Abstract
Upon light irradiation, fluorophore-CPP conjugates can disrupt the integrity of biological membranes. This activity can in turn be used to photo-induce the disruption of endocytic organelles and promote the delivery of entrapped macromolecules such as proteins or RNAs into live cells. Recent mechanistic studies have shown that ROS production by the fluorophore and a latent lytic ability of CPPs act in synergy to elicit photolysis. However, how the structure of fluorophore-CPP conjugates impacts this synergistic activity remains unclear. Herein, using red blood cells as a model of biological membranes, we show that the number of arginine residues in a CPP as well as the position of fluorophore with respect to the CPP dramatically affect the photolytic activity of a fluorophore-CPP conjugate. These factors should therefore be considered for the development of effective photo-inducible delivery agents.
Graphical Abstract
INTRODUCTION
The intracellular delivery of macromolecules such as proteins and nucleic acids is useful for various cell biology and therapeutic applications (1–3). Macromolecules however are unable to spontaneously translocate into cells and many strategies have been developed to circumvent this problem. For instance, cell-penetrating peptides (CPPs) or lipid formulations have been used to deliver proteins, DNA and RNA inside cells, both in vitro and in vivo. With these approaches, cellular uptake is typically initiated by endocytosis. Only a small number of endocytosed macromolecules are able to escape into the cytosol and trigger biological effects (4). In contrast, a large fraction of macromolecules are trapped inside endosomes, unable to reach their cytosolic targets and thus carry out their functions (4). As a consequence, approaches that can improve the endosomal escape of macromolecules have been pursued as a means to improve overall delivery efficiency.
One of the strategies used to improve endosomal escape is a light-based approach called Photochemical Internalization (PCI). Introduced by Berg and co-workers (5), PCI uses photosensitizer molecules that localize inside endosomes along with macromolecules of interest. Upon irradiation, photosensitizers produce reactive oxygen species that damage endosomal membrane components. Oxidative damage then results in endosomal leakage and subsequent release of macromolecules into the cell cytosol. PCI is in principle very attractive because light provides spatial and temporal control over the delivery process. Photosensitizers such as Rose Bengal, porphyrins (TPPS2a or TPPS4a), phthalocynanines (AlPcS) and chlorins (TPCS2a) (6, 7, 5, 8, 9) were originally chosen as PCI agents based on their propensity to accumulate inside endosomes as well as their high efficiencies of ROS generation. These photosensitizers have been used to deliver immunotoxins, therapeutic drugs (bleomycin and doxorubicin) (10) and oligonucleotides (11) both in vitro and in vivo. In recent years, CPPs have also been conjugated to photosensitizers that do not accumulate inside endosomes on their own (12). In these studies, the CPP is used to transport the photosensitizer inside the endocytic pathway of cells prior to light irradiation.
Interestingly, fluorophores (Fl) that do not typically generate ROS in high yields and that are commonly used for live-cell imaging applications, also display a light-induced endosomal release upon conjugation to CPPs (Fl-CPPs) (13–15). Arginine-rich peptides conjugated to the fluorophore fluorescein and Alexa-Fluor633 were, for instance, shown to escape from endosomes and enter the cytoplasm of cells upon irradiation with 488 nm and 633 nm laser light respectively (13). Fl-CPPs have been used as PCI agents to successfully deliver p53 protein (14), shRNA (15) and dextran-Rhodamine (16) (3 kDa) into cells. The mechanisms by which Fl-CPPs disrupt endosomal membranes have been recently investigated with the model compounds TMR-TAT and TMR-R9, two arginine-rich CPPs conjugated to the fluorophore tetramethylrhodamine (TMR). TMR-TAT and TMR-R9 are both capable of causing endosomal leakage in HeLa cells, HaCaT cells and COLO316 cells extremely rapidly and efficiently upon light irradiation at 556 nm (17). Using red blood cells and liposomes as model systems for the membrane of endosomes, several key insights have been gained in the photolytic activity mediated by TMR-CPPs (18, 19). Upon irradiation, TMR-CPPs produce the ROS singlet oxygen and superoxide in relatively low yield (19). By binding to negatively charged phospholipids, the CPPs moieties however bring TMR in close proximity to the lipid bilayer. The ROS generated from the TMR molecules residing at the lipid bilayer then oxidize unsaturated lipids with efficiency significantly greater than the ROS that are generated from TMR present in solution (19). Oxidized lipids might subsequently destabilize the lipid bilayer as these species are well known to have detergent-like properties (20). However, the generation of oxidized lipids by TMR-CPPs was not sufficient to fully account for the photolytic activity observed (19). For instance, the control compound TMR-K9 did not display a significant photolytic activity despite being a positively charged peptide that can also bind negatively charged phospholipids and cause lipid oxidation with an efficiency similar to TMR-R9. Instead, experiments performed with pre-oxidized membranes established that the CPP moiety accelerates lysis. While non-oxidized membranes did not lyse in the presence of CPPs, membranes that were partially oxidized were readily lysed after addition of CPPs (18). The presence of arginine residues in the peptide sequence appears to be necessary for this activity as the peptide K9 did not cause the lysis of oxidized membranes (18). Together, these results therefore support the notion that a CPP acts in a synergistic manner with ROS-generating compounds to greatly enhance their photolytic activities.
While the presence of arginine residues in the CPP appears to be of importance to accelerate the lysis of photo-oxidized membranes, the structural features of the peptide that are required to maximize photolysis efficiency remain unclear. This is important because optimizing the structure of photosensitizer-CPP or Fl-CPP constructs could facilitate PCI-based delivery approaches. For instance, optimally engineered constructs could be active at lower concentrations or might require less light than current compounds. These compounds could, in turn, be more appropriate for in vivo applications (21). In this report, we aimed to identify how the number of arginine residues of a TMR-peptide conjugate affects photolysis. In addition, because proximity of the TMR moiety to the membrane is important, we also test the hypothesis that the positioning of TMR in the peptide sequence impacts photolysis. We use red blood cells as a model system to establish structure-activity relationships that should permit the future optimization of CPP-based PCI.
MATERIALS AND METHODS
Peptide synthesis reagents including amino acids and carboxy-tetramethylrhodamine (TMR) were obtained from Novabiochem. Tiron, nitro blue tetrazolium (NBT), p-nitrosodimethylaniline (RNO), imidazole and Rose Bengal (RB) reagents were purchased from Sigma-Aldrich. Whole blood was ordered from the Gulf Coast Regional Blood Center (Houston, TX).
Solid phase peptide synthesis (SPPS)
The Fl-CPP conjugates TMR-TAT, eosin-TAT, TMR-R1, TMR-R3, TMR-R5, TMR-R7, TMR-R9, TMR-R11, TMR-R13, R9-K(TMR), K(TMR)-R9 and R4-K(TMR)-R5 were synthesized by SPPS synthesis (Fmoc chemistry). Rink amide MHBA resin was used on a 0.72 mmol scale to synthesize the conjugates. The amino acids used for the making the peptide were Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Lys(Mtt)-OH, Fmoc-Gln-OH. Fluorophores used were 5(6)-carboxytetramethylrhodamine and 5(6)-carboxyeosin Y. Reactions were all carried out in a glass peptide synthesis vessel in the presence of N2 gas at room temperature. First, the resin was treated with 20% piperidine in DMF to remove the Fmoc group (deprotection step). This step was carried out twice for 5 min and 15 min respectively with DMF washes after every reaction. Amino acids were then added to the resin (coupling step). Coupling reaction mixtures contained Fmoc-amino acid (2.88 mmol), HBTU (1.06 g, 2.80 mmol) and DIEA (1.25 mL, 7.2 mmol) in DMF for 4 hr. After every amino acid coupling, the resin was rinsed with DMF thoroughly and deprotected for Fmoc removal. For the preparation of TMR-Rn, TMR-TAT and eosin-TAT conjugates, peptides were first assembled using Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Gln-OH. The Fmoc-group on the N-terminal residue was then removed using a deprotection reaction while keeping intact the side chain protecting groups of the other residues. The fluorophores were coupled to the N-terminus of the peptides using a mixture of 5(6)-carboxy-tetramethylrhodamine or 5(6)-carboxyeosin Y (2.88 mmol), HBTU (1.06 g, 2.80 mmol) and DIEA (1.25 mL, 7.2 mmol) in DMF overnight. For R9-K(TMR), K(TMR)-R9 and R4-K(TMR)-R5, the amino acids Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH and Fmoc- Fmoc-Lys(Mtt)-OH were used for the peptide chain synthesis. The resin was then washed with DCM and treated with a solution containing 1% TFA and 1% triisopropylsilane in DCM (4 times) to remove the Mtt protecting group from the lysine side chain. The resin was rinsed with DMF. The fluorophore was then coupled to the lysine side chain using a mixture of 5(6)-carboxytetramethylrhodamine (2.88 mmol), HBTU (1.06 g, 2.80 mmol) and DIEA (1.25 mL, 7.2 mmol) in DMF overnight. The Fmoc on the N-terminal amino acid was then removed by a deprotection reaction.
Following assembly of the conjugates, the resin was treated with a reaction mixture containing TFA (95%), H2O (2.5%) and triisopropylsilane (2.5%) for 2 h. This reaction causes simultaneous removal of the side chain deprotection groups on all amino acid residues and cleavage of the Fl-CPP conjugate from the resin. Crude Fl-CPPs in the TFA solution were then subjected to a cold anhydrous Et2O washes to obtain precipitates of Fl-CPP conjugates. The crude peptides were then solubilized in acetonitrile and lyophilized. The conjugates were purified by semi-preparative HPLC and their identity was confirmed by MALDI-TOF analysis. Pure Fl-CPPs were lyophilized and then dissolved in water to obtain a 1 mM stock solution. Concentrations of Fl-CPPs were calculated by measuring the absorbance of the TMR (ε = 91,500 M−1cm−1)(obtained from Molecular probes) or eosin (ε = 83,000 M−1cm−1) (22) at 556 nm and 525 nm respectively. Working solutions were made by diluting the Fl-CPP stocks in PBS.
Analytical results
TMR-TAT; calculated mass: 1865.07 Da, observed mass: 1865.94 Da; TMR-R1; calculated mass: 642.29 Da, observed mass: 642.61 Da; TMR-R3; calculated mass: 954.49 Da, observed mass: 955.51 Da; TMR-R5; calculated mass: 1266.70 Da, observed mass: 1267.74 Da; TMR-R7; calculated mass: 1578.90 Da, observed mass: 1579.99 Da; TMR-R9; calculated mass: 1891.10 Da, observed mass: 1892.96 Da; TMR-R11; calculated mass: 2203.30 Da, observed mass: 2204.82 Da; TMR-R13; calculated mass: 2515.50 Da, observed mass: 2516.95 Da; eosin-TAT; calculated mass: 2121.61 Da, observed mass: 2126.95 Da; R9-K(TMR); calculated mass: 2020.21 Da, observed mass: 2021.32 Da ; K(TMR)-R9; calculated mass: 2020.21 Da, observed mass: 2022.23 Da; R4-K(TMR)-R5; calculated mass: 2020.21 Da, observed mass: 2020.24 Da.
Photohemolysis assay
Whole blood was centrifuged for 15 min at 1500 g to separate the erythrocytes from other blood components. Erythrocyte pellet was resuspended in PBS and centrifuged (4 times) to remove plasma and the buffy coat completely. Equal volume of PBS was added to the erythrocyte pellet to obtain a 50% stock of RBCs. Fl-CPPs (2 μM) and a working solution of erythrocytes (0.1% in PBS) were then mixed in PBS and added to a 384-well plate. Cells were then incubated for 15 min and allowed to settle to the bottom of the dish prior to imaging. Following incubation, the sample was irradiated using green light (560 nm) on the microscope (RFP channel, Ex = 560±20 nm / Em= 630±35 nm) at periodic intervals. Images were captured after each irradiation using time-lapse imaging using Slidebook 4.2 software. Using bright field images, the number of lysed cells was counted after each irradiation. Lysed RBCs on bright field appear as transparent ghosts (no hemoglobin inside cells) while intact RBCs have a dark contrast due to the presence of hemoglobin (Figure 1D). For each Fl-CPP conjugate, a photohemolysis plot was generated. A minimum of 300 cells were analyzed for each Fl-CPP. Data represents the average lysis from 3 experiments. The corresponding standard deviations have also been shown.
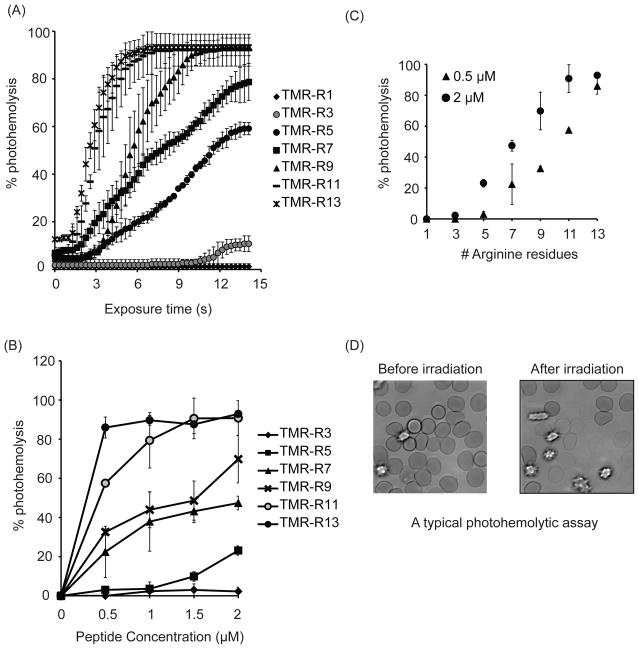
Figure 1.
The photohemolytic activity of TMR-Rn increases arginine content. A) Photohemolysis curves of TMR-Rn conjugates (n=1,3,5,7,9,11,13) at 2 μM concentration. B) Comparison of photohemolytic activity of TMR-Rn conjugates at 0.5 μM and 2 μM respectively after 7 s of light irradiation. C) Concentration dependence on the photohemolytic activity of TMR-Rn conjugates. Each data point represents the average from 3 experiments. The corresponding standard deviations are included.
Microscopy Imaging
Samples on a 384-well dish were placed on a temperature controlled stage at 37 °C, on an inverted epifluorescence microscope (Model IX81, Olympus, Center Valley, PA). Imaging was performed using a Rolera-MGI Plus back-illuminated EMCCD camera (Qimaging, Surrey, BC, Canada). Images were captures at bright field and using the RFP filter set (Ex = 560±20 nm / Em= 630±35 nm). The light source used was a 100W mercury lamp (Leeds Precision Instruments # L202 Osram). Light passes through filter cubes and the 100X objective prior to being incident on the sample. The amount of light transmitted to the sample was controlled using neutral density filters (ND 1, 2, 3 and 4 correspond to 100, 25, 12.5 and 5% transmittance). Irradiances were measured using a monochromic photometer (model 840-c, Newport, Irvine, CA). At 100X, the irradiances is approximately 21 or 420 W/cm2 with ND4 or ND1, respectively.
RNO assay to detect singlet oxygen production
A spectrophotometric RNO assay was performed to determine the production of singlet oxygen (1O2) from the TMR moiety of TMR-peptide conjugates upon light irradiation (23). The photosensitizer Rose Bengal (RB) has a reported 1O2 quantum yield of 0.76 in aqueous solution (23). Since RB (24) has spectral properties that are comparable to TMR (Ex (max) = 550 nm, Em (max) = 580 nm) (25), RB was used as a positive control for the assay. The compounds p-nitrosodimethylaniline (RNO) (50 μM) and imidazole (10 μM) were mixed with RB or TMR-CPPs in PBS on a 96-well plate and then irradiated using a halogen lamp set-up. First, a peroxide intermediate is formed upon reaction of 1O2 with imidazole. Reaction of the chromophore RNO with the intermediate results in bleaching (26). As a result, there is a loss of absorbance of RNO at 450 nm wavelength which is used to determine the rate of production of 1O2 (23). In order to ensure that both RB and TMR-CPPs absorb the same amount of light, the concentration of all compound was a adjusted such that their absorbance was 0.9 at 556 nm prior to irradiation (27). Upon irradiation, the decrease in the absorbance of RNO at 450 nm was recorded at periodic intervals using a plate reader.
Detection of superoxide formation using NBT method
A photometric assay that uses nitro blue tetrazolium (NBT) was performed to monitor the formation of superoxide (O2−) by TMR-CPPs (28–30). Eosin Y is a photosensitizer that produces superoxide radicals upon light irradiation (31). Hence, eosin-TAT was used as a positive control for this assay (conjugation of eosin Y to TAT was performed to improve the solubility of eosin Y in water). Singlet oxygen produced during the irradiation of the TMR-CPPs does not interfere with the NBT assay (28). NBT (80 μM), NADH (10 mM) and EDTA (1 mM) were mixed with TMR-CPPs (10 μM) in PBS buffer (32). The samples were then irradiated using the halogen lamp set-up. NBT gets reduced upon reaction with superoxide radicals and forms an insoluble purple compound formazan (28). Following irradiation, samples were diluted 5-fold in DMSO and absorbance at 600 nm was measured. The contribution of TMR or eosin to absorbance at 600 nm was negligible.
RESULTS
Length of the CPP affects the lytic ability of Fl-CPPs of Red blood cell membranes
To determine whether the length of the CPPs and its arginine content affects TMR-CPP mediated membrane photolysis, the lytic activity of TMR-Rn conjugates, with n = 3,5,7,9,11,13 was tested with red blood cells (RBCs) using a photohemolysis assay. For this assay, 0.1% RBCs was incubated with 2 μM TMR-Rn conjugates. The mixture was irradiated with green light at 560±20 nm. The light induced lysis of RBCs was monitored using time-lapse bright-field imaging using a microscope with a 100X objective. Photohemolysis curves were generated for each conjugate by counting the number of lysed ghosts in images obtained at each time point.
As shown in Figure 1A, TMR-R1 and TMR-R3 (2 μM) were not significantly photohemolytic. In comparison, increasing the length of the peptide to R5, R7, and R9 caused an increase in the photohemolytic response. In particular, the total percentage of lysed cells at each time point increased with peptide length (Figure 1A). The irradiation time required to initiate lysis also reduced as arginine content was increased. Interestingly, photohemolysis did not further increase when the conjugates TMR-R11 and TMR-R13 were tested (Figure 1A).
Because the saturation in the lytic activity of TMR-R11 and TMR-R13 might be a result of a saturating concentration of the Fl-CPP, a concentration dependent study of the photolysis activity with all TMR-Rn conjugates was performed. Photohemolysis was measured as a function of the number of arginine residues at 0.5 μM, 1 μM, 1.5 μM and 2 μM TMR-Rn concentration (Figure 1B). At a concentration of 0.5 μM, the photohemolytic activity of TMR-Rn increased linearly with ‘n’. However, at concentrations 1.5 μM and above, TMR-Rn lytic activity reached saturation for n>9 (Figure 1C). Interestingly, 100% photohemolysis was not achieved under these conditions, indicating that saturation does not happen simply because all RBCs in the sample are lysed. Overall, these results suggest that lytic activity of a TMR-Rn conjugates can be varied by altering the concentration of conjugate in solution as well as the number of arginine residues in the CPP.
The position of fluorophore with respect to CPP affects the lytic ability of Fl-CPPs
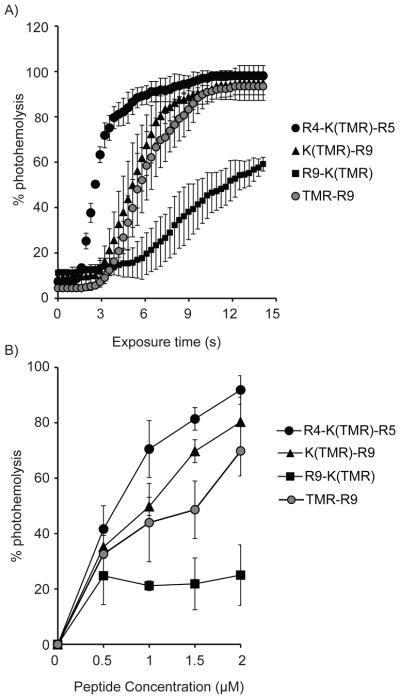
TMR-R9 was chosen as a template to investigate how positioning of the fluorophore with respect to the peptide might affect lytic activity. In order to obtain compounds that would differ in this specific structural parameter, the peptides K(TMR)-R9, R4-K(TMR)-R5, and R9-K(TMR) were synthesized using SPPS. For each peptide, TMR was conjugated to the side chain amino group of the lysine residue. The labeled lysine was placed at the N-terminus of the peptide, at the central position in the peptide sequence as well as at the C-terminus. The photohemolytic activity of each peptide (2 μM) was then measured and compared to that of the control TMR-R9 (where TMR is conjugated to the N-terminal amino group of the peptide). As shown in Figure 2A, both TMR-R9 and K(TMR)-R9 showed similar photolytic activity. However, shifting the TMR fluorophore from N to C terminus had a dramatic effect as R9-K(TMR) displayed a light-induced activity significantly lower than TMR-R9 and K(TMR)-R9. In contrast, shifting TMR to the center of the peptide improved lytic activity as R4-K(TMR)-R5 was the most photolytic conjugate tested. In particular, 50% photohemolysis is achieved with R4-K(TMR)-R5 with only half the light dose required to obtain similar results with TMR-R9. Interestingly, it is worth noting that both K(TMR)-R9 and R9-K(TMR) were more hemolytic than TMR-R9 in the dark (Figure 2A, time t=0). To compare the relative activities of these TMR-peptides further, photohemolysis was monitored at different concentrations of these conjugates. As shown in Figure 2B, K(TMR)-R9, R4-K(TMR)-R5, and TMR-R9 followed a similar dose response. In contrast, the lytic activity of R9-K(TMR) was not significantly affected by concentration. While surprising, these data nonetheless confirm that placing TMR at the C-terminus of the peptide greatly diminishes the photolytic activity of the conjugate. Overall, these results highlight how the position of the fluorophore in respect to the peptide sequence is an important structural factor in the conjugate’s lytic activity and how alterations in structure can both lead to increase or loss in activity.
Figure 2.
Photohemolysis is affected by the position of the fluorophore in the peptide sequence. A) Photohemolysis curves were generated TMR-R9, K(TMR)-R9, R9-K(TMR) and R4-K(TMR)-R5 at 2 μM. B) Concentration dependence on the photolytic activity. The reported percent hemolysis is obtained after 7 s of light irradiation. Each data point represents the average from 3 experiments. The corresponding standard deviations are included.
Effect of conjugation on singlet oxygen and superoxide generation
In principle, one can envision how the ROS generation of TMR might be affected by the peptide to which the fluorophore is attached. This would then potentially explain the different behaviors observed for the Fl-CPP conjugates tested. It was previously determined that the photolytic activity of TMR-R9 is oxygen dependent and mediated by formation of both singlet oxygen and superoxide (19). The generation of these ROS by all TMR conjugates was therefore measured. A RNO assay was used to establish the rate of formation of singlet oxygen. Rose Bengal was used as a positive control in this assay (the reported 1O2 quantum yield for RB is 0.76) (27). As shown in Figure 3A, 1O2 formation by the TMR-CPPs upon light irradiation was detectable, although very inefficient in comparison to Rose Bengal. This data is consistent with the low values of triplet state quantum yield of TMR (0.001–0.003) reported in the literature (33, 34). The singlet oxygen quantum yield of TMR thus, should be lower than the reported TMR triplet state quantum yield. From the RNO assay, the production of singlet oxygen was similar among all TMR conjugates, indicating that conjugation to different CPPs does not affect this activity significantly. Next, a NBT assay was used to detect superoxide formation. In this assay, eosin-TAT, a known superoxide producer, was used as a positive control. The samples were irradiated at 560 nm and the reduction of NBT was monitored spectroscopically. Here again, the rates of superoxide formation for all TMR-CPPs were found to be identical (Figure 3B–C). This in turn indicates that superoxide radical production is not affected by conjugation to CPPs. Overall, these results suggest that the observed differences in photolytic activities are not caused by differences in ROS production.
Figure 3.
Effect of conjugation of TMR to different peptides on singlet oxygen and superoxide production A) Detection of singlet oxygen formation by TMR-CPPs. The formation of singlet oxygen upon irradiation of TMR-CPPs was monitored using the RNO assay with Rose Bengal as a positive control (all at 10 μM). B,C) Detection of photo-generation of superoxide radicals by TMR-CPPs. using the NBT assay with eosin-TAT as the positive control (all at 10 μM). The control sample contained all reagents necessary for NBT assay but no peptide.
Increasing the length and arginine content of CPPs improves its lytic activity on oxidized membranes
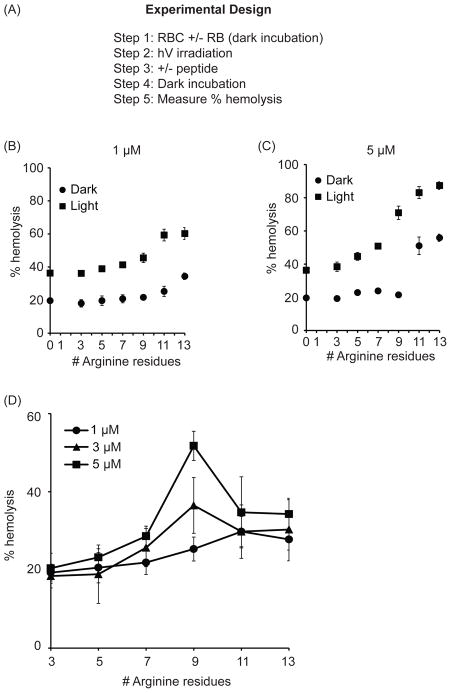
While R9 and TAT are not significantly hemolytic in the dark, these arginine-rich CPPs destabilize the membrane of RBCs upon photo-oxidation of membrane components (18). We therefore reasoned that the photohemolytic efficiencies observed with the various conjugates tested might in part be due to differences in this particular activity. To test this hypothesis, the effect of K(TMR)R9, R9K(TMR), R4K(TMR)R5 and the TMR-Rn peptides on photo-oxidized membranes was investigated. In this assay, 1.25% RBCs were first incubated with 1 μM of the lipophilic photosensitizer Rose Bengal (RB) and exposed to a single dose of light irradiation. RB, like TMR, produces singlet oxygen and superoxide (19). It also binds to the plasma membrane of RBCs and lyses these cells upon irradiation (18). RB-mediated membrane oxidation might therefore be similar to what is obtained upon TMR-CPP irradiation. In this experiment, RB irradiation was limited to a few seconds so as to avoid premature lysis (irradiation of RB alone lyses RBCs if irradiation is extended, data not shown). After irradiation, the TMR-CPP conjugates were added to the RB and RBC mix, and incubated for 15 min in the dark at 37 °C (Figure 4A). The mixture was then spun down and the absorbance of the supernatant was measured at 450 nm to calculate the extent of hemolysis. As shown in Figure 4, addition of the CPPs to RBCs partially photo-oxidized by RB enhanced the lysis of the RBCs. This enhancement in lysis after Fl-CPP addition in the dark increased with the length of the peptide and the arginine content (Figure 4B). Hemolysis also increased with Fl-CPP concentration (Figure 4C). Interestingly, the TMR-R11 and TMR-R13 peptides showed significant lysis in the dark (hemolysis obtained after RBCs are treated with RB and TMR-Rn in the dark) (Figure 4C). This suggests that damage of membranes by a mixture of RB and TMR-R11 or TMR-R13 can occur without a light trigger.
Figure 4.
The propensity of TMR-Rn to disrupt photo-oxidized RBCs is optimal for n=9. A, B) In the dark, TMR-Rn peptides lyse membrane of RBCs photo-oxidized by Rose Bengal. RBCs incubated with 1 μM RB were irradiated followed by addition of 1 μM and 5 μM TMR-Rn peptide in the dark. This is represented as light conditions. Alternately, the ability if Rn CPPs to disrupt un-oxidized (no irradiation but RB added) at 1 μM and 5 μM is also represented and referred to as dark conditions. High arginine content in CPPs resulted in dark lysis of un-oxidized RBC membranes but also enhanced disruption of photo-oxidized RBCs membranes. Hemolysis was measured at 450 nm and plotted against number of arginine residues on the CPP. The data shown represents the average of 3 experiments and the corresponding standard deviations. C) Net lysis of RBCs caused by Rn CPPs at 1 μM, 3 μM and 5 μM is shown here. The lysis caused by CPPs under dark conditions was subtracted from lysis caused by CPPs of photo-oxidized RBCs. 100% hemolysis was determined by addition of Triton X-100 to RBCs.
A plot comparing the ability of TMR-Rn peptides to lyse photo-oxidized RBCs (lysis obtained in the dark was subtracted from the lysis obtained after partial photosensitization) (Figure 4D). At 1 μM, the Rn CPPs enhanced the lysis of partially photodamaged RBCs proportionally to the number of arginine residue n. At higher concentrations, the disruption of photo-oxidized membrane was instead optimal at n=9 arginines residues. In particular, while R11 and R13 are more hemolytic in the dark than R9, R11 and R13 led to a relatively smaller improvement in lysis after light exposure. Overall, these data suggest that increasing arginine content in a CPP (n>9) does not necessarily increase the disruption of oxidized membranes.
Unlike the TMR-Rn series, the conjugates R9-K(TMR), K(TMR)-R9, R4-K(TMR)-R5 did not cause an increase in hemolysis of RBC incubated with RB in the dark. However, upon membrane photo-oxidation by RB, the conjugate R9-K(TMR), K(TMR)-R9 increased hemolysis in a manner similar to that obtained with TMR-R9 (Figure 5). Interestingly, R4-K(TMR)-R5, the most efficient photolytic compound in the experiment presented in Figure 3, displayed a slightly lower capacity to damage partially oxidized RBCs. Overall, these results suggests that these conjugates are all capable of disrupting partially-oxidized membranes. However, this activity does not account for the differences observed in Figure 2.
Figure 5.
Fl-CPPs with TMR at different positions enhance disruption of RBC membranes partially photo-oxidized by Rose Bengal with comparable efficiencies. For each Fl-CPP conjugate, the disruption of un-oxidized (no irradiation step but RB added) and photo-oxidized (irradiation in the presence of RB) RBC membranes is compared at different concentrations. The protocol used is the same as for the experiment in Figure 4.
Discussion
While CPP-mediated PCI is potentially useful in a number of cellular delivery applications, structure-activity relationships (SARs) for Fl-CPPs have not been established. In this study, our goal was to establish how the structure of CPPs might impact the lytic activity of Fl-CPPs. Measuring and comparing the photo-endosomolytic activity of Fl-CPPs, however, is complex. For instance, multiple parameters such as the amount of ROS generated, membrane binding and peptide-induced membrane disruption contribute to the photo-endosomolytic activity of Fl-CPPs. Direct assessment of the lytic activity of Fl-CPPs in live cells is also challenging because the concentration of Fl-CPPs inside endosomes will be influenced by the endocytic uptake of the peptide. In addition, the lumen of an endosome contains proteases that can degrade Fl-CPPs. Overall, quantitatively comparing the photo-endosomolytic activity of Fl-CPPs that have different propensities to be endocytosed and degraded is therefore difficult. To circumvent these complications and measure the intrinsic photolytic activity of Fl-CPPs more simply, the photohemolysis rather than photo-endosomolytic activity of Fl-CPPs was determined. Admittedly, many differences exist between the membranes of endosomes and that of RBCs. Topology and surface curvature are, for instance, quite different between these two systems. Yet, the plasma membrane of RBC and the membrane of endosomes have several features in common. In particular, both membrane systems display an asymmetry in the distribution of phosphatidylserine between cytoplasmic and external/luminal leaflets (35, 36). This asymmetry has been proposed to be important for the membrane translocation activity of CPPs (37). In addition, parallels between the lysis of endosomal and that of RBCs have previously been observed (17). For instance, TMR-R9, is both photo-endosomolytic and photohemolytic. In contrast, TMR-K9 is much less efficient then TMR-R9 at causing endosomal leakage upon irradiation, even when a greater amount of TMR-K9 than TMR-R9 accumulates inside endosomes (17). Similarly, TMR-K9 is poorly photohemolytic. These results therefore support the notion that the photohemolytic activity of Fl-CPP conjugates mirror their photo-endosomolytic activity.
Two aspects of the structure of Fl-CPPs were tested herein: the arginine residue content and the respective positioning between Fl and CPP moieties. Based on previous reports, both arginine residues and proximity between Fl and CPPs were suggested to be of importance for the photolytic activity of Fl-CPPs (18, 19). To establish SARs, a series of polyarginine Rn (n=1,3,5,7,9,11,13) peptides were labeled with the fluorophore TMR at the N-terminus to obtain TMR-Rn conjugates. In addition, TMR was conjugated to the side chain of a lysine residue positioned in the center or N and C-termini of the peptide R9. Overall, several clear trends could be identified from these SAR studies: 1) increasing the number of arginine residues accelerates photolysis, 2) R9 shows the highest propensity to disrupt photo-oxidized membranes, 3) positioning TMR in the center of the R9 peptide sequence increases the photolytic activity of the conjugate when compared to peptides conjugated at the termini, 4) N and C-terminus labeling are not equivalent. These simple rules should provide a guiding framework for the development future peptide-based photo-endosomolytic agents. Yet, our studies also highlight complex behaviors that are currently still poorly understood. For instance, the saturation behavior of TMR-R11 and TMR-R13, their hemolytic activity in the dark or reduced propensity to disrupt oxidized membranes are non-trivial. Along the same line, the reason why R9-K(TMR) is so poorly active when all R9 conjugates are photolytic is surprising. It is however interesting to note that R9-K(TMR) shows higher percentage of RBC lysis even before irradiation (t = 0s) as compared to K(TMR)-R9 and R4-K(TMR)-R5. The conjugation of TMR to the C- terminus of the CPP seems to confer a dark lytic activity while reducing the photolytic ability of R9-K(TMR). This behavior of R9-K(TMR) could be due to positioning of K(TMR)-R9 in the membranes in such a manner that the CPP moiety is able to cause destabilization of the membrane in the dark but the fluorophore moiety is not in very close proximity to the membrane. As a result, the oxidative damage of the membrane might be low and hence the low photolytic activity. Moreover, differences could arise in the activity of TMR-CPPs upon coupling of TMR to CPPs using different linker molecules. This could be because linker molecules may distance the TMR from the CPP moiety, in turn affecting reducing the proximity of TMR from the membranes which is an important factor for Fl-CPP lytic activity. Furthermore, because all conjugates were found to generate ROS in similar yield, a possible explanation for some of these observations is that membrane binding is very different among these species. For example, the sites of TMR-R13 binding on the surface of RBCs could be saturated even at all the concentrations tested. This could then explain why the activity TMR-R13 is relatively concentration independent in our assays. However, the binding of these TMR-CPPs to RBCs is relatively weak and we could not experimentally quantify how binding contributes to photohemolysis (17). While this represents a limitation of our study, this is in itself very interesting. In particular, previous studies have revealed that binding of Fl-CPPs to membranes is required for photolysis (19). For instance, liposomes containing negatively charged phospholipids that bind to CPPs are photo-destroyed by TMR-CPPs while liposomes containing neutral lipids that do not bind CPPs, but otherwise identical in the number of oxidizable sites, do not (19). The fact that little binding is detected would then suggest that the binding sites available on the surface of RBCs, while important for TMR-CPP mediated lysis, are either few in numbers or not strongly associating with the TMR-CPP conjugates. Yet, these interactions are sufficient to permit membrane photo-oxidation and disruption. It should also be noted that poor binding has limited our ability to identify the membrane components that participate in Fl-CPP mediated photo-damage. It is therefore at present difficult to obtain molecular insights about what contributes to the differences in activities of the various Fl-CPPs presented herein. Future studies with model systems such as liposomes, while not fully reflecting the complexity of a biological membrane, might help provide such explanations. In particular, the effect of oxidative damage on lipids and lipid bilayers has been well documented (38–41). Lipid peroxidation has for instance been shown to promote permeabilization by formation of hydrophobic or water defects (38, 39), induce raft formation (40), stimulate flip-flop (42), and cause a decrease in the bilayer thickness (39). One can speculate that the CPPs used in this study might interact with oxidized lipids and alter these processes to various degrees.
Our results highlight how the photolytic activity of TMR labeled oligoarginine conjugates can be modulated by changing the structure of the peptide moiety while keeping ROS-generation yields from TMR constant. We have focused on peptides containing only arginine residues because we have already demonstrated that arginine residues present an activity towards oxidized membranes that other residues do not reproduce (18) and because many CPPs have high arginine residue content (43, 44). The SAR established herein might therefore apply to arginine-rich CPPs in general. We have also used TMR because it is a relatively hydrophilic fluorophore that does not bind to lipid bilayers and biological membranes (19). TMR should therefore not contribute significantly to TMR-CPP/membrane interactions. Consequently, TMR might be representative of other fluorophores or photosensitizers that can generate ROS but that otherwise do not participate in membrane binding. On the other hand, one might expect that the activities reported would be significantly different if hydrophobic ROS-generators that strongly associate with membranes were used in place of TMR. In such cases, these compounds could have a dominant effect on membrane binding and the role played by the CPP moiety could be dramatically diminished. Finally, it should be noted that TMR is zwitterionic and that it presumably does not interact electrostatically with a positively charged CPP. In contrast, negatively charged fluorophores could bind to arginine side chains and alter the conformation of a CPP (the polyarginine peptides used in this report form random coils) (45). This would then give rise to Fl-CPP conjugates that display SAR very distinct from what is reported herein.
Overall, our studies provide several possible paths to optimizing CPP-mediated PCI. Optimizing the photolytic activity of ROS generators with CPPs could in turn have several benefits. One can, for instance, envision how optimal compounds would achieve endosomal lysis at low light doses. This would then permit PCI protocols to be performed with a low cost irradiation set-up. In the context of in vivo experiments, PCI is currently limited by the fact that visible light does not penetrate tissues deeply. In principle, compounds that are active at low light doses could also facilitate PCI delivery deeper into tissues. In addition, as ROS can damage many different biomolecules in a cell, PCI agents that can trigger endosomal leakage at low ROS levels should lead to fewer undesirable off-target reactions. This might in turn be beneficial to the physiology and viability of cells. Our SAR studies suggest that optimization of the photolytic activity Fl-CPPs is a viable path toward solving these problems.
Acknowledgments
This work was supported by Award Number R01GM087227 and R01GM087981 from the National Institute of General Medical Sciences, the Norman Ackerman Advanced Research Program, and the Robert A. Welch foundation (Grant A-1769).
References
- 1.Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–49. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annual review of biomedical engineering. 2006;8:343–75. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- 3.Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci. 2000;21:45–8. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 4.Liou JS, Liu BR, Martin AL, Huang YW, Chiang HJ, Lee HJ. Protein transduction in human cells is enhanced by cell-penetrating peptides fused with an endosomolytic HA2 sequence. Peptides. 2012;37:273–84. doi: 10.1016/j.peptides.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad O, Kjolsrud S, Anholt H, Rodal GH, Rodal SK, Hogset A. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59:1180–3. [PubMed] [Google Scholar]
- 6.Mathews MS, Blickenstaff JW, Shih EC, Zamora G, Vo V, Sun CH, Hirschberg H, Madsen SJ. Photochemical internalization of bleomycin for glioma treatment. J Biomed Opt. 2012;17:058001. doi: 10.1117/1.JBO.17.5.058001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selbo PK, Weyergang A, Bonsted A, Bown SG, Berg K. Photochemical internalization of therapeutic macromolecular agents: a novel strategy to kill multidrug-resistant cancer cells. J Pharmacol Exp Ther. 2006;319:604–12. doi: 10.1124/jpet.106.109165. [DOI] [PubMed] [Google Scholar]
- 8.Berg K, Nordstrand S, Selbo PK, Tran DT, Angell-Petersen E, Hogset A. Disulfonated tetraphenyl chlorin (TPCS2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2011;10:1637–51. doi: 10.1039/c1pp05128h. [DOI] [PubMed] [Google Scholar]
- 9.Selbo PK, Hogset A, Prasmickaite L, Berg K. Photochemical internalisation: a novel drug delivery system. Tumour Biol. 2002;23:103–12. doi: 10.1159/000059713. [DOI] [PubMed] [Google Scholar]
- 10.Berg K, Weyergang A, Prasmickaite L, Bonsted A, Hogset A, Strand MT, Wagner E, Selbo PK. Photochemical internalization (PCI): a technology for drug delivery. Methods in molecular biology. 2010;635:133–45. doi: 10.1007/978-1-60761-697-9_10. [DOI] [PubMed] [Google Scholar]
- 11.Berg K, Berstad M, Prasmickaite L, Weyergang A, Selbo PK, Hedfors I, Hogset A. Photochemical internalization: a new tool for gene and oligonucleotide delivery. Top Curr Chem. 2010;296:251–81. doi: 10.1007/128_2010_63. [DOI] [PubMed] [Google Scholar]
- 12.Wang JT, Giuntini F, Eggleston IM, Bown SG, MacRobert AJ. Photochemical internalisation of a macromolecular protein toxin using a cell penetrating peptide-photosensitiser conjugate. Journal of controlled release : official journal of the Controlled Release Society. 2012;157:305–13. doi: 10.1016/j.jconrel.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Maiolo JR, 3rd, Ottinger EA, Ferrer M. Specific redistribution of cell-penetrating peptides from endosomes to the cytoplasm and nucleus upon laser illumination. J Am Chem Soc. 2004;126:15376–7. doi: 10.1021/ja044867z. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita M, Noguchi H, Lu YF, Tomizawa K, Michiue H, Li ST, Hirose K, Bonner-Weir S, Matsui H. Photo-acceleration of protein release from endosome in the protein transduction system. FEBS Lett. 2004;572:221–6. doi: 10.1016/j.febslet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Endoh T, Sisido M, Ohtsuki T. Spatial regulation of specific gene expression through photoactivation of RNAi. Journal of controlled release : official journal of the Controlled Release Society. 2009;137:241–5. doi: 10.1016/j.jconrel.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Muthukrishnan N, Johnson GA, Lim J, Simanek EE, Pellois JP. TAT-mediated photochemical internalization results in cell killing by causing the release of calcium into the cytosol of cells. Biochimica et biophysica acta. 2012;1820:1734–43. doi: 10.1016/j.bbagen.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan D, Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Lim J, Simanek EE, Pellois JP. Conjugation to the cell-penetrating peptide TAT potentiates the photodynamic effect of carboxytetramethylrhodamine. PloS one. 2011;6:e17732. doi: 10.1371/journal.pone.0017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthukrishnan N, Johnson G, Erazo-Oliveras A, Pellois JP. Synergy Between Cell-Penetrating Peptides and Singlet Oxygen Generators Leads to Efficient Photolysis of Membranes. Photochem Photobiol. 2012 doi: 10.1111/php.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meerovich I, Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Pellois JP. Photodamage of lipid bilayers by irradiation of a fluorescently labeled cell-penetrating peptide. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbagen.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niki E, Yamamoto Y, Komuro E, Sato K. Membrane damage due to lipid oxidation. The American journal of clinical nutrition. 1991;53:201S–205S. doi: 10.1093/ajcn/53.1.201S. [DOI] [PubMed] [Google Scholar]
- 21.Berg K, Prasmickaite L, Selbo PK, Hellum M, Bonsted A, Hogset A. Photochemical internalization (PCI)--a novel technology for release of macromolecules from endocytic vesicles. Oftalmologia. 2003;56:67–71. [PubMed] [Google Scholar]
- 22.Hulspas R, Krijtenburg PJ, Keij JF, Bauman JG. Avidin-EITC: an alternative to avidin-FITC in confocal scanning laser microscopy. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1993;41:1267–72. doi: 10.1177/41.8.7687265. [DOI] [PubMed] [Google Scholar]
- 23.Kraljić I, Mohsni SE. A new method for the detection of singlet oxygen in aqueous solutions. Photochem Photobiol. 1978;28:577–581. [Google Scholar]
- 24.Sugioka K, Nakano M. A possible mechanism of the generation of singlet molecular oxygen in nadph-dependent microsomal lipid peroxidation. Biochimica et biophysica acta. 1976;423:203–16. doi: 10.1016/0005-2728(76)90179-1. [DOI] [PubMed] [Google Scholar]
- 25.Geisow MJ, Evans WH. pH in the endosome. Measurements during pinocytosis and receptor-mediated endocytosis. Experimental cell research. 1984;150:36–46. doi: 10.1016/0014-4827(84)90699-2. [DOI] [PubMed] [Google Scholar]
- 26.Kraljic I, Mohsni SE. New Method for Detection of Singlet Oxygen in Aqueous-Solutions. Photochemistry and Photobiology. 1978;28:577–581. [Google Scholar]
- 27.Kochevar IE, Redmond RW. Photosensitized production of singlet oxygen. Methods Enzymol. 2000;319:20–8. doi: 10.1016/s0076-6879(00)19004-4. [DOI] [PubMed] [Google Scholar]
- 28.Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, Janjua S, Gad F, Hamblin MR. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–10. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi HS, Kim JW, Cha YN, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem. 2006;27:31–44. doi: 10.1080/15321810500403722. [DOI] [PubMed] [Google Scholar]
- 30.Umezawa N, Arakane K, Ryu A, Mashiko S, Hirobe M, Nagano T. Participation of reactive oxygen species in phototoxicity induced by quinolone antibacterial agents. Archives of biochemistry and biophysics. 1997;342:275–81. doi: 10.1006/abbi.1997.0124. [DOI] [PubMed] [Google Scholar]
- 31.Natera JE, Massada WA, Amat-Guerri F, García NA. Elementary processes in the eosin-sensitized photooxidation of 3,3′-diaminobenzidine for correlative fluorescence and electron microscopy. Journal of Photochemistry and Photobiology A: Chemistry. 2011;220:25–30. [Google Scholar]
- 32.Seki S, Flavahan NA, Smedira NG, Murray PA. Superoxide anion scavengers restore NO-mediated pulmonary vasodilation after lung transplantation. The American journal of physiology. 1999;276:H42–6. doi: 10.1152/ajpheart.1999.276.1.H42. [DOI] [PubMed] [Google Scholar]
- 33.Eggeling C, Widengren J, Rigler R, Seidel CA. Photobleaching of Fluorescent Dyes under Conditions Used for Single-Molecule Detection: Evidence of Two-Step Photolysis. Anal Chem. 1998;70:2651–9. doi: 10.1021/ac980027p. [DOI] [PubMed] [Google Scholar]
- 34.Geissbuehler M, Spielmann T, Formey A, Marki I, Leutenegger M, Hinz B, Johnsson K, Van De Ville D, Lasser T. Triplet imaging of oxygen consumption during the contraction of a single smooth muscle cell (A7r5) Biophys J. 2010;98:339–49. doi: 10.1016/j.bpj.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor J, Gillum K, Schroit AJ. Maintenance of lipid asymmetry in red blood cells and ghosts: effect of divalent cations and serum albumin on the transbilayer distribution of phosphatidylserine. Biochimica et biophysica acta. 1990;1025:82–6. doi: 10.1016/0005-2736(90)90193-r. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Jiang Y, Zeng S, Yan J, Li X, Zhang Y, Zou W, Wang X. Endocytic sorting and recycling require membrane phosphatidylserine asymmetry maintained by TAT-1/CHAT-1. PLoS Genet. 2010;6:e1001235. doi: 10.1371/journal.pgen.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriques ST, Castanho MA. Consequences of nonlytic membrane perturbation to the translocation of the cell penetrating peptide pep-1 in lipidic vesicles. Biochemistry. 2004;43:9716–24. doi: 10.1021/bi036325k. [DOI] [PubMed] [Google Scholar]
- 38.Kotova EA, Kuzevanov AV, Pashkovskaya AA, Antonenko YN. Selective permeabilization of lipid membranes by photodynamic action via formation of hydrophobic defects or pre-pores. Biochimica et biophysica acta. 2011;1808:2252–7. doi: 10.1016/j.bbamem.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L. Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J. 2007;93:4225–36. doi: 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayuyan AG, Cohen FS. Lipid peroxides promote large rafts: effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys J. 2006;91:2172–83. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villacara A, Spatz M, Dodson RF, Corn C, Bembry J. Effect of arachidonic acid on cultured cerebromicrovascular endothelium: permeability, lipid peroxidation and membrane “fluidity”. Acta neuropathologica. 1989;78:310–6. doi: 10.1007/BF00687761. [DOI] [PubMed] [Google Scholar]
- 42.Volinsky R, Cwiklik L, Jurkiewicz P, Hof M, Jungwirth P, Kinnunen PK. Oxidized phosphatidylcholines facilitate phospholipid flip-flop in liposomes. Biophys J. 2011;101:1376–84. doi: 10.1016/j.bpj.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madani F, Lindberg S, Langel U, Futaki S, Graslund A. Mechanism of Celullar Uptake of Cell-Penetrating Peptides. Journal of Biophysics. 2011 doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herce HD, Garcia AE, Litt J, Kane RS, Martin P, Enrique N, Rebolledo A, Milesi V. Arginine-rich peptides destabilize the plasma membrane, consistent with a pore formation translocation mechanism of cell-penetrating peptides. Biophys J. 2009;97:1917–25. doi: 10.1016/j.bpj.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takechi Y, Yoshii H, Tanaka M, Kawakami T, Aimoto S, Saito H. Physicochemical mechanism for the enhanced ability of lipid membrane penetration of polyarginine. Langmuir. 2011;27:7099–107. doi: 10.1021/la200917y. [DOI] [PubMed] [Google Scholar]