Abstract
Designer natriuretic peptides (NPs) represent an active area of drug development. In canine and human studies, the designer natriuretic peptide CD-NP demonstrated more desirable therapeutic potential than recombinant B-type NP (BNP), which is known as nesiritide and is approved for treatment of acute decompensated heart failure. However, why CD-NP is more effective than BNP is not known. We previously reported that CD-NP is a poorer activator of human guanylyl cyclase-A (GC-A) and a better activator of human guanylyl cyclase-B than BNP. Here, guanylyl cyclase bioassays were used to compare the susceptibility of CD-NP verses ANP, BNP, CNP and DNP to inactivation by human kidney membranes. The half time (t1/2) for CD-NP inactivation was increased by factors of 13, 3 and 4 compared to ANP, BNP and CNP, respectively, when measured in the same assay. Surprisingly, DNP failed to undergo complete inactivation and was the most degradation resistant of the peptides tested. The neutral endopeptidase (NEP) inhibitor, phosphoramidon, blocked inactivation of CNP and CD-NP, but not BNP or DNP. In contrast, the general serine and cysteine protease inhibitor, leupeptin, completely blocked the degradation of BNP and CD-NP, but did not block CNP inactivation unless phosphoramidon was included in the assay. Thus, NPs with shorter carboxyl tails (ANP and CNP) are degraded by phosphoramidon-sensitive proteases and NPs with extended carboxyl tails (BNP, DNP and CD-NP) are resistant to NEP degradation and degraded by leupeptin-sensitive proteases. We conclude that DNP and CD-NP are highly resistant to proteolysis and that proteolytic resistance contributes to the beneficial cardiovascular properties of CD-NP. We suggest that this property may be exploited to increase the half-life of NP-based drugs.
Keywords: Natriuretic peptide receptor A, Carperitide, Nesiritide, Natriuretic peptide, Heart failure, cGMP
1. Introduction
Humans express three structurally similar but genetically distinct natriuretic peptides: atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) [1]. ANP decreases cardiac preload by inducing vasorelaxation, natriuresis, diuresis and endothelial permeability, whereas gene inactivation studies in mice indicate that BNP signals in a paracrine manner to inhibit cardiac fibrosis but not blood pressure. CNP has been shown to stimulate venodilation [2,3], but it does not decrease blood pressure at physiologic or pathologic concentrations [4]. ANP and BNP activate the transmembrane receptor, guanylyl cyclase A (GC-A), while CNP activates guanylyl cyclase B (GC-B) [5]. Both receptors synthesize cGMP in response to natriuretic peptide binding. The vasorelaxing properties of ANP and BNP decrease blood pressure acutely. However, over time, decreased blood pressure reduces renal perfusion pressure, which is hypothesized to decrease blood flow through the kidney, and ultimately inhibit renal fluid and salt secretion. Thus, the acute vasorelaxing properties of recombinant BNP, which is known as nesiritide and is approved for the treatment of acute decompensated heart failure in many countries, may ultimately limit the clinical effectiveness of this drug.
New chimeric natriuretic peptides exhibit reduced vascular effects, and therefore, maintain renal stimulating and volume unloading functions. CD-NP is a chimeric molecule composed of full length human CNP fused to the 15-amino acid carboxyl-terminal tail of Dendroaspis natriuretic peptide (DNP), an endogenous GC-A agonist isolated from the venom of the green mamba [6]. Immunological evidence supporting the existence of human DNP is conflicting, but no cDNA for human DNP has been reported [7,8]. CNP is advantageous because it is less hypotensive than ANP or BNP [9,10] and the signaling receptor for CNP is not downregulated in the failing heart [11]. However, CNP lacks the diuretic and natriuretic actions of ANP and BNP, which are critical for the improvement of patients with congestive heart failure. Since DNP possesses both natriuretic and hypotensive activities [10], CD-NP was engineered to combine the venodilatory and cardiac properties of CNP with the beneficial renal properties of DNP. Animal and human studies suggest that CD-NP possesses superior clinical properties compared to nesiritide. Infusion of CD-NP into dogs increased natriuresis and diuresis while being significantly less hypotensive than BNP [10]. Additionally, human Phase I clinical trials are consistent with CD-NP possessing natriuretic and aldosterone-suppressing properties without inducing excessive hypotension [9].
We reported that the carboxyl terminus of DNP increases the affinity of CNP for GC-A, which may account for the renal effects observed in the canine and human studies [12]. In this report, we examined the proteolytic inactivation of CD-NP. Human kidney membranes were used as the protease source because the kidney is a major natriuretic peptide target organ, and inhibitors of the renal protease, neutral endopeptidase that is also known as neprilysin or NEP, increase serum natriuretic peptide concentrations [13–15]. Our hypothesis was that CD-NP is more resistant to proteolysis than CNP or ANP because studies have indicated that natural or mutant natriuretic peptides with longer carboxyl termini are more resistant to degradation than peptides with shorter carboxyl termini [16–19]. We found that peptides containing the C-terminal tail of DNP (DNP and CD-NP) are highly resistant to proteolytic degradation.
2. Materials and methods
2.1. Reagents
Human wild-type ANP, BNP, and CNP were purchased from Sigma-Aldrich (St. Louis, MO). CD-NP and DNP were from Dr. John Burnett (Mayo Clinic, Rochester, MN). 125I-cGMP radioimmunoassay kits were from Perkin Elmer (Waltham, MA). Protease inhibitors were from Roche Applied Science (Indianapolis, IN) (Complete Roche protease inhibitor cocktail tablet), MP Biomedicals, LLC (Solon, OH) (phosphoramidon), Sigma Aldrich (St. Louis, MO) (actinonin) and Fisher Scientific (Pittsburgh, PA) (leupeptin).
2.2. Cells
Human embryonic 293 cells lacking any known natriuretic peptide receptor and stably expressing a single human natriuretic peptide receptor were maintained as described [12].
2.3. Proteolysis bioassay
Human kidney samples were obtained from the Tissue Procurement Program at the University of Minnesota and stored at −80 °C until used as previously described [16]. Crude membranes were prepared as reported [16]. Due to the small amount of tissue obtained at each collection, multiple individual kidney samples were used for the assays. Proteolysis experiments were performed as described where cells expressing human GC-A or GC-B were used to assess the activity of ANP, BNP and DNP or CNP and CD-NP, respectively [16,20].
2.4. Statistical analysis
The bioassay standard curves were generated using a nonlinear regression model. Proteolytic half-lives were determined using Prism data analysis software according to a one- or two-site exponential decay models. The formula for the one site model was Y=span*exp(−K*X)+plateau and the formula for the two-site model was Y=span1*exp(−K1*X)+span2*exp(−K2*X)+plateau. Data are presented as the mean±standard error of the mean (SEM). Significance was determined by two-tailed t-test where p≤0.05.
3. Results
3.1. Peptides containing the C-terminal tail of DNP are resistant to degradation
The ability of human kidney membranes to inactivate CD-NP versus ANP, BNP, CNP and DNP was investigated using a coupled proteolysis-cGMP elevation bioassay [16,20]. Because of variations in the human kidney samples, a single natriuretic peptide was always compared to CD-NP in the same assay. The natriuretic peptides were incubated with human kidney membranes for increasing periods of times at 37 °C to facilitate peptide proteolysis. The degradation assay was stopped with acid and the remaining natriuretic peptide activity was determined by measuring the ability of the neutralized extracts to elevate cGMP in HEK293 cells expressing either human GC-A or human GC-B.
Initial studies compared the time required for human kidney membranes to inactivate CNP versus CD-NP as determined by GC-B activation (Fig. 1A). The activity loss associated with CNP was best fit by a one-phase exponential decay model, whereas the activity loss associated with CD-NP was best fit by a two-phase exponential decay model (dotted line). The one-phase model indicated half times for peptide inactivation of 4.3 and 17.9 min for CNP and CD-NP, respectively. The two-phase model indicated half times of 4.4 and 30 min for the fast and slow components of CD-NP degradation.
Fig. 1.
The C-terminal tail of DNP confers proteolytic resistance. The indicated natriuretic peptides were incubated with human kidney membranes at 37 °C for the indicated periods of time. Remaining peptide concentrations were determined as described under Materials and methods. The solid lines represent peptide concentrations determined using a single-phase exponential decay model. The broken line represents a two-phase exponential decay model. (A) Degradation of CNP and CD-NP where n=8. (B) Degradation of ANP and CD-NP where n=4. (C) Degradation of BNP and CD-NP where n=4. (D) Degradation of DNP and CD-NP where n=8.
The degradation of CD-NP was also compared to ANP by measuring the ability of the membrane-exposed peptides to elevate cGMP concentrations in 293 cells expressing human GC-A (Fig. 1B). ANP was rapidly inactivated in this assay with a t1/2 of 1.3 min. ANP inactivation was best fit by a one-phase model. In contrast, the t1/2 for CD-NP degradation was 17 min when estimated with a one-phase model and 9 and 92 min when estimated with a two-phase model.
Time-dependent proteolytic inactivation of CD-NP was also compared to human BNP by measuring cGMP elevations in 293 cells expressing GC-A (Fig. 1C). The t1/2 for BNP was 8 min, whereas the t1/2 for CD-NP was 23 min when estimated using a one-phase decay model. A two-phase model did not improve the fit of the curve for CD-NP in this assay.
Proteolytic inactivation of DNP was also measured. In this assay, the t1/2 for CD-NP degradation was 17.9 min. However, DNP was extremely resistant to degradation (Fig. 1D). After two hours, 46% of the biological activity of DNP remained. The t1/2 for DNP degradation was estimated at 159 min, but correlation coefficient was very low (0.18).
3.2. CD-NP, BNP and DNP are degraded by a leupeptin-, not neprilysin, sensitive protease
To characterize the proteases that degrade CD-NP in the human kidney membranes, protease inhibitors were included in the degradation cocktail. In the absence of inhibitors, incubation for 30 min at 37 °C decreased the bioactivity of CNP to less than 5% of initial values (Fig. 2A). Identical treatment of CD-NP only decreased activity to 35% of initial activity. Boiling the membranes prior to peptide exposure abolished membrane-dependent inactivation of both CNP and CD-NP, consistent with a protease-dependent mechanism. The NEP inhibitor, phosphoramidon, blocked about 40% of the activity loss associated with CNP but had no significant effect on CD-NP activity. The meprin inhibitor, actinonin, did not block inactivation of CNP or CD-NP when added alone and when added together with phosphoramidon, it did not further increase activity above that seen with phosphoramidon alone. In contrast, the general serine–threonine–cysteine protease inhibitor, leupeptin, did not statistically increase the bioactivity of CNP but completely blocked inactivation of CD-NP. The addition of phosphoramidon, actinonin and leupeptin to the cocktail yielded similar CNP or CD-NP bioactivities as leupeptin alone.
Fig. 2.
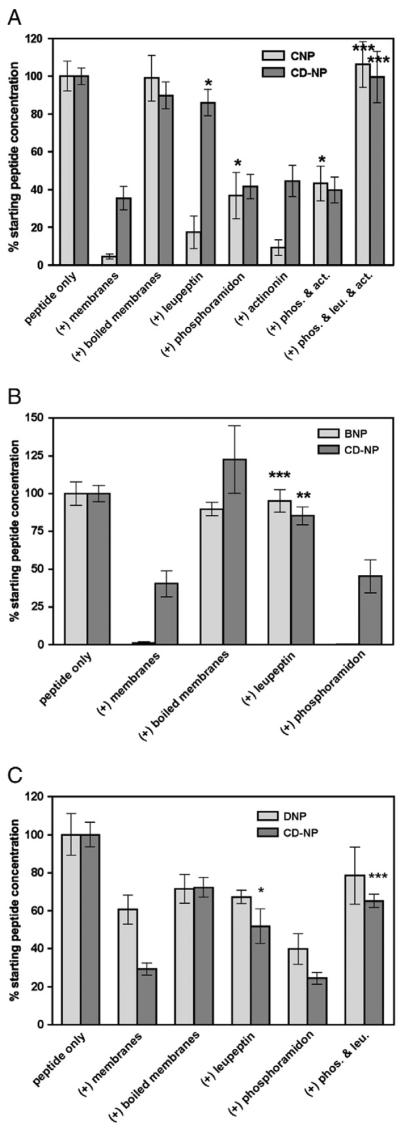
Leupeptin, but not phosphoramidon, blocks renal membrane degradation of CD-NP and BNP. The indicated natriuretic peptides were incubated with crude human kidney membranes at 37 °C for 30 min for panels A and B and 120 min for panel C in the absence or presence of the indicated protease inhibitors. Remaining peptide concentrations were determined as described under Materials and methods. (A) Proteolytic inactivation of CNP and CD-NP where n=8. (B) Proteolytic inactivation of BNP and CD-NP where n=4. (C) Proteolytic inactivation of DNP and CD-NP where n=4. Abbreviations: act, actinonin; leu, leupeptin; phos, phosphoramidon. *p≤0.05, **p≤0.005, ***p≤0.0001.
The ability of the protease inhibitors to block BNP and CD-NP degradation during a 30 min incubation with human kidney membranes was tested using 293-GC-A cells (Fig. 2B). BNP was completely inactivated by human kidney membranes, whereas CD-NP activity was reduced to 40% of initial values. Boiling the membranes before peptide exposure blocked inactivation of both BNP and CD-NP. In contrast to CNP, leupeptin completely blocked the degradation of BNP and CD-NP, whereas phosphoramidon failed to preserve the activity of either peptide.
The ability of the various protease inhibitors to block DNP degradation was tested by incubating the peptides with membranes at 37 °C for 120 min in order to maximize DNP degradation (Fig. 2C). However, a four-fold increase in the proteolysis period did not result in a statistically significant reduction in the ability of DNP to activate human GC-A (compare membranes to boiled membrane in Fig. 2C). Inclusion of leupeptin in the assay slightly increased activity but the difference was not statistically significant. Activity measured in the presence of phosphoramidon was significantly less than control values.
4. Discussion
CD-NP is a new natriuretic peptide derivative with similar but unique cardiovascular properties to nesiritide, but how CD-NP functionally differs from nesiritide and other natriuretic peptides is not known. We determined that the DNP carboxyl-terminal tail decreases the degradation of CNP by kidney proteases. CD-NP was more resistant to proteolytic degradation than any mammalian natriuretic peptide having degradation half times 13, 3 and 4 times greater than ANP, BNP and CNP, respectively, when compared head-to-head in the same assay. The only peptide more resistant to degradation was DNP, which suggests that the DNP-tail is the primary determinant regulating renal natriuretic peptide proteolysis. However, the fact that DNP was more resistant to degradation than CD-NP indicates that regions besides the C-terminal tail also play a role in degradation.
Previous studies demonstrated that NEP efficiently degrades CNP in vitro and that NEP inhibitors elevate CNP concentrations in whole animals [13,14,17]. Here, we found that inhibition of NEP in kidney membranes increased the bioactivity of CNP 8-fold but had no effect on the bioactivity of CD-NP even though CD-NP has the same amino-terminal and ring structure as CNP. Hence, the addition of the DNP tail markedly decreased the degradation of CNP by NEP. Interestingly, both leupeptin and phosphoramidon were required to maximally preserve CNP activity, which is consistent with a CNP inactivation mechanism that requires multiple proteases.
A mutation in ANP that adds 12 residues to the carboxyl-terminus is elevated in patients with familial atrial fibrillation [21]. We found that the carboxyl-terminally extended from of ANP was resistant to degradation by NEP [16], and Ralat and colleagues found that this C-terminally extended form of ANP was resistant to degradation by insulin degrading enzyme [18]. Thus, natriuretic peptides with the longer carboxyl-terminal tails are poor substrates for NEP and are degraded by leupeptin-sensitive proteases. Whether the specific amino acid sequence of the DNP tail is required for optimal inhibition of NEP cleavage or whether any sequence of amino acids that extends the carboxyl terminus will block NEP degradation is not known. It should be noted that while our studies clearly document biological inactivation of these peptides, additional mass spectrometry based studies are required to determine the exact proteolytic cleavage sites.
Our data indicate that leupeptin is the most effective inhibitor of BNP and CD-NP. Leupeptin also increased the activity of DNP but the difference was not statistically significant, probably because the magnitude of the inactivation was low in our assay when boiled membranes were used as the control. We do not know why activity observed with peptide alone was significantly higher than activity measured in extracts exposed to boiled membranes (Fig. 2C). It is possible that the variation is related to the unique human kidney membrane sample that was used for this assay because a similar difference was also observed for CD-NP. In contrast, NEP clearly did not degrade DNP in our assay, which is consistent with the data showing that NEP inhibitors do not alter the renal response to DNP in dogs [22].
The development of designer natriuretic peptides that maintain beneficial renal effects in the absence of negative hypotensive effects is progressing [9]. One approach is to generate natriuretic peptides that are more susceptible to proteolysis in the vasculature and less resistant to proteolysis in the kidney, which would decrease vasorelaxation and increase fluid excretion, respectively. It is theoretically possible to selectively increase natriuretic peptide concentrations using protease inhibitors, but we believe this approach will be problematic due to the broad substrate specificity of the natriuretic peptide degrading enzymes. The current study characterizes the proteases that cleave currently available natriuretic peptides in human kidney preparations. We found marked differences in the susceptibility of various natriuretic peptides to degradation as well as evidence for degradation by unique proteases. We conclude that DNP is the most proteolytically resistant natriuretic peptide identified to date and that the addition of the C-terminal tail of DNP confers dramatic proteolytic resistance to CNP. We suggest that reduced susceptibility to proteolysis contributes to the beneficial properties of CD-NP and that peptides containing the C-terminal tail from DNP will have extended half-lives in humans.
Acknowledgments
We are grateful to Dr. John Burnett for supplying DNP and CD-NP.
Role of the funding source This work was supported by National Institutes of Health Grant R21HL093402 to L.R.P.
Footnotes
Disclosure statement D.M.D has nothing to declare. L.R.P. received lecture fees from Medtronic Inc. and Eli Lilly, Inc.
References
- [1].Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009:341–66. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Drewett JG, Fendly BM, Garbers DL, Lowe DG. Natriuretic peptide receptor-B (guanylyl cyclase-B) mediates C-type natriuretic peptide relaxation of precontracted rat aorta. J Biol Chem. 1995;270:4668–74. doi: 10.1074/jbc.270.9.4668. [DOI] [PubMed] [Google Scholar]
- [3].Wei CM, Aarhus LL, Miller VM, Burnett JC., Jr Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol. 1993;264:H71–3. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]
- [4].Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab. 1994;78:1428–35. doi: 10.1210/jcem.78.6.8200946. [DOI] [PubMed] [Google Scholar]
- [5].Potter LR. Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol Ther. 2011;130:71–82. doi: 10.1016/j.pharmthera.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps) J Biol Chem. 1992;267:13928–32. [PubMed] [Google Scholar]
- [7].Richards AM, Lainchbury JG, Nicholls MG, Cameron AV, Yandle TG. Dendroaspis natriuretic peptide: endogenous or dubious? Lancet. 2002;359:5–6. doi: 10.1016/S0140-6736(02)07270-7. [DOI] [PubMed] [Google Scholar]
- [8].Schirger JA, Heublein DM, Chen HH, Lisy O, Jougasaki M, Wennberg PW, et al. Presence of Dendroaspis natriuretic peptide-like immunoreactivity in human plasma and its increase during human heart failure. Mayo Clin Proc. 1999;74:126–30. doi: 10.4065/74.2.126. [DOI] [PubMed] [Google Scholar]
- [9].Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, et al. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009;49:668–73. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–8. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dickey DM, Flora DR, Bryan PM, Xu X, Chen Y, Potter LR. Differential regulation of membrane guanylyl cyclases in congestive heart failure: natriuretic peptide receptor (NPR)-B, not NPR-A, is the predominant natriuretic peptide receptor in the failing heart. Endocrinology. 2007;148:3518–22. doi: 10.1210/en.2007-0081. [DOI] [PubMed] [Google Scholar]
- [12].Dickey DM, Burnett JC, Jr, Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–9. doi: 10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brandt RR, Mattingly MT, Clavell AL, Barclay PL, Burnett JC., Jr Neutral endopeptidase regulates C-type natriuretic peptide metabolism but does not potentiate its bioactivity in vivo. Hypertension. 1997;30:184–90. doi: 10.1161/01.hyp.30.2.184. [DOI] [PubMed] [Google Scholar]
- [14].Charles CJ, Espiner EA, Nicholls MG, Richards AM, Yandle TG, Protter A, et al. Clearance receptors and endopeptidase 24.11: equal role in natriuretic peptide metabolism in conscious sheep. Am J Physiol. 1996;271:R373–80. doi: 10.1152/ajpregu.1996.271.2.R373. [DOI] [PubMed] [Google Scholar]
- [15].Olins GM, Spear KL, Siegel NR, Zurcher-Neely HA. Inactivation of atrial natriuretic factor by the renal brush border. Biochim Biophys Acta. 1987;901:97–100. doi: 10.1016/0005-2736(87)90260-4. [DOI] [PubMed] [Google Scholar]
- [16].Dickey DM, Yoder AR, Potter LR. A familial mutation renders atrial natriuretic peptide resistant to proteolytic degradation. J Biol Chem. 2009;284:19196–202. doi: 10.1074/jbc.M109.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–8. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ralat LA, Guo Q, Ren M, Funke T, Dickey DM, Potter LR, et al. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. J Biol Chem. 2011;286:4670–9. doi: 10.1074/jbc.M110.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dickey DM, Potter LR. Human B-type natriuretic peptide is not degraded by meprin A. Biochem Pharmacol. 2010;80:1007–11. doi: 10.1016/j.bcp.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–65. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen HH, Lainchbury JG, Burnett JC., Jr Natriuretic peptide receptors and neutral endopeptidase in mediating the renal actions of a new therapeutic synthetic natriuretic peptide Dendroaspis natriuretic peptide. J Am Coll Cardiol. 2002;40:1186–91. doi: 10.1016/s0735-1097(02)02127-7. [DOI] [PubMed] [Google Scholar]



