Abstract
Caffeine is a potent psychostimulant that can have significant and widely variable effects on the activity of multiple neuronal pathways. The most pronounced caffeine-induced behavioral effect seen in rodents is to increase locomotor activity which has been linked to a dose-dependent inhibition of A1 and A2A receptors. The effects of caffeine at the level of the lumbar spinal central pattern generator (CPG) network for hindlimb locomotion are lacking. We assessed the effects of caffeine to the locomotor function of the spinal CPG network via extracellular ventral root recordings using the isolated neonatal mouse spinal cord preparation. Addition of caffeine and of an A1 receptor antagonist significantly decreased the cycle period accelerating the ongoing locomotor rhythm, while decreasing burst duration reversibly in most preparations suggesting the role of A1 receptors as the primary target of caffeine. Caffeine and an A1 receptor antagonist failed to stimulate ongoing locomotor activity in the absence of dopamine or in the presence of a D1 receptor antagonist supporting A1/D1 receptor-dependent mechanism of action. The use of caffeine or an A1 receptor blocker failed to stimulate an ongoing locomotor rhythm in the presence of a blocker of the cAMP-dependent protein kinase (PKA) supporting the need of this intracellular pathway for the modulatory effects of caffeine to occur. These results support a stimulant effect of caffeine on the lumbar spinal network controlling hindlimb locomotion through the inhibition of A1 receptors and subsequent activation of D1 receptors via a PKA-dependent intracellular mechanism.
Keywords: caffeine, locomotion, spinal cord, adenosine, mouse, dopamine
Graphical Abstract
Introduction
Caffeine is the most consumed psychoactive substance worldwide (Nehlig et al. 1992; Fredholm, 1999). It is considered a stimulant drug that can have significant and widely variable effects on the activity of neuronal pathways in the central and peripheral nervous system including the modulation of behaviors such as vigilance, attention, arousal, and locomotor activity (Fredholm et al. 1999; Griffiths et al. 2003; Ferré, 2008). Caffeine is a known inhibitor of the cAMP degrading enzyme cyclic nucleotide phosphodiesterase but its major neural action is as a nonselective blocker of adenosine receptors (specifically A1 and A2A receptors) both pre- and post-synaptically (Ferré, 2008; Ferré, 2010). Caffeine blocks the access of endogenous adenosine at these receptors, which in rodents is reflected by an increase in locomotor behavior (Ferré, 2008; Fredholm et al. 1999; Griffiths et al. 2003). Caffeine’s behavioral effects are dose-dependent, with low doses causing behavioral activation and high doses causing suppression (Ferré, 2008; Ferré, 2010).
In animal models, caffeine has been shown to cause motor sensitization by increasing motor activity to a stimulus that previously did not induce a motor response (Tronci et al. 2006; Hsu et al. 2009; Simola et al. 2009). Caffeine also induced conditioned place preference, which is a form of Pavlovian conditioning used to measure the motivational effects of objects or experiences (Bedingfield et al. 1998; Patkina et al. 1998 Hsu et al. 2009). Additionally, the application of caffeine was shown to have an effect on cross-sensitization. These animals became sensitized to substances different from the ones they were already sensitized to during locomotion when exposed to nicotine and amphetamines (Celik et al. 2006; Simola et al. 2006). Furthermore, a recent study demonstrated that caffeine and SCH58261, a selective adenosine A2A receptor antagonist, but not DPCPX, a selective A1 eceptor antagonist, can induce reward and behavioral sensitization, which refers to a repeated exposure to a drug that enhances the motor-stimulant response to this drug (Hsu et al. 2009). Increased locomotor activity of a freely behaving rodent in the central region of an open field or a greater ratio to total locomotion is used as an indicator for evaluating anxiogenic or anxiolytic properties (Prut and Belzung, 2003). Caffeine has been shown to promote anxiogenic-like behavior in the light–dark preference test (Uchiyama et al., 2010). Additionally, caffeine was shown to increase locomotor activity in the open field (Kuribara et al., 1992; Nehlig et al., 1992; Haghgoo et al., 1995; Soares et al., 2009) in a bell-shaped dose-dependent manner. Caffeine exerts a stimulating effect on locomotor activity at low to moderate doses, but less stimulating and even depressive effects at higher doses (Mumford and Holtzman, 1991; El Yacoubi et al., 2000; Malec and Poleszak, 2006; Uchiyama et al., 2010; Zhang et al. 2011).
Most of the studies assessing the effects of caffeine and/or adenosine receptor agonists and antagonists on locomotor behavior have been performed on freely behaving rodents using systemic administration of these drugs (e.g. intravenous or intraperitoneal injection). As previously mentioned, the modulatory effects of caffeine on locomotor behavior have been attributed to mostly its blockade of A1 and A2A receptors located in brain regions such as the hippocampus, cortex, and cerebellum, these containing high levels of A1 receptors (with relatively low expression in the basal ganglia), and the striatum, nucleus accumbens, and olfactory tubercle which contain high levels of A2A receptors (Xie et al. 2007). The highly interconnected nature of the locomotor-regulatory circuitry of the brain, which includes (among others) the dorsal striatum, thalamus, and (ultimately) cortex linked by means of the globus pallidus, has created much debate regarding which of the two adenosine receptors is the principal target for caffeine exerting its motor stimulatory effects (Ferré et al. 2008). The use of a more isolated neural network controlling a repetitive motor behavior led us to study effects of caffeine at the level of the central pattern generator (CPG) network for hindlimb locomotion, located within the ventromedial region at the lower thoracic and upper lumbar levels of the mammalian spinal cord (Kjaerulff et al. 1994; Kjaerulff and Kiehn 1996; Kiehn et al. 1996; Raastad et al. 1996; Tresch and Kiehn 1999; Butt et al. 2002; Butt and Kiehn 2003). Thus this study was aimed at assessing the effects of caffeine and specific agonists and antagonists of adenosine receptors to locomotor output using the neonatal mouse isolated spinal cord preparation. A recent study by Witts et al looking at the modulatory effects of purinergic receptor activation on mammalian locomotor behavior showed that the application of adenosine led to a reduction in the frequency of locomotor activity recorded from ventral roots (Witts et al. 2012). This effect by adenosine was not present on slow rhythmic activity recorded upon blockade of all inhibitory transmission, suggesting that adenosine may act via the modulation of inhibitory transmission. Additionally, the application of the A1-specific antagonist cyclopentyl dipropylxanthine (DPCPX) but not SCH58261, a A2A-receptor antagonist, led to an increase in the frequency of locomotor activity suggesting that purinergic neuromodulation of locomotor behavior could be acting through the blockade of A1 but not A2A receptors (Witts et al. 2012). The results prompted us to explore if caffeine can modulate locomotor behavior when applied directly to the isolated spinal cord of mice, and if so, is this effect through the antagonism of adenosine receptors as previously reported in the brain? (El Yacoubi et al., 2000; Malec and Poleszak, 2006; Mumford and Holtzman, 1991; Uchiyama et al., 2010; Zhang et al. 2011). We report that addition of caffeine to the superfusate of an isolated lumbar spinal cord preparation, in the presence of a locomotor-like pattern induced by a combination of serotonin (5-TH), the glutamate analog n-methyl-d-aspartate (NMDA) and dopamine, had a stimulating effect by significantly decreasing the cycle period of the locomotor pattern, while decreasing motorneuron burst duration in most preparations in a reversible manner. Perfusion of an A1 but not and A2A receptor blocker mimicked the effects of caffeine confirming previous results regarding the mode of action of caffeine at low doses. Addition of adenosine or an A1 receptor agonist decreased the cycle period and burst duration reversibly in most preparations. Additionally, the use of caffeine or an A1 receptor antagonist failed to stimulate locomotor activity in the absence of dopamine in the superfusate, in the presence of a D1 but not a D2 receptor antagonist, and in the presence of a protein kinase A (PKA) inhibitor supporting an A1/D1/PKA-dependent mechanism through which caffeine stimulates locomotor activity in the neonatal mouse spinal cord.
Material and Methods
Experiments were performed using spinal cords of 0- to 3-day-old (P0–P3) ICR mice (Charles River, Wilmington, MA). The animal protocol was approved by the University of Puerto Rico Institutional Animal Care and Use Committee and was in accordance with National Institutes of Health guidelines. Animals were killed by rapid decapitation. The spinal cord was isolated by ventral laminectomy under ice-cold (4°C) oxygenated (95% O2-5% CO2) low-calcium Ringer solution (in mM: 128 NaCl, 4.7 KCl, 1.2 KH2PO4, 0.25 CaCl2, 1.3 MgCl2, 3.25 MgSO4, 25 NaHCO3, and 22 D-glucose) or glycerol-based artificial cerebrospinal fluid (aCSF) composed of (in Mm): 222 Glycerol, 3.08 KCl, 1.25MgSO4, KH2PPO4, 2.52 CaCl2, 25 NaCO3, and 11 D-glucose. The isolated spinal cord from segments C5 to S3 was removed and pinned ventral-side up and superfused with oxygenated normal Ringer solution (in nM) 118 NaCl, 4.69 KCl, 25.0 NaHCO3, 1.18 KH2PO4, 1.25 MgSO4, 2.52 CaCl2, 11.0 D-glucose or normal aCSF composed of (in mM) 111 NaCl, 3.08 KCl, 25 NaHCO3, 1.18 KH2PO4, 1.25 MgSO4, 2.52 CaCl2, and 11 D-glucose.
Locomotor-like activity (termed fictive locomotion which is defined as occurring without overt movement) was evoked by perfusion with mouse Ringer solution containing a combination of 5-HT (9 μM), NMDA (6 μM) and dopamine (18 μM). Small-diameter suction electrodes were placed either: (1) on both L2 ventral roots to monitor alternating flexor activity in the motor pattern from the right and left sides, (2) in both L5 ventral roots to monitor alternating extensor activity or (3) in the L2 and L5 ventral roots to record alternating motor activity between flexor and extensor motorneuron pools as previously done (Kiehn and Kjaerulff 1996; Figure 1A). Ventral root recordings were band-pass-filtered (100 Hz to 1 kHz) and recorded using an AC amplifier (Model 1600 from A-M systems). The locomotor rhythm was allowed to stabilize over 20 minutes, after which Caffeine or adenosine receptors agonists or antagonists were added to the bath. A washout was performed using the control mouse ringer solution containing the above mentioned concentrations of 5-HT, NMDA and dopamine.
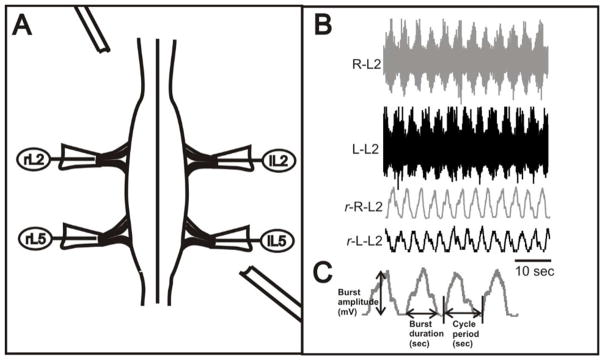
Figure 1. Experimental setup.
A: Suction recording electrodes were placed to monitor motor activity from ventral root nerve before, during and after perfussion of drugs. B: Extracellular recordings from rL2, and lL2 ventral roots after application of 6μM NMDA and 9μM 5-HT and 18μM dopamine (DA), showing locomotor-like activity characterized by left–right alternation in a P2 spinal cord. Raw (upper two traces) and rectified (lower two traces) recordings are shown. C: Representative segment of a rectified and smoothed trace from an actual control ventral root recording showing the locomotor-related parameters and how there were measured.
Drugs
Serotonin (5-HT), N-methyl-d-aspartate (NMDA) and dopamine were purchased from Sigma and stocks and prepared in de-ionized water and later diluted in regular mouse ringer or normal aCSF. Caffeine, the A1 receptor antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine), the A2A receptor antagonist SCH58261 (5-amino-7-(β-phenylethyl)-2-(8-furyl) pyrazolol [4,3-e] - 1,2,4 - triazolol [1,5-c] pyrimidine), adenosine, the A1 receptor agonist N-Cyclopentyladenosine (CPA), the A2A receptor agonist CGS21680, the D1 receptor antagonist SCH23390 ((R)-(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride) hydrochloride, the D2 receptor antagonist sulpiride ((S)-(−)-5-Aminosulfonyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide), the cAMP-dependent protein kinase (PKA) inhibitor 14–22 amide myristoylated and the adenylyl cyclase activator Forskolin were purchased from Tocris (St. Louis, Missouri). Caffeine was prepared in de-ionized water and later dissolved in regular mouse ringer or normal aCSF whereas the adenosine and dopamine receptor agonists and antagonists were prepared as stock solutions in Dimethyl Sulphoxide (DMSO) or de-ionized water (depending on solubility) and later diluted in de-ionized water.
Data analysis
Locomotor-like activity was recorded in the intact spinal cord preparation during bath application of 5-HT, NMDA and dopamine. Clampfit 9.0 (Molecular Devices), Excel (Microsoft, Seattle, WA), and Spike 2 (Cambridge Electronic Design, Cambridge, UK) were used for data analysis. A cycle of motor nerve activity started at the onset of either an L2 or L5 ventral root burst and ended at the onset of the next L2 or L5 ventral root burst recorded from the same nerve; these onsets were determined by a custom-made program in Spike2 (courtesy of Dr. Thomas Cleland, Cornell University) to detect when the rectified signal exceeded the average noise level between bursts by a preset amount (Figure 1B). Measurements of cycle period (defined as the interval between onset of burst n and burst n + 1), burst duration (defined as time between onset of burst n and offset of burst n) and burst amplitude (measured from trough to crest) were determined by analysis of rectified and normalized L2 or L5 activity using Spike 2 software (Figure 1C). Averages of cycle period, burst duration and burst amplitude were determined from all locomotor bursts that occurred once a stable pattern of locomotor-like activity had been established (after a minimum of 15–20 minutes of stable locomotor activity was recorded). Circular statistics (Zar, 1974) were used, together with a custom-made program made in MatLab (courtesy of Dr. Alex Kwan, Yale University) to determine the coupling strength between opposing L2 and L5 ventral roots. Left or right L2 bursts occurring over a continuous 5 min interval were selected, and their phase values were calculated in reference to either the onsets of each left or right L5 burst, respectively (they were always located in the same side in order to have alternation). Phase values were determined by dividing the latency between the onset of the first L2 burst and the following burst in L5 by the cycle period (time between the reference L2 burst and the next L2 burst). Locomotor steps in which the ispilateral L2 and L5 roots were completely out of phase (i.e., appropriate flexor-extensor alternation) had phase values of approximately 0.5. Those completely in phase (cobursting) had phase values of around 0. The r values are a measure of the concentration of phase values around the mean value for alternation (0.5). An r value of 1 indicates all the phase values are 0.5, whereas an r value of 0 indicates the phase values are distributed randomly.
Statistical comparisons between experimental conditions were made using one-way repeated measures ANOVA followed by a Fisher LSD post-hoc test if the data were normally distributed and had equal variance. Otherwise, the data were compared with a Mann-Whitney rank sum test. Results were considered statistically significant at p < 0.05. Data are expressed as mean ± SD. Figures were compiled using Sigma Plot 10, Photoshop and Corel Draw.
Results
Effects of caffeine application on locomotor-related motorneuron output
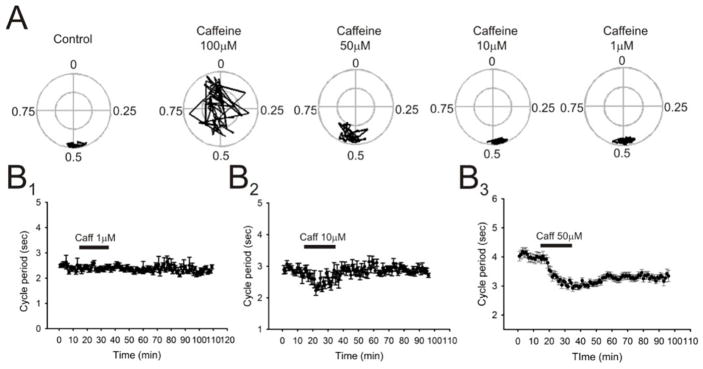
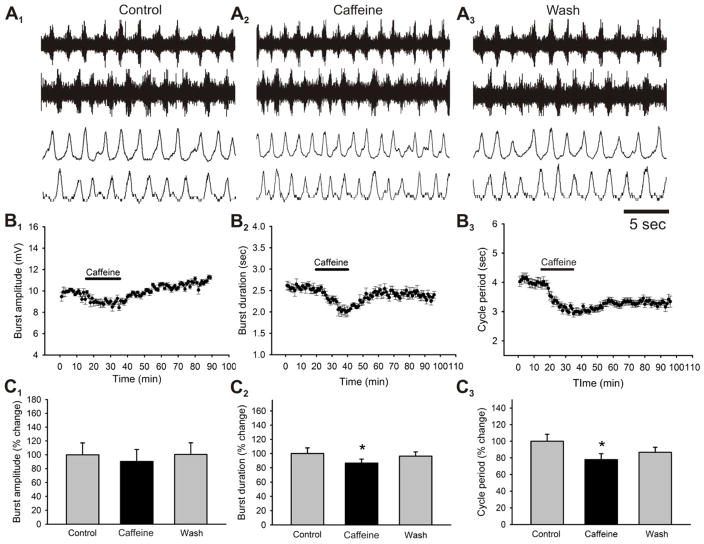
We started by assessing the effects of physiologically relevant doses of caffeine on drug-induced (5-HT/NMDA/DA) fictive locomotor behavior in the neonatal mouse lumbar spinal cord. Previous studies have demonstrated that physiologically relevant concentrations of caffeine are found between 50 to 100 micromoles per liter (Spyridopoulos et al. 2008; Fisone et al. 2004), thus based on these numbers we first assessed the effects of bath-applied caffeine on the locomotor pattern at concentrations ranging from 1μM to 100μM. We bath-applied caffeine at concentrations of 1, 10, 50 and 100μM to isolated spinal cords of neonatal mice ranging from 0 to 3 days of age and monitored its effects on the phasing of the rhythmic motor pattern and potential modulatory effects on the speed of the drug-induced fictive locomotor rhythm by measuring changes in the cycle period. The application of caffeine at a concentration of 100μM disrupted ongoing locomotor activity in 7 of 8 preparations within 10 minutes after its perfusion to the bath (Fig. 2A). The application of caffeine at concentrations of 1 (Fig. 2B1), 10 (Fig. 2B2) and 50μM (Fig. 2B3) did not disrupt locomotor activity, but it was caffeine applied at the concentration of 50μM which produce the most robust and reversible effect on the locomotor pattern (Fig. 2B3). After confirming that 50μM was the concentration which most reliably modulated locomotor behavior without disrupting the rhythm, we began characterizing the modulatory effects of applying 50μM caffeine to motor output. Application of caffeine (50μM; 20 minutes) led to no significant changes in the amplitude of bursts of locomotor-related activity recorded from ventral nerve roots although a consistent trend was seen to decrease the recorded burst amplitude (n = 7; p = 0.062; Fig. 3A1, B1). Application of caffeine caused a significant decrease in the duration of bursts of locomotor-related activity with a maximal effect at around 15 minutes after application (13.4 ± 5.5% reduction; n = 7; p < 0.05; Fig. 3A2, B2) before partially returning to control levels within 45 minutes of drug washout in most experiments. Additionally, application of caffeine caused a significant decrease in the cycle period between the recorded bursts thereby speeding up the locomotor rhythm with a maximal effect at around 15 minutes after application (22 ± 6.4% reduction; n = 7; p < 0.05; Fig. 3A3, B3) before partially returning to control levels within 60 minutes of drug washout in most experiments. As previously mentioned, no significant changes were seen in the actual phase relationship between the nerve recordings (mostly L2 and L5) suggesting that the modulatory effects of caffeine on motorneuron output are primarily at the pattern generating level of the CPG network responsible for transmitting the information to the motorneuron pools and not at the rhythm generating level which controls the operation of the pattern generating network (McCrea and Rybak, 2008).
Figure 2. Effects of different concentrations of caffeine on locomotor behavior.
A: Circular plots showing the effects of increasing concentrations of caffeine (1, 10, 50 and 100μM) to the phasing between rL2 and lL2 or rL2 and rL5 ventral roots (Note: cycles of motor burst activity located at 0.5 are considered in alternation while 0 is considered synchronous activity). Cycles of activity located outside the inner circle have a correlation coefficient (r value) which is significant with the type of locomotor-like activity to which it is related (alternation/synchronization). Concentrations of caffeine up to 50μM did not disrupt ongoing drug-induced locomotor behavior in most preparations (7/8). B panels: Time-course plots showing the effects of the application increasing concentrations of caffeine (1, 10 and 50μM) on locomotor cycle period. Each point represents 1 minute worth of recording. Comparing concentrations that did not disrupt ongoing locomotor behavior, application of 50μM consistently and reversibly decreased cycle period in most preparations (7/8).
Figure 3. Effects of caffeine on locomotor-related output parameters.
A panels: raw (top), rectified and integrated (bottom) traces showing the effects of caffeine on pharmacologically induced (9μM 5-HT, 6μM NMDA, 18μM dopamine) locomotor activity recorded from the lumbar ventral roots of an isolated neonatal mouse spinal cord preparation. B panels: time-course plots showing a decrease in locomotor burst amplitude (B1) which is not statistically significant and a decrease in motor burst duration (B2) and cycle period (B3) which was statistically significant (50μM, 20 minutes; n = 7). Each point represents 1 minute worth of recording. C: pooled data, averaged of 5 minutes worth of recordings in each condition, showing a significant decrease in burst duration and cycle period after the application of caffeine (n = 7). *Significantly different from control.
Effects of selective adenosine receptor antagonists and agonists on locomotor output
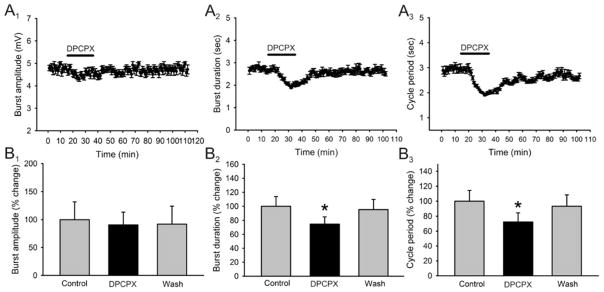
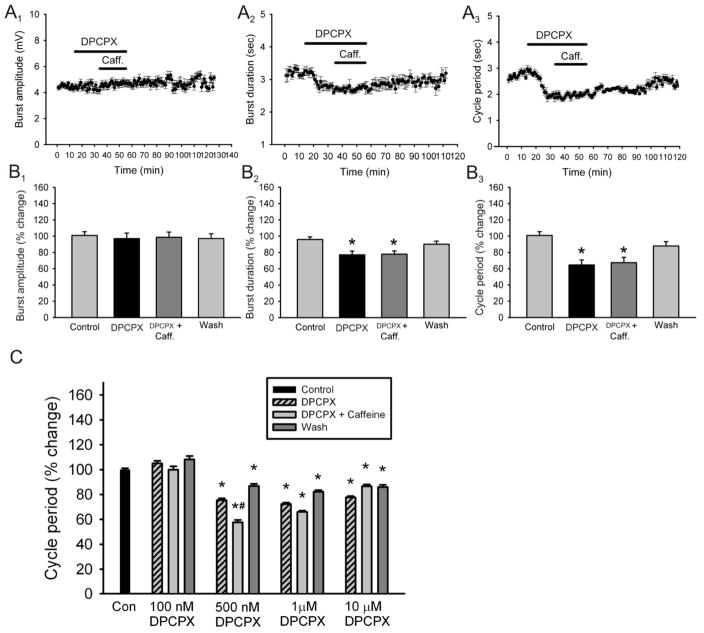
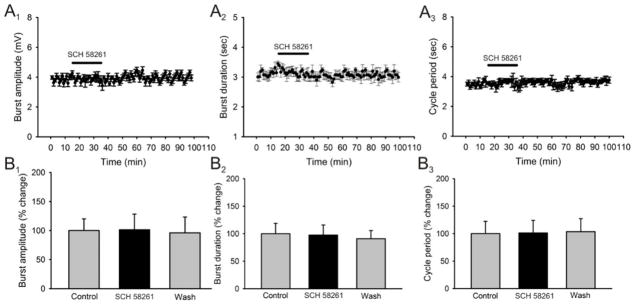
The primary mechanism of caffeine action is antagonism of adenosine receptors (Nehlig, 1992). To date, four adenosine receptors have been identified: the A1, A2A, A2B, and A3 (Fredholm, 1999). Since the A1 and A2A receptors bind caffeine at low doses, but the A2B receptor only binds caffeine at high doses and the A3 is caffeine insensitive (Fredholm, 1999), we wanted to assess if the effects of caffeine are through the antagonism of A1 and/or A2A receptors. We first used the selective A1 receptor antagonist DPCPX and applied it to the superfusate for a total of 20 minutes. Application of DPCPX (1μM) had very similar effects to those seen by caffeine. Application of DPCPX did not produce a significant change in burst amplitude (n = 5; Fig. 4A1, B1) but did have a significant decrease in burst duration (25 ± 4 % reduction; n = 5; p < 0.05; Fig. 4A2, B2) and cycle period (27.6 ± 12 % reduction; n = 4; p < 0.05; Fig. 4A3, B3). We additionally tested the effects of caffeine in the presence of DPCPX. The application of DPCPX produced a reduction in burst duration (18± 3.7 % reduction; n = 5; Fig. 5A2, B2) and cycle period (30 ± 5.8 % reduction; n = 5; p < 0.05; Fig. 5A3, B3) but the addition of caffeine (50μM) in the presence of DPCPX (1μM) did not produce any additional effects on burst duration (18 ± 2.6 % reduction; n = 5; p < 0.05; Fig. A2, B2) or cycle period (27 ± 5.1 % reduction; n = 5; p < 0.05; Fig. 5A3, B3). In contrast, application of the A2A receptor antagonist SCH58261 (1μM) had not significant effects on either: burst amplitude, burst duration or cycle period on all preparations (n = 4; Fig. 6, all panels). In order to confirm that the concentration of DPCPX used was not maximal, which would not have allowed another substance to produce an additional effect, we conducted a dose-response analysis of the effects of DPCPX on the parameter of cycle period. A drug-induced locomotor pattern was elicited and the effect of DPCPX was tested before and during the addition of caffeine to the perfusate in order to choose the most appropriate concentration. Our analysis demonstrated that it was the use of DPCPX at a concentration of 1μM which produced the most significant effect and also occluded the effects of caffeine in a reversible manner. These results suggest that caffeine is producing its stimulating effects primarily through the blockade of A1 receptors (n=3 in each concentration; Fig. 5C).
Figure 4. Effects of DPCPX, an A1 receptor antagonist, on locomotor-related output parameters.
A: time-course plots showing a decrease in locomotor burst amplitude which is not statistically significant and a significant decrease in motor burst duration and cycle period (1μM, 20 minutes; n = 5). Each point represents 1 minute worth of recording. B: pooled data, averaged of 5 minutes worth of recordings in each condition, showing a significant decrease in burst duration and cycle period after the application of DPCPX (n = 5). *Significantly different from control.
Figure 5. Effects of DPCPX, an A1 receptor antagonist, and caffeine on locomotor-related output parameters.
A: time-course plots not showing an effect on locomotor burst amplitude and a significant decrease in motor burst duration and cycle period after the application of DPCPX however caffeine did not exert any additional effects on any of the parameters measured (50μM caffeine; 1μM DPCPX; 20 minutes; n = 5). Each point represents 1 minute worth of recording. B: pooled data, average of 5 minutes worth of recordings in each condition, showing a significant decrease in burst duration and cycle period after the application of DPCPX but no additional effects after the application of caffeine in the presence of DPCPX (n = 5). C: Dose-response analysis of the effects of DPCPX on cycle period before and after the addition of caffeine showing that a concentration of DPCPX of 1μM produced the most significant effect while occluding the effects of caffeine in a reversible manner (n = 3 in each concentration). *Significantly different from control; #Significantly different from DPCPX.
Figure 6. Effects of SCH58261, an A2A adenosine receptor antagonist, on locomotor-related output parameters.
A: time-course plots showing no effects on either locomotor burst amplitude, motor burst duration and cycle period after the application of SCH58261 (1μM, 20 minutes; n = 5). Each point represents 1 minute worth of recording. B: pooled data, averaged of 5 minutes worth of recordings in each condition, showing no effects on either locomotor burst amplitude, motor burst duration and cycle period after the application of SCH58261 (1μM, 20 minutes; n = 5). *Significantly different from control.
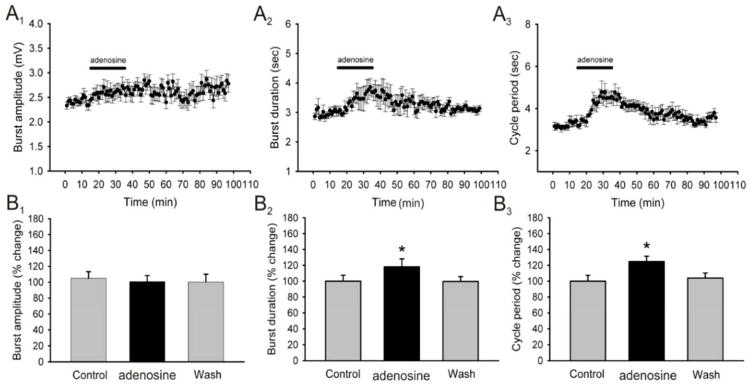
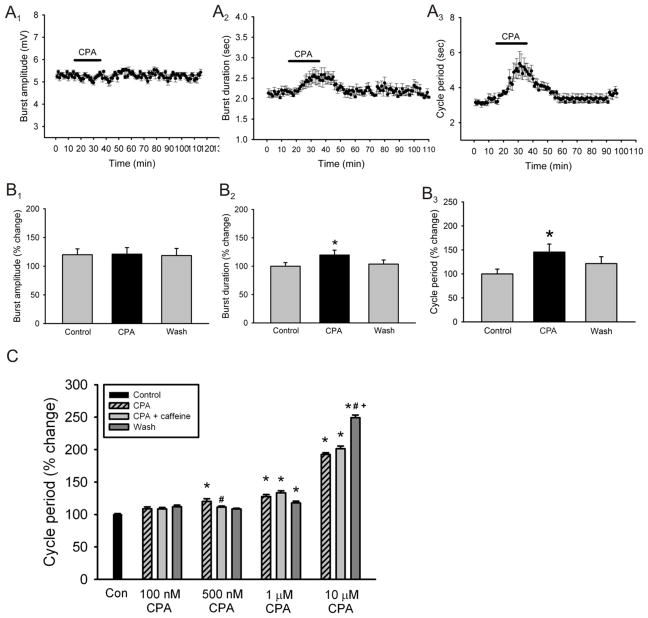
We next wanted to validate the results obtained using blockers of the A1 and A2A receptors by using adenosine receptor agonists and test their effects on locomotor output. We began by using adenosine (100μM) as a broad non-selective receptor agonist and applied it to the superfusate for a total of 20 minutes. Application of adenosine failed to produce a significant change in burst amplitude (Fig. 7A1, B1; n = 4) but did have a significant increase in burst duration (19.8 ± 8.2 % increase; n = 4; p < 0.05; Fig. 7A2, B2) and cycle period (24.7 ± 6.8 % increase; n = 4; p < 0.05; Fig. 7A3, B3). Since adenosine binds to all adenosine receptors, which could lead to non-specific effects, we wanted to test a more specific adenosine receptor agonist which is why we used the selective A1 receptor agonist N-Cyclopentyladenosine (CPA). Application of CPA (1μM) had no significant effects on burst amplitude (Fig. 8A1, B1; n = 4) but did produce a notable increase in burst duration (18.3 ± 6.1 % increase; n = 4; p < 0.05; Fig. 8A2, B2) and cycle period in 4 of 5 preparations (45.5 ± 8.3 % increase; n = 5; p < 0.001; Fig. 8A3, B3) significantly slowing down rhythmic motor output in a reversible manner. The use of the selective A2A receptor agonist CGS21680 had no effects on any of the parameters measured (data not shown). In order to confirm that the concentration of CPA used was not maximal, which would not have allowed another substance to produce an additional effect, we conducted a dose-response analysis of the effects of CPA on cycle period of a drug-induced locomotor pattern before and after the addition of caffeine to the perfusate in order to choose the most appropriate concentration. Our analysis demonstrated that it was the use of CPA at a concentration of 1μM which produced a reversible effect which is statistically significant and occluded the effects of caffeine (partial wash; n = 3 in each concentration; Fig. 8C). These results further support our findings which suggest that caffeine stimulates locomotor behavior by decreasing burst duration and cycle period acting mostly through the inhibition of A1 receptors.
Figure 7. Effects of adenosine, a broad spectrum adenosine receptor agonist, on locomotor-related output parameters.
A: time-course plots showing no significant effects on locomotor burst amplitude and a significant increase in motor burst duration and cycle period after the application of adenosine (100μM, 20 minutes; n = 4). Each point represents 1 minute worth of recording. B: pooled data, average of 5 minutes worth of recordings in each condition, showing a significant increase in burst duration and cycle period after the application of adenosine (n = 4). *Significantly different from control.
Figure 8. Effects of CPA, a specific A1 adenosine receptor agonist, on locomotor-related output parameters.
A: time-course plots showing no effect in locomotor burst amplitude and a significant increase in motor burst duration and cycle period after the application of CPA (1μM; 20 minutes; n = 4). Each point represents 1 minute worth of recording. B: pooled data, average of 5 minutes worth of recordings in each condition, showing a significant increase in burst duration and cycle period after the application of CPA (n = 4). *Significantly different from control. C: Dose-response analysis of the effects of CPA on cycle period before and after the addition of caffeine showing that a concentration of CPA of 1μM produced the most significant effect while occluding the effects of caffeine in a reversible manner (n = 3 in each concentration). * Significantly different from control; # Significantly different from CPA; + Significantly different from CPA + caffeine.
The role of dopamine receptors in the stimulating effects of caffeine
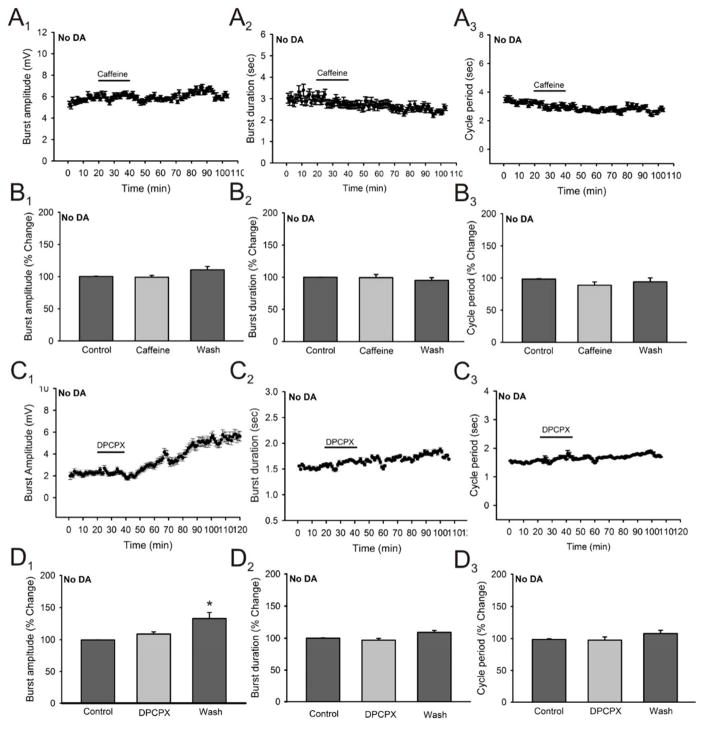
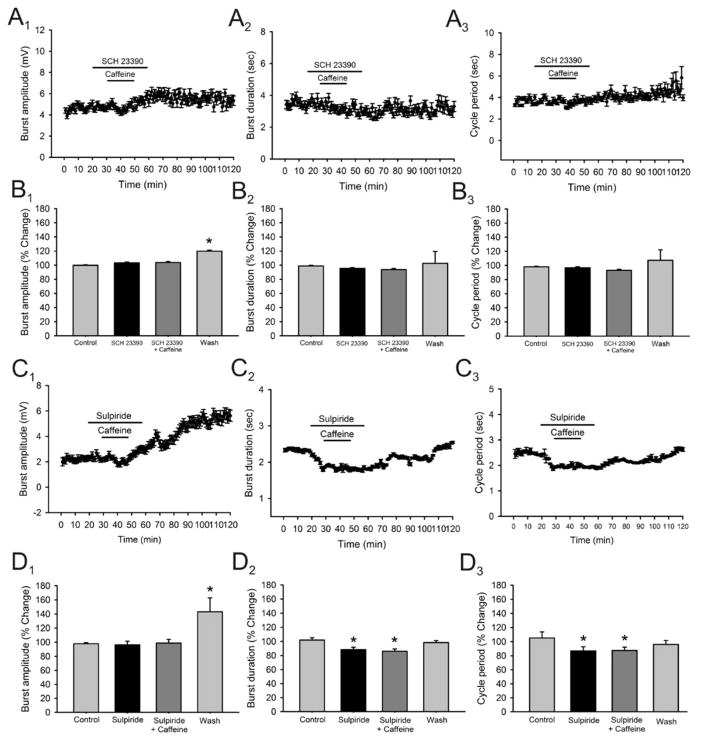
It has been shown that in brain areas such as the striatum dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes which have been suggested to be the main targets responsible for the pshychostimulant effects of Caffeine (Ferré et al. 1992; Ferré et al. 1997; Agnati et al. 2004, Ferré et al. 2008). Dopamine is not produced locally in the spinal cord being supplied by supraspinal centers such as the diencephalon where DA receptors and fibers projecting from the A11 region are present in the ventral horn of the adult spinal cord (Bjorklund and Skagerberg, 1979; Weil-Fugazza and Godefroy, 1993; Yoshida and Tanaka, 1988; Holstege et al., 1996; Qu et al, 2006). Thus, we can assess the modulatory effects of caffeine in the absence of dopamine since it has been shown that dopamine acts mainly as an excitatory modulator of spinal locomotor circuits working mainly through D1 receptors (Maitra et al. 1993; Seth et al. 1993; Barriere et al. 2004; Madriaga et al. 2004; Han et al. 2007). We proceeded to elicit locomotor activity using 5-HT and NMDA only, which has been shown to elicit locomotor activity reliably in rodents (see Kiehn, 2006), and then added caffeine (50μM) to the superfusate. The addition on caffeine in the absence of dopamine failed to produce significant changes in burst amplitude (Fig. 9A1, B1; n = 6), burst duration (Fig. 9A2, B2; n = 6) or cycle period (Fig. 9A3, B3; n = 6) in all preparations tested. We then proceeded to test the A1 receptor antagonist DPCPX in the absence of dopamine and it also failed to produce significant changes in burst duration (Fig. 9C2, D2; n = 6) and cycle period (Fig. 9C3, D3; n = 6) in all preparations but it did produce a significant non-reversible increase in burst amplitude after washout (33.2 ± 10% increase; n = 6; p < 0.05; Fig. 9C1, D1). These results suggest that the modulatory effects of caffeine on spinal motor circuits are dopamine-dependent. We then tested which specific dopamine receptor could be mediating these effects. We applied caffeine (50μM) in the presence of SCH23390, a specific D1 receptor antagonist, and it failed to produce significant changes in burst amplitude (Fig. 10A1, B1; n = 4), burst duration (Fig. 10A2, B2; n = 4), and cycle period (Fig. 10A2, B2; n = 4). In order to test for the possible involvement of the D2 receptor in the modulatory effects of caffeine we used sulpiride, a D2 receptor antagonist, and added it to the perfusion followed by caffeine (50μM). To our surprise, the application of sulpiride (15–20μM) alone significantly decreased burst duration (13.4 ± 7.9 % decrease; p < 0.05, Fig. 10C2, D2; n = 4) and cycle period (18.3 ± 4..8 % decrease; p < 0.05, Fig. 10C3, D3; n = 4) while having no significant effects on burst amplitude (Fig. 10C1, D1; n = 4) with no additional effects caused by the application of caffeine when added in the presence of sulpiride (Fig. 10, C and D panels). Although initially puzzling, it has been shown that dopamine has higher affinity for D2 versus D1 receptors in various areas in the central nervous system of mammals such as the striatum and nucleus accumbens (Marcellino et al. 2012; Vanderschuren et al. 1999) as well as in other vertebrate systems such as the lamprey and Xenopus embryos (see Clemens et al. 2012). The blockade of D2 receptors could have allowed more dopamine to bind to the less sensitive D1 receptors thus activating this intracellular pathway which is supported by our data regarding the interaction of between A1 and D1, but not A1 and D2, receptors as necessary for the modulatory effects of caffeine on locomotor activity to occur.
Figure 9. Effects of caffeine and DPCPX in the absence of dopamine on locomotor-related output parameters.
A: time-course plots from a locomotor rhythm elicited with 5-HT and NMDA in the absence of dopamine showing no effects in locomotor burst amplitude, burst duration or cycle period after the application of caffeine (50μM; 20 minutes; n = 4). Each point represents 1 minute worth of recording. B: pooled data, average of 5 minutes worth of recordings in each condition, showing no significant effects on any of the parameters measured after the application of caffeine in the absence of dopamine (n = 4). C: time-course plots from a locomotor rhythm elicited with 5-HT and NMDA in the absence of dopamine showing no effects in locomotor burst amplitude, burst duration or cycle period after the application of the A1 adenosine receptor antagonist DPCPX (1μM; 20 minutes; n = 4). Each point represents 1 minute worth of recording. D: pooled data, average of 5 minutes worth of recording in each condition, showing no significant effects on any of the parameters measured after the application of DPCPX in
Figure 10. Effects of caffeine in the presence of SCH23390, a specific D1 receptor antagonist and sulpiride, a specific D2 receptor antagonist, on locomotor-related output parameters.
A: time-course plots showing no effect in locomotor burst amplitude, burst duration and cycle period after the application of caffeine (50μM; 20 minutes; n = 4) in the presence of SCH23390. Each point represents 1 minute worth of recording. B: pooled data, average of 5 minutes worth of recordings in each condition, showing no significant effects on burst amplitude, burst duration and cycle period after the application of caffeine in the presence of SCH23390 (n = 4). C: time-course plots showing no effect in locomotor burst amplitude, while producing a statistically significant decrease in burst duration and cycle period after the application of sulpiride with not additional effects produced by the application of caffeine (50μM; 20 minutes; n = 4) in the presence of sulpiride. Each point represents 1 minute worth of recording. Each point represents 1 minute worth of recording. D: pooled data, average of 5 minutes worth of recordings in each condition, showing a significant decrease in burst duration and cycle period with no significant effects on burst amplitude after the application of sulpiride with no additional effects produced by the subsequent application of caffeine in the presence of sulpiride (n = 4).* Significantly different from control.
Cellular mechanisms underlying the neuromodulatory effects of caffeine
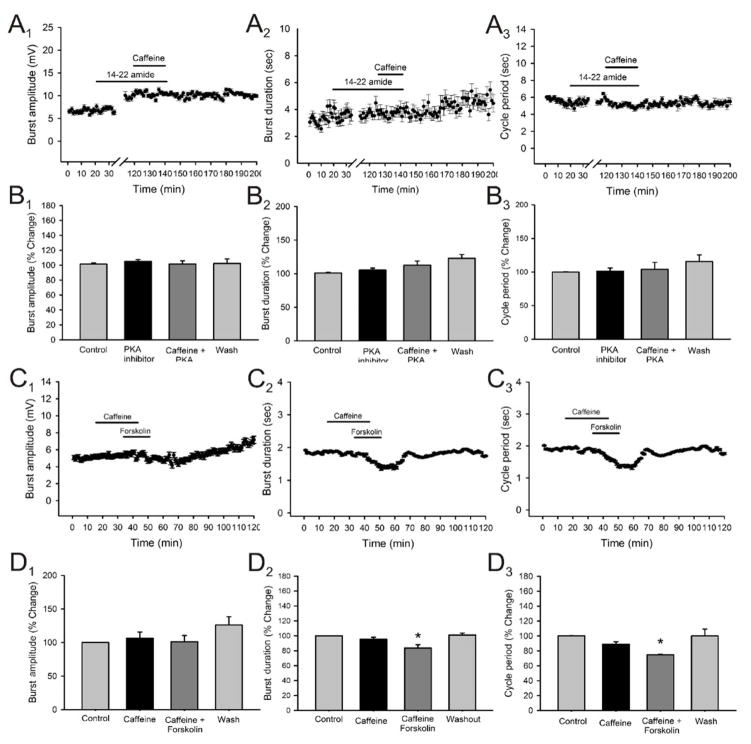
After establishing an interaction between A1 and D1 receptor subtypes as necessary for the neuromodulatory actions of caffeine on locomotor activity, we proceeded to test if these effects are mediated through intracellular second messenger mechanisms. We proceeded to use an inhibitor of the second messenger pathway related to protein kinase A (PKA) which has been related to adenosine receptor activity. Adenosine receptors are known to influence cyclic adenosine monophosphate (cAMP) levels, affecting the function of protein kinase A (PKA). We proceeded to use the PKA inhibitor, 14–22 amide, and tested if caffeine still produced its modulatory effects on the locomotor rhythm. After establishing a stable drug-induced locomotor rhythm, we applied 14–22 amide (1μM) to the perfusate for 90 minutes prior to the application of caffeine to allow for the cellular internalization of the drug. The application of caffeine in the presence of 14–22 amide did not produce any significant effects on burst amplitude, duration or cycle period (Fig. 11A and B panels; n = 3). We wanted to confirm that the abolishment of the modulatory effects of effects on locomotor activity where actually through the inhibition of PKA activity. Since caffeine does not modulate locomotor activity in the absence of dopamine (Fig. 9) and we have shown that PKA activity is essential for the modulatory effects to occur, we proceeded to directly activate adenylyl cyclase with forskolin in the absence of dopamine to confirm that the modulatory effects of caffeine are through this second messenger pathway. We proceeded to apply caffeine in the absence of dopamine which did not produce any significant effects on any of the parameters measured (Fig. 11, C and D panels). Then, in the presence of caffeine, we applied forskolin (5–10μM) which produced a significant decrease in burst duration (16.2 ± 6.1 % decrease; p < 0.05, Fig. 11 C2, D2; n = 3) and cycle period (25.4 ± 7.5 % decrease; p < 0.05, Fig. 11 C3, D3; n = 3) while not producing significant effects on burst amplitude (Fig. 11 C1, D1; n = 3). These results suggest that the modulatory effects of caffeine require the inhibition of the A1 receptor which allows the sustained activation of the D1 receptor leading to a PKA-dependent stimulation of locomotor activity.
Figure 11. Effects of caffeine in the presence of 14–22 amide, a specific phosphate kinase A (PKA) inhibitor, and of forskolin, a specific activator of the protein adenylyl cyclase, on locomotor-related output parameters.
A: time-course plots showing no effect in locomotor burst amplitude, burst duration and cycle period after the application of caffeine (50μM; 20 minutes; n = 4) in the presence of 14–22 amide (1μM). Each point represents 1 minute worth of recording. B: pooled data, average of 5 minutes worth of recording in each condition, showing no significant effects on burst amplitude, burst duration and cycle period after the application of caffeine in the presence of 14–22 amide (n = 4). C: time-course plots showing no effect in locomotor burst amplitude, while producing a statistically significant decrease in burst duration and cycle period after the application of caffeine (50μM; 20 minutes; n = 4) in the absence of dopmine from the locomotor-activating cocktail of drugs (5-HT and NMDA only) with significant effects on burst duration and cycle period by the application of forskolin (5–10μM; 20 minutes; n = 4) in the presence of caffeine. Each point represents 1 minute worth of recording. Each point represents 1 minute worth of recording. D: pooled data, average of 5 minutes worth of recording in each condition, showing a significant decrease in burst duration and cycle period with no significant effects on burst amplitude after the application of forskolin with no additional effects produced by the previous application of caffeine in the absence of dopamine in the perfusate (n = 4).* Significantly different from control.
Discussion
Our findings support a novel cellular mechanism by which caffeine stimulates locomotor activity in the mammalian spinal cord. Pharmacological characterization of these effect using caffeine and other adenosine receptor antagonists (and agonists) suggest that the cellular mechanism of action depends on previously described functional interactions between adenosine A1 and dopamine D1 receptors (See Ferré et al. 1996a,b). These A1/D1-specific effects have been mainly localized to striatal and nucleus accumbens D1 receptor-expressing GABAergic neurons (Wong et al. 1999; Yabuuchi et al. 2006; O’Neill et al. 2007). Our current results indicate that the same type of interactions exist in the lumbar spinal cord of mammals. Additionally, our findings support the blockade of principally A1 receptors in the spinal cord as accounting for the motor-stimulating effects in the neonatal mouse spinal cord. These results contrasts with previous studies that support mainly the blockade of the A2A or both A1 and A2A receptors as the primary mechanisms mediating motor activation in mammals (Svenningsson et al. 1997; Popoli et al. 1998; El Yacoubi et al. 2000; Chen et al. 2001; Kuzmin et al. 2006; Lerner et al. 2010; Tozzi et al. 2012). We now discuss our current findings and provide possible functional implications as well as comparisons to previous studies in other CNS regions and other animal model systems.
First, we found that the application of caffeine to an ongoing drug-induced (5HT/NMDA/DA) locomotor rhythm induced a decrease in the cycle period (increase in frequency of locomotor-related activity) and in the burst duration of motorneuron output without disrupting the phasing of the locomotor rhythm. We found that caffeine at a concentration of 50μM had the most reproducible and reversible effects on locomotor behavior without disrupting the actual rhythm (figure 2). We did see more robust effects at concentrations of 100μM or above in some preparations but this effect eventually ceased locomotor-like activity within 10 minutes of bath perfusion in over 70% of these preparations (data not shown). The cessation of locomotor activity at higher concentrations of caffeine raises the possibility that caffeine can have an “inverted U shape” dose dependent-effect in the spinal locomotor function similar to reported effects of systemic application of this drug producing an increase in locomotor activity in freely behaving animals (Kuribara et al., 1992; Nehlig et al., 1992; Haghgoo et al., 1995; Soares et al., 2009). These studies suggested that caffeine exerts a stimulating effect on locomotor activity at low to moderate doses, but less stimulating and even depressive effects at higher doses (Mumford and Holtzman, 1991; El Yacoubi et al., 2000; Malec and Poleszak, 2006; Uchiyama et al., 2010; Zhang et al. 2011).
The effects induced by caffeine in the neonatal mouse spinal cord were similar to those seen in studies looking at the effects of the activation of group I metabotropic glutamate receptors on locomotor activity in mice (Igawaki and Miles, 2011), and the activation of endogenous metabotropic glutamate receptors (Krieger et al. 1998), the application of nitric oxide (Kyriakatos et al. 2009), low concentrations of dopamine (McPherson and Kemnitz, 1994) and acetylcholine (Quinlan et al. 2004) to the spinal cord of lampreys. The decrease in the cycle period and burst duration with no consistent changes in burst amplitude of the locomotor pattern suggests that caffeine modulates the activity of component neurons of the spinal CPG network involved in the generation of the locomotor rhythm (Griener et al. 2013; Dougherty et al. 2013). The application of caffeine had no effects on the amplitude of bursts of locomotor-related motorneuron output recorded from ventral roots. Thus caffeine does not appear to recruit new motoneurons to participate in the locomotor rhythm but apparently raises the excitability of motoneurons which were already active during locomotion. Caffeine has been shown to modulate the excitability and calcium transients of neonatal rat hypoglossal motoneurons in vitro (Donato et al. 2003).
It is known that throughout the mammalian brain, caffeine acts as a broadly acting antagonist of adenosine receptors (Ferré et al. 1992; Ferré 2008, 2010). We found that the application of an A1 receptor antagonist (DPCPX) mimicked the effects of caffeine on fictive locomotor behavior while the application of an A2A receptor antagonist (SCH58261) failed to produce any significant effects on any of the parameters measured. These results contrast with previous studies which supported the blockade of A2A receptors as a primary mechanism leading to motor activation such as the use of a A2A receptor knockout mouse were the application of caffeine dose-dependently decreased locomotion (El Yacoubi et al. 2000), an arousal behavioural test were rats injected with caffeine and SCH58261 were found to significantly increase locomotion and rearing behaviors, whereas DPCPX did not alter locomotion and reduced rearing (Svenningsson et al. 1997a) and a study were mice bilaterally injected via cannulae into the nucleus accumbens with CGS 21680, a potent and selective agonist at striatal adenosine A2A receptors, elicited pronounced dose-related reductions in locomotor activity whereas similar bilateral dosages of CPA, a selective agonist at adenosine A1 receptors, did not significantly affect locomotor activity (Barraco et al. 1994), among others. Although these experiments support A2A receptors as a primary target for caffeine and other adenosine receptor antagonists leading to motor activation, more recent studies support the A1 receptor, as well as A2A receptors, as a key target for the motor activating effects caused by caffeine and other A1 receptor antagonists. The administration of DPCPX increased motility and locomotion in freely behaving C57BL/6J mice (Kuzmin et al. 2006). However, the effects were not as strong as when administering caffeine, but when a combination of both DPCPX and SCH58261 were used, its effects mimicked those seen by the administration of caffeine (Kuzmin et al. 2006). Also, a study looking at the counteraction of the motor-depressant effects of the selective A1 receptor agonist CPA and the A2A receptor agonist CGS 21680 by caffeine, the selective A1 receptor antagonist CPT, and the A2A receptor antagonist MSX-3 showed that caffeine counteracted motor depression induced by CPA and CGS 21680 at doses that produced motor activation (Karcz-Kubicha et al. 2003). However, caffeine was less effective than CPT at counteracting CPA and even less effective than MSX-3 at counteracting CGS 21680. On the other hand, when administered alone in habituated animals, caffeine produced stronger motor activation than CPT or MSX-3. An additive effect on motor activation was obtained when CPT and MSX-3 were co-administered. Altogether, these results suggest that the motor-activating effects of acutely administered caffeine in rats involve the central blockade of both A1 and A2A receptors (Karcz-Kubicha et al. 2003). Additionally, another study comparing the motor effects induced by caffeine with those induced by selective A1 and A2A receptor antagonists in rats showed that the pattern of behavioral activation induced by caffeine was better mimicked by CPT (A1 receptor antagonist) than by MSX-3 (A2A receptor antagonist). Co-administration of CPT and MSX-3 gave different results depending on the dose and the type of behavioral response. CPA was more effective at decreasing the activating effects of caffeine and CPT than those of CGS 21680 (A2A receptor agonist). On the other hand, CGS 21680 was more effective at decreasing the activating effects of MSX-3 than those of caffeine or CPT. Factor analysis revealed a complex three-dimensional behavioral profile for caffeine that was similar to the profile for CPT and was different from the profile for MSX-3. These results suggested a predominant role for A1 receptors in the motor-activating effects of acutely administered caffeine (Antoniou et al. 2005).
Consistent with a primary role for A1 receptors in adenosine-receptor mediated modulation, significant expression of A1 receptors has been reported throughout the rodent spinal cord, including in the ventral horn (Deuchars et al. 2001). A recent study showed that the application of either ATP or adenosine to isolated spinal cords of mice led to a reduction in the frequency of locomotor activity (Witts et al. 2012). They also showed that an A1 receptor antagonist (cyclopentyl Dipropylxanthine), but not an A2A receptor antagonist (SCH58261), caused an increase in locomotor burst frequency. These findings correlate with our results supporting that endogenously derived adenosine activates primarily A1 receptors during locomotor network activity. Additionally, Witts et al. demonstrated that in the presence of blockers of inhibitory neurotransmission, adenosine showed no effects on locomotor frequency suggesting that adenosine may act via the modulation of inhibitory transmission (Witts et al. 2012). The role of A1 receptors within the spinal cord as mediators of inhibitory neurotransmission correlates with previous studies that have shown coupling between A1 receptors and inihibitory G proteins within cells (Fredholm et al. 1999). These findings and our own results suggest a modulatory role of caffeine acting through the inhibition of A1 receptors. This interaction could lead to a reduction of the inhibitory control exerted by the adenosine system which produces an increase in excitatory drive translating into a faster locomotor rhythm.
We then assessed the source of this inhibitory control by the adenosine system focusing on the possible interaction with dopamine receptors. Our findings established that in the absence of dopamine in the locomotor-inducing “cocktail” of excitatory neurotransmitters (thus only using 5-HT and NMDA as activators of the lumbar CPG network), the application of caffeine or the A1 receptor blocker DPCPX failed to produce any significant effects on the locomotor pattern. We then explored further the role of specific dopamine receptors on these actions and found that the application of a specific D1 receptor blocker (SCH23390) abolished the reduction of burst duration and cycle period in the locomotor pattern produced by the application of caffeine. Futhermore, the use of a specific blocker of D2 receptors (sulpiride) mimicked the modulatory effects of caffeine which was confirmed by the subsequent application of caffeine in the presence of sulpiride showing no additional “stimulating” effects on locomotor-like activity. Previous studies have shown compelling evidence regarding the role of adenosine receptors as regulators of dopaminergic and/or of dopamine receptor-mediated neurotransmission. Studies in the rat strioentopenduncular pathway showed that dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission is modulation by adenosine A1 receptor-mediated mechanisms thus supporting the strioentopeduncular neurons as an important loci for adenosine-dopamine interactions in the brain (Ferré et al. 1996). Additionally, studies in nucleus accumbens neurons using whole-cell patch-clamp recordings have shown that activation of postsynaptic D1 receptors enhanced the synaptic activation of NMDA receptors in nucleus accumbens neurons, thereby promoting a trans-synaptic feedback inhibition of glutamatergic synaptic transmission via release of adenosine binding primarily to A1 receptors (Harvey and Lacey, 1997). Additional studies showed that the application of caffeine mediated the induction of c-fos, zif-268 and arc expression through A1 receptors in the striatum and that these effects were abolished when a lesion of the nigrostriatal pathway was induced or when they applied the D1 antagonist SCH23390 to striatonigral neurons but not to striatopallidal neurons thus supporting the role of adenosine acting at the A1 receptor as potentially influential on a broad range of neuronal functions in the striatum (Le Moine et al. 1997).
Other studies looking into the interaction of A1 and D1 receptors mediating motor behaviors also support our current findings. A study looking at motor behavior in rodents showed that the systemic administration of the adenosine A1 antagonist 8-cyclopentyl-1,3-dimethylxanthine (8-CPT) significantly potentiated the motor activating properties of the systemically administered dopamine D1 agonist SKF 38393, but not the D2 agonist quinpirole, in both reserpinized mice and unilaterally 6-hydroxy-dopamine-lesioned rats thus supporting an antagonistic interaction between adenosine A1 and dopamine D1 receptors mediating the motor activating effects of adenosine antagonists, like caffeine (Popoli et al. 1996). Another study supported the presence of an inhibitory A1/D1 receptor interaction through the regulation of GABA release in the substantia nigra pars reticulata as the use of the A1 agonist CCPA and the D1 antagonist SCH3390 produced ipsilateral turning whereas the A1 antagonist DPCPX caused contralateral turning via unilateral intranigral injections into rats challenged with systemic methamphetamine. These results supported other experiments which established that the inhibition exerted by A1 receptors on GABAergic striatonigral transmission were due exclusively to blockade of the facilitation resulting from activation of D1 receptors (Florán et al. 2012). A more recent study demonstrated that adenosine A1 receptor stimulation reduces D1 receptor-mediated GABAergic transmission from striato-nigral terminals and attenuates L-DOPA-induced dyskinesia in dopamine-denervated mice (Mango et al. 2014). Although these previous findings are our own results do not discard a role of the striatal A1-D1 receptor interactions, they establish a direct connection between spinal cord A1-D1 antagonistic interactions and caffeine-induced hindlimb locomotion in mammals.
The potential involvement of second messenger systems in the neuromodulatory actions of caffeine on locomotor activity were then assessed. The use of an inhibitor (14–22 amide) of phosphate kinase A activity (PKA) onto the spinal cord of rodents was able to abolish the modulatory effects of caffeine on locomotor activity. Furthermore, the use of forskolin which is an activator of adenylyl cyclase, a producer of cAMP and upstream effector of PKA, in the absence of dopamine (known to not be produced locally in the spinal cord but being supplied supraspinally as previously stated) mimicked the effects of caffeine by significantly decreasing burst duration and cycle period in all preparations tested. The relation between activity of the A1 receptor and PKA-dependent signaling has been previously shown. It is known that the adenosine A1 receptor is a G-protein alpha-i and G-protein alpha-15 coupled receptor (Liu and Wong, 2004). When the G-protein alpha-i pathway is activated through A1 receptor binding, it inhibits activity of several adenylyl cyclase isoforms which leads to the decrease of cAMP levels and attenuation of cAMP responsive element binding protein 1 (CREB1) phosphorylation by PKA and other cAMP-dependent mechanisms (Defer et al. 2000). Studies looking at purinergic modulation of spontaneous GABAergic miniature inhibitory postsynaptic currents in mechanically dissociated immature rat hippocampal CA1 and rat tuberomammillary nucleus neurons showed that the activation of A1 receptors decreased GABAergic transmission through presynaptic inhibition via a cAMP- and PKA-dependent pathway (Jeong et al. 2003; Yum et al. 2008). Another study investigating the effects of the dopamine D1 receptor activation on excitatory neurotransmission in post-ischemic striatal neurons found that D1 receptor activation pre-synaptically depressed excitatory synaptic transmission in striatal neurons after ischemia through activation of PKA and A1 receptors as shown by using specific blockers of PKA and A1 receptors (Zhang et al. 2008). These studies could support a possible cellular mechanism of action in which the application of adenosine or an A1 receptor agonist (CPA) binds to A1 receptors located within the lumbar spinal cord they could depress locomotor activity by reducing PKA signaling through a G-protein alpha-i-dependent pathway. But this potential mechanism of action was negated when in the absence of dopamine the use of caffeine or the A1 receptor antagonist DPCPX failed to modulate locomotor activity then pointing toward an interaction with dopamine neurotransmission. D1 receptors enhance the currents evoked by NMDA agonists (Cepeda et al., 1993; Harvey and Lacey, 1997; Hernandez-Lopez et al., 1997; Cepeda and Levine, 1998) and this effect is mediated through a PKA-dependent pathway (Colwell and Levine, 1995; Blank et al., 1997). The relationship between D1 receptor activation and PKA signaling has been shown to be important in many fundamental processes such as learning (see Beninger and Miller, 1998), functional modification of glutamate receptor channels (see Hatt, 1999), dopaminergic control of synaptic plasticity (see Centonze et al. 2001; Wolf et al. 2003) and dendritic spine remodeling in normal and pathophysiological conditions (see Penzes et al. 2011), among others.
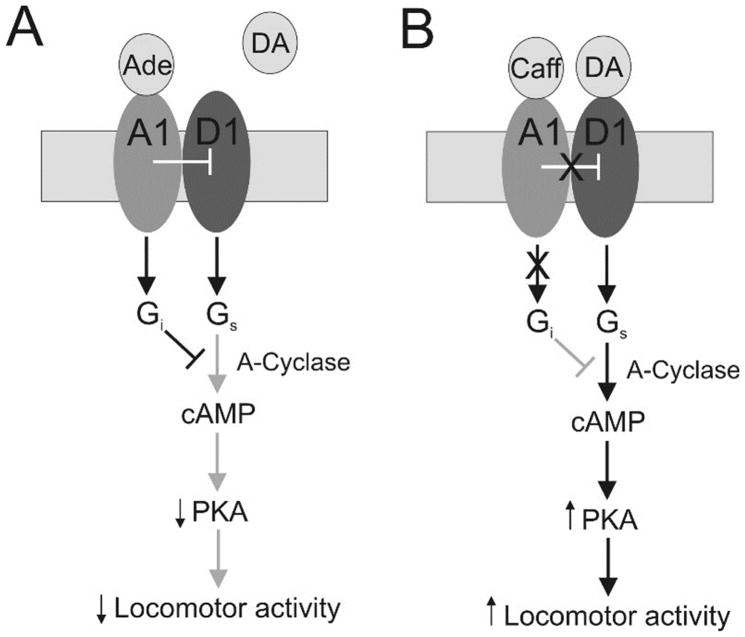
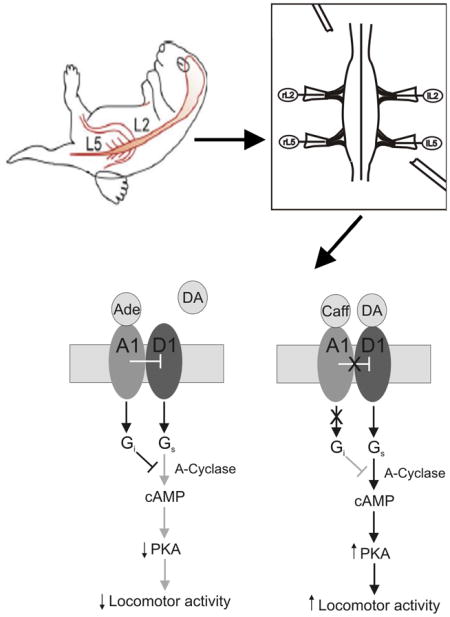
Taken together, our current findings support the role of caffeine as a stimulant of the spinal CPG network for hindlimb locomotion through a previously uncharacterized novel cellular mechanism (Cowley and Schmidt 1997; Kremer and Lev-Tov 1997; Kjaerulff and Kiehn 1996). This effect is mediated through the blockade of A1 receptors leading to enhanced D1 receptor signaling which enhances locomotor activity through a PKA-dependent second messenger mechanism (Figure 12).
Figure 12. Proposed cellular mechanisms mediating the modulation of locomotor activity through the activation or inhibition of the A1 adenosine receptor in the mammalian spinal cord.
A: The binding of adenosine to the spinal A1 adenosine receptor coupled to a Gi protein inhibits D1 dopamine receptor activity and of adenylyl cyclase lowering the levels of cAMP and thus decreasing the activity of PKA leading to a depression of locomotor activity. B: The binding of caffeine to the A1 adenosine receptor leads to inhibition of adenosine signaling which no longer suppress the activity of the D1 receptor leading to the activation of adenylyl cyclase through its coupling to a GS protein increasing the levels of intracellular cAMP which augments PKA-dependent activity leading to the stimulatory effects of caffeine on locomotor activity.
Highlights.
We used the neonatal mouse spinal cord to study the effects of caffeine.
We examined changes in fictive locomotor activity focusing on bursting activity.
Caffeine stimulated locomotion through blockade of A1 receptors.
The effects of caffeine were found to depend on the activation of D1 receptors.
Caffeine stimulation of locomotion is dependent on PKA intracellular signaling.
Acknowledgments
We thank Thomas Cleland (Cornell University) for providing Spike 2 scripts used in data analysis. Additionally we thank Alex Kwan (Yale University) for providing MatLab scripts used in data analysis.
GRANTS
This work was supported by NIH grant 1P20GM103642-01A1, Craig Nielsen Foundation grant 124554, NSF grant 1026061, NSF-URM grant 0932955, RCMI-UPR-MSC grant G12RR03051 and a NIH-RISE predoctoral fellowship (grant R25GM061838) to JeanMarie Acevedo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. doi: 10.1124/pr.55.3.2. [DOI] [PubMed] [Google Scholar]
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Panlilio L, Müller CE, Goldberg SR, Ferré S. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology (Berl) 2005;183(2):154–62. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Martens KA, Parizon M, Normile HJ. Role of adenosine A2A receptors in the nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18(3):545–53. doi: 10.1016/0278-5846(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Bedingfield JB, King DA, Holloway FA. Cocaine and caffeine: conditioned place preference, locomotor activity, and additivity. Pharmacol Biochem Behav. 1998;61:291–296. doi: 10.1016/s0091-3057(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Miller R. Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev. 1998;22(2):335–45. doi: 10.1016/s0149-7634(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Skagerberg G. Evidence for a major spinal cord projection from the diencephalic A11 dopamine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res. 1979;177:170–175. doi: 10.1016/0006-8993(79)90927-2. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Teichert U, Kugler H, Behrsing H, Fienberg A, Greengard P, Spiess J. The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proc Natl Acad Sci USA. 1997;94:14859–14864. doi: 10.1073/pnas.94.26.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci. 2002;22(22):9961–71. doi: 10.1523/JNEUROSCI.22-22-09961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38(6):953–63. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cauli O, Morelli M. Caffeine and the dopaminergic system. Behav Pharmacol. 2005;16:63–77. doi: 10.1097/00008877-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Celik E, Uzbay IT, Karakas S. Caffeine and amphetamine produce crosssensitization to nicotine-induced locomotor activity in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:50–55. doi: 10.1016/j.pnpbp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13(6):1071–7. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc Natl Acad Sci. 2001;98(4):1970–5. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol. 2012;107:2250–2259. doi: 10.1152/jn.00366.2011. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Levine MS. Excitatory synaptic transmission in neostriatal neurons: regulation by cAMP-dependent mechanisms. J Neurosci. 1995;15:1704–1713. doi: 10.1523/JNEUROSCI.15-03-01704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77(1):247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279(3):F400–16. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Brooke RE, Deuchars J. Adenosine A1 receptors reduce release from excitatory but not inhibitory synaptic inputs onto lateral horn neurons. J Neurosci. 2001;21:6308–6320. doi: 10.1523/JNEUROSCI.21-16-06308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Canepari M, Lape R, Nistri A. Effects of caffeine on the excitability and intracellular Ca(2+) transients of neonatal rat hypoglossal motoneurons in vitro. Neurosci Lett. 2003;346(3):177–81. doi: 10.1016/s0304-3940(03)00568-8. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron. 2013;80(4):920–33. doi: 10.1016/j.neuron.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Duan L, Yang J, Slaughter MM. Caffeine inhibition of ionotropic glycine receptors. J Physiol. 2009;587(Pt 16):4063–75. doi: 10.1113/jphysiol.2009.174797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129(7):1465–73. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Ferré S. Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis. 2010;20(Suppl 1):S35–49. doi: 10.3233/JAD-2010-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fuxe K, von Euler G, Johansson B, Fredholm BB. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–9. doi: 10.2741/2852. Review. [DOI] [PubMed] [Google Scholar]
- Ferré S, O’Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, Stromberg I, Goldstein M, Ogren SO, Ungerstedt U, Fredholm BB, Fuxe K. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci. 1996;8(7):1545–53. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Tinner-Staines B, Fuxe K. Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neurosci Lett. 1996;208(2):109–12. doi: 10.1016/0304-3940(96)12577-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Rimondini R, Popoli P, Reggio R, Pèzzola A, Hansson AC, Andersson A, Fuxe K. Stimulation of adenosine A1 receptors attenuates dopamine D1 receptor-mediated increase of NGFI-A, c-fos and jun-B mRNA levels in the dopamine-denervated striatum and dopamine D1 receptor-mediated turning behaviour. Eur J Neurosci. 1999;11(11):3884–92. doi: 10.1046/j.1460-9568.1999.00810.x. [DOI] [PubMed] [Google Scholar]
- Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61(7–8):857–72. doi: 10.1007/s00018-003-3269-3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Svenningsson P. Adenosine-dopamine interactions: development of a concept and some comments on therapeutic possibilities. Neurology. 2003;61:S5–9. doi: 10.1212/01.wnl.0000095204.89871.ff. [DOI] [PubMed] [Google Scholar]
- Frostholm A, Rotter A. Glycine receptor distribution in mouse CNS: autoradiographic localization of [3H] strychnine binding sites. Brain Res Bull. 1985;15(5):473–86. doi: 10.1016/0361-9230(85)90038-3. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; Amsterdam: 2004. pp. 445–508. [Google Scholar]
- Goodman RR, Synder SH. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982;2(9):1230–41. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griener A, Dyck J, Gosgnach S. Regional distribution of putative rhythm-generating and pattern-forming components of the mammalian locomotor CPG. Neurosci. 2013;250:644–50. doi: 10.1016/j.neuroscience.2013.07.070. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Juliano LM, Chausmer A. Caffeine: Pharmacology and Clinical Effects. In: Graham AW, Schultz TK, Mayo-Smth MF, Ries RK, Wilford BB, editors. Principles of Addiction Medicine. 3. American Society of Addiction Medicine; Chevy Chase: 2003. pp. 193–224. [Google Scholar]
- Haghgoo S, Hasegawa T, Nadai M, Wang L, Ishigaki T, Miyamoto K, Nabeshima T. Brain distribution characteristics of xanthine derivatives and relation to their locomotor activity in mice. J Pharm Pharmacol. 1995;47(5):412–9. doi: 10.1111/j.2042-7158.1995.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci. 2007;27(48):13192–204. doi: 10.1523/JNEUROSCI.1279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17(14):5271–80. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H. Modification of glutamate receptor channels: molecular mechanisms and functional consequences. Naturwissenschaften. 1999;86(4):177–86. doi: 10.1007/s001140050593. [DOI] [PubMed] [Google Scholar]
- Hernández-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca21 conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege JC, Van Dijken H, Buijs RM, Goedknegt H, Gosens T, Bongers CM. Distribution of dopamine immunoreactivity in the rat, cat and monkey spinal cord. J Comp Neurol. 1996;376:631–652. doi: 10.1002/(SICI)1096-9861(19961223)376:4<631::AID-CNE10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Chen CY, Wang CS, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75-DARPP-32 in mice. Psychopharmacology (Berl) 2009;204:313–325. doi: 10.1007/s00213-009-1461-3. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Nabekura J, Akaike N. Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J Neurophysiol. 2003;89(3):1214–22. doi: 10.1152/jn.00516.2002. [DOI] [PubMed] [Google Scholar]
- Jursky F, Nelson N. Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J Neurochem. 1995;64(3):1026–33. doi: 10.1046/j.1471-4159.1995.64031026.x. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Müller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28(7):1281–91. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Barajon I, Kiehn O. Sulphorhodamine-labelled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro. J Physiol. 1994;478(Pt 2):265–273. doi: 10.1113/jphysiol.1994.sp020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77(3):1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- Krieger P, Grillner S, El Manira A. Endogenous activation of metabotropic glutamate receptors contributes to burst frequency regulation in the lamprey locomotor network. Eur J Neurosci. 1998;10:3333–3342. doi: 10.1046/j.1460-9568.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Gimenez L, Ogren SO, Fredholm BB. Combination of adenosine A1 and A2A receptor blocking agents induces caffeine-like locomotor stimulation in mice. Eur Neuropsychopharmacol. 2006;16(2):129–36. doi: 10.1016/j.euroneuro.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kyriakatos A, Molinari M, Mahmood R, Grillner S, Sillar KT, El Manira A. Nitric oxide potentiation of locomotor activity in the spinal cord of the lamprey. J Neurosci. 2009;29(42):13283–91. doi: 10.1523/JNEUROSCI.3069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H, Asahi T, Tadokoro S. Behavioral evaluation of psycho-pharmacological and psychotoxic actions of methylxanthines by ambulatory activity and discrete avoidance in mice. J Toxicol Sci. 1992;17(2):81–90. doi: 10.2131/jts.17.81. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Svenningsson P, Fredholm BB, Bloch B. Dopamine-adenosine interactions in the striatum and the globus pallidus: inhibition of striatopallidal neurons through either D2 or A2A receptors enhances D1 receptor-mediated effects on c-fos expression. J Neurosci 1997. 1997;17(20):8038–48. doi: 10.1523/JNEUROSCI.17-20-08038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci. 2010;30(6):2160–4. doi: 10.1523/JNEUROSCI.5844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan LC, McVittie LD, Smyk-Randall EM, Nakata H, Monsma FJ, Jr, Gerfen CR, Sibley DR. Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol. 1991;40(1):1–7. [PubMed] [Google Scholar]
- Malec D, Poleszak E. Involvement of adenosine receptors in dizocilpine-induced motor activity in mice. Pharmacol Rep. 2006;58(1):101–6. [PubMed] [Google Scholar]
- Mango D, Bonito-Oliva A, Ledonne A, Cappellacci L, Petrelli R, Nisticò R, Berretta N, Fisone G, Mercuri NB. Adenosine A1 receptor stimulation reduces D1 receptor-mediated GABAergic transmission from striato-nigral terminals and attenuates l-DOPA-induced dyskinesia in dopamine-denervated mice. Exp Neurol. 2014;261:733–43. doi: 10.1016/j.expneurol.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Kehr J, Agnati LF, Fuxe K. Increased affinity of dopamine for D(2) -like versus D(1) -like receptors. Relevance for volume transmission in interpreting PET findings. Synapse. 2012;66(3):196–203. doi: 10.1002/syn.21501. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57(1):134–46. doi: 10.1016/j.brainresrev.2007.08.006. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson DR, Kemnitz CP. Modulation of lamprey fictive swimming and motoneuron physiology by dopamine, and its immunocytochemical localization in the spinal cord. Neurosci Lett. 1994;166(1):23–6. doi: 10.1016/0304-3940(94)90831-1. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Holtzman SG. Qualitative differences in the discriminative stimulus effects of low and high doses of caffeine in the rat. J Pharmacol Exp Ther. 1991;258(3):857–65. [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17:139–70. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- O’Neill C, Nolan BJ, Macari A, O’Boyle KM, O’Connor JJ. Adenosine A1 receptor-mediated inhibition of dopamine release from rat striatal slices is modulated by D1 dopamine receptors. Eur J Neurosci. 2007;26(12):3421–8. doi: 10.1111/j.1460-9568.2007.05953.x. [DOI] [PubMed] [Google Scholar]
- Patkina NA, Zvartau EE. Caffeine place conditioning in rats: comparison with cocaine and ethanol. Eur Neuropsychopharmacol. 1998:287–291. doi: 10.1016/s0924-977x(97)00086-2. [DOI] [PubMed] [Google Scholar]
- Penzes P, Woolfrey KM, Srivastava DP. Epac2-mediated dendritic spine remodeling: implications for disease. Mol Cell Neurosci. 2011;46(2):368–80. doi: 10.1016/j.mcn.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli P, Giménez-Llort L, Pezzola A, Reggio R, Martínez E, Fuxe K, Ferré S. Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neurosci Lett. 1996;218(3):209–13. doi: 10.1016/s0304-3940(96)13143-8. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res. 2006;168:152–156. doi: 10.1007/s00221-005-0075-1. [DOI] [PubMed] [Google Scholar]
- Quinlan KA, Placas PG, Buchanan JT. Cholinergic modulation of the locomotor network in the lamprey spinal cord. J Neurophysiol. 2004;92(3):1536–48. doi: 10.1152/jn.01053.2003. [DOI] [PubMed] [Google Scholar]
- Rath M. Energy drinks: what is all the hype? The dangers of energy drink consumption. J Am Acad Nurse Pract. 2012;24(2):70–6. doi: 10.1111/j.1745-7599.2011.00689.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Oliver MS, Alvarez-Bagnarol Y, Díaz-Ríos ME. The modulatory effects of caffeine on the intrinsic properties of spinal lateral motoneurons. Society for Neuroscience abstract: # 2015-S-12106-SfN 2015 [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401(2):163–86. [PubMed] [Google Scholar]
- Simola N, Cauli O, Morelli M. Sensitization to caffeine and crosssensitization to amphetamine: influence of individual response to caffeine. Behav Brain Res. 2006;172:72–79. doi: 10.1016/j.bbr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Soares PM, Patrocínio MC, Assreuy AM, Siqueira RC, Lima NM, de Arruda MO, de Souza Escudeiro S, de Carvalho KM, Sousa FC, Viana GS, Vasconcelos SM. Aminophylline (a theophylline-ethylenediamine complex) blocks ethanol behavioral effects in mice. Behav Pharmacol. 2009;20(4):297–302. doi: 10.1097/01.FBP.0000358355.88022.fa. [DOI] [PubMed] [Google Scholar]
- Solinas M, Ferré S, Antoniou K. Involvement of adenosine A1 receptors in the discriminative-stimulus effects of caffeinein rats. Psychopharmacology. 2005;179:576–586. doi: 10.1007/s00213-004-2081-6. [DOI] [PubMed] [Google Scholar]
- Spyridopoulos I, Fichtlscherer S, Popp R, Toennes SW, Fisslthaler B, Trepels T, Zernecke A, Liehn EA, Weber C, Zeiher AM, Dimmeler S, Haendeler J. Caffeine enhances endothelial repair by an AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2008;28(11):1967–74. doi: 10.1161/ATVBAHA.108.174060. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Ongini E, Fredholm BB. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience. 1997a;79(3):753–64. doi: 10.1016/s0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997b;27(4):322–35. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Marsili V, Romano R, Tantucci M, Di Filippo M, Costa C, Napolitano F, Mercuri NB, Borsini F, Giampà C, Fusco FR, Picconi B, Usiello A, Calabresi P. A2A adenosine receptor antagonism enhances synaptic and motor effects of cocaine via CB1 cannabinoid receptor activation. PLoS One. 2012;7(6):e38312. doi: 10.1371/journal.pone.0038312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Coding of locomotor phase in populations of neurons in rostral and caudal segments of the neonatal rat lumbar spinal cord. J Neurophysiol. 1999;82(6):3563–3574. doi: 10.1152/jn.1999.82.6.3563. [DOI] [PubMed] [Google Scholar]
- Tronci E, Simola N, Carta AR, De Luca MA, Morelli M. Potentiation of amphetamine-mediated responses in caffeine-sensitized rats involves modifications in A2A receptors and zif-268 mRNAs in striatal neurons. J Neurochem. 2006;98:1078–1089. doi: 10.1111/j.1471-4159.2006.03943.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama H, Toda A, Imoto M, Nishimura S, Kuroki H, Soeda S, Shimeno H, Watanabe S, Eyanagi R. The stimulatory effects of caffeine with oseltamivir (Tamiflu) on light-dark behavior and open-field behavior in mice. Neurosci Lett. 2010;469(2):184–8. doi: 10.1016/j.neulet.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Wardeh G, De Vries TJ, Mulder AH, Schoffelmeer AN. Opposing role of dopamine D1 and D2 receptors in modulation of rat nucleus accumbens noradrenaline release. J Neurosci. 1999;19(10):4123–31. doi: 10.1523/JNEUROSCI.19-10-04123.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Fugazza J, Godefroy F. Dorsal and ventral dopaminergic innervation of the spinal cord: functional implications. Brain Res Bull. 1993;30:319–324. doi: 10.1016/0361-9230(93)90259-e. [DOI] [PubMed] [Google Scholar]
- Witts EC, Panetta KM, Miles GB. Glial-derived adenosine modulates spinal motor networks in mice. J Neurophysiol. 2012;107(7):1925–34. doi: 10.1152/jn.00513.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003;1003:241–9. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Wong AC, Shetreat ME, Clarke JO, Rayport S. D1- and D2-like dopamine receptors are co-localized on the presynaptic varicosities of striatal and nucleus accumbens neurons in vitro. Neuroscience. 1999;89(1):221–33. doi: 10.1016/s0306-4522(98)00284-x. [DOI] [PubMed] [Google Scholar]
- Xie X, Ramkumar V, Toth LA. Adenosine and Dopamine Receptor Interactions in Striatum and Caffeine-induced Behavioral Activation. Comparative Med. 2007;57(6):538–545. [PubMed] [Google Scholar]
- Yabuuchi K1, Kuroiwa M, Shuto T, Sotogaku N, Snyder GL, Higashi H, Tanaka M, Greengard P, Nishi A. Role of adenosine A1 receptors in the modulation of dopamine D1 and adenosine A2A receptor signaling in the neostriatum. Neuroscience. 2006;141(1):19–25. doi: 10.1016/j.neuroscience.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Tanaka M. Existence of new dopaminergic terminal plexus in the rat spinal cord: assessment by immunohistochemistry using anti-dopamine serum. Neurosci Lett. 1988;94:5–9. doi: 10.1016/0304-3940(88)90261-3. [DOI] [PubMed] [Google Scholar]
- Yum DS, Cho JH, Choi IS, Nakamura M, Lee JJ, Lee MG, Choi BJ, Choi JK, Jang IS. Adenosine A1 receptors inhibit GABAergic transmission in rat tuberomammillary nucleus neurons. J Neurochem. 2008;106(1):361–71. doi: 10.1111/j.1471-4159.2008.05400.x. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Engelwood Cliffs, NJ: Prentice Hall; 1974. [Google Scholar]
- Zhang Y, Deng P, Ruan Y, Xu ZC. Dopamine D1-like receptors depress excitatory synaptic transmissions in striatal neurons after transient forebrain ischemia. Stroke. 2008;39(8):2370–6. doi: 10.1161/STROKEAHA.107.506824. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu YP, Ye YL, Zhang JT, Zhang WP, Wei EQ. Spatiotemporal properties of locomotor activity after administration of central nervous stimulants and sedatives in mice. Pharmacol Biochem Behav. 2011;97(3):577–85. doi: 10.1016/j.pbb.2010.09.011. [DOI] [PubMed] [Google Scholar]