Abstract
Despite the importance of host attributes for the likelihood of associated microbial transmission, individual variation is seldom considered in studies of wildlife disease. Here, we test the influence of host phenotypes on social network structure and the likelihood of cuticular bacterial transmission from exposed individuals to susceptible group-mates using female social spiders (Stegodyphus dumicola). Based on the interactions of resting individuals of known behavioural types, we assessed whether individuals assorted according to their behavioural traits. We found that individuals preferentially interacted with individuals of unlike behavioural phenotypes. We next applied a green fluorescent protein-transformed cuticular bacterium, Pantoea sp., to individuals and allowed them to interact with an unexposed colony-mate for 24 h. We found evidence for transmission of bacteria in 55% of cases. The likelihood of transmission was influenced jointly by the behavioural phenotypes of both the exposed and susceptible individuals: transmission was more likely when exposed spiders exhibited higher ‘boldness’ relative to their colony-mate, and when unexposed individuals were in better body condition. Indirect transmission via shared silk took place in only 15% of cases. Thus, bodily contact appears key to transmission in this system. These data represent a fundamental step towards understanding how individual traits influence larger-scale social and epidemiological dynamics.
Keywords: bacterial transmission, boldness, social network analysis, assortativity, social spider, transmission heterogeneity
1. Background
The transmission of microorganisms from exposed to susceptible hosts represents one of the most crucial forces regulating infectious disease dynamics. Recent attention [1,2], however, has scrutinized historical attempts to quantify and predict transmission dynamics. For example, a foundational principle of epidemiology, ‘the mass action principle’, relies on the assumption that the course of an epidemic is determined by the rate of random contacts between infected and susceptible individuals [3]. Of course, populations are not comprised individuals who interact randomly, and interactions during epidemics do not always result in transmission [4]. Variation in host behavioural phenotypes and social interactions remain putative explanations of the immense transmission heterogeneity observed in human and wildlife diseases [5–7]. Consequently, an important topic of current discourse in disease ecology has focused on understanding how consistent behavioural variation among individuals (i.e. behavioural types, syndromes or personality [8,9]) may influence the dynamics of microbial transmission [10].
Many of the most prevalent and deadly diseases threatening wildlife and human populations are characterized by intense variation in the degree to which infected individuals produce secondary cases of infection [5]. In fact, host heterogeneity in infection susceptibility [11] and pathogen transmission [5,6,12] are becoming increasingly emphasized in disease ecology and epidemiology. In addition, a group's behavioural composition may also have consequences for the spread of infectious agents [13]. A recent study on behavioural variation and disease dynamics in house finches found that an individuals' propensity to use experimental feeders increased both the acquisition and transmission of a bacterial pathogen [14]. Such studies are sorely needed because they employ experimental infections and characterize interindividual variation in behaviours that are predicted to influence transmission dynamics.
Variation among individuals in how frequently and with whom they interact has important implications for epidemiological dynamics. For example, individuals that interact with others more frequently can have an inordinately large effect on driving the spread of an epidemic [5]. Both individual-level (e.g. infection status) and group-wide processes (e.g. disease outbreaks) are influenced by the connectivity of individuals [15–17]. The incorporation of social network theory into models of disease dynamics has shown that the structure of the interaction network and the processes that underlies its formation can affect transmission rate [18–20]. Therefore, empirical studies of the processes that drive social network formation are crucial for our understanding of disease dynamics. A common mechanism of network formation is assortativity according to a certain trait, that is, individuals often tend to interact with others of similar phenotypes [21]. Just as common is the complementary mechanism, disassortativity, where individuals tend to interact with individuals of opposing phenotypes [22,23]. These interaction preferences will shape network structure and may influence the transmission rate of microbes among individuals [7,18].
Some prior studies have identified natural relationships between host personality type and infection status [24–26], though fewer studies have experimentally differentiated behaviour-mediated infection from parasite manipulation of behaviour (notable exceptions include: [27,28]) (reviewed in [29]). Others report evidence for associations between host behavioural traits and virus transmission [30], and experimental infections of laboratory animals have generated enormous variation in pathogen shedding rates associated with host traits like co-infection status and immunocompetence [31,32]. Despite these advances, studies rarely, if ever, evaluate the role of behavioural phenotypes in both infected and susceptible individuals simultaneously to test their joint effects on multiple modes of transmission.
Here, we examine how variation among individuals in consistent behavioural traits influences contact network formation and bacterial transmission in the social spider Stegodyphus dumicola (Araneae, Eresidae). In colonies of S. dumicola, the execution of collective behaviours such as prey capture [33–36] and antipredator defences [37] is associated with heterogeneity among individuals in their boldness, suggesting that boldness is a reliable indicator of individuals' role in these societies. Therefore, we examined assortativity according to boldness by observing marked individuals in experimental colonies. To examine how the boldness of both exposed and susceptible individuals influences the likelihood of direct and indirect (i.e. environmental) transmission, we experimentally exposed individual spiders with a green fluorescent protein (GFP)-transformed cuticular bacterium. We focused on cuticular bacteria, because the integument represents the barrier between the host and constant bombardment by microbes from its environment, representing a primary line of defence against invading microbes, and body contact represents a likely mode of transmission for cuticle-associated microbes [38,39]. We hypothesize that cuticular bacterial transmission will be more likely to occur between individuals who are more likely to interact in observed contact networks. That is, if colony contact networks are assortative, then cuticular bacterial transmission should be more likely between similar individuals. In contrast, if colony networks are disassortative, then transmission should be more likely to occur between dissimilar individuals. Lastly, we hypothesize that cuticular bacteria will be primarily transmitted through direct interactions (i.e. bodily contact) rather than indirectly through the environment.
2. Material and methods
(a). Animal collection and maintenance
Stegodyphus dumicola is a southwestern African social spider that lives in age-structured colonies of up to several hundred individuals that exhibit cooperative behaviours and alloparental care, and spend the majority of their time in close contact with colony-mates, either in the colony retreat or during co-feeding [40–43]. We collected 16 S. dumicola colonies along roadside Acacia trees in the Northern Cape of South Africa in January 2015. After transport to the laboratory, individual adult females were isolated into 30 ml plastic cups containing a piece of chicken wire to facilitate web-building. Only adult female spiders were used in the present study. Spiders were each fed one two-week-old cricket weekly until the onset of behavioural assays.
(b). Behavioural assays
In Stegodyphus, ‘boldness' (the latency to resume movement after experiencing an aversive stimulus [44]) and aggressiveness are highly consistent behavioural metrics (repeatability ≈0.63 and 0.55, respectively; [45]) and are linked with an individual's propensity to participate in a variety of collective tasks [45–48].
To determine individuals' boldness, we subjected them to an antipredator behaviour assay developed by Riechert & Hedrick [49]. The spider is placed in a clear plastic arena (12 cm diameter), given a 30 s acclimation period, and then administered two rapid puffs of air to the anterior prosoma using an infant nose-cleaning bulb which causes them to ‘huddle’ by halting movement and pulling the legs close to the body. We then measured the latency for spiders to ‘unhuddle’ and move one full body length. ‘Bold’ individuals unhuddle and resumed movement more quickly, whereas ‘shy’ individuals have longer latencies to resume activity. We subtract the latency for a spider to resume movement from the maximum latency allowed (600 s) such that a higher boldness score represents bolder behaviour.
For the bacterial transmission experiments, we also assessed individuals' aggressiveness by placing the spider in a plastic arena (12 cm diameter), allowing it a 30 s acclimation period, and then prodding their foremost left leg with a blunt metal probe. We scored their immediate response to this stimulus with a nominal categorization described previously [45,46]. ‘Non-aggressive’ behaviours included a ‘huddle’ response and moving away from stimulus, whereas ‘aggressive’ behaviours included turning or walking towards the stimulus, raising their anterior legs and shifting the abdomen in place. Aggressiveness assays took place the same day as boldness assays, approximately 6 h later.
(c). Social interactions
We observed interactions in laboratory colonies to determine whether individuals assort according to boldness and whether there is a relationship between boldness and the number of associates of resting spiders. Nine colonies of 10–30 adult female spiders of known boldness (see boldness assay above), and individually marked with acrylic paint dots atop their dorsal abdomen, were kept in plastic containers with chicken wire that allowed them to build a retreat and a capture web. Each experimental colony was constructed from a different source colony, without mixing individuals from multiple source colonies. These spiders spend much of their time resting in the colony retreat, often in groups. We defined interactions between resting group members as a physical contact between any body parts of two spiders. We manually noted the resting interaction patterns of all individuals in each colony two to four times a week (figure 1). Repeated observations of the same colony occurred either on different days or on the same day if the colony had an opportunity to re-assort. For example, an observation was conducted before a colony was fed or provided with water and, if the colony responded to the prey or water, another resting network observation was conducted after the collective response ended and the spiders resumed resting (electronic supplementary material, table S1).
Figure 1.
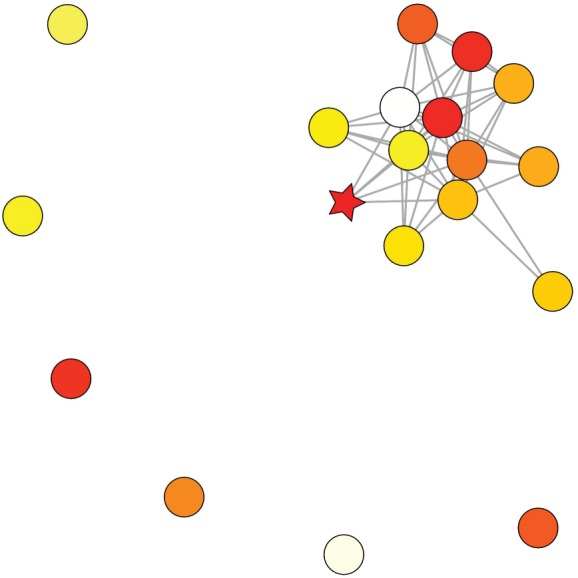
Interaction patterns among 19 spiders in an experimental colony of marked individuals. Darker colours represent bolder individuals and the boldest individual in the group is denoted by a star. The assortativity value of this network is −0.1 which is more disassortative than random at a probability of 0.0001. That is, individuals were more likely to interact with others of different boldness phenotypes than expected at random. (Online version in colour.)
(d). Bacterial exposure
We used electroporation [50] to transform a strain of Pantoea (CNK01) collected from the cuticle of an adult female spider in the field in January 2014 (methods described in Keiser et al. 2016 – in Current Zoology) with the pGLO plasmid (BioRad, Hercules, CA) that encodes β-lactamase (conferring ampicillin resistance) and green fluorescent protein (henceforth CNK02; electronic supplementary material, text S1). Experimental bacterial cultures were prepared by selecting a single colony of CNK02 and growing it in 1 ml LB broth supplemented with 100 µg ml−1 ampicillin and 20% arabinose (‘LB amp/ara’; electronic supplementary material, text S2) for 15 h with agitation. This solution was vortexed at 2500 r.p.m. for 25 min, washed with 1 ml phosphate-buffered saline (PBS, pH 7.4; Sigma-Aldrich, St Louis, MO 63103), and then diluted in 1 ml PBS. Ninety spiders marked with a blue paint dot were placed in 1 ml of liquid culture of CNK02 at approximately 109 CFU ml−1, and shaken at 1500 r.p.m. for 3 s with a vortex to disrupt the hydrophobic barrier of the spider's cetae. These bacteria remain viable and detectable on the cuticles of spiders at least 72 h after exposure (electronic supplementary material, text S3). Henceforth, we will refer to experimentally exposed individuals as ‘exposed’ and those that were exposed only to PBS and marked with a green paint dot as ‘susceptible’. Prior to bacterial exposure, we measured each spider's mass and prosoma width. All bacterial exposures were carried out by the same two researchers (C.N.K. and D.A.A.) to minimize methodological inconsistencies.
(e). Transmission experiments
To test for the transmission of bacteria between exposed and susceptible individuals when allowed to interact directly (i.e. via cuticle-to-cuticle contact), we exposed one spider as described above, and allowed it 24 h in its housing container to dry. We then transferred the exposed individual to the housing container of a susceptible individual with a different boldness value than the exposed individual, and allowed them to interact naturally for 24 h (n = 66 pairs). The identities of the spiders chosen for each pair were chosen using a random-number generator, and paired spiders always originated from the same source colony. Care was taken to place the exposed individual away from the susceptible individual in its home container to allow natural contact between spiders. After 24 h, we removed the susceptible individual and vortexed it in 1 ml sterile LB amp/ara broth for 10 s. We removed the spider, transferred 40 µl of this solution onto LB amp/ara agar, and incubated this plate and the remaining solution (960 µl) at 30°C for 20 h. We visually counted the number of LB amp/ara broth solutions that fluoresced under a long-wave UV light pen (BioRad) to assess successful transmission, and counted the number of colony forming units (CFUs) that grew and fluoresced on each LB amp/ara plate to approximate the relative bacterial load that had been transferred to susceptible individuals. We performed a series of side experiments to verify that (i) the pGLO plasmid is required to observe fluorescence from spider-collected bacterial cultures, (ii) that amplification at 30°C is required to visually detect fluorescence from GFP-transformed Pantoea collected from spiders, (iii) that 20 h of amplification under 30°C is sufficient to detect fluorescence of bacteria collected from bold and shy spiders and that (iv) fluorescent bacteria are detectable on the cuticles of spiders for at least 72 h after exposure (electronic supplementary material, text S3).
To test for the occurrence of bacterial transmission from spider to spider indirectly via silk, we exposed a spider to CNK02 as above and allowed it 24 h to dry in its housing container. During this time, a susceptible individual was housed in a different container and allowed to build a web. We then removed the susceptible individual and transferred an exposed individual to its housing container. We allowed the exposed individual to interact with the silk of the susceptible individual for 24 h (n = 33 pairs). After 24 h, we removed the exposed individual, replaced it with the susceptible individual, and allowed it to interact with the exposed silk for 24 h. Then, we removed the susceptible individual and tested for the presence of CNK02 on its cuticle as described above. We also gathered the silk from the susceptible individuals' containers with sterile wooden rods and placed them in LB amp/ara at 30°C for 20 h to test for the presence of viable CNK02 on the silk.
(f). Statistical analyses
(i). Social interactions
To determine assortativity according to boldness of the resting networks, we used the igraph package in R [51]. Positive assortativity values indicate assortative networks (i.e. homophily) and negative assortativity values indicate disassortative networks. To determine whether the observed assortativity values are different than expected at random, we created 10 000 randomized networks for each observed network maintaining the connectivity (degree) of each individual (node) and permuting who it interacted with. We then calculated the probability that an observed assortativity value differed from the 10 000 randomized assortativity values as the proportion of cases in which the absolute value of the observed assortativity was smaller than the absolute value of the randomized assortativity. To determine whether the difference between observed and randomized assortativity values was statistically significant, we compared the average assortativity of all observed networks with the average assortativity of each of the 10 000 sets of randomized networks. We deemed the observed assortativity significantly different from random if the absolute value of the observed average assortativity was smaller than the absolute value of the average randomized assortativity in less than 0.05 of the 10 000 randomizations. We examined the relationship between connectivity (degree) and boldness in each interaction network using Pearson's correlation and Bonferroni correction for multiple hypotheses testing which set the significant p-value at 0.002.
(ii). Transmission experiments
We used two nominal logistic regressions and one GLMM with a log link function with identical independent variables to test for transmission via direct interaction, indirect transmission and the number of CFUs ml−1 collected from susceptible individuals in the direct interaction experiment, respectively. The independent variables in each model were the difference in boldness between the two individuals, the aggressiveness of the exposed individual, the aggressiveness of the susceptible individual, the body condition of the susceptible individual and the body condition of the exposed individual. Values for the difference in boldness between exposed and susceptible individuals ranged from −600 to 600 (electronic supplementary material, figure S2). Body condition was estimated using the residuals of a linear regression of individual body mass and body size [52]. We treated aggressiveness as a categorical variable here (following the methods of [45]). In a supplemental analysis (electronic supplementary material, text S4), we treated aggressiveness as an ordinal variable (following the methods of [46]), and used a ‘difference in aggressiveness' value for each spider pair. We tested for correlations between measures of boldness and aggressiveness with nominal logistic regression. Experimental pair ID nested in source colony ID was included as a random effect in each model.
3. Results
(a). Social interactions
Observed networks were significantly more disassortative than expected at random. The observed average assortativity value was significantly smaller than that obtained from 10 000 randomizations (figure 2). Of the 36 resting interaction networks, 35 exhibited disassortative mixing, where individuals preferentially interacted with individuals of different boldness than their own. The probability that an observed assortativity value differed from the randomized assortativity for each network is provided in electronic supplementary material, table S1. We did not detect a significant relationship between the boldness of an individual and the number of individuals it contacted while resting in any of the interaction networks (electronic supplementary material, table S1).
Figure 2.
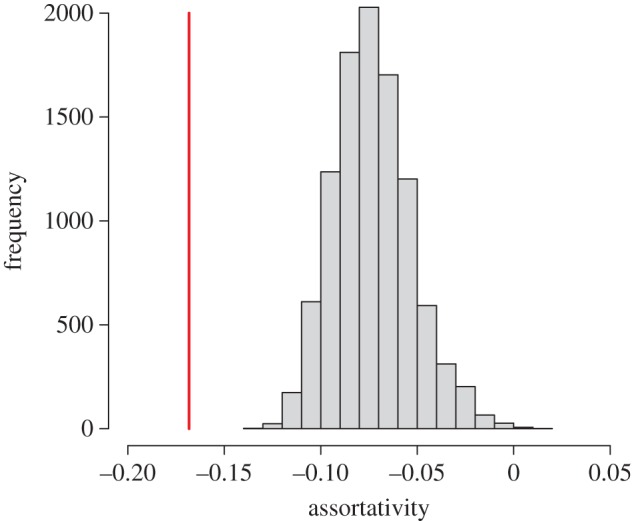
Average assortativity of all observed networks (red vertical line) is significantly smaller than the average assortativity of each of the 10 000 sets of randomized networks (histogram). (Online version in colour.)
(b). Bacterial transmission
Transmission of cuticular bacteria via direct interaction was influenced by the boldness of both the exposed and susceptible individuals. In the direct contact experiment, we detected the presence of CNK02 on the cuticles of 36/66 susceptible spiders (55%) that were allowed to interact with exposed spiders (figure 3). We detected interindividual transmission of cuticular bacteria in 20/29 (69%) of the cases where the exposed individual was bolder, compared with 15/36 (42%) of the cases where the susceptible individual was bolder (figure 4a). We found evidence that interindividual transmission was more likely to occur when exposed spiders had higher boldness than their paired susceptible individual (nominal logistic regression: χ2 = 7.58, d.f. = 1, p = 0.006; table 1 and figure 4b). Further, an additional analysis verified that the absolute value of the difference in boldness between spiders did not predict the likelihood of transmission (electronic supplementary material, text S4). Transmission was also more likely when susceptible individuals were in better body condition, that is, they weighed more than predicted based on their body size (nominal logistic regression: χ2 = 5.70, d.f. = 1, p = 0.02; table 1 and figure 4c). The aggressiveness of the exposed individual did not predict the likelihood of bacterial transmission (nominal logistic regression: p = 0.25; table 1), though there was a non-significant trend for a greater incidence of bacterial transmission to occur with more aggressive susceptible spiders (nominal logistic regression: p = 0.06, table 1). Aggressiveness was not correlated with boldness in this study (nominal logistic regression: χ2 = 5.39, d.f. = 8; p = 0.72), although a negative relationship between these traits has been described previously [45]. Although not influenced by any independent variables (nominal logistic regression, all p > 0.07; table 1), we estimated a vast range from 25 to 16 100 CFUs ml−1 of CNK02 on the cuticles of susceptible spiders after having cohabitated with an exposed individual (26 samples, x̄ = 3513 CFUs ml−1, s.d. = 5161).
Figure 3.
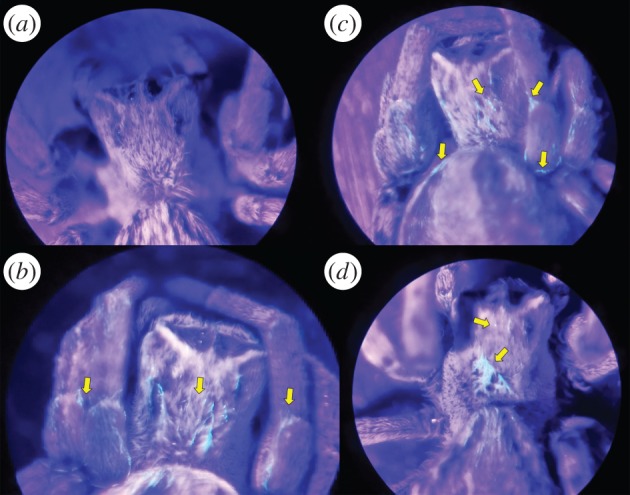
Spiders under long-wave UV light: (a) exposed to sterile PBS (control); (b) 4 h after exposure to CNK02; and (c) 48 h after exposure to CNK02. (d) An unexposed spider that has interacted with an exposed spider for 24 h. Areas of green fluorescence, suggesting the presence of viable CNK02 cells, are pointed out with arrows. (Online version in colour.)
Figure 4.
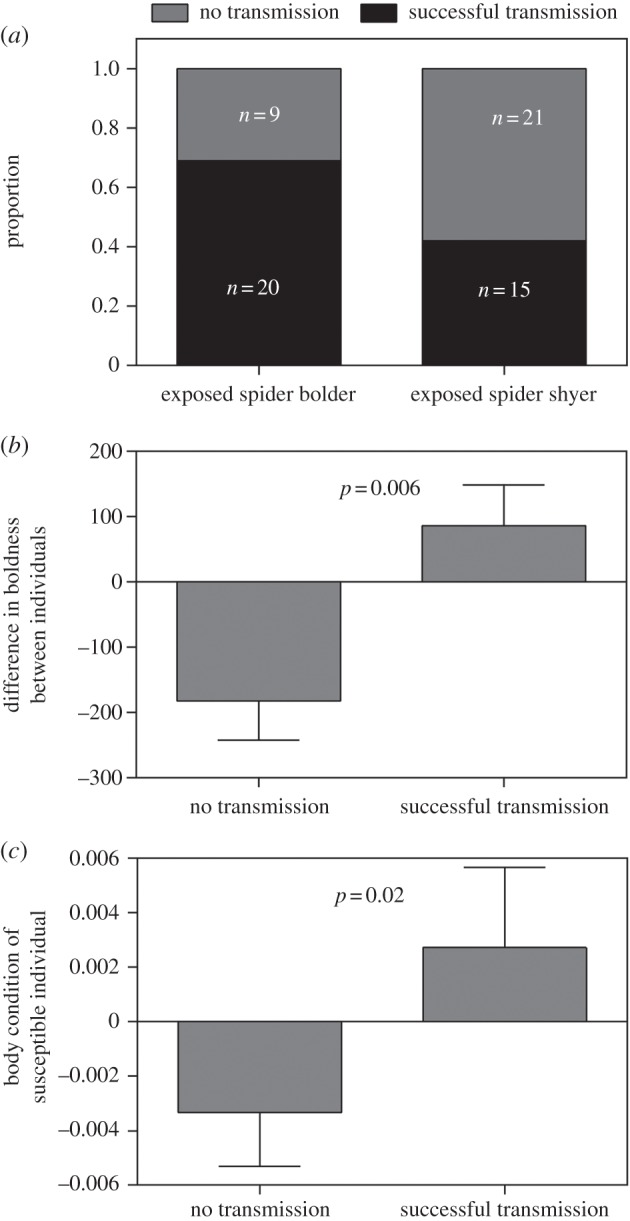
Direct transmission of cuticular bacteria is influenced by the phenotypes of both exposed and susceptible individuals. (a) A greater proportion of pairs in which the exposed individual was bolder resulted in successful transmission and (b) transmission was more likely when exposed individuals were bolder than their susceptible colony-mates (positive values denote a bolder exposed individual, while negative values denote a bolder susceptible). (c) Transmission was also more likely when susceptible individuals had a positive body condition, i.e. weighed more than predicted based on their body size.
Table 1.
(a,b) Two nominal logistic regressions predicting the presence or absence of CNK02 on the cuticles of susceptible individuals after interacting with exposed individuals directly (i.e. co-habitating) or indirectly (i.e. only in contact with the same substrate 1 day later). (c) A GLMM predicting the estimated bacterial load collected from the cuticles of susceptible spiders after directly interacting with exposed spiders. Effects with significant p-values are denoted with an asterisk.
| effect | d.f. | χ2 | p-value |
|---|---|---|---|
| (a) transmission after direct interaction | |||
| difference in boldness between individuals | 1 | 7.58 | 0.006** |
| aggressiveness of exposed individual | 7 | 9.00 | 0.25 |
| aggressiveness of susceptible individual | 9 | 16.74 | 0.06 |
| body condition of susceptible individual | 1 | 5.70 | 0.02* |
| body condition of exposed individual | 1 | 0.51 | 0.47 |
| (b) transmission after indirect interaction | |||
| difference in boldness between individuals | 1 | 0.53 | 0.46 |
| aggressiveness of exposed individual | 6 | 10.58 | 0.10 |
| aggressiveness of susceptible individual | 7 | 8.63 | 0.28 |
| body condition of susceptible individual | 1 | 0.81 | 0.37 |
| body condition of exposed individual | 1 | 0.39 | 0.53 |
| (c) estimated bacterial load transmitted (direct interaction) | |||
| difference in boldness between individuals | 1 | 0.42 | 0.52 |
| aggressiveness of exposed individual | 6 | 9.30 | 0.16 |
| aggressiveness of susceptible individual | 5 | 6.95 | 0.22 |
| body condition of susceptible individual | 1 | 0.37 | 0.54 |
| Body condition of exposed individual | 1 | 3.33 | 0.07 |
*p < 0.05, **p < 0.01.
We detected CNK02 on the cuticles of susceptible spiders in only 5/33 cases (15%) when exposed spiders interacted with a susceptible spider's silk alone and never with the susceptible spider directly. We did, however, detect CNK02 on the silk with which exposed spiders interacted in 12/33 cases (36%). Thus, 5/12 cases (42%) where the silk became contaminated with CNK02 resulted in exposure to the susceptible individual. Evidence of indirect transmission was not influenced by the traits of either the exposed or susceptible individual (logistic regression: all p > 0.10; table 1).
4. Discussion
Interindividual variation in behaviour and contact networks of infected and susceptible individuals have vast consequences for many emerging diseases in wildlife [53] and humans [5]. Here, we did not find support for our original hypothesis, but rather found that S. dumicola contact networks are behaviourally disassortative, and that the transmission of cuticular bacteria is more likely when exposed individuals were bolder than their susceptible colony-mates. Thus, under some conditions, this system might be poised for rapid and widespread transmission of cuticular microbes, harmful or otherwise. Presuming that at least a subset of resident cuticular bacteria can be harmful under some circumstances, as is the case for this species [54], the observed social network pattern may help to explain the high incidence of idiopathic colony extinction in S. dumicola and other species of social spiders [41,55,56].
(a). Network patterns and bacterial transmission
In our observed spider contact networks, behavioural disassortativity was more prevalent than expected at random. Many animal and human networks, however, are characterized by positive assortativity, (i.e. homophily) [22,23,57], where individuals preferentially interact with others like themselves. It has been suggested that infectious agents spread more slowly when hosts engage in disassortative networks [22]. For instance, simulated outbreaks of foot and mouth disease in livestock are shortened owing to disassortative contacts [58]. Additionally, individuals often tend to avoid visibly infected conspecifics [59] whose recognition may be heightened via their altered behaviours, further reducing the likelihood of transmission. Whether, and to what degree, our observed social interaction patterns in S. dumicola would afford colonies reduced overall transmission among individuals (i.e. a form of ‘social immunity’; [20,60,61]), or alternatively, enhanced susceptibility to transmission is yet unresolved; we discuss both possibilities below.
Our data suggest that the ability of the disassortative nature of these spiders' interactions to prevent or facilitate the transmission of cuticular microbes would depend on the traits of the exposed and susceptible individuals. Transmission was more likely when there was a difference in the boldness of the exposed versus susceptible individual, but more so in one direction. When the exposed individual was bolder than the susceptible individual, transmission was more likely (69%). Conversely, when the susceptible individual was bolder than the exposed individual, the incidence of transmission was lower (42%). Thus, disassortative contact networks in S. dumicola colonies could, depending on the situation considered, either intensify or reduce the incidence of bacterial transmission throughout the colony. If the index case (aka ‘patient zero’) for a transmission event is a bold individual, this could beget rapid transmission to shyer colony members, which is the more common behavioural phenotype within these societies. However, if the index case is a shy individual, bacterial transmission to bolder individuals could be constrained. Although reduced, the observed incidence of transmission from shyer to bolder spiders was still considerable, and colony-wide bacterial transmission would not be completely quelled if the index case were a shy spider.
The aggregate effects that these interaction networks and patterns of transmission have on colony performance would thus depend on whether bold individuals or shy individuals are more likely to be the index case, and whether different behavioural phenotypes are differentially likely to encounter, and become colonized by, novel environmental microbes. In S. dumicola, the available data suggest that bold individuals more readily interact with the environment outside of the colony's nest during foraging [33,62], suggesting that these individuals may be the pathway by which environmental microbes are subsequently transmitted to shy colony-mates.
(b). Transmission through contact versus via shared silk
Notably, we found that the incidence of transmission was greater when individuals were allowed to interact directly compared to cases where individuals only interacted indirectly via shared experience with the same silk. This suggests an important role of body contact or affiliative behavioural interactions in the transmission of cuticular bacteria. A recent experiment using freshwater snails demonstrated that bodily contact is a major determinant of the dispersal of a defensive symbiont from ‘donor’ to ‘receiver’ hosts, and similar (in terms of trait disassortativity) to the data presented here, the degree of transmission was greater when donor hosts were larger than their unexposed receivers, with the opposite being true if receivers were larger than donors [63]. This highlights the need to test the generality of trait disassortativity and transmission across many different host–symbiont systems.
(c). Future directions
For studies of wildlife disease, variation in the behavioural traits of infected and susceptible individuals is rarely explored in conjunction, despite ample evidence of its importance from the biomedical and human epidemiological fields. More comprehensive experiments should test how syndromes of behavioural traits can combine to influence the likelihood of individuals' acquisition, colonization and transmission of microbes (i.e. ‘behavioural competence’; [64]). Future experiments could also incorporate how the composition of resident cuticular microbial communities can combine with behavioural traits to drive the likelihood of bacterial colonization and transmission [65,66]. More specifically in regards to this system, it would be informative to identify the role that males play in social contact networks, including sexual interactions, and their influence on bacterial transmission. Although colony sex ratios are strongly female-biased [39], males could have a high impact on bacterial transmission if they are highly interactive and/or susceptible to exposure. Further, it would be informative to observe matched pairs of exposed and susceptible individuals and record the frequency and nature of their interactions to determine if bacteria are transmitted simply by bodily contact or if other affiliative/aggressive interactions are linked to transmission. Our data here verify that the behavioural traits of exposed and susceptible individuals jointly influence the likelihood of interindividual transmission of cuticular bacteria, and represent a fundamental step towards understanding how individual traits can explain larger-scale epidemiological processes. These data thus reinforce the growing sentiment that comprehensive models of epidemiological processes must account for behavioural variation at the level of the individual.
Supplementary Material
Acknowledgements
We thank the South Africa Department of Tourism, Environment, and Conservation for providing permits for animal collection (FAUNA 1690/2014). We thank Jenna Zalewski and Andy VanDemark for assistance with preparing electrocompetent cells. We thank Reut Berger-Tal, and Andrew Le for assistance in collecting interaction data for the network analysis.
Data accessibility
All data associated with this manuscript have been deposited at Dryad digital repository: http://dx.doi.org/10.5061/dryad.60nn7.
Author's contributions
C.N.K. transformed the bacteria under the advisement of J.G.L. and performed statistical analyses; C.N.K., D.A.A. and M.J.Z. carried out experiments; N.P.W. and L.H. performed colony observations and network analyses; J.N.P. assisted in experimental design and manuscript preparation. All authors gave final approval for publication.
Competing interests
We declare that we have no competing interests.
Funding
Funding for this research was provided by the University of Pittsburgh and the National Science Foundation IOS grants 1352705 and 1455895 to J.N.P. and 1456010 to N.P.W.
References
- 1.Begon M, Bennett M, Bowers RG, French NP, Hazel S, Turner J. 2002. A clarification of transmission terms in host–microparasite models: numbers, densities and areas. Epidemiol. Infect. 129, 147–153. ( 10.1017/S0950268802007148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCallum H, Barlow N, Hone J. 2001. How should pathogen transmission be modelled? Trends Ecol. Evol. 16, 295–300. ( 10.1016/S0169-5347(01)02144-9) [DOI] [PubMed] [Google Scholar]
- 3.Regoes RR, Hottinger JW, Sygnarski L, Ebert D. 2003. The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass-action principle. Epidemiol. Infect. 131, 957–966. ( 10.1017/S0950268803008793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blyton MDJ, Banks SC, Peakall R, Lindenmayer DB, Gordon DM. 2014. Not all types of host contacts are equal when it comes to E. coli transmission. Ecol. Lett. 17, 970–978. ( 10.1111/ele.12300) [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz W. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359. ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolhouse ME, et al. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338–342. ( 10.1073/pnas.94.1.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White LA, Forester JD, Craft ME. 2015. Using contact networks to explore mechanisms of parasite transmission in wildlife. Biol. Rev. ( 10.1111/brv.12236) [DOI] [PubMed] [Google Scholar]
- 8.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 9.Barber I, Dingemanse NJ. 2010. Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088. ( 10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kortet R, Hedrick AV, Vainikka A. 2010. Parasitism, predation and the evolution of animal personalities. Ecol. Lett. 13, 1449–1458. ( 10.1111/j.1461-0248.2010.01536.x) [DOI] [PubMed] [Google Scholar]
- 11.Dwyer G, Elkinton JS, Buonaccorsi JP. 1997. Host heterogeneity in susceptibility and disease dynamics: tests of a mathematical model. Am. Nat. 150, 685–707. ( 10.1086/286089) [DOI] [PubMed] [Google Scholar]
- 12.Galvani AP, May RM. 2005. Epidemiology: dimensions of superspreading. Nature 438, 293–295. ( 10.1038/438293a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pike TW, Samanta M, Lindström J, Royle NJ. 2008. Behavioural phenotype affects social interactions in an animal network. Proc. R. Soc. B 275, 2515–2520. ( 10.1098/rspb.2008.0744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adelman JS, Moyers SC, Farine DR, Hawley DM. 2015. Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc. R. Soc. B 282, 20151429 ( 10.1098/rspb.2015.1429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sih A, Hanser SF, McHugh KA. 2009. Social network theory: new insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 63, 975–988. ( 10.1007/s00265-009-0725-6) [DOI] [Google Scholar]
- 16.Gardy JL, et al. 2011. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N. Engl J. Med. 364, 730–739. ( 10.1056/NEJMoa1003176) [DOI] [PubMed] [Google Scholar]
- 17.Perkins SE, Cagnacci F, Stradiotto A, Arnoldi D, Hudson PJ. 2009. Comparison of social networks derived from ecological data: implications for inferring infectious disease dynamics. J. Anim. Ecol. 78, 1015–1022. ( 10.1111/j.1365-2656.2009.01557.x) [DOI] [PubMed] [Google Scholar]
- 18.Fefferman NH, Ng KL. 2007. How disease models in static networks can fail to approximate disease in dynamic networks. Phys. Rev. E 76, 031919 ( 10.1103/PhysRevE.76.031919) [DOI] [PubMed] [Google Scholar]
- 19.Bansal S, Grenfell BT, Meyers LA. 2007. When individual behaviour matters: homogeneous and network models in epidemiology. J. R. Soc. Interface 4, 879–891. ( 10.1098/rsif.2007.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hock K, Fefferman NH. 2012. Social organization patterns can lower disease risk without associated disease avoidance or immunity. Ecol. Complex 12, 34–42. ( 10.1016/j.ecocom.2012.09.003) [DOI] [Google Scholar]
- 21.Croft DP, Krause J, Darden SK, Ramnarine IW, Faria JJ, James R. 2009. Behavioural trait assortment in a social network: patterns and implications. Behav. Ecol. Sociobiol. 63, 1495–1503. ( 10.1007/s00265-009-0802-x) [DOI] [Google Scholar]
- 22.Newman ME. 2002. Assortative mixing in networks. Phys. Rev. Lett. 89, 208701 ( 10.1103/PhysRevLett.89.208701) [DOI] [PubMed] [Google Scholar]
- 23.Newman ME. 2003. Mixing patterns in networks. Phys. Rev. E 67, 026126 ( 10.1103/PhysRevE.67.026126) [DOI] [PubMed] [Google Scholar]
- 24.Dizney L, Dearing MD. 2013. The role of behavioural heterogeneity on infection patterns: implications for pathogen transmission. Anim. Behav. 86, 911–916. ( 10.1016/j.anbehav.2013.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seaman B, Briffa M. 2015. Parasites and personality in periwinkles (Littorina littorea): infection status is associated with mean-level boldness but not repeatability. Behav. Process 115, 132–134. ( 10.1016/j.beproc.2015.03.014) [DOI] [PubMed] [Google Scholar]
- 26.Boyer N, Reale D, Marmet J, Pisanu B, Chapuis JL. 2010. Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J. Anim. Ecol. 79, 538–547. ( 10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- 27.Kekäläinen J, Lai Y-T, Vainikka A, Sirkka I, Kortet R. 2014. Do brain parasites alter host personality?—Experimental study in minnows. Behav. Ecol. Sociobiol. 68, 197–204. ( 10.1007/s00265-013-1634-2) [DOI] [Google Scholar]
- 28.DiRienzo N, Niemelä PT, Skog A, Vainikka A, Kortet R. 2015. Juvenile pathogen exposure affects the presence of personality in adult field crickets. Front. Ecol. Evol. 3, 36 ( 10.3389/fevo.2015.00036) [DOI] [Google Scholar]
- 29.Poulin R. 2013. Parasite manipulation of host personality and behavioural syndromes. J. Exp. Biol. 216, 18–26. ( 10.1242/jeb.073353) [DOI] [PubMed] [Google Scholar]
- 30.Pontier D, Fromont E, Courchamp F, Artois M, Yoccoz NG. 1998. Retroviruses and sexual size dimorphism in domestic cats (Felis catus L.). Proc. R. Soc. Lond. B 265, 167–173. ( 10.1098/rspb.1998.0278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lass S, Hudson PJ, Thakar J, Saric J, Harvill E, Albert R, Perkins SE. 2013. Generating super-shedders: co-infection increases bacterial load and egg production of a gastrointestinal helminth. J. R. Soc. Interface 10, 20120588 ( 10.1098/rsif.2012.0588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopinath S, Hotson A, Johns J, Nolan G, Monack D. 2013. The systemic immune state of super-shedder mice is characterized by a unique neutrophil-dependent blunting of TH1 responses. PLoS Pathog. 9, e1003408 ( 10.1371/journal.ppat.1003408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruitt JN, Keiser CN. 2014. The personality types of key catalytic individuals shape colonies’ collective behaviour and success. Anim. Behav. 93, 87–95. ( 10.1016/j.anbehav.2014.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keiser CN, Pruitt JN. 2014. Personality composition is more important than group size in determining collective foraging behaviour in the wild. Proc. R. Soc. B 281, 20141424 ( 10.1098/rspb.2014.1424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruitt JN, Grinsted L, Settepani V. 2013. Linking levels of personality: personalities of the ‘average’ and ‘most extreme’ group members predict colony-level personality. Anim. Behav. 86, 391–399. ( 10.1016/j.anbehav.2013.05.030) [DOI] [Google Scholar]
- 36.Pruitt JN, Pinter-Wollman N. 2015. The legacy effects of keystone individuals on collective behavior scale to how long they remain within a group. Proc. R. Soc. B 282, 20151766 ( 10.1098/rspb.2015.1766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright C, Keiser C, Pruitt J. 2016. Behavioral composition can facilitate collective defenses and alter colony-level behavioral plasticity in a social spider. Anim. Behav. ( 10.1016/j.anbehav.2016.03.002) [DOI] [Google Scholar]
- 38.Brey PT, Lee W-J, Yamakawa M, Koizumi Y, Perrot S, Francois M, Ashida M. 1993. Role of the integument in insect immunity: epicuticular abrasion and induction of cecropin synthesis in cuticular epithelial cells. Proc. Natl Acad. Sci. USA 90, 6275–6279. ( 10.1073/pnas.90.13.6275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallet-Gely I, Lemaitre B, Boccard F. 2008. Bacterial strategies to overcome insect defences. Nat. Rev. Microbiol. 6, 302–313. ( 10.1038/nrmicro1870) [DOI] [PubMed] [Google Scholar]
- 40.Henschel J, Lubin Y, Schneider J. 1995. Sexual competition in an inbreeding social spider, Stegodyphus dumicola (Araneae: Eresidae). Insect. Soc. 42, 419–426. ( 10.1007/BF01242170) [DOI] [Google Scholar]
- 41.Henschel JR. 1998. Predation on social and solitary individuals of the spider Stegodyphus dumicola (Araneae, Eresidae). J. Arachnol. 61–69. [Google Scholar]
- 42.Avilés L, Varas C, Dyreson E. 1999. Does the African social spider Stegodyphus dumicola control the sex of individual offspring? Behav. Ecol. Sociobiol. 46, 237–243. ( 10.1007/s002650050615) [DOI] [Google Scholar]
- 43.Bilde T, Coates K, Birkhofer K, Bird T, Maklakov A, Lubin Y, Aviles L. 2007. Survival benefits select for group living in a social spider despite reproductive costs. J. Evol. Biol. 20, 2412–2426. ( 10.1111/j.1420-9101.2007.01407.x) [DOI] [PubMed] [Google Scholar]
- 44.Sloan Wilson D, Clark AB, Coleman K, Dearstyne T. 1994. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442–446. ( 10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 45.Keiser CN, Jones DK, Modlmeier AP, Pruitt JN. 2014. Exploring the effects of individual traits and within-colony variation on task differentiation and collective behavior in a desert social spider. Behav. Ecol. Sociobiol. 68, 839–850. ( 10.1007/s00265-014-1696-9) [DOI] [Google Scholar]
- 46.Grinsted L, Pruitt JN, Settepani V, Bilde T. 2013. Individual personalities shape task differentiation in a social spider. Proc. R. Soc. B 280, 20131407 ( 10.1098/rspb.2013.1407) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Settepani V, Grinsted L, Granfeldt J, Jensen JL, Bilde T. 2013. Task specialization in two social spiders, Stegodyphus sarasinorum (Eresidae) and Anelosimus eximius (Theridiidae). J. Evol. Biol. 26, 51–62. ( 10.1111/jeb.12024) [DOI] [PubMed] [Google Scholar]
- 48.Wright CM, Keiser CN, Pruitt JN. 2015. Personality and morphology shape task participation, collective foraging and escape behaviour in the social spider Stegodyphus dumicola. Anim. Behav. 105, 47–54. ( 10.1016/j.anbehav.2015.04.001) [DOI] [Google Scholar]
- 49.Riechert SE, Hedrick AV. 1993. A test for correlations among fitness-linked behavioural traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim. Behav. 46, 669–675. ( 10.1006/anbe.1993.1243) [DOI] [Google Scholar]
- 50.Li Y, Shen Q, Zhao C, Li X, Zhao L. 1999. Transformation of biological control strain of Pantoea agglomerans by electroporation. Wei sheng wu xue tong bao 27, 245–249. [Google Scholar]
- 51.R Core T. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 52.Jakob EM, Marshall SD, Uetz GW. 1996. Estimating fitness: a comparison of body condition indices. Oikos 77, 61–67. ( 10.2307/3545585) [DOI] [Google Scholar]
- 53.Jankowski MD, Williams CJ, Fair JM, Owen JC, Ren X. 2013. Birds shed RNA-viruses according to the Pareto principle. PLoS ONE 8, e72611 ( 10.1371/journal.pone.0072611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keiser CN, Shearer TA, DeMarco AE, Brittingham HA, Knutson KA, Kuo C, Zhao K, Pruitt JN. In press. Cuticular bacteria appear lethal to social spiders in mixed but not monoculture inoculations. Curr. Zool. ( 10.1093/cz/zow015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pruitt JN, Goodnight CJ. 2014. Site-specific group selection drives locally adapted group compositions. Nature 514, 359–362. ( 10.1038/nature13811) [DOI] [PubMed] [Google Scholar]
- 56.Aviles L. 1996. Causes and consequences of cooperation and permanent-sociality in spiders. In The Evolution of Social Behaviour in Insects and Arachnids (eds Jae C. Choe, Bernard Crespi), pp. 476–498 Cambridge University Press. [Google Scholar]
- 57.Lusseau D, Newman MEJ. 2004. Identifying the role that animals play in their social networks. Proc. R. Soc. Lond. B 271, S477–S481. ( 10.1098/rsbl.2004.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiss IZ, Green DM, Kao RR. 2006. The network of sheep movements within Great Britain: network properties and their implications for infectious disease spread. J. R. Soc. Interface 3, 669–677. ( 10.1098/rsif.2006.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zylberberg M, Klasing KC, Hahn TP. 2013. House finches (Carpodacus mexicanus) balance investment in behavioural and immunological defences against pathogens. Biol. Lett. 9, 20120856 ( 10.1098/rsbl.2012.0856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traniello JF, Rosengaus RB, Savoie K. 2002. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc. Natl Acad. Sci. USA 99, 6838–6842. ( 10.1073/pnas.102176599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cremer S, Armitage SA, Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, R693–R702. ( 10.1016/j.cub.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 62.Hopkins SR, Boyle LJ, Belden LK, Wojdak JM. 2015. Dispersal of a defensive symbiont depends on contact between hosts, host health, and host size. Oecologia 1–12. ( 10.1007/s00442-015-3333-3) [DOI] [PubMed] [Google Scholar]
- 63.Barron DG, Gervasi SS, Pruitt JN, Martin LB. 2015. Behavioral competence: how host behaviors can interact to influence parasite transmission risk. Curr. Opin. Behav. Sci. 6, 35–40. ( 10.1016/j.cobeha.2015.08.002) [DOI] [Google Scholar]
- 64.Harris RN, James TY, Lauer A, Simon MA, Patel A. 2006. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 3, 53–56. ( 10.1007/s10393-005-0009-1) [DOI] [Google Scholar]
- 65.Harris RN, et al. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 3, 818–824. ( 10.1038/ismej.2009.27) [DOI] [PubMed] [Google Scholar]
- 66.Cogen A, Nizet V, Gallo R. 2008. Skin microbiota: a source of disease or defence? Br. J. Dermatol. 158, 442–455. ( 10.1111/j.1365-2133.2008.08437.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this manuscript have been deposited at Dryad digital repository: http://dx.doi.org/10.5061/dryad.60nn7.


