The hydrophobic effect, which is used to describe the aversion of oil for water or the affinity of oily objects for one another in water, plays an important role in diverse disciplines (1). For example, by segregating to the oil–water interface, amphiphilic molecules that possess both hydrophobic and hydrophilic groups can mitigate unfavorable oil–water interactions, thereby stabilizing emulsions and facilitating detergency. Because roughly half the amino acids, which form the basic building blocks of proteins, are hydrophobic, the hydrophobic effect also plays a central role in biophysics. Owing to its ubiquity and its multifaceted nature (2, 3), being able to accurately model the hydrophobic effect is both important and challenging. In PNAS, Vaikuntanathan et al. (4) provide important insights into the essential ingredients required in a minimal model of the hydrophobic effect.
The hydrophobic effect characteristically manifests itself in very different ways at microscopic and macroscopic length scales (1). Macroscopically, the aversion of oil and water for one another is captured by the large surface tension associated with the oil–water interface. At this scale, the hydrophobic effect drives the minimization of the unfavorable interfacial area (for example, by the coalescence of oil droplets in water). Although the macroscopic hydrophobic effect is governed by the relatively straightforward physics of interfaces, it can nevertheless lead to complex phenomena, such as the nanobubble-mediated long-ranged forces between extended hydrophobic surfaces (5), or the assembly of anisotropic particles at curved interfaces (6). Interestingly, the thermodynamic driving force for such hydrophobic assembly, which is dictated by interfacial tension, decreases with increasing temperature.
In contrast, at the molecular scale, the driving force for hydrophobic assembly famously increases with increasing temperature (1). In fact, whenever biomolecular assembly occurs upon increasing temperature, in seeming disregard for entropic considerations, the hydrophobic effect is often the first suspect. A classic example is the cold denaturation of proteins. Whereas the denaturation or unfolding of proteins upon heating is common, and is ascribed to the configurational entropy of the macromolecule prevailing over the favorable energy of the folded state, certain proteins also denature upon cooling. Such cold denaturation is explained by the fact that the molecular hydrophobic effect, which stabilizes the folded state of the protein, is weakened upon cooling, causing the protein to unravel (7).
To understand these contrasting manifestations of the hydrophobic effect, it is instructive to consider how water structure is perturbed near small (molecular) and large (macroscopic) hydrophobic solutes. Near a macroscopic hydrophobic object, such as an oil droplet, interfacial water molecules are unable to participate in four hydrogen bonds like their bulk counterparts (8). This energetically unfavorable scenario is responsible for the large oil–water surface tension and drives the minimization of interfacial area. In the vicinity of molecular hydrophobes such as methane, the hydration waters are able to maintain all their hydrogen bonds, but accommodating the hydrophobe severely constrains the hydrogen bonding network of water (1). This strain on the hydrogen bonding network is reflected in a large negative entropy of hydration; it is this entropic penalty that causes molecular hydrophobes to assemble more strongly as temperature is increased. Thus, hydrophobic hydration and assembly at the molecular level are not governed by interfacial physics, but are fundamentally different. Given these differences in hydrophobic hydration in the molecular and macroscopic limits, at what size do we cross over from one regime to the other?
Theory, simulation, and experiment all agree that the cross-over occurs for solutes that are about 1 nm in size (2, 9–13). This length scale emerges from a product of the surface tension of water and its isothermal compressibility, properties that respectively influence the hydration of large and small solutes (1, 10, 12). The 1-nm length scale also plays a key role in numerous molecular assemblies; for example, most amino acid side chain residues are smaller than 1 nm, but folded proteins are larger than 1 nm. Being able to quantitatively capture the length scale cross-over in hydrophobic hydration is thus critical for understanding biophysical phenomena such as protein folding and aggregation, and must be a central requirement of any comprehensive theory of the hydrophobic effect.
In a seminal contribution, Lum, Chandler, and Weeks (LCW) introduced the key theoretical principles necessary for reconciling hydrophobic hydration at small and large length scales (2). The LCW theory of hydrophobicity prescribed decomposing the spatial variations in water density, which arise from the presence of a hydrophobic solute, into two separate components. The first component, , is governed by interfacial physics and displays only gentle variations over molecular length scales; LCW used a Landau–Ginzburg density functional to describe the free energetics of this component. The second component, , incorporates the small-amplitude, short-wavelength fluctuations in molecular density. Importantly, the statistics of such small fluctuations in water density are Gaussian, a fact that underpins liquid-state theories (14, 15) and has been confirmed by molecular simulations (16). LCW theory leverages the Gaussian statistics of the small length scale fluctuations to integrate out , thereby providing a renormalized and coarse-grained description of the free energetics of . In particular, remains close to its bulk liquid value, , within and near molecular solutes, because the small fluctuations are sufficient to vacate the region occupied by the solute. In contrast, vacating waters from a region larger than 1 nm (for the solute to occupy) requires nucleation of an interface, with vanishing within the solute region and approaching outside.
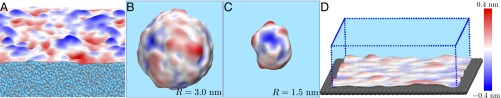
The original LCW paper used a variational formalism to evaluate in the mean-field limit (2). To incorporate fluctuations in , subsequent LCW-inspired treatments (17, 18) used simple models capable of capturing interfacial physics, such as the lattice gas model. In the lattice gas model, variations in density between adjacent lattice sites carry an energetic penalty, ε, which along with the lattice spacing, δ, determines the interfacial tension, γ. However, the formation of a water–vapor interface at ambient conditions is associated not only with an energetic penalty, but also with entropically favorable interfacial fluctuations (Fig. 1A); indeed, the statistics of such long-wavelength capillary wave fluctuations are well understood (19). Vaikuntanathan and Geissler (20) recognized that for the lattice gas model to appropriately capture capillary wave fluctuations, only a small range of ε values could be used. If ε is too small, we approach the critical point, and the bulk liquid and vapor phases display large density fluctuations; however, if ε is too large, interfacial fluctuations are suppressed. With the optimally parameterized lattice gas for , the authors’ LCW-inspired model was able to quantitatively capture, without any adjustable parameters, the length scale cross-over in the hydration free energies of idealized hydrophobes of various shapes. Remarkably, their model was also able to capture subtle features of the interfacial free energetics, such as a preference for droplets over bubbles (20, 21), highlighting the importance of capillary fluctuations even for nanoscale interfaces (Fig. 1 B and C).
Fig. 1.
Long-wavelength interfacial fluctuations can be seen in simulation snapshots of (A) the liquid–vapor interface, (B and C) nanoscopic vapor bubbles in bulk water, and (D) at a hydrophobic surface. The work of Vaikuntanathan et al. (4) and Vaikuntanathan and Geissler (20) highlights the importance of such fluctuations and provides a way to incorporate them into a minimal model of the hydrophobic effect. The average interface in each case is either flat (A and D) or spherical (B and C), and the color represents deviation away from the average interface toward (red) or away (blue) from water (cyan). The instantaneous interfaces were computed using the algorithm outlined in ref. 22.
In their recent study, Vaikuntanathan et al. (4) subject their LCW-inspired model to another stringent test, that of quantifying the statistics of water density fluctuations; such fluctuations, characterized by the probability, , of observing N waters in a probe volume, v, have provided numerous insights into the multifaceted nature of the hydrophobic effect (3, 16, 23, 24). For example, low-N fluctuations are significantly enhanced near a hydrophobic surface, situating the interfacial waters at the edge of dewetting transition (Fig. 1D) and making them sensitive to perturbations (2, 24). Using their LCW-inspired model, Vaikuntanathan et al. (4) obtain not just qualitative, but also quantitative, agreement with molecular simulations, in their estimates of for probe volumes in bulk water, at interfaces, and in hydrophobic confinement. Furthermore, this quantitative agreement is lost if either the small length-scale Gaussian fluctuations or the large length-scale interfacial fluctuations are not included in the model.
By identifying capillary fluctuations as an essential ingredient of a minimal model of the hydrophobic effect, Vaikuntanathan et al. (4) have laid the groundwork for studying aqueous systems using a coarse-grained representation of water, which has the accuracy of molecular simulations but exacts a tiny fraction of the computational cost. Given the importance of solvent fluctuations in modulating dewetting barriers and the kinetics of hydrophobic interactions (25–27), their model could conceivably be used to study not only the thermodynamics, but also the kinetics of complex self-assemblies. Another important application is the study of biomolecules, which are too large to model using explicit water. Along with hydrophobic interactions, electrostatic interactions also play an important role in the biomolecular context. We hope that the success of LCW-inspired models motivates the development of minimal models that efficiently incorporate fluctuations in both the short- and long-wavelength water polarization response to charged moieties (28, 29).
Acknowledgments
This work was supported by National Science Foundation Grants UPENN MRSEC DMR 11-20901 and CBET 1511437, and the Charles E. Kaufman Foundation Grant KA2015-79204.
Footnotes
The authors declare no conflict of interest.
See companion article on page E2224 in issue 16 of volume 113.
References
- 1.Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature. 2005;437(7059):640–647. doi: 10.1038/nature04162. [DOI] [PubMed] [Google Scholar]
- 2.Lum K, Chandler D, Weeks JD. Hydrophobicity at small and large length scales. J Phys Chem B. 1999;103:4570–4577. [Google Scholar]
- 3.Patel AJ, et al. Extended surfaces modulate hydrophobic interactions of neighboring solutes. Proc Natl Acad Sci USA. 2011;108(43):17678–17683. doi: 10.1073/pnas.1110703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaikuntanathan S, Rotskoff G, Hudson A, Geissler PL. Necessity of capillary modes in a minimal model of nanoscale hydrophobic solvation. Proc Natl Acad Sci USA. 2016;113(16):E2224–E2230. doi: 10.1073/pnas.1513659113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducker WA, Mastropietro D. Forces between extended hydrophobic solids: Is there a long-range hydrophobic force? Curr Opin Colloid Interface Sci. 2016;22:51–58. [Google Scholar]
- 6.Cavallaro M, Jr, Botto L, Lewandowski EP, Wang M, Stebe KJ. Curvature-driven capillary migration and assembly of rod-like particles. Proc Natl Acad Sci USA. 2011;108(52):20923–20928. doi: 10.1073/pnas.1116344108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matysiak S, Debenedetti PG, Rossky PJ. Role of hydrophobic hydration in protein stability: A 3D water-explicit protein model exhibiting cold and heat denaturation. J Phys Chem B. 2012;116(28):8095–8104. doi: 10.1021/jp3039175. [DOI] [PubMed] [Google Scholar]
- 8.Lee CY, McCammon JA, Rossky PJ. The structure of liquid water at an extended hydrophobic surface. J Chem Phys. 1984;80:4448–4455. [Google Scholar]
- 9.Stillinger FH. Structure in aqueous solutions of nonpolar solutes from the standpoint of scaled-particle theory. J Solution Chem. 1973;2:141–158. [Google Scholar]
- 10.Ashbaugh HS, Pratt LR. Colloquium: Scaled particle theory and the length scales of hydrophobicity. Rev Mod Phys. 2006;78:159. [Google Scholar]
- 11.Southall NT, Dill KA. The mechanism of hydrophobic solvation depends on solute radius. J Phys Chem B. 2000;104:1326–1331. [Google Scholar]
- 12.Rajamani S, Truskett TM, Garde S. Hydrophobic hydration from small to large lengthscales: Understanding and manipulating the crossover. Proc Natl Acad Sci USA. 2005;102(27):9475–9480. doi: 10.1073/pnas.0504089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li IT, Walker GC. Signature of hydrophobic hydration in a single polymer. Proc Natl Acad Sci USA. 2011;108(40):16527–16532. doi: 10.1073/pnas.1105450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratt LR, Chandler D. Theory of the hydrophobic effect. J Chem Phys. 1977;67:3683–3704. [Google Scholar]
- 15.Chandler D. Gaussian field model of fluids with an application to polymeric fluids. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993;48(4):2898–2905. doi: 10.1103/physreve.48.2898. [DOI] [PubMed] [Google Scholar]
- 16.Hummer G, Garde S, García AE, Pohorille A, Pratt LR. An information theory model of hydrophobic interactions. Proc Natl Acad Sci USA. 1996;93(17):8951–8955. doi: 10.1073/pnas.93.17.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ten Wolde PR, Sun SX, Chandler D. Model of a fluid at small and large length scales and the hydrophobic effect. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;65(1 Pt 1):011201. doi: 10.1103/PhysRevE.65.011201. [DOI] [PubMed] [Google Scholar]
- 18.Varilly P, Patel AJ, Chandler D. An improved coarse-grained model of solvation and the hydrophobic effect. J Chem Phys. 2011;134(7):074109. doi: 10.1063/1.3532939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weeks JD. Structure and thermodynamics of the liquid–vapor interface. J Chem Phys. 1977;67:3106–3121. [Google Scholar]
- 20.Vaikuntanathan S, Geissler PL. Putting water on a lattice: The importance of long wavelength density fluctuations in theories of hydrophobic and interfacial phenomena. Phys Rev Lett. 2014;112(2):020603. doi: 10.1103/PhysRevLett.112.020603. [DOI] [PubMed] [Google Scholar]
- 21.Sedlmeier F, Netz RR. The spontaneous curvature of the water-hydrophobe interface. J Chem Phys. 2012;137(13):135102. doi: 10.1063/1.4755753. [DOI] [PubMed] [Google Scholar]
- 22.Willard AP, Chandler D. Instantaneous liquid interfaces. J Phys Chem B. 2010;114(5):1954–1958. doi: 10.1021/jp909219k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godawat R, Jamadagni SN, Garde S. Characterizing hydrophobicity of interfaces by using cavity formation, solute binding, and water correlations. Proc Natl Acad Sci USA. 2009;106(36):15119–15124. doi: 10.1073/pnas.0902778106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel AJ, et al. Sitting at the edge: How biomolecules use hydrophobicity to tune their interactions and function. J Phys Chem B. 2012;116(8):2498–2503. doi: 10.1021/jp2107523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setny P, Baron R, Michael Kekenes-Huskey P, McCammon JA, Dzubiella J. Solvent fluctuations in hydrophobic cavity-ligand binding kinetics. Proc Natl Acad Sci USA. 2013;110(4):1197–1202. doi: 10.1073/pnas.1221231110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remsing RC, et al. Pathways to dewetting in hydrophobic confinement. Proc Natl Acad Sci USA. 2015;112(27):8181–8186. doi: 10.1073/pnas.1503302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwary P, Mondal J, Morrone JA, Berne BJ. Role of water and steric constraints in the kinetics of cavity-ligand unbinding. Proc Natl Acad Sci USA. 2015;112(39):12015–12019. doi: 10.1073/pnas.1516652112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hummer G, Pratt LR, García AE. Multistate gaussian model for electrostatic solvation free energies. J Am Chem Soc. 1997;119:8523–8527. [Google Scholar]
- 29.Remsing RC, Liu S, Weeks JD. Long-ranged contributions to solvation free energies from theory and short-ranged models. Proc Natl Acad Sci USA. 2016;113:2819–2826. doi: 10.1073/pnas.1521570113. [DOI] [PMC free article] [PubMed] [Google Scholar]