Significance
The rise of agriculture during the Neolithic period has paradoxically been associated with worldwide population growth despite increases in disease and mortality. We examine the effects of sedentarization and cultivation on disease load, mortality, and fertility among Agta foragers. We report increased disease and mortality rates associated with sedentarization alongside an even larger increase in fertility associated with both participation in cultivation and sedentarization. Thus, mothers who transition to agriculture have higher reproductive fitness. We provide the first empirical evidence, to our knowledge, of an adaptive mechanism behind the expansion of agriculture, explaining how we can reconcile the Neolithic increase in morbidity and mortality with the observed demographic expansion.
Keywords: quality–quantity trade-off, epidemiological transition, hunter-gatherers, Neolithic revolution, Neolithic demographic transition
Abstract
The Neolithic demographic transition remains a paradox, because it is associated with both higher rates of population growth and increased morbidity and mortality rates. Here we reconcile the conflicting evidence by proposing that the spread of agriculture involved a life history quality–quantity trade-off whereby mothers traded offspring survival for increased fertility, achieving greater reproductive success despite deteriorating health. We test this hypothesis by investigating fertility, mortality, health, and overall reproductive success in Agta hunter-gatherers whose camps exhibit variable levels of sedentarization, mobility, and involvement in agricultural activities. We conducted blood composition tests in 345 Agta and found that viral and helminthic infections as well as child mortality rates were significantly increased with sedentarization. Nonetheless, both age-controlled fertility and overall reproductive success were positively affected by sedentarization and participation in cultivation. Thus, we provide the first empirical evidence, to our knowledge, of an adaptive mechanism in foragers that reconciles the decline in health and child survival with the observed demographic expansion during the Neolithic.
Agriculture appeared in multiple locations from around 12–10,000 y B.P. before slowly spreading to become the dominant subsistence system by 5,000 y B.P. (1–5). The Neolithic transition was associated with sedentarization, food storage, wealth accumulation, and wealth inequality as well as increasing population size (3, 4, 6, 7). It has been suggested that cultivation increased calorie availability which, combined with a reduction in energy expenditure resulting from sedentarization, led to increased energy availability for reproduction (8–10). As a result, although exact estimates vary, it has been argued that average population growth rates rose from <0.001% to ∼0.04% per year during the early Neolithic (6, 8, 11–17).
On the other hand, studies reveal significant increases in morbidity and mortality associated with a reduction in dietary breadth and sedentarization (6, 18–22). Research reveals increased prevalence of tuberculosis, syphilis, and the plague (6, 23–25), overall immunological stress (26), and a deterioration in oral health (16, 27, 28). Farming led to higher population densities, sedentarization, increased contact with neighboring populations, the presence of rodents attracted by food stores, the domestication of animals, and fecal pollution (29–31). All those factors facilitated the spread of virulent bacterial and viral pathogens as well as soil-borne helminths (roundworm, hookworm, and whipworm) (32–38). Although some argue that Paleolithic foragers experienced high helminth loads (37, 39, 40), archaeological data instead show an increase in helminths in farming populations (33, 41–45) as compared with mobile, low-density hunter-gatherers. In summary, the overall effect of agriculture on health was a trend toward increasing morbidity and mortality (16, 19, 28, 46), although the intensity of the trend exhibits some regional variation and inconsistencies (47–56).
Given this significant increase in disease burden, why farming arose independently and expanded in diverse geographical regions (57, 58) remains a puzzle. Here we propose that settled agriculture was accompanied by a life history trade-off, whereby mothers switch strategies to invest in offspring quantity rather than quality and thereby increase their reproductive fitness. A quantity–quality trade-off is an adaptive process based on a principle of optimal distribution of limited resources: Parents must allocate finite resources either to a few higher-quality offspring or to multiple lower-quality ones (59–64). By proposing that a quantity–quality trade-off provides an explanation for the transition to agriculture, we provide an adaptive mechanism that reconciles the decline in population health with increases in fertility during the Neolithic transition. We test this hypothesis with data from extant Palanan Agta foragers in the Philippines (henceforth referred to as “Agta”), a diverse group varying in mobility, foraging, wealth accumulation, and camp permanence, all traits attributed to the Neolithic revolution (1, 3, 6, 7). Residing completely within the Northern Sierra Madre Natural Park, the Palanan Agta have remained mostly separated from other Agta populations who reside outside the park limits and further south in Casiguran (65–67). By measuring fertility and mortality alongside viral, bacterial, and helminth markers in different Agta camps, we found that sedentarization is associated with increased morbidity and mortality, but both sedentarization and involvement in nonforaging activities are associated with even further increases in fertility, resulting in higher reproductive fitness. Our results provide evidence that a quality–quantity trade-off is adaptive and suggest a pathway through which, despite resulting poor health, farming could have become the dominant subsistence system after its origin in the Neolithic. Thus, we demonstrate how exploring the mechanisms that lead extant foragers to seek increased settlement and food production is a theoretically informative approach to test key archaeological predictions (6, 8, 19).
Results
Palanan Agta Exhibit a Suite of Traits Attributed to the Neolithic Transition.
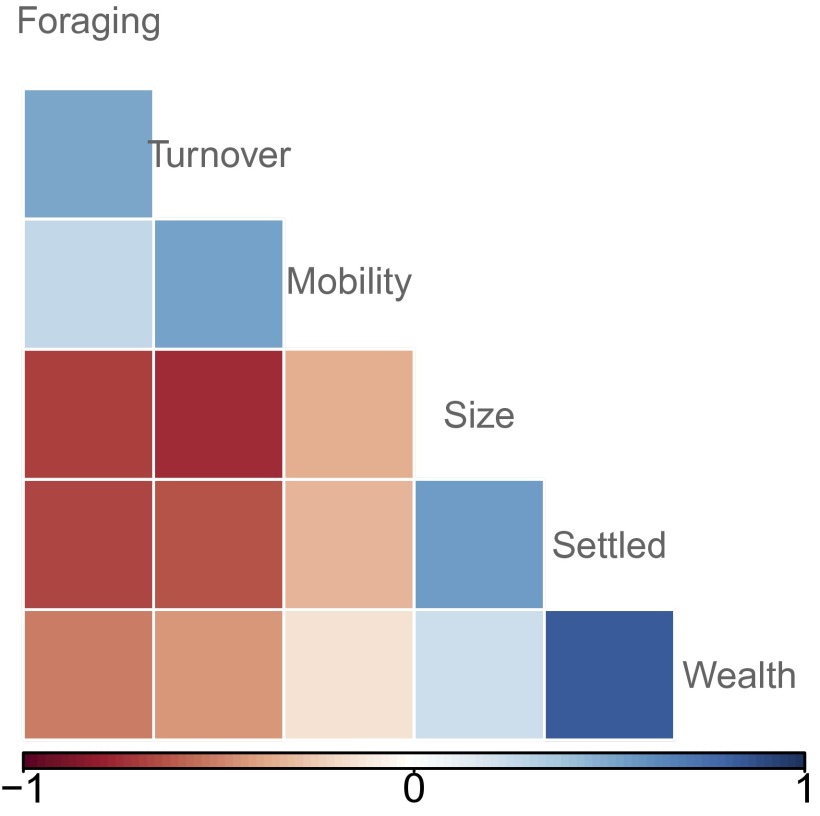
Archaeological studies have shown that the Neolithic transition from hunting-gathering to agriculture was associated with a suite of traits (3, 7) that also are present across Agta camps. Fig. 1 shows significant correlations between the proportion of food produced by cultivation and key transition traits. These correlations indicate that some Agta camps are characterized by extensive foraging, small size, high mobility, and few belongings, whereas others exhibit more food production, larger camp size, little mobility, and many belongings. Thus the variability present within the Agta today allows us to test the influence of these traits associated with transition to agriculture on fitness-related outcomes.
Fig. 1.
Correlation plot for six key predictor variables (n = 345). Positive correlations are marked in blue shades, and negative correlations are marked in red shades. All correlations are statistically significant at P < 0.05. Variables are settled camp (number of permanent structures within a camp); wealth (an index quantifying household belongings); mobility (whether an individual was ever witnessed to move camp during a 2-y period, with 0 indicating never moved); turnover (number of people leaving a camp); and foraging (proportion of food that individuals acquired from foraging activities versus food production and wage labor).
We used these transition variables associated with the Neolithic revolution in multilevel analyses to examine fertility, morbidity, and mortality. Because of covariance, two models were produced for each analysis, one exploring the effects of household mobility (whether or not a household moved camp at least once during the 2 y of fieldwork) and the second exploring the effects of household foraging (a binary measure in which 1 represents more than 75% of maternal time spent in foraging as opposed to cultivation and wage labor). Both these models included one camp settlement variable, chosen based on lowest Akaike Information Criterion (AIC) score in univariate regressions (SI Appendix, Table S4), and included (i) camp housing type (temporary, semipermanent, or permanent); (ii) a binary sedentarized camp variable denoted by the presence of permanent camp infrastructures; and (iii) out-of-camp mobility over 2 y, with 0 representing camp abandonment. AICs and full model results are reported in SI Appendix, Tables S8–S14.
Settled Agta Exhibit Increased Fertility.
The total fertility rate (TFR) among the Agta based on 41 live births in 2013–2014 was 7.7, one of the highest reported in foragers (68). For the 117 women we interviewed (age 16–75 y), the mean number of live births was 4.4 (ranging from 0 to 13). Residuals from a linear regression of offspring count and maternal age, including a logarithmic term, were used to control for age effects on fertility and reproductive success, and an exponential term was included for child mortality (SI Appendix, Text). Settled mothers had significantly higher fertility than women who moved camp at least once (β = 1.3 ± 0.6, P = 0.04). Correspondingly, increases in household belongings were positively associated with fertility (β = 0.5 + 0.2, P = 0.005). This relationship is confirmed further by examining the TFR: Settled mothers had a 16.7% higher TFR (7.7) than mobile mothers TFR (6.6). Similarly, for all live births settled mothers had 20.5% more offspring (5.3) than mobile mothers (4.4) (SI Appendix, Table S6). A possible pathway may be that settled mothers are able to accumulate more somatic resources for reproduction. Accordingly, settlement was a significant predictor of maternal body mass index (BMI) (i.e., for mothers never witnessed to move camp, β = 1.7 ± 0.6, P = 0.004), and BMI positively predicts fertility (β = 0.3 ± 0.1, P = 0.02).
Mothers Who Forage More Exhibit Lower Fertility.
A high degree of foraging also significantly predicts fertility (β = −1.4 ± 0.7, P = 0.04). As a result, mothers who spent less than 75% of their time foraging experience 0.23 higher fertility residuals than expected for their age. Mothers who spent more than 75% of their time foraging had 0.85 less offspring, given their age (SI Appendix, Table S7). Women who spent more time foraging also had marginally lower BMI (β = −1.5 ± 0.9, P = 0.08). Therefore the transition to farming as measured by both increasing cultivation and sedentarization is positively associated with fertility, perhaps because of increased somatic resources.
Sedentarization Is Associated with Health Deterioration.
We examined the effects of cultivation and sedentarization on three white blood cell types—lymphocytes, neutrophils, and eosinophils—as proxies for different immunological attacks: viral, bacterial, and helminthic, respectively (SI Appendix, Text). Our sample consisted of 345 Agta, of whom 140 were adults (48.6% males) and 205 were children under age 16 y (60% males). Because of inconclusive test results, missing data reduced the sample to 293 persons, of whom 117 were adults (47% males) and 176 were children (59% males). Table 1 shows that people in large camps with low out-of-camp mobility had a 2.8× higher chance of presenting with lymphocytosis than people residing in mobile camps. People residing in camps with permanent and semipermanent houses had significantly higher eosinophil concentrations than individuals residing in temporary camps. Severe eosinophilia (>5 × 109/L) is indicative of extreme helminth infestations and is found only in sedentarized camps. In contrast, people residing in sedentarized camps with some form of infrastructure (i.e., church or water pump) had lower odds (by 80%) of neutrophilia. One major bacterial disease among the Agta is Mycobacterium tuberculosis infection (TB): 41.7% of the 24 individuals suffering from neutrophilia had clinically diagnosed or suspected TB. In contrast, the percentage of time spent foraging had little effect on health. Individuals in households who foraged more had a 76% reduction in the odds of presenting with lymphocytosis as compared with persons in households who foraged less. However, indicators of helminthic or bacterial infections were not affected by the percentage of time spent foraging (SI Appendix, Tables S11–S13).
Table 1.
White blood cell types, medically abnormal thresholds, frequency, predictors, and health implications
| Measure | Abnormal values | Affected, % | Explanatory variables | Indication of |
| Lymphocytosis | Lymphocytes >3 × 109/L | 67 | Out-of-camp mobility: OR = 2.8, P = 0.03 | Viral infection |
| >75% foraging: OR = 0.24, P = 0.04 (baseline < 75% foraging) | ||||
| Neutrophilia | Neutrophils >7 × 109/L | 6.5 | Settled camp: OR = 0.2 P = 0.01 | Bacterial infection |
| (baseline: mobile camp) | ||||
| Eosinophilia | Eosinophils >0.5 × 109/L | 71.3 | Semipermanent housing: β = 0.53 + 0.2, P = 0.004 | Helminthic infection |
| Permanent housing: β = 0.45 + 0.2 P = 0.007 | ||||
| (baseline: temporary housing) |
Eosinophilia is a continuous analysis so to differentiate between mild, medium, and extreme eosinophilia, as these reflect significant variance in helminth load (see SI Appendix). All other analyses are logistic; n = 293 because of missing data regarding household wealth and inconclusive test results. OR, odds ratio.
To explore the helminth burden further, we collected 30 fecal samples from Agta who had presented with eosinophilia. All 30 samples tested positive for helminth ova. The mean number of species was 1.6 ± 0.7 and included roundworm (Ascaris lumbricoides; 83.3%), hookworm (Ancylostoma duodenale or Necator americanus; 46.7%), and whipworm (Trichuris trichiura; 30%). Because of the small sample size nonparametric Kruskal–Wallis tests were run on three measures of transition. Residence in a sedentarized camp was associated with a 0.65 mean increase in parasite species number compared with residence in mobile camps (χ2 = 5.9, df = 1, P = 0.02). Household participation in cash labor decreased helminth loads by 37.4% (χ2 = 8.9, df = 1, P = 0.003), indicating that families who interact with the wider economy may be more able, financially and/or behaviorally, to acquire anthelmintic medicines. Although cash labor is a positive predictor, the proportion of time spent foraging is not (χ2 = 3.3, df = 1, P = 0.2). Our results indicate that although increased helminthic load is likely to be a consequence of the transition to agriculture, it is settlement, rather than cultivation, that predicts helminth infestation.
Child Mortality Is Increased in Settled Camps.
We examined whether the negative effects of settlement on health also extend to child mortality rates (including all postnatal deaths until age 16 y). Of 520 reported live births from 124 mothers, 19% of children died before age 1 y, 13.9% died between the ages of 1 and 5 y, and 6% died between the ages of 5 and 15 y. Infant mortality at 19% is significantly lower than the 28–29% previously reported for neighboring Agta (65, 69). Mothers residing in camps with permanent housing experienced significantly higher childhood mortality rates than mothers living in camps with temporary housing (β = 0.7 ± 0.3, P = 0.005). The relationship for semipermanent camps was in the same direction but of marginal significance (β = 0.5 ± 0.3, P = 0.08). This relationship results in a 63.2% increase in mortality rates for settled mothers living in sedentarized camps (0.93) compared with nomadic mothers in temporary camps (0.57). Thus the sedentarized camps with the highest helminth and viral disease rates also experience the highest childhood mortality rates. As with the morbidity results, the proportion of time spent in cultivation had no significant relationship with child mortality (β = 0.01 ± 0.04, P = 0.8).
A Quantity–Quality Trade-Off Explains Higher Reproductive Success in Transitioning Agta.
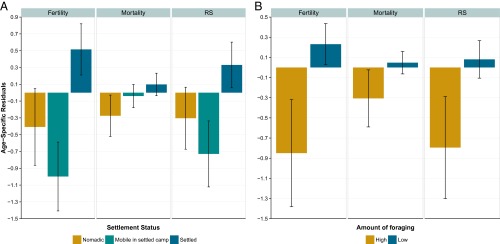
Our results show that women who settle down trade child survival for fertility, increasing their overall reproductive success (Fig. 2A). Settled women (β = 0.8 ± 0.4, P = 0.03) had significantly more children surviving to age 16 y (our measure of reproductive fitness) than did mobile women. Although these settled women faced higher child mortality rates, they also experienced extremely high fertility. As a result of the negative morbidity effect of sedentarized camps compared with the positive fertility effect of settled households, women residing in permanent camps suffered lower child survival (β = −1.2 ± 0.5, P = 0.01) than women in temporary camps after we controlled for household mobility. Fig. 2A demonstrates that mobile women living in sedentarized camps faced the worst situation, because they experienced both the higher mortality rates associated with permanent camps and the lower fertility rates associated with high mobility. Thus, they experienced 0.73 lower fitness, given their age. Overall, our results show that, despite the higher mortality rates, settled mothers had an extra 0.59 offspring (4.4) surviving to age 16 y as compared with mobile women (3.8), a relative increase of 15.8% in reproductive fitness.
Fig. 2.
Relationship between measures of transition and age-specific fertility, mortality, and reproductive success (RS) separated by individual settlement status (A) and degree of foraging (B). Error bars represent SEMs; n = 90.
Our other indicator of transition, the percentage of time spent in foraging, also predicted reproductive success (β = −1.2 ± 0.6, P = 0.05). We find that mothers who foraged more than 75% of the time had 0.74 less children surviving to age 16 y given their age, whereas mothers who spent less time foraging experienced 0.04 more children surviving to age 16 y (Fig. 2B). Thus, although abandoning foraging does not significantly affect child mortality rates, it does increase fertility and, consequently, the mother’s reproductive success. Thus cultivation is associated with increased fertility, whereas sedentarization is associated with both increased fertility and increased morbidity and mortality. The overall consequence of this combined process is a reproductive trade-off whereby settled mothers involved in cultivation have the highest reproductive fitness, despite increased morbidity and mortality.
Discussion
The variability of lifestyles currently observed within the Agta allows the testing of a hypothesis proposed by archaeologists to explain the demographic processes taking place during the Neolithic transition (8, 29). Current evidence from archaeology and other sources suggests a Neolithic paradox in which fertility increased alongside morbidity (58). We have tried to shed light on this debate by offering an adaptive explanation for the Neolithic transition. We propose that a life history trade-off between offspring quantity and quality can explain a suite of traits including the higher fertility, mortality, morbidity, and overall reproductive success observed in sedentarized Agta practicing cultivation. To the degree that the variability observed within the Agta is reflective of Neolithic trends, our results illuminate the adaptive mechanisms involved in the transition to sedentarized food production and provide empirical evidence that a quantity–quality trade-off can explain the demographic changes associated with cultivation and sedentism.
Our data indicate that sedentarization was associated with an increased risk of infectious disease. Viral infections were higher in the larger, less mobile camps with increased cultivation. Camp size and population density (measured here through out-of-camp mobility) were significantly correlated with viral infections in our data. This finding is consistent with the expectations of transmission of viruses such as Caliciviruses and Rotaviruses, causing gastroenteritis and influenza (38), which are common among settled Agta and are dependent on higher population densities (70). Our results mirror the finding of increased risk of high C-reactive protein concentrations (indicative of an inflammatory response) in children among more acculturated Tsimane forager-horticulturalists (71). In particular, cultivation may independently predict increased viral load (indicated by the association between lymphocytosis and foraging) because of the poorer nutritional quality of agricultural staples. Increased consumption of rice with cultivation entails a loss of dietary breadth and macro- and micronutritional quality (72, 73), leading to malnutrition, which is a significant predictor of disease and mortality (74).
As with viral infections, Agta living in more permanent camps (as predicted by camp housing type) also were worse off in terms of helminthic infections, matching theoretical predictions (29, 33, 43, 44, 75). Contrary to this finding, some studies have argued that high helminth burden was a major selective pressure before the Neolithic (37, 39, 76) because of extreme helminth rates in many semi-sedentarized, farmer-foragers (31, 37, 77). However, these groups are no longer fully mobile. Supporting our interpretation and findings, researchers observed a near absence of helminths in an isolated Amazonian group (78), Hadza children (79), and lower helminth loads among isolated and mobile Saluma and Yanomamo groups (80). Although some parasitic species were hosted by our hominid ancestors (29, 81, 82), there is little archaeological evidence for an extreme intestinal parasite burden predating agriculture (33, 41–43, 45, 81). Thus, increased viral and helminth burdens are costs associated with sedentarization. It should be noted that settled Agta did not show an increased incidence of neutrophilia, which in our sample is often caused by TB. Chronic bacterial infections, such as TB, have evolved to survive at low population densities and thus likely predate agriculture (83, 84). The lower incidence of neutrophilia in settled Agta camps is likely the result of the limited access to medical care, which also might explain the finding that cash labor decreased helminth loads by 37.4%. Because westernized medicine is a recent occurrence, the overall increased helminthic load in sedentarized camps may underestimate the effect that settlement had on human populations during the Neolithic transition.
We found that more permanent camps had significantly higher childhood mortality rates, matching archaeological evidence from the Neolithic (6). Our results also revealed significantly higher fertility rates in settled women, particularly those transitioning from foraging to cultivation. Agriculture has long been associated with increases in fertility (85, 86), because the reduction in energy expenditure with settlement (87) and increased carbohydrate consumption (10, 88) are associated with increased BMI, which correlates with shorter interbirth intervals and higher fertility (89). The association between BMI and transition suggests a pathway through which increased cultivation could lead to increased fertility. An additional pathway, as suggested by Kramer and Boone (90), considers the increased economic productivity of children in agriculture, which reduces maternal constraints and thus increases a woman’s fertility. Our study remains correlative, leaving unanswered the question of the causal direction between fertility, sedentarization, and food production. Because sedentarization correlates with food production in the Agta, with more-sedentarized households abandoning foraging, these two process seem to be interdependent and thus are treated here as part of a process that involved a cluster of transition traits. This correlation is likely to have been the case for the Neolithic transition as well, because many have reported the interplay between sedentarization and food production leading to the demographic changes associated with the origins of agriculture (4, 7). Further limitations stem from using a cross-sectional study design to explore longer-term trends; thus, it is unclear to what degree sedentism is reflective of mobility trends beyond our 2-y sample period. However, a cross-sectional study design does permit the exploration of intrapopulation variability without requiring this variability to be recent or unilineal. Furthermore, foraging and mobility behaviors are likely altered during the heavy rainy and typhoon season, circumstances that are not captured here because data collection occurred during the dry period. However, this collection period does avoid systemic temporal bias in the data, because camps were visited within the same season. Finally, more detailed dietary data would be required to explore these questions in greater depth and to allow us to separate better the effects of transition.
In summary, our study of the Agta provides evidence supporting the hypothesis that, although the transition to agriculture in the Neolithic significantly depressed health, the overall fitness payoff was greater. Therefore, the proposed quality–quantity trade-off provides an adaptive mechanism that reconciles deteriorating health, increased mortality, and demographic expansion following the spread of agriculture in the Neolithic. Finally, because high fertility rates were accompanied by relatively high mortality rates, the trade-off also explains why population numbers did not explode during the Neolithic but instead increased relatively slowly (8), perhaps because large increases in fertility were matched by increases in mortality (17).
Methods
Data collection occurred over two field seasons from April to June 2013 and February to October 2014 during the Isabela dry season. There are around 1,000 Agta living in Palanan municipality, Isabela Province, located in northeastern Luzon, Philippines. The Agta are primarily fisher-foragers, residing either at the coast or in inland watersheds (91). Hunting does occur, but fishing is far more common: of 2,168 work activities 54% were spent fishing, 20% in gathering, and 3% hunting. The remaining nonforaging activities consisted of cultivation (13.5%) and wage labor (9.5%). However, households varied significantly in diet (SI Appendix, Fig. S1) and activities: Mobile households spent only 3.5% of their work activities in cultivation and 0% of time in wage labor. Comparatively settled households spent 11.6% and 10% of their work activities in cultivation and wage labor, respectively. We found that 26% of Agta moved camp at least once during our fieldwork, resulting in an average camp move of once every 10 d. Similarly, the degree of camp sedentarization varies drastically, because some camps change daily and consist of temporary shelters, whereas others consist of permanent wooden structures built around churches and cultivation plots. Some Agta living in permanent camps closer to town have limited access to medical care and government provisioning. However, these sporadic interventions have limited effects on Agta health (see SI Appendix, Text for further ethnographic descriptions). We stayed ∼14 d in each of the 13 camps and conducted reproductive histories to quantify demographics and interviews to produce an index of household belongings. After creating a list of household belongings based on their importance for the Agta, we asked each household whether it had each of the 14 objects, and if so how many. This response was weighted based on the number of each of the items a household owned (SI Appendix, Table S1). We also conducted four daily camp scans at staggered time intervals based on spot observation techniques (SI Appendix, Fig. S4) (92). We categorized each individual’s activity at the allocated time, separating hunting, fishing, foraging, wage labor, and cultivation. Thus we can quantify the proportion of time individuals spent in foraging activities versus cultivation and wage labor. Finally, we recorded key features of the camp and its changing composition as we visited each camp two to three times (depending on whether it had dissolved into new camps).
These data created the transition variables which were broken into (a) camp sedentarization measured by (i) camp housing type (temporary, semipermanent, or permanent), (ii) a binary sedentarized camp variable denoted by the presence of church and/or water pump, and (iii) out-of-camp mobility between three visits, with 0 representing camp abandonment, 0.5 when 50% of individuals remained, and 1 when no one left; (b) household mobility established by whether a household moved camp or not during the 2 y of fieldwork; (c) household belongings based on a list of common objects; and (d) household foraging, a binary variable in which 1 represents mothers spending more than 75% of food-production activities in foraging rather than in cultivation and wage labor. Because of multicollinearity, only one of the variables in the camp sedentarization group was entered into each model, chosen by the lowest AIC in univariate regressions. White blood cell composition analysis was conducted using the HemoCue WBC DIFF system, and we collected fecal samples from 30 individuals with intestinal parasites symptoms. Further information and descriptive statistics for these variables can be found in SI Appendix, Tables S2 and S3. Logistic and linear multilevel models were conducted in R version 3.1.2 (93) using the lme4 package (94). All models were originally run with level 1 as the individual, level 2 as the household, and level 3 as the camp. Levels that failed to improve the model were removed to produce the most parsimonious model. Only theoretically informed variables were entered into the model; however, because many variables covary, the maximal model suffered from multicollinearity [variance inflation factor scores of more than 2.5 (95)]. Therefore, two models were produced, separating household mobility from household foraging, to correct for any remaining covariance. Otherwise all models contained all appropriate controls (age, sex, mean relatedness, household dependents, and belongings) and the best predictor variable for camp sedentarization. Because the proportion of each leukocyte is dependent on the circulating levels of other leukocytes, we controlled for whether an individual had abnormally high levels of any white blood cell types as well as total white blood cell count in each analysis. Because of the smaller sample size, nonparametric Kruskal–Wallis tests were used to examine the data on polyparasitic infections. All model betas are reported in the text alongside SEs. This research and fieldwork were approved by the University College London (UCL) Ethics Committee (UCL Ethics Code 3086/003) and were carried out with permission from local government and tribal leaders in Palanan. Informed consent was obtained from all participants, and parents signed the informed consents for their children (after group and individual consultation and explanation of the research objectives in the Agta language). As part of the process, people identified as having any disease were provided with medical care in partnership with the local government.
Supplementary Material
Acknowledgments
We thank the Human Evolutionary Ecology and Hunter-Gatherer groups at University College London and Matt Thomas for their comments on earlier drafts, the three reviewers for their constructive critique, our assistants in the Philippines, Palanan Mayor Angelo Bernardo, the Palanan field hospital, Dr. Chung, and most importantly, the Agta. This project was funded by Leverhulme Trust Grant RP2011-R-045 (to A.B.M. and R.M.). R.M. received funding from European Research Council Advanced Grant AdG 249347.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data presented in this paper have been deposited in the Dryad Digital Depositary (datadryad.org).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524031113/-/DCSupplemental.
References
- 1.Bar-Yosef O, Meadow RH. The origins of argiculture in the near east. In: Price D, Gebauer AB, editors. Last Hunters-First Farmers. School of American Research Press; Santa Fe, NM: 1995. pp. 39–94. [Google Scholar]
- 2.Bollongino R, et al. 2000 years of parallel societies in Stone Age Central Europe. Science. 2013;342(6157):479–481. doi: 10.1126/science.1245049. [DOI] [PubMed] [Google Scholar]
- 3.Piperno D, Pearsall DM. The Origins of Agriculture in the Lowland Neotropics. Academic; London: 1998. [Google Scholar]
- 4.Price DT, Gebauer AB. New perspectives on the transition to agriculture. In: Price DT, Gebauer AB, editors. Last Hunters-First Farmers. School of American Research Press; Santa Fe, NM: 1995. pp. 3–20. [Google Scholar]
- 5.Richerson PJ, Boyd R, Bettinger RL. Was agriculture impossible during the Pleistocene but mandatory during the Holocene? A climate change hypothesis. Am Antiq. 2001;66(3):387–411. [Google Scholar]
- 6.Cohen MN, Crane-Kramer GMM. Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification. University Press of Florida; Gainesville, FL: 2007. [Google Scholar]
- 7.Hayden B. A new overview of domestication. In: Price D, Gebauer AB, editors. Last Hunters-First Farmers. School of American Research Press; Santa Fe, NM: 1995. pp. 215–242. [Google Scholar]
- 8.Bocquet‐Appel J, Naji S. Testing the hypothesis of a worldwide Neolithic demographic transition: Corroboration from American cemeteries. Curr Anthropol. 2006;47(2):341–365. [Google Scholar]
- 9.Starling AP, Stock JT. Dental indicators of health and stress in early Egyptian and Nubian agriculturalists: A difficult transition and gradual recovery. Am J Phys Anthropol. 2007;134(4):520–528. doi: 10.1002/ajpa.20700. [DOI] [PubMed] [Google Scholar]
- 10.Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000;54(Suppl 1):S47–S51. doi: 10.1038/sj.ejcn.1600984. [DOI] [PubMed] [Google Scholar]
- 11.Wells JCK, Stock JT. The biology of the colonizing ape. Am J Phys Anthropol. 2007;50(Suppl 45):191–222. doi: 10.1002/ajpa.20735. [DOI] [PubMed] [Google Scholar]
- 12.Bandy MS. New World settlement evidence for a two-stage Neolithic demographic transition. Curr Anthropol. 2005;46(S):S109–S115. [Google Scholar]
- 13.Bellwood P, Oxenham M. The expansions of farming societies and the role of the Neolithic Demographic Transition. In: Bocquet‐Appel J, Bar-Yosef O, editors. The Neolithic Demographic Transition and Its Consequences. Springer; London: 2008. pp. 13–34. [Google Scholar]
- 14.Downey SS, Bocaege E, Kerig T, Edinborough K, Shennan S. The Neolithic demographic transition in Europe: Correlation with juvenility index supports interpretation of the summed calibrated radiocarbon date probability distribution (SCDPD) as a valid demographic proxy. PLoS One. 2014;9(8):e105730. doi: 10.1371/journal.pone.0105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershkovitz I, Gopher A. Demographic, Biological and Cultural Aspects of the Neolithic revolution: A view from the southern Levant. In: Bocquet‐Appel J, Bar-Yosef O, editors. The Neolithic Demographic Transition and Its Consequences. Springer; London: 2008. pp. 441–482. [Google Scholar]
- 16.Willis A, Oxenham MF. The neolithic demographic transition and oral health: The Southeast Asian experience. Am J Phys Anthropol. 2013;152(2):197–208. doi: 10.1002/ajpa.22343. [DOI] [PubMed] [Google Scholar]
- 17.Zahid HJ, Robinson E, Kelly RL. Agriculture, population growth, and statistical analysis of the radiocarbon record. Proc Natl Acad Sci USA. 2016;113(4):931–935. doi: 10.1073/pnas.1517650112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armelagos GJ, Goodman AH, Harper KN, Blakey ML. Enamel hypoplasia and early mortality: Bioarcheological support for the Barker hypothesis. Evol Anthropol. 2009;18(6):261–271. [Google Scholar]
- 19.Cohen MN, Armelagos GJ. Paleopathology at the Origins of Agriculture. Academic; Gainesville, FL: 1984. [Google Scholar]
- 20.Cohen MN. Health and the Rise of Civilisation. Yale Univ Press; London: 1989. [Google Scholar]
- 21.Kohler TA, Varien MD. A Scale Model of Seven Hundred Years of Farming Settlements in Southwestern Colorado. In: Bandy MS, Fox JR, editors. Becoming Villagers. Univ of Arizona Press; Tucson, AZ: 2010. pp. 37–61. [Google Scholar]
- 22.Richards MP. A brief review of the archaeological evidence for Palaeolithic and Neolithic subsistence. Eur J Clin Nutr. 2002;56(12):1270–1278. doi: 10.1038/sj.ejcn.1601646. [DOI] [PubMed] [Google Scholar]
- 23.Hershkovitz I, et al. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS One. 2008;3(10):e3426. doi: 10.1371/journal.pone.0003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenblatt CL, Spigelman M. In: Emerging Pathogens: Archaeology, Ecology and Evolution of Infectious Disease. Greenblatt CL, Spigelman M, editors. Oxford Univ Press; Oxford, UK: 2003. [Google Scholar]
- 25.Rasmussen S, et al. Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell. 2015;163(3):571–582. doi: 10.1016/j.cell.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathieson I, et al. 2015. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528(7583):499–503.
- 27.Pechenkina EA, Benfer RA, Jr, Zhijun W. Diet and health changes at the end of the Chinese Neolithic: The Yangshao/Longshan transition in Shaanxi province. Am J Phys Anthropol. 2002;117(1):15–36. doi: 10.1002/ajpa.10014. [DOI] [PubMed] [Google Scholar]
- 28.Danford ME, Jacobi KP, Wrobel GD, Glassman S. Health and the transition to horticulture in the south-central United States. In: Cohen MN, Crane-Kramer GMM, editors. Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification. University Press of Florida; Gainesville, FL: 2007. pp. 65–79. [Google Scholar]
- 29.Barrett R, Kuzawa CW, McDade T, Armelagos GJ. Emerging and re-emerging infectious diseases: The third epidemiologic transition. Annu Rev Anthropol. 1998;27(1):247–271. [Google Scholar]
- 30.Cockburn TA. Infectious diseases in ancient populations. In: Landy D, editor. Culture, Disease and Healing. Studies in Medical Anthropology. Macmillan Publishing; New York: 1977. pp. 83–96. [Google Scholar]
- 31.Froment A. Human biology and health of African rainforest inhabitants. In: Hewlett BS, editor. Hunter-Gatherers of the Congo Basin: Cultures, Histories and Biology of African Pygmies. Transaction Publishers; London: 2014. pp. 117–164. [Google Scholar]
- 32.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: Coevolutionary legacies? Immunology. 2009;126(1):18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leles D, et al. A parasitological paradox: Why is ascarid infection so rare in the prehistoric Americas? J Archaeol Sci. 2010;37(7):1510–1520. [Google Scholar]
- 34.Bethony J, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 35.Dounias E, Froment A. When forest-based hunter-gatherers become sedentary: Consequences for diet and health. Unasylva. 2006;224(57):26–33. [Google Scholar]
- 36.Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P. 2005. Hookworm: “The great infection of mankind.” PLoS Med 2(3):e67. [DOI] [PMC free article] [PubMed]
- 37.Hurtado M, Frey MA, Hurtado I, Hill KR, Baker J. The role of helminthes in human evolution. In: Elton S, O’Higgins P, editors. Medicine and Evolution: Current Applications, Future Prospects. CRC; London: 2008. pp. 153–180. [Google Scholar]
- 38.Van Blerkom LM. Role of viruses in human evolution. Am J Phys Anthropol. 2003;122(Suppl 37):14–46. doi: 10.1002/ajpa.10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rook GAW. Review series on helminths, immune modulation and the hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunology. 2009;126(1):3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stearns SC. Evolutionary medicine: Its scope, interest and potential. Proc R Soc B Biol Sci. 2012;279(1746):4305–4321. doi: 10.1098/rspb.2012.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hugot JP, Reinhard KJ, Gardner SL, Morand S. Human enterobiasis in evolution: Origin, specificity and transmission. Parasite. 1999;6(3):201–208. doi: 10.1051/parasite/1999063201. [DOI] [PubMed] [Google Scholar]
- 42.Reinhard K, Ambler JR, Mcguffie M. Diet and parasitism at Dust Devil cave. Am Antiq. 1985;50(4):819–824. [Google Scholar]
- 43.Reinhard KJ, Araujo A. Synthesining parasitology with archaeology in paleopathology. In: Buikstra JE, Roberts C, editors. The Global History of Paleapathology. Oxford Univ Press; Oxford, UK: 2012. pp. 751–764. [Google Scholar]
- 44.Reinhard KJ. Cultural ecology of prehistoric parasitism on the Colorado Plateau as evidenced by coprology. Am J Phys Anthropol. 1988;77(3):355–366. doi: 10.1002/ajpa.1330770308. [DOI] [PubMed] [Google Scholar]
- 45.Santoro C, Vinton SD, Reinhard KJ. Inca expansion and parasitism in the lluta valley: Preliminary data. Mem Inst Oswaldo Cruz. 2003;98(Suppl 1):161–163. doi: 10.1590/s0074-02762003000900024. [DOI] [PubMed] [Google Scholar]
- 46.Cohen MN. Rethinking the origins of agriculture. Introduction. Curr Anthropol. 2009;50(5):591–595. doi: 10.1086/603548. [DOI] [PubMed] [Google Scholar]
- 47.Hutchinson DL, Norr L, Teaford MF. Outer Coast foragers and Inner Coast farmers in Late Prehistoric North Carolina. In: Cohen MN, Crane-Kramer GMM, editors. Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification. University Press of Florida; Gainesville, FL: 2007. pp. 52–64. [Google Scholar]
- 48.Pinhasi R, Stock JT, editors. 2011. Introduction: Changing paradigms in our understanding of the transition to agriculture. Human Bioarchaeology of the Transition to Agriculture (Wiley, Oxford, UK), pp 1–16.
- 49.Douglas MT. Subsistence change and dental health in the people of Non Nok Tha, northeast Thailand. In: Oxenham MF, Tayles N, editors. Bioarchaeology of Southeast Asia. Cambridge Univ Press; Cambridge, UK: 2006. pp. 191–219. [Google Scholar]
- 50.Humphrey LT, et al. Earliest evidence for caries and exploitation of starchy plant foods in Pleistocene hunter-gatherers from Morocco. Proc Natl Acad Sci USA. 2014;111(3):954–959. doi: 10.1073/pnas.1318176111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukacs JR. Dental paleopathology and agricultural intensification in South Asia: New evidence from Bronze Age Harappa. Am J Phys Anthropol. 1992;87(2):133–150. doi: 10.1002/ajpa.1330870202. [DOI] [PubMed] [Google Scholar]
- 52.Oxenham MF, Tayles N. Synthesising Southeast Asian population history and palaeohealth. In: Oxenham MF, Tayles N, editors. Bioarchaeology of Southeast Asia. Cambridge Univ Press; Cambridge, UK: 2006. pp. 335–349. [Google Scholar]
- 53.Oxenham MF, Nguyen LC, Nguyen KD. Oral health in northern Vietnam. Bull Indo-Pacific Prehist Assoc. 2008;22(6):121–134. [Google Scholar]
- 54.Pietrusewsky M, Ikehara-Quebral R. The bioarchaeology of the Vat Kommou cemetry, Angkor Borei, Cambodia. Bull Indo-Pacific Prehist Assoc. 2007;26:86–97. [Google Scholar]
- 55.Tayles N, Domett KM, Halcrow S. Can dental caries be interpreted as evidence of farming? The Asian experience. In: Koppe T, Meyer G, Alt KW, editors. Comparative Dental Morpohology. Karger; Basel: 2009. pp. 162–166. [DOI] [PubMed] [Google Scholar]
- 56.Walker P, Hewlett B. Dental health diet and social status among Central African foragers and farmers. Am Anthropol. 1990;92(2):383–398. [Google Scholar]
- 57.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418(6898):700–707. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- 58.Lambert PM. Health versus fitness: Competing themes in the origins and spread of agriculture? Curr Anthropol. 2009;50(5):603–608. doi: 10.1086/605354. [DOI] [PubMed] [Google Scholar]
- 59. Borgerhoff Mulder M (2000) Optimizing offspring: The quantity-quality tradeoff in agropastoral Kipsigis. Evol Hum Behav 21(6):391–410. [DOI] [PubMed]
- 60.Draper P, Hames R. Birth order, sibling investment, and fertility among Ju/'Hoansi (!Kung) Hum Nat. 2000;11(2):117–156. doi: 10.1007/s12110-000-1016-0. [DOI] [PubMed] [Google Scholar]
- 61.Gillespie DO, Russell AF, Lummaa V. When fecundity does not equal fitness: Evidence of an offspring quantity versus quality trade-off in pre-industrial humans. Proc R Soc B Biol Sci. 2008;275(1635):713–722. doi: 10.1098/rspb.2007.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagen EH, Barrett HC, Price ME. Do human parents face a quantity-quality tradeoff?: Evidence from a Shuar community. Am J Phys Anthropol. 2006;130(3):405–418. doi: 10.1002/ajpa.20272. [DOI] [PubMed] [Google Scholar]
- 63.Hill K, Hurtado AM. Aché Life History: The Ecology and Demography of a Foraging People. Aldine de Gruyter; New Haven, CT: 1996. [Google Scholar]
- 64.Lawson DW, Alvergne A, Gibson M. The life-history trade-off between fertility and child survival. Proc R Soc B Biol Sci. 2012;279(1748):4755–4764. doi: 10.1098/rspb.2012.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minter T. 2010. The Agta of the Northern Sierra Madre. Dissertation (Leiden University, Leiden, The Netherlands)
- 66.Rai NK. Living in a Lean-To: Philippine Negrito Foragers in Transition. Univ of Michigan Press; Ann Arbor, MI: 1990. [Google Scholar]
- 67.Peterson JT. The Ecology of Social Boundaries: Agta Foragers of the Philippines. Univ of Illinois Press; Champaign, IL: 1978. [Google Scholar]
- 68.Kelly RL. The Lifeways of Hunter-Gatherers: The Foraging Spectrum. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 69.Early JD, Headland TN. Population Dynamics of a Philippine Rain Forest People: The San Ildefonso Agta. University Press of Florida; Gainesville, FL: 1998. [Google Scholar]
- 70.Norrby E, Kövamees J, Blixenkrone-Möller M, Sharma B, Orvell C. Humanized animal viruses with special reference to the primate adaptation of morbillivirus. Vet Microbiol. 1992;33(1-4):275–286. doi: 10.1016/0378-1135(92)90055-x. [DOI] [PubMed] [Google Scholar]
- 71.McDade TW, et al. Predictors of C-reactive protein in Tsimane’ 2 to 15 year-olds in lowland Bolivia. Am J Phys Anthropol. 2005;128(4):906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- 72.Hurrell RF. Influence of vegetable protein sources on trace element and mineral bioavailability. J Nutr. 2003;133(9):2973S–2977S. doi: 10.1093/jn/133.9.2973S. [DOI] [PubMed] [Google Scholar]
- 73.Mcdonell A, Oxenham MF. Localised primary canine hypoplasia: Implications for maternal and infant health at Man Bac, Vietnam, 4000-3500 years BP. Int J Osteoarchaeol. 2014;24(4):531–539. [Google Scholar]
- 74. WHO (2012) WHO Children reducing mortality. WHO Media Cent:1. Available at: www.who.int/mediacentre/factsheets/fs178/en/. Accessed November 13, 2015.
- 75.Mascie-Taylor CGN. The biological anthropology of disease. In: Mascie-Taylor CGN, editor. The Anthropology of Disease. Oxford Univ Press; Oxford, UK: 1993. pp. 1–72. [Google Scholar]
- 76.Cooper PJ. Intestinal worms and human allergy. Parasite Immunol. 2004;26(11-12):455–467. doi: 10.1111/j.0141-9838.2004.00728.x. [DOI] [PubMed] [Google Scholar]
- 77.Tanner S, et al. Influence of helminth infections on childhood nutritional status in lowland Bolivia. Am J Hum Biol. 2009;21(5):651–656. doi: 10.1002/ajhb.20944. [DOI] [PubMed] [Google Scholar]
- 78.London D, Hruschka D. Helminths and human ancestral immune ecology: What is the evidence for high helminth loads among foragers? Am J Hum Biol. 2014;26(2):124–129. doi: 10.1002/ajhb.22503. [DOI] [PubMed] [Google Scholar]
- 79.Jelliffe DB, Woodburn J, Bennett FJ, Jelliffe EF. The children of the Hadza hunters. J Pediatr. 1962;60:907–913. doi: 10.1016/s0022-3476(62)80176-0. [DOI] [PubMed] [Google Scholar]
- 80.Confalonieri U, Ferreira LF, Araújo A. Intestinal helminths in lowland South American Indians: Some evolutionary interpretations. Hum Biol. 1991;63(6):863–873. [PubMed] [Google Scholar]
- 81.Gonçalves MLC, Araújo A, Ferreira LF. Human intestinal parasites in the past: New findings and a review. Mem Inst Oswaldo Cruz. 2003;98(Suppl 1):103–118. doi: 10.1590/s0074-02762003000900016. [DOI] [PubMed] [Google Scholar]
- 82.Sprent JFA. Helminth “zoonoses”: An analysis. Helminthol Abstr. 1969;38:333–351. [Google Scholar]
- 83.Armelagos GJ, Harper KN. Genomics at the origins of agriculture. Evol Anthropol. 2005;14(3):109–121. [Google Scholar]
- 84.Sreevatsan S, et al. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94(18):9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bentley GR, Jasienska G, Goldberg T. Is the fertility of agriculturalists higher than that of nonagriculturalists? Curr Anthropol. 1993;34(5):778–785. [Google Scholar]
- 86.Sellen D, Mace R. Fertility and mode of subsistence: A phylogenetic analysis. Curr Anthropol. 1997;3(5):878–889. [Google Scholar]
- 87.Blurton Jones N. Bushman birth spacing: Direct tests of some simple predictions. Ethol Sociobiol. 1987;8(3):183–203. [Google Scholar]
- 88.Hardy K, Brand-Miller J, Brown KD, Thomas MG, Copeland L. The importance of dietary carbohydrate in human evolution. Q Rev Biol. 2015;90(3):251–268. doi: 10.1086/682587. [DOI] [PubMed] [Google Scholar]
- 89.Tracer DP. Fertility-related changes in maternal body composition among the Au of Papua New Guinea. Am J Phys Anthropol. 1991;85(4):393–405. doi: 10.1002/ajpa.1330850404. [DOI] [PubMed] [Google Scholar]
- 90.Kramer KL, Boone JL. Why intensive agriculturalists have higher fertility: A household energy budget approach. Curr Anthropol. 2002;43(3):511–517. [Google Scholar]
- 91.Dyble M, et al. Human behavior. Sex equality can explain the unique social structure of hunter-gatherer bands. Science. 2015;348(6236):796–798. doi: 10.1126/science.aaa5139. [DOI] [PubMed] [Google Scholar]
- 92.Gibson M, Mace R. Helpful grandmothers in rural Ethiopia: A study of the effect of kin on child survival and growth. Evol Hum Behav. 2005;26(6):469–482. [Google Scholar]
- 93.R Core Team 2015. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria). Available at https://www.R-project.org/
- 94.Bates D, Bolker B, Maechler M, Walker S. 2013. lme4 R Package.
- 95.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1(1):3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.