Abstract
Reef-building corals begin as non-calcifying larvae that, upon settling, rapidly begin to accrete skeleton and a protein-rich skeletal organic matrix that attach them to the reef. Here, we characterized the temporal and spatial expression pattern of a suite of biomineralization genes during three stages of larval development in the reef-building coral Pocillopora damicornis: stage I, newly released; stage II, oral-aborally compressed and stage III, settled and calcifying spat. Transcriptome analysis revealed 3882 differentially expressed genes that clustered into four distinctly different patterns of expression change across the three developmental stages. Immunolocalization analysis further reveals the spatial arrangement of coral acid-rich proteins (CARPs) in the overall architecture of the emerging skeleton. These results provide the first analysis of the timing of the biomineralization ‘toolkit’ in the early life history of a stony coral.
Keywords: acidic proteins, coral development, biomineralization, gene expression
1. Introduction
(a). Calcification in corals
Although carbonates from corals abound in the fossil record, how these organisms precipitate their mineral skeletons remains enigmatic. Corals (Cnidaria) belong to one of the most anciently diverged invertebrate phyla and are among the first known metazoans to precipitate calcium carbonate [1]. Their radial body plan consists of only two cell layers: an ectoderm and an endoderm, separated by the mesoglea, a non-cellular gelatinous matrix [2]. In stony symbiotic corals, the endoderm contains intracellular photosynthetic dinoflagellates of the genus Symbiodinium. The interface between the aboral ectoderm and the skeleton, referred to as the calicoblastic epithelium [3], is the site where extracellular aragonite crystals (orthorhombic CaCO3) are precipitated and organized to form a macroscopic biomineral structure [4].
The biomineralization process in corals is associated with desmocytes, the differentiated calicoblastic cells [5] that secrete proteins [6], phospholipids [7] and polysaccharides [8]. These cells appear to control the extracellular precipitation of calcium carbonate as microscopic fibrils that develop into a skeletal framework with structural features that are genetically derived. The secreted biomolecules, collectively termed the skeletal organic matrix (SOM), have long been hypothesized to aid in the stabilization, nucleation, growth, spatial orientation and structural integrity of the skeleton (e.g. [9]). However, the temporal and spatial patterns of the molecules responsible for skeletal formation and organization are very poorly understood. To elucidate these patterns, we followed the temporal succession of gene expression during metamorphosis and settlement of coral planulae.
(b). Earliest stages
Corals have a biphasic life cycle with planktonic larval stages and benthic adults [10]. These two phases are separated by settlement and metamorphosis, which are critical stages in coral development during which planulae undergo substantial morphological changes involving the building and rearrangement of tissues, initiation of aragonite precipitation, and in some cases, uptake of photosynthetic symbionts. Planulae, the planktonic larvae of stony corals, are non-calcifying until they settle. Three clear morphological stages of larvae have been described: (i) planktonic larvae that are long and thin in shape; (ii) partially metamorphosed larvae that are flattened along the oral to aboral axis in a globular shape prior to settlement; and (iii) clearly metamorphosed, settled and calcifying spat [11] (figure 1). The morphological changes during metamorphosis are well documented [11]; however, few studies have related the molecular events underlying metamorphosis and settlement to calcification. Those have focused mainly on carbonic anhydrase and galaxin-like proteins that are differentially expressed over these life stages [13,14], or on proteins not retained in the skeleton but instead used in metabolism and stress response [15]. Whereas most studies on coral biomineralization have focused on adults, the settling stage of coral larvae provides a unique system for studying the mechanism of skeletogenesis because calcification is initiated immediately upon settlement, thereby potentially allowing insight into a synchronized pattern of gene expression.
Figure 1.
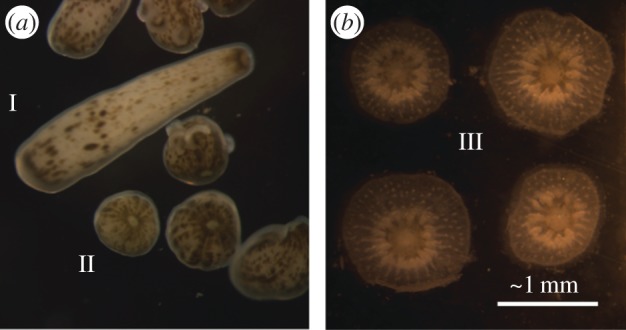
Stages of the brooded P. damicornis larvae used for our comparative analyses [12]. Pictured are: (a) stages I and II, motile larvae and (b) stage III, settled and calcifying spat.
Here we (i) analyse the transcriptome of the coral animal in each of the three early life-history stages with a focus on adhesion and structural proteins involved in calcification; (ii) assess the expression of putative biomineralization proteins identified by their high content of acidic residues; and (iii) characterize the immunolocalization patterns of distinct skeletal matrix proteins during settlement and metamorphosis. Based on these data, we were able to elucidate the timing and composition of an integral portion of the biomineralization ‘toolkit’ in early life-history stages of a stony coral.
2. Results
(a). Host differential gene expression
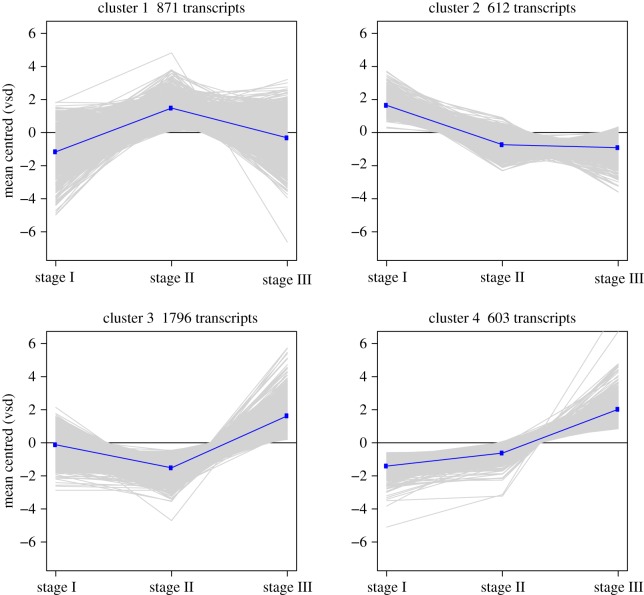
Differential gene expression (DE) analysis and hierarchical clustering revealed 3882 life-history stage-specific DE genes, of which 74 were characterized as highly ‘acidic’. Both groups clustered into four gene expression patterns relative to the planktonic larval stage (stage I): (i) genes that are upregulated during metamorphosis and subsequently by downregulated upon settling; (ii) genes that are downregulated during metamorphosis and settlement; (iii) genes that are downregulated during metamorphosis and subsequently upregulated upon settling; and (iv) genes that are upregulated during metamorphosis and after settling (figures 2 and 3).
Figure 2.
Gene expression clusters from RNAseq analysis of 3882 DE genes in P. damicornis. VSD, variance stabilizing transformation. (Online version in colour.)
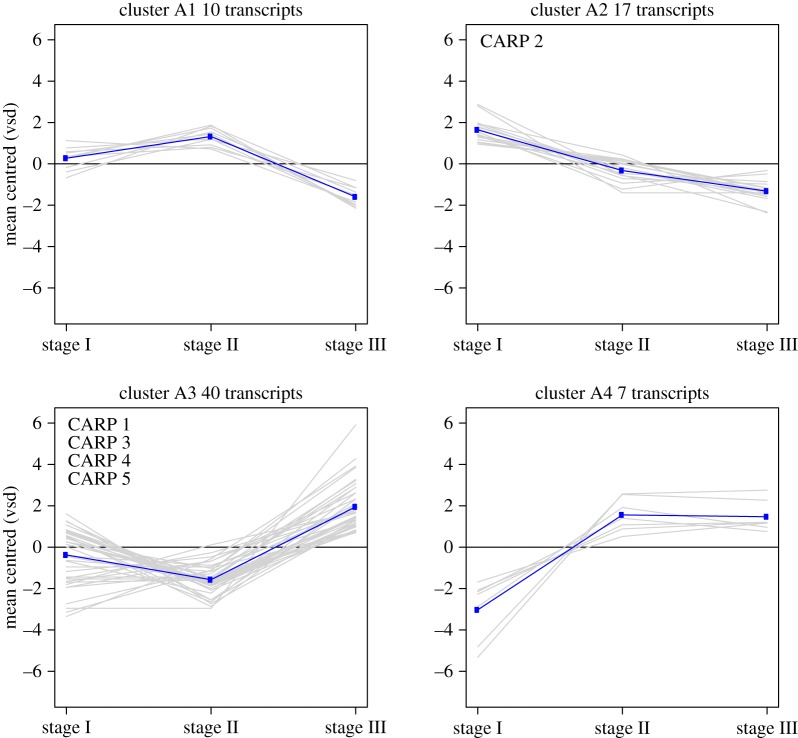
Figure 3.
Gene expression clusters from RNAseq analysis of 74 novel acidic proteins. VSD, variance stabilizing transformation. (Online version in colour.)
Nineteen genes, previously described as components of the biomineralization ‘toolkit’ (electronic supplementary material, table S3) [16–18], encoding proteins containing one or more trans-membrane domains, extracellular matrix proteins and coral acid-rich proteins (CARPs) were among those differentially expressed. Eighteen of these genes were upregulated upon settlement (clusters 3 and 4, figure 2; electronic supplementary material, table S3). Both galaxin-like and amgalaxin-like genes identified in this study were upregulated in stages II and III and various paralogues of biomineralization toolkit genes were expressed in all stages (electronic supplementary material, table S3). In addition, three genes previously described by Grasso et al. [13] to be involved in immunity and cell adhesion, and apoptosis during larvae metamorphosis in the genus Acropora, were upregulated during metamorphosis (Cluster 1; CL84Contig1, CL18594Contig1, contig_27772), reinforcing the importance of these genes in the process of metamorphosis and suggesting a role in the initiation of biominaralization. We also searched the P. damicornis transcriptome for the SLC4γ gene, a coral-specific transporter of bicarbonate ions to the site of calcification [19]. The P. damicornis SLC4γ homologue (contig_797) showed significant upregulation in stage III, compared with stages I and II (i.e. 9.2-fold and 12.8-fold, respectively).
(b). Novel ‘acidic’ proteins
Analysis of the coral transcriptome for acidic proteins (see §4) identified 74 candidates that may be involved in biomineralization (i.e. 95% acidic and 5% basic; electronic supplementary material, table S2). Repeated sequences were found in 91% of those genes. By contrast, secretion peptides were found in only ten proteins, which might be explained by incomplete gene prediction at their 5′ termini. The proteins were complex and significantly different in their repeat types and numbers (electronic supplementary material, figure S5). A BLASTP analysis of these genes against available coral genome and transcriptome data (http://comparative.reefgenomics.org/) [20] revealed that 88% of them have orthologues in most stony coral species with greater than 50% protein identity (electronic supplementary material, table S2). Analysis of the expression pattern clusters of the acidic proteins revealed that at the early stages (I and II) prior to the initiation of biomineralization, only approximately 14% and approximately 23% were upregulated, respectively. By contrast, during active calcification in stage III, approximately 63% of the acidic proteins including the previously described CARPs 1, 3, 4 and 5 [16,18] were upregulated, whereas approximately 36% of them, including CARP 2, were downregulated at this stage. The search criteria for novel acidic proteins identified four proteins with more than 25% acidic amino acids (Asp and Glu) that were net-basic. These four novel basic proteins were upregulated relative to stage I, two during metamorphosis (CL16466Contig1, CL14732Contig1) and two (contig_91645, contig_43693) upon settlement (electronic supplementary material, table S2).
(c). Microscopy and immunohistochemistry
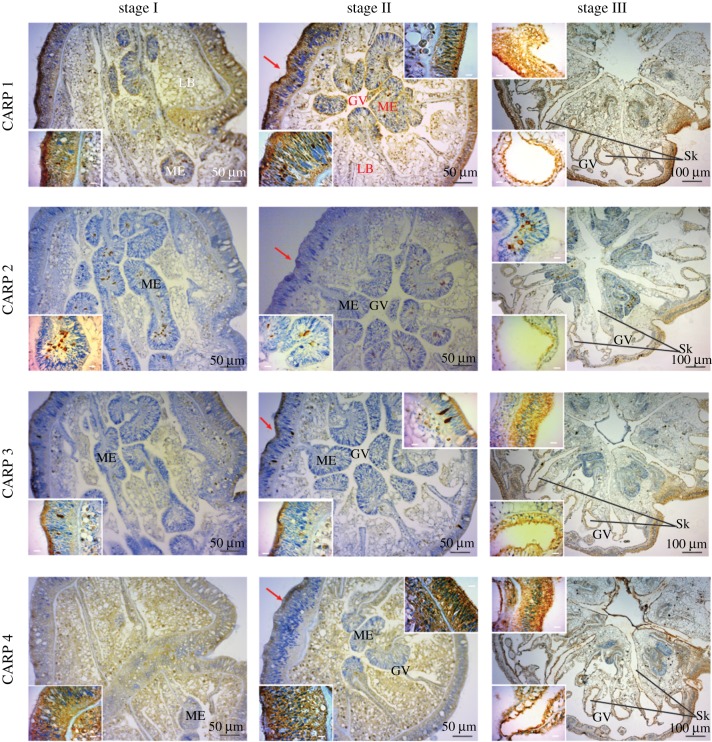
The first calcareous elements that are visible upon settling are circular platelets and rod-shaped granules (electronic supplementary material, figure S3) that aggregate to form the primary septa (electronic supplementary material, figure S3), followed by the formation of the basal disc [12]. Immunohistochemical (IHC) analysis revealed that cadherin and actin are ubiquitous in the tissues at all stages (electronic supplementary material, figure S4). By contrast, the CARPs display a differential localization pattern. CARP 2 expression was most abundant at the base of the mesentery of the pre-settled P. damicornis planulae (stages I, II, figure 4; ME), whereas CARPs 1, 3 and 4 were abundant at the aboral epidermis of the pre-settled planulae (stage II, figure 4; red arrow), which is the epithelium at this stage and will form the coral skeleton. In the settled and calcifying spat (stage III), CARPs were localized around the skeletal features corresponding to the septa and the basal plate [21] in the calicodermis layer (figure 4; Sk).
Figure 4.
Immunolabelling of P. damicornis larvae (embedded in paraffin) at the three developmental stages studied. Immunohistochemistry of four CARPs (1–4) reveals labelling at distinct intracellular locations for each protein (brown), counterstained with hematoxylin (blue) to show nuclei (insets). Sk, skeleton side; GV, gastrovascular canals; LB, lipid bodies; ME, mesentery; red arrow, aboral epidermis in stage II. Stage II sections are cut on an oblique plane that shows the basal body wall on the left, which is thicker and contains granules that stain brown; on the right is to the thinner surface body wall, which does not have stained cytoplasmic granules (insets). Inset scale bars are all 10 µm.
3. Discussion
Matrix proteins play crucial roles in the skeletal formation of corals. Two functionally distinct components of coral SOM can be distinguished [22]: (i) structural, adhesive and metalloproteins that enable cell–cell and cell–substrate adhesion and modify the calcifying environment [16,23] and (ii) proteins with acidic residues (e.g. CARPs) that catalyse the nucleation of the biomineral [18]. Our results reveal that a structural scaffold develops between stages II and III, followed by precipitation of aragonite in the immediate proximity of CARPs localization post-settlement in stage III (figure 5).
Figure 5.
Summary of the major findings of our study on gene differential expression (DE) and protein immunolocalization during early development in P. damicornis. (a) The identity of structural proteins with DE during the three stages of coral development, with red text indicating previously described biomineralization toolkit genes; (b) the per cent of novel acidic proteins upregulated in the DE analysis; (c) the relative expression levels of CARPs during development (mean of the triplicate samples following variant stabilizing transformation); letters above indicate the novel acidic protein expression cluster (see figure 3 and electronic supplementary material, table S1); (d) cartoon summary of immunolocalization patterns of CARPs across the three life-history stages; and (e) the proposed roles of these proteins in the animal. CARP 4 and 5 have high protein similarity and our polyclonal antibody epitope region shares 68% identity and 79% similarity; therefore, they may cross-react with the same antibody. VSD, variance stabilizing transformation.
Multiple SOM adhesion genes, several structural genes, carbonic anhydrase and galaxins are upregulated in planulae after settling (figures 2 and 3; electronic supplementary material, table S3). Although some of these proteins have previously been observed in cells other than the calicoblastic ectoderm [21,24], carbonic anhydrase and (am)galaxins were also reported to be upregulated at the transition from coral larva to polyp [13,14], as are several adhesion proteins [15]. Although the role of galaxin in biomineralization remains unresolved, the protein shows sequence similarity to usherin, a type IV collagen binding protein. Type IV collagen is also found in the coral skeleton [16,17] and four orthologues of collagen IV are upregulated post-settlement in this study (Cluster 4; contig_35764, contig_48298, CL4100Contig1, CL5938Contig1). We suggest that galaxin is recruited to cement individual aragonite crystals to form a macroscopic skeletal structure. Furthermore, the expression of paralogues of SOM genes in the pre-settled stages (I, II) encoding proteins containing one or more trans-membrane domains, adhesion and structural proteins suggests these proteins may have general cellular functions related to development, in addition to skeletogenesis.
Twenty-seven acidic proteins, including CARP 2, are upregulated in planulae prior to metamorphosis and settlement (Clusters A2, figures 3 and 5). This group of acidic proteins is remarkably rich in glutamic acid, and is downregulated following settlement (figure 5c). It has been previously suggested that glutamic acid-rich macromolecules are associated with amorphous calcium carbonate phases in ascidians and sponges and are responsible for both inhibition of crystallization and stabilization of an amorphous phase [25]. We propose a role (figure 5) for this group of proteins in the inhibition of crystal nucleation and growth in corals prior to settlement, analogous to the roles of osteopontin and ostenoectin in bone formation [26,27]. This hypothesis will need to be tested using cell biological or functional genetic methods; the latter are, as yet, unavailable for corals.
The coral acidic gene family is the only one that lacks homologues among other non-calcifying taxa. Although functional analogues (not homologues) are found across the biomineralizing tree of life, the genes described in this study appear to be unique to Scleractinia. Moreover, the fact that within this group, approximately 64% are upregulated and approximately 36% are downregulated in planulae after settling (figure 3), suggests specific and critical roles for those proteins in coral biomineralization. This inference is supported by the localization pattern of the CARPs. Polyclonal antibodies raised against CARPs 1–4 showed that CARPs 1, 3 and 4 are localized to the cells around the skeleton in the calicodermis layer after planula settlement (figures 4 and 5). This expression pattern is similar to that in the adult coral [21].
The composition of genes encoding acidic coral proteins identified in this and other studies [16–18,28] having putative roles in biomineralization is remarkable. Like other invertebrates and vertebrates, the amino acid composition of the coral SOM is rich in glutamic and aspartic acids (e.g. [29–31]). This feature is considered convergent because no significant sequence similarities are observed among acidic skeletal proteins from different phyla [32]. Lysine and serine are also abundant, allowing for protein phosphorylatation and glycosylation (electronic supplementary material, table S2). These characteristics suggest the capacity to bind calcium ions [18]; in fact, similar proteins have a low affinity and high capacity for calcium binding [33]. This distinguishes them from the typical ‘high-affinity, low-capacity’ canonical calcium-binding domains, such as EF-hands [34].
Our search for proteins with more than 25% acidic amino acids (Asp and Glu) also revealed four novel basic hypothetical proteins that are DE during coral development. In addition to a high content of Asp and Glu, their primary structure is composed of more than 26% arginine and lysine (electronic supplementary material, table S2). Under the classical model of biologically controlled calcium carbonate precipitation, acidic proteins in the SOM play key roles including, but not limited to, nucleation [18], inhibition of crystal growth [35], crystal extension or termination [36] and amorphous phase stabilization [37]. Therefore, proteins with a basic pI (isoelectric point) are unexpected components of the coral SOM and are reported here for the first time for hexacorals. Basic proteins were reported to be a key accelerator in the control of crystal growth in mollusc nacre [38,39], and are present in echinoderm spicules [40,41]. The high content of arginine and lysine can interact with negatively charged ions (bicarbonate) or acidic matrix proteins.
The temporal and spatial patterns of host gene expression and protein localization across the transition from non-calcifying to calcifying tissues in developing larvae reveals how CARPs are switched on and off in the three developmental stages (electronic supplementary material, figure S1; figure 2). These results clarify the timeline and key components required for biomineralization in corals (figure 5). The initial DE of structural and adhesive proteins, limited upregulation of acidic proteins and low expression of CARPs 1, 3, 4/5, suggest that stage I is dominated by processes involved in cell–cell adhesion and maintenance of cellular and tissue structure. As the larvae transition to an oral–aboral compressed stage and begin metamorphosis, however, expression of a set of structural and adhesive proteins, including those previously documented to have a direct role in biomineralization (e.g. galaxin-like, [23]) change significantly; i.e. the percentage of upregulated acidic proteins increases, and CARPs localize aborally near the future site of the basal plate, preparing the epithelium for adhesion to the substrate (figure 5).
Consistent with a direct role in biomineralization is the strong DE of structural and adhesion proteins. The majority of the acidic proteins (63%, clusters A3 and A4, figure 3), including the CARPs 1, 3–5, are upregulated and CARP localization is in immediate contact with the precipitated aragonite at stage III (figures 4 and 5). Together, this analysis indicates there are key players during the different stages of the initiation of calcification exemplified by the strong separation of the gene expression patterns by stage and tight grouping of expression patterns within the stage (electronic supplementary material, figure S1, figure 5). These key players appear to prevent premature calcification, provide adhesion to the substrate, enhance the nucleation of CaCO3 and direct or facilitate precipitation of the aragonite skeleton (figure 5e).
In conclusion, our results demonstrate that CARPs play a key role in the formation of the coral skeleton during development, in addition to their known role in the later life of the adult animal. These proteins are unique to corals. We have found that there is a range of acidic to alkaline pI values for the skeletal matrix proteins (electronic supplementary material, table S2), although the net pI of the SOM as a whole is acidic [31,42,43]. Furthermore, the DE pattern of the acidic proteins together with their distinct amino acid composition suggest they act as a central hub that both enhances and inhibits mineral formation in addition to stabilizing the amorphous phase [25,27,44,45]. In addition, the coral proteome is composed of paralogues of adhesion, structural and functional proteins. The fact that 11 proteins were previously reported to be part of the biomineralization toolkit [16,17] and the encoding genes are upregulated in stage III, suggests that corals co-opt pre-existing genes for biomineralization. The novel characterization of the temporal and spatial pattern of SOM proteins presented here (figure 5) illuminates the timeline and key players in a critical biological function, biomineralization. Here, with the use of developmental stages as natural on–off gene switches, we have confirmed the role of key biomineralization proteins (CARPs) and identified novel unannotated acidic proteins for further testing through targeted functional genomics analysis of each candidate gene. The greatest threat to reefs is loss of the biomineralizing ecosystem engineers and resulting habitat diversity. Our results reveal the temporal expression patterns of key biomineralization proteins that may aid in predicting and supporting persistence of stony corals at critical early larval stages. How gene expression may be altered as these organisms face the compounding anthropogenic stressors of ocean acidification, sea surface temperature warming, eutrophication and others remains to be elucidated.
4. Experimental procedures
(a). Sample collection
Larvae were collected from adult Pocillopora damicornis colonies located in the south end of Kaneohe Bay, Hawaii. Nets were placed on 10 adult corals for each of 2 days to generate a pool of larvae for stage selection. Larvae were collected during 21 and 22 November 2013 ((lunar days 19 and 20, following peak release at approximately full moon [46]), returned to the laboratory and held in controlled incubator conditions in 0.2 µm filtered seawater. The temperature was maintained at approximately 27°C in the incubator, which is close to seawater values for November and December (26.92 ± 0.02°C, hourly NOAA buoy data November–December 2013, n = 1464; http://tidesandcurrents.noaa.gov/physocean.html?id=1612480) and light was set to 115.2 µmol photons m−2 s−1 on a 12 L : 12 D cycle.
Three clear morphological stages of larvae were targeted for analysis (figure 1). Seawater was refreshed and larvae were checked every 2 days for the presence of replicate groups of 15 larvae at stages I and II and 10 individuals at stage III. The metamorphosis and settlement processes were not induced, but proceeded naturally. Each replicate group was collected on the day when a minimum number of targeted larvae were available to generate the RNA pool for RNAseq analysis [47]. This resulted in replicate groups that were collected 24 h, 24–48 h and 11 days post release for stages I, II and III, respectively. Larvae were flash frozen in liquid nitrogen and stored at −80°C for RNA extraction. Additional larvae were fixed in Z-fix (Anatech) for use in microscopy and IHC analyses [21].
(b). RNA extraction, processing and sequencing
Total RNA was extracted using TRI-Reagent (Sigma) following the manufacturer's protocol with some modification at the homogenization step. Briefly, 10–15 larvae and 550 µl of TRI-Reagent were transferred to a QiaShredder cartridge and centrifuged at top speed for 1 min. Then, 450 µl of TRI-Reagent were added to the collection tube to bring the volume up to 1 ml and incubated at room temperature for 5 min. Following the RNA wash in 70% ethanol, we performed on-column DNase digestion using Qiagen RNase-free DNaseI kit, following the manufacturer's protocol. Three independent samples were generated for each growth stage. The cDNA libraries were constructed using the Illumina TruSeq RNA Sample Prep kit v2 according to the manufacturer's protocol, and libraries were pooled in groups of three per run.
(c). Sequencing and assembly
RNAseq data were generated using triplicate samples of the three P. damicornis developmental stages (figure 1). The triplicates were indexed and sequenced on flow cells using the Illumina MiSeq with 150 bp single-end reads and 150-cycle V3 reagent kits (nine indexes on three flow cells). The three runs yielded 115 142 502 raw reads (BioProject PRJNA306839). After adapter- and quality-trimming using CLC Genomics Workbench (CLC Inc, Aarhus, Denmark), 104 402 226 of these reads were kept for downstream analysis.
The 39 genes recently shown to be part of the biomineralization toolkit in the stony coral Stylophora pistillata [16,30] were used to guide assembly of their homologues in P. damicornis. However, two of these genes (g2385 and g19762.t1 (both encoding hypothetical proteins) with GenBank accession numbers AGC24391 and AGG36359, respectively) had less than 20% coverage over their length using the P. damicornis RNAseq data and were therefore not included in the differential gene expression (DE) analysis. All reads that did not align to the remaining 37 genes (104 077 224) were used for de novo transcriptome assembly. This assembly yielded 260 644 cDNA contigs; after clustering the transcripts with TGICL [48] they were then clustered at the amino acid level with CD-HIT [49,50]. After applying a threshold of 85% identity and 5× average coverage cut-off, and addition of the 37 separately assembled biomineralization genes, the entire combined assembly consisted of 135 265 contigs with N50 = 1104 bp.
(d). Filtering for host genes
Because we were primarily interested in genes related to biomineralization control by the coral host, we conducted a BLASTP analysis against the NCBI non-redundant protein database setting the e-value cut-off less than 10−3, and retained only sequences that hit a metazoan sequence. This step excluded hits to contaminating DNA including, Alveolata (4903 contigs), Stramenopiles (1740 contigs) and Bacteria (1583 contigs), thereby providing an animal-centric output for interpretation. Using this approach, out of the 135 265 contigs, 30 103 had a BLAST hit to metazoans; this dataset was then used for DE analysis.
We compared our P. damicornis transcriptome assembly to existing data (http://cnidarians.bu.edu/PdamBase/cgi-bin/). The PdamBase assembly, consisting of 70 786 contigs, was used as query for a BLAST search against the NR (non redundant protein sequences) database to filter out all non-metazoan sequences. The 25 837 contigs that had a hit to a metazoan homologue were then analysed with CEGMA to evaluate the completeness of the assembly, whereby 60% of the 248 most highly conserved Core Eukaryotic Genes (CEGs) were found in the assembly. By contrast, 76% of the 248 CEGs were present in our assembly. We also did a reciprocal BLAST analysis between both assemblies, which showed that 13 396 contigs were shared between them. These analyses suggest that our assembly is robust and comparatively more representative of the P. damicornis gene inventory than PdamBase. The relatively small number of shared genes between these two assemblies likely indicates that different tissues/development stages were used to generate the data. Given these results, we chose to use our assembly as the reference because it is directly derived from the RNAseq reads that were generated to address DE in the three larval developmental stages.
(e). Differential gene expression
The 9 libraries (3 stages × 3 replicates) were individually mapped to the transcriptome assembly and the number of unique reads mapped per contig were counted and used as input to DESeq2 [51], an R bioconductor package for DE. Using DESeq2 we performed a principal components analysis to test if the triplicates provided biological replicates (electronic supplementary material, figure S1). These results show a strong correlation in gene expression within each developmental stage database (electronic supplementary material, figure S1), which supports the existence of stage-specific (i.e. useful) signal (electronic supplementary material, figure S2). For identifying DE genes and examining their expression in the three stages, we made three comparisons between the three developmental stages: stage III versus stage II, stage III versus stage I and stage II versus stage I. Our goal was to collect all genes that showed differential expression in at least one of the comparisons; therefore, we applied a fold-change (FC) cut-off of log2 (FC) > 3 (i.e. eightfold difference) and log2(1/FC) < −3 and a false discovery rate (FDR) < 0.01. Using this approach, out of the 30 103 genes tested, 3882 were found to be DE (electronic supplementary material, table S1), of which 47 were DE in all three comparisons and 1676 genes were DE in two comparisons (electronic supplementary material, figure S6). We found 346 genes that had hits with e-value <1 × 10−20 to skeletal matrix proteins previously reported in Stylophora pistillata and Acropora millepora [16–18] (electronic supplementary material, table S3). The expression values of 3882 genes in the three stages were exported from DESeq2 following transformation using the variant stabilizing transformation (VST; provides a statistical basis for comparing expression values between libraries). This dataset was mean centred and clustered (hclust package in R) to identify expression patterns conserved across the life stages. A more comprehensive annotation of SOM structural proteins was completed based on previous proteomic analysis of coral SOM [16].
(f). Identification of novel ‘acidic’ proteins
Based on prior identification of acidic proteins involved in biomineralization within the organic matrix, a search for novel SOM proteins was performed via a transcriptome-wide search for acidic sequences containing: (i) high content (more than 25%) of acidic amino acids (Asp and Glu), (ii) comprising at least 100 amino acid residues and (iii) differentially expressed in the three life-history stages. The rationale for this approach was that the soluble fraction of the SOM is known to be rich in acidic amino acids [6,29,30] and five CARPs have been suggested to play a major role in calcium carbonate nucleation [16,18]. This dataset was clustered (hclust package in R) to identify expression patterns conserved across the life-history stages that may be important in organizing and promoting calcification within the organic matrix. We conducted a BLASTP analysis of the resulting proteins against the most comprehensive coral genome and transcriptome database to date (http://comparative.reefgenomics.org/) with an e-value cut-off of less than −10−5 to determine conservation of proteins across biomineralizing cnidarians. In addition, the presence of a signal peptide was investigated using SignalP3.0 server and repeated sequences detected by Radar [52].
(g). Immunolocalization
We focused on the taxonomically restricted proteins that have high similarity within Cnidaria but are absent in non-calcifying phyla. These were previously described as biomineralization toolkit genes [16–18] and may play specific roles in this process. In order to elucidate their putative role in coral biomineralization, six genes (four acidic proteins: CARPs 1–4, and two biomineralization toolkit genes: cadherin and actin) were selected for spatial localization analysis across the life-history stages.
Fixed larvae were embedded in paraffin and cross-sectioned (4 µm thickness). All immunohistochemistry was performed using a Ventana Medical Systems Discovery XT automated immunostainer. Custom polyclonal rabbit antibodies raised against conserved regions of CARPs 1–4, cadherin from S. pistillata [21] and commercially available β-actin (PA5-16914; Pierce) were applied at 1 : 800, 1 : 50, 1 : 200, 1 : 2000, 1 : 500 and 1 : 200 dilution, respectively, followed by application of pre-diluted universal secondary antibody (no. 760–4205; Ventana Medical Systems) and then a chromogenic detection kit (DABMap; no. 760–124; Ventana Medical Systems) [28]. Haematoxylin was used as a counterstain. Control experiments were performed similarly without the first antibody step and diluted as described above (electronic supplementary material, figure S4). Microscopic imaging was carried out with an upright epifluorescent microscope (Zeiss SteREO Discovery.V12) and field emission scanning electron microscopy with energy-dispersive X-ray spectrometry.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank L. Cong (histopathology and imaging services, Cancer Institute of New Jersey), N. Wagner, E.C. Peters, N. Sher, S. Murali and C. Vidito.
Data accessibility
The Pocillopora damicornis transcriptome data generated in this study are available for download at http://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA306839.
Authors' contributions
T.M., J.L.D. and H.M.P. carried out the laboratory and fieldwork, participated in data analysis, participated in the design of the study and drafted the manuscript; E.Z. carried out the bioinformatics analyses and helped draft the manuscript; P.G.F., D.B. and R.D.G. conceived of the project, designed and coordinated the study, and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was made possible by a grant from the National Science Foundation (EF-1416785) awarded to P.G.F., D.B. and T.M. and to H.M.P. (OCE-PRF-1323822).
References
- 1.Knoll AH. 2003. Biomineralization and evolutionary history. Rev. Miner.Geochem. 54, 329–356. ( 10.2113/0540329) [DOI] [Google Scholar]
- 2.Pochon X, Stat M, Takabayashi M, Chasqui L, Chauka LJ, Logan DDK, Gates RD. 2010. Comparison of endosymbiotic and free-living Symbiodinium (Dinophyceae) diversity in a Hawaiian reef environment. J. Phycol. 46, 53–65. ( 10.1111/j.1529-8817.2009.00797.x) [DOI] [Google Scholar]
- 3.Von Heider A. 1881. Die Gattung Cladocora Ehrenb. Sber. Akad. Wiss. Wien 84, 634–637. [Google Scholar]
- 4.Johnston IS. 1980. The ultrastructure of skeletogensis in hermatypic corals. Int. Rev. Cytol. 67, 171–214. ( 10.1016/S0074-7696(08)62429-8) [DOI] [Google Scholar]
- 5.Tambutte E, Allemand D, Zoccola D, Meibom A, Lotto S, Caminiti N, Tambutte S. 2007. Observations of the tissue–skeleton interface in the scleractinian coral Stylophora pistillata. Coral Reefs 26, 517–529. ( 10.1007/s00338-007-0263-5) [DOI] [Google Scholar]
- 6.Mitterer RM. 1978. Amino acid composition and metal binding capability of the skeletal protein of corals. Bull. Mar. Sci. 28, 173–180. [Google Scholar]
- 7.Isa Y, Okazaki M. 1987. Some observations on the Ca2+ binding phospholipid from scleractinian coral skeletons. Compar. Biochem. Physiol. B 87, 507–512. ( 10.1016/0305-0491(87)90045-9) [DOI] [Google Scholar]
- 8.Cuif JP, Dauphin Y, Doucet J, Salome M, Susini J. 2003. XANES mapping of organic sulfate in three scleractinian coral skeletons. Geochim. Cosmochim. Acta 67, 75–83. ( 10.1016/s0016-7037(02)01041-4) [DOI] [Google Scholar]
- 9.Addadi L, Joester D, Nudelman F, Weiner S. 2006. Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem. Eur. J. 12, 980–987. ( 10.1002/chem.200500980) [DOI] [PubMed] [Google Scholar]
- 10.Grasso L, Maindonald J, Rudd S, Hayward D, Saint R, Miller D, Ball E. 2008. Microarray analysis identifies candidate genes for key roles in coral development. BMC Genomics 9, 540 ( 10.1186/1471-2164-9-540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandermeulen JH. 1975. Studies on reef corals. III. Fine structural changes of calicoblast cells in Pocillopora damicornis during settling and calcification. Mar. Biol. 31, 69–77. ( 10.1007/BF00390649) [DOI] [Google Scholar]
- 12.Vandermeulen JH, Watabe N. 1973. Studies on reef corals. I. Skeleton formation by newly settled planula larva of Pocillopora damicornis. Mar. Biol. 23, 47–57. ( 10.1007/BF00394111) [DOI] [Google Scholar]
- 13.Grasso LC, Negri AP, Fôret S, Saint R, Hayward DC, Miller DJ, Ball EE. 2011. The biology of coral metamorphosis: molecular responses of larvae to inducers of settlement and metamorphosis. Dev. Biol. 353, 411–419. ( 10.1016/j.ydbio.2011.02.010) [DOI] [PubMed] [Google Scholar]
- 14.Reyes-Bermudez A, Lin Z, Hayward D, Miller D, Ball E. 2009. Differential expression of three galaxin-related genes during settlement and metamorphosis in the scleractinian coral Acropora millepora. BMC Evol. Biol. 9, 178 ( 10.1186/1471-2148-9-178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayward D, Hetherington S, Behm C, Grasso L, Foret S, Miller D, Ball E. 2011. Differential gene expression at coral settlement and metamorphosis—a subtractive hybridization study. PLoS ONE 6, e26411 ( 10.1371/journal.pone.0026411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake JL, Mass T, Haramaty L, Zelzion E, Bhattacharya D, Falkowski PG. 2013. Proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. Proc. Natl Acad. Sci. USA 110, 3788–3793. ( 10.1073/pnas.1301419110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos-Silva P, Kaandorp J, Huisman L, Marie B, Zanella-Cléon I, Guichard N, Miller DJ, Marin F. 2013. The skeletal proteome of the coral Acropora millepora: the evolution of calcification by cooption and domain shuffling. Mol. Biol. Evol. 30, 2099–2112. ( 10.1093/molbev/mst109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mass T, Drake JL, Haramaty L, Kim JD, Zelzion E, Bhattacharya D, Falkowski PG. 2013. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Curr. Biol. 23, 1126–1131. ( 10.1016/j.cub.2013.05.007) [DOI] [PubMed] [Google Scholar]
- 19.Zoccola D, et al. 2015. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 5, 9983 ( 10.1038/srep09983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya D, et al. In press. Basis for ecological success of reef-forming corals elucidated using comparative genomics. eLife ( ) [DOI] [PMC free article] [PubMed]
- 21.Mass T, Drake JL, Peters EC, Jiang W, Falkowski PG. 2014. Immunolocalization of skeletal matrix proteins in tissue and mineral of the coral Stylophora pistillata. Proc. Natl Acad. Sci. USA 111, 12 728–12 733. ( 10.1073/pnas.1408621111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helman Y, Natale F, Sherrell RM, LaVigne M, Starovoytov V, Gorbunov MY, Falkowski PG. 2008. Extracellular matrix production and calcium carbonate precipitation by coral cells in vitro. Proc. Natl Acad. Sci. USA 105, 54–58. ( 10.1073/pnas.0710604105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moya A, Tambutte S, Bertucci A, Tambutte E, Lotto S, Vullo D, Supuran CT, Allemand D, Zoccola D. 2008. Carbonic anhydrase in the scleractinian coral Stylophora pistillata—characterization, localization, and role in biomineralization. J. Biol. Chem. 283, 25 475–25 484. ( 10.1074/jbc.M804726200) [DOI] [PubMed] [Google Scholar]
- 24.Bertucci A, Tambutté S, Supuran C, Allemand D, Zoccola D. 2011. A new coral carbonic anhydrase in Stylophora pistillata. Mar. Biotechnol. 13, 992–1002. ( 10.1007/s10126-011-9363-x) [DOI] [PubMed] [Google Scholar]
- 25.Aizenberg J, Addadi L, Weiner S, Lambert G. 1996. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 8, 222–226. ( 10.1002/adma.19960080307) [DOI] [Google Scholar]
- 26.Sodek J, Ganss B, McKee MD. 2000. Osteopontin. Crit. Rev. Oral Biol. Med. 11, 279–303. ( 10.1177/10454411000110030101) [DOI] [PubMed] [Google Scholar]
- 27.Doi Y, Okuda R, Takezawa Y, Shibata S, Moriwaki Y, Wakamatsu N, Shimizu N, Moriyama K, Shimokawa H. 1989. Osteonectin inhibiting de novo formation of apatite in the presence of collagen. Calcif. Tissue Int. 44, 200–208. ( 10.1007/BF02556565) [DOI] [PubMed] [Google Scholar]
- 28.Shinzato C, et al. 2011. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476, 320–323. ( 10.1038/nature10249) [DOI] [PubMed] [Google Scholar]
- 29.Constantz B, Weiner S. 1988. Acidic macromolecules associated with the mineral phase of scleractinian coral skeletons. J. Exp. Zool. 248, 253–258. ( 10.1002/jez.1402480302) [DOI] [Google Scholar]
- 30.Mass T, Drake JL, Haramaty L, Rosenthal Y, Schofield OME, Sherrell RM, Falkowski PG. 2012. Aragonite precipitation by ‘Proto-polyps’ in coral cell cultures. PLoS ONE 7, e35049 ( 10.1371/journal.pone.0035049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarashina I, Endo K. 2006. Skeletal matrix proteins of invertebrate animals: comparative analysis of their amino acid sequences. Paleontol. Res. 10, 311–336. ( 10.2517/prpsj.10.311) [DOI] [Google Scholar]
- 32.Drake JL, Mass T, Falkowski PG. 2014. The evolution and future of carbonate precipitation in marine invertebrates: witnessing extinction or documenting resilience in the Anthropocene? Elementa 2, 000026 ( 10.12952/journal.elementa.000026) [DOI] [Google Scholar]
- 33.Maurer P, Hohenester E, Engel J. 1996. Extracellular calcium-binding proteins. Curr. Opin. Cell Biol. 8, 609–617. ( 10.1016/S0955-0674(96)80101-3) [DOI] [PubMed] [Google Scholar]
- 34.Kretsinger RH. 1976. Calcium-binding proteins. Annu. Rev. Biochem. 45, 239–266. ( 10.1146/annurev.bi.45.070176.001323) [DOI] [PubMed] [Google Scholar]
- 35.Treccani L, Mann K, Heinemann F, Fritz M. 2006. Perlwapin, an abalone nacre protein with three four-disulfide core (Whey Acidic Protein) domains, inhibits the growth of calcium carbonate crystals. Biophys. J. 91, 2601–2608. ( 10.1529/biophysj.106.086108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerbaud V, et al. 2000. Mechanism of calcite crystal growth inhibition by the N-terminal undecapeptide of lithostathine. J. Biol. Chem. 275, 1057–1064. ( 10.1074/jbc.275.2.1057) [DOI] [PubMed] [Google Scholar]
- 37.Politi Y, Mahamid J, Goldberg H, Weiner S, Addadi L. 2007. Asprich mollusk shell protein: in vitro experiments aimed at elucidating function in CaCO3 crystallization. CrystEngComm 9, 1171–1177. ( 10.1039/B709749B) [DOI] [Google Scholar]
- 38.Fang D, Pan C, Lin HJ, Lin Y, Zhang GY, Wang HZ, He MX, Xie LP, Zhang RQ. 2012. Novel basic protein, PfN23, functions as key macromolecule during nacre formation. J. Biol. Chem. 287, 15 776–15 785. ( 10.1074/jbc.M112.341594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blank S, Arnoldi M, Khoshnavaz S, Treccani L, Kuntz M, Mann K, Grathwohl G, Fritz M. 2003. The nacre protein perlucin nucleates growth of calcium carbonate crystals. J. Microsc. 212, 280–291. ( 10.1111/j.1365-2818.2003.01263.x) [DOI] [PubMed] [Google Scholar]
- 40.Killian CE, Wilt FH. 1996. Characterization of the proteins comprising the integral matrix of Strongylocentrotus purpuratus embryonic spicules. J. Biol. Chem. 271, 9150–9159. ( 10.1074/jbc.271.15.9150) [DOI] [PubMed] [Google Scholar]
- 41.Wilt FH, Killian CE, Livingston BT. 2003. Development of calcareous skeletal elements in invertebrates. Differentiation 71, 237–250. ( 10.1046/j.1432-0436.2003.7104501.x) [DOI] [PubMed] [Google Scholar]
- 42.Gorski J. 1992. Acidic phosphoproteins from bone matrix: a structural rationalization of their role in biomineralization. Calcif. Tissue Int. 50, 391–396. ( 10.1007/BF00296767) [DOI] [PubMed] [Google Scholar]
- 43.Kawasaki K, Buchanan AV, Weiss KM. 2009. Biomineralization in humans: making the hard choices in life. Annu. Rev. Genet. 43, 119–142. ( 10.1146/annurev-genet-102108-134242) [DOI] [PubMed] [Google Scholar]
- 44.Marin F, Luquet G, Marie B, Medakovic D. 2007. Molluscan shell proteins: primary structure, origin, and evolution. In Current topics in developmental biology (ed. Gerald PS.), pp. 209–276. New York, NY: Academic Press. [DOI] [PubMed] [Google Scholar]
- 45.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. 1981. Osteonectin, a bone-specific protein linking mineral to collagen. Cell 26, 99–105. ( 10.1016/0092-8674(81)90037-4) [DOI] [PubMed] [Google Scholar]
- 46.Jokiel PL. 1985. Lunar periodicity of planula release in the reef coral Pocillopora damicornis in relation to various environmental factors. In Proc. 5th Int. Coral Reef Congress, Tahiti, 27 May–1 June 1985, Vol. 4: Symposia and Seminars (B) (eds C Gabrie, B Salvat), pp. 307–312.
- 47.Putnam HM, Mayfield AB, Fan TY, Chen CS, Gates RD. 2013. The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2. Mar. Biol. 160, 2157–2173. ( 10.1007/s00227-012-2129-9) [DOI] [Google Scholar]
- 48.Pertea G, et al. 2003. TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics 19, 651–652. ( 10.1093/bioinformatics/btg034) [DOI] [PubMed] [Google Scholar]
- 49.Li W, Godzik A. 2006. CD-HIT: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659. ( 10.1093/bioinformatics/btl158) [DOI] [PubMed] [Google Scholar]
- 50.Fu L, Niu B, Zhu Z, Wu S, Li W. 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152. ( 10.1093/bioinformatics/bts565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21. ( 10.1186/s13059-014-0550-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emanuelsson O, Brunak S, von Heijne G, Nielsen H. 2007. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protocols 2, 953–971. ( 10.1038/nprot.2007.131) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Pocillopora damicornis transcriptome data generated in this study are available for download at http://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA306839.