Abstract
Purpose
We tested the feasibility of using titanium to enhance adhesion of the Boston Keratoprosthesis (B-KPro), ultimately to decrease the risk of implant-associated complications.
Methods
Cylindrical rods were made of poly(methyl methacrylate) (PMMA), PMMA coated with titanium dioxide (TiO2) over a layer of polydopamine (PMMATiO2), smooth (Ti) and sandblasted (TiSB) titanium, and titanium treated with oxygen plasma (Tiox and TiSBox). Topography and surface chemistry were analyzed by scanning electron microscopy (SEM), atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS). Adhesion force between rods and porcine corneas was measured ex vivo. Titanium sleeves, smooth and sandblasted, were inserted around the stem of the B-KPro and implanted in rabbits. Tissue adhesion to the stem was assessed and compared to an unmodified B-Kpro after 1 month.
Results
X-ray photoelectron spectroscopy demonstrated successful deposition of TiO2 on polydopamine-coated PMMA. Oxygen plasma treatment did not change the XPS spectra of titanium rods (Ti and TiSB), although it increased their hydrophilicity. The materials did not show cell toxicity. After 14 days of incubation, PMMATiO2, smooth titanium treated with oxygen plasma (Tiox), and sandblasted titanium rods (TiSB, TiSBox) showed significantly higher adhesion forces than PMMA ex vivo. In vivo, the use of a TiSB sleeve around the stem of the B-KPro induced a significant increase in tissue adhesion compared to a Ti sleeve or bare PMMA.
Conclusions
Sandblasted titanium sleeves greatly enhanced adherence of the B-KPro to the rabbit cornea. This approach may improve adhesion with the donor cornea in humans as well.
Translational Relevance
This approach may improve adhesion with donor corneas in humans.
Keywords: keratoprosthesis, Boston Keratoprosthesis, artificial cornea, titanium coating, titanium oxide, titanium skirt, biointegration, corneal adhesion, titanium adhesion
Introduction
The Boston keratoprosthesis (B-Kpro; Massachusetts Eye and Ear Infirmary, Boston, MA) is currently (as of 2015) the most widely used artificial cornea, with more than 12,000 units implanted worldwide. Its current configuration consists of a carrier donor corneal graft sandwiched between a poly(methyl methacrylate) (PMMA) front plate and a PMMA or titanium back plate.1–4 Since the stem of the B-Kpro crosses the cornea, inadequate attachment with corneal tissue could create a space5 through which microorganisms can penetrate into the eye and induce endophthalmitis.6,7 Consequently, the recommended protocol after B-Kpro implantation includes the use of daily antibiotic drops, which have markedly decreased the risk of endophthalmitis. Nonetheless, severe infections still can occur if compliance with the antibiotic regimen is not observed.6
We have hypothesized that modifying the surface of the B-Kpro stem could enhance adhesion with the donor corneal carrier, which could decrease the risk of endophthalmitis. We selected titanium to test this hypothesis, due to its long history of successful use in bone or dental prostheses,8–12 and the fact that it is biologically very well tolerated, as it induces relatively little inflammation and foreign body reaction.13–15 Furthermore, by modifying their topography, titanium surfaces can induce proliferation or differentiation of osteocytes, promoting better apposition and adhesion between the material and bone.12,16 In vitro studies have shown very low toxicity to human corneal epithelial cell lines.17,18 In addition, titanium has been used as a locking ring and back plate in the B-Kpro,2,19 as an intrastromal haptic in the Fyodorov-Zuev keratoprosthesis,19 and in other experimental corneal devices in rabbits.19,20
We assessed the effectiveness of titanium-based materials in improving adherence to corneal tissue ex vivo and in vivo.
Materials and Methods
In Vitro Surface Characterization of the Candidate Materials
We studied several surface modifications of titanium as candidate materials for the B-Kpro in an attempt to enhance adhesion between the device and the cornea, and compared them to PMMA, the current manufacturing material of the device. All experimental samples were obtained from and processed by a local vendor (JG Machine Co., Inc., Wilmington, MA). For the in vitro surface characterization and cell viability studies, we used discs (0.2 mm thick × 10 mm diameter) made either of 100% medical-grade PMMA (Spartech Townsend, Pleasant Hill, IA) or titanium-grade 23 (Ti-6AL-4V-ELI or Ti 6-4 ELI), which contains 6% aluminum and 4% vanadium (ISO 9001:2008 certification; President Titanium Co., Inc., Hanson, MA). Before proceeding with any experiments, all the samples were cleaned ultrasonically with Alconox powdered detergent (Alconox, Inc., White Plains, NY) in two cycles of 1 minute each, after which they were rinsed profusely with deionized water to remove any remaining debris, and blow-dried with air.
Titanium initially was chosen based on its long-term compatibility and safety when used inside the eye.2,14,19,20 In addition, we chose to manufacture the titanium samples with a rough surface achieved through sand blasting (TiSB), and compared them to samples with a smooth surface (Ti). The sand blasting of the surface was intended to promote and enhance the adhesion between the titanium and corneal tissue, in the same way that previous studies have shown an enhanced tissue adhesion between implanted titanium and bone or tooth after increasing the surface roughness of the implant.16 Sand blasting currently is used on the back plate of the B-Kpro to eliminate its shiny-metallic appearance.17 Some samples underwent plasma oxidization (Tiox, and TiSBox) in an attempt to make the surfaces more hydrophilic, which also is believed to promote better cellular and even extracellular matrix adhesion.21 For this purpose, the samples were placed in a plasma oxidizer chamber at ≈ 250 millitorrs, for 5 minutes. Additionally, we included unmodified PMMA samples (PMMA) as well as plasma-treated PMMA (PMMAox) as group controls.
Finally, we studied another surface modification by coating PMMA with titanium dioxide (PMMATiO2), which has been shown to promote keratocyte adhesion in cell culture.13 The rationale for using this material can be explained by the fact that TiO2 implants possess a high corrosion resistance in vitro and in vivo with low inflammatory reaction.14 Furthermore, a TiO2 coating can be easily applied directly on PMMA through liquid phase deposition,22 without adding additional components to the current design of the B-Kpro, which could potentially raise questions on safety. For this purpose, TiO2 was deposited over a base-layer of polydopamine (PDA), which in turn allows the deposition of TiO2 on virtually any surface, even those with complex topographies.22 Briefly, the PMMA rods were immersed in a dilute aqueous solution of dopamine (2 mg/mL dopamine in 10 mM Tris buffer, pH 8.5) and shaken with a vortex mixer overnight to create a layer of PDA. After a thorough wash with deionized water the next day, the PDA-coated rods were immersed in 0.1 M ammonium hexafluorotitanate ([NH4]2TiF6) and 0.3 M boric acid (H3BO3) solution at pH 3.9 and were left overnight on a shaker at room temperature. Finally the samples were rinsed carefully with deionized water and blow-dried with air.
For the initial characterization of the PMMATiO2 samples, we used a PHI VersaProbe II Scanning X-ray photoelectron spectroscope (XPS; Physical Electronics, Chanhassen, MN) equipped with a monochromatized Al Kα source. Furthermore, we used a drop shape analyzer (Drop Shape Analyzer DSA100B, Model: FM3200; Krüss GmbH, Hamburg, Germany) to measure the hydrophilicity of all the samples in our study by registering the water contact angle on their surface, while atomic force microscopy (AFM; MFP-3D AFM; Asylum Research, Santa Barbara, CA) was used to characterize their surface nano-topography. Finally, we analyzed the surface morphology with scanning electron microscopy (SEM; JEOL 5600LV; JEOL Inc., Peabody, MA), after coating the samples with gold-palladium.
In Vitro Cell Viability Assay
To assess cell viability, we performed an alamarBlue assay (Invitrogen, Life Technologies, Grand Island, NY). This assay allows establishing relative cell toxicity by using resazurin and the reducing power of living cells to quantitatively measure proliferation. For this purpose, discs were cleaned, placed individually in 24-well plates, and seeded with primary human corneal fibroblasts at 5 × 103 cells/cm2 (courtesy of James Zieske, PhD, from Schepens Eye Research Institute, Harvard Medical School, Boston, MA) in Minimum Essential Medium Eagle (Sigma-Aldrich Corp., St. Louis, MO) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. At 7 and 14 days after seeding, we added alamarBlue reagent to each well (10% vol/vol), incubated for 2.5 hours, and measured the absorbance of the media at 570/600 nm. The cellular proliferation was measured by the degree of reduction of the reagent, which was calculated by the following equation provided in the manufacturer's manual:
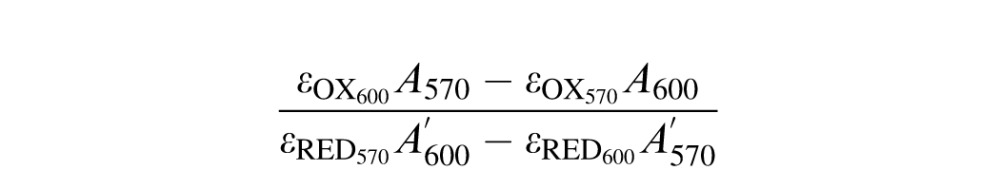 |
εOX and εRED are the molar extinction coefficients of alamarBlue in oxidized and reduced form, and A and A′ are the absorbances of each well and of media only, respectively. The data were normalized to the cells cultured on tissue culture polystyrene (TCPS).
Ex Vivo Pullout Force Technique
For the pullout ex vivo experiments we used rods (15 mm long × 3.35 mm diameter) made of the different materials, which previously were cleaned following the aforementioned procedure. To test the adhesion between the cornea and candidate materials, we used a mechanical “pullout” technique developed in our lab.23 Porcine eyes were obtained from the vendor, transported to the laboratory in a moisture chamber with ice, and processed within 24 hours of animal death. Before manipulation, the eyes were rinsed with 10% povidone iodine solution, and washed with phosphate-buffered saline (PBS 10X, pH 7.4; Invitrogen, Life Technologies). The porcine cornea then was trephined with a 10-mm diameter surgical trephine, and an additional 3-mm hole was punched through the center with an Acu·Punch biopsy punch (Acuderm, Inc., Fort Lauderdale, FL). The cornea then was slid over one of the 3.35-mm diameter rods, and placed individually in a 12-well plate filled with 2 mL of Chen cornea organ culture medium, which consists of Modified Medium 199 (Invitrogen), 1% penicillin-streptomycin, 7% dextran, 25 mM Hepes, and 10 nM D,L-β-hydroxybutyrate (Sigma-Aldrich Corp.).24,25 Specimens were incubated at 37°C in 5% carbon dioxide for 2 weeks, and during that time half of the media was replaced every 12 hours.
After 14 days, the specimens were placed in a custom-designed holder,23 to fit a universal tensiometer (Instron 5542; Instron, Norwood, MA). The cornea was placed in a cavity between two plastic holders (acrylonitrile butadiene styrene; ABSPlus; Dimension, Inc., Eden Prairie, MN), with the rod protruding through the top of the holder. The space between the parts of the holder was kept fixed (500–1000 μm approximately) with the help of two screws, so the cornea would not be compressed. The Instron device gripped the holder from its base and the rod was pulled out from above at a constant velocity (10 mm/min), while the force required to sustain the pullout was measured. The test was considered finished when the cylinder was completely removed from the cornea. After completion of the mechanical testing, the rods were submerged in 4% formalin, and processed for SEM. Before imaging, the samples were dried in a critical point dryer, and 5 nm of gold/palladium were deposited on the surface to avoid unnecessary electric charging.
In Vivo B-Kpro Implantation
All animal studies were approved by the Animal Care Committee of the Massachusetts Eye and Ear Infirmary and performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
A total of 7 New Zealand White albino rabbits were used (male, 2.5–3.5 Kg), each one having a type 1 B-Kpro surgically implanted into the right eye, using the same standard technique as used in humans. Briefly, in deeply anesthetized animals, the cornea was trephined (9 mm), excised and set aside, and the lens was removed through an open sky extracapsular extraction. The excised cornea then was further trephined centrally with a 3 mm-diameter punch. The device then was slid through the cornea carrier, and a titanium back plate was clicked-on. The corneal graft with the B-Kpro then was sutured back in the eye with twelve 10-0 interrupted nylon sutures. Two animals were implanted with a smooth Ti clad (Ti) around the stem of the B-Kpro, three animals received sand blasted titanium clads (TiSB), and the remaining two animals received a standard B-Kpro. The titanium ring was inserted easily around the stem of the B-Kpro after placing the prosthesis in dry ice for 30 seconds. This was performed before sending the implants for sterilization. After the implantation, the animals were treated with topical antibiotics (polymyxin B/trimethoprim) and steroids (prednisolone acetate 1%), every 12 hours for the first week, and once daily for the following weeks. Prednisolone was discontinued at 2 weeks postoperatively. Six animals were monitored for 1 month while one of the TiSB clad animals was kept for 10 months. At the end time point, the animals were euthanized and enucleated, and the B-Kpro pulled away manually from the cornea. The devices then were immersed in half-strength Karnovsky's fixative (2% paraformaldehyde; 2.5% glutaraldehyde) in 0.1 M phosphate buffer pH 7.4 overnight, dried in a critical point dryer, and coated with gold/palladium for SEM imaging.
Statistics
In general, data are presented as means ± SD and were analyzed accordingly. For all statistical tests, 2-tailed values of P < 0.05 were considered statistically significant. All data analysis was performed using the statistical tool provided by IGOR Pro (version 6.3; WaveMetrics, Inc., Portland, OR) and GraphPad Prism for Mac OS X (version 5.0c, GraphPad Software, Inc., La Jolla, CA).
Results
Production and Characterization Of Materials
The aim of this study was to assess the potential for titanium-based materials to enhance adhesion between the PMMA B-KPro stem and the adjacent donor cornea. For in vitro studies, titanium samples were manufactured as discs (0.2 mm thick × 10 mm diameter), and for ex vivo work as rods (15 mm long × 3.35 mm diameter). Sample surfaces were either smooth (Ti) or were roughened by sand blasting (TiSB), since rougher surfaces are reported to promote cellular adhesion.16 Both types of experimental samples also were oxidized by oxygen plasma treatment (Tiox, and TiSBox) to make the surfaces more hydrophilic, which also is believed to promote better cellular attachment.21 Their performance was compared to that of unmodified PMMA (PMMA) as well as plasma-treated PMMA (PMMAox), and PMMA coated with TiO2 by deposition on top of PDA (PMMATiO2). Polydopamine films can form on a wide range of substrates by the polymerization of dopamine in an alkaline solution. The quinone groups in PDA can chelate metal ions and have been used for the deposition of hydroxyapatite and titanium oxide.22,23
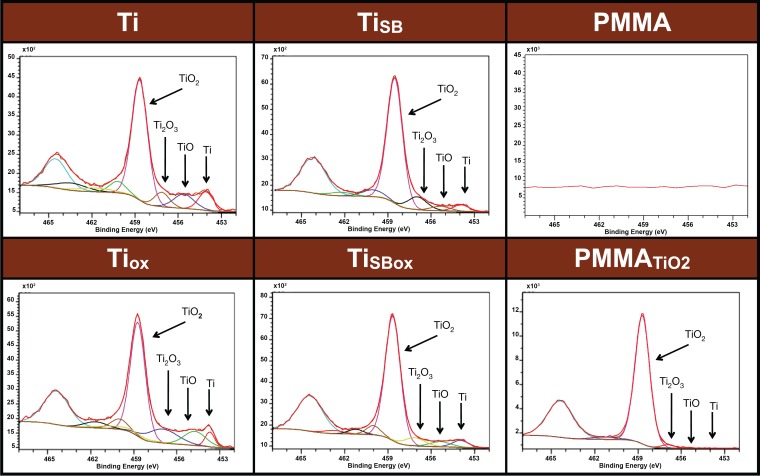
The surface chemistry of materials was characterized by their high-resolution XPS spectra to confirm the presence of titanium and its oxidation states (Fig. 1). The binding energies for titanium and its various oxidation states were identified: 453.9 eV = Ti, 455.5 eV = TiO, 457.3 eV = Ti2O3, and 458.7 eV = TiO2.26 High-resolution spectra of all titanium samples, regardless of surface roughness (smooth and sandblasted) and oxygen plasma treatment, could be assigned mainly to TiO2 with minor variations of Ti, TiO, and Ti2O3 peaks. Coating of PMMA with TiO2 (PMMATiO2) was demonstrated by the presence of a TiO2 peak, along with negligible peaks for Ti and other oxidized states of titanium (TiO, Ti2O3). The PMMA samples did not show any traces of titanium.
Figure 1.
X-ray photoelectron spectroscopy of various materials. Peaks corresponding to Ti (453.9 eV), TiO (455.5 eV), Ti2O3 (457.3 eV), and TiO2 (458.7 eV) are indicated.
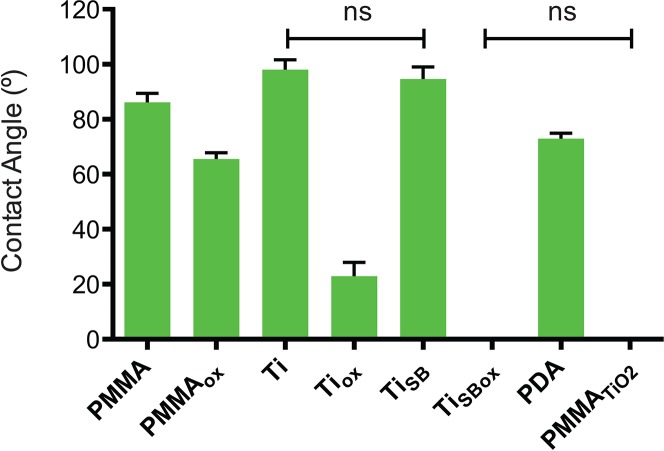
The hydrophilicity of the materials was characterized by measuring water contact angles. Samples treated with oxygen plasma showed a marked decrease in the contact angle measurements, indicating an increase in hydrophilicity due to oxidization of the outermost surface of the samples (Fig. 2). PMMATiO2 had a zero contact angle because it was coated with a highly hydrophilic TiO2 layer (Fig. 1). All the groups in Figure 2 were statistically significantly different from each other (P < 0.05), except where indicated.
Figure 2.
Water contact angle measurements. The graph shows the mean value of the contact angle measurements from every group. All samples were statistically significantly different from each other (P < 0.05 by ANOVA with Tukey's test), except where indicated (ns). Data are means ± SD (N = 4).
Atomic force microscopy imaging was used to quantify the nano-scale topography and the surface roughness of the materials, as shown in Figure 3A. The TiSB samples were the roughest materials, while the Ti samples were the smoothest. The effect of the addition of TiO2 on the roughness of the PMMA (PMMATiO2 samples) was not statistically significant (P > 0.05). Oxygen plasma treatment did not have a statistically significant effect on the surface roughness of PMMA, Ti, or TiSB (P = 0.9593). Scanning electron microscopy imaging of the materials showed no obvious effect of oxidation on surface roughness, but confirmed that the sand blasted groups (TiSB, TiSBox) had greater surface roughness (Fig. 3B).
Figure 3.
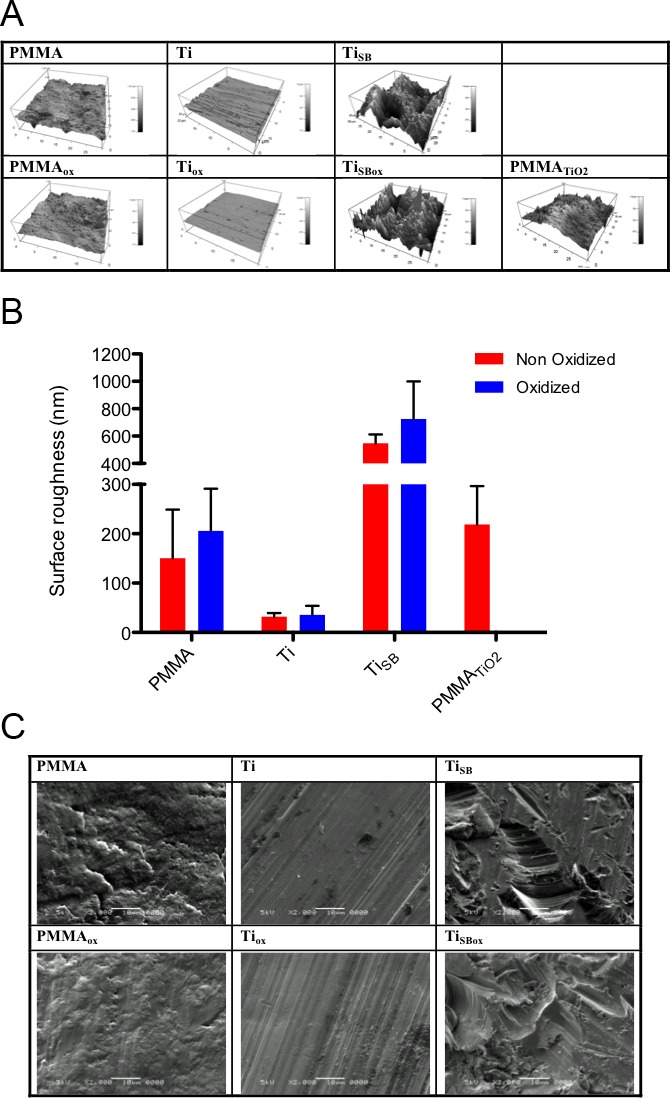
Surface imaging. (A) Atomic force microscopy imaging provided quantitative data on the surface topography and roughness of the materials, summarized in (B) the graph. Data are means ± SD (N = 4, t-test). Oxidation did not show any statistical differences in any of the materials (P > 0.05). (C) Scanning electron microscopy of materials before (top row) and after plasma oxidization (bottom row).
In Vitro Cell Viability Assay
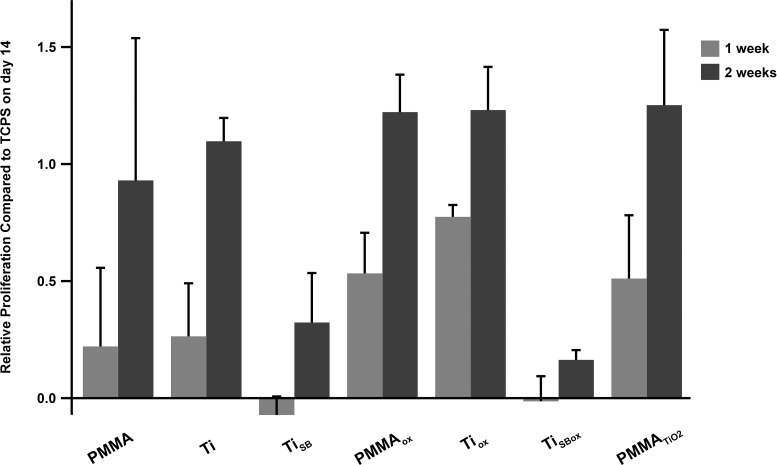
Cell viability on the various surfaces was assessed by the alamarBlue assay. Human corneal fibroblasts proliferated on all the samples over 2 weeks (Fig. 4). In the TiSB and TiSBox groups, the number of cells that attached was lower than in other groups, possibly because of the high roughness of the materials.27,28 Nonetheless, these results demonstrated that cell growth occurred on all the testing surfaces, indicating that the materials were not toxic to cells.
Figure 4.
Alamar blue assay for cell viability (human cornea fibroblasts). Data are means ± SD.
Ex Vivo Measurements of Adhesion
We measured the strength of the adhesion between the cornea and the materials ex vivo. Rods of the various materials were implanted in holes punched into porcine corneas (see Methods). The assembly was maintained in corneal tissue culture for 14 days and then mounted in a device developed in our lab to measure the force required to pull the rods from the corneas (Figs. 5A, 5B).23 After 14 days of incubation, the force required to pull Tiox, PMMATiO2, TiSB, and TiSBox rods out of the porcine corneas was statistically significantly higher than rods of plain PMMA or smooth titanium (P < 0.05; Fig. 5C). The increased adhesion after treating Ti surfaces with oxygen can be attributed to increased surface hydrophilicity, which improves adhesion by osteoblasts and keratocytes.13,16,21 In general, PMMA is a poor substrate for cell adhesion29 and no improvement was observed after treating it with oxygen plasma. In the case of sandblasted titanium, oxygen plasma did not further improve adhesion, most likely because the surface roughness dominated the enhanced adhesion. Scanning electron microscopy imaging of the surfaces of the rods following the pull out experiments (Fig. 6) showed more tissue adherent to the samples that required a higher force to be pulled out from the corneas (Tiox, PMMATiO2, TiSB, and TiSBox).
Figure 5.
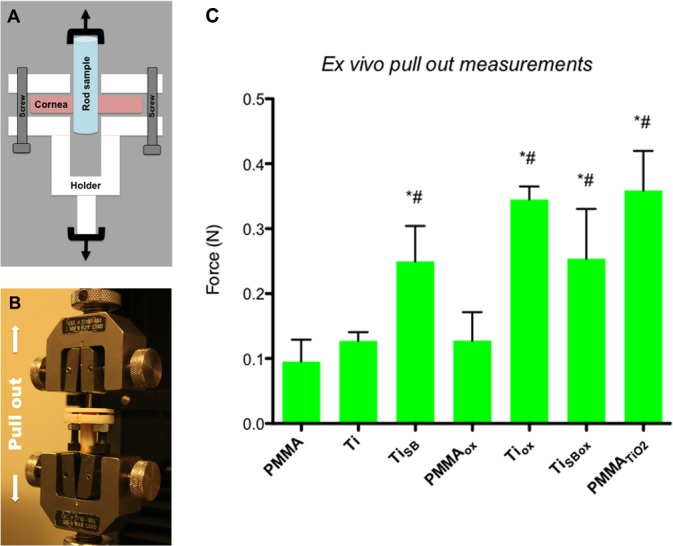
Ex vivo pull out measurements. (A) Schematic of the customized holder containing a cornea-rod assembly. (B) Image of the customized holder within a universal (mechanical) testing machine. (C) Force required to pull rods of various materials from porcine corneas. * and # indicate P < 0.05 (by ANOVA with Tukey's test) in comparison to plain PMMA and smooth Ti, respectively. Data are means ± SD (N = 4).
Figure 6.
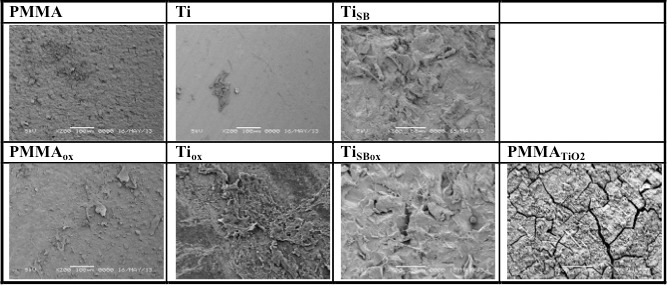
Representative scanning electron microscopy of implant surfaces after the ex vivo pullout experiments.
Our ex vivo results showed that when increasing the hydrophilicity of the surface of titanium, or when its surface roughness is increased, the corneal tissue attachment was enhanced. This is not the case for PMMA.
In Vivo Results With a Ti-Clad Around the B-Kpro Stem
The four groups that showed the strongest adhesion to corneas ex vivo were Tiox, PMMATiO2, TiSB, and TiSBox. Although plasma oxidation greatly increased sample adhesion to the cornea ex vivo, we did not pursue that approach in vivo because the effect of plasma oxidation on titanium (Tiox and TiSBox) is only temporary in air, and would be lost during the manufacturing and storing stages before implantation in humans.21 We also did not proceed with the PMMATiO2 coating because we realized that it was easily scratched even with careful handling, which would present a problem during surgical implantation. Consequently, we decided to use solid titanium instead (Ti and TiSB) for in vivo experimentation. These materials have the additional advantage of having well established and simple means of manufacture.
Boston keratoprostheses, unmodified or with a smooth Ti or TiSB sleeve around the stem, were implanted in two rabbits each, using the same technique used in humans. All the devices were retained, without signs of infection or inflammation. One month after implantation, animals were euthanized, the eyes were enucleated and a corneal-scleral ring was excised from the globe. A full thickness tangential cut was performed on the cornea, and the tissue was removed manually from the stem. In the rabbits implanted with either a standard B-Kpro or with a smooth Ti sleeve, the cornea was removed easily from the stem. In the rabbits implanted with a TiSB sleeve, the force required to remove the tissue was clearly (albeit subjectively) greater.
Scanning electron microscopy imaging of the explanted devices (Fig. 7) showed few tissue remnants on the standard B-Kpro (Fig. 7A) or the B-Kpro with a smooth Ti sleeve (Fig. 7B), with larger areas without any tissue residue. The B-Kpros with the TiSB sleeves (Fig. 7C) showed much more tissue residue, covering the implants.
Figure 7.
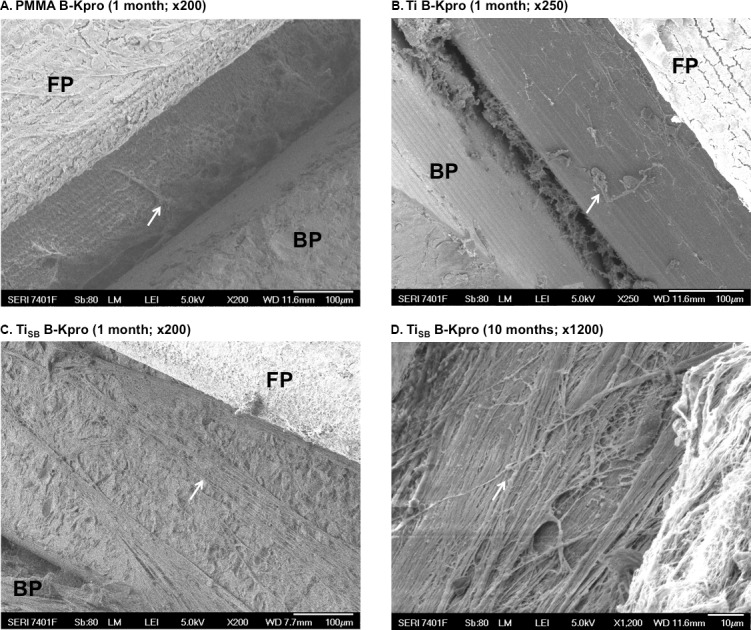
Scanning electron microscopy of B-Kpros after implantation in rabbits. FP, front plate; BP, back plate. Arrows indicate the remains of corneal tissue attached to the stem.
One additional animal implanted with B-Kpro with a TiSB sleeve around the stem was followed for 10 months. As at 1 month, this B-Kpro required greater force to remove. On SEM image (Fig. 7D), the sleeve was completely covered with a thick layer of fibrous material.
Discussion
Since its introduction in 1974,1 the B-Kpro has become an important surgical option for cases where a standard corneal transplantation has an increased risk of graft failure. However, despite providing immediate restoration of visual acuity, thanks to its almost perfect optical qualities (it can allow vision of 20/15), B-Kpro implantation still carries important risks, including glaucoma progression,30 corneal necrosis or keratolysis,31–35 and bacterial endophthalmitis.6,7 Among these complications, tissue necrosis has a major role and is of high importance since it may lead to gaps in the interface between the B-Kpro and the carrier cornea,33 which in turn can affect retention and facilitate the entry of bacteria or other microorganisms into the eye, ultimately leading to endophthalmitis.
Although microbial endophthalmitis after B-Kpro has been drastically reduced in the last decade by use of daily low-dose prophylactic antibiotics,6,36 infectious complications still occur, especially in the developing world.32,37 It has long been suspected that the main contributing factor facilitating the increase in the risk of endophthalmitis could be inadequate integration between the B-KPro and the surrounding corneal tissue. Shapiro et al.5 have recently documented this phenomenon by imaging B-Kpro patients with anterior segment optical coherence tomography (AS-OCT), in which a significant gap could be observed between the stem of the device and the corneal graft tissue. The aim of this study has been to identify titanium-based materials that would improve the attachment (or biointegration)29 between the corneal donor tissue and the B-Kpro.
Biointegration or a tight attachment between implanted medical devices and body tissues directly influences clinical outcomes, including tissue breakdown and infection, ultimately having an impact on safety and efficacy.8–10 The strength of attachment will depend on the chemical composition and the topography of the surface of the implanted material. It has been demonstrated that materials with a highly hydrophilic surface and high surface roughness induce better tissue attachment in vivo.16,21 The ex vivo and in vivo data in our study also found that the materials with the most hydrophilic surface or the highest roughness demonstrated a stronger cellular attachment.
Titanium is very well tolerated in the eye.19 In 1993, the first successful use of titanium in a keratoprosthesis was reported. The device consisting of a PMMA optic with a tantalum-titanium alloy haptic was implanted in humans.19 The extrusion rate in this study was 3% to 13% depending upon the operative techniques used, and aseptic corneal necrosis presented in 11% of the cases. Three years later, an experimental keratoprosthesis was developed that included an intrastromal haptic made of titanium.20 The mild inflammatory reaction in rabbit corneas was consistent with that found after any medical device implantation. A more recent study has evaluated the use of titanium oxide in vitro. In this study, TiO2 enhanced keratocyte cell integration and cell adhesion, while at the same time presenting antibacterial properties, suggesting it could be used as candidate material for an artificial corneal skirt.13 Titanium has recently become a major component of the B-Kpro; the most recent version includes a back plate made of medical grade titanium alloy (also containing aluminum and vanadium).2 This experience informed our decision to use titanium in this study. However, none of the previous studies addressed the potential for titanium to enhance mechanical adherence between cornea and keratoprosthesis in vivo.
Titanium can be found in several oxidative states, the most common one being TiO2. This was our first choice for the study, based on the fact that TiO2 is highly hydrophilic, possesses a high corrosion resistance in vitro and in vivo, and causes little inflammatory response in vivo.14 We coated PMMA with PDA, which served as a base-layer for subsequently depositing TiO2 through liquid phase deposition22 (PMMATiO2). X-ray photoelectron spectroscopy analysis of the PMMATiO2 samples was consistent with previous publications,38 and confirmed the successful coating of TiO2. The high hydrophilicity of the coating, verified by the contact angle measurements, was predictive of the ex vivo findings that PMMATiO2 showed increased adhesion to corneal tissue. These results also correlated well with previous work on corneal keratocytes13 and osteoblasts,39 in which the increased hydrophilicity of TiO2 facilitated cell attachment. Unfortunately, the PMMATiO2 coating was easily scratched with handling, making it less practical for use in vivo. Solid titanium is structurally robust and has the advantage of having well established and simple means of manufacture. Therefore, we decided to use solid titanium instead.
Here solid titanium was used in the form of a sleeve around the stem of the B-Kpro in in vivo studies. The sand blasted titanium sleeve was used because it had performed well in the ex vivo studies of adhesion to corneal tissue. Those results had been consistent with previous studies where osteoblast and corneal keratocyte proliferation and differentiation in vitro were promoted by an increase in surface roughness.15,16,27,40
Plasma oxidation increases the hydrophilicity of material surfaces and promotes cellular attachment.21 In our study, plasma-oxidation of titanium significantly increased adhesion to cornea tissue compared to nonoxidized titanium. However, the effects of plasma oxidation are short-lived and the equipment for it is not widely available, which would make it less practical to implement this approach in clinical situations. Our ex vivo study revealed that simple sand-blasting on titanium even without the plasma oxidation process enhanced adhesion as effectively as titanium oxide deposition or plasma oxidation on titanium. In vivo, the TiSB sleeve around the B-Kpro stem had a tighter attachment to the cornea than did the unmodified PMMA stem or the smooth titanium sleeve, and had more tissue remnants adherent to the surface of the sleeve compared to the two other groups.
In conclusion, our findings supported the hypothesis that titanium-based materials would enhance adhesion between the B-Kpro and corneal tissue in vivo. Our ex vivo and in vivo results showed better tissue attachment when titanium was manufactured with a rough surface through sand blasting, compared to a smooth surface titanium sleeve or PMMA. Incorporating a sand blasted titanium sleeve around the stem of the B-Kpro has the potential to improve attachment to the carrier corneal graft in humans, and reduce the likelihood of postoperative complications such as keratolysis and endophthalmitis.
Acknowledgments
The authors thank Ann Tisdale, M.S., Staff Associate at Ilene K. Gipson laboratory, Schepens Eye Research Institute of Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA, USA, for providing the SEM images of the B-Kpro devices; and John Granney, JG Machine, Precision Machining, the manufacturer of the B-Kpro, for providing all the necessary materials for this project.
Supported by the Keratoprosthesis Fund, Massachusetts Eye and Ear Infirmary, Boston, MA, USA, and R01 GM073626; DSK. The authors alone are responsible for the content and writing of this paper.
Borja Salvador-Culla and Kyung Jae Jeong contributed equally to this work.
B. Salvador-Culla and K.J. Jeong designed and conducted the study, B. Salvador-Culla, K.J. Jeong and H.H. Chiang collected the data; B. Salvador-Culla, K.J. Jeong, C.H. Dohlman, and D.S. Kohane were responsible for management; B. Salvador-Culla, J.J. Jeong, and P.E. Kolovou performed analysis; B. Salvador-Culla, K.J. Jeong, P.E. Kolovou, J. Chodosh, C.H. Dohlman, and D.S. Kohane interpreted the data; B. Salvador-Culla and P.E. Kolovou helped with manuscript preparation; all authors reviewed and approved the manuscript.
Disclosure: B. Salvador-Culla (E); K.J. Jeong, None; P.E. Kolovou (E); H.H. Chiang, None; J. Chodosh (E); C.H. Dohlman (E); D.S. Kohane, None
References
- 1. Dohlman CH,, Schneider HA,, Doane MG. Prosthokeratoplasty. Am J Ophthalmol. 1974; 77: 694–670. [DOI] [PubMed] [Google Scholar]
- 2. Todani A,, Ciolino JB,, Ament JD,, et al. Titanium back plate for a PMMA keratoprosthesis: clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2011. 249; 10: 1515–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doane MG,, Dohlman CH,, Bearse G. Fabrication of a keratoprosthesis. Cornea. 1996; 15: 179–184. [DOI] [PubMed] [Google Scholar]
- 4. Colby KA,, Koo EB. Expanding indications for the Boston keratoprosthesis. Curr Opin Ophthalmol. 2011; 22: 267–273. [DOI] [PubMed] [Google Scholar]
- 5. Shapiro BL,, Cortés DE,, Chin EK,, et al. High-resolution spectral domain anterior segment optical coherence tomography in type 1 Boston keratoprosthesis. Cornea. 2013; 32: 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Behlau I,, Martin KV,, Martin JN,, et al. Infectious endophthalmitis in Boston keratoprosthesis: incidence and prevention. Acta Ophthalmol. 2014; 92: 546–555. [DOI] [PubMed] [Google Scholar]
- 7. Chan CC,, Holland EJ. Infectious endophthalmitis after Boston type 1 keratoprosthesis implantation. Cornea. 2012; 31: 346–349. [DOI] [PubMed] [Google Scholar]
- 8. Henry PJ. Clinical experiences with dental implants. Adv Dent Res. 1999; 13: 147–152. [DOI] [PubMed] [Google Scholar]
- 9. Theis JC,, Gambhir S,, White J. Factors affecting implant retention in infected joint replacements. ANZ J Surg. 2007; 77: 877–879. [DOI] [PubMed] [Google Scholar]
- 10. Hacking SA,, Pauyo T,, Lim L,, et al. Tissue response to the components of a hydroxyapatite-coated composite femoral implant. J Biomed Mater Res A. 2010; 94: 953–960. [DOI] [PubMed] [Google Scholar]
- 11. Branemark PI,, Hansson BO,, Adell R,, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977; 16: 1–132. [PubMed] [Google Scholar]
- 12. Baril E,, Lefebvre LP,, Hacking SA. Direct visualization and quantification of bone growth into porous titanium implants using micro computed tomography. J Mater Sci Mater Med. 2011; 22: 1321–1332. [DOI] [PubMed] [Google Scholar]
- 13. Tan XW,, Perera AP,, Tan A,, et al. Comparison of candidate materials for a synthetic osteo-odonto keratoprosthesis device. Invest Ophthalmol Vis Sci. 2011; 52: 21–29. [DOI] [PubMed] [Google Scholar]
- 14. Tan XW,, Beuerman RW,, Shi ZL,, et al. In vivo evaluation of titanium oxide and hydroxyapatite as an artificial cornea skirt. J Mater Sci Mater Med. 2012; 23: 1063–1072. [DOI] [PubMed] [Google Scholar]
- 15. Hacking SA,, Harvey E,, Roughley P,, et al. The response of mineralizing culture systems to microtextured and polished titanium surfaces. J Orthop Res. 2008; 26: 1347–1354. [DOI] [PubMed] [Google Scholar]
- 16. Hacking SA,, Boyraz P,, Powers BM,, et al. Surface roughness enhances the osseointegration of titanium headposts in non-human primates. J Neurosci Methods. 2012; 211: 237–244. [DOI] [PubMed] [Google Scholar]
- 17. Paschalis EI,, Chodosh J,, Spurr-Michaud S,, et al. In vitro and in vivo assessment of titanium surface modification for coloring the backplate of the Boston keratoprosthesis. Invest Ophthalmol Vis Sci. 2013; 54: 3863–3873. [DOI] [PubMed] [Google Scholar]
- 18. Ament JD,, Spurr-Michaud SJ,, Dohlman CH,, et al. The Boston Keratoprosthesis: comparing corneal epithelial cell compatibility with titanium and PMMA. Cornea. 2009; 28: 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salvador-Culla B,, Dohlman CH. Keratoprosthesis. Lemp M,, Benítezdel Castillo J, Ocular Surface Disorders. London: JP Medical Ltd; 2013. [Google Scholar]
- 20. Linnola RJ,, Happonen RP,, Andersson OH,, et al. Titanium and bioactive glass-ceramic coated titanium as materials for keratoprosthesis. Exp Eye Res. 1996; 63: 471–478. [DOI] [PubMed] [Google Scholar]
- 21. Aronsson BO,, Lausmaa J,, Kasemo B. Glow discharge plasma treatment for surface cleaning and modification of metallic biomaterials. J Biomed Mater Res. 1997; 35: 49–73. [DOI] [PubMed] [Google Scholar]
- 22. Ou J,, Wang J,, Zhang D,, et al. Fabrication and biocompatibility investigation of TiO(2) films on the polymer substrates obtained via a novel and versatile route. Colloids Surf B Biointerfaces. 2010; 76: 123–127. [DOI] [PubMed] [Google Scholar]
- 23. Wang L,, Jeong KJ,, Chiang HH,, et al. Hydroxyapatite for keratoprosthesis biointegration. Invest Ophthalmol Vis Sci. 2011; 52: 7392–7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen CH,, Chen SC. The efficacy of non-lactate-generating metabolites as substrates for maintaining donor tissues. Transplantation. 1994; 57: 1778–1785. [PubMed] [Google Scholar]
- 25. Chen CH,, Rama P,, Chen SC,, et al. Efficacy of organ preservation media enriched with nonlactate-generating substrate for maintaining tissue viability: a transplantation study. Transplantation. 1997; 63: 656–663. [DOI] [PubMed] [Google Scholar]
- 26. Wagner D,, Naumkin AV,, Kraut-Vass A,, et al. Rumble, NIST Standard Reference Database 20, version 3.4 (web version), 2003. Available at: http:/srdata.nist.gov/xps/.
- 27. Martin JY,, Schwartz Z,, Hummert TW,, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995; 29: 389–401. [DOI] [PubMed] [Google Scholar]
- 28. Kim MJ,, Kim CW,, Lim YJ,, et al. Microrough titanium surface affects biologic response in MG63 osteoblast-like cells. J Biomed Mater Res A. 2006; 79: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 29. Jeong KJ,, Kohane DS. Surface modification and drug delivery for biointegration. Ther Deliv. 2011; 2: 737–752. [DOI] [PubMed] [Google Scholar]
- 30. Crnej A,, Paschalis EI,, Salvador-Culla B,, et al. Glaucoma progression and role of glaucoma surgery in patients with Boston keratoprosthesis. Cornea. 2014; 33: 349–354. [DOI] [PubMed] [Google Scholar]
- 31. Zerbe BL,, Belin MW,, Ciolino JB,, et al. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006; 113: 1779.e1–e7. [DOI] [PubMed] [Google Scholar]
- 32. Aldave AJ,, Sangwan VS,, Basu S,, et al. International results with the Boston type I keratoprosthesis. Ophthalmology. 2012; 119: 1530–1538. [DOI] [PubMed] [Google Scholar]
- 33. Sivaraman KR,, Hou JH,, Allemann N,, et al. Retroprosthetic membrane and risk of sterile keratolysis in patients with type I Boston Keratoprosthesis. Am J Ophthalmol. 2013; 155: 814–822. [DOI] [PubMed] [Google Scholar]
- 34. Srikumaran D,, Munoz B,, Aldave AJ,, et al. Long-term outcomes of Boston Type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology. 2014; 121: 2159–2164. [DOI] [PubMed] [Google Scholar]
- 35. Ciolino JB,, Belin MW,, Todani A,, et al. Retention of the Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. 2013; 120: 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Durand ML,, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea. 2009; 28: 896–901. [DOI] [PubMed] [Google Scholar]
- 37. Jain V,, Mhatre K,, Shome D,, et al. Fungal keratitis with the type 1 Boston keratoprosthesis: early Indian experience. Cornea. 2012; 31: 841–843. [DOI] [PubMed] [Google Scholar]
- 38. Andrei C,, Lestini E,, Crosbie S,, et al. Plasmonic enhancement of dye sensitized solar cells via a tailored size-distribution of chemically functionalized gold nanoparticles. PLoS One. 2014; 9: e109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terriza A,, Díaz-Cuenca A,, Yubero F,, et al. Light induced hydrophilicity and osteoblast adhesion promotion on amorphous TiO2. J Biomed Mater Res A. 2013; 101: 1026–1035. [DOI] [PubMed] [Google Scholar]
- 40. Teixeira AI,, Nealey PF,, Murphy CJ. Responses of human keratocytes to micro- and nanostructured substrates. J Biomed Mater Res A. 2004; 71: 369–376. [DOI] [PubMed] [Google Scholar]