Summary
The generation of B cell responses to proteins requires a functional thymus to produce CD4-positive T cells which help in the activation and differentiation of B cells. Because the mature T cell repertoire has abundant cells with helper phenotype, one might predict that in mature individuals the generation of B cell memory would proceed independently of the thymus. Contrary to that prediction, we show here that removal of the thymus after the establishment of the T cell compartment or sham surgery without removal of the thymus impairs affinity maturation of antibodies. Because removal or manipulation of the thymus did not decrease the frequency of mutation of the Ig variable heavy chain exons encoding antigen specific antibodies, we conclude that the thymus controls affinity maturation of antibodies in the mature individual by facilitating selection of B cells with high affinity antibodies.
Keywords: T cell repertoire, immunoglobulin, antibody secreting cells, thymus, affinity maturation
Introduction
B cell memory confers lasting immunity to microorganisms and their products by ensuring rapid production of high affinity antibodies of switched isotype(s) (particularly immunoglobulin G (IgG)), distinct from those that predominate in the “natural” immune response. Antibodies opsonize microbes and neutralize toxins and viruses, precluding cell entry and damage. The high affinity of recall antibodies may be the most critical property for effective neutralization of toxins since those are toxic at very low concentrations. Production of high affinity class switched antibodies requires that activated B cells undergo somatic hypermutation and class switch recombination, followed by antigen selection of B cells expressing the receptors with enhanced affinity. B cell memory is manifested by recall antibody responses the result of plasma cells generated from B memory cells upon re-exposure to the antigen and by persisting antigen-specific antibodies secreted by long-lived plasma cells in the bone marrow [1].
The generation of B cell memory requires T cells. Thus, removal of the thymus in newborn mice (during the first 16 hours of life) causes severe cellular immunity defects [2] and abolishes antibody responses to protein antigens [3]. However, removal of the thymus of mature mice (between 5 and 8 weeks of age) has no immediate effect on the primary antibody responses to protein antigens or cellular immune responses [4-6]. Whether or not removal of the thymus in mature individuals perturbs B cell memory is not known.
T cells promote B cell responses to protein antigens by directly interacting with B cells. Thus, deficiencies in the CD40 or CD154 or blocking their interaction by antibodies impairs antibody responses to protein antigens, immunoglobulin isotype class switch, somatic hypermutation and B cell memory [7-9]. Because primary responses to protein antigens proceed to establish memory, the specific requirements for the generation and/or maintenance of B cell memory cannot be exploited in their absence. We have recently found that individuals with severe contraction of the T cell repertoire owing to removal of the thymus and depletion of mature T cells before cardiac transplantation in infancy do not develop hyper immunoglobulin M (IgM) syndrome and/or hypo-gammaglobulinemia, indicating some level of T cell help. Preliminary studies in subjects of cardiac transplantation in infancy suggested defective B cell memory to vaccination with protein antigens in spite of normal primary antibody responses. These observations suggested that the T cell help required to generate primary antibody responses might differ in some respects from T cell help necessary to establish and/or evoke B cell memory responses. [10]. Here we report that selection of affinity mature antibodies generated in response to protein antigens requires the integrity of the thymus.
Results
Removal of the thymus of mature mice causes a persistent decrease in the number of CD4+ or CD8+ T cells without contracting T cell receptor diversity
To explore the role of the thymus in B cell memory responses we removed the thymus of mice at 5 weeks of age reasoning that at this age mice already have an established T cell compartment and competent cellular immunity [4]. Removal of the thymus at 5 weeks of age completely abrogated recent thymic emigrants because mice lacking the thymus (in the manuscript referred to as athymic mice) lacked any measurable T cell receptor excision circles (TRECs) at 5 and 10 weeks following thymectomy (supplemental figure 1). Consistent with absent thymic function, athymic mice had reduced CD4+, CD25+, Foxp3 cells, at 10 weeks of age (supplemental figure 3).
Mice from which the thymus had been removed (athymic mice) at 5 weeks of age had fewer T cells in the spleen 5 and 10 weeks after the surgery, compared to control mice. Figures 1A, 1B and Table 1 show that thymectomy caused a persistent 2.8 or 3.0 fold decrease in the number of CD4+ or CD8+ T cells respectively, 10 weeks after surgery. Sham operation of the thymus also decreased the number of CD4+ or CD8+ T cells 10 weeks after surgery, albeit less profoundly than removal of the thymus (Figures 1 A, 1 B and Table 1). Results from other laboratories are consistent with ours showing close to 2 fold reduction in the number of CD4 T cells in the spleen. Thus, Gagnerault et al. [11] found a 2 fold reduction in the number of CD4 T cells in the spleen following thymectomy in 3- week-old mice; and Bourgeois et al. [12] found a reduction of almost 2 fold in the number of peripheral CD4-positive T cells 15 weeks after interrupting thymic output in a model of chemical thymectomy.
Figure 1. Number of lymphocytes in spleens harvested from mice lacking the thymus, sham-operated or non-manipulated controls at 5 and at 10 weeks of age.
Thymectomies were performed 2 days before the mice turned 5 weeks old. Numbers of lymphocytes were calculated by multiplying the respective percentage as defined in a flow cytometry dot plot analysis, with specific monoclonal antibodies, by the total number of white blood cells (WBC) in athymic (T), sham-operated (S) or unmanipulated control (C) mice at 5 and 10 weeks (W) after surgery. (A) Number of CD4+ splenocytes or, (B) Number of CD8+ splenocytes. (C, D) Number of memory-like T cells in spleens defined as (CD4+or CD8+)/CD44hi/CD62L- by FACS analysis. In the graphs, the bar represents the average of each distribution. Means were compared by a paired two-tailed T test. Statistically significant differences are denoted by an asterisk and indicate P <0.05.
TABLE 1.
Numbers of lymphocytes in the spleen of athymic (T), sham-operated (S) or non-manipulated (C) control mice
| 5 Weeks | 10 Weeks | |||||
|---|---|---|---|---|---|---|
| Number (average ± SD) |
T | S | C | T | S | C |
| CD4+ | 4.77 × 106 ± 0.85 × 106 |
7.92 × 106 ± 0.82 × 106 |
8.37 × 106 ± 1.73 × 106 |
3.6 × 106 ± 0.97 × 106 |
8.8 × 106 ± 2.96 × 106 |
10.1 × 106 ± 1.78 × 106 |
| CD8+ | 2.42 × 106 ± 0.47 × 106 |
4.32 × 106 ± 0.41 × 106 |
2.59 × 106 ± 0.39 × 106 |
2.52 × 106 ± 0.72 × 106 |
5.74 × 106 ± 2.14 × 106 |
7.68 × 106 ± 2.55 × 106 |
| CD4+ CD44hiCD62L− |
1.3 × 106 ± 0.1 × 106 |
1.4 × 106 ± 0.4 × 106 |
1.7 × 106 ± 0.45 × 106 |
1.04 × 106 ± 0.44 × 106 |
1.6 × 106 ± 0.53 × 106 |
1.2 × 106 ± 0.23 × 106 |
| CD8+ CD44hiCD62L− |
0.74 × 106 ± 0.05 × 106 |
0.94 × 106 ± 0.3 × 106 |
1.1 × 106 ± 0.26 × 106 |
1.15 × 106 ± 0.46 × 106 |
1.44 × 106 ± 0.3 × 106 |
1.5 × 106 ± 0.34 × 106 |
| CD19+CD21+CD23− | 1.29 × 106 ± 0.32 × 106 |
1.36 × 106 ± 0.45 × 106 |
1.53 × 106 ± 0.3 × 106 |
4.3 × 106 ± 2.5 × 106 |
3.6 × 106 ± 01.1 × 106 |
5.93 × 106 ± 1.6 × 106 |
| CD19+CD21+CD23+ | 21.59 × 106 ± 2.11 × 106 |
20.50 × 106 ± 3.89 × 106 |
24.32 × 106 ± 4.9 × 106 |
27.31 × 106 ± 11.33 × 106 |
24.49 × 106 ± 6.9 × 106 |
25.12 × 106 ± 5.7 × 106 |
To determine whether the removal of the thymus caused compensatory proliferation, we enumerated CD4+ or CD8+ cells with a memory-like phenotype (CD62L-negative and with high expression of CD44). Figures 1C, 1D and Table 1 show that athymic mice and controls had similar numbers of CD4+ or CD8+ T cells with a “memory” like phenotype in the spleen. However, the proportion of CD4+ memory-like T cells was significantly increased in athymic mice (14%) compared to sham-operated (11%) or control (9%) mice. Likewise, the proportion of CD8+ memory-like T cells was increased in athymic mice (45%) versus sham-operated (25%) or non-manipulated control mice (19%). Since the absolute number of “memory-like” T cells is similar in athymic and control mice, the increased proportion of “memory-like” T cells brought about by removal of the thymus probably reflects the decrease in the absolute number of naïve T cells rather than compensatory proliferation of T cells brought about by removal of the thymus. The apparent lack of compensatory proliferation in athymic mice might partly reflect a decrease in IL-7 which is produced by thymic epithelial cells [13].
Because cellular immunity depends in part on the diversity of T cell receptors we analyzed TCR diversity in athymic mice and in controls 10 weeks after surgery. We used a novel approach to quantify TCR beta transcript diversity using a real-time polymerase chain reaction (PCR)-based method [14]. Briefly, the method amplifies TCR V beta (β) transcripts using combinations of primers specific for a total of 240 Vβ-Jβ combinations. Cycle threshold (Ct) values were determined for each Vβ-Jβ combination for each RNA template and mean Ct values were calculated. Results shown in table 2 indicate that Ct values did not significantly differ in control (17.8), sham-operated (17.9) or athymic mice (18.7) suggesting that removal of the thymus or sham operation did not cause significant decrease in TCR diversity or oligoclonal expansions. These results were supported by Shannon entropy calculated for each Vβ-Jβ matrix in each set of mice [15] (Table 2). An estimate of entropy (H) was calculated by the equation H=Σ (p log 2 p)/log 2 (1/240) where p was the probability of abundance calculated for each Vβ-Jβ combination by the equation p=2–y/Σ2–y where y was the Ct value for each Vβ-Jβ primer pair and p=0 when Ct>40 cycles. Entropy ranges from zero to one with one representing maximal diversity. Control mice had an average entropy of 0.85, sham-operated mice had an average entropy of 0.84 and athymic mice had an average entropy of 0.85. These results agree with values reported for wild-type repertoires (0.88 on average) and contrast with values obtained in SCID-nude mice (0.76) (Dr. Wettstein, personal communication).
TABLE 2.
Cycle threshold values (Ct values) were estimated for all Vβ-Jβ combinations for each RNA template and mean Ct values were calculated. Ninety-five percent confidence intervals (CI) are shown. Diversities of expressed Vβ-Jβ pairs were calculated with Shannon entropy [15]. An estimate of scaled entropy (H) was calculated for each Vβ-Jβ matrix by the equation H=Σ (p log 2 p)/log 2 (1/240) where p was the probability of abundance calculated for each Vβ-Jβ combination by the equation p=2–y/Σ2–y where y was the Ct value for the Vβ-Jβ primer pair and p=0 when Ct>40 cycles. Scaled entropy ranges from zero to one with one representing maximal diversity
| Sample | Entropy | Mean Ct | 95% CI |
|---|---|---|---|
| C-spleen #1 | 0.85 | 17.4 | 17.1-17.4 |
| C-spleen #2 | 0.84 | 18.1 | 17.8-18.4 |
| S-spleen #1 | 0.83 | 18.0 | 17.7-18.4 |
| S-spleen #2 | 0.84 | 17.8 | 17.4-18.2 |
| T-spleen #1 | 0.84 | 18.8 | 18.4-19.1 |
| T-spleen #2 | 0.85 | 18.5 | 18.2-18.8 |
T=athymic mice, S=sham-operated mice, C=non-manipulated mice
Thymectomy does not impair T cell memory
To determine whether and how removal of the thymus might impair memory T cell responses we used delayed type hypersensitivity (DTH) to ovalbumin as an index. Figure 2A shows that challenge of athymic mice, produced larger foot-pad swelling than challenge of control mice, indicating that removal of the thymus did not impair and may instead enhance memory T cell responses. To determine whether removal of the thymus impairs primary T cell responses and test whether memory T cell responses are enhanced in athymic mice, we tested the rate of rejection of male to female skin grafts. Figure 2B shows that removal of the thymus slows the kinetics of skin graft rejection in athymic female recipients to male antigens since the median survival time of male skin grafts was 37 days in athymic mice and only 25 days in sham-operated and control mice, respectively. This result suggests that primary T cell responses were impaired. However T cell memory responses were intact as second set grafts were rejected with accelerated kinetics by all recipients, including those lacking the thymus. The results demonstrated that generation of T cell memory does not require an intact thymus.
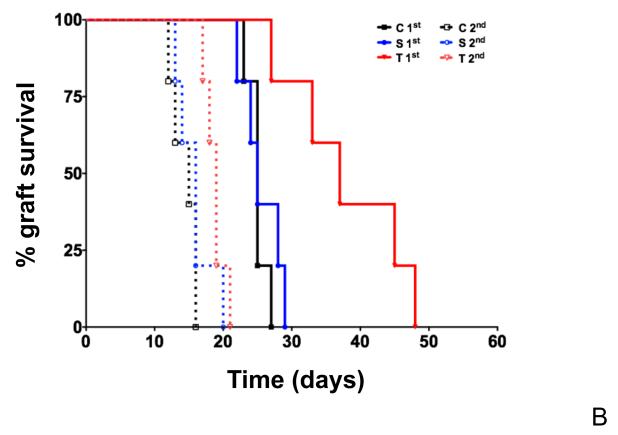
Figure 2. Primary cellular immune responses are delayed in mice lacking the thymus but T cell memory responses are maintained.
(A) Delayed Type Hypersensitivity (DTH) responses to ovalbumin in control (C), sham-operated (S) or athymic (T) mice. DTH responses to intradermic injection of 20 μg ovalbumin were examined in the footpad of mice 6 days after priming by subcutaneous injection with 100 μg of ovalbumin (priming) or PBS (control). Footpad swelling measured in mm is indicated on the y-axis. Mice lacking the thymus produced significantly larger swelling (15 mm, on average) in response to challenge than sham-operated mice (6.0 mm, on average) or control mice (6.5 mm, on average). Footpad swelling was compared by a paired two-tailed T test. (B) Kaplan Meier survival curves for H-Y incompatible skin grafts in athymic (T), sham-operated (S) or control (C) mice. x-axis, days following surgery; y-axis, skin graft survival fraction. Grafts were considered rejected when 90% or more of the graft lacked any viable signs: hair, pigment and scale pattern. The median survival time of first set grafts was 25 days in control mice, 25 days in sham-operated mice and 37 days in mice lacking the thymus. Skin graft rejection by athymic mice was significantly delayed compared to rejection in controls (p=0.0052, log-rank, Mantel-Cox test). Secondary transplants were done 8 to 12 weeks after rejection of the first transplant. The median survival time of initial transplants was 15 days in control mice, 16 days in sham-operated mice and 19 days in athymic mice. Secondary graft survival in athymic recipients did not significantly differ from graft survival in control or sham-operated recipients.
Removal of the thymus in adult mice does not impair primary or secondary antibody responses but increases long-lived antibody secreting cells in the bone marrow
Manifest B cell memory requires antigen specific antibody production at times remote from primary antigen stimulation. At least some B cells engaged in a primary response must survive and some must have the ability to respond upon re-exposure. These antibody responses require T cell help [16]. Whether the thymus is necessary to generate B cell memory responses beyond generating a diverse T cell repertoire is not known. To answer that question we tested B cell memory in mice from which the thymus had been removed or manipulated without removal 5 weeks before.
To exclude the possibility that thymectomy imposed a B cell autonomous defect independent of T cells we asked whether athymic mice mounted antibody responses to NP-Ficoll, a T-independent antigen. Figures 3A and 3B show comparable concentrations of NP-specific IgM and IgG3, 21 days following immunization, in athymic mice (230 μg/ml IgM and 64 μg/ml IgG3, on average) and sham-operated mice (167 μg/ml IgM and 86 μg/ml IgG3, on average). Hence, thymectomy did not perturb T-independent antibody production. Indeed B cells developed normally in mice lacking the thymus compared to sham-operated mice (Supplemental Figure 2). Supplemental figure 2 shows that the average number of mature CD19-positive B cells is comparable in mice lacking the thymus, sham-operated or control mice at 5 weeks and at 10 weeks of age, respectively. There were no population unbalances, as the number of marginal zone B cells (CD19+ and CD21+) or follicular (CD19+, CD21+ and CD23+) B cells was comparable in athymic, sham-operated and control mice.
Figure 3. T-independent antibody responses maintained in athymic mice.
(A, B) T-independent antibody responses to NP-Ficoll in athymic mice (T) or in sham-operated (S) mice. Figures 3A and 3B represent the concentrations of NP-specific IgM (A) or NP-specific IgG3 (B), in μg/ml (y-axis) prior to and 21 days after immunization. Mice lacking the thymus and sham-operated mice had on average 4.0μg/ml and 5.0 μg/ml NP-specific IgM respectively, and non-detectable NP-specific IgG3, prior to immunization. Athymic and sham-operated mice had 230μg/ml and 167μg/ml NP-specific IgM, on average, 21 days after immunization, respectively. Mice lacking the thymus and sham-operated mice had on average 64μg/ml and 86 μg/ml NP-specific IgG3, 21 days after immunization, respectively. The concentrations of NP-specific IgM or IgG3 in athymic mice and in sham-operated mice did not significantly differ.
A hallmark of B cell memory is the rapid production of high affinity antibodies upon re-exposure [16]. These properties reflect the survival of fully differentiated antigen specific B cells and plasma cells. To determine whether B cell memory responses were impaired in mature athymic mice, we studied responses to immunization with 4-hydroxy-3-nitrophenyl acetyl (NP), conjugated to ovalbumin. Figures 4A and 4B show that athymic mice produced as much NP-specific IgM or IgG1 as sham-operated mice indicating that removal of the thymus did not impair antigen-specific antibody primary or secondary antibody responses to vaccination with proteins. Consistent with that conclusion we found that the number of antibody secreting cells present in the bone marrow 6 months after immunization was maintained in sham-operated mice and increased by 2 fold in athymic mice compared to non-manipulated controls (Figure 5). In fact, since the number of ASC in athymic mice was significantly increased compared to the number of ASC in control or sham-operated mice, our results suggest that the thymus in the adult may inhibit either the differentiation or the maintenance of long-lived antibody secreting cells in the bone marrow.
Figure 4. Removal of the thymus or sham operation maintained IgG1 specific antibody responses.
(A, B) T-dependent responses to NP-ovalbumin. Figures 4A and 4B represent the concentrations of NP-specific IgM or NP-specific IgG1, in μg/ml (y-axis) prior to (PI) and the 21 days after primary (D21) or booster immunization (21PB), respectively. (A) Athymic mice (T) and sham-operated mice (S) had an average of 8.4μg/ml and 4.4μg/ml NP-specific IgM, prior to immunization (PI), 76 μg/ml and 111μg/ml, 21 days after immunization (D21), 293 μg/ml and 296 μg/ml 21 days after boosting (21PB), respectively. There were no significant differences between athymic and sham-operated mice. (B) Mice lacking the thymus or sham-operated mice had no detectable NP-specific IgG1 prior to immunization, but produced on average, 121μ μg/ml and 231μg/ml NP-specific IgG1 21 days after immunization, 596 μg/ml and 622 μg/ml 21 days after boosting, respectively. T test analysis revealed no significant differences between athymic and sham-operated mice.
Figure 5. Number of NP-specific IgG1 antibody secreting cells (ASC) in the bone marrow of mice lacking the thymus or control mice, 6 months after boost immunization.
Mice lacking the thymus (T) had on average 198 ASC per 106 B cells while sham-operated mice (S) had an average of 113 ASC per 106 B cells and, control mice (C) had an average of 100 ASC per 106 B cells NP-specific IgG1 antibody secreting cells in the bone marrow. The number of ASC in athymic mice was significantly increased compared to the number of ASC in control (P=0.0042) or sham-operated mice (P=0.0075) (unpaired T test). The number of ASCs was calculated from 4 mice per group.
Removal or manipulation of the thymus impairs the generation of Ig heavy chains associated with high affinity to NP
The most significant function associated with antibody recall responses is selection of cells bearing receptors with increased affinity for the antigen. To determine if affinity maturation requires the integrity of the thymus in the adult, we sampled antibody heavy chain variable region nucleotide and protein sequences of IgG1-positive B cells obtained from mice that had their thymus removed, manipulated (sham operation) or of non-manipulated controls, 10 days following booster immunization. Sequences were obtained from cloned PCR gene products amplified with VH186.2-specific primers (NP selects antibodies encoding the VH186.2 canonical germline sequence rearranged to DFL16.1 and JH2 [17]) and Cγ1 reverse primers in a nested PCR reaction and with Pfu proof-reading polymerase. Two sequences were obtained per clone and a consensus was generated. To determine if selection of antigen responsive B cells was perturbed in athymic or sham-operated mice we first determined the frequency of the VH186.2, DFL16.1 and JH2 joins in all the unique VH186.2 encoding HC sequences obtained for each group of mice. Out of 76 sequences encoding VH186.2 exons obtained from athymic mice, 19 had different joins (25%) and 12 of used DFL16.1 and JH2 (63%). In a total of 70 sequences encoding VH186.2 exons obtained from sham-operated mice, 37 had different joins (53%), and 20 used DFL16.1 and JH2 (54%). In 48 sequences encoding VH186.2 exons obtained from control mice, we found 17 different joins (35%) and 11 used DFL16.1 and JH2 (65%). These results suggested that removal of the thymus decreased, while sham operation increased, clonal diversity of NP responding B cells in comparison to controls even-though the majority of clones encoding the VH186.2 gene segment also encoded DFL16.1 and JH2 in all the three groups of mice.
Next we compared the aminoacid sequences of CDR3 regions encoded by each unique join. NP-binding antibodies often encode Tyr or Gly at position 95 [18]. While 94% CDR3 joins sequenced from control mice had Y or G at position 95 only 68% of the unique CDR3 joins obtained from sham-operated mice had Y or G at position 95 and 84% that of the unique CDR3 joins sequenced from athymic mice had Y or G at position 95. These results suggest that removal of the thymus and sham operation disturbs selection of NP-reactive clones. These results are consistent with defective selection of NP-specific antibodies in sham-operated and athymic mice.
Because defective selection of NP-specific antibodies could result from defective somatic hypermutation we measured the mutation frequency of the unique VH gene segments obtained from athymic, sham-operated or control mice in relation to the VH186.2 germline sequence. The VH mutation frequencies were 2.6%, 3% and 2.3% in athymic, sham-operated and control mice, respectively, suggesting that manipulation or removal of the thymus in the adult did not impair somatic hypermutation, per se. However, the frequency of mutation in the CDR1 region of VH186.2 encoding antibodies obtained from control mice was 13.6% and consisted of very focused changes at mostly 3 positions (Figure 6A), but the frequency of mutation in the CDR1 region of antibodies obtained from athymic and sham-operated mice was only 8.5% and 7.2%, respectively, and less focused (Figures 6B and 6C). Decreased frequencies of mutations in the CDR1 regions of the VH186.2 exons in athymic or sham-operated mice compared to CDR1 sequences obtained from non-manipulated mice suggested a defect in the selection of antigen-specific antibodies. In fact, the fraction of sequences containing the W33L NP-affinity enhancing mutation was decreased in athymic mice (87%, figure 7A) and in sham-operated mice (21%, figure 7B), compared to that fraction (98%) in sequences obtained from control mice in which all sequences except for one contained the W33L mutation (figure 6C). Contingency analysis (Chi-square test) revealed the reduction in the number of W33L mutations in athymic or sham-operated mice relative to control mice to be significant (p< 0.05, p< 0.0001, respectively). Remarkably, manipulation of the thymus caused a significant reduction in the number of the W33L mutations compared to that number in athymic mice (p<0.0001), suggesting that manipulation of the thymus without its removal compromises affinity maturation more seriously than its removal. Because the W33L mutation in the VH186.2 exon by itself causes a 10 fold increase on affinity to NP [19] the reduction in the frequency of the W33L mutation in athymic and in sham-operated mice indicates that the integrity of the thymus is necessary for the production of high affinity antibodies.
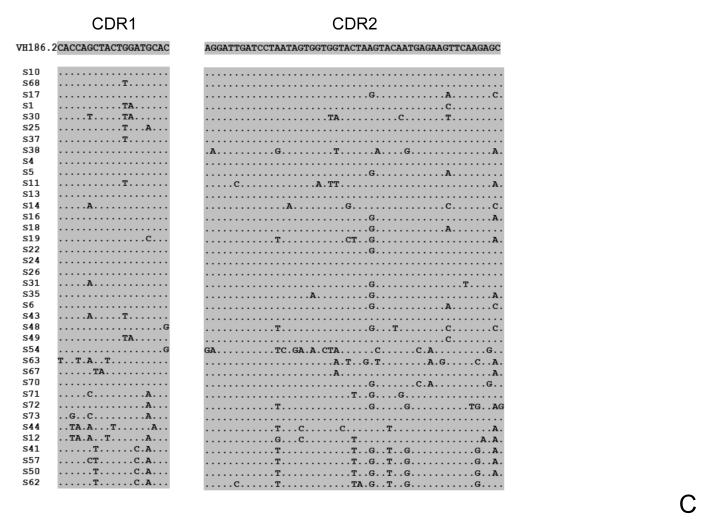
Figure 6. Heavy chain VH CDR1 and CDR 2 DNA sequences of IgG1-B cells obtained from the spleen of mice lacking the thymus (T), sham-operated (S) or control (C) mice, 10 days following boost immunization.
Figures show the CDR1 or CDR2 sequences of all the distinct VH sequences aligned to the germline VH186.2 segments. Shadowed are CDR1 and CDR2 regions. (A) Sequences obtained from control non-manipulated mice; (B) Sequences obtained from athymic mice; (C) Sequences obtained from sham-operated mice.
Figure 7. Heavy chain VH aminoacid sequences of IgG1-B cells obtained from the spleen of mice lacking the thymus (A), sham-operated (B) or control (C) mice, 10 days following boost immunization.
Figures show the aligned translation of all the distinct VH186.2 segments obtained from each group of mice. Shadowed are CDR1 and CDR2 regions and in a darker grey shade residue #33 is indicated. Antibodies with high affinity to NP often encode a W to L mutation in this position.
Discussion
Our results show that the thymus contributes to priming T cell responses (as expected), and to affinity maturation of antibodies. Surprisingly, in spite of compromised selection of B cells bearing high affinity B cell receptors, production of long lived antibody secreting cells is not defective in athymic or sham-operated mice. In fact, our results suggest the possibility that the thymus may inhibit the generation and/or maintenance of long-lived antibody secreting cells. Since removal of the thymus did not critically contract the T cell receptor diversity or decrease the number of T cells in a substantive manner, these results suggest that affinity maturation of antibodies is critically dependent on the integrity of the thymus.
Recent studies support the idea that T cell help and the B cell receptor (BCR) strength determine B cell fate in response to T-dependent antigen activation. Thus Paus et al. [20] and Phan et al. [21] suggested that high BCR affinity for antigen dictates differentiation to extra-follicular antibody secreting cells causing primary antibody responses. O’Connor et al. [22] proposed that B cells with a low affinity BCR typically undergo somatic hypermutation, while B cells with BCR with moderate affinity for antigen produced mostly long-lived antibody secreting cells. BCR affinity and T cell help are interdependent since B cells present antigens to T cells following Ig-dependent internalization [23]. Thus B cells that have a competitive advantage to bind antigen owing to higher affinity receptors may also better compete for limiting T cell “help” which in turn may determine their fate. The interdependence between BCR affinity and B cell antigen presentation to T cells has made it difficult to dissociate the contributions of each to B cell selection and differentiation. In a “tour de force”, Victora et al. [24] showed that enhancing antigen B cell presentation without engaging the BCR promoted migration of B cells from the light zone to the dark zone of the germinal center, clonal expansion and plasmablast differentiation. These authors concluded that T cell help limits expansion and differentiation of B cells in the germinal center independently of BCR engagement. However, enhancing B cell antigen presentation by germinal center B cells did not induce antibody affinity maturation, suggesting that the combined signals provided by BCR ligation and engagement of T cells determine the B cell fate.
Our results showing normal or enhanced antibody responses to protein antigens suggested that T cell help in athymic or sham-operated mice is adequate to activate and promote differentiation of antibody secreting cells short- and long-term. However since removal or manipulation of the thymus compromised affinity maturation of antibodies our results suggest that disruption of thymic integrity selectively impairs affinity maturation of antibodies much in the same way as enhancing antigen presentation independently of the BCR as reported by Victora et al. [24]. Because removal of the thymus interrupts the flux of new T cells we considered the possibility that the availability of cognate help may be reduced to a greater extent than non-cognate help, enhancing BCR-independent antigen presentation which in turn would impair selection of high affinity B cells. We propose that absence of optimal cognate T cell help owing to interruption of thymic emigration or following manipulation of the thymus abrogates competition for B cells expressing B cell receptors with high affinity for antigen randomizing differentiation and apoptosis. Other functions of the thymus such as production of IL7 or production of regulatory T cells could also contribute to the regulation of immunity. We observed that removal of the thymus impairs production or maintenance of T regulatory cells (Supplementary figure 3). Whether or not decreased production of T regulatory cells in athymic mice contributes to defective affinity maturation of antibodies in these mice is not known and this question will be an interesting one to resolve.
Our findings concur with those of Ahuja et al. [25] who proposed that the long-lived antibody secreting cell compartment is maintained independently of the memory B cell compartment because it does not decline when memory B cells are abrogated. Our results indicate that differentiation of long-lived antibody secreting cells occurs independently of affinity maturation that normally accompanies B cell memory responses. Our work suggests that strategies to immunize individuals with congenital or acquired thymic defects (such as following cardiac transplantation or cardiac surgery in infancy), or with contracted T cell repertoires (such as in aging or after T cell depletion to treat cancer) would benefit from new vaccine designs including surrogates of cognate T cell help.
Materials and Methods
Thymectomy
Thymuses were removed surgically from mice or sham-surgery was performed at 5 weeks of age. Mice were anesthetized with ketamine (120-200 mg/kg) + xylazine (10 mg/kg) i.p. An incision was made on the ventral neck midline extending from 0.5 cm cranial of the sternal notch. The clavicle was cut along the sternum to the second rib and retracted to expose the trachea, sternohyoid and sternothyroid muscles which were gently separated to expose the superior end of the thymic lobes. The thymus was then gently dissected with blunt instruments and excised by vacuum. The thorax was closed using 6-0 absorbable suture placed through the dorsal thorax to draw the clavicle and ribs together. The fat pad with the submaxillary gland was returned to its original position and held in place by liquid skin adhesive. Skin was closed using 6-0 absorbable suture. Mice were monitored every 12h for the first 48h, and daily thereafter. Sham-operated mice underwent the same surgical procedure, except for the fact that the thymus was not excised, just manipulated with the tip of blunt scissors.
Blood collection
Done following the recommendations of the University of Michigan Committee on Use and Care of Animals (UCUCA).
Immunizations
T-independent immunizations were performed as explained by Mantchev et al. [26] by injecting mice i.p. with 30 μg of NP-Ficoll (NP41- AECM-Ficoll; Biosearch Technologies, Novato, CA, USA) diluted in 100 μl PBS once. Primary T-dependent immunizations were performed by i.p. injection of 100 μl of an emulsion of incomplete Freund’s adjuvant containing 100 μg NP(25)- ovalbumin (Biosearch Technologies, Novato, CA, USA) and boost immunizations performed by i.p. injection of 100 μl of a PBS solution containing 10 μg NP(25)-ovalbumin. To obtain RNA from memory B cells, mice were boosted a second time by i.v. injection of 50 μg NP(25)-ovalbumin dissolved in 100 μl of PBS.
Strains of mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in a specific pathogen-free facility at the University of Michigan. All mice were between 5 and 25 weeks of age and all experiments were carried out in accordance with protocols approved by UCUCA.
Ig gene analysis
RNA was obtained from spleen cells and extracted with QIAGEN RNeasy (Qiagen, Inc., Valencia, CA, USA). cDNA was obtained from 0.2 μg of RNA using oligo(dT) primed reverse transcription. VH186.2 gene sequences joined to the IgG1 constant region were amplified with VH186.2 and Cγ1 specific primers in a nested reaction and with Pfu polymerase, followed by cloning with pCR4-TOPO (Invitrogen, USA). Sequencing of cloned PCR fragments was done by the Mayo Clinic Sequencing Core. Forward primer: CATGCTCTTCTTGGCAGCAACAGC (specific for VH186.2), reverse primer: GTGCACACCGCTGGACAGGGATCC (specific for Cγ 1). PCR was performed for 30 cycles of 1 minute at 94° Celsius, 2 minutes at 55° Celsius, and 3 minutes at 72° Celsius. Nested PCR amplification forward primer: CAGGTCCAACTGCAGCAG, and reverse primer, AGTTTGGGCAGCAGA. Sequences were aligned and analyzed using Sequencher software (Gene Codes, MI, USA) and with a software program developed by Dr. Calvacoli at the University of Michigan Sequencing Core. VH, D and JH gene CDR3 sequence assignments were done according to the international ImMunoGeneTics (IMGT) system software developed by Dr Lefrank at the CNRS, France, [27]. Complementary determining regions were determined according to Kabat et al. [28].
FACS analysis and antibodies
Splenocytes were obtained and prepared for flow cytometry analysis as in [29]. Fluorescently conjugated or biotynilated antibodies were purchased from BD Biosciences unless noted: rat anti-mouse CD4 (GK 1.5), rat anti-mouse CD21a/CD35 (7G6), rat anti-mouse CD3ε (145-2C11), rat anti-mouse IgDb (AMS9.1), rat anti-mouse CD4 (GK1.5), rat anti-mouse CD44 (Pgp-1, Ly-24), rat anti-mouse CD23 (Fc ε RII), rat anti-mouse IgMb (DS-1), rat anti-mouse CD8α (Ly-2), rat anti-mouse CD19 (1D3), rat anti-mouse CD62L (LECAM-1, Ly22), rat anti-mouse CD25 (IL-2R α chain, p55) and rat anti mouse Foxp3 (FJK-16S, eBiosciences). Biotynilated antibodies (Abs) were revealed by streptavidin-PE-Cy5 purchased from BD Biosciences, USA. Data were collected on a FACScalibur (BD Biosciences) and analyzed with CellQuest software (BD Biosciences). Isotype controls were used to define gates.
Delayed type hypersensitivity (DTH) assay
Mice were primed by subcutaneous injection of 100 μg of ovalbumin dissolved in PBS, and challenged by intra-dermal injection of 20 μg of ovalbumin in PBS in the footpad, 6 days after priming. Non-primed mice challenged with PBS were included as controls. Footpad swelling was measured with a caliper. Effective swelling indicates the difference in the thickness of footpads in the same mice (one injected with the antigen, another injected with PBS). Responses were recorded at 24 and 48 hours post-challenge.
Skin Grafts
Skin grafts were performed according to a modified technique of Billingham et al. [30]. Secondary transplants were 30 days after the primary graft was shed.
TRECS
DNA was obtained from splenocytes with a DNeasy Blood and Tissue Kit (Qiagen, Inc., Valencia, CA, USA). PCR amplification of sjTREC DNA was done from 100 ng of DNA in a Mastercycler ep realplex real-time PCR system (Eppendorf) using specific primers targeting murine δRec-ψJα excision circles. Real-time PCR cycler conditions were set for 95°C for 10′ followed by 40 cycles of 95°C for 15″ and 60°C for 1′ with 5μM forward and reverse primers and 0.05μl of 100μM FAM-QSY probe. Forward primer (upstream of ψJα segment): 5′CAT TGC CTT TGA ACC AAG CTG3′; Reverse primer (downstream of the δRec1 segment): 5′TTA TGC ACA GGG TGC AGG TG3′ according to [31]. A fluorescent probe for RT-PCR: FAM - CAG GGC AGG TTT TTG TAA AGG TGC TCA CTT - QSY (Applied Biosystems). The mouse transferring receptor gene Tfrc gene (TaqMan Copy Number Reference Assay, Applied Biosystems) was amplified to quantify cell number in mouse DNA samples. Each sample was run in triplicate. Standard curves were created with either serial dilutions sjTREC plasmid DNA or of C57BL/6J DNA followed by Tfrc gene amplification.
Statistical analysis
Performed using Prism software (Prism Software Corporation, Irvine, CA). Group comparisons were performed using the unpaired, two-sided Student’s t test after testing the global difference with a one-way analysis of variance (ANOVA). Comparison of skin graft survival was performed by a log rank test. A value of p < 0.05 was considered significant.
ELISA (Enzyme-linked immunosorbent assay)
MaxiSorp-treated or PolySorp-treated polystyrene 96- well plates (Thermo Scientific, Rochester, NY, USA) were coated with 4 μg/mL of goat anti-mouse Ig (SouthernBiotech, Birmingham, AL, USA) in PBS to measure total Ig, or with 5 μg/mL of NP-BSA in borate saline buffer to detect NP-specific antibodies, for 1 hour at room temperature. ELISA was performed according to previously described protocols [29, 32]. Plates were developed with ABST (SouthernBiotech, Birmingham, AL, USA) read at 405 nm in microplate reader Synergy 2 (BioTec Laboratories Ltd., Suffolk, UK) and analyzed using Gen 5 software version 1.04.5 (BioTek, VT, USA). The 17.2.25 IgGl was used as a standard for quantification.
ELISPOT
Done according to standard procedures in the laboratory [26]. MultiScreen HTS-HA 96- well plates (Millipore, Billerica, MA, USA) were coated with 5 μg/mL NP-BSA or 5 μg/mL BSA in sodium carbonate buffer overnight at 4°C and blocked with 5% milk in TBS-Tween for 2 hours at 37°C. B cells isolated from the spleen by negative selection were serially diluted, seeded in the wells and cultured in complete RPMI-1640, overnight at 37°C in 5% CO2 atmosphere. ELISPOT analyses of antibody secreting cells obtained from adoptively transferred recipients were done with splenocytes. To detect NP-specific antibody secreting cells, each well was washed and incubated with AP-conjugated goat anti-mouse IgM or IgG antibody (SouthernBiotech, Birmingham, AL, USA) for 2 hours at 37°C. Each well was developed with BCIP/NBT (Sigma-Aldrich, St. Louis, MO, USA). The number of spots of NP-specific IgM or IgG secreting cells was counted by ImmunoSpot Professional Analyzer version 5.0.9 (Cellular Technology Ltd., Shaker Heights, OH, USA) and confirmed by direct observation.
TCR beta chain diversity analysis
TCR beta chain diversity analysis was done as reported [14]. Briefly, RNA was obtained from spleens using a RNeasy Protect Minikit (Qiagen, CA). Residual DNA was removed from RNA samples using a RNase-Free DNase Set (Qiagen). cDNA was produced from 15 ng of RNA with a 20 pmol of a 5′biotynilated TCR Cββb primer and pools of 21 different TCR Vβ primers homologous to the CDR 1 region providing 66 pmol of each (three pools of 5 and one pool of 6 primers), at 50°C for 32′ followed by incubation at 94°C to inactivate the reverse transcriptase. cDNA synthesis was followed by PCR amplification at 1′ at 94°C, 30″at 60°C, and 1′ at 72°C for 25 cycles. RT-PCR products were purified by QIAquick PCR Purification Kit (Qiagen) and biotynilated products separated with MyOneTM Streptavidin C1 Dynabeads (Dynal Biotech ASA, Oslo, Norway) according to the manufacturers’ instructions. TCR Vβ diversity was determined by real time PCR in a total of 240 individual reactions using combinations of 20 TCR Vβ and 20 TCR Jβ primers, as described [14]. Reactions were performed in a 10 ml volume containing 10 pmol of a nested TCR Vβ primer homologous to TCR Vβ CDR2, 10 pmol of a TCR Jβ primer, l μl of purified PCR products and 5 μl of Power SYBR Green PCR master mix (2x) (Applied Biosystems). Cycling was preceded by incubation at 50°C for 2′ and at 95°C for 10′, followed by 40 cycles of 15″ at 95°C and 1′ at 60°C. Data were analyzed with the 7900HT Sequence Detection System Version 2.3 software (Applied Biosystems) to estimate the cycle threshold (Ct) for all reactions. Ct values are fractional cycle numbers at which fluorescence passes the threshold set to be within the exponential region of the amplification curve corresponding to a linear relationship between the log of change in fluorescence and cycle number. Primers were as published [14] and synthesized by Invitrogen (Carlsbad, CA, USA).
Supplementary Material
Acknowledgements
We thank Bruce Knudsen and Karen Lien for technical assistance. This work is in partial fulfillment of the PhD requirements to MA by the Charles University, Faculty of Medicine (Hradec Králové) Prague, Czech Republic. We also thank Dr. James Cavalcoli who produced software to identify repeat sequences.
This work was supported by NIH grant P01 HL79067. Principal funding recipients, Jeffrey L Platt and Marilia Cascalho.
Abbreviations
- ASC
antibody secreting cells
- CDR3
complementary determining region 3
- DTH
delayed type hypersensitivity
- TREC
T cell receptor excision circle(s)
Footnotes
Conflict of Interest There are no financial or commercial conflicts of interest to disclose.
References
- 1.Manz RA, Arce S, Cassese G, Hauser AE, Hiepe F, Radbruch A. Humoral immunity and long-lived plasma cells. Curr Opin Immunol. 2002;14:517–521. doi: 10.1016/s0952-7915(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 2.Miller JF. Immunologic function of the thymus. Lancet. 1961;2:748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 3.Miller JF, De Burgh PM, Grant GA. Thymus and the production of antibody-plaque-forming cells. Nature. 1965;208:1332–1334. doi: 10.1038/2081332a0. [DOI] [PubMed] [Google Scholar]
- 4.Miller JF. Effect of thymectomy in adult mice on immunological responsiveness. Nature. 1965;208:1337–1338. doi: 10.1038/2081337a0. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf D. Delayed effect of thymectomy in adult life on immunological competence. Nature. 1965;208:1336. doi: 10.1038/2081336a0. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RB. Decay of immunological responsiveness after thymectomy in adult life. Nature. 1965;208:1334–1335. doi: 10.1038/2081334a0. [DOI] [PubMed] [Google Scholar]
- 7.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 8.Korthauer U, Graf D, Mages HW, Briere F, Padayachee M, Malcolm S, Ugazio AG, et al. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 9.Davies EG, Thrasher AJ. Update on the hyper immunoglobulin M syndromes. British Journal of Haematology. 2010;149:167–180. doi: 10.1111/j.1365-2141.2010.08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogle BM, West LJ, Driscoll DJ, Strome SE, Razonable RR, Paya CV, Cascalho M, Platt JL. Effacing of the T cell compartment by cardiac transplantation in infancy. Journal of Immunology. 2006;176:1962–1967. doi: 10.4049/jimmunol.176.3.1962. [DOI] [PubMed] [Google Scholar]
- 11.Gagnerault MC, Lanvin O, Pasquier V, Garcia C, Damotte D, Lucas B, Lepault F. Autoimmunity during thymectomy-induced lymphopenia: role of thymus ablation and initial effector T cell activation timing in nonobese diabetic mice. Journal of Immunology. 2009;183:4913–4920. doi: 10.4049/jimmunol.0901954. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeois C, Hao Z, Rajewsky K, Potocnik AJ, Stockinger B. Ablation of thymic export causes accelerated decay of naive CD4 T cells in the periphery because of activation by environmental antigen. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8691–8696. doi: 10.1073/pnas.0803732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alves NL, Richard-Le Goff O, Huntington ND, Sousa AP, Ribeiro VS, Bordack A, Vives FL, et al. Characterization of the thymic IL-7 niche in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1512–1517. doi: 10.1073/pnas.0809559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wettstein P, Strausbauch M, Therneau T, Borson N. The application of real-time PCR to the analysis of T cell repertoires. Nucleic Acids Res. 2008;36:e140. doi: 10.1093/nar/gkn634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shannon C, Warren W. The mathematical theory of communication. University of Illinois; Urbana: 1949. [Google Scholar]
- 16.Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–150. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 17.Bothwell AL, Paskind M, Reth M, Imanishi-Kari T, Rajewsky K, Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981;24:625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. Journal of Experimental Medicine. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen D, Simon T, Sablitzky F, Rajewsky K, Cumano A. Antibody engineering for the analysis of affinity maturation of an anti-hapten response. Embo J. 1988;7:1995–2001. doi: 10.1002/j.1460-2075.1988.tb03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. Journal of Experimental Medicine. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. Journal of Experimental Medicine. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor BP, Vogel LA, Zhang W, Loo W, Shnider D, Lind EF, Ratliff M, et al. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. Journal of Immunology. 2006;177:7723–7732. doi: 10.4049/jimmunol.177.11.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annual Review of Immunology. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 24.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4802–4807. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantchev GT, Cortesao C, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. The Journal of Immunology. 2007;179:2282–2288. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 27.Giudicelli V, Chaume D, Lefranc MP. IMGT/GENE-DB: a comprehensive database for human and mouse immunoglobulin and T cell receptor genes. Nucleic Acids Res. 2005;33:D256–261. doi: 10.1093/nar/gki010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5 Edn NIH; Bethesda, MD: 1991. [Google Scholar]
- 29.Cascalho M, Ma A, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- 30.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 31.Sempowski GD, Rhein ME. Measurement of mouse T cell receptor excision circles. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im1031s63. Chapter 10: Unit 10 31. [DOI] [PubMed] [Google Scholar]
- 32.Cascalho M, Wong J, Wabl M. VH gene replacement in hyperselected B cells of the quasimonoclonal mouse. Journal of Immunology. 1997;159:5795–5801. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.