Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal disease without any cure. Both human disease and animal models demonstrate dysregulated wound healing and unregulated fibrogenesis in a background of low-grade chronic T lymphocyte infiltration. Tissue-resident memory T cells (Trm) are emerging as important regulators of the immune microenvironment in response to pathogens, and we hypothesized that they might play a role in regulating the unremitting inflammation that promotes lung fibrosis. Herein, we demonstrate that lung-directed immunotherapy, in the form of i.n. vaccination, induces an antifibrotic T cell response capable of arresting and reversing lung fibrosis. In mice with established lung fibrosis, lung-specific T cell responses were able to reverse established pathology — as measured by decreased lung collagen, fibrocytes, and histologic injury — and improve physiologic function. Mechanistically, we demonstrate that this effect is mediated by vaccine-induced lung Trm. These data not only have implications for the development of immunotherapeutic regimens to treat IPF, but also suggest a role for targeting tissue-resident memory T cells to treat other tissue-specific inflammatory/autoimmune disorders.
Administration of a vaccinia-based vaccine induces lung niche memory T cells that arrest and reverse established lung fibrosis and improve pulmonary function.
Introduction
Pulmonary fibrosis is a progressive, fatal disease that primarily affects elderly patients with no clear etiology and no effective treatments to prevent, arrest, or reverse the fibrosis. Although the cause of pulmonary fibrosis is unknown, it is associated with dysregulated wound healing and unregulated fibrogenesis in a background of low-grade chronic inflammation (1, 2). Current therapies at best have only slowed the ongoing fibrosis, rather than halting or reversing it (3, 4). One reason for the absence of effective therapies may be the lack of understanding of the pathophysiology leading to fibrosis. What is becoming clear is the importance of the immune system in both the development and resolution of this disease. There are numerous observations in animals and humans that demonstrate a persistent association between pulmonary fibrosis and lung T lymphocyte infiltration (5–7). However, how this inflammatory milieu determines if T cells will promote a fibrotic response, as opposed to a healing response, is unclear.
Recently an important role for tissue-resident memory T cells (Trm) in the recall response to pathogens has been described (8, 9). Numerous studies have demonstrated the essential role for Trm in mediating protection against tissue-specific challenges such as viral, bacterial, and parasitic infections (8, 9). For example, in the lungs, both CD4+ and CD8+ Trm are important for protection against influenza (10). In this regard, these tissue-specific memory T cells interact with cells of the innate immune response to collectively promote immunity. Through the elaboration of cytokines, they are able to greatly influence the tissue immune microenvironment, leading to the activation of DCs, macrophages, NK cells, and the local upregulation of antimicrobial or antiviral genes.
While the precise mechanisms leading to pulmonary fibrosis are unknown, it is clear that unremitting dysregulated lung inflammation promotes its development (1, 2). Our group and others have identified a role for IL17 and Th17 cells in promoting the inflammation leading to fibrosis (11–14). Alternatively, Th1 immune responses in the lung are associated with resolution of inflammation (12). To this end, we have previously demonstrated that promotion of a Th1 environment in the lung abrogates and protects against the development of pulmonary fibrosis in an acute model of bleomycin-induced pulmonary fibrosis (12). That is, by skewing the inflammatory milieu of the lung to a Th1 environment, we can mitigate the development of acute bleomycin-induced lung injury. These studies serve to clarify the immunologic components of lung inflammation leading to resolution versus progressive fibrosis. Additionally, there are numerous reports demonstrating the ability of a variety of inhibitors, antibodies, and cytokines to prevent the development of bleomycin-induced intratracheal (i.t.) fibrosis (15–17). The true benefit of these studies, however, is confounded by the fact that it is unclear whether the interventions prevent fibrosis or whether they merely inhibit the initial acute inflammatory process prior to the initiation of the fibrogenic programs.
In an effort to determine if immunotherapy in the form of Th1-promoting vaccines can treat/reverse already established disease, we utilized a model of i.p. injections of bleomycin over the course of 4 weeks that leads to subsequent progressive lung fibrosis at 42–72 days. This model differs from an acute i.t. bleomycin model by causing subacute inflammation, which progresses to lung fibrosis (18, 19). The i.p. bleomycin model appears to more closely approximate the human fibrotic disease idiopathic pulmonary fibrosis (IPF) in that, like the human disease, there is no acute inflammatory phase and the fibrosis progresses slowly and continuously over time. In addition, just like in human disease, i.p. bleomycin induces significant low-grade immune cell infiltration, collagen deposition, and fibrotic changes in the mouse lung. This model facilitates the analysis of potential treatments that may interrupt or reverse ongoing fibrosis, as opposed to treatments that merely prevent fibrosis, by preventing the acute inflammation leading to fibrosis. Thus, this model allows us to separate the effects of acute inflammation and subacute inflammation on the development and progression of lung fibrosis, better allowing for therapeutic interventions.
In this report, we demonstrate that i.n. administration of a vaccinia-based vaccine, after fibrosis has already been established, is effective at reversing established pathology, as measured by decreased lung collagen deposition and histologic damage and improved lung function. Furthermore, mechanistically, we show that the interruption and reversal of the disease is mediated by Trm. Thus, we demonstrate the ability of Trm to regulate the tissue-specific immune microenvironment in order to protect/reverse pathologic inflammation and fibrosis. Overall, these data not only have implications for the development of an immunotherapeutic regimen to treat IPF, but also suggest a role for targeting Trm in order to treat a wide array of tissue-specific inflammatory/autoimmune disorders.
Results
Immunotherapy with vaccinia vaccination interrupts the development of pulmonary fibrosis.
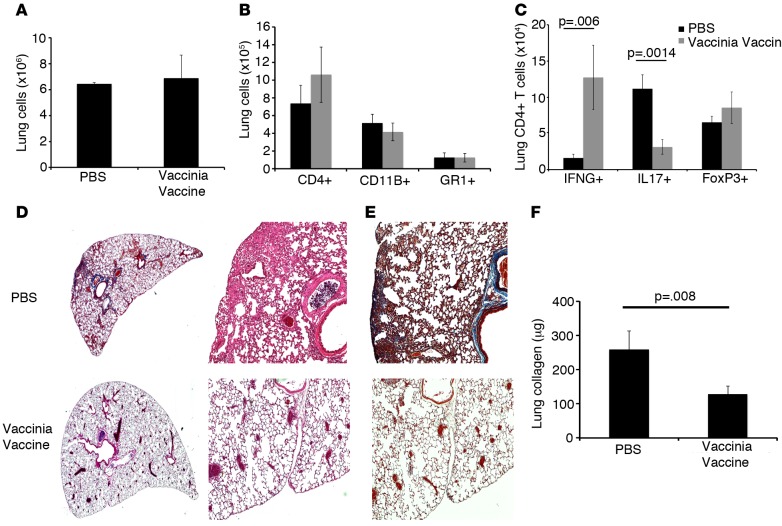
To test the hypothesis that immunotherapy in the form of i.n. vaccination could arrest or reverse subacute progressive fibrosis, we injected WT C57B6 mice i.p. with bleomycin on days 0, 3, 7, 10, 14, 21, and 28. On day 14, mice received either i.n. PBS or vaccinia vaccine (small pox vaccine; 2 million pock-forming units [pfu]). Our vaccine consisted of a genetically modified vaccinia that contains the full-length ovalbumin protein but lacks lytic ability; thus, the vaccinia cannot spread. Once the cells are infected, the virus will replicate in that cell but cannot release further virus to perpetuate an infection. Previously, we have demonstrated that the vaccinia vaccination causes no sustained damage to the mouse lungs, as evidenced by no mortality, sustained weight loss, no change in total lung cell count, and no histologic damage after vaccination (12). Four weeks following vaccination (day 42 after first i.p. injection of bleomycin), mice were sacrificed and lungs were harvested and processed into single cell suspensions for flow cytometry analysis. Total lung cell numbers and total numbers of lung CD4+ T cells, CD11B+ macrophages, and GR1+ neutrophils were not significantly different between PBS and vaccinated mice (Figure 1, A and B). These data indicate that vaccination was not inducing additional inflammation in the lungs of treated mice. However, in keeping with the known Th1 skewing effect of vaccinia, mice that received the vaccine had significantly increased numbers of IFNG+CD4+ T cells (Figure 1C). We also observed a significant decrease in IL17+CD4+ T cells in the lungs of vaccinia-treated mice but no significant difference in Foxp3+ regulatory T cells between PBS and vaccinia vaccine–treated mice. Histologic examination of the lungs revealed less inflammation, distortion, and collagen staining in the vaccinated bleomycin injured mice, as compared with PBS-treated bleomycin-injured mice (Figure 1, D and E). Finally, Figure 1F demonstrates that vaccination was able to reverse the accumulation of collagen in the lungs of bleomycin-injured mice. These data indicate that, while not increasing overall lung inflammation, i.n. vaccination effectively drives the CD4+ T cell response away from detrimental IL17 skewing and promotes a protective Th1 response. Interestingly, when we treated mice with i.p. vaccinia, we observed no protective effect and no significant increase in IFNG producing CD4+ T cells in the lungs (data not shown). These data indicate that tissue site–specific administration of vaccinia vaccine appears to enhance lung immunity and provide a protective effect against fibrotic development.
Figure 1. Immunotherapy with vaccinia vaccine inhibits the development of pulmonary fibrosis.
(A) Day-42 total lung cell numbers of mice treated with i.p. bleomycin and either i.n. PBS or vaccinia vaccine on day 14. (B) Flow cytometric analysis of total lung CD4+ T cells, CD11b+ cells, and Gr1+ cells. (C) Intracellular cytokine analysis of total lung IFNG+, IL17+, and FoxP3+ CD4+ T cells. Histological analysis of lungs 42 days following i.p. bleomycin. (D) H&E staining of lung sections at ×20 (left panels) and ×100 (right panels) magnification. (E) Masson’s trichrome staining of lung sections at ×100 magnification. (F) Total lung collagen 42 days following i.p. bleomycin. Error bars represent one standard deviation of the mean. Experiments were performed 3 times with 10 mice per group. Paired Student’s t tests were performed with Bonferroni correction when indicated.
Vaccinia immunotherapy reduces fibrocyte recruitment from the BM.
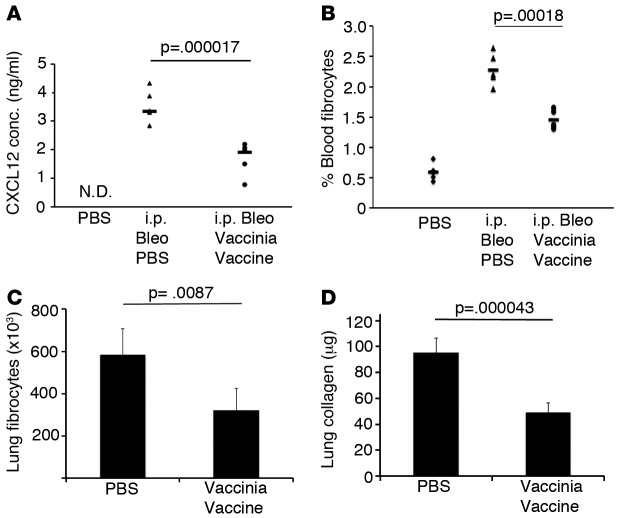
As we observed a beneficial effect of vaccination in interrupting developing fibrosis in the lung, we investigated the effect of vaccination on circulating fibrocytes in the blood. Fibrocytes, which are CD45+collagen 1+ BM–derived cells, are believed to be recruited to the lungs and contribute to the development of pulmonary fibrosis (20, 21). Increased circulating fibrocytes in the blood have been shown to correlate with progressive disease in patients with IPF (22). It has been proposed that these BM-derived circulating fibrocytes migrate to the injured lungs in part due to increased levels of the chemokines such as CXCL12, CCL2, CCL12, CCL1, and CCL3 (21, 23–33). As peripheral levels of CXCL12, peripheral fibrocytes, and lung fibrocytes have been measured in IPF patients and correlate with worsening disease, we sought to determine if vaccination would affect CXCL12 levels in the blood and ultimately decrease circulating fibrocytes (20, 21). C57BL/6 mice were treated with a 4-week course of i.p. bleomycin as described above. On day 14, mice received either PBS or i.n. vaccinia vaccine, and 2 weeks later, the mice were sacrificed and blood was collected by cardiac puncture. Peripheral blood mononuclear cells (PBMC) were stained for the fibrocyte markers CD45 and collagen 1 and analyzed by FACS. Concomitantly, serum CXCL12 concentrations were determined by ELISA. Immunotherapy in the form of vaccination resulted in a significant decrease in serum levels of CXCL12 (Figure 2A). Furthermore, percentages of circulating blood fibrocytes were also significantly decreased by vaccinia treatment (2.5%–1.5%), (Figure 2B). Analysis of lungs 42 days following i.p. bleomycin revealed a significantly decreased number of lung fibrocytes in the vaccinated mice (Figure 2C). In addition, total lung collagen was significantly decreased by nearly 50% in bleomycin-injured mice that received i.n. vaccine when compared with PBS control mice (Figure 2D). Thus, immunotherapy with vaccinia vaccination may in part reverse the fibrosis by decreasing CXCL12 serum concentrations, circulating fibrocytes in the blood and lung, and ultimately collagen deposition in the lung. These data demonstrate the ability of i.n. vaccination to interrupt the development of fibrosis even after the fibrotic process has already begun.
Figure 2. Vaccinia vaccine immunotherapy abrogates fibrocyte recruitment and lung collagen deposition.
(A) ELISA of blood serum CXCL12 28 days following i.p. bleomycin (Bleo) treatment. (B) Flow cytometric analysis of circulating blood fibrocytes (CD45+, Col I+) on day 28 following i.p. Bleo treatment. (C) Flow cytometric analysis of lung fibrocytes (CD45+, Col I+) 42 days following i.p. Bleo treatment. (D) Total lung collagen 42 days following i.p. Bleo treatment. Error bars represent one standard deviation of the mean. Experiments were performed 4 times with 5 mice per group (A and B). Experiments were performed 3 times with 10 mice per group (C and D). Paired Student’s t test were performed on samples with ANOVA. P < 0.05. Col I, collagen I.
Vaccinia immunotherapy reverses established lung fibrosis.
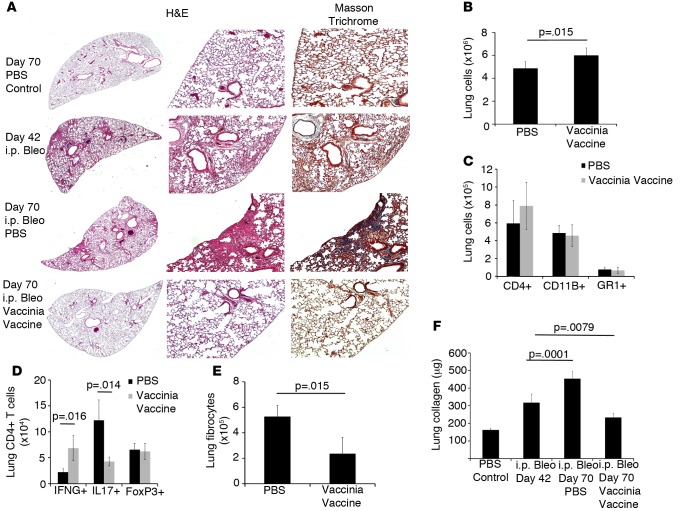
In humans, IPF is a slow, progressive disease of insidious onset, and we have no clear understanding of what initiates or perpetuates the fibrosis. The vast majority of patients have lost lung function prior to presentation. Therefore, any effective treatment strategy would have to not only halt progression of the fibrosis, but also ideally reverse the fibrosis. To date, the potential treatments for IPF have at best only slowed the progression of the fibrosis, not stopping and certainly not reversing the disease (3, 4). C57BL/6 mice were injected for 4 weeks with i.p. bleomycin, and after 2 additional weeks (day 42 when fibrosis is well established), the mice were given either i.n. PBS or vaccinia vaccine. Four weeks later (70 days after the first dose of bleomycin), lungs were harvested for flow cytometry and histologic analysis. Lung histologic analysis displayed increased inflammation, collagen, and fibrosis in bleomycin-PBS–treated mice, as compared with bleomycin-vaccinia–treated mice (Figure 3A). By day 42, all bleomycin-treated mouse lungs already had substantial fibrosis and collagen deposition. On day 70, bleomycin-treated mice that received PBS therapy demonstrated increased inflammation and collagen deposition; however, bleomycin-treated mice that received immunotherapy displayed reduced inflammation and collagen deposition even when compared with lungs from day-42 bleomycin-injured mice prior to therapy (Figure 3A). On day 70, there was a small but significant increase in total lung cells in vaccinated mice; however, total numbers of CD4+ T cells, CD11B+ macrophages, and GR1+ neutrophils were not significantly different (Figure 3, B and C). As previously noted, vaccinated mice displayed a significant increase in IFNG+CD4+ T cells while also having significantly decreased numbers of IL17+CD4+ T cells when compared with PBS-treated mice (Figure 3D). Again, there was not a significant difference in total Foxp3+ regulatory T cell numbers, indicating that vaccinia vaccination treatment does not affect regulatory T cell numbers in this model. Flow cytometry of lung fibrocytes showed that vaccination led to reduced fibrocyte numbers to approximately half of bleomycin-PBS–treated mice (Figure 3E). In addition, we found that vaccinated mice had significantly decreased concentrations of collagen in their lungs when compared with day 42 harvested mice (Figure 3F). Interestingly, mice that received PBS on day 42 after bleomycin continued to have increased collagen deposition and had significantly increased collagen levels in their lungs on day 70 compared with day-42 animals, indicating that ongoing fibrosis was occurring between days 42 and 70. However, mice that received vaccinia vaccine on day 42 after bleomycin had collagen levels that were below both day-42 animals and day-70 PBS-treated animals. Thus, vaccinia vaccine not only blocked progression of fibrosis, but it also substantially reversed lung fibrosis. Overall, these data indicate that i.n. vaccinia vaccination immunotherapy, even after a full course of i.p. bleomycin and established fibrosis, is able to stop and reverse the progression of pulmonary fibrosis.
Figure 3. Vaccinia immunotherapy reverses lung fibrosis.
(A) Histological analysis of lungs 70 days following i.p. bleomycin (Bleo). H&E staining of lung sections at ×20 (left panels) and ×100 (right panels) magnification; Masson’s trichrome staining at ×100 magnification. (B) Total lung cells 70 days following i.p. Bleo. (C) Flow cytometric analysis of total lung CD4+ T cells, CD11b+, and GR1+ cells. (D) Intracellular cytokine analysis of total lung IFNG+, IL17+, and FoxP3+ CD4+ T cells. (E) Flow cytometric analysis of lung fibrocytes. (F) Total Lung collagen at either 42 or 70 days following i.p. Bleo. Error bars represent one standard error of the mean. Experiments were performed at 3 times with 10 mice per group. Significance determined by a paired Student’s t tests or 1-way ANOVA followed by Tukey’s test when indicated.
Vaccinia immunotherapy improves lung function.
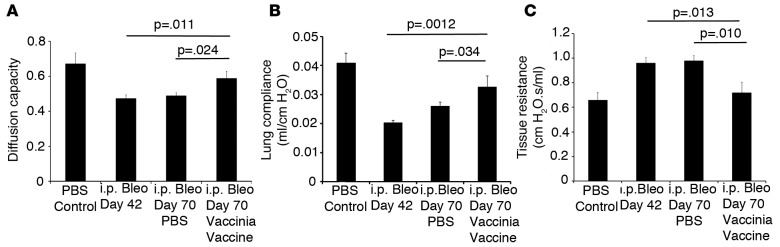
Next, we wanted to determine if the ability of vaccinia immunotherapy to arrest and reverse the pathologic fibrosis correlated with improved lung function. Pulmonary fibrosis is characterized by a stiffening of the lung, which causes an increase in lung resistance and a decrease in lung compliance. In addition, due to increased collagen deposition and thickening of the interstitium, there is a substantial decrease in gas exchange in the lung. By employing pulmonary function testing in humans, it is possible to follow the progression of disease. Therefore, we sought to determine if vaccinia immunotherapy was capable of improving lung function in our animal model.
To accomplish this, bleomycin was administered by i.p. injection for 4 weeks (days 1–28). Two weeks later (day 42), pulmonary function testing was performed on part of the cohort of mice to establish the baseline decrease in lung function due to the bleomycin-induced fibrosis. Lung gas exchange was assessed by diffused capacity of carbon monoxide (CO), and tissue resistance and compliance were measured by conventional forced oscillation technique (34). Mice that didn’t receive pulmonary function testing received either PBS or vaccinia vaccine i.n. at this time (day 42). On day 70, pulmonary function testing was performed on all remaining mice to examine the effect of vaccinia vaccine on reversing functional lung damage due to pulmonary fibrosis.
Bleomycin treatment alone resulted in a decrease in lung gas exchange on day 42, as measured by diffusing capacity of CO compared with uninjured mice (Figure 4A). Mice that were harvested on day 70 and received PBS i.n. on day 42 also had significantly decreased gas exchange, with values similar to that seen by mice harvested on day 42. However, mice that received vaccinia vaccine on day 42 had a 20% increase in gas exchange when compared with mice harvested on day 42 or mice treated with PBS and harvested on day 70. Similar findings were obtained for lung mechanic measurements where vaccinated mice displayed a 35% decrease in lung resistance (Figure 4C) and a 40% increase in lung compliance (Figure 4B) when compared with either day-42 analyzed mice or day-70 mice that received PBS i.n. These data indicate that not only is vaccinia vaccine immunotherapy effective at abrogating collagen deposition, but it is also effective at improving lung function.
Figure 4. Vaccinia immunotherapy improves lung function.
(A) Diffusion capacity of mice on days 42 and 70 following i.p. bleomycin (Bleo) treatment with or without vaccinia vaccine immunotherapy. Pulmonary function testing for (B) tissue resistance and (C) lung compliance of mice on days 42 and 70 following i.p. Bleo treatment with or without vaccinia vaccine immunotherapy. Error bars represent one standard deviation of the mean. Experiments were performed 3 times with 10 mice per group. Significance determined by 1-way ANOVA followed by Tukey’s test when indicated.
The establishment of targeted lung tissue Th1 responses is critical for the ability of vaccinia immunotherapy to reverse lung fibrosis and improve lung function.
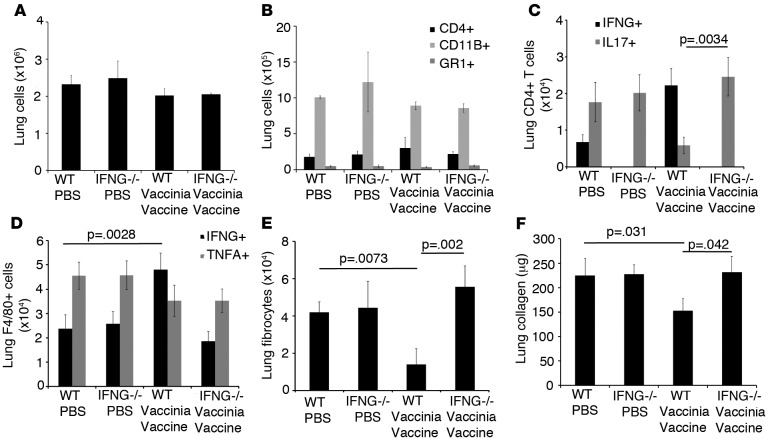
The inhibition and reversal of pulmonary fibrosis by vaccinia vaccine therapy correlates with increases in Th1 CD4+ T cells in the lung. Therefore, we sought to determine if Th1 T cells were required for the beneficial effects seen by vaccinia treatment. To accomplish this, C57BL/6 Rag–/– mice were reconstituted with CD4+ and CD8+ T cells from WT or mice lacking Th1 T cells (IFNG–/– C57BL/6 mice). Equivalent reconstitution was confirmed by flow cytometric analysis of blood 2 weeks later (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/jci.insight.83116DS1). Two weeks later, mice received 4 weeks of i.p. bleomycin injections. On day 35, mice received either PBS or vaccinia vaccine i.n. On day 70, mice were sacrificed and flow cytometry was performed. Total lung cells numbers were not significantly different between mice that received WT or IFNG–/– T cells (Figure 5A). Total CD4+ T cells, CD11B+ macrophages, and GR1+ neutrophils were also not significantly different between groups (Figure 5B).
Figure 5. Reversal of pulmonary fibrosis by vaccinia immunotherapy requires Th1 CD4+ T cells.
(A) Total lung cells on day 70 following transfer of either WT or IFNG-null CD4+ and CD8+ T cells into RAG–/– recipients followed by i.p. bleomycin with or without vaccinia vaccine immunotherapy. (B) Flow cytometric analysis of CD4+ T cells, CD11b+ cells, and Gr1+ cells on day 70 following i.p. bleomycin. (C) Intracellular cytokine analysis of IFNg- and IL17-producing CD4+ T cells. (D) Intracellular cytokine analysis of F4/80+ IFNG+ and TNFΑ+ lung cells. (E) Flow cytometric analysis of lung fibrocytes (CD45+, Col I+). (F) Total lung collagen 70 days following i.p. bleomycin. Error bars represent one standard deviation of the mean. Experiments were performed at least 3 times with 10 mice per group. Significance determined by 1-way ANOVA followed by Tukey’s test when indicated. Col I, collagen I.
Mice that received PBS treatment had increased numbers of IL17+CD4+ T cells when compared with mice that received WT T cells and vaccinia vaccine therapy. In addition, mice that received IFNG–/– T cells and vaccinia therapy also had increased numbers of IL17+CD4+ T cells, indicating that IFNG promotes CD4+ T cells skewing toward Th1 and away from Th17 differentiation in the lung (Figure 5C). We also observed that, even though all mice had macrophages capable of producing IFNG, only mice that received WT CD4+ T cells and vaccinia vaccine therapy had increased numbers of IFNG+ macrophages in the lung, indicating that Th1-skewed T cells were driving macrophage-derived IFNG production (Figure 5D). Mice that received WT T cells and vaccinia vaccine had decreased numbers of lung fibrocytes (Figure 5E) and lung collagen (Figure 5F), indicating that Th1 skewing inhibits fibrocyte recruitment to the lung. These data were confirmed by lung histology that demonstrated dramatically decreased lung infiltration and collagen staining only in mice that received WT T cells and vaccinia vaccine (Supplemental Figure 2). Overall, these data indicate that the lung tissue–specific Th1 response is the driving force behind the ability of i.n. vaccination to abrogate fibrosis, perhaps by skewing the immune response away from the pathologic role of Th17 cells.
Vaccinia immunotherapy induces the formation of a lung niche of memory CD4+ T cells.
Recently, it has been demonstrated that inflammation in the lung in the setting of viral infection leads to the establishment of a lung niche that contains activated and memory T cells (10). These lung niche T cells establish a pseudolung lymph node within the interstitium of the lung and are subsequently protected from blood circulation. It is therefore possible to differentiate circulating lymphocytes from protected lung lymphocytes by in vivo staining of circulating lymphocytes and in vitro staining of lung niche lymphocytes (10).
In light of our observations, we hypothesized that vaccinia immunotherapy was altering the makeup of lung niche lymphocytes. To address this, bleomycin was administered i.p. on days 0, 3, 7, 10, 14, 21, and 28. On day 42, mice received either PBS or vaccinia vaccine i.n. On day 70, mice were injected i.v. with anti-CD4 FITC and lungs were removed 10 minutes later. Lungs were then rinsed with PBS, removed, processed to single cell suspensions, and stained with anti-CD4 phycoerythrin (PE) in order to identify protected lung niche lymphocytes.
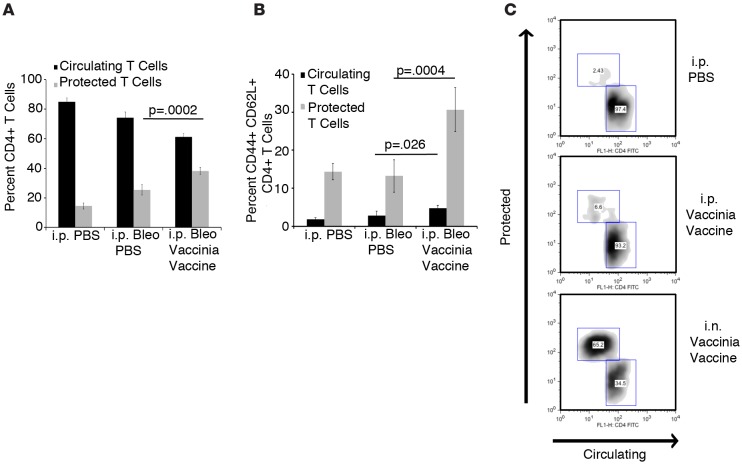
Bleomycin-treated mice that received PBS had an increased percentage of protected CD4+ T cells, indicating that bleomycin alone is sufficient to induce a lung niche. However, mice that received vaccinia vaccine had a significantly greater percentage of protected CD4+ T cells than PBS-treated mice (Figure 6A). In addition, when protected CD4+ T cells were stained with the cell surface markers CD44 and CD62L, the mice that received i.n. vaccination had a significantly increased percentage of memory T cells (CD44+ CD62L+) when compared with PBS-treated mice (Figure 6B.) These data indicate that vaccinia therapy following bleomycin treatment results in the development of a lung niche that contains a significant number of memory T cells. In addition, these lung niche memory CD4+ T cells may be important for the resolution and perhaps reversal of pulmonary fibrosis induced by bleomycin.
Figure 6. Vaccinia immunotherapy creates a lung niche of memory CD4+ T cells.
(A) Flow cytometric analysis of circulating (blood) vs. protected (lung niche) CD4+ T cells on day 70 following i.p. bleomycin (Bleo) with or without vaccinia vaccine treatment. (B) Percentage of memory (CD44+ CD62L+) marker expressing CD4+ T cells in the circulating and protected pool of i.p. Bleo-treated mice. (C) Flow cytometric plots of circulating (blood) vs. protected (lung niche) CD4+ T cells on day 14 from mice treated with i.p. PBS, i.p. vaccinia vaccine, or i.n. vaccinia vaccine. Error bars represent one standard deviation of the mean. Experiments were performed 3 times with 10 mice per group. Significance determined by 1-way ANOVA followed by Tukey’s test when indicated.
As we previously noted, vaccinia immunotherapy was only effective if the vaccinia vaccine was administered i.n. We sought to determine whether the method of vaccinia vaccine administration was important for the development of the protective lung niche. To address this, mice were treated with i.n. PBS, i.n. vaccinia vaccine, or i.p. vaccinia vaccine. Two weeks later, mice were injected i.v. with anti-CD4 FITC. Lungs were then rinsed in PBS, removed, processed to single cell suspensions, and stained with anti-CD4 allophycocyanin (APC) in order to identify protected lung niche lymphocytes. As previously seen, we observed a significant increase in lung-resident CD4+ T cells in mice treated with i.n. vaccinia vaccine (Figure 6C). However, mice that received i.p. vaccinia vaccine had only a slight increase in lung-resident CD4+ T cells, indicating that the development of a lung niche requires a site-specific treatment of vaccinia vaccine (Figure 6C).
The induction of tissue-resident lung CD4+ T cells by vaccinia vaccine is required to mitigate bleomycin-induced pulmonary fibrosis.
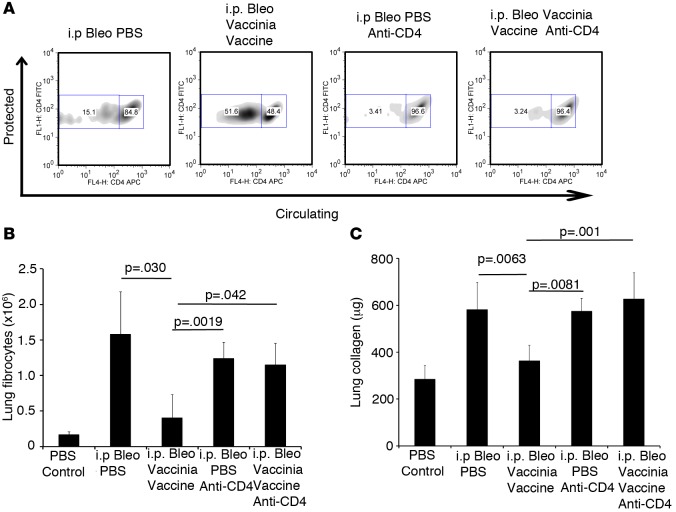
We have demonstrated that vaccinia immunotherapy induces lung tissue–resident memory CD4+ T cells, and we hypothesize that these lung tissue–resident T cells are required for vaccination-induced arrestment and reversal of lung fibrosis. Thus, we wanted to design an experiment whereby we could selectively eliminate the vaccine-induced lung Trm and then determine the efficacy of our immunotherapy strategy. To achieve this goal, we took advantage of previous findings of Laidlaw et al., who demonstrated that the depletion of CD4+ T cells during the response to infection (vaccine) eliminated the generation of the lung-specific Trm (35). To this end, mice received i.p. bleomycin on days 0, 3, 7, 10, 14, 21, and 28. On days 15 and 16, some mice received i.p. injections of the CD4-depleting antibody GK1.5. Also on day 16, mice received either i.n. PBS or vaccinia vaccine. On day 21, mice were analyzed in order to determine the effectiveness of T cell depletion. As seen in Supplemental Figure 3, CD4 T cells were completely absent from blood, BAL, and lung tissue. On day 42, mice were injected i.v. with anti-CD4 FITC, and 10 minutes later, lungs were harvested, rinsed with PBS, processed to single cell suspensions, and stained with CD4 APC in order to distinguish circulating lymphocytes from protected lymphocytes. Mice that received i.n. vaccinia vaccine had a significant increase in protected lung cells when compared with PBS-treated mice. However, mice that received anti-CD4 depleting antibody and vaccinia vaccine had substantially fewer protected lung cells than mice that did not received depleting antibody (Figure 7A). Importantly, while the protected T cells were markedly diminished, the overall T cell compartment had returned to normal in the GK1.5-treated mice.
Figure 7. Lung-resident memory CD4+ T cells are required for the vaccinia vaccination to mitigate fibrosis.
(A) Flow cytometric analysis of circulating vs. protected CD4+ T cells on day 42 following i.p. bleomycin (Bleo) with or without vaccinia vaccine treatment and T cell depletion. (B) Flow cytometric analysis of lung fibrocytes. (C) Total lung collagen on day 42 following i.p. Bleo. Error bars represent one standard deviation of the mean. Experiments were performed 3 times with 5 mice per group. Significance determined by 1-way ANOVA followed by Tukey’s test when indicated.
When lung fibrocytes and lung collagen were analyzed, mice that received vaccinia vaccine demonstrated protection from bleomycin-induced fibrosis. However, when the generation of lung Trm was prevented, the protective effects of vaccination were lost (Figure 7, B and C). These data are further supported by lung histology, which showed similar cell infiltration and collagen levels in mice that received PBS or vaccinia vaccine if both received depleting antibody (Supplemental Figure 4). These data indicate that the ability of vaccinia vaccine to mitigate bleomycin-induced lung fibrosis requires the generation of resident memory T cells that produce the Th1 cytokine IFNG and, in doing so, inhibit and reverse the unremitting inflammation that promotes fibrosis.
Discussion
Being in continuous contact with the environment, the lung immune microenvironment is designed to respond robustly and rapidly to pathogens. Trm are emerging as critical mediators of this response (10). Equally as important to antipathogen responses in the lung is the resolution of inflammation in the lung such that lung architecture and function remain intact. In this regard, IPF represents dysregulated, unremitting, low-grade inflammation that fails to resolve and, thus, leads to fibrosis (1). In this report, we demonstrate the ability of immunotherapy in the form of an i.n. vaccine as a means of interrupting and reversing this process. In doing so, we established a previously unappreciated role of Trm in regulating this pathologic inflammation and reestablishing lung homeostasis. We observe that vaccinia-induced IFNG-producing lung niche memory T cells are able to both arrest and reverse fibrosis. Mechanistically, we observed that our immunotherapeutic approach significantly affected the lung immune microenvironment. Specifically, we observed decreases in Th17 cells and the production of CXCL12 — both of which have been implicated as playing important roles in promoting fibrosis. Similarly, we observed decreased recruitment of fibrocytes and subsequent decreased collagen deposition. That is, our immunotherapy approach is able to inhibit several mechanisms that have been previously shown to promote lung fibrosis (Th17 cells, M2 macrophages, CXCL12, and fibrocytes) (12, 20, 21, 36). Perhaps most remarkably was not only the ability of vaccine-induced lung niche memory T cells to alter the tissue immune microenvironment and thereby arrest the development of fibrosis, but also its ability to promote the appropriate resolution and healing, leading to improved lung function.
A critical distinguishing feature about our work is that we demonstrate the ability of immunotherapy to interrupt and reverse established fibrosis. Many studies using various KO mice and drugs have been shown to be able to block the induction of fibrosis by using the acute i.t. bleomycin model (37–46). However, in such cases, it is unclear if these specific genes or drugs regulate the development of fibrosis per se or rather block the initial acute generic inflammation that begins the process that ultimately leads to fibrosis. In our studies, we allow the mice to develop fibrosis over 42 days before initiating immunotherapy. In doing so, not only do we demonstrate the ability of vaccine therapy to arrest the developing fibrosis, but we also promote the reestablishment of nonpathogenic Th1-mediated immunity, leading to partial resolution of fibrosis. This resolution is not only quantified by histology, but also by improvement in lung function. To this end, our data have great relevance to clinical IPF in which patients initially present with ongoing established disease. Interestingly, while these current studies employ vaccinia vaccine to induce local Th1 responses in the lungs, pilot studies using an engineered listeria vaccine to induce Th1 responses also appear to robustly arrest/reverse disease (data not shown). Of note, these listeria vaccine constructs are currently being safely administered to patients as cancer immunotherapy vaccines (47–49). It is important to note that our strategies employing vaccinia (or listeria) vaccination represent nonreplicating genetically altered vaccines, not infections. This is in stark contrast to, for example, γ herpes virus infections in which persistent viral replication and epithelial cell death leads to an increase in TGFB production and a worsening of pulmonary fibrosis (50, 51).
Previous studies by the Farber group have phenotypically and functionally defined lung Trm in response to viral infection in the lung (52). By injecting Fl-labeled anti-CD4 i.v., they were able to elegantly demonstrate that the fluorescein-negative CD4+ T cells in the lung were Trm. Indeed, our studies demonstrate a similar upregulation of these cells in response to vaccinia. Furthermore, when we prevent the development of the lung niche memory T cells, vaccinia vaccination is no longer protective. Based on these data, we propose a model whereby lung Trm derived from vaccination in the lung are able to promote an immune microenvironment that can arrest and reverse the chronic processes mediating fibrosis. While further studies will be needed to define the precise details of this model, our findings support the development of immunotherapy as a means of treating IPF.
Recently, 2 drugs have been approved for use in patients with IPF. Pirfenidone has been shown to have antifibrotic and antiinflammatory properties in animal models, and it slows the loss of lung function in clinical trials (3). Nintedanib, a tyrosine kinase inhibitor, has also been associated with reducing the decline in lung function in clinical trials (4). Although systemic IFNG treatment for patients with IPF initially showed intriguing promise in a phase II clinical trial, it ultimately was shown to have no beneficial effect in a phase III trial (53). These results are consistent with our findings that systemic vaccinia vaccination does not mitigate disease. That is, the key to successful immunotherapy to arrest/reverse disease is dependent upon the establishment of a local Trm-mediated Th1 response within the lung. It is not the ability of vaccinia vaccination to induce IFNG that promotes the therapeutic effect, but rather it is the ability of vaccinia vaccination to promote a local Th1 response that results in the reversal of the fibrosis. Thus, our data would also predict that systemic administration of IFNG would not be effective, as it would not promote a Trm-mediated Th1 lung response. Of note, our studies were performed on 8- to 20-week-old mice, whereas IPF affects mostly older individuals. However, we and others have demonstrated that bleomycin injury in mice is greater as mice age, and even mice 16–20 weeks old have increased fibrosis and mortality compared with young mice (12, 54). Indeed, we have already demonstrated that, despite the increasing mortality due to bleomycin with age, vaccinia vaccination can prevent bleomycin-induced mortality in older mice (12). Further studies will be necessary in very aged mice (>24 months) to evaluate the benefits of immunotherapy in this population.
Finally, our findings demonstrating a role for local Trm have potentially important implications for employing immunotherapy to treat inflammatory/autoimmune diseases. For example, in the case of idiopathic liver fibrosis, it is possible that inducing IFNG-producing Trm in the liver might mitigate disease. Similarly, other investigators are employing Th1-inducing parasites as immunotherapy to treat allergic disorders of the lungs (55–57). Based on our studies, we would predict that such an approach could mediate its effects through lung Trm.
Methods
Mice.
C57BL/6, B6.129S7-Rag1tm1Mom/J, and B6.129S7-Ifngtm1Ts/J were purchased from The Jackson Laboratory. Eight- to 10-week-old female mice (20 g) were utilized.
Reagents.
Modified vaccinia ankara virus was generated as previously described (58). This modified vaccinia ankara virus is a modified vaccinia that contains the full-length ovalbumin protein but lacks lytic ability. Bleomycin was purchased from App Pharmaceuticals. PMA and Ionomycin were purchased from Sigma-Aldrich. Flow cytometry reagents were purchased from BD Biosciences. Antibodies utilized were from eBioscience: IFNG FITC (clone XMG1.2), IL17A PE (clone ebio17B7), Foxp3 APC (clone FJK-16s), CD11B APC (clone M170), and CD8 APC (clone 53-6.7); from BD Pharmidgen: CD4 Percp (clone 4-5) and CD4 FITC (clone RM4-5); and from BioLegend: TNFA FITC (clone MP6-XT22), GR1 Percp (clone RB6-8C5), CD45 Percp (clone 30-F11), and CD4 APC (clone RM4-4). Collagen 1 biotin antibody was purchased from Rockland (part number 600-406-103). Strepavidin FITC was purchased BioLegend (catalog 405202).
Pulmonary fibrosis model.
Mice were injected i.p. with 0.8 U bleomycin on days 0, 3, 7, 10, 14, 21, and 28 to induce pulmonary fibrosis. Vaccinia vaccine was administered at a dose of 2 million pfu per mouse.
ELISA.
CXCL12 ELISA (R&D Systems) was performed according to manufacturer’s protocols.
Collagen assay.
Hydroxyproline (Biovision) assay was performed according to manufacturer’s protocols.
Histology.
Lungs were inflated to pressure with formalin and sectioned and stained for H&E and Masson’s trichrome. Samples were analyzed by microscope at ×20 and ×100 magnification.
In vivo antibody labeling and flow cytometry.
For in vivo antibody labeling, mice were injected i.v. with 2.5 μg FITC-conjugated anti-CD4 antibody (clone RM4-5), and after 10 minutes, lungs were isolated and rinsed in PBS, and cells were isolated. Isolated lymphocytes were then stained in vitro with a different, noncompeting clone of APC–anti-CD4 (clone RM4-4), along with antibodies to other surface markers with fluorochrome-conjugated antibodies. Stained cells were analyzed using a BD FacsCaliber (BD Biosciences), and analyzed using with FlowJo software (Tree Star Inc.).
In vivo T cell depletion.
Mice were injected i.p. with 250 μg GK1.5 antibody (BioXcell) on days 15 and 16 following the initiation of i.p. bleomycin injections (35).
Reconstitution experiments.
T cells (CD4+ and CD8+), were purified from spleen cells from C57BL/6 or B6.129S7-Ifngtm1Ts/J mice by MACS employing negative selection (Miltenyl Biotec). The WT or IFNg–/– CD4+ and CD8+ T cells were mixed and injected i.v. into B6.129S7-Rag1tm1Mom mice, which were then challenged with bleomycin and treated with or without vaccinia-vaccination.
Diffusion factor for CO measurement.
To assess overall functional changes in the lungs following bleomycin-induced injury, measurement of the diffusion factor for CO (DFCO) was performed as described previously (59). Briefly, mice were anesthetized with a mixture of ketamine (100 mg/kg)/xylazine (15 mg/kg) via i.p. injection. Once sedated, a tracheostomy was performed, and an 18-gauge cannula was inserted. Mouse lungs were quickly inflated with a 0.8 ml gas mixture (0.5% neon, 1% CO and balance air). After a 9-second breath hold, 0.8 m of gas was quickly withdrawn from the lung and diluted to 2 ml with room air. The neon and CO concentrations in the diluted air were measured by gas chromatography (INFICON, Model 3000A) to assess DFCO. The dilution to 2 ml was needed, since the gas chromatograph required a minimal sample size of 1 ml.
Pulmonary mechanics measurements.
After DFCO assessment, mice were connected to a flexi-VentTM ventilator (SCIREQ) and ventilated with a tidal volume of 0.2 ml of 100% oxygen at a rate of 150 Hz. with a positive end–expiratory pressure (PEEP) of 3 cmH2O. Mice were paralyzed with an i.p. injection of succinylcholine (75 mg/kg), subjected to deep inspiration at 30 cmH2O for 5 seconds and returned to normal ventilation for 1 minute. Baseline measurements of respiratory system resistance (Rrs), compliance (Crs) and elastance (Ers) were measured during a 2-second breath hold with a 2.5-Hz sinusoidal oscillation using the single compartment model (34). The impedance of the respiratory system was also obtained using a constant phase model to provide measurements of airway resistance (Raw), tissue damping (G), and tissue elastance (H) (60).
Statistics.
Statistical analyses were conducted using paired, Student’s t test for single comparisons. For analysis when multiple comparisons, 1-way ANOVA followed by Tukey’s test was used to analyze the differences between the groups. Bonferroni correction was performed on P values for experiments when greater then 3 variables were tested. Statistical significant values were those where P < 0.05.
Study approval.
All animal studies were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University (Baltimore, Maryland, USA).
Author contributions
MRH, SCL, WM, and JDP designed the research. SLC, YCL,CLV, NL, and MO conducted experiments. SLC, YCL,CLV, NL, and MO acquired data. MRH, SLC, and JDP prepared the manuscript.
Supplementary Material
Acknowledgments
Funding for this manuscript: NIH PO1 HL010342 (to M.R. Horton), NIH R21 HL111783 (to M.R. Horton), FAMRI (to M.R. Horton), and the Osborne Family Research Fund (to M.R. Horton).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:JCI Insight. 2016;1(4):e83116. doi:10.1172/jci.insight.83116.
Contributor Information
Yee Chan-Li, Email: ychanli1@jhmi.edu.
Nathachit Limjunyawong, Email: nlimjuny@jhsph.edu.
Wayne Mitzner, Email: wmitzner@jhsph.edu.
References
- 1.Kuhn C. The pathogenesis of pulmonary fibrosis. Monogr Pathol. 1993;(36):78–92. [PubMed] [Google Scholar]
- 2.Martinet Y, Menard O, Vaillant P, Vignaud JM, Martinet N. Cytokines in human lung fibrosis. Arch Toxicol Suppl. 1996;18:127–139. doi: 10.1007/978-3-642-61105-6_14. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Jr, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.Fireman E, et al. Predictive value of response to treatment of T-lymphocyte subpopulations in idiopathic pulmonary fibrosis. Eur Respir J. 1998;11(3):706–711. [PubMed] [Google Scholar]
- 6.Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol. 2008;83(2):237–244. doi: 10.1189/jlb.0707504. [DOI] [PubMed] [Google Scholar]
- 7.Papiris SA, et al. Relationship of BAL and lung tissue CD4+ and CD8+ T lymphocytes, and their ratio in idiopathic pulmonary fibrosis. Chest. 2005;128(4):2971–2977. doi: 10.1016/S0012-3692(15)52722-0. [DOI] [PubMed] [Google Scholar]
- 8.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107(42):17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13(5):309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 10.Turner DL, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7(3):501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson MS, et al. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins SL, Chan-Li Y, Hallowell RW, Powell JD, Horton MR. Pulmonary vaccination as a novel treatment for lung fibrosis. PLoS One. 2012;7(2):e83116. doi: 10.1371/journal.pone.0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonian PL, Roark CL, Born WK, O’Brien RL, Fontenot AP. γΔ T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl Res. 2009;154(5):222–227. doi: 10.1016/j.trsl.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakao A, et al. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104(1):5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grygielko ET, et al. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther. 2005;313(3):943–951. doi: 10.1124/jpet.104.082099. [DOI] [PubMed] [Google Scholar]
- 17.Higashiyama H, et al. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp Mol Pathol. 2007;83(1):39–46. doi: 10.1016/j.yexmp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, et al. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol. 2011;186(2):1097–1106. doi: 10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le TT, et al. Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. J Immunol. 2014;193(7):3755–3768. doi: 10.4049/jimmunol.1302470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36(4):598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Phillips RJ, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller A, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 23.Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara A, et al. Correlation between circulating fibrocytes, and activity and progression of interstitial lung diseases. Respirology. 2012;17(4):693–698. doi: 10.1111/j.1440-1843.2012.02167.x. [DOI] [PubMed] [Google Scholar]
- 25.Antoniou KM, et al. Expression analysis of angiogenic growth factors and biological axis CXCL12/CXCR4 axis in idiopathic pulmonary fibrosis. Connect Tissue Res. 2010;51(1):71–80. doi: 10.3109/03008200903056150. [DOI] [PubMed] [Google Scholar]
- 26.Andersson-Sjoland A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40(10):2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 28.Kalderen C, et al. CCL2 mediates anti-fibrotic effects in human fibroblasts independently of CCR2. Int Immunopharmacol. 2014;20(1):66–73. doi: 10.1016/j.intimp.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Singh SR, et al. CCL2 release by airway smooth muscle is increased in asthma and promotes fibrocyte migration. Allergy. 2014;69(9):1189–1197. doi: 10.1111/all.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carulli MT, Handler C, Coghlan JG, Black CM, Denton CP. Can CCL2 serum levels be used in risk stratification or to monitor treatment response in systemic sclerosis? Ann Rheum Dis. 2008;67(1):105–109. doi: 10.1136/ard.2006.067967. [DOI] [PubMed] [Google Scholar]
- 31.Ekert JE, Murray LA, Das AM, Sheng H, Giles-Komar J, Rycyzyn MA. Chemokine (C-C motif) ligand 2 mediates direct and indirect fibrotic responses in human and murine cultured fibrocytes. Fibrogenesis Tissue Repair. 2011;4(1):e83116. doi: 10.1186/1755-1536-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore BB, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galindo M, Santiago B, Rivero M, Rullas J, Alcami J, Pablos JL. Chemokine expression by systemic sclerosis fibroblasts: abnormal regulation of monocyte chemoattractant protein 1 expression. Arthritis Rheum. 2001;44(6):1382–1386. doi: 10.1002/1529-0131(200106)44:6<1382::AID-ART231>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 34.Ewart S, Levitt R, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol (1985) 1995;79(2):560–566. doi: 10.1152/jappl.1995.79.2.560. [DOI] [PubMed] [Google Scholar]
- 35.Laidlaw BJ, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41(4):633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gharib SA, et al. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J Leukoc Biol. 2014;95(1):9–18. doi: 10.1189/jlb.1112587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters-Golden M, et al. Protection from pulmonary fibrosis in leukotriene-deficient mice. Am J Respir Crit Care Med. 2002;165(2):229–235. doi: 10.1164/ajrccm.165.2.2104050. [DOI] [PubMed] [Google Scholar]
- 38.Nakatani-Okuda A, et al. Protection against bleomycin-induced lung injury by IL-18 in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L280–L287. doi: 10.1152/ajplung.00380.2004. [DOI] [PubMed] [Google Scholar]
- 39.Izbicki G, et al. Bleomycin-induced lung fibrosis in IL-4-overexpressing and knockout mice. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1110–L1116. doi: 10.1152/ajplung.00107.2002. [DOI] [PubMed] [Google Scholar]
- 40.Tang YJ, et al. Latent transforming growth factor-beta1 protects against bleomycin-induced lung injury in mice. Am J Respir Cell Mol Biol. 2014;51(6):761–771. doi: 10.1165/rcmb.2013-0423OC. [DOI] [PubMed] [Google Scholar]
- 41.Horikawa M, et al. E- and P-selectins synergistically inhibit bleomycin-induced pulmonary fibrosis. Am J Pathol. 2006;169(3):740–749. doi: 10.2353/ajpath.2006.060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makino H, et al. Antifibrotic effects of CXCR4 antagonist in bleomycin-induced pulmonary fibrosis in mice. J Med Invest. 2013;60(1–2):127–137. doi: 10.2152/jmi.60.127. [DOI] [PubMed] [Google Scholar]
- 43.Hamblin MJ, et al. Lovastatin inhibits low molecular weight hyaluronan induced chemokine expression via LFA-1 and decreases bleomycin-induced pulmonary fibrosis. Int J Biomed Sci. 2014;10(3):146–157. [PMC free article] [PubMed] [Google Scholar]
- 44.Chen ES, Greenlee BM, Wills-Karp M, Moller DR. Attenuation of lung inflammation and fibrosis in interferon-γ-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol. 2001;24(5):545–555. doi: 10.1165/ajrcmb.24.5.4064. [DOI] [PubMed] [Google Scholar]
- 45.Besnard AG, et al. CXCL6 antibody neutralization prevents lung inflammation and fibrosis in mice in the bleomycin model. J Leukoc Biol. 2013;94(6):1317–1323. doi: 10.1189/jlb.0313140. [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto H, Zhao LH, Jain F, Kradin R. IL-12p40(–/–) mice treated with intratracheal bleomycin exhibit decreased pulmonary inflammation and increased fibrosis. Exp Mol Pathol. 2002;72(1):1–9. doi: 10.1006/exmp.2001.2409. [DOI] [PubMed] [Google Scholar]
- 47.Le DT, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18(3):858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le DT, Dubenksy TW, Jr, Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol. 2012;39(3):311–322. doi: 10.1053/j.seminoncol.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toussaint B, Chauchet X, Wang Y, Polack B, Gouellec AL. Live-attenuated bacteria as a cancer vaccine vector. Expert Rev Vaccines. 2013;12(10):1139–1154. doi: 10.1586/14760584.2013.836914. [DOI] [PubMed] [Google Scholar]
- 50.Ashley SL, Jegal Y, Moore TA, van Dyk LF, Laouar Y, Moore BB. γ-Herpes virus-68, but not Pseudomonas aeruginosa or influenza A (H1N1), exacerbates established murine lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2014;307(3):L219–L230. doi: 10.1152/ajplung.00300.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mora AL, et al. Lung infection with γ-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L711–L721. doi: 10.1152/ajplung.00007.2005. [DOI] [PubMed] [Google Scholar]
- 52.Turner DL, Farber DL. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol. 2014;5:e83116. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raghu G, et al. A placebo-controlled trial of interferon γ-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350(2):125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 54.Ganster RW, Taylor BS, Shao L, Geller DA. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-κB. Proc Natl Acad Sci U S A. 2001;98(15):8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dittrich AM, et al. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol. 2008;180(3):1792–1799. doi: 10.4049/jimmunol.180.3.1792. [DOI] [PubMed] [Google Scholar]
- 56.Mo HM, et al. Schistosoma japonicum infection modulates the development of allergen-induced airway inflammation in mice. Parasitol Res. 2008;103(5):1183–1189. doi: 10.1007/s00436-008-1114-1. [DOI] [PubMed] [Google Scholar]
- 57.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202(9):1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Earl PL, Cooper N, Wyatt LS, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. Curr Protoc Protein Sci. 2001;Chapter 5:e83116. doi: 10.1002/0471140864.ps0512s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fallica J, Das S, Horton M, Mitzner W. Application of carbon monoxide diffusing capacity in the mouse lung. J Appl Physiol (1985) 2011;110(5):1455–1459. doi: 10.1152/japplphysiol.01347.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol (1985) 1992;72(1):168–178. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.