Introduction
A male patient with history of Sézary syndrome (SS) developed an isolated cutaneous plaque of large CD30+ T cells directly over a port device containing silicone. The histology was consistent with large cell transformation (LCT), which may occur in various hematologic malignancies, including the SS form of cutaneous T-cell lymphoma (CTCL). SS that has transformed into CD30+ large T-cell cutaneous lymphoma has been distinguished from other primary cutaneous CD30+ lymphoproliferative disorders (eg, anaplastic large cell lymphoma [ALCL] or lymphomatoid papulosis). An association between silicone implants and anaplastic lymphoma kinase-1 (ALK-1) –negative ALCL prompted the US Food and Drug Administration to issue a Medical Device Safety Communication on January 26, 2011.1 The case report presented in our article raises the consideration of a pathophysiologic connection between the presence of silicone and the observed CD30+ T-cell lymphoma, akin to the development of extranodal ALCL localized to silicone breast implants.
Case Report
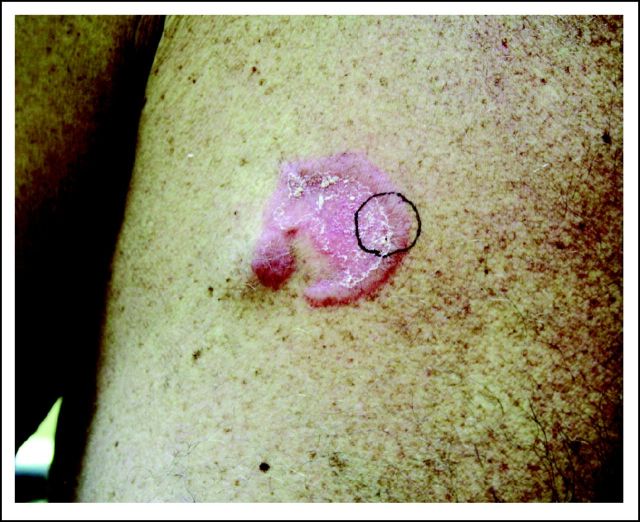
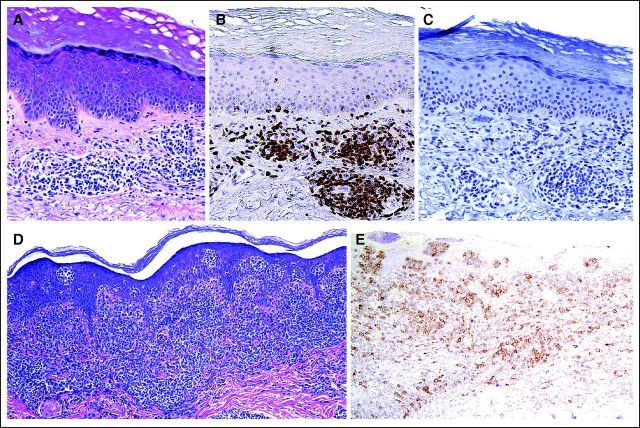
A 53-year-old white man with an approximately 2-year history of SS and previous allogeneic stem-cell transplantation was evaluated for a new right-chest plaque. This plaque was situated directly over a chemotherapy port, which was implanted 13 months earlier to treat his SS using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy. It was a SlimPort MRI Ultra-Low Profile port (product #0655640, Bard Access Systems, Salt Lake City, UT), containing a silicone catheter and polyurethane components. He described steady plaque enlargement above his port over the previous 4 months. Physical examination revealed a 3.5 cm × 3.5 cm pink-red round plaque whose periphery was arcuate, firm, and nonscaly but had a centrally overlying fine, white scale (Fig 1). Skin biopsy was performed, with histopathology demonstrating a dense lymphoid infiltrate composed of atypical large cells in the dermis with prominent overlying epidermotropism (Fig 2D). Immunohistochemistry was similar to that performed during the patient's SS work-up, except for CD30 expression by the majority of the large cells (Fig 2E). ALK-1 and epithelial membrane antigen staining were negative, as was Epstein-Barr virus–encoded RNA in situ hybridization.
Fig 1.
Fig 2.
Two years before the chest plaque developed, the patient was first evaluated for an erythematous, pruritic rash involving the face and torso. Peripheral T-cell lymphoma not otherwise specified was initially diagnosed by his oncologist following biopsy of an enlarged inguinal lymph node. The patient also had lymphadenopathy of the head and neck. Skin biopsy revealed a patchy small lymphocytic infiltrate located predominantly in the superficial dermis (Fig 2A). The T cells expressed CD2, CD3 (Fig 2B), CD4, and CD5, and notably had loss of T-cell marker CD7 (Fig 2C); Ki-67 nuclear staining was positive on approximately 15% of cells. Polymerase chain reaction (PCR) identified monoclonal T-cell receptor (TCR) gene rearrangement in the skin. Computed tomography (CT) showed axillary and inguinal lymphadenopathy and positron emission tomography (PET) revealed low-grade fluorodeoxyglucose (FDG) avidity in the axilla, neck, left lower chest wall, and bilateral inguinal nodes. Bone marrow biopsy demonstrated an atypical T-cell population expressing CD2, CD3, CD4, and CD5; there was no expression of CD7 or CD8. Fluorescence in situ hybridization did not reveal an ALK gene translocation, and karyotype analysis of the bone marrow was normal.
Directed by his oncologist, the patient completed CHOP therapy (five 21-day cycles), and repeat PET and CT showed near complete resolution of previously noted lymphadenopathy. However, peripheral blood flow cytometry performed after CHOP revealed a markedly elevated CD4:CD8 ratio (45:1) with expanded CD4+CD26− compartment. The patient became erythrodermic 1 month after CHOP and was diagnosed with SS by International Society of Cutaneous Lymphoma classification criteria.2
The patient subsequently received vorinostat and total-skin electron-beam radiation (a total 24 Gy in 24 fractions), followed by reduced intensity allogeneic stem-cell transplantation using pentostatin and low-dose total-body irradiation (cumulative, 6 Gy) as a conditioning regimen. He received tacrolimus after transplantation.
Repeat PET, CT, and bone marrow biopsies post-transplantation did not reveal evidence of residual T-cell lymphoma. Approximately 4 months post-transplantation, the patient first noticed a solitary cutaneous lesion over the implanted port. Skin biopsy histology and immunohistochemistry demonstrated that approximately 50% of the infiltrate was composed of CD30+ large-cell T lymphocytes. PCR analysis of TCR gene rearrangements revealed a monoclonal population, and comparison with earlier PCR analysis obtained during his initial SS work-up identified that each population of T cells contained the same TCR gene rearrangement. Two weeks thereafter, he began localized electron-beam therapy (cumulative, 36 Gy). Over a 10-month follow-up period, he did not develop any new skin lesions or extracutaneous involvement after completing localized radiotherapy. Repeat PET scans and bone marrow biopsies were negative for disease progression. Most recent peripheral blood flow cytometry revealed no evidence of a T-cell lymphoproliferative disorder.
Discussion
Although CD30+ LCT in CTCL has been described in the literature,3–9 our patient is unique in that he developed a single plaque of CD30+ large T cells directly over a silicone-containing chemotherapy port. The rapid plaque growth, features of LCT adjacent to his port, and phenotypic changes different from his baseline neoplastic T cells together suggest that a port component, such as silicone, might be implicated in the recruitment, stimulation, and/or transformation of T cells. The pathogenesis underlying transformation in this patient may therefore share similarities to the pathogenesis of ALCL associated with silicone prostheses.
In the current lymphoma classification, given the patient's history of CTCL and identification of identical TCR gene rearrangements between the previous SS biopsy and the new port-associated lesion, the histopathologic diagnosis is consistent with LCT, as atypical large cells exceeded 25% of the total lymphoid infiltrate.4,8,10 In addition, according to current criteria, primary cutaneous ALCL would not be a diagnostic option given the previous history of CTCL and CD30 expression of less than 75%.10
Transformed cells in LCT are T cells,4 and multiple reports indicate that LCT represents the progression of a single malignant T-cell clone in CTCL.11,12 Expression of new antigens such as CD30, Leu-M1, LN1, LN2, and CD8 by transformed cells may occur4; CD30 expression is variable (estimated at 40%).8 LCT is associated with an extremely poor prognosis.5,8,13 Reported survival ranges between 213 to 37 months,4 compared with 163 months in patients without transformation.5 Factors associated with unfavorable outcomes include transformation within 2 years of diagnosis and an advanced disease stage at transformation, including SS.14
The question of whether the large CD30+ T cells in our patient's tumor are representative of a silicone-induced activation of T cells rather than progression of a single malignant T-cell clone might have prognostic implications. This is particularly significant because breast implant–associated ALCL generally has a favorable prognosis relative to LCT. Duvic et al15 published a case series of three women with breast implants who developed non-Hodgkin's lymphoma of the breast and had associated skin involvement overlying the implants. Subsequent reports recognized ALK-1–negative, indolent ALCL in patients with breast prostheses.16,17 ALK-1 negativity usually indicates a worse prognosis in systemic ALCL18 and portends a 5-year survival rate of less than 40%18 compared with a 5-year overall survival rate of 71% to 79% in ALK-1–positive cases.19 Alternatively, primary cutaneous ALCL, which has a 5-year survival rate greater than 90%,20 is generally ALK-1–negative.10,21 A recent review of prosthesis-associated breast lymphomas found 17 of 28 women with ALK-1–negative ALCL were alive without disease 4 to 108 months after treatment.22 Whether these cases of ALCL represent the cutaneous form of the disease, a less aggressive systemic form, or a new form altogether has yet to be deciphered.17,22
The important attention to a possible link between silicone and ALCL raises the question in our case whether a common pathogenic mechanism may be at work leading to an apparent LCT. As we described, our patient is classified as having LCT by current diagnostic criteria; however, given his clinical presentation and favorable outcome to date, we speculate that this patient's lesion may be silicone-induced, analogous to silicone-induced ALCL in breast tissue, and not a true LCT of his systemic lymphoma. Although exact mechanisms linking silicone breast implants to ALCL are unknown, several theories are proposed, including direct antigenic stimulation from silicone components23,24 and production of a cytokine milieu incited from a chronic inflammatory response to silicone, thereby supporting growth and transformation of a malignant T-cell clone.17,22 Rupture of saline-filled implants appears unnecessary for development of breast prosthesis-related ALCL, as many patient implants were intact and presence of a silicone-coated foreign object may be sufficient to stimulate inflammation. Indeed, implantable access ports have been associated with chronic inflammation.25 Our observation of apparent LCT directly over a port with silicone parts but no internal silicone gel also suggests that interaction between the malignant T cells and silicone may play a role in transformation.
Because implant-associated ALCL tends to improve on device removal, it is possible that our patient benefited from removal of his port; however, because he received local radiotherapy before port removal, it cannot be known whether removal alone would have sufficed to resolve his plaque. Although ALCL associated with breast prostheses tends to be less aggressive compared with ALCL unrelated to prostheses, it is not clear whether to expect an indolent course for our patient. We believe that increased attention and investigation into the potential link between silicone and CD30+ T-cell disorders is warranted.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.US Food and Drug Administration Center for Devices and Radiological Health: FDA medical device safety communication: Reports of anaplastic large cell lymphoma (ALCL) in women with breast implants, 2011. http://www.fda.gov/MedicalDevices/Safety/alertsandNotices/ucm240000.htm. [Google Scholar]
- 2.Vonderheid EC, Bernengo MG, Burg G, et al. Update on erythrodermic cutaneous T-cell lymphoma: Report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46:95–106. doi: 10.1067/mjd.2002.118538. [DOI] [PubMed] [Google Scholar]
- 3.Greer JP, Salhany KE, Cousar JB, et al. Clinical features associated with transformation of cerebriform T-cell lymphoma to a large cell process. Hematol Oncol. 1990;8:215–227. doi: 10.1002/hon.2900080406. [DOI] [PubMed] [Google Scholar]
- 4.Salhany KE, Cousar JB, Greer JP, et al. Transformation of cutaneous T cell lymphoma to large cell lymphoma: A clinicopathologic and immunologic study. Am J Pathol. 1988;132:265–277. [PMC free article] [PubMed] [Google Scholar]
- 5.Diamandidou E, Colome-Grimmer M, Fayad L, et al. Transformation of mycosis fungoides/Sezary syndrome: Clinical characteristics and prognosis. Blood. 1998;92:1150–1159. [PubMed] [Google Scholar]
- 6.Braverman IM. Transformation in cutaneous T-cell lymphoma. J Invest Dermatol. 1993;101:249–250. doi: 10.1111/1523-1747.ep12365124. [DOI] [PubMed] [Google Scholar]
- 7.Cerroni L, Rieger E, Hödl S, et al. Clinicopathologic and immunologic features associated with transformation of mycosis fungoides to large-cell lymphoma. Am J Surg Pathol. 1992;16:543–552. doi: 10.1097/00000478-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Arulogun SO, Prince HM, Ng J, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112:3082–3087. doi: 10.1182/blood-2008-05-154609. [DOI] [PubMed] [Google Scholar]
- 9.Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow S, Campo E, Harris N, et al. ed 4. Lyon, France: World Health Organization; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, IARC WHO Classification of Tumours. [Google Scholar]
- 11.Wolfe JT, Chooback L, Finn DT, et al. Large-cell transformation following detection of minimal residual disease in cutaneous T-cell lymphoma: Molecular and in situ analysis of a single neoplastic T-cell clone expressing the identical T-cell receptor. J Clin Oncol. 1995;13:1751–1757. doi: 10.1200/JCO.1995.13.7.1751. [DOI] [PubMed] [Google Scholar]
- 12.Wood GS, Bahler DW, Hoppe RT, et al. Transformation of mycosis fungoides: T-cell receptor beta gene analysis demonstrates a common clonal origin for plaque-type mycosis fungoides and CD30+ large-cell lymphoma. J Invest Dermatol. 1993;101:296–300. doi: 10.1111/1523-1747.ep12365416. [DOI] [PubMed] [Google Scholar]
- 13.Dmitrovsky E, Matthews MJ, Bunn PA, et al. Cytologic transformation in cutaneous T cell lymphoma: A clinicopathologic entity associated with poor prognosis. J Clin Oncol. 1987;5:208–215. doi: 10.1200/JCO.1987.5.2.208. [DOI] [PubMed] [Google Scholar]
- 14.Diamandidou E, Colome M, Fayad L, et al. Prognostic factor analysis in mycosis fungoides/Sézary syndrome. J Am Acad Dermatol. 1999;40:914–924. doi: 10.1016/s0190-9622(99)70079-4. [DOI] [PubMed] [Google Scholar]
- 15.Duvic M, Moore D, Menter A, et al. Cutaneous T-cell lymphoma in association with silicone breast implants. J Am Acad Dermatol. 1995;32:939–942. doi: 10.1016/0190-9622(95)91328-9. [DOI] [PubMed] [Google Scholar]
- 16.Roden AC, Macon WR, Keeney GL, et al. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: An indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21:455–463. doi: 10.1038/modpathol.3801024. [DOI] [PubMed] [Google Scholar]
- 17.Thompson PA, Lade S, Webster H, et al. Effusion-associated anaplastic large cell lymphoma of the breast: Time for it to be defined as a distinct clinico-pathological entity. Haematologica. 2010;95:1977–1979. doi: 10.3324/haematol.2010.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ten Berge RL, Oudejans JJ, Ossenkoppele GJ, et al. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J Clin Pathol. 2000;53:445–450. doi: 10.1136/jcp.53.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gascoyne RD, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999;93:3913–3921. [PubMed] [Google Scholar]
- 20.Querfeld C, Khan I, Mahon B, et al. Primary cutaneous and systemic anaplastic large cell lymphoma: Clinicopathologic aspects and therapeutic options. Oncology (Williston Park) 2010;24:574–587. [PubMed] [Google Scholar]
- 21.Lamant L, Pileri S, Sabattini E, et al. Cutaneous presentation of ALK-positive anaplastic large cell lymphoma following insect bites: Evidence for an association in five cases. Hematologica. 2010;95:449–455. doi: 10.3324/haematol.2009.015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazzeri D, Agostini T, Bocci G, et al. ALK-1-negative anaplastic large cell lymphoma associated with breast implants: A new clinical entity. Clin Breast Cancer. 2011;11:283–296. doi: 10.1016/j.clbc.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 23.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 24.Bonzheim I, Geissinger E, Roth S, et al. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood. 2004;104:3358–3360. doi: 10.1182/blood-2004-03-1037. [DOI] [PubMed] [Google Scholar]
- 25.Narducci F, Jean-Laurent M, Boulanger L, et al. Totally implantable venous access port systems and risk factors for complications: A one-year prospective study in a cancer centre. Eur J Surg Oncol. 2011;37:913–918. doi: 10.1016/j.ejso.2011.06.016. [DOI] [PubMed] [Google Scholar]