems using only those calls for which we had examples from both sexes (electronic supplementary material, Results and figure S3). Patterns were similar across analyses, indicating that the observed pattern in our primary results does not merely reflect sex differences in the use of particular call types, which may differ in F0. Our data also indicate that sex differences in F0 result mainly from selection on males rather than females: we observed greater F0 dimorphism in polygynous species, where male sexual selection is stronger, and F0 affected components of men's but not women's mating success. These results thus provide a plausible explanation for the prior finding that F0 predicted men's but not women's reproductive success among Hadza foragers [55]. If low F0 augments male reproductive success by increasing access to mates in some other anthropoids, as appears to be the case in humans, then we would expect males to evolve relatively lower F0 when mating competition intensifies during transitions to polygyny, as we found.
By contrast, F0 dimorphism appears unlikely to be a by-product of greater male size: with mating system controlled, F0 dimorphism decreased with relative male size. Although F0 dimorphism probably facilitates sex identification, if it evolves primarily for this function, then one might expect it to be greater in arboreal species, where visibility is obscured, and in monogamous species, where the sexes are otherwise less dimorphic [38], yet in both cases we found the opposite.
Previous work indicates that lower-frequency male loud calls are selected for propagation over distance [36], but our finding that male F0 is relatively lower in terrestrial species than in arboreal species suggests that selection for long-distance male calls is not the only or primary influence on F0 dimorphism across species’ vocal repertoires. Lower frequencies should, ceteris paribus, aid long-distance transmission to a greater degree in arboreal habitats than in terrestrial ones for the heights at which primates normally communicate. Although we view our measure of environmental influence on acoustic transmission (arboreal versus terrestrial) as a safe parsing for such a broad sample encompassing a panoply of subtly different habitat types, it is admittedly crude, and environmental effects on acoustic transmission are complex. Other variables such as type of call, location in an arboreal environment and ambient noise are likely to play a role [37], and an exploration of these influences should be taken up more fully as more precise data accumulate. However, such possible environmental influences might be expected to weaken any relationship between F0 dimorphism and arboreal versus terrestrial habitat rather than producing an effect that is opposite the predicted direction. Our results thus challenge the hypothesis that overall F0 dimorphism primarily evolves when male calls are selected for long-distance transmission, and our findings are more consonant with elevated male–male competition in terrestrial compared with arboreal species [56,57].
Inspection of figure 1 indicates an increase in F0 dimorphism from the last common ancestor of the apes to modern humans, culminating in humans exhibiting the greatest F0 dimorphism of all apes. These results contrast sharply with moderate human body mass dimorphism and negligible canine length dimorphism, which some have suggested indicate weak sexual selection in ancestral humans [58]. However, unlike other primates, in humans, female adiposity greatly exceeds that of males, producing modest overall mass dimorphism despite the fact that males possess 60% more muscle mass, and men fight with handheld weapons and fists rather than teeth in combat [57,59]. These unique features preclude conclusions about the strength of human sexual selection based on overall mass or canine size dimorphism [60,61]. Yet, if mating competition also tends to decrease male F0 relative to female F0 across primates, then F0 dimorphism has the potential to elucidate human sexual selection in ways that comparisons of body mass or canine size cannot. Our results suggest that, despite widespread pair bonding and contrary to some claims [58,62], ancestral human mating should not be viewed as fundamentally monogamous [52].
When phylogeny and mating system were statistically controlled, evolutionary changes towards greater F0 dimorphism were associated with changes towards less body size dimorphism and vice versa. Perhaps where mates are won mainly through direct fighting, males receive less benefit from exaggerating size acoustically and must instead invest in mass that is useful in contests. Conversely, acoustic threats and displays may be more effective when body mass or fights are especially costly, or where female choice is more important to male fitness. In humans, male F0 was indeed important in mate attraction, yet F0 more strongly predicted perceptions of men's dominance, consistent with previous experimental evidence [22,39]. Masculinity in men's faces is similarly perceived as aggressive across human societies, whereas the influence on attractiveness is more variable in magnitude and direction [63]. While such male traits appear better designed to function in male contests than in female choice [57], it remains possible that female choice is relatively more important in humans compared with other polygynous primates, and that stronger female choice tends to favour lower male F0 and more modest size dimorphism among polygynous primates. It is also possible that the unprecedented lethality imbued to human fighting with the advent of handheld and projectile weapons elevated the importance of threats and deference in relation to physical attacks [52,57]. Finally, in arboreal primates, large body mass may impose additional energetic costs, as well as increasing the risk of injury from falls. Although the relatively limited number of species in our sample prohibited exploring interactions between mating system, habitat and body mass dimorphism, we note that the single species in our sample with the greatest F0 dimorphism, Cercopithecus campbelli, is polygynous, partly arboreal and exhibits body mass dimorphism comparable to that of humans. In other words, one can speculate that male C. campbelli achieve polygyny partly via acoustic threats and/or mate attraction, whereas body mass is constrained by partial arboreality.
In many species, males exaggerate size to intimidate conspecifics, but attention to these exaggerations is likely to be maintained by a continued association between apparent size and formidability [4]. Although F0 decreased with increasing body size in both sexes across primate species, body size only weakly predicts F0 in adult humans [5,7]. However, our data show that cortisol and testosterone interact to predict men's F0 in a pattern that has previously been found to predict men's dominance [35], attractiveness [34] and immunocompetence [34], and hence that F0 is likely to reveal male condition to same-sex competitors and potential mates.
Our results thus not only demonstrate a likely influence of sexual selection in the origins and maintenance of sexual dimorphism in F0 across anthropoids, but also suggest that male contests, and to a lesser degree female mate choice, favour low male F0 as a signal of condition, shedding new light on the intensity and mechanisms of sexual selection in humans and other primates. Future research should extend these comparative investigations to vocal tract resonance frequencies, which have been implicated in mating competition and size exaggeration in several mammals [1,4,7], including humans [11,15].
Supplementary Material
Acknowledgements
This paper is dedicated to our colleague and friend Michael Owren, an exemplary scientist and human being. We thank Robert Montgomerie, George H. Perry, David Reby and two anonymous reviewers for comments on previous drafts of this manuscript.
Ethics
Participants provided informed written consent. The ethics committees of Michigan State University and Pennsylvania State University approved these studies, and all procedures adhered to the Declaration of Helsinki.
Data accessibility
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.r0160.
Authors' contributions
Conception and design of the experiments: D.A.P., A.K.H., R.C., R.P.B.; collection, analysis and interpretation of data: D.A.P., A.K.H., D.H.B., R.S.W., D.R., J.R.W., L.L.M.W., R.P.B., A.R.L., C.L.A., M.J.O., C.B., M.E.G., G.R-F.; drafting the article or revising it for important intellectual content: D.A.P., A.K.H. All authors approved the final version of the manuscript.
Competing interests
We declare we have no conflict of interest.
Funding
D.A.P. was supported by a National Institutes of Mental Health T32 MH70343-05 fellowship. J.R.W. was supported by a National Science Foundation predoctoral fellowship.
References
- 1.Harris TR, Fitch WT, Goldstein LM, Fashing PJ. 2006. Black and white colobus monkey (Colobus guereza) roars as a source of both honest and exaggerated information about body mass. Ethology 112, 911–920. ( 10.1111/j.1439-0310.2006.01247.x) [DOI] [Google Scholar]
- 2.Schön MA. 1971. The anatomy of the resonating mechanism in howling monkeys. Folia Primatol. 15, 117–132. ( 10.1159/000155371) [DOI] [PubMed] [Google Scholar]
- 3.Fitch WT, Giedd J. 1999. Morphology and development of the human vocal tract: a study using magnetic resonance imaging. J. Acoust. Soc. Am. 106, 1511–1522. ( 10.1121/1.427148) [DOI] [PubMed] [Google Scholar]
- 4.Fitch WT, Reby D. 2001. The descended larynx is not uniquely human. Proc. R. Soc. Lond. B 268, 1669–1675. ( 10.1098/rspb.2001.1704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puts DA, Apicella CL, Cárdenas RA. 2012. Masculine voices signal men's threat potential in forager and industrial societies. Proc. R. Soc. B 279, 601–609. ( 10.1098/rspb.2011.0829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado RA. 2006. Sexual selection in the loud calls of male primates: signal content and function. Int. J. Primatol. 27, 5–25. ( 10.1007/s10764-005-9001-4) [DOI] [Google Scholar]
- 7.Rendall D, Kollias S, Ney C, Lloyd P. 2005. Pitch (F0) and formant profiles of human vowels and vowel-like baboon grunts: the role of vocalizer body size and voice-acoustic allometry. J. Acoust. Soc. Am. 117, 944–955. ( 10.1121/1.1848011) [DOI] [PubMed] [Google Scholar]
- 8.Fant G. 1960. Acoustic theory of speech production. The Hague: Mouton. [Google Scholar]
- 9.Titze IR. 2000. Principles of voice production. Iowa City, IA: National Center for Voice and Speech. [Google Scholar]
- 10.Eitan Z, Schupak A, Gotler A, Marks LE. 2014. Lower pitch is larger, yet falling pitches shrink: interaction of pitch change and size change in speeded discrimination. Exp. Psychol. 61, 273–284. ( 10.1027/1618-3169/a000246) [DOI] [PubMed] [Google Scholar]
- 11.Feinberg DR, Jones BC, Little AC, Burt DM, Perrett DI. 2005. Manipulations of fundamental and formant frequencies affect the attractiveness of human male voices. Anim. Behav. 69, 561–568. ( 10.1016/j.anbehav.2004.06.012) [DOI] [Google Scholar]
- 12.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: Murray. [Google Scholar]
- 13.Snowdon CT. 2004. Sexual selection and communication. In Sexual selection in primates: new and comparative perspectives (eds Kappeler P, van Schaik CP), pp. 57–70. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Mitani JC. 1985. Sexual selection and adult male orangutan long calls. Anim. Behav. 33, 272–283. ( 10.1016/S0003-3472(85)80141-X) [DOI] [Google Scholar]
- 15.Puts DA, Hodges C, Cárdenas RA, Gaulin SJC. 2007. Men's voices as dominance signals: vocal fundamental and formant frequencies influence dominance attributions among men. Evol. Hum. Behav. 28, 340–344. ( 10.1016/j.evolhumbehav.2007.05.002) [DOI] [Google Scholar]
- 16.Puts DA, Barndt JL, Welling LLM, Dawood K, Burriss RP. 2011. Intrasexual competition among women: vocal femininity affects perceptions of attractiveness and flirtatiousness. Pers. Indiv. Diff. 50, 111–115. ( 10.1016/j.paid.2010.09.011) [DOI] [Google Scholar]
- 17.Apicella CL, Feinberg DR. 2009. Voice pitch alters mate-choice-relevant perception in hunter–gatherers. Proc. R. Soc. B 276, 1077–1082. ( 10.1098/rspb.2008.1542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisanski K, Mishra S, Rendall D. 2012. The evolved psychology of voice: evaluating interrelationships in listeners’ assessments of the size, masculinity, and attractiveness of unseen speakers. Evol. Hum. Behav. 33, 509–519. ( 10.1016/j.evolhumbehav.2012.01.004) [DOI] [Google Scholar]
- 19.Pisanski K, et al. 2014. Vocal indicators of body size in men and women: a meta-analysis. Anim. Behav. 95, 89–99. ( 10.1016/j.anbehav.2014.06.011) [DOI] [Google Scholar]
- 20.Cartei V, Bond R, Reby D. 2014. What makes a voice masculine: physiological and acoustical correlates of women's ratings of men's vocal masculinity. Horm. Behav. 66, 569–576. ( 10.1016/j.yhbeh.2014.08.006) [DOI] [PubMed] [Google Scholar]
- 21.Sell A, Bryant GA, Cosmides L, Tooby J, Sznycer D, von Rueden C, Krauss A, Gurven M. 2010. Adaptations in humans for assessing physical strength from the voice. Proc. R. Soc. B 277, 3509–3518. ( 10.1098/rspb.2010.0769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puts DA, Gaulin SJC, Verdolini K. 2006. Dominance and the evolution of sexual dimorphism in human voice pitch. Evol. Hum. Behav. 27, 283–296. ( 10.1016/j.evolhumbehav.2005.11.003) [DOI] [Google Scholar]
- 23.Leongómez JD, Binter J, Kubicová L, Stolařová P, Klapilová K, Havlíček J, Roberts SC. 2014. Vocal modulation during courtship increases proceptivity even in naive listeners. Evol. Hum. Behav. 35, 489–496. ( 10.1016/j.evolhumbehav.2014.06.008) [DOI] [Google Scholar]
- 24.Weusthoff S, Baucom BR, Hahlweg K. 2013. The siren song of vocal fundamental frequency for romantic relationships. Front. Psychol. 4, 439 ( 10.3389/fpsyg.2013.00439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts ML, Buchanan KL, Hasselquist D, Evans MR. 2007. Effects of testosterone and corticosterone on immunocompetence in the zebra finch. Horm. Behav. 51, 126–134. ( 10.1016/j.yhbeh.2006.09.004) [DOI] [PubMed] [Google Scholar]
- 26.Bortolotti GR, Mougeot F, Martinez-Padilla J, Webster LM, Piertney SB. 2009. Physiological stress mediates the honesty of social signals. PLoS ONE 4, e4983 ( 10.1371/journal.pone.0004983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore FR, Cornwell RE, Smith MJ, Al Dujaili EA, Sharp M, Perrett DI. 2011. Evidence for the stress-linked immunocompetence handicap hypothesis in human male faces. Proc. R. Soc. B 278, 774–780. ( 10.1098/rspb.2010.1678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. [DOI] [PubMed] [Google Scholar]
- 29.Burnstein KL, Maiorino CA, Dai JL, Cameron DJ. 1995. Androgen and glucocorticoid regulation of androgen receptor cDNA expression. Mol. Cell. Endocrinol. 115, 177–186. ( 10.1016/0303-7207(95)03688-1) [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Wang J, Yu G, Liu W, Pearce D. 1997. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J. Biol. Chem. 272, 14 087–14 092. ( 10.1074/jbc.272.22.14087) [DOI] [PubMed] [Google Scholar]
- 31.Smith RG, Syms AJ, Nag A, Lerner S, Norris JS. 1985. Mechanism of the glucocorticoid regulation of growth of the androgen-sensitive prostate-derived R3327H-G8-A1 tumor cell line. J. Biol. Chem. 260, 12 454–12 463. [PubMed] [Google Scholar]
- 32.Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. 1992. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev. 16, 115–130. ( 10.1016/S0149-7634(05)80175-7) [DOI] [PubMed] [Google Scholar]
- 33.Tilbrook AJ, Turner AI, Clarke IJ. 2000. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev. Reprod. 5, 105–113. ( 10.1530/ror.0.0050105) [DOI] [PubMed] [Google Scholar]
- 34.Rantala MJ, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. 2012. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nat. Commun. 3, 694–698. ( 10.1038/ncomms1696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta PH, Josephs RA. 2010. Testosterone and cortisol jointly regulate dominance: evidence for a dual-hormone hypothesis. Horm. Behav. 58, 898–906. ( 10.1016/j.yhbeh.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 36.Mitani JC, Stuht J. 1998. The evolution of nonhuman primate loud calls: acoustic adaptation for long-distance transmission. Primates 39, 171–182. ( 10.1007/BF02557729) [DOI] [Google Scholar]
- 37.Waser PM, Brown CH. 1986. Habitat acoustics and primate communication. Am. J. Primatol. 10, 135–154. ( 10.1002/ajp.1350100205) [DOI] [PubMed] [Google Scholar]
- 38.Mitani JC, Gros-Louis J, Richards AF. 1996. Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am. Nat. 147, 966–980. ( 10.1086/285888) [DOI] [Google Scholar]
- 39.Puts DA, Jones BC, DeBruine LM. 2012. Sexual selection on human faces and voices. J. Sex Res. 49, 227–243. ( 10.1080/00224499.2012.658924) [DOI] [PubMed] [Google Scholar]
- 40.Puts D, Doll L, Hill A. 2014. Sexual selection on human voices. In Evolutionary perspectives on human sexual psychology and behavior (eds Weekes-Shackelford V, Shackelford T), pp. 69–86. Berlin, Germany: Springer. [Google Scholar]
- 41.Leutenegger W, Cheverud J. 1982. Correlates of sexual dimorphism in primates: ecological and size variables. Int. J. Primatol. 3, 387–402. ( 10.1007/BF02693740) [DOI] [Google Scholar]
- 42.Wade MJ, Shuster SM. 2004. Sexual selection: harem size and the variance in male reproductive success. Am. Nat. 164, E83–E99. ( 10.1086/424531) [DOI] [PubMed] [Google Scholar]
- 43.Clutton-Brock TH. 1989. Mammalian mating systems. Proc. R. Soc. Lond. B 236, 339–372. ( 10.1098/rspb.1989.0027) [DOI] [PubMed] [Google Scholar]
- 44.Clutton-Brock TH, Harvey PH, Rudder B. 1977. Sexual dimorphism, socionomic sex ratio and body weight in primates. Nature 269, 797–800. ( 10.1038/269797a0) [DOI] [PubMed] [Google Scholar]
- 45.Clutton-Brock TH, Harvey PH. 1977. Primate ecology and social organization. J. Zool. 183, 1–39. ( 10.1111/j.1469-7998.1977.tb04171.x) [DOI] [Google Scholar]
- 46.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 47.Clutton-Brock TH, Harvey PH. 1984. Comparative approaches to investigating adaptation. In Behavioral ecology: a evolutionary approach (eds Krebs JR, Davies NB), pp. 7–29, 2nd edn Oxford, UK: Blackwell. [Google Scholar]
- 48.Harcourt AH, Purvis A, Liles L. 1995. Sperm competition: mating system, not breeding season, affects testes size of primates. Funct. Ecol. 9, 468–476. ( 10.2307/2390011) [DOI] [Google Scholar]
- 49.Arnold C, Matthews LJ, Nunn CL. 2010. The 10 k trees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118. ( 10.1002/evan.20251) [DOI] [Google Scholar]
- 50.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. Caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5.2 ed. See http://CRANRproject.org/package=caper.
- 51.Hauser MD. 1993. The evolution of nonhuman primate vocalizations: effects of phylogeny, body weight and social-context. Am. Nat. 142, 528–542. ( 10.1086/285553) [DOI] [PubMed] [Google Scholar]
- 52.Puts D. 2016. Human sexual selection. Curr. Opin. Psychol. 7, 28–32. ( 10.1016/j.copsyc.2015.07.011) [DOI] [Google Scholar]
- 53.Fairbanks G. 1960. Voice and articulation drillbook, 2nd edn New York, NY: Harper & Row. [Google Scholar]
- 54.Mrosovsky N. 2003. Beyond the suprachiasmatic nucleus. Chronobiol. Int. 20, 1–8. ( 10.1081/CBI-120017811) [DOI] [PubMed] [Google Scholar]
- 55.Apicella CL, Feinberg DR, Marlowe FW. 2007. Voice pitch predicts reproductive success in male hunter-gatherers. Biol. Lett. 3, 682–684. ( 10.1098/rsbl.2007.0410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheverud JM, Dow MM, Leutenegger W. 1985. The quantitative assessment of phylogenetic constraints in comparative analyses: sexual dimorphism in body-weight among primates. Evolution 39, 1335–1351. ( 10.2307/2408790) [DOI] [PubMed] [Google Scholar]
- 57.Puts DA. 2010. Beauty and the beast: mechanisms of sexual selection in humans. Evol. Hum. Behav. 31, 157–175. ( 10.1016/j.evolhumbehav.2010.02.005) [DOI] [Google Scholar]
- 58.Lovejoy CO. 2009. Reexamining human origins in light of Ardipithecus ramidus. Science 326, 74e1–74e8. ( 10.1126/science.1175834) [DOI] [PubMed] [Google Scholar]
- 59.Carrier DR, Morgan MH. 2014. Protective buttressing of the hominin face. Biol. Rev. Camb. Phil. Soc. 90, 330–346. ( 10.1111/brv.12112) [DOI] [PubMed] [Google Scholar]
- 60.Marlowe FW, Berbesque JC. 2012. The human operational sex ratio: effects of marriage, concealed ovulation, and menopause on mate competition. J. Hum. Evol. 63, 834–842. ( 10.1016/j.jhevol.2012.09.004) [DOI] [PubMed] [Google Scholar]
- 61.Plavcan JM. 2012. Sexual size dimorphism, canine dimorphism, and male–male competition in primates: where do humans fit in? Hum. Nat. 23, 45–67. ( 10.1007/s12110-012-9130-3) [DOI] [PubMed] [Google Scholar]
- 62.Stanyon R, Bigoni F. 2014. Sexual selection and the evolution of behavior, morphology, neuroanatomy and genes in humans and other primates. Neurosci. Biobehav. Rev. 46, 579–590. ( 10.1016/j.neubiorev.2014.10.001) [DOI] [PubMed] [Google Scholar]
- 63.Scott IM, et al. 2014. Human preferences for sexually dimorphic faces may be evolutionarily novel. Proc. Natl Acad. Sci. USA 111, 14 388–14 393. ( 10.1073/pnas.1409643111) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.r0160.