Significance
Asthmatics depend on β-agonist bronchodilator drugs, but a majority develop resistance to these drugs in their lifetime, and new ways to bronchodilate are needed. We show that brochodilation can be triggered in normal human and asthmatic mouse airways through an alternative signaling pathway, using new pharmacologic agents that directly activate the soluble guanylate cyclase (sGC) enzyme. Because an sGC-based drug was recently approved to treat pulmonary arterial hypertension, our findings imply that such drugs could become new bronchodilators in asthma. Our work also provides insight on how the sGC signaling enzyme becomes desensitized toward NO in inflammatory asthma, and thus helps to explain why NO is an ineffective bronchodilator in this disease.
Keywords: bronchodilation, bronchoconstriction, S-nitrosylation, nitric oxide, heme protein
Abstract
Asthma is defined by airway inflammation and hyperresponsiveness, and contributes to morbidity and mortality worldwide. Although bronchodilation is a cornerstone of treatment, current bronchodilators become ineffective with worsening asthma severity. We investigated an alternative pathway that involves activating the airway smooth muscle enzyme, soluble guanylate cyclase (sGC). Activating sGC by its natural stimulant nitric oxide (NO), or by pharmacologic sGC agonists BAY 41–2272 and BAY 60–2770, triggered bronchodilation in normal human lung slices and in mouse airways. Both BAY 41–2272 and BAY 60–2770 reversed airway hyperresponsiveness in mice with allergic asthma and restored normal lung function. The sGC from mouse asthmatic lungs displayed three hallmarks of oxidative damage that render it NO-insensitive, and identical changes to sGC occurred in human lung slices or in human airway smooth muscle cells when given chronic NO exposure to mimic the high NO in asthmatic lung. Our findings show how allergic inflammation in asthma may impede NO-based bronchodilation, and reveal that pharmacologic sGC agonists can achieve bronchodilation despite this loss.
Asthma is an inflammatory disease that causes airway hyperreactivity (AHR) and bronchoconstriction, which impedes daily life activities and, when severe, can cause death. It is the most common chronic disease of childhood, accounts for one in three emergency department visits daily, and asthma diagnoses are increasing worldwide (1). The leading treatment for relief and acute care is bronchodilation, which relies heavily on the β-adrenergic receptor-cAMP pathway. Nearly 70% of patients, however, develop resistance or tachyphylaxis to the existing β-agonist therapy (2), underscoring a need for new bronchodilators that can act through a different pharmacologic principle.
The nitric oxide-soluble guanylate cyclase-cGMP pathway (NO-sGC-cGMP) is the primary signal transduction pathway for relaxing vascular smooth muscle (3). In contrast, a role for the NO-sGC-cGMP pathway in relaxing airway smooth muscle is less clear (4, 5), and bronchodilation was instead suggested to depend on glutathione nitrosothiol levels in the lung (6, 7). However, recent studies have shown that inflammation can desensitize sGC toward its natural activator, NO (8), and new drugs have become available that directly activate sGC, independent of NO (9). These developments encouraged us to re-examine the NO-sGC-cGMP pathway regarding its role in bronchodilation, its becoming damaged in inflammatory asthma, and its potential for alternative bronchodilator development under this circumstance.
Results
The NO-sGC-cGMP Pathway Bronchodilates Human Lung.
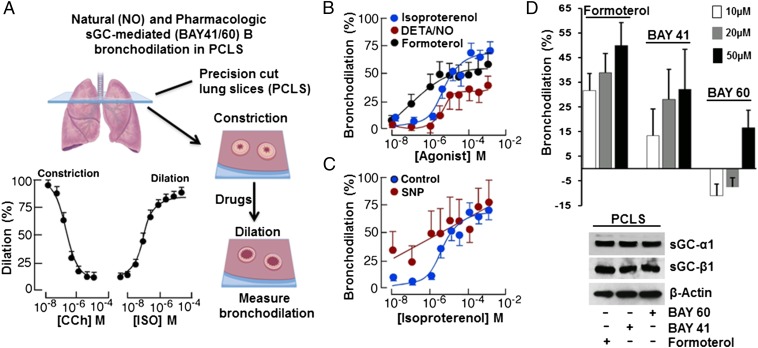
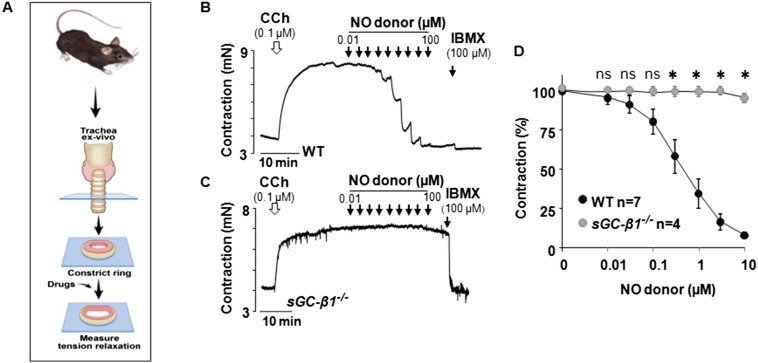
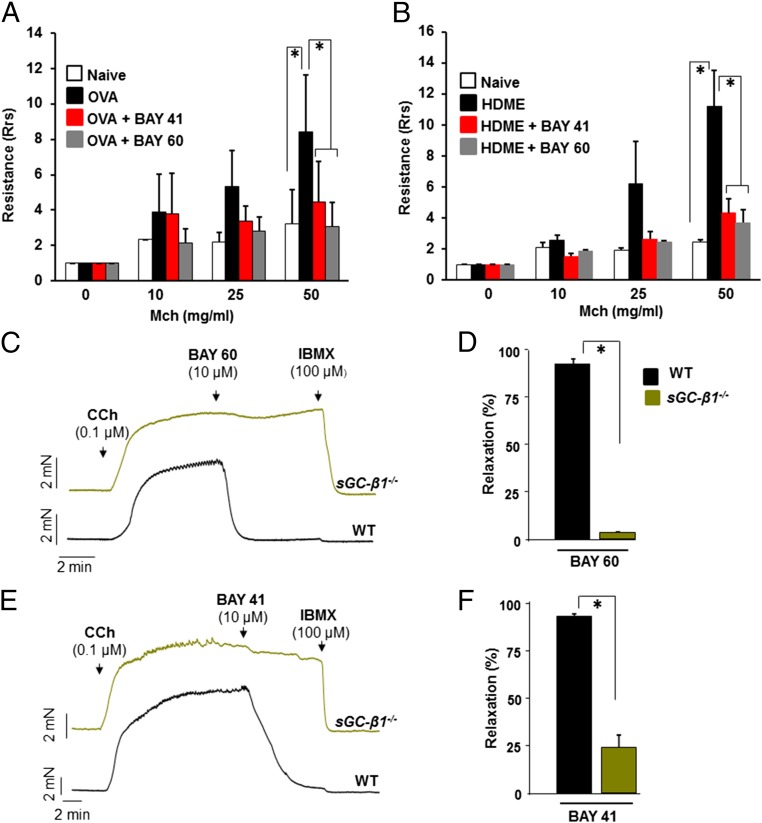
We first tested if stimulating the NO-sGC-cGMP pathway would dilate preconstricted small airways in human precision-cut lung slices (PCLS) obtained from healthy donor lungs (Fig. 1A and Table S1). Graded doses of the slow-release NO donor DETA/NO [3,3-Bis(aminoethyl)-1-hydroxy-2-oxo-1-triazene] produced bronchodilation in human PCLS similar to what was induced by a standard β-agonist bronchodilator, Formoterol (Fig. 1B). In addition, having a NO donor (sodium nitroprusside, SNP) present at a subeffective concentration made the preconstricted human small airways more responsive to lower doses of the β-agonist bronchodilator Isoproterenol (Fig. 1C). This finding shows that triggering the NO-sGC-cGMP pathway bronchodilated the preconstricted human lung, and could also synergize with β-adrenergic-based bronchodilators in doing so. We confirmed a similar role for the NO-sGC-cGMP pathway in bronchodilating the mouse airway, using tracheal rings that we isolated from normal or sGC-β1−/− mice (Fig. S1).
Fig. 1.
NO donors and sGC stimulators and activators bronchodilate human lung slices. (A) Small airways in human PCLS were contracted with CCh followed by addition of the indicated compounds and image collection and processing to determine bronchiole lumen area, expressed as percent compared with baseline. (B) NO donor (DETA/NO) caused a dose-dependent bronchodilation in a manner similar to the β-agonist Formoterol. (C) Coadministering a subthreshold dose of NO donor (SNP, 1 µM) enhanced Isoproterenol (ISO) dilation of PCLS. (D) Differing capacity of the BAY sGC activators to dilate PCLS relative to Formoterol, with the final sGC-α1 and β1 expression levels in the slices compared below. For A, n = 3; for B–D, n = 2; mean ± SD; four to seven slices per condition.
Table S1.
Characteristics of population used for the study
| Control/patient | Pathology | Age/race/gender | BMI | Drugs taken/cause of death | Experimental use |
| 1. | Normal | 11/C/M | 39.86 | Unknown/head trauma | Bronchodilation on lung slices (Fig. 1B) |
| 2. | Normal | 22/C/F | 32.56 | Unknown/anoxia | Bronchodilation on lung slices (Fig. 1C) |
| 3. | Normal | 49/C/F | 29.76 | Estrogen, Tylenol and migrane drugs/CVA | Bronchodilation on lung slices and WB (Fig. 1D) |
| 4. | Normal | 33/AA/F | 27.49 | Amlodipine, Doxazosin, Lisinopril/CVA | Bronchodilation on lung slices and WB (Fig. 1D) |
| 5. | Normal | 49/AA/M | 53.42 | Clonidine, Lisinopril/CVA | SNO, IPs, and WB (Fig. 3 F–H) |
| 6. | Normal | 49/AA/M | 30.07 | Diovan/CVA | SNO, IPs, and WB (Fig. 3 F–H) |
| 7. | Normal | 22/C/M | 23.6 | Unknown/head injury | WB on lung tissue sample (Fig. S4A, Control 1) |
| 8. | Normal | 21/H/M | 21.5 | Unknown/CVA | WB on lung tissue sample (Fig. S4A, Control 2) |
| 9. | Asthma | 13/AA/M | 15.6 | Flovent, Advair and Albuterol/anoxia | WB on lung tissue sample (Fig. S4A, Asthma 1′) |
| 10. | Asthma | 48/C/F | 26.7 | Advair, Albuterol and Synthroid/anoxia | WB on lung tissue sample (Fig. S4A, Asthma 2′) |
| 11. | Normal | 52/C/F | 28.82 | Nexium, Topomax/AVM | WB on HASMCs (Fig. S4B, Control 1) |
| 12. | Normal | 22/AA/F | 22.77 | Unknown/head trauma | SNO, IPs, and WB on HASMCs (Fig. 4 E and F, Fig. S4B, Control 2, and Fig. S6) |
| 13. | Normal | 31/AA/F | 27.25 | Unknown/aneurysm | WB on HASMCs (Fig. S4B, Control 3) |
| 14. | Normal | 11/C/F | 39.86 | Unknown/head trauma | WB on HASMCs (Fig. S4B, Control 4) |
| 15. | Asthma | 19/C/F | 26.11 | Albuterol/anoxia | WB on HASMCs (Fig. S4B, Asthma 1′) |
| 16. | Asthma | 22/AA/M | 36.29 | Albuterol, Advair/anoxia | WB on HASMCs (Fig. S4B. Asthma 2′) |
| 17. | Asthma | 9/C/M | 11.25 | Albuterol/asthma | WB on HASMCs (Fig. S4B, Asthma 3′) |
| 18. | Asthma | 26/C/F | Unknown | Unknown/anoxia | WB on HASMCs (Fig. S4B, Asthma 4′) |
Abbreviations: AA, African American; AVM, arteriovenous malformation; BMI, body mass index; C, Caucasian; CVA, cerebrovascular accident; F, female; H, Hispanic; HASMCs, Human airway smooth muscle cells; IPs, immunoprecipitations; M, male; SNO, S-nitrosylation; WB, Western blots.
Fig. S1.
Mouse trachea response to NO requires sGC. (A) Tracheal rings prepared from normal (WT) or sGC-β1−/− mice were precontracted with CCh and then their relaxation in response to compounds was measured. (B and C) Representative traces indicating the relaxation achieved to the indicated concentrations of an NO donor (DEA-NO) and subsequently to the NO-independent relaxing agent IBMX (100 µM) to achieve maximal dilation. (D) Averaged data derived from replicate experiments with wild-type n = 7 rings and sGC-β1−/− n = 4 (*P < 0.05, by one-way ANOVA; ns, not statistically significant).
Direct-Acting Pharmacologic sGC Agonists Bronchodilate Human Lung.
The pharmacologic agent Riociguat is an NO-independent sGC stimulator (10) that was recently approved to treat patients with different forms of pulmonary hypertension. Riociguat, and it’s structural analog BAY 41–2272 (BAY 41), only stimulate the NO-responsive, heme-containing form of sGC (9), whereas Cinaciguat or its structural analog BAY 60–2770 (BAY 60) only stimulate the oxidatively damaged, heme-free and NO-insensitive forms of sGC (11), which may accumulate in cells and tissues under inflammatory oxidative stress (10, 12). We found that BAY 41–2272, and to a lesser extent BAY 60–2770, bronchodilated the preconstricted human PCLS obtained from healthy donors (Fig. 1D). Thus, pharmacologic agents that act directly on sGC can bronchodilate constricted airways in human lung.
sGC Agonist Drugs Eliminate Airway Hyperresponsiveness in Mouse Models of Allergic Asthma.
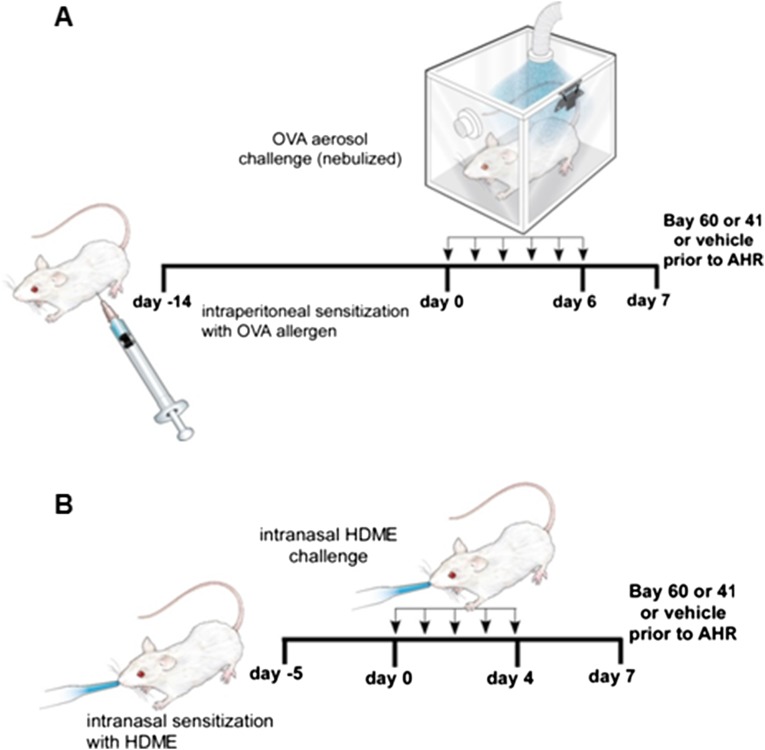
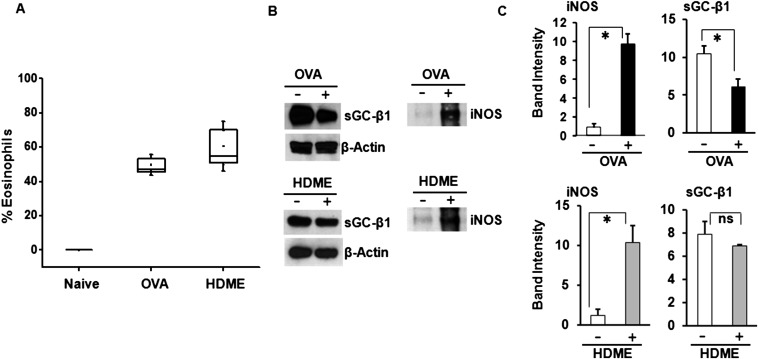
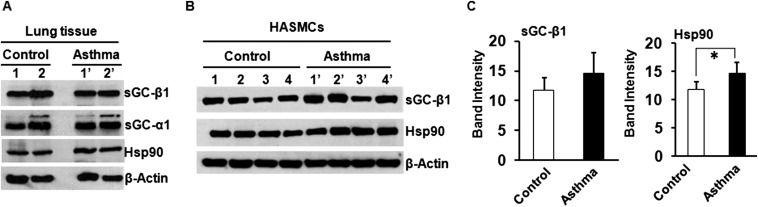
We next tested if the sGC stimulator BAY 41–2272 and sGC activator BAY 60–2770 would act as bronchodilators in vivo in two different mouse models of asthma, one in which the mice are sensitized and challenged to ovalbumin protein (OVA), and another in which mice are exposed to house dust mite allergen extract (HDME) (Fig. S2). OVA is a standard experimental model of mouse allergic airway inflammation, whereas HDME more closely mimics human sensitization via airway epithelial cells, and dust mites are a common worldwide asthmatic allergen (13). The allergen sensitizations and subsequent 7-d exposure caused an expected asthma-like inflammation to develop in the mouse lungs for both models, as judged by an increase in eosinophil numbers and an increased expression of the NO-generating enzyme, inducible NO synthase (iNOS) (Fig. S3). The inflammation only partly diminished (OVA) or did not alter (HDME) the sGC protein expression levels in mouse lungs (Fig. S3), which correlates with our finding a near normal sGC expression level in lung tissues and cell samples that we recovered from human asthmatics (Fig. S4).
Fig. S2.
Mice models of allergic asthma. (A and B) Mice were treated to develop an inflammatory asthma toward either OVA or HDME and then received a single intratracheal administration of vehicle or BAY drug (50 µL; 30 µg/kg BAY 41–2272 or 90 µg/kg BAY 60–2770) at 30 min before testing airway resistance.
Fig. S3.
Changes in mouse lung associated with the allergic inflammatory asthma. (A) Percentage of eosinophils in bronchial lavage fluid from naïve mice and from mice with allergic asthma toward OVA or HDME (n = 3). (B) Comparative sGC-β1 and iNOS protein expression levels in lung supernatants and insoluble particulate fractions, respectively, from naïve and asthmatic mice. (C) Densitometric quantification of the iNOS and sGCβ1 protein expression. n = 3 each for naïve or allergic asthmatic mice (OVA/HDME) for iNOS and n = 4 for naive or OVA/HDME mice for sGC-β1 (*P < 0.05, by one-way ANOVA; ns, not statistically significant).
Fig. S4.
Expression of sGC and hsp90 in human lung tissue and in HASMCs derived from control or asthmatic lungs. (A) Expression of sGC-α1, β1, and hsp90 in control and asthmatic lung tissue. (B) Expression of sGC-β1 and hsp90 in control and asthmatic HASMCs. (C) Densitometric quantification of the sGC-β1 and hsp90 expression as depicted in B. Values are mean ± SD, and n = 4 each for control or asthmatic HASMCs (*P < 0.05, by one-way ANOVA).
The OVA and HDME mice displayed a typical hyperresponsiveness in their airway resistance toward the administered airway constrictor methacholine (Mch) (Fig. 2 A and B), as is also seen in human asthmatics during testing of their airway function. Remarkably, administering a single dose of BAY 41–2272 or BAY 60–2770 into the trachea of the asthmatic mice, 30 min before testing, greatly reduced or eliminated the hyperresponsiveness in both mouse models of asthma (Fig. 2 A and B); and here BAY 60–2770 was equally or somewhat more effective than BAY 41–2272 in both asthma models. Experiments using tracheal rings of sGC-β1−/− mice confirmed that both drugs bronchodilated through sGC and not through off-target effects (Fig. 2 C–F). Because the ability of BAY 41–2272 and BAY 60–2770 to resolve AHR in these murine models of asthma is similar to the therapeutic effect of β-agonist drugs, it implies that sGC-targeted drugs could be effective for inducing bronchodilation in asthmatics.
Fig. 2.
sGC agonists abolish airway hyper-response in two models of allergic asthma. Mice were treated to develop an inflammatory asthma toward either OVA or HDME and then received an intratracheal administration of vehicle or BAY drug (50 µL; 30 µg/kg BAY 41–2272 or 90 µg/kg BAY 60–2770) at 30 min before testing airway resistance. (A and B) Airway resistance recorded for groups of naïve and asthmatic mice in response to methacholine bronchoconstrictor (Mch), showing the hypersensitive response of asthmatic mice was alleviated by either BAY compound. n = 6 for OVA-challenged mice at dosage 0 and 50 of Mch and n = 3 at other doses and for control mice. For HDME model, n = 4 for treated or control mice. Values are mean ±SD and are normalized with respect to their 0 Mch (*P < 0.05, by one-way ANOVA). (C and D) Test of sGC agonist specificity. Representative traces of mechanical tension versus time for tracheal rings from normal (WT) or sGC-β1−/− mice that were precontracted with CCh, then given BAY 60–2770 or BAY 41–2272, with IBMX. (E and F) Averaged data derived from replicate experiments with n = 5 rings for WT or sGC-β1−/− using BAY 60–2770 and n = 6 with BAY 41–2272 (*P < 0.05, by Mann–Whitney test two-tailed).
Biomarkers of sGC Damage Manifest in the Mouse Asthmatic Lung and Correlate with an Altered sGC Drug-Response Profile.
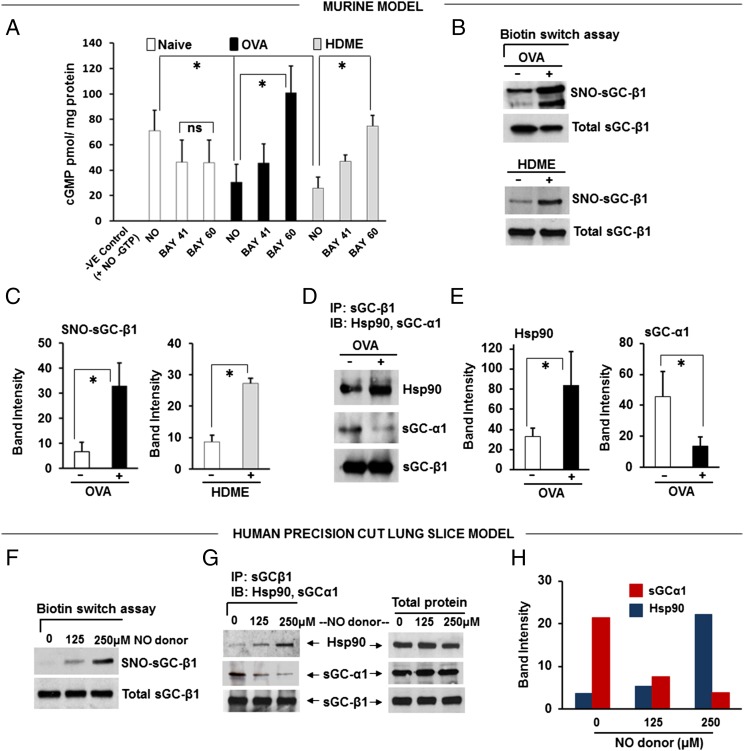
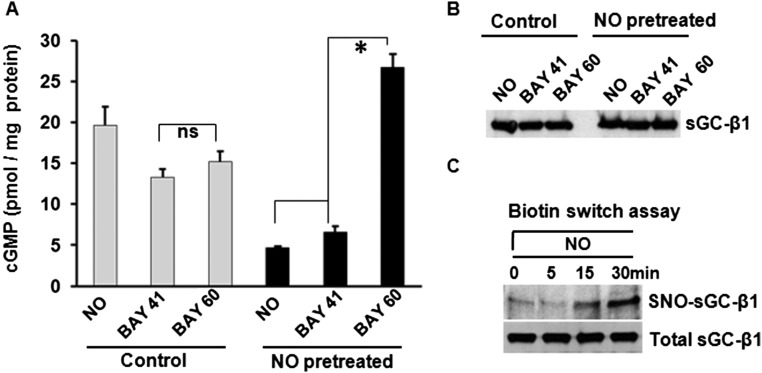
To explore this mechanism, we analyzed lung tissues that were harvested from the naïve and OVA or HDME asthmatic mice. The lung sGC from both the OVA and HDME mice had a decreased catalytic response (cGMP production) toward NO and BAY 41–2272 and an increased catalytic response toward BAY 60–2770, relative to lung sGC from naïve mice (Fig. 3A). This response pattern matches what we saw in the live asthmatic mice regarding their nearly equivalent airway responses toward BAY 41–2272 and BAY 60–2770 (Fig. 2), and implies that the allergic inflammation caused a significant portion of the lung sGC to accumulate in an oxidatively damaged and NO-unresponsive—but BAY 60–2270-responsive—form. Indeed, the sGC-β1 from the asthmatic lungs exhibited three biochemical hallmarks of being damaged and NO-unresponsive (14, 15): an increased level of cysteine S-nitrosylation (Fig. 3 B and C), a diminished association with its partner sGC-α1 subunit, and an enhanced association with the cellular chaperone hsp90 (Fig. 3 D and E).
Fig. 3.
Asthmatic mouse lungs and NO-treated human PCLS contain altered sGC. (A) Activation profiles of lung supernatant sGC in response to NO donor (SNAP), BAY 41–2272, and BAY 60–2770. cGMP values are mean ± SD, n = 3 experiments. (B) Relative levels of total and SNO-sGC-β1 in lung supernatants from asthmatic and naïve mice. (C) Quantification of the SNO-sGC-β1 levels. (D) Change in sGC-β1 association with sGC-α1 and hsp90 as determined by immunoprecipitation. (E) Quantification of associated sGC-α1 and hsp90. Values are mean ± SD and n = 3 (for C) to 5 (for E) each for naive, OVA, or HDME mice. (*P < 0.05, by one-way ANOVA; ns, not statistically significant). (F) Groups of human PCLS (six to seven slices) were cultured overnight with three concentrations of NO donor (NOC-18) and their SNO-sGC and total sGC levels were determined. (G) Relative sGC-β1 associations with sGC-α1 versus hsp90 as determined by immunoprecipitation. (H) Densitometric quantification of the sGC-α1 or hsp90 associated with sGC-β1 from two independent experiments, as described in G.
Biomarkers of sGC Damage Manifest in Human PCLS Exposed to Chronic NO.
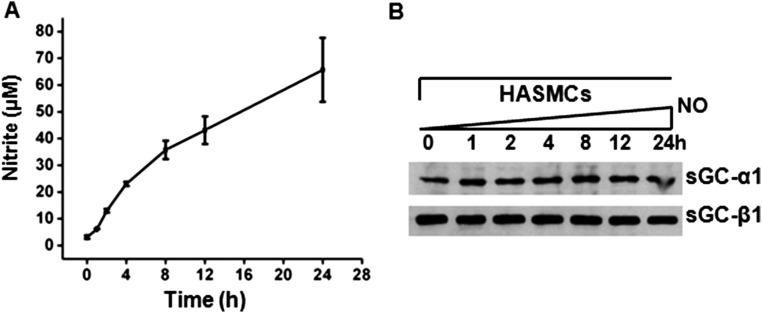
To see if similar changes occur in human lung under nitrosative stress, we exposed human PCLS overnight to constant NO generation by a slow-release NO donor (DETA/NO) to mimic the chronic NO exposure that occurs naturally in asthmatic human lung (16, 17). This treatment did not diminish expression of sGC-β1 (Fig. 3 F and G) but did increase its S-nitrosylation (SNO) level, decrease its sGC-α1 association, and increase its hsp90 association (Fig. 3 F–H). We also found similar sGC protein expression levels in asthmatic and normal human lung tissues, and in primary cultures of human airway smooth muscle cells (HASMC) that were isolated from either asthmatic or healthy human donor lungs (Fig. S4). Thus, neither an asthma-like airway inflammation nor chronic NO exposure significantly diminished sGC protein expression, but both circumstances did cause sGC to show hallmarks of oxidative damage that are associated with its NO-insensitive form. These biochemical transitions of sGC likely explain why BAY 60–2770 became a more effective bronchodilator in the asthmatic mice.
Cell Coculture Experiments Reveal Dynamics and Links Between NO Production, sGC Damage, and sGC Drug Response.
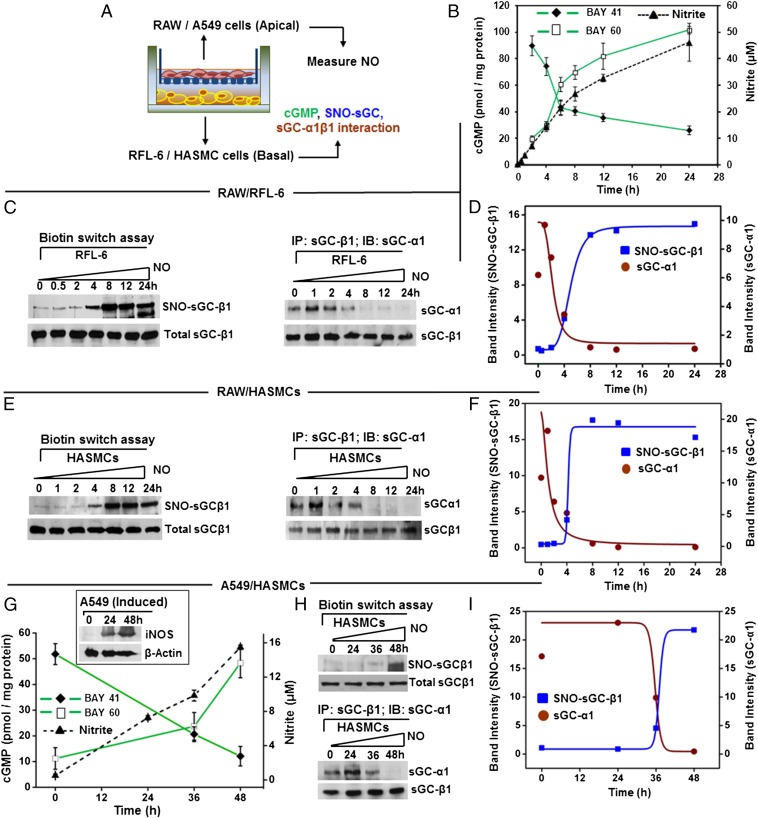
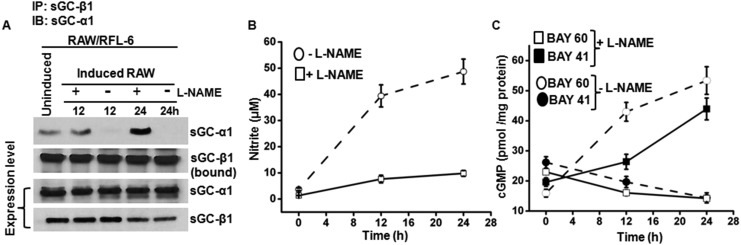
In small airways, a layer of epithelial cells that express iNOS and generate NO (18, 19) overlie a layer of smooth muscle cells that express sGC and are responsible for constricting or relaxing the bronchial passages (4, 5). To probe the mechanism within a similar setting, we performed Transwell coculture experiments with spatially separated NO-generating and sGC-expressing cells, and examined if this would recapitulate the changes in sGC that we observed in the mouse asthmatic lungs or in the NO-exposed human PCLS. We grew two types of NO-generating cells on apical membranes (a mouse macrophage cell line RAW 264.7 or a human bronchial epithelial cell line A549), induced them with cytokines to express iNOS, and then cultured them above monolayers of two types cells that naturally express sGC, a rat fetal lung fibroblast cell line (RFL-6) or primary HASMCs (14, 20) (Fig. 4A). We observed uniform NO generation in the cocultures, as evidenced by nitrite accumulation over time. However, the NO production (nitrite accumulation) supported by RAW cells differed in its timing and amount compared with the A549 cells, with the latter supporting a more gradual and diminished extent of NO production (Fig. 4 B and G and Fig. S5). Nevertheless, the time courses of NO synthesis correlated with (i) a switch in the pharmacologic sGC activation-response profile to ultimately favor BAY 60–2770 and disfavor BAY 41–2272 (Fig. 4 B and G), and (ii) a corresponding accumulation of SNO-sGC-β1, and dissociation of sGC-β1 from its sGC-α1 partner (Fig. 4 C–F, H, and I). Similarly, exposure of RFL-6 cells alone to a slow-release chemical NO donor caused a buildup in SNO-sGC-β1 and a shift in the drug response profile to favor activation by BAY 60–2770 and disfavor activation by BAY 41–2272 or NO (Fig. S6). RFL-6 or HASMC cultured alone did not show any of these sGC changes, nor did RFL-6 cells cultured with unactivated RAW cells or with induced RAW cells in the presence of the NOS inhibitor NOS inhibitor nitro l-arginine methyl ester (l-NAME) (Fig. S7). Thus, both human and murine NO-generating cells eventually caused the rat (RFL-6) and human (HASMC) sGC-expressing cells to accumulate SNO-sGC-β1, dissociate their sGC-α1β1 heterodimer, and shift their sGC activation response profile to favor BAY 60–2770, thereby mimicking the changes in sGC that we had observed in the asthmatic mouse lungs.
Fig. 4.
Immuno-activated inflammatory cells alter sGC in responder cells. (A) Transwell coculture containing combinations of NO-producing cells (RAW/A549) and sGC-containing cells (RFL-6/HASMC) mimics the spatial relationship in the bronchial airways. (B) NO production by IFN-γ/LPS-activated RAW cells in the Transwell cultures, as indicated by nitrite accumulation, and the corresponding change in RFL-6 sGC activity over time in response to BAY 41–2272 or BAY 60–2770. Values are mean ± SD from three experiments. (C) Corresponding SNO-sGC-β1 buildup in the RFL-6 cells during the coculture (Left) and loss in sGC-α1β1 heterodimerization (Right) with time. (D) Quantification of SNO-sGC-β1 levels and sGC-α1 association versus time from two independent experiments. (E) Corresponding SNO-sGC-β1 build-up in HASMC (Left) in response to NO from activated RAW cells and loss in sGC-α1β1 heterodimerization (Right) with time. (F) HASMC SNO-sGC-β1 levels and sGC-α1 association with sGC-β1 versus time for two independent experiments. (G) Kinetics of NO production by cytokine-stimulated human A549 cells as indicated by nitrite accumulation, iNOS expression (Inset), and corresponding change in HASMC sGC responses to BAY 41–2272 or BAY 60–2770. Values are mean ± SD from three experiments. (H) Corresponding SNO-sGC-β1 buildup in the HASMC (Upper) and loss in sGC-α1β1 heterodimerization (Lower) with time. (I) Comparative SNO-sGC-β1 levels and sGC-α1 association versus time from two independent experiments.
Fig. S5.
NO produced from activated RAW cells does not alter expression of sGC in HASMCs. A Transwell coculture system as described in Fig. 4 with activated RAW cells in the apical and HASMCs in the basal chamber was used to study effects of NO produced from RAW cells on smooth muscle sGC. (A) NO production by IFN-γ/LPS-activated RAW cells in the Transwell cultures, as indicated by nitrite accumulation in the media. Values are mean ± SD of three independent experiments. (B) Representative Western blots depicting expression level of sGC-α1 and β1 in the airway smooth muscle cells under indicated time points.
Fig. S6.
Chronic NO exposure alters sGC in cells. (A) RFL-6 cells were treated with vehicle or an NO donor (NOC-12) for 12 h, washed, and then their sGC activity was tested by measuring cGMP production in response to activators NO (SNAP), BAY 41–2272, or BAY 60–2770. Values are mean (n = 3) ± SD (*P < 0.05, by one-way ANOVA; ns, not statistically significant). (B) sGC-β1 protein expression level in cells from A. (C) Levels of the total sGC-β1 and SNO-sGC-β1 in RFL-6 cells after treating with NO donor (NOC-12) for the indicated times, as determined by biotin switch and Western analysis.
Fig. S7.
Inflammatory cell-mediated changes in sGCα1β1 are NO-dependent. Transwell cocultures contained cytokine-activated RAW cells (on apical membrane) and RFL-6 cells and were cultured for the indicated times in the absence or presence of the NOS inhibitor l-NAME. (A) Representative immunoprecipitationss depicting the sGCα1β1 interaction and the total sGC protein expression versus time in coculture. (B) NO production by the activated RAW cells in the Transwell cultures ± l-NAME as indicated by the nitrite accumulation. (C) Corresponding changes in RFL-6 activity response profile over time (cGMP production) in response to BAY 41–2272 or BAY 60–2770. Values are mean ± SD from three experiments.
Working Model.
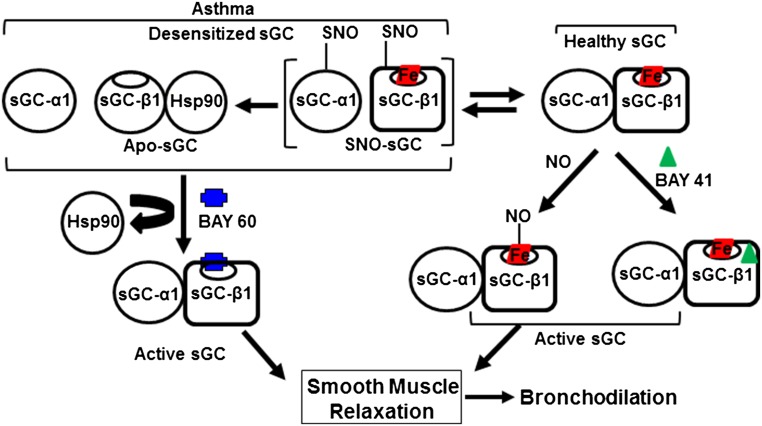
Taken together, our findings support a mechanism whereby the inflamed asthmatic airway and associated excessive NO production damages the sGC in airway smooth muscle, and thereby diminishes the capacity of the NO-sGC-cGMP pathway to participate in bronchodilation or to relieve bronchoconstriction, unless sGC stimulators like BAY 41–2272 or activators like BAY 60–2770 are provided. A model summarizing these concepts is presented in Fig. 5.
Fig. 5.
Model for sGC-based bronchodilation and basis for its alteration in asthma. sGC expressed in healthy airway smooth muscle exists primarily as a heme-containing (red boxed) α1β1 heterodimer that is activated naturally by airway NO or by compounds like BAY 41–2272 to enable bronchodilation. In inflammatory asthma, significant sGC becomes SNO-modified or heme-deficient. Such NO-unresponsive or “desensitized” forms are characterized by sGC-α1β1 heterodimer dissociation and sGC-β1 association with hsp90. Desensitized sGC can still be activated by BAY 60–2770 to trigger airway smooth muscle relaxation. In this way, pharmacologic sGC stimulators and activators can restore normal bronchodilatory function in inflamed asthmatic lung.
Discussion
Our study highlights the potential of targeting the NO-sGC-cGMP pathway to achieve bronchodilation, particularly in the asthmatic inflamed lung, by administering drugs that bypass NO and instead directly act on the sGC. Although the NO-sGC-cGMP pathway is demonstrably able to bronchodilate preconstricted human lung slices (Fig. 1), the utility of using NO to induce bronchodilation in healthy humans may be inherently masked by the continuous NO generation that takes place in normal human airway (19). This endogenous NO is likely to fully activate sGC on its own and thus would mask any potential bronchodilation response to externally provided NO or to NO-releasing compounds (20). In asthmatic lungs, our study suggests that a significant portion of airway sGC actually becomes NO-unresponsive, which would also render administration of NO or NO donors less effective (21, 22). However, the advent of direct-acting sGC agonists like BAY 41–2272 and BAY 60–2770 provide a new chance to assess the NO-sGC-cGMP pathway, and we found that these pharmacologic agents can act as powerful bronchodilators in two established mouse models of allergic asthma. We envision that these findings may spur further development that will ultimately help initiate clinical trials in human asthmatics, to treat the significant patient subpopulation (23) that are inherently resistant toward existing β-agonist bronchodilator drugs or who become resistant during the course of their disease management. Although systemic circulatory effects have sometimes been reported for sGC agonist drugs (24), this issue has already been managed for Riociguat in treating pulmonary hypertension, and it could be minimized in asthma, for example, by creating inhalable formulations of the sGC agonist drugs.
Historically, the hallmark of sGC oxidative damage has been a pharmacologic test; that is, demonstrating the existence of (or a shift toward) a sGC population whose activation is NO-insensitive but responsive toward an sGC activator like BAY 60–2770. Our study revealed that this shift occurs for lung sGC during development of allergic asthma. Thus, allergic asthma joins an expanding group of inflammatory diseases that are associated with compromised sGC function and response toward NO (10). Determining the sGC pharmacologic response profile could likely help in guiding a wide variety of disease treatments and in following patient management outcomes, and for fashioning treatment protocols for individual patients. Beyond this, our study provides information on other changes in sGC that accompany its shift in pharmacologic behavior. Specifically, they are a loss in the mature sGC heterodimer population (i.e., the amount of sGC-β1 associated with its partner sGC-α1 subunit), and a concomitant gain in the population of sGC-β1 that is complexed instead with hsp90. We found this change in sGC-β1 protein association occurred in lung samples from the asthmatic mice, and also in human lung slices or HASMC that had been exposed to either chemical- or cell-derived NO in a manner that mimicked the chronic airway NO exposure that is seen in asthma. Our previous work (15) showed that BAY 60–2770 could convert the inactive, hsp90-associated form of sGC-β1 back into active sGC-α1β1 heterodimers, and our current findings are consistent with this conversion likely being its mode of action for activating the NO-resistant sGC subpopulation that built up in the asthmatic airways. Regarding BAY 41–2272, we saw that it remained effective in causing bronchodilation in our asthmatic mouse models, but its relative efficacy is likely tied to the extent to which a mature sGC subpopulation remains intact in the inflammed lungs, which in turn may be inversely related to the degree of lung inflammation. In any case, our results show that the asthmatic mouse lungs contained sGC subpopulations that were responsive toward either class of sGC agonist (stimulators and activators), and thus continued investigation for their use in bronchodilation is warranted.
Our cell coculture experiments reveal that tight kinetic relationships exist between NO production, the SNO modification of sGC-β1, the shift in sGC-β1 protein partners, and the relative abilities of NO/BAY 41–2272 and BAY 60–2770 to activate sGC catalysis. Although the kinetic relationships suggest the processes could be linked mechanistically, further work will be needed to help decipher their relationships. Regardless, the current study shows how these changes in sGC behavior are tied to the flux of NO production in the system. For example, the changes occur in a much more gradual manner with the slower NO generation by the A549 cells compared with faster NO generation by the RAW cells (Fig. 4). Furthermore, in all cases, there is an initial NO exposure period where the changes associated with sGC damage do not occur. This delay suggests that the RLF-6 cells and HASMC have protective mechanisms that enable them to respond to NO in a manner that does not damage their sGC, but after some time of chronic NO exposure, a point is reached where the protections break down and the hallmarks of sGC damage begin to accumulate. We believe this scenario as demonstrated in our coculture experiments may also take place in the inflamed asthmatic lung, where increased NO and possibly other oxidants ultimately tip the balance and compromise the ability of airway smooth muscle cells to protect their sGC and allow it to remain NO-responsive and able to participate in bronchodilation. However, because this problem can be bypassed by administering direct-acting sGC agonists, they provide a novel therapeutic approach to achieve bronchodilation in asthma, despite the persistent airway inflammation and an attenuation of β-agonist efficacy.
Materials and Methods
Reagents.
All chemicals were purchased from Sigma or Fischer chemicals. NO donors, DETANONOate or DETA/NO, 3-Ethyl-3-(ethylaminoethyl)-1-hydroxy-2-oxo-1-triazene (NOC-12), SNP, S-Nitroso-N-Acetyl-D,l-Penicillamine (SNAP), phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX), and l-NAME were purchased from Sigma. BAY 60–2770 (BAY 60) and BAY 41–2272 (BAY 41) were obtained from Bayer AG. RFL-6, macrophage (RAW 264.7), and human bronchial epithelial (A549) cell lines were purchased from ATCC. HASMCs were obtained from R.A.P. (University of Pennsylvania, Philadelphia). cGMP ELISA kit was obtained from Cell Signaling Technology. For animal experiments, wild-type naïve female 6- to 8-wk-old BALB/c mice were purchased from the Jackson Laboratory.
Antibodies.
Rabbit polyclonal sGC-β1 and sGC-α1 antibodies were obtained from Cayman Chemicals and Abcam, respectively. Rabbit and goat polyclonal hsp90 antibodies were obtained from Cell Signaling Technology and Santa Cruz Biotechnology, whereas mouse-specific iNOS antibody was purchased from Cell Signaling Technology. Mouse monoclonal β-actin antibody was purchased from Sigma.
Generation of PCLS and Bronchoconstriction Assays.
Human PCLS were prepared as previously described (25–28). Briefly, whole human lungs from nonasthma and asthma donors were dissected and inflated using 2% (wt/vol) low melting-point agarose. Once the agarose set, the lobe was sectioned, and cores of 8-mm diameter were made. The cores that contained a small airway by visual inspection were sliced at a thickness of 350 μm (Precisionary Instruments VF300 Vibratome) and collected in wells containing supplemented Ham’s F-12 medium. Suitable airways (≤1-mm diameter) on slices were selected on the basis of the following criteria: presence of a full smooth muscle wall, presence of beating cilia, and unshared muscle walls at airway branch points to eliminate possible counteracting contractile forces. Each slice contained ∼98% parenchyma tissue; hence, all airways situated on a slice had sufficient parenchymal tissue to impart basal tone. Adjacent slices containing contiguous segments of the same airway were paired and served as controls and were incubated at 37 °C in a humidified air-CO2 (95–5%) incubator. Sections were placed in fresh media every 2–3 h during the remainder of day 1 and all of day 2 to remove agarose and endogenous substances released that variably confound the production of inflammatory mediators or alter airway tone. To assess luminal area, lung slices were placed in a 12-well plate in media and held in place using a platinum weight with nylon attachments. The airway was located using a microscope (Nikon Eclipse; model no. TE2000-U; magnification, 40×) connected to a live video feed (Evolution QEi; model no. 32–0074A-130 video recorder). Airway luminal area was measured using Image-Pro Plus software (v6.0; Media Cybernetics) and represented in units of square micrometers (25–28). To study bronchoconstriction and subsequent bronchodilation the PCLS were contracted with carbachol (CCh; 10−8−10−5 M) followed by addition of agonists like NO donors (DETANONOate or SNP), isoproterenol (10−9−10−5 M), or formoterol to induce bronchodilation. In another set of experiments slices were bronchoconstricted with CCh and then bronchodilated with formoterol or BAY compounds (BAY 41–2272 or BAY 60–2770), at indicated concentrations (10, 20, or 50 μM). Formoterol dose was given every 5 min; BAY compounds were given after every 30 min. Images were collected 10 min after the addition of test compound and the area of each airway at baseline and at the end of the time course or dose of agonist was calculated using Image-Pro Plus software. The human lung tissue samples and HASMCs obtained from R.A.P.’s laboratory are commercially obtained from anonymous donors and are therefore exempt from requiring Institutional Review Board approval. Although these samples have demographic information about the donors, there are no unique identifiers that will link the subject’s identification to the tissue sample.
Mouse Models of Allergic Asthma.
Allergic airway disease was performed as described in details elsewhere (29, 30). Briefly, animals were sensitized by intraperitoneal injection with OVA (Sigma Chemicals) [10 µg, adsorbed in Al(OH)3] and after 2 wk challenged with aerosolized OVA [1% (wt/vol) in sterile PBS] for 6 d. Lung mechanics were measured 24 h after the last OVA exposure using the FlexiVent ventilator (FlexiVent, Scireq); where indicated animals were treated intratracheally with 90 µg/kg BAY 60 (in 0.09% DMSO, 99.9% 0.2% citric acid buffer) or 30 µg/kg of BAY 41 (in 0.1% DMSO, 1.7% ethanol, 98.2% 0.2% citric acid) or vehicle alone before AHR measurements. AHR and lung mechanics in response to increasing doses of inhaled methacholine were quantified as previously described (31, 32). After this procedure, bronchoalveolar lavage fluid was collected and differential count for eosinophils, lymphocytes, neutrophils, or alveolar macrophages were performed. All counts were performed by a single observer blinded to study groups. For the house dust mite model mice were anesthetized by isoflurane inhalation and intranasally sensitized with 100 µg HDME in 50 µL saline. Five days later animals received five daily intranasal challenges of 10 µg HDME in 50 µL saline (32, 33). Seventy-two hours after the final challenge, BAY treatments and AHR measurements were performed as described for the OVA model. All animal experiments were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Preparation of Murine Tissues and Isometric Force Studies.
For isometric force studies, wild-type and sGC-β1−/− animals were killed by cervical dislocation. Mice were opened, the trachea was isolated and transferred to Krebs-Henseleit (K-H) solution bubbled with 95% O2/5% CO2 (vol/vol). Tracheal rings were mounted longitudinally on fixed segment support pins in two four-chamber myographs (Myograph 610, Danish Myo Technology) containing 5 mL K-H solution. Resting tension was set to 4 mN. Rings were precontracted with CCh (0.1 µM). Relaxation was induced with DEA-NO or BAY compounds as indicated.
Western Blots and Immunoprecipitations.
Standard protocols were followed as previously mentioned (14, 15). For immunoprecipitations (IP), 500 μg of the total mouse/human PCLS lung or RFL-6 cell supernatant was precleared with 20 µL of protein G-Sepharose beads (Amersham) for 1 h at 4 °C, beads were pelleted, and the supernatants incubated overnight at 4 °C with 3 μg of anti–sGC-β1 antibody. Protein G-Sepharose beads (20 µL) were then added and incubated for 1 h at 4 °C. The beads were microcentrifuged (3,220 × g), washed three times with wash buffer (50 mM Hepes pH 7.6, 100 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40), and then boiled with SDS-buffer and centrifuged. The supernatants were then loaded on SDS/PAGE gels and Western blotted with specific antibodies. Band intensities on Westerns were quantified using ImageJ quantification software (NIH).
cGMP ELISA.
The cGMP concentration in various cell supernatants made from intact cells that had been given sGC activators was estimated using the cGMP ELISA kit (Cell Signaling Technology). In certain other cases, sGC activity in the cell supernatants was determined by first passing them through the desalting G-25 PD-spin trap columns. Enzymatic activity (15, 34) was determined in 10-min incubations containing the protein solution, 500 μM GTP, 20 µM of sGC activators BAY 41–2272 or BAY 60–2770, and buffer containing 50 mM triethanolamine/HCl pH 7.4, 3 mM MgCl2, 3 mM DTT, and 250 μM IBMX at 37 °C. Reactions were quenched by addition of 10 mM Na2CO3 and Zn (CH3CO3)2. The cGMP concentration was then determined by ELISA.
Biotin Switch Assay.
The biotin switch assay was performed on tissue or cell supernatants to determine SNO proteins, as described previously (35), and the presence of the S-nitrosated target protein was assayed by immunoblotting with specific antibodies.
Monolayer Cell Culture and Transwell Coculture.
All cell lines were grown and harvested as previously described (15, 36). Cultures (50–60% confluent) of RFL-6 cells were treated with NO donor NOC-12 (70 μM) for 12 h, with media changed every 6 h and fresh NOC-12 added. The cells were then treated with 0.5 mM IBMX for 10 min followed by treatment with NO donor for 5 min (SNAP, 50 μM) or with a heme-dependent (BAY 41, 10 μM) or a heme-independent (BAY 60, 10 μM) sGC activator for 30 min before being harvested. Cell supernatants were assayed for cGMP content by ELISA and for protein content and sGC expression. Similarly, parallel cultures of RFL-6 were treated with NOC-12 for time points between 0 and 30 min and harvested for SNO-sGC determination by biotin switch assay. For Transwell coculture experiments a macrophage cell line (RAW 264.7) or a human bronchial epithelial cell line (A549) was grown in six-well Transwell plates in the apical part. RAW cells were induced to express iNOS with IFN-γ and LPS in the presence or absence of 3 mM l-NAME (36) for 12 h, and parallel cultures of RFL-6 or HASMCs were maintained in six-well plates. The induced RAW 264.7 cells in the Transwell baskets were then placed above the RFL-6 (±l-NAME) or HASMC cultures and incubated at varying time points between 0 and 24 h before being harvested. The A549 cells in the apical chamber were cocultured with HASMCs in the basal chamber and were simultaneously induced with IFN-γ, IL-1β, and TNF-α (37) from 0 to 48 h and HASMCs in the basal chamber were harvested at indicated time points. For all Transwell cocultures, nitrite in the cell media measured by Griess assay, with cells being harvested for cGMP measurement, determination of protein content and iNOS or sGC expression, SNO-sGC determination, or for immunoprecipitation assays.
Acknowledgments
We thank K. Queisser, D. Durra, Jennifer Rodgers, A. Majors, P. Sandner, and the Lerner Research Institute Biological Resource Unit for excellent assistance or discussions; and D. Schumick for illustrations. K.A. is a Scholar of the International Society for Advancement of Cytometry. This study is supported by National Institutes of Health Grants HL103453 (to S.C.E. and M.A.A.), HL081064 (to S.C.E., D.J.S., M.A.A., K.A., and A.G.), GM097041 (to D.J.S.), and HL114471 (to R.A.P.); a Bayer grants for target award (to D.J.S.); and Deutsche Forschungsgemeinschaft FR 1725/1-5 (to A.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524398113/-/DCSupplemental.
References
- 1.Olin JT, Wechsler ME. Asthma: Pathogenesis and novel drugs for treatment. BMJ. 2014;349:g5517. doi: 10.1136/bmj.g5517. [DOI] [PubMed] [Google Scholar]
- 2.Lin R, et al. Chronic treatment in vivo with β-adrenoceptor agonists induces dysfunction of airway β(2) -adrenoceptors and exacerbates lung inflammation in mice. Br J Pharmacol. 2012;165(7):2365–2377. doi: 10.1111/j.1476-5381.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(1) Suppl 1:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis JL. Role of soluble guanylyl cyclase in the relaxations to a nitric oxide donor and to nonadrenergic nerve stimulation in guinea pig trachea and human bronchus. J Pharmacol Exp Ther. 1997;280(3):1215–1218. [PubMed] [Google Scholar]
- 5.Papapetropoulos A, Simoes DC, Xanthou G, Roussos C, Gratziou C. Soluble guanylyl cyclase expression is reduced in allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L179–L184. doi: 10.1152/ajplung.00330.2005. [DOI] [PubMed] [Google Scholar]
- 6.Que LG, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308(5728):1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Que LG, Yang Z, Stamler JS, Lugogo NL, Kraft M. S-nitrosoglutathione reductase: An important regulator in human asthma. Am J Respir Crit Care Med. 2009;180(3):226–231. doi: 10.1164/rccm.200901-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. J Clin Invest. 2006;116(9):2330–2332. doi: 10.1172/JCI29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evgenov OV, et al. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5(9):755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Follmann M, et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl. 2013;52(36):9442–9462. doi: 10.1002/anie.201302588. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, et al. Insights into BAY 60-2770 activation and S-nitrosylation-dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes. Biochemistry. 2013;52(20):3601–3608. doi: 10.1021/bi301657w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendes-Silverio CB, et al. Activation of haem-oxidized soluble guanylyl cyclase with BAY 60-2770 in human platelets lead to overstimulation of the cyclic GMP signaling pathway. PLoS One. 2012;7(11):e47223. doi: 10.1371/journal.pone.0047223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nials AT, Uddin S. Mouse models of allergic asthma: Acute and chronic allergen challenge. Dis Model Mech. 2008;1(4-5):213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh A, Stuehr DJ. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc Natl Acad Sci USA. 2012;109(32):12998–13003. doi: 10.1073/pnas.1205854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh A, Stasch JP, Papapetropoulos A, Stuehr DJ. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J Biol Chem. 2014;289(22):15259–15271. doi: 10.1074/jbc.M114.559393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dweik RA, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181(10):1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silkoff PE, Sylvester JT, Zamel N, Permutt S. Airway nitric oxide diffusion in asthma: Role in pulmonary function and bronchial responsiveness. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1218–1228. doi: 10.1164/ajrccm.161.4.9903111. [DOI] [PubMed] [Google Scholar]
- 18.Robbins RA, et al. Expression of inducible nitric oxide in human lung epithelial cells. Biochem Biophys Res Commun. 1994;203(1):209–218. doi: 10.1006/bbrc.1994.2169. [DOI] [PubMed] [Google Scholar]
- 19.Guo FH, et al. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA. 1995;92(17):7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britt RD, Jr, et al. Soluble guanylate cyclase modulators blunt hyperoxia effects on calcium responses of developing human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2015;309(6):L537–L542. doi: 10.1152/ajplung.00232.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redington AE. Modulation of nitric oxide pathways: Therapeutic potential in asthma and chronic obstructive pulmonary disease. Eur J Pharmacol. 2006;533(1-3):263–276. doi: 10.1016/j.ejphar.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 22.Hamad AM, Clayton A, Islam B, Knox AJ. Guanylyl cyclases, nitric oxide, natriuretic peptides, and airway smooth muscle function. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L973–L983. doi: 10.1152/ajplung.00033.2003. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein Y, Bournissen FG, Hutson JR, Shannon M. Polymorphism of the ADRB2 gene and response to inhaled beta- agonists in children with asthma: A meta-analysis. J Asthma. 2009;46(9):900–905. doi: 10.3109/02770900903199961. [DOI] [PubMed] [Google Scholar]
- 24.Mittendorf J, et al. Discovery of riociguat (BAY 63-2521): A potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem. 2009;4(5):853–865. doi: 10.1002/cmdc.200900014. [DOI] [PubMed] [Google Scholar]
- 25.Cooper PR, et al. Formoterol and salmeterol induce a similar degree of β2-adrenoceptor tolerance in human small airways but via different mechanisms. Br J Pharmacol. 2011;163(3):521–532. doi: 10.1111/j.1476-5381.2011.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper PR, et al. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L530–L537. doi: 10.1152/ajplung.00133.2009. [DOI] [PubMed] [Google Scholar]
- 27.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122(4):734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 28.Cooper PR, et al. C-027 inhibits IgE-mediated passive sensitization bronchoconstriction and acts as a histamine and serotonin antagonist in human airways. Allergy Asthma Proc. 2011;32(5):359–365. doi: 10.2500/aap.2011.32.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asosingh K, Hanson JD, Cheng G, Aronica MA, Erzurum SC. Allergen-induced, eotaxin-rich, proangiogenic bone marrow progenitors: A blood-borne cellular envoy for lung eosinophilia. J Allergy Clin Immunol. 2010;125(4):918–925. doi: 10.1016/j.jaci.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronica MA, et al. Susceptibility to allergic lung disease regulated by recall responses of dual-receptor memory T cells. J Allergy Clin Immunol. 2004;114(6):1441–1448. doi: 10.1016/j.jaci.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Jonasson S, Hedenstierna G, Hedenström H, Hjoberg J. Comparisons of effects of intravenous and inhaled methacholine on airway physiology in a murine asthma model. Respir Physiol Neurobiol. 2009;165(2-3):229–236. doi: 10.1016/j.resp.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Debeer A, et al. Preterm rabbit lung tissue mechanics: Maturational changes and effect of antenatal steroids. Pediatr Pulmonol. 2010;45(4):349–355. doi: 10.1002/ppul.21191. [DOI] [PubMed] [Google Scholar]
- 33.Plantinga M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Fernhoff NB, Derbyshire ER, Underbakke ES, Marletta MA. Heme-assisted S-nitrosation desensitizes ferric soluble guanylate cyclase to nitric oxide. J Biol Chem. 2012;287(51):43053–43062. doi: 10.1074/jbc.M112.393892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001(86):pl1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 36.Waheed SM, et al. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic Biol Med. 2010;48(11):1548–1558. doi: 10.1016/j.freeradbiomed.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo FH, et al. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100(4):829–838. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]