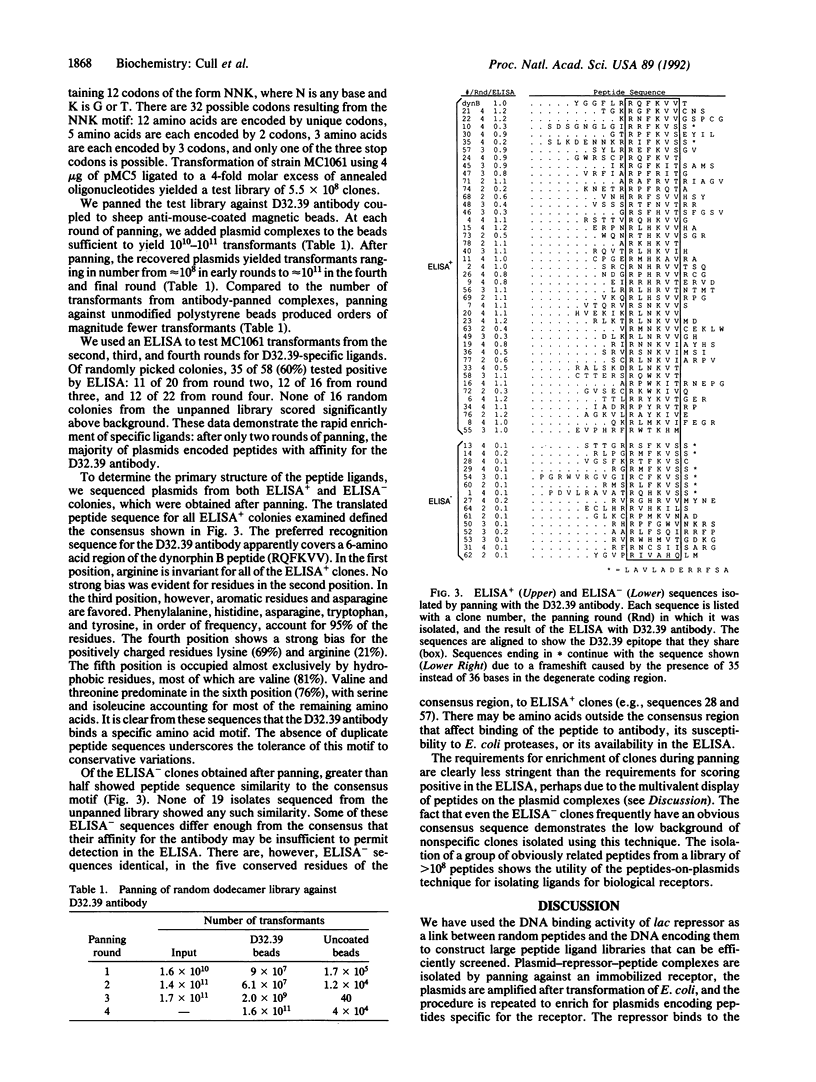
Abstract
We have constructed a large library of random peptides fused to the C terminus of the lac repressor. The DNA binding activity of the repressor protein physically links the peptides to the plasmid encoding them by binding to lac operator sequences on the plasmid. This linkage allows efficient enrichment for specific peptide ligands in the random population of peptides by affinity purification of the peptide-repressor-plasmid complexes with an immobilized receptor. After transformation of Escherichia coli with recovered plasmids, the library can be amplified for additional rounds of affinity enrichment or specific plasmids can be sequenced to determine the primary structure of the peptides. We used a monoclonal antibody specific for the peptide dynorphin B as a model receptor to screen a random dodecamer library. After only two rounds of enrichment, the majority of the plasmids in the selected population encoded fusion peptides that bound specifically to the antibody. These peptides contain a consensus sequence similar to a segment of dynorphin B (RQFKVV). This technique should be useful to find peptide ligands for a variety of biological receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett R. W., Goldstein A. A monoclonal antibody specific for a dynorphin precursor. Neuropeptides. 1985 Apr;6(2):113–120. doi: 10.1016/0143-4179(85)90102-7. [DOI] [PubMed] [Google Scholar]
- Besse M., von Wilcken-Bergmann B., Müller-Hill B. Synthetic lac operator mediates repression through lac repressor when introduced upstream and downstream from lac promoter. EMBO J. 1986 Jun;5(6):1377–1381. doi: 10.1002/j.1460-2075.1986.tb04370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly T. J., Matthews K. S. Characterization and modification of a monomeric mutant of the lactose repressor protein. Biochemistry. 1986 Sep 23;25(19):5474–5478. doi: 10.1021/bi00367a019. [DOI] [PubMed] [Google Scholar]
- Devlin J. J., Panganiban L. C., Devlin P. E. Random peptide libraries: a source of specific protein binding molecules. Science. 1990 Jul 27;249(4967):404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington A. D., Szostak J. W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990 Aug 30;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- England B. P., Ackerman M. S., Barrett R. W. A chimeric D2 dopamine/m1 muscarinic receptor with D2 binding specificity mobilizes intracellular calcium in response to dopamine. FEBS Lett. 1991 Feb 11;279(1):87–90. doi: 10.1016/0014-5793(91)80257-4. [DOI] [PubMed] [Google Scholar]
- Flashner Y., Gralla J. D. Dual mechanism of repression at a distance in the lac operon. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8968–8972. doi: 10.1073/pnas.85.23.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Read J. L., Pirrung M. C., Stryer L., Lu A. T., Solas D. Light-directed, spatially addressable parallel chemical synthesis. Science. 1991 Feb 15;251(4995):767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- Gold L., Stormo G. D. High-level translation initiation. Methods Enzymol. 1990;185:89–93. doi: 10.1016/0076-6879(90)85009-d. [DOI] [PubMed] [Google Scholar]
- Hsieh W. T., Whitson P. A., Matthews K. S., Wells R. D. Influence of sequence and distance between two operators on interaction with the lac repressor. J Biol Chem. 1987 Oct 25;262(30):14583–14591. [PubMed] [Google Scholar]
- Hu M. C., Davidson N. Targeting the Escherichia coli lac repressor to the mammalian cell nucleus. Gene. 1991 Mar 15;99(2):141–150. doi: 10.1016/0378-1119(91)90120-z. [DOI] [PubMed] [Google Scholar]
- Kania J., Brown D. T. The functional repressor parts of a tetrameric lac repressor-beta-galactosidase chimaera are organized as dimers. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3529–3533. doi: 10.1073/pnas.73.10.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleina L. G., Miller J. H. Genetic studies of the lac repressor. XIII. Extensive amino acid replacements generated by the use of natural and synthetic nonsense suppressors. J Mol Biol. 1990 Mar 20;212(2):295–318. doi: 10.1016/0022-2836(90)90126-7. [DOI] [PubMed] [Google Scholar]
- Koob M., Szybalski W. Cleaving yeast and Escherichia coli genomes at a single site. Science. 1990 Oct 12;250(4978):271–273. doi: 10.1126/science.2218529. [DOI] [PubMed] [Google Scholar]
- Krämer H., Niemöller M., Amouyal M., Revet B., von Wilcken-Bergmann B., Müller-Hill B. lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J. 1987 May;6(5):1481–1491. doi: 10.1002/j.1460-2075.1987.tb02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M. A., Baim S. B., Shenk T., Levine A. J. Conversion of the lac repressor into an allosterically regulated transcriptional activator for mammalian cells. Mol Cell Biol. 1990 Jul;10(7):3343–3356. doi: 10.1128/mcb.10.7.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens D., Howley P. M. Novel method for identifying sequence-specific DNA-binding proteins. Mol Cell Biol. 1985 Sep;5(9):2307–2315. doi: 10.1128/mcb.5.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeberg J., Wahlberg J., Uhlén M. Rapid colorimetric quantification of PCR-amplified DNA. Biotechniques. 1991 Jan;10(1):68–75. [PubMed] [Google Scholar]
- Mossing M. C., Record M. T., Jr Upstream operators enhance repression of the lac promoter. Science. 1986 Aug 22;233(4766):889–892. doi: 10.1126/science.3090685. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Kania J. Lac repressor can be fused to beta-galactosidase. Nature. 1974 Jun 7;249(457):561–563. doi: 10.1038/249561a0. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Fontaine A., Bãckman S. Biosynthesis of a repressor/nuclease hybrid protein. J Biol Chem. 1989 Sep 5;264(25):15066–15069. [PubMed] [Google Scholar]
- Parmley S. F., Smith G. P. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988 Dec 20;73(2):305–318. doi: 10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Sadler J. R., Sasmor H., Betz J. L. A perfectly symmetric lac operator binds the lac repressor very tightly. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6785–6789. doi: 10.1073/pnas.80.22.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Simons A., Tils D., von Wilcken-Bergmann B., Müller-Hill B. Possible ideal lac operator: Escherichia coli lac operator-like sequences from eukaryotic genomes lack the central G X C pair. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1624–1628. doi: 10.1073/pnas.81.6.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiesen H. J., Bach C. Target Detection Assay (TDA): a versatile procedure to determine DNA binding sites as demonstrated on SP1 protein. Nucleic Acids Res. 1990 Jun 11;18(11):3203–3209. doi: 10.1093/nar/18.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990 Aug 3;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Influence of supercoiling and sequence context on operator DNA binding with lac repressor. J Biol Chem. 1987 Oct 25;262(30):14592–14599. [PubMed] [Google Scholar]




