Abstract
Background
Squamous cell carcinoma of the head and neck can present as a cervical metastasis from an unknown primary site. Recently, transoral robotic surgery (TORS) and transoral laser microsurgery (TLM) have been incorporated in the workup of unknown primary tumors.
Methods
We searched MEDLINE, EMBASE, Cochrane, and CINAHL from inception to June 2015 for all English-language studies that utilized TORS, TLM, or lingual tonsillectomy in the approach to an unknown primary.
Results
Of 217 identified studies, eight were reviewed. TORS/TLM identified the primary tumor in 111/139 (80 %) patients overall, and 36/54 (67 %) patients with no remarkable findings following physical exam, radiologic imaging, and panendoscopy with directed biopsies. Lingual tonsillectomy identified the primary tumor in 18/25 (72 %) patients with no findings. Hemorrhage (5 %) was the most common perioperative complication.
Conclusion
Lingual tonsillectomy using new approaches such as TORS/TLM may improve the identification of occult primary tumors.
Keywords: Unknown primary, TORS, TLM, Lingual tonsillectomy, Cervical metastases
Background
Cervical metastases from an unknown primary tumor site account for 2 to 5 % of all squamous cell carcinoma of the head and neck [1, 2]. Identification of the primary site may have an impact on disease control and survival, in addition to potentially minimizing treatment-related toxicity from large volume head and neck mucosal irradiation [2–7].
The standard workup of an unknown primary consists of a history, physical examination with flexible endoscopy, and diagnostic imaging such as computed tomography (CT) and/or magnetic resonance imaging (MRI). Positron-emission tomography (PET), alone or fused with CT images (PET-CT), may improve the diagnostic sensitivity when traditional imaging modalities fail to localize a primary tumor [1, 4, 8, 9]. When the primary tumor remains elusive despite these modalities, examination under anesthesia with panendoscopy and directed biopsies of the nasopharynx, hypopharynx, and oropharynx has been the traditional approach. The definition of an unknown primary is neither absolute nor static; a primary tumor site identified by any diagnostic modality is, by definition, no longer an unknown primary. Despite this extensive workup, however, over 50 % of primary tumors remain undiscovered [1, 3, 10, 11].
In the absence of a visible or palpable lesion, a palatine tonsillectomy may improve the diagnostic yield of an occult primary tumor compared to deep tonsil biopsies [12–14], as many occult primaries maybe hidden deep in tonsillar crypts. [12] Given that 80–90 % of occult primary tumors are eventually localized in the palatine tonsil and tongue base, palatine and lingual tonsillectomies have been recognized as important additions to the diagnostic workup of an unknown primary [1, 5, 10].
Recently, Transoral Laser Microsurgery (TLM) and Transoral Robotic Surgery (TORS) have emerged as effective modalities to aid in the identification and treatment of an unknown primary tumor. These techniques provide enhanced visualization and maneuverability, allowing for a complete resection of the entire tongue base mucosa and lingual tonsils, a procedure which is challenging to perform using traditional instrumentation and visualization [15, 16]. Recent case series of occult primary tumors have reported high rates of detection ranging from 86 to 94 % using TLM [16, 17], and 72 to 90 % using TORS [15, 18, 19]. However, these studies contain small, heterogeneous patient populations with variable preoperative investigations and findings, and thus cannot be directly compared.
The present study aims to conduct a systematic review of the literature to determine the incremental benefit of lingual tonsillectomy using TORS/TLM in localizing the primary tumor site of regionally metastatic head and neck squamous cell carcinoma of unknown origin.
Methods
Search strategy
A systematic review of published reports on TORS or TLM for the workup of CUP was performed. MEDLINE, EMBASE, Cochrane Central Register, and CINAHL were searched from inception to June 2015 for all relevant English-language studies. Medical Subject Headings and keywords specifying histopathology (e.g. squamous cell carcinoma), location (e.g. head and neck, cervical metastases), unknown primary, and diagnostic approach (e.g. TORS, TLM, or lingual tonsillectomy) were used to identify studies. Bibliographies of all included studies were also searched for relevant articles.
Selection criteria
Two reviewers (T.F. & A.F.) independently screened all identified studies by title and abstract for further full text review, and then independently reviewed these studies for eligibility (Fig. 1). Studies were included if they used TORS, TLM, or lingual tonsillectomy via TORS/TLM in the diagnostic approach to head and neck squamous cell carcinoma of unknown primary origin. Non-English and non-original studies (i.e. reviews) were excluded. When multiple studies were published by a single institution, only the most recent study was included to avoid inclusion of the same patients more than once in the review. Disagreements were resolved by consensus.
Fig. 1.
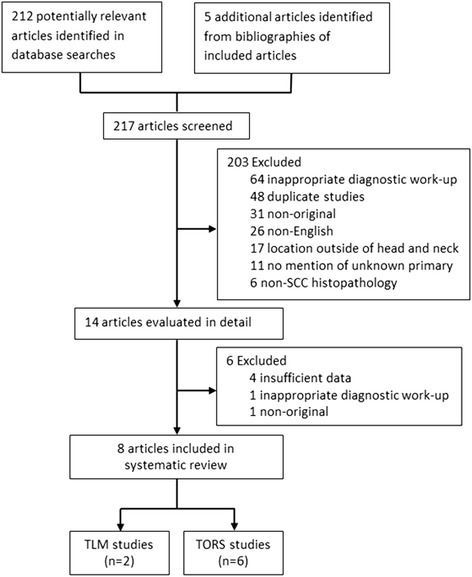
Selection of studies for systematic review
Data extraction & statistical analysis
Data were extracted in duplicate by two reviewers (T.F. & A.F.). The primary outcome was the identification rate of an unknown primary site using TORS, TLM, or lingual tonsillectomy performed using TORS/TLM. Information on study design, patient and tumor characteristics, diagnostic workup, margin status, and perioperative complications was also extracted. Subgroup analysis of identification rates were performed based on the presence or absence of positive findings on preoperative investigations including [1]: physical examination (PE) [2], diagnostic imaging (DI) consisting of computed tomography or magnetic resonance imaging (CT or MRI) [3], positron emission tomography-computed tomography (PET-CT) [4], a combination of PE/DI/PET-CT, and [5] examination under anesthesia (EUA) with directed biopsies of the nasopharynx, hypopharynx, tonsil, and base of tongue. Data were aggregated using Microsoft Excel 2010 (Microsoft Corp., Redmond, Washington), and all statistical analyses were conducted using SPSS version 21.0 (SPSS Inc., Chicago, Illinois).
Results
Study selection
The literature search identified a total of 217 articles (Fig. 1). Excluded studies included those that did not use TORS, TLM, or lingual tonsillectomy (64), duplicates (48), non-original studies (31), non-English studies (26), studies of non-head and neck neoplasms (17), studies without mention of unknown primary (11), and those reporting on non-SCC histopathology (6). Of the 14 remaining studies, three were follow-up studies [20–22] from the same institution, one study [23] was excluded due to insufficient data, one study [24] did not use TORS or TLM in the diagnostic workup, and one study [25] was a review paper. Inter-rater agreement for study inclusion was excellent (κ = 0.92).
Eight studies containing a total of 139 patients met the final inclusion criteria [15–19, 26–28]. Of these eight studies, six studies [15, 18, 19, 26–28] reported outcomes for 85 patients undergoing TORS for workup of an unknown primary, and two studies [16, 17] reported outcomes for 54 patients undergoing TLM.
Study characteristics
Characteristics of the eight included studies are summarized in Table 1. Included studies were case series or case reports published between 2011 and 2014. All were single-institution studies aside from one study [15] which pooled data from six institutions.
Table 1.
Summary of studies included in systematic review
| Authors | Year | Institution | No. Pts (N = 139) |
|---|---|---|---|
| Abuzeid et al. [26] | 2011 | University of Michigan | 1 |
| Blanco et al. [28] | 2013 | Johns Hopkins School of Medicine | 4 |
| Durmus et al. [18] | 2013 | Ohio State University Wexner Medical Center | 22 |
| Karni et al. [16] | 2011 | Washington University School of Medicine | 18 |
| Mehta et al. [19] | 2013 | University of Pittsburgh Medical Center | 10 |
| Mourad et al. [27] | 2013 | Albert Einstein College of Medicine | 1 |
| Nagel et al. [17] | 2014 | Mayo Clinic Arizona | 36 |
| Patel et al. [15] | 2013 | University of Washington Medical Center, University of Texas MD Anderson Cancer Center, University of Alabama-Birmingham Hospital, University of Texas Medical School at Houston, Johns Hopkins Hospital, Oregon Health Sciences University | 47 |
Patient characteristics are summarized in Table 2. The mean age of patients undergoing TORS or TLM was 57.3 years (standard deviation [SD] 2.1, range 44–78 years). Patients were predominantly male (88 %), and the majority (82 %) of the 65 patients with a reported p16 status were positive [15, 18, 19, 26, 27]. Of the 94 patients with known nodal status, 19 (20 %) were N1, 62 (66 %) were N2, and 13 (14 %) were N3. The mean diameter of identified primary tumors was 1.15 cm (SD 0.79 cm, range 0.2 to 3.0 cm). Of 71 patients with known margin status, 44 (62 %) had negative margins [15, 18, 26, 27].
Table 2.
Characteristics of patients from included studies
| Characteristic | No. Pts (%) (N = 139) |
|---|---|
| Age, mean (SD) | 57.3 (2.1) |
| Sex | |
| Female | 16 (12 %) |
| Male | 119 (88 %) |
| na | 4 |
| HPV | |
| + | 53 (82 %) |
| - | 12 (18 %) |
| na | 74 |
| Nodal status | |
| N1 | 19 (20 %) |
| N2 | 62 (66 %) |
| N3 | 13 (14 %) |
| na | 45 |
| Size, mean cm (SD) | 1.15 (79 %) |
| Negative Margins | 44 (62 %) |
Diagnostic workup of unknown primary
The diagnostic workup for an unknown primary was highly variable between institutions as shown in Table 3. PE findings were suspicious for a primary tumor in 24 of 135 (18 %) patients. Nine of 89 (10 %) patients had suspicious findings on DI, and 17 of 39 (44 %) patients had findings on PET-CT scan. Of the 78 patients undergoing a full diagnostic workup including PE/DI/PET-CT, 43 (55 %) had suspicious findings. EUA with biopsies of the nasopharynx, hypopharynx, and oropharynx revealed remarkable findings in 12 of 52 (23 %) patients. All 12 patients with findings on EUA received a lingual tonsillectomy using TORS.
Table 3.
Diagnostic workup and proportion of patients with suspicious findings (n = 139)
| Investigation | Proportion of patients with suspicious findings | Proportion of patients without suspicious findings | No. Patients with Missing Data |
|---|---|---|---|
| Physical Exam | 24/135 (18 %) | 111/135 (82 %) | 4 |
| DI (CT/MRI) | 9/89 (10 %) | 80/89 (90 %) | 41 |
| PET-CT | 17/39 (44 %) | 22/39 (56 %) | 100 |
| PE/DI/PET-CT | 43/78 (55 %) | 35/78 (45 %) | 61 |
| EUA with biopsy | 12/52 (23 %) | 40/52 (77 %) | 87 |
Abbreviations: DI diagnostic imaging, CT computed tomography, MRI magnetic resonance imaging, PE physical examination, PET positron emission tomography, EUA panendoscopic examination under anesthesia
A total of 108 of 139 patients (78 %) underwent lingual tonsillectomy by TORS or TLM. Of the 90 patients with available information, 36 (40 %) had ipsilateral lingual tonsillectomy and 54 (60 %) had bilateral lingual tonsillectomy. Three studies [17, 19, 26] explicitly described the procedure for performing lingual tonsillectomy. The procedure was generally consistent across all three institutions and involved complete resection of the lingual tonsil from the midline of the tongue to the lateral pharyngeal wall, and from the circumvallate papillae to the vallecula, using the muscular layer as the deep plane of dissection.
A total of 70 of 103 (68 %) patients underwent palatine tonsillectomy by TORS or TLM. Palatine tonsillectomy was either not performed or not reported in the remainder of the patients (36 of 139) for the following reasons: (i) 20 patients in one series [15] did not undergo palatine tonsillectomy, (ii) at least ten patients had a previous childhood tonsillectomy [18, 19, 26], and (iii) one study [17] did not report the frequency of palatine tonsillectomies in the TORS/TLM group. Among the 55 patients undergoing palatine tonsillectomy with available information, 24 (44 %) had ipsilateral tonsillectomy while 31 (56 %) had bilateral tonsillectomy.
Identification of unknown primary using TORS/TLM
Overall, TORS/TLM successfully localized the primary tumor in 111 of 139 (80 %) patients, as shown in Table 4. An occult primary was identified in 60 of 108 (56 %) patients undergoing lingual tonsillectomy and 34 of 70 (49 %) patients undergoing palatine tonsillectomy using TORS or TLM. One patient undergoing TORS had synchronous primary tumors found in the palatine and lingual tonsils [15]. The location of the primary tumor was not specified in the remaining 18 of 111 patients with an occult tumor found on TLM [17].
Table 4.
Overall identification rate of unknown primary with TORS/TLM
| Author | Method | Proportion identified with TORS/TLM | Proportion identified with lingual tonsillectomy using TORS/TLM | Proportion identified with palatine tonsillectomy using TORS/TLM |
|---|---|---|---|---|
| Abuzeid et al. [26] | TORS | 1/1 (100 %) | 1/1 (100 %) | 0/0 (0 %)a |
| Blanco et al. [28] | TORS | 1/4 (25 %) | 0/4 (0 %) | 1/4 (25 %) |
| Durmus et al. [18] | TORS | 17/22 (77 %) | 4/14 (29 %) | 13/17 (76 %) |
| Karni et al. [16] | TLM | 17/18 (94 %) | 11/18 (61 %) | 6/18 (33 %) |
| Mehta et al. [19] | TORS | 9/10 (90 %) | 9/10 (90 %) | 0/3 (0 %)b |
| Mourad et al. [27] | TORS | 1/1 (100 %) | 1/1 (100 %) | 0/1 (0 %) |
| Nagel et al. [17] | TLM | 31/36 (86 %) | 13/19 (68 %) | - |
| Patel et al. [15] | TORS | 34/47 (72 %) | 21/41 (51 %) | 14/27 (52 %)c |
| Total | TORS/TLM | 111/139 (80 %) | 60/108 (56 %) | 34/70 (49 %) |
aPatient had childhood tonsillectomy
bSeven of ten patients had childhood tonsillectomy
cOne patient had synchronous lingual/palatine tonsil tumors
Identification rates for subgroups of patients with positive or negative findings during preoperative investigations are shown in Fig. 2 and Table 5. In some studies, identification rates were reported for the entire cohort and not stratified by subgroup of patients with or without abnormal findings, thus limiting the extractable data. Only one study [26] described the identification rate in patients with positive physical exam findings. The occult primary was eventually localized in this patient (100 %) with suspicious findings on PE. In contrast, the identification rate was 86 % (75 of 87) among patients without exam findings [16–19, 27]. The primary tumor was also identified in 1 of 1 (100 %) patient with suspicious findings on DI [26], and 43 of 50 (86 %) patients without DI findings [16, 18, 19]. A primary tumor was identified in six of six (100 %) patients with remarkable findings on PET-CT [19, 26, 27], and five of six (83 %) patients without PET-CT findings [19]. TORS/TLM localized the primary tumor site in 34 of 43 (79 %) patients with remarkable findings on either PE, DI, or PET-CT [15, 18, 19, 26, 27], and 25 of 35 (71 %) patients without findings on these investigations [15, 18, 19]. In addition, a primary tumor was identified in 11 of 12 (92 %) patients with findings on EUA with directed biopsies [18, 27], but only 36 of 54 (67 %) patients without EUA findings [15, 17–19, 26]. Although a total of 34 palatine tonsil primaries were identified, the location was specified in only 13 cases [18]. Of these 13 cases, 11 (85 %) were identified in the ipsilateral tonsil, and 2 (15 %) were found in the contralateral tonsil.
Fig. 2.
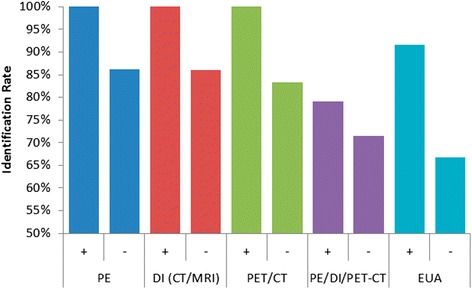
Identification of unknown primary using TORS/TLM in the presence (+) or absence (-) of other findings
Table 5.
Identification rate of TORS/TLM in the presence of other findings
| Author | Physical Exam | DI (CT/MRI) | PET/CT | PE/DI/PET-CT | EUA with biopsy | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | - | + | - | + | - | + | - | + | - | |
| Abuzeid et al. [26] | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 0/0 (0 %) | 1/1 (100 %) |
| Blanco et al. [28] | - | - | - | - | - | - | - | - | - | - |
| Durmus et al. [18] | 0/0 (0 %) | 17/22 (77 %) | 0/0 (0 %) | 17/22 (77 %) | - | - | 10/11 (91 %) | 7/11 (64 %)a | 10/11 (91 %) | 7/11 (64 %) |
| Karni et al. [16] | 0/0 (0 %) | 17/18 (94 %) | 0/0 (0 %) | 17/18 (94 %) | - | - | - | - | - | - |
| Mehta et al. [19] | 0/0 (0 %) | 9/10 (90 %) | 0/0 (0 %) | 9/10 (90 %) | 4/4 (100 %) | 5/6 (83 %) | 4/4 (100 %) | 5/6 (83 %) | 0/0 (0 %) | 9/10 (90 %) |
| Mourad et al. [27] | 0/0 (0 %) | 1/1 (100 %) | - | - | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) |
| Nagel et al. [17] | 0/0 (0 %) | 31/36 (86 %) | - | - | - | - | - | - | - | 8/14 (57 %) |
| Patel et al. [15] | - | - | - | - | - | - | 18/26 (69 %)b | 13/18 (72 %) | 0/0 (0 %) | 11/18 (61 %)c |
| Total | 1/1 (100 %) | 75/87 (86 %) | 1/1 (100 %) | 43/50 (86 %) | 6/6 (100 %) | 5/6 (83 %) | 34/43 (79 %) | 25/35 (71 %) | 11/12 (92 %) | 36/54 (67 %) |
Abbreviations: DI diagnostic imaging, CT computed tomography, MRI magnetic resonance imaging, PE physical examination, PET positron emission tomography, EUA panendoscopic examination under anesthesia
aNo suspicious findings on PET/CT, EUA, directed biopsies, or robotic exam
bDenominator was calculated as 47 total patients minus 18 patients without positive findings minus three patients who did not undergo radiographic imaging before TORS
cFailed deep tongue base biopsy
Identification of unknown primary using lingual tonsillectomy
Similarly, identification rates were recorded for a subgroup of patients who underwent lingual tonsillectomy performed using TORS or TLM (Fig. 3 and Table 6). A primary tumor site was localized in 1 of 1 (100 %) patient with suspicious PE findings [26], and 38 of 62 (61 %) of patients without suspicious findings [16–19, 27]. The primary tumor was also identified in the same 1 of 1 (100 %) patient who also had findings on DI [26], and 24 of 42 (57 %) patients without findings on DI [16, 18, 19]. Lingual tonsillectomy identified the primary tumor in six of six (100 %) patients with remarkable findings on PET-CT [19, 26, 27], and five of six (83 %) without PET-CT findings [19]. Of the 31 patients with suspicious findings on either PE, DI, or PET-CT, 19 (61 %) were successfully identified [15, 19, 26, 27], while 13 of 22 (59 %) patients without findings were identified [15, 19]. A primary tumor was identified in 1 of 1 (100 %) patient with positive findings on EUA with biopsy [27], and 18 of 25 (72 %) patients without findings on EUA [17, 19, 26]. Although a total of 60 primaries were identified in the lingual tonsils, the location was specified for only 49 patients [15, 17–19, 26, 27]. Of these 49 tumors, 46 (94 %) were identified in the ipsilateral base of tongue and 3 (6 %) were found in the contralateral base of tongue.
Fig. 3.
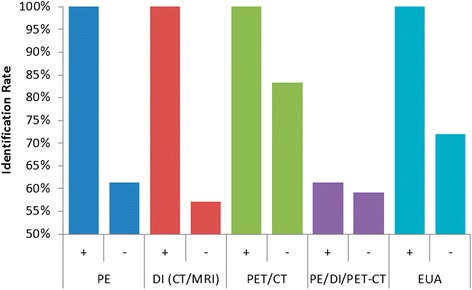
Identification of unknown primary using lingual tonsillectomy in the presence (+) or absence (-) of other findings. Abbreviations: PE, physical examination; DI, diagnostic imaging; CT, computed tomography; MRI, magnetic resonance imaging; PET, position emitted tomography; EUA, examination under anesthesia with directed biopsy
Table 6.
Identification rate of lingual tonsillectomy in the presences of other findings
| Author | Physical Exam | DI (CT/MRI) | PET/CT | PE/DI/PET-CT | EUA with biopsy | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | - | + | - | + | - | + | - | + | - | |
| Abuzeid et al. [26] | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 0/0 (0 %) | 1/1 (100 %) |
| Blanco et al. [28] | - | - | - | - | - | - | - | - | - | - |
| Durmus et al. [18] | 0/0 (0 %) | 4/14 (29 %) | 0/0 (0 %) | 4/14 (29 %) | - | - | - | - | - | - |
| Karni et al. [16] | 0/0 (0 %) | 11/18 (61 %) | 0/0 (0 %) | 11/18 (61 %) | - | - | - | - | - | - |
| Mehta et al. [19] | 0/0 (0 %) | 9/10 (90 %) | 0/0 (0 %) | 9/10 (90 %) | 4/4 (100 %) | 5/6 (83 %) | 4/4 (100 %) | 5/6 (83 %) | 0/0 (0 %) | 9/10 (90 %) |
| Mourad et al. [27] | 0/0 (0 %) | 1/1 (100 %) | - | - | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) | 1/1 (100 %) | 0/0 (0 %) |
| Nagel et al. [17] | 0/0 (0 %) | 13/19 (68 %) | - | - | - | - | - | - | - | 8/14 (57 %) |
| Patel et al. [15] | - | - | - | - | - | - | 13/25 (52 %) | 8/16 (50 %) | - | - |
| Total | 1/1 (100 %) | 38/62 (61 %) | 1/1 (100 %) | 24/42 (57 %) | 6/6 (100 %) | 5/6 (83 %) | 19/31 (61 %) | 13/22 (59 %) | 1/1 (100 %) | 18/25 (72 %) |
Abbreviations: DI diagnostic imaging, CT computed tomography, MRI magnetic resonance imaging, PE physical examination, PET positron emission tomography, EUA panendoscopic examination under anesthesia
Adverse events for TORS/TLM
Table 7 shows the adverse events reported in studies of TORS or TLM. In total, six studies [15, 17–19, 26, 27] reported overall perioperative complication rates for 117 patients and all eight studies [15–19, 26–28] reported perioperative mortality rates for 139 patients. Additionally, four studies [15, 17, 19, 28] reported hemorrhage rates for 97 patients, one study [18] reported tracheostomy rates for 22 patients, three studies [18, 19, 28] reported gastrostomy rates for 36 patients, and five studies [17–19, 26, 28] commented on return to diet for 73 patients.
Table 7.
Adverse events following TORS/TLM
| Author | Hemorrhage | Tracheostomy | Gastrostomy | No Return to diet | Other | Deaths | Total Complications |
|---|---|---|---|---|---|---|---|
| Abuzeid et al. [26] | - | - | - | 0/1 (0 %) | - | 0/1 (0 %) | 0/1 (0 %) |
| Blanco et al. [28] | 0/4 (0 %) | - | 0/4 (0 %) | 0/4 (0 %) | 0/4 (0 %)a | 0/4 (0 %) | - |
| Durmus et al. [18] | 0/22 (0 %) | 0/22 (0 %) | 0/22 (0 %) | - | 0/22 (0 %) | 0/22 (0 %) | |
| Karni et al. [16] | - | - | - | - | - | 0/18 (0 %) | - |
| Mehta et al. [19] | 0/10 (0 %) | - | 1/10 (10 %)b | 1/10 (10 %)b | - | 0/10 (0 %) | 2/10 (20 %) |
| Mourad et al. [27] | - | - | - | - | - | 0/1 (0 %) | 0/1 (%) |
| Nagel et al. [17] | 1/36 (3 %)c | - | - | 0/36 (0 %) | 0/36 (0 %) | 0/36 (0 %) | 1/36 (3 %) |
| Patel et al. [15] | 4/47 (9 %)d | - | - | - | 1/47 (2 %)e | 0/47 (0 %) | 5/47 (11 %) |
| Total | 5/97 (5 %) | 0/22 (0 %) | 1/36 (3 %) | 1/73 (1 %) | 1/87 (1 %) | 0/139 (0 %) | 8/117 (7 %) |
aNo patients developed esophageal strictures
bPatient was a heavy smoker (60 packs/year) with an identified HPV-negative 2.0 cm submucosal tongue base tumor
cPostoperative tonsil bleed requiring return to OR
dTwo patients required return to OR
eOne patient had tongue swelling requiring one additional day of observation before discharge
The most common complication was hemorrhage in 5 of 97 (5 %) patients, of which three (3 %) required return to the operating room for hemostasis. None (0 %) of the 22 patients with available outcomes required tracheostomy, and only 1 of 36 (3 %) patients required a gastrostomy tube. In this single patient, the requirement for a permanent gastrostomy tube was due to adjuvant chemoradiation and heavy tobacco use in the post-operative period [19]. Furthermore, only 1 of 73 (1 %) patients did not tolerate return to diet within 24 h post-operatively. Other perioperative complications such as tongue swelling [15] occurred in 1 of 87 (1 %) patients. There were no perioperative deaths resulting from TORS or TLM.
Discussion
Localization of the primary tumor in patients with cervical metastasis of unknown origin remains a challenging yet important goal. When available diagnostic modalities fail to detect a primary tumor, treatment typically consists of large volume radiation to the neck as well as potential primary mucosal sites with or without chemotherapy, or neck dissection with or without adjuvant chemoradiation [2–7]. Head and neck irradiation may be associated with dysphagia, xerostomia, mucosal atrophy, and osteoradionecrosis of the jaw [11, 29, 30]. Identification of the primary tumor site may mitigate these risks by minimizing radiotherapy volumes and also allowing for more directed radiation, potentially sparing the pharyngeal constrictors, salivary glands, and mandible. Furthermore, depending on the margin status and pathological features of the primary tumor identified (and resected) by any of these approaches, one may elect to avoid radiotherapy to mucosal surfaces and manage the neck disease in isolation. The implications of this strategy warrant further study.
The goal of this systematic review was to determine the effectiveness of TORS and TLM in localizing an occult primary tumor and to elucidate the role of these techniques within the traditional diagnostic paradigm. Our findings demonstrated that TORS/TLM can increase the detection of occult primary tumors at all stages of the diagnostic workup. We also aimed to determine the incremental benefit of using these techniques by analyzing the identification rate of unknown primaries in a subgroup of patients undergoing lingual tonsillectomies. Many of the patients who are managed with TORS and TLM undergo a palatine tonsillectomy in addition to lingual tonsillectomy. While a palatine tonsillectomy can be performed using more cost-effective traditional approaches, a lingual tonsillectomy, on the other hand, may require the superior visualization and exposure afforded by these techniques. In the present study, the identification rate of a primary tumor using lingual tonsillectomy was 60/108 (56 %).
Currently, there is no standard diagnostic algorithm for an unknown primary tumor. The typical workup includes physical examination and diagnostic imaging consisting of CT and/or MRI. The addition of PET and PET/CT have resulted in improved detection rates ranging from 15 to 28 % [1, 4, 9, 11, 31] and 32 to 44 % [4, 8, 32], respectively. Studies have also reported successful primary tumor identification using PET/CT in the presence of unremarkable findings on physical examination, imaging, and panendoscopy, with identification rates ranging from 28 to 37 % [4, 33, 34]. However, PET and PET/CT does not reliably detect tumors smaller than 8 to 10 mm in diameter [35]. Interestingly, in our study of 111 identified primary tumors, we reported an average tumor diameter of 1.15 cm, with 57 % of primary tumors less than 10 mm in diameter. This finding may suggest that many of the primary tumors in this setting are below the detection level of PET-CT imaging. Another limitation of PET imaging is the high false-positive rate due to physiologic uptake in the lymphoid tissue of Waldeyer’s ring. Recent reviews have reported false-positive rates as high as 39 % for PET and 37 % for PET/CT [8, 9]. These false positives may (incorrectly) guide treating physicians to target treatment volumes based on the areas of uptake. Histopathologic corroboration with tissue is needed prior to making treatment decisions.
Surgical evaluation of an unknown primary involves the use of examination under anesthesia with biopsies of clinically and radiologically suspicious sites. Studies also show that palatine tonsillectomy improves the detection rate compared to tonsil biopsy in patients with demonstrable tonsillar tissue [12, 14]. Overall, a comprehensive diagnostic workup including physical examination, imaging, and panendoscopy with directed biopsies and/or tonsillectomy reveals a primary tumor site in 19 to 53 % of patients [1, 3–5, 10, 36]. However, in the absence of remarkable physical examination or radiological findings, detection rates are only 17 to 29 % [5, 10, 36].
In comparison, our present study demonstrates significantly higher identification rates using TORS/TLM compared to traditional diagnostic techniques, suggesting that evaluation of the lingual tonsil with the aid of TORS/TLM has clinical benefit in the work-up of unknown primary tumors. In contrast to the detection rates reported above, our review of the literature revealed an identification rate of 79 % in the presence of remarkable findings on physical examination and imaging, and a 92 % detection rate in the presence of remarkable findings on panendoscopy. Most importantly, the detection rate remained high at 71 % in the absence of findings on physical examination and imaging (including PET/CT), and 67 % even after failed EUA with directed biopsies. This highlights a potential role for TORS/TLM in the diagnostic algorithm of these patients as a “final step” after failed panendoscopy.
Similar findings were noted in the subgroup of patients undergoing lingual tonsillectomy using TORS or TLM. The detection rate was 61 % among patients with remarkable findings on physical examination and radiological imaging, and remained at 59 % among patients with unremarkable findings. Furthermore, lingual tonsillectomy was successful in identifying the primary tumor in 18 of 25 (72 %) patients even after failed EUA with biopsies. These data also support the use of TORS and TLM to perform a lingual tonsillectomy as a “last resort” when all other diagnostic modalities have failed to localize a primary tumor site.
Some authors advocate for upfront lingual tonsillectomy in the initial management of occult primary tumors rather than awaiting the results of directed biopsies of the pharynx [16, 17]. This approach may reduce the delay to diagnosis and definitive treatment, and also obviate the need for a second operation in the event of positive biopsy results. Our data showed that lingual tonsillectomy identified the primary tumor site in 60 of 108 patients (56 %) overall, supporting a potential role for upfront lingual tonsillectomy in select patients with unknown primary tumors. Disadvantages of this approach include a longer initial operation, and exposure to potentially unnecessary surgery and associated risks of perioperative complications. Our review of the literature revealed that the complication rate of TORS/TLM, while relatively low (7 %), was not zero [15, 17–19, 26]. The potential impact on quality of life (QOL) is another important consideration, with a recent study demonstrating a significant decline in multiple QOL domains such as speech, eating, aesthetics, and social disruption up to 12 months post-treatment with TORS [20]. Further research is needed to evaluate long-term QOL outcomes following TORS/TLM and investigate the role of lingual tonsillectomy in the initial work up of occult primary tumors.
Our findings corroborate previous studies that suggest that subsites of the oropharynx such as the palatine tonsil and tongue base are the most common sites of occult primary tumors [5, 10, 12, 15]. This is likely due to the fact that small primary tumors can be hidden in areas that are difficult to visualize such as the palatine and lingual tonsillar crypts. The justification for performing a palatine tonsillectomy for detection of a hidden primary tonsillar cancer can similarly be applied to the tongue base, where a lingual tonsillectomy is necessary to identify small hidden primaries.
The issue of “bilaterality” or contralateral tumor resection is one that warrants discussion. In our study, we report a contralateral primary tumor in the tongue base in 6 % of the contralateral tongue base and 15 % in the contralateral tonsil. This proportion is comparable to previous reports which have found bilateral or contralateral palatine tonsil disease in 10 to 23 % of identified primary tumors [13, 37, 38]. However, it remains unclear whether these represent multiple primary tumors or multicentric disease that has been described in human papillomavirus (HPV) mediated oropharyngeal carcinoma compared to isolated contralateral disease [24, 39, 40]. Regardless, these findings support the use of bilateral palatine and/or lingual tonsillectomy as part of a comprehensive diagnostic workup or staged resection of the contralateral palatine tonsil and lingual tonsil in the event that no primary is found on the ipsilateral side. However, clinical judgment is required to weigh potential benefits and risks, and determine the optimal approach for each individual patient [13–16].
This study is limited by the small sample size of included studies, particularly for the subgroup of patients receiving lingual tonsillectomy, as well as the heterogeneity between diagnostic workup performed at different institutions. This is not surprising given the relatively recent advent of this expanded surgical paradigm in the investigation of head and neck carcinoma of unknown primary. Our study highlights the need for a standardized diagnostic and treatment approach to unknown primary tumors that also considers emerging transoral surgical procedures such as TORS and TLM. Inter-institutional and inter-surgeon variation in the technique used to perform lingual tonsillectomy could have also affected our findings, particularly given that only three studies [17, 19, 26] provided a general description of this procedure. More frequent and detailed reporting on surgical technique is needed to further investigate the impact of inter-institutional variation on identification rates. Another potential limitation is publication bias, as institutions with more favorable results may be more likely to publish their findings, particularly for the newer TORS/TLM techniques. Despite these limitations, this is the first systematic review to comprehensively evaluate the use of TORS/TLM and lingual tonsillectomy specifically for the identification of primary head and neck squamous cell carcinoma with an unknown primary site. By pooling data from multiple institutions with varying methods of preoperative assessment, we were able to gather a relatively large sample size and minimize single-surgeon and single-institution biases. Future prospective studies with more patients and standardized diagnostic and treatment protocols are needed to further investigate the usefulness and cost-effectiveness of these newer transoral surgical techniques, and corroborate the encouraging results presented in this study.
Conclusion
This systematic review supports the use of TORS and TLM to aid in the identification of a primary head and neck squamous cell carcinoma of unknown origin, with superior detection rates compared to the traditional diagnostic workup. We also demonstrate that the addition of formal lingual tonsillectomy using TORS/TLM is a safe and effective option that can increase the yield of localizing an occult primary tumor. Identification of the primary tumor using minimally-invasive transoral techniques reduces treatment-induced morbidity and permits directed management, thereby potentially improving survival and functional outcomes.
Abbreviations
- CT
computed tomography
- DI
diagnostic imaging
- EUA
examination under anesthesia
- HPV
human papillomavirus
- MRI
magnetic resonance imaging
- PE
physical examination
- PET
positron-emission tomography
- QOL
quality of life.
- TLM
transoral laser microsurgery
- TORS
transoral robotic surgery
Footnotes
Competing interests
The authors declare that they have no financial or non-financial competing interests.
Authors’ contributions
All authors contributed extensively to the work presented in this article. TSF and JRD jointly conceived the study design. TSF collected and analyzed the data. TSF and AF interpreted the results and prepared the manuscript under the supervision of JRD AF and DPG provided technical support and conceptual advice. All authors read and approved the final manuscript.
References
- 1.Waltonen JD, Ozer E, Hall NC, Schuller DE, Agrawal A. Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg. 2009;135(10):1024–9. doi: 10.1001/archoto.2009.145. [DOI] [PubMed] [Google Scholar]
- 2.Strojan P, Ferlito A, Langendijk JA, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: II. a review of therapeutic options. Head Neck. 2013;35(2):286–93. doi: 10.1002/hed.21899. [DOI] [PubMed] [Google Scholar]
- 3.Pattani KM, Goodier M, Lilien D, Kupferman T, Caldito G, Nathan CO. Utility of panendoscopy for the detection of unknown primary head and neck cancer in patients with a negative PET/CT scan. Ear Nose Throat J. 2011;90(8):E16–20. doi: 10.1177/014556131109000818. [DOI] [PubMed] [Google Scholar]
- 4.Keller F, Psychogios G, Linke R, et al. Carcinoma of unknown primary in the head and neck: comparison between positron emission tomography (PET) and PET/CT. Head Neck. 2011;33(11):1569–75. doi: 10.1002/hed.21635. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall WM, Mancuso AA, Amdur RJ, Stringer SP, Villaret DB, Cassisi NJ. Squamous cell carcinoma metastatic to the neck from an unknown head and neck primary site. Am J Otolaryngol. 2001;22(4):261–7. doi: 10.1053/ajot.2001.24820. [DOI] [PubMed] [Google Scholar]
- 6.Koivunen P, Laranne J, Virtaniemi J, et al. Cervical metastasis of unknown origin: a series of 72 patients. Acta Otolaryngol. 2002;122(5):569–74. doi: 10.1080/00016480260092435. [DOI] [PubMed] [Google Scholar]
- 7.Oen AL, de Boer MF, Hop WC, Knegt P. Cervical metastasis from the unknown primary tumor. Eur Arch Otorhinolaryngol. 1995;252(4):222–8. doi: 10.1007/BF00179915. [DOI] [PubMed] [Google Scholar]
- 8.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Rad. 2009;19(3):731–44. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101(11):2641–9. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]
- 10.Cianchetti M, Mancuso AA, Amdur RJ, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119(12):2348–54. doi: 10.1002/lary.20638. [DOI] [PubMed] [Google Scholar]
- 11.Regelink G, Brouwer J, de Bree R, et al. Detection of unknown primary tumours and distant metastases in patients with cervical metastases: value of FDG-PET versus conventional modalities. Eur J Nuc Med Mol Imaging. 2002;29(8):1024–30. doi: 10.1007/s00259-002-0819-0. [DOI] [PubMed] [Google Scholar]
- 12.Waltonen JD, Ozer E, Schuller DE, Agrawal A. Tonsillectomy vs. deep tonsil biopsies in detecting occult tonsil tumors. Laryngoscope. 2009;119(1):102–6. doi: 10.1002/lary.20017. [DOI] [PubMed] [Google Scholar]
- 13.Koch WM, Bhatti N, Williams MF, Eisele DW. Oncologic rationale for bilateral tonsillectomy in head and neck squamous cell carcinoma of unknown primary source. Otolaryngol Head Neck Surg. 2001;124(3):331–3. doi: 10.1067/mhn.2001.114309. [DOI] [PubMed] [Google Scholar]
- 14.McQuone SJ, Eisele DW, Lee DJ, Westra WH, Koch WM. Occult tonsillar carcinoma in the unknown primary. Laryngoscope. 1998;108(11 Pt 1):1605–10. doi: 10.1097/00005537-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Patel SA, Magnuson JS, Holsinger FC, et al. Robotic surgery for primary head and neck squamous cell carcinoma of unknown site. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1203–11. doi: 10.1001/jamaoto.2013.5189. [DOI] [PubMed] [Google Scholar]
- 16.Karni RJ, Rich JT, Sinha P, Haughey BH. Transoral laser microsurgery: a new approach for unknown primaries of the head and neck. Laryngoscope. 2011;121(6):1194–201. doi: 10.1002/lary.21743. [DOI] [PubMed] [Google Scholar]
- 17.Nagel TH, Hinni ML, Hayden RE, Lott DG. Transoral laser microsurgery for the unknown primary: role for lingual tonsillectomy. Head Neck. 2014;36(7):942–6. doi: 10.1002/hed.23372. [DOI] [PubMed] [Google Scholar]
- 18.Durmus K, Rangarajan SV, Old MO, Agrawal A, Teknos TN, Ozer E. Transoral robotic approach to carcinoma of unknown primary. Head Neck. 2014;36(6):848–52. doi: 10.1002/hed.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta V, Johnson P, Tassler A, et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope. 2013;123(1):146–51. doi: 10.1002/lary.23562. [DOI] [PubMed] [Google Scholar]
- 20.Durmus K, Patwa HS, Gokozan HN, et al. Functional and quality-of-life outcomes of transoral robotic surgery for carcinoma of unknown primary. Laryngoscope. 2014;124(9):2089–95. doi: 10.1002/lary.24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrd JK, Albergotti WG, Davis K, et al. Transoral robotic surgery and the unknown primary: a highly effective technique for identifying the tumor. Otolaryngol Head Neck Surg. 2013;149(2):80. doi: 10.1177/0194599813495815a140. [DOI] [Google Scholar]
- 22.Byrd JK, Smith KJ, de Almeida JR, et al. Transoral robotic surgery and the unknown primary: a cost-effectiveness analysis. Otolaryngol Head Neck Surg. 2014;150(6):976–982. doi: 10.1177/0194599814525746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galloway TJ, Davis KS, Burtness B, et al. HPV association and the increasing incidence of unknown primary head-and-neck squamous cell carcinoma: epidemiology and prevention. Int J Radiat Oncol Biol Phys. 2014;88(2):494. doi: 10.1016/j.ijrobp.2013.11.102. [DOI] [Google Scholar]
- 24.Roeser MM, Alon EE, Olsen KD, Moore EJ, Manduch M, Wismayer DJ. Synchronous bilateral tonsil squamous cell carcinoma. Laryngoscope. 2010;120(4):S181. doi: 10.1002/lary.21645. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh L, Dahut W, Kakar S, et al. Management of patients with metastatic cancer of unknown primary. Curr Probl Surg. 2005;42(1):12–66. doi: 10.1067/j.cpsurg.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Abuzeid WM, Bradford CR, Divi V. Transoral robotic biopsy of the tongue base: a novel paradigm in the evaluation of unknown primary tumors of the head and neck. Head Neck. 2013;35(4):E126–30. doi: 10.1002/hed.21968. [DOI] [PubMed] [Google Scholar]
- 27.Mourad WF, Blakaj DM, Kabarriti R, et al. Lack of adjuvant radiotherapy may increase risk of retropharyngeal node recurrence in patients with squamous cell carcinoma of the head and neck after transoral robotic surgery. Case Rep Oncol Med. 2013;2013:727904. doi: 10.1155/2013/727904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco RG, Fakhry C, Ha PK, et al. Transoral robotic surgery experience in 44 cases. J Laparoendosc Adv Surg Tech A. 2013;23(11):900–7. doi: 10.1089/lap.2013.0261. [DOI] [PubMed] [Google Scholar]
- 29.Jansma J, Vissink A, Spijkervet FK, et al. Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer. 1992;70(8):2171–80. doi: 10.1002/1097-0142(19921015)70:8<2171::AID-CNCR2820700827>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Madani I, Vakaet L, Bonte K, Boterberg T, De Neve W. Intensity-modulated radiotherapy for cervical lymph node metastases from unknown primary cancer. Int J Radiat Oncol Biol Phys. 2008;71(4):1158–66. doi: 10.1016/j.ijrobp.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 31.Al-Ibraheem A, Buck A, Krause BJ, Scheidhauer K, Schwaiger M. Clinical Applications of FDG PET and PET/CT in Head and Neck Cancer. J Oncol. 2009;2009:208725. doi: 10.1155/2009/208725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roh JL, Kim JS, Lee JH, et al. Utility of combined (18)F-fluorodeoxyglucose-positron emission tomography and computed tomography in patients with cervical metastases from unknown primary tumors. Oral Oncol. 2009;45(3):218–24. doi: 10.1016/j.oraloncology.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Nassenstein K, Veit-Haibach P, Stergar H, et al. Cervical lymph node metastases of unknown origin: primary tumor detection with whole-body positron emission tomography/computed tomography. Acta Radiol. 2007;48(10):1101–8. doi: 10.1080/02841850701581768. [DOI] [PubMed] [Google Scholar]
- 34.Wartski M, Le Stanc E, Gontier E, et al. In search of an unknown primary tumour presenting with cervical metastases: performance of hybrid FDG-PET-CT. Nucl Med Commun. 2007;28(5):365–71. doi: 10.1097/MNM.0b013e3280708edf. [DOI] [PubMed] [Google Scholar]
- 35.Rumboldt Z, Gordon L, Gordon L, Bonsall R, Ackermann S. Imaging in head and neck cancer. Curr Treat Options Oncol. 2006;7(1):23–34. doi: 10.1007/s11864-006-0029-2. [DOI] [PubMed] [Google Scholar]
- 36.Haas I, Hoffmann TK, Engers R, Ganzer U. Diagnostic strategies in cervical carcinoma of an unknown primary (CUP) Eur Arch Otorhinolaryngol. 2002;259(6):325–33. doi: 10.1007/s00405-002-0470-1. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446–9. doi: 10.1002/1097-0142(197206)29:6<1446::AID-CNCR2820290604>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 38.Kothari P, Randhawa PS, Farrell R. Role of tonsillectomy in the search for a squamous cell carcinoma from an unknown primary in the head and neck. Br J Oral Maxillofac Surg. 2008;46(4):283–7. doi: 10.1016/j.bjoms.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 39.McGovern SL, Williams MD, Weber RS, et al. Three synchronous HPV-associated squamous cell carcinomas of Waldeyer’s ring: case report and comparison with Slaughter’s model of field cancerization. Head Neck. 2010;32(8):1118–24. doi: 10.1002/hed.21171. [DOI] [PubMed] [Google Scholar]
- 40.Caley A, Evans M, Powell N, et al. Multicentric human papillomavirus-associated head and neck squamous cell carcinoma. Head Neck. 2015;37(2):202–8. doi: 10.1002/hed.23584. [DOI] [PubMed] [Google Scholar]


