Significance
Cleavage and polyadenylation specificity factor 30 (CPSF30) is part of a complex of proteins, collectively called CPSF, that direct pre-mRNA processing. CSPF30 is classified as a “zinc-finger” protein because it contains five repeats of three cysteine and one histidine residue (CCCH) within its amino acid sequence, which are sequences known to bind zinc in other proteins. We report that CPSF30 contains an unexpected 2Fe–2S cluster, at one of the CCCH domains, in addition to zinc. We also demonstrate that CPSF30 selectively recognizes the AU-rich “hexamer” sequence present in all pre-mRNA via a cooperative, metal-dependent binding mechanism. These findings identify a functional role for CPSF30 in pre-mRNA recognition and identify a previously unidentified Fe–S cluster in this zinc-finger protein.
Keywords: zinc, iron–sulfur, RNA, protein, binding
Abstract
Cleavage and polyadenylation specificity factor 30 (CPSF30) is a key protein involved in pre-mRNA processing. CPSF30 contains five Cys3His domains (annotated as “zinc-finger” domains). Using inductively coupled plasma mass spectrometry, X-ray absorption spectroscopy, and UV-visible spectroscopy, we report that CPSF30 is isolated with iron, in addition to zinc. Iron is present in CPSF30 as a 2Fe–2S cluster and uses one of the Cys3His domains; 2Fe–2S clusters with a Cys3His ligand set are rare and notably have also been identified in MitoNEET, a protein that was also annotated as a zinc finger. These findings support a role for iron in some zinc-finger proteins. Using electrophoretic mobility shift assays and fluorescence anisotropy, we report that CPSF30 selectively recognizes the AU-rich hexamer (AAUAAA) sequence present in pre-mRNA, providing the first molecular-based evidence to our knowledge for CPSF30/RNA binding. Removal of zinc, or both zinc and iron, abrogates binding, whereas removal of just iron significantly lessens binding. From these data we propose a model for RNA recognition that involves a metal-dependent cooperative binding mechanism.
Zinc-finger proteins (ZFs) are a large class of proteins that use zinc as structural cofactors (1–4). ZFs perform a variety of functions ranging from the modulation of gene expression through specific interactions with DNA or RNA to the control of signaling pathways via protein–protein interactions. The general feature that defines a ZF protein is the presence of one or more domains that contain a combination of four cysteine and/or histidine residues that serve as ligands for zinc. When zinc binds to these ligands, the domain adopts the structure necessary for function (1–4).
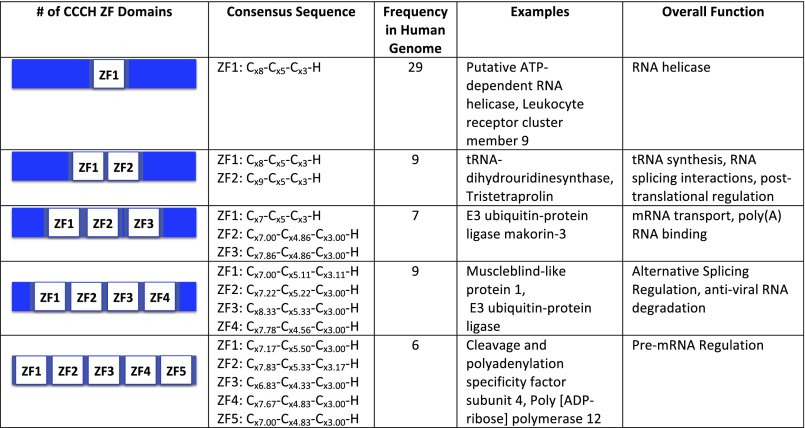
ZFs are typically identified by the presence of cysteine and histidine residues in regular repeats and are categorized into classes based upon the number of cysteine and histidine residues, and the spacing between the residues (1, 2). At least 14 distinct classes of ZFs have been identified to date. ZFs are highly abundant, with more than 3% of the proteins in the human genome annotated as ZFs, based upon their sequences (1, 5–9). In some cases, there are considerable in vitro and in vivo data that support the annotation of proteins as ZFs, whereas in other cases the only evidence that a protein is a ZF comes from its amino acid sequence. The best-studied class of ZFs comprises the “classical” ZFs. This class is composed of ZFs that contain a Cys2His2 domain (CysX2–5CysX12–13HisX3–5His). Classical ZFs adopt an alpha-helical/antiparallel beta-sheet structure when zinc is coordinated and bind DNA in a sequence-specific manner (2, 4). The remaining classes of ZFs are collectively called “nonclassical” ZFs (1). One class of nonclassical ZFs is the Cys3His class (CysX7–9CysX4–6CysX3His). The first protein of this class to be identified was tristetraprolin, which contains two Cys3His domains and regulates cytokine mRNAs via a specific ZF domain/RNA binding interaction (1). With the publication of genome sequences this domain has been found in a myriad of proteins. The National Center for Biotechnology Information (NCBI) conserved domain architecture tool identifies 404 distinct proteins (both hypothetical and experimentally validated) that contain this domain, and humans contain at least 60 (Fig. 1). As a class, these proteins are predicted to be involved in RNA regulation; however, the function(s) of most of these proteins have not yet been established (1, 2, 10, 11).
Fig. 1.
Survey of the CCCH domain containing proteins in H. sapiens.
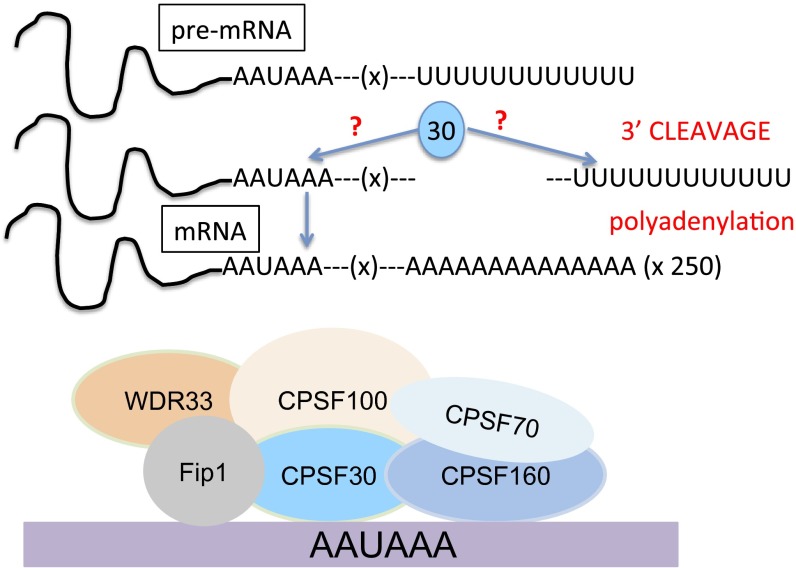
One important Cys3His ZF protein is cleavage and polyadenylation specificity factor 30 (CPSF30) (2, 12). CPSF30 contains five Cys3His domains. CPSF30 is part of a complex of proteins, collectively called CPSF, that are involved in the polyadenylation step of pre-mRNA processing (16). The other members of CPSF are CPSF160, CPSF73, CPSF100, Fip1, and Wdr33 (16). Polyadenylation is a 3′ end maturation step that all eukaryotic mRNAs (except histones) undergo (Fig. 2) (12). It involves endonucleolytic cleavage of the pre-mRNA followed by addition of a polyadenosine tail. Polyadenylation occurs at a specific region of the pre-mRNA called the polyadenylation cleavage site (PAS). The PAS consists of an upstream element with the conserved sequence AAUAAA (also called the AU-hexamer), a stretch of bases where cleavage occurs, after which a conserved GU-rich or U-rich sequence is present (usually between 40–60 nt after the cleavage site) (12, 13). CPSF73 is the endonuclease that cleaves the RNA; the roles of the other CPSF proteins are less clear (12, 13). Initially, CPSF160 was identified as the protein within the CPSF complex that recognizes the AU-hexamer (14–16); however, two recent studies using cell-based methods found that CPSF160 does not play this role (17, 18). Instead, CPSF30 and Wdr33 were identified as the proteins involved in AU-hexamer recognition (17, 18). These findings are intriguing in light of evidence that the H1N1 human influenza virus protein NS1A targets CPSF30 to obstruct cellular mRNA processing (19–21), suggesting that the link between NS1A and cellular mRNA processing is RNA recognition by CPSF30.
Fig. 2.
Cartoon of pre-mRNA processing, with possible roles of CPSF30 highlighted in blue.
Given these cell-based results that CPSF30 is involved in recognition of the AU-hexamer of pre-mRNA along with our emerging understanding that CCCH-type ZFs are a general ZF motif involved in AU-rich RNA sequence recognition, we sought to determine whether CPFS30 directly recognizes the pre-mRNA AU-hexamer sequence via its CCCH domains by isolating CPSF30 and examining its RNA binding properties at the molecular level. CPSF30 contains five CCCH domains, and our hypothesis was that CPSF30 would bind five zinc ions at these CCCH domains and selectively recognize the AU-rich hexamer of pre-mRNA. To our surprise, CPSF30 was a reddish-colored protein upon isolation and purification, which suggested the presence of an iron cofactor. Here, we report that CPSF30 contains a 2Fe–2S site, with a CCCH ligand set, in addition to zinc. We also report that CPSF30 selectively recognizes the polyadenylation hexamer (AAUAAA) of pre-mRNA in a cooperative and metal-dependent manner. These findings are discussed in the context of CCCH “zinc” domains, iron, and recognition of AU-rich RNA sequences.
Results and Discussion
CCCH ZF Proteins.
CPSF30 belongs to a class of proteins that are annotated as ZFs in genome databases. To determine how frequently this “CCCH ZF domain” occurs in eukaryotes a search using UniProt was performed (22). This resulted in 516 reviewed proteins and 25,610 total proteins when all organisms were considered. To provide further context, the search was narrowed to include only “CCCH ZF proteins” found in Homo sapiens. This led to the identification of 60 reviewed and 222 total proteins. The 60 reviewed proteins were then grouped based upon the number of CCCH domains present and a consensus sequence for each domain within the context of the number of domains was determined (Fig. 1) The proteins with CCCH ZF domains had between one and five domains, with the proteins with one domain being the most abundant (30 of the 66 proteins had only one domain). The organization of the domains (i.e., spacing between cysteine and histidine ligands) was generally invariant.
CPSF30 Contains Iron.
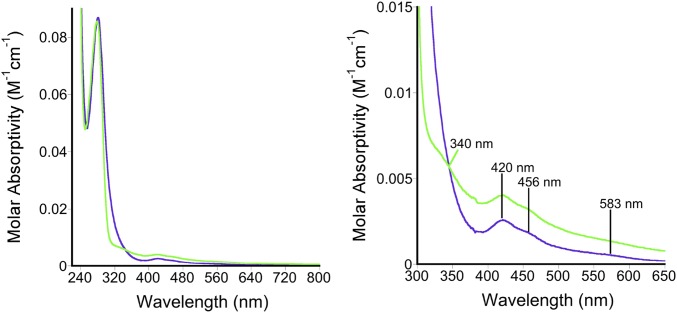
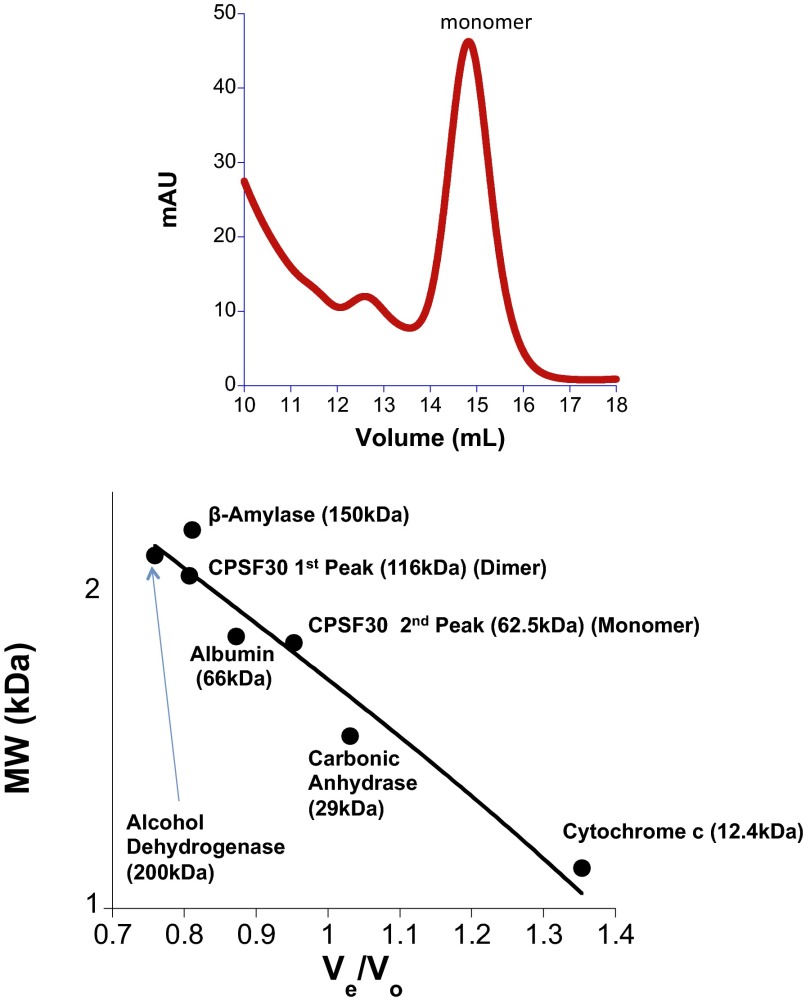
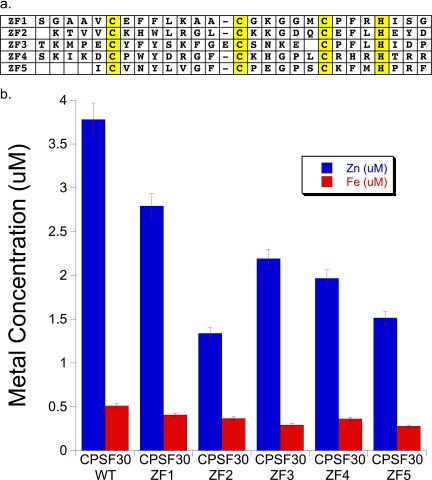
A series of CPSF30 constructs that contained just the five CCCH ZF domains were prepared recombinantly and expressed under standard conditions. Unexpectedly, all of the constructs turned red upon protein overexpression, suggesting that iron was coordinating to the protein in addition to or in lieu of zinc (Fig. S1). The most soluble construct, which contained a maltose-binding fusion tag (MBP), was purified and used for subsequent studies. The UV-visible spectrum of the purified CPSF30 exhibited peaks at 420, 456, and 583 nm, which are indicative of iron (particularly iron–sulfur clusters), in addition to the expected peaks around 220–280 for protein backbone and aromatic amino acid peaks around 280 nm (Fig. 3) (23). Inductively coupled plasma mass spectrometry (ICP-MS) of CPSF30 was performed to measure the metal content. In a given preparation, both zinc and iron were observed, on average 3.78 ± 0.02 zinc and 0.51 ± 0.01 iron per protein. A ferrozine assay independently confirmed the presence of iron (24). The metal content of MBP alone was also measured by ICP-MS and a ferrozine assay. No metal was found to be present in these samples, ruling out MBP as a site for metal binding. The oligomerization state of CPSF30 was measured by size-exclusion chromatography and CPSF30 was found to be primarily a monomer (Fig. S2). Efforts to prepare just iron-loaded, zinc-loaded, and apo-CPSF30 were made. Incubation with EDTA produced the iron-loaded protein (0.29 ± 0.13 zinc and 0.45 ± 0.08 iron) and incubation with o-phenanthroline or dipyridyl in the presence of DTT produced apo-CPSF30 (0.11 ± 0.08 zinc and 0.10 ± 0.05 iron with o-phenanthroline and 0.10 ± 0.08 zinc and 0.15 ± 0.05 iron with dipyridyl). The zinc-loaded protein was obtained by overexpressing the protein in iron-deplete minimal media that was supplemented with zinc upon induction (3.71 ± 0.2 zinc and 0.07 ± 0.003 iron). Together, these studies revealed that CPSF30’s CCCH ZF domains have both zinc and iron cofactors, of which zinc is more readily chelated.
Fig. S1.
CPSF30 is a red-colored protein.
Fig. 3.
Optical spectrum of CPSF30 (purple) and ‟apo”-CPSF30 (blue, after addition of EDTA) in 20 mM Tris, 100 mM NaCl, pH 8. (Left) full spectrum. (Right) Close-up between 300–650 nm.
Fig. S2.
Size-exclusion chromatography data.
X-Ray Absorption Spectroscopy of the Iron Sites.
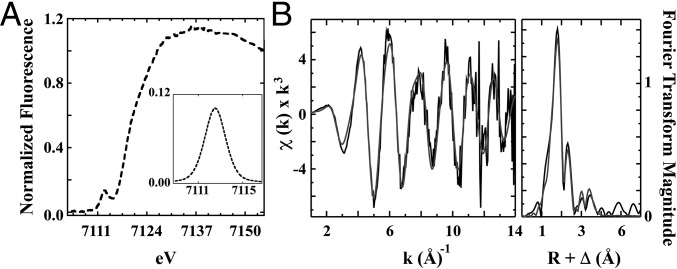
X-ray absorption spectroscopy (XAS) was used to characterize the protein-bound Fe coordination geometry and ligand environment. The X-ray absorption near edge structure (XANES) portion of the XAS spectrum for Fe bound to CPSF30 is shown in Fig. 4A. A Fe pre-edge 1s-3d electronic transition peak, observed at ∼7,112.4 eV with a corresponding peak area of 23.7 × 10−2 eV2, is consistent with a four-coordinate tetrahedral ferric complex (25, 26). The Fe edge energy, determined from the first inflection point of the rising edge, occurs at 7,120 eV and is again consistent with a Fe(III) species characteristic of an oxidized 2Fe(III)–2S cluster (27). The Fe extended X-ray absorption fine structure (EXAFS) (Fig. 4B) was best simulated in the nearest-neighbor environment with ca. three S ligands at an average bond length of 2.26 Å and ca. one O/N ligand at 2.03 Å (Table S1). Long-range scattering includes an Fe–Fe vector at a bond length of 2.67 Å. In addition, multiple Fe–C interactions were observed at 3.17, 3.44, and 3.95 Å. These bond lengths represent average values obtained from two independent samples and are consistent with reported values for oxidized 2Fe–2S clusters bound by three Cys and one His residues (28). Thus, we speculate that iron is bound to CPSF30 as a 2Fe–2S cluster with a CCCH ligand set.
Fig. 4.
(A) Fe XANES for CPSF30. (Inset) An expansion of the individual 1s-3d transition peak for CPSF30 Fe site. (B) Iron EXAFS and Fourier transform of EXAFS data for CPSF30 Fe site. (Left) Raw EXAFS data displayed in black and best fit in gray. (Right) Corresponding Fourier transform plot of raw EXAFS data in black and best fit in gray.
Table S1.
Summary of best-fit simulation analysis of raw Fe and Zn CPSF30 EXAFS data
| Nearest-neighbor ligand environment* | Long-range ligand environment* | ||||||||
| Metal | Atom† | R,‡ Å | CN§ | s2¶ | Atom† | R,‡ Å | CN§ | s2¶ | F′# |
| Fe | O/N | 2.03 | 1.0 | 2.66 | Fe | 2.67 | 0.5 | 2.51 | 1.33 |
| S | 2.26 | 2.5 | 4.67 | C | 3.17 | 0.5 | 4.42 | ||
| C | 3.44 | 1.5 | 5.30 | ||||||
| C | 3.95 | 4.0 | 4.61 | ||||||
| Zn | O/N | 2.10 | 1.0 | 4.25 | C | 3.07 | 3.0 | 2.88 | 0.31 |
| S | 2.31 | 2.5 | 4.71 | C | 3.26 | 3.5 | 1.05 | ||
| C | 3.42 | 3.0 | 1.73 | ||||||
| C | 4.03 | 2.0 | 5.03 | ||||||
Data were fit over a k range of 1–14 Å−1.
Independent metal–ligand scattering environment.
Scattering atoms: N (nitrogen), oxygen (O), sulfur (S), and carbon (C).
Average metal–ligand bond length from 9 (Fe) and 10 (Zn) scans per sample.
Average metal–ligand coordination number from 9 (Fe) and 10 (Zn) scans per sample.
Average Debye–Waller factor in Å2 x 103 from 9 (Fe) and 10 (Zn) scans per sample.
Number of degrees of freedom weighted mean square deviation between data and fit.
XAS of the Zinc Sites.
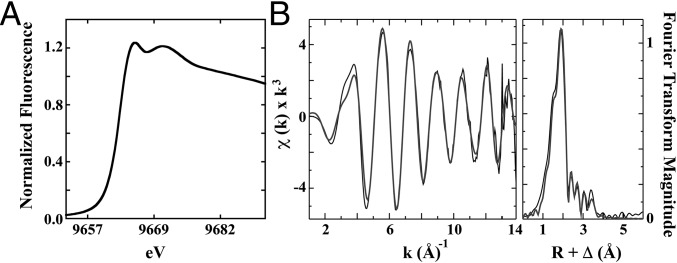
XAS was also used to characterize protein-bound Zn coordination geometry and metrical parameters. The Zn edge inflection energy at 9,662.5 eV is consistent with Zn(II) (Fig. 5A). The postedge region of the spectrum shows features at both 9,665.7 eV and 9,671 eV, consistent with independent sulfur and oxygen/nitrogen ligand environments published for ZnN1S3 peptides (29, 30), suggesting similar coordination environments. The Zn EXAFS region (Fig. 5B) was best fit in the nearest-neighbor ligand environment with ca. one O/N ligand at 2.01 Å and ca. 2.5 S ligands at 2.31 Å (Table S1). The Fourier transform of the Zn EXAFS for CPSF30 is consistent with a ZnN1S3 peptide system published previously (30). Long-range carbon scattering was also observed at 3.07, 3.26, 3.43, and 4.03 Å. These data are consistent with Zn coordinated to CPSF30 in a tetrahedral CCCH ligand environment.
Fig. 5.
(A) Zn XANES for CPSF30. (B) Zinc EXAFS and Fourier transform of EXAFS data for CPSF30 Zn site. (Left) Raw EXAFS data displayed in black and best fit in gray. (Right) Corresponding Fourier transform plot of raw EXAFS data in black and best fit in gray.
RNA Binding Studies: CPSF30 Binds to the AU-Rich Hexamer of α-Synuclein Pre-mRNA in a Cooperative Manner.
CPSF30 is part of a complex of proteins, collectively called CPSF, that regulate pre-mRNA processing. CPSF30 has been proposed to be involved in recognition of the PAS present in pre-mRNA, because the signal contains an AU-rich sequence, which is a favored recognition signal for certain CCCH-type ZF proteins (1, 2). Recently, two laboratories simultaneously reported studies to identify the role of CPSF30 in a cellular setting. In one set of studies, Shi and coworkers (18) immunoprecipitated the entire CPSF complex then performed cross-linking with a pre-mRNA (viral SLV-4) that included the AU-hexamer and digested the complex to identify the specific protein or proteins that bound to the pre-mRNA. From these studies, along with subsequent iCLIP studies, CPSF30 was identified as one of two proteins that directly interact with the pre-mRNA at the AU-hexamer (the other is a newly identified protein, Wrd33). Schönemann et al. (17) reported that they could reconstitute CPSF160, CPSF30, hfip1, and WDR33 as a complex that bound to pre-mRNA (via a filter binding assay) and cross-linking/immunoprecipitation studies implicated CPSF30 and WDR33 as the proteins that directly bind to pre-mRNA. Together, these studies suggest that CPSF30 directly binds to pre-RNA. We sought to evaluate this interaction at the molecular level by measuring the affinity of isolated CPFS30 for a pre-mRNA target.
A 38-nt segment of the human alpha-synuclein transcript (NCBI reference NM_001146055.1) was used to study this interaction (31). In the sequence, the AU-rich hexamer (AAUAAA) is centrally located within the 38-nt RNA, where it is flanked by 16 nt on the 5′ and 3′ ends (from now on referred to as αSyn38) (Table 1). Shorter RNA oligomers were also evaluated: αSyn30 and αSyn24. αSyn30 and αSyn24 maintain the polyadenylation signal in the center of the oligomer and are both derived from αSyn38, with either 12 or 9 nt flanking the polyadenylation signal, respectively (Table 1). A fragment of rabbit betaglobin (Rβ) mRNA was used as a negative control RNA oligomer because it does not contain the polyadenylation hexamer and is not enriched in adenosine and uridine nucleotides (Table 1).
Table 1.
RNA oligomers tested
| RNA oligomer | Length, nt | Sequence (5′–3′) |
| α-Syn38 | 38 | CCCAUCUCACUUUAAUAAUAAAAAUCAUGCUUAUAAGC |
| α-Syn30 | 30 | UCUCACUUUAAUAAUAAAAAUCAUGCUUAU |
| α-Syn24 | 24 | CACUUUAAUAAUAAAAAUCAUGCU |
| GU-rich24 | 24 | CCAGAAGUGUGUUUUGGUAUGCAC |
| Poly U24 | 24 | UUUUUUUUUUUUUUUUUUUUUUUU |
| Poly C24 | 24 | CACUUUAAUCCCCCCAAUCAUGCU |
| Rβ31 | 31 | UGGCCAAUGCCCUGGCUCACAAAUACCACUG |
The AU hexamer region is shown in bold.
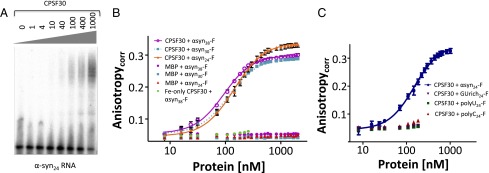
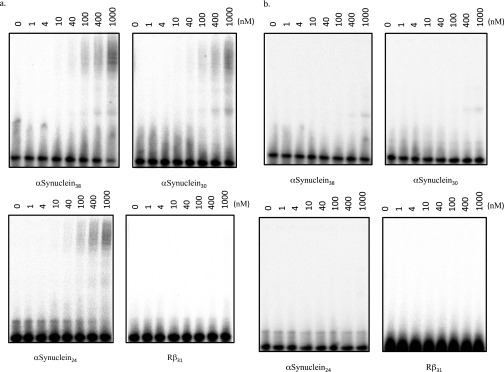
Electrophoretic mobility shift assays (EMSA) were performed with the four αSyn oligomers and the Rβ control. CPSF30 formed complexes with all of the αSyn oligomers interrogated, as evidenced by shifting of the RNA oligomers (Fig. 6A and Fig. S3A), whereas no binding was observed for CPSF30 with Rβ or with the MBP tag with any of the RNA oligomers (Fig. S3B).
Fig. 6.
(A) EMSA of α-syn38 with CPSF30 (concentration: 0–1,000 nM). (B) FA monitored binding of CPSF30 with αSyn38-Fl (open magenta circles), αSyn30-Fl (teal squares), and αSyn24-Fl (orange triangles). A titration of EDTA generated ‟apo”-CPSF30-5FE with αSyn38-Fl (closed green circles) and MBP with αSyn38-Fl (closed magenta circles), αSyn30-Fl (light blue squares), and αSyn24-Fl (red triangles). (C) FA monitored binding of CPSF30 with αSyn24-Fl (blue circles), polyU24 (green squares), GUrich24 (purple triangles), and polyC24 (red triangles). Data are fit to a cooperative binding equilibrium.
Fig. S3.
(A) EMSA of CPSF30 with different-length RNA (αsyn38, αsyn30, and αsyn24 versus a random RNA Rβ31). (B) EMSA of MBP different-length RNA (αsyn38, αsyn30, and αsyn24 versus a random RNA Rβ31). No binding was observed to any of the sequences.
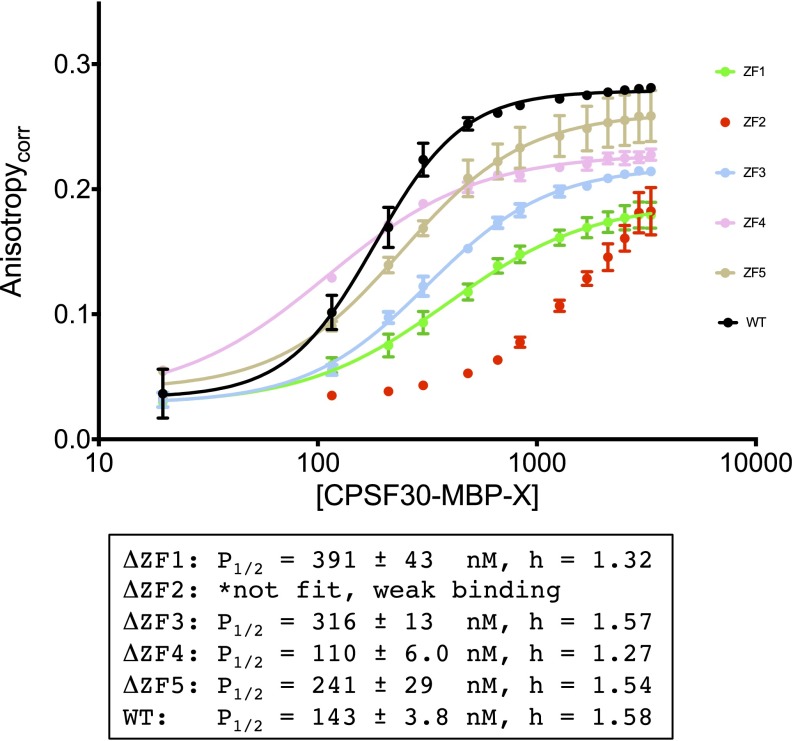
Fluorescence anisotropy (FA) was performed to determine the binding affinity of CPSF30 for the αSyn38, αSyn30, and αSyn24 oligomers. Binding was observed for all three αSyn RNA targets, but not for Rβ. Anisotropy values, corrected for the change in quantum yield, were plotted versus the concentration of CPSF30 protein titrated. The data were fit to two models: a 1:1 binding model and a cooperative binding model (32). The cooperative binding model gave the best fit, with [P]1/2 values of 93.5 ± 2.7 nM, 115.0 ± 3.6 nM, and 143.8 ± 3.8 nM, with Hill coefficients of 1.67 ± 0.07, 1.63 ± 0.08, and 1.58 ± 0.07 for αSyn38, αSyn30, and αSyn24, respectively (Fig. 6B). Binding was not observed when these RNA targets were titrated with either apo-CPSF30 or iron-loaded (zinc-deplete) CPSF30. The zinc-loaded (iron-deplete) CPSF30 exhibited significantly weakened affinity compared with the iron- and zinc-loaded CPSF30 ([P]1/2 570.5 ± 23.4) nM with αSyn38. No binding was observed for MBP alone with any of the RNA targets.
To determine whether the AU-rich sequence is the site of RNA binding, titrations of CPSF30 with three altered RNA sequences were performed (Table 1). The sequences all lacked the AU-hexamer. In two cases the AU-hexamer was replaced with polyC (CCCCCC) or polyU (UUUUUU), and in the third the GU-rich sequence (UGUUUU) near the polyuridine site that has been proposed as an alternative target sequence for CPSF30 (33). CPSF30 showed no binding to any of these sequences (in the Fe/Zn, Fe-only, Zn-only, or apo forms) (Fig. 6C).
Identification of Iron and Zinc Domains.
The five CCCH domains of CPSF30 are highly homologous (Fig. S4A), and from sequence comparison it is not apparent which domain is loaded with the Fe–S cluster and which is loaded with zinc. A series of mutants in which each domain was modified at the coordinating cysteine and histidine ligands (CCCH to AAAA named ΔZF1, ΔZF2, ΔZF3, ΔZF4, and ΔZF5) were prepared to identify where iron and zinc bind. The mutant proteins were overexpressed and purified following the identical conditions used for WT-CPSF30. All of the mutants were reddish in color, and analysis by ICP-MS (Fig. S4B) revealed that they all were loaded with iron, but lost between one and two equivalents of Zn, with ΔZF2 losing the most Zn (2.4 equivalents) and ΔZF1 the least (1.3 equivalents). The affinities of these mutants for RNA (αSyn24) was subsequently measured (Fig. S5) ΔZF4 retained comparable binding affinity to WT CPSF30, ΔZF1, ΔZF3, and ΔZF5 exhibited two- to threefold weaker affinities, and the ΔZF2 mutant did not bind to αSyn24 RNA with any appreciable affinity. Like WT-CPSF30, none of the mutants exhibited any affinity for polyC RNA. Taken together, these data support a model in which the sites of Fe and Zn binding are flexible, with iron loading occurring first. The data also suggest that ZF2 is critical for tight RNA binding, whereas the other ZFs seem to be less important.
Fig. S4.
(A) Sequence alignment of the five CCCH ZF domains of Bos taurus CPSF30. Cysteine and histidine ligands are highlighted in yellow. (B) ICP-MS analysis of CPSF30 protein and mutants expressed and purified as described in Detailed Experimental Protocols. Metal concentrations are per 1 μM protein.
Fig. S5.
FA data for the five CPSF30 mutants with αSyn24 compared with WT-CPSF30. The data are shown fit to a cooperative binding model (described in Detailed Experimental Protocols); data were also fit to 1:1 binding models, for comparison, but the data fit better to the cooperative model.
Model of CPSF30/RNA Binding.
The data for CPSF30/RNA binding were best fit to a cooperative binding model with a minimum stoichiometry of 2 CPSF30:1 RNA and an average Hill coefficient of 1.63 ± 0.07 (Fig. 6 B and C). CPSF30 alone is a monomer, as evidenced by gel filtration chromatography data (Fig. S2), yet exhibits positive cooperativity upon RNA recognition, perhaps indicating RNA-induced protein dimerization. Dimerization of proteins that bind to RNA is not unprecedented; for example, the Cys3His ZF ZAP is a monomer in solution that then dimerizes upon binding to the ZAP-responsive RNA element (34). Other RNA-binding proteins that recognize their targets in a cooperative manner are the human zipcode-binding protein IMP-1, HIV-1 Rev protein, hordeiviral γb protein, human La protein, and tomato bushy stunt virus p33 protein (35–38). There are also other AU-rich RNA-binding proteins such as AUF-1 (p42), Hsp70, and HuR, which bind to their respective RNA partners in a cooperative manner (39–41). Moreover, a two-domain construct of CPSF30 has been crystallized bound to the NS1 influenza A virus protein, where it is present as a dimer (21).
Conclusions
Several important conclusions can be drawn from the work presented here: (i) CPSF30 is the protein within the CPSF complex that directly recognizes PAS RNA; (ii) CPSF30 contains an unexpected 2Fe–2S cluster, in addition to zinc; (iii) CPSF30 is always loaded with iron first, then zinc; and (iv) the sites of iron and zinc binding are flexible but high-affinity RNA binding requires that one Fe site and at least two Zn sites be occupied. The CPSF complex directs mRNA 3′ maturation via a mechanism of RNA recognition, cleavage, and polyadenylation (Fig. 2); however, the roles of the proteins that make up the CPSF complex are not clearly defined. Studies of the complex in a cellular setting have provided tantalizing support for CPSF30 as the protein that binds to the PAS signal. The work described here provides direct evidence that CPSF30 selectively recognizes the AU-rich hexamer of pre-RNA via its CCCH domains. Unexpectedly, the work also shows that CPSF30 contains a 2Fe–2S site with a CCCH ligand set, in addition to its predicted zinc sites.
These 2Fe-2S sites with CCCH ligand sets are rare; 2Fe-2S sites were first identified in the 1960s, with two principal types identified: ferredoxin (1962), which uses a CCCC ligand set, and rieske type (1964), which use a CCHH ligand set. However, it was not until the late 2000s that a 2Fe–2S site with a CCCH ligand set was identified (23). This site was found in a protein called mitoNEET, a mitochondrial protein that is a target of the type-2 diabetes drug pioglitazone (23). Remarkably, like CPSF30, mitoNEET was annotated as a ZF protein based on the presence of a CCCH domain (with spacing of CXCX9CX3H compared with CX7–9CX4–5CX3H for CPSF30 and its homologs) and turned red upon protein expression and purification (42). MitoNEET contains a singular 2Fe–2S cluster with a CCCH ligand set and no zinc sites. The two homologs of MitoNEET, Miner1 (or NAF1) and Miner2, also contain between one and two 2Fe–2S clusters bound to CCCH sites (43–45). This suggests that the annotation of a protein as a ZF protein simply based upon its amino acid sequence (i.e., repeats of cysteine and histidine residues) may not always be correct and care must be taken in defining a ZF protein based just upon amino acid sequence. There is also evidence for a CCCH ligated 2Fe–2S cluster in proteins involved in iron–sulfur cluster assembly in Escherichia coli IscR and IscU (46, 47). In addition, in yeast, the Grx3/4/Fra2 signaling proteins have been shown to interact via a 2Fe–2S cluster that has a CCHX ligand set (48).
The biological significance of the 2Fe–2S cluster identified in CPSF30 is not yet known. Fe–S sites have been shown to play roles in electron transfer, oxygen sensing, iron sensing, substrate activation, and catalysis (49). Similarly, in mitoNEET there is not yet a definitive role for the 2Fe–2S site; however, there is evidence that it may be involved in trafficking iron via a redox sensing mechanism (50–54). Redox sensing may also be important for CPSF30: The CPSF30 yeast homolog, YtH1, responds to hypoxic stress (loss of O2) by shuttling to the cytoplasm from the nucleus (55), and the Fe–S cluster may facilitate the protein localization (50–54). Additionally, the Arabidopsis homolog of CPSF30, which contains just three CCCH domains, has been shown to be involved in redox signaling, via its cysteine ligands (56). Another possible role for the 2Fe–2S cluster of CPSF30 is to regulate oligonucleotide binding a redox-dependent manner; Fe–S clusters are emerging as key cofactors in a range of regulatory proteins. In some proteins for which the Fe–S cluster plays a regulatory role, such as the base excision repair proteins, the Fe–S cluster’s DNA binding affinity is dependent on the oxidation state of the Fe–S cluster and modulated by DNA charge transfer (57–62). In others, such as Aft2 (which also contains a structural zinc site), it is the presence or absence of the Fe–S cluster that drives DNA binding (63).
Taken together, our experimental data for CPSF30 reveal that CPSF30 contains both a 2Fe–2S site and a zinc site. Both sites are important for sequence-specific RNA binding, with the Zn site playing a larger role. CPSF30 selectively recognizes and binds to the polyadenylation AU-rich hexamer of α-synuclein pre-mRNA in a cooperative manner with high affinity. The location of the zinc and iron sites within the 5CCCH domains of CPSF30 seem to be flexible; however, iron is loaded before zinc. CPSF30 is a target of the human influenza virus, NS1A, which binds F2 and F3 of CPSF30 to obstruct cellular pre-mRNA processing, and we speculate that these two CCCH domains may be involved in direct pre-mRNA processing.
Materials and Methods
CPSF30 (33–170) was cloned into pMAL-c5e, expressed in BL21-DE3 cells at 37 °C for 3 h and purified via amylose and SP Sepharose chromatography. Metal content was measured via ICP-MS and oligomerization state by size-exclusion chromatography (Supadex 200 100/300 gel). Metals ions were removed by addition of EDTA, dipyridyl, or phenanthroline. XAS was used to identify metal ion sites and EMSA and FA to characterize RNA binding. Detailed experimental protocols are described in Supporting Information.
Detailed Experimental Protocols
Protein Preparation and Purification.
The cDNA encoding for CPSF30 was a kind gift of Professor Walter Keller, University of Basel. DNA encoding the 5 Cys3His domains of CPSF30 was cloned into the pMAL-c5e plasmid (New England Biolabs) using the NdeI and BamHI restriction sites and the DNA sequence was confirmed at the University of Maryland’s Biopolymer-Genomics Core Facility. The resultant plasmid was transformed into the chemically competent E.coli cell line, BL21-DE3 (Invitrogen), via heat shock. Cultures were grown at 37 °C on LB-Agar (Sigma) plates containing 100 μg/mL ampicillin (Sigma). A single colony was picked to inoculate 50 mL of LB Lennox broth (American Bioanalytical Inc.) containing 100 μg/mL ampicillin at 37 °C with shaking, overnight. Overnight cultures were used to inoculate 1 L of LB Lennox broth containing 100 μg/mL ampicillin. Cell cultures were grown at 37 °C until reaching an OD600 of 0.5–0.6, where protein expression was initiated with 50 μM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Research Products International Corp.). Cell culture media was supplemented with 1 mM ZnCl2 (Sigma) immediately before induction. CPSF30 protein expression was allowed to continue for 3 h postinduction at 37 °C, followed by pelleting the cells at 7,800 × g, 4 °C for 15 min (Avanti J-20 XPI; Beckman-Coulter). Cell pellets were stored at −20 °C for up to 2 wk. CPSF30 was purified on an amylose column followed by a SP Sepharose column (New England Biolabs). SDS/PAGE and matrix-assisted laser desorption ionization MS verified purity.
Preparation of Fe-CPSF30, Apo-CPSF30, and Zn-CPSF30.
Fe-CPSF30 was prepared by incubating of CPSF30 under anaerobic conditions with 50 mM EDTA in 20 mM Tris and 100 mM NaCl, pH 7.5, followed by extensive dialysis. Apo-CPSF30 was prepared by incubation with either dipyridyl or o-phenanthroline along with 10 mM DTT in 20 mM Tris and 100 mM NaCl, pH 7.5, under anaerobic conditions, followed by extensive dialysis. Zn-CPSF30 was prepared via a minimal media approach. BL21-DE3 cells inoculated with the CPSF30-pMAL-c5e plasmid were grown overnight in 50 mL of LB Lennox Broth containing 100 µg/mL ampicillin at 37 °C. The overnight cultures were used to inoculate 250 mL of M9 minimal media (20× M9 salts, 1 M MgSO4⋅7H2O, 1 M CaCl2⋅2H2O, 1 M glucose, and MilliQ water). Cell cultures were grown at 37 °C until reaching an OD600 of 0.5–0.6, where the culture was passaged to a flask containing 1 L of M9 minimal media with the same content. As the cell cultures reached an OD600 of ∼0.3, 1 mM ZnCl2 was added to the flask. As the cell cultures reached an OD600 of 0.5–0.6, protein expression was initiated with 1 mM IPTG. CPSF30 protein expression was allowed to continue for 3 h postinduction at 37 °C, followed by pelleting the cells at 7,800 × g, 4 °C for 15 min (Avanti J-20 XPI; Beckman-Coulter). Purification followed the procedure described in the preceding section (Protein Preparation and Purification).
Mutant CPSF30 Proteins.
Five mutant plasmids (CPSF30-pMAL-c5e) in which each CCCH domain was mutated to an AAAA sequence were designed and purchased (GenScript). Each DNA sequence was confirmed the University of Marylands Biopolymer-Genomics Core Facility. The expression and purification of the mutant proteins (ΔZF1, ΔZF2, ΔZF3, ΔZF4, and ΔZF5) exactly followed the experimental protocol described above for WT-CPSF30 (Protein Preparation and Purification).
ICP-MS.
One-micromolar samples of protein (CPSF30, metal-removed, and the five mutants) were prepared in 2% trace metal grade nitric acid (HNO3; Fisher) to a total volume of 15 mL. For each experiment, 150 µL internal standard (100µg/mL Bi, Ge, In, Li, Lu, Rh, Sc, and Tb; Agilent Technologies) was added to samples to ensure accuracy. Zinc and iron levels were detected using the octopole reaction system in HE mode, an rf power of 1,550 W, an argon carrier gas flow of 1.0 L/min, argon make-up gas flow of 0.1 L/min, helium gas flow of 4.5 mL/min, octopole rf of 160 V, QP bias of −15 V, and OctP bias of −18 V. Zinc and Iron concentrations in samples were derived from a calibration curve generated by a series of iron and zinc atomic absorption standard dilutions (Fluka Analytical). These dilutions were prepared using the same method as for the protein samples. ICP-MS data were recorded using an Agilent 7700x ICP-MS instrument. Data analysis used Agilent Mass Hunter software.
Gel Filtration Chromatography.
A 100-µL sample of 250 µM CPSF30 was injected onto an analytical Superdex 200 10/300 gel filtration column (GE) on an Akta FPLC system (GE) at a flow rate of 0.40 mL/min. The column was equilibrated with a 20 mM Tris, pH 7, and 50 mM NaCl buffer. The molecular weight of CPSF30 was determined using a calibration curve using a molecular weight standard kit from Sigma.
XAS.
Independent reproducible protein samples were prepared in 20 mM Tris, 50 mM NaCl, and 30% glycerol, pH 7. Metal concentrations for all protein samples were confirmed by ICP-MS analysis, with metal concentrations at ca. 0.7 mM Fe and 6 mM Zn. Samples were loaded into lucite XAS cells prewrapped with kapton tape, flash-frozen in liquid nitrogen, and stored in liquid nitrogen until data collection. Fe and Zn XAS data were collected at both the Stanford Synchrotron Radiation Light Source (SSRL), on beamline 9-3, and the National Synchrotron Light Source (NSLS), on beamline X3-B. Beamline 9-3 was equipped with a Si[220] double crystal monochromator and beamline X3-B was equipped with a Si[111] monochromator; both beamlines were equipped with a mirror for focusing and harmonic rejection. During data collection, samples at SSRL were maintained at 10 K using an Oxford Instruments continuous-flow liquid helium cryostat, and samples at NSLS were maintained at 24 K using a helium Displex cryostat. Protein fluorescence excitation spectra were measured using Canberra germanium solid-state detectors with 100 channels at SSRL and 31 channels at NSLS. Soller slits and Mn and Cu filters (for Fe and Zn XAS, respectively) at 0.6-mm (SSRL) or 0.3-mm (NSLS) thickness were placed between the cryostat and detector to reduce scattering and background signals in the Fe and Zn XAS, respectively. At both facilities, XAS spectra were collected in 5-eV increments in the pre-edge region, 0.25-eV increments in the edge region, and 0.05 Å−1 increments in the EXAFS region to k = 14 Å−1, integrated from 1 to 25 s in a k3-weighted manner for a total scan length of ∼40 min. X-ray energies were individually calibrated by collecting a foil absorption spectrum simultaneously with protein data. The first inflection point of the Fe foil spectrum was assigned to 7,111.2 eV and that of Zn was assigned to 9,659 eV. To minimize the occurrence of Fe photoreduction, only two or three scans were collected per spot on each sample. Scans were collected at an average of three new spots per sample over a total of four samples, obtaining an average of 10–12 first scans for Fe. For Zn, 8–10 scans were collected per sample. Replicates samples were analyzed independently to ensure spectral reproducibility and all spectra were closely monitored for other spectral anomalies during data collection and analysis.
XAS data were processed using the Macintosh OS X version of EXAFSPAK (ssrl.slac.stanford.edu/∼george/exafspak/mac.htm) integrated with Feff v8 for theoretical model generation. Normalized XANES data were subjected to pre-edge and edge analysis. Only spectra collected at SSRL were used for pre-edge analysis. The Fe 1s-3d pre-edge peak analysis was completed as described previously (64); peak areas was determined over the energy range of 7,110–7,116 eV using the program Kaleidagraph and areas are represented in units of 10−2 eV2 (65). Oxidation states for both elements were determined as a function of the first inflection energy at the edge, measured based on edge half-height in the normalized XANES (66). EXAFS data reduction used a polynomial function in the pre-edge region and a four point cubic spline throughout the EXAFS region for background signal removal. Data were converted to k space using E0 values of 7,130 eV (Fe) and 9,680 eV (Zn). For both elements, data were collected to k = 14 Å−1, which corresponds to a spectral resolution of 0.121 Å for all metal–ligand interactions (66); therefore, only independent scattering environments at distances >0.121 Å were considered resolvable in the EXAFS fitting analysis. Only raw data were used for EXAFS fitting analysis and data were fit using both single and multiple scattering model amplitudes and phase functions to simulate Fe- and Zn–O/N, S, and Fe–Fe ligand interactions. During Fe data simulations, a scale factor (Sc) of 0.95 and threshold shift (ΔE0) value of −10 eV (Fe–O/N/C), −12 eV (Fe–S) and −15 eV (Fe–Fe) were used, whereas for Zn an Sc of 1 and E0 value of −15.25 eV (Zn–O/N/C and Zn–S) were used. These values were obtained from fitting crystallographically characterized small molecule Fe and Zn compounds (64, 67). When simulating empirical data, Sc, E0, and ligand coordination numbers were held constant and only absorber–scatterer bond length (R) and Debye–Waller factor (σ2) were allowed to freely vary. Criteria for judging the best-fit simulation to the data were determined from both the lowest mean-square deviation between data and fit (F′), corrected for the number of degrees of freedom and reasonable Debye–Waller factors (σ2 < 0.0056 Å2) (68).
Protein/RNA Binding Assays.
EMSA (for CPSF30) and FA assays (for CPSF30 and the mutants, ΔZF1, ΔZF2, ΔZF3, ΔZF4, and ΔZF5) were performed with the RNA oligonucleotides (fluorescein functionalized on the 3′ end for FA) listed in Fig. 4. 32P-labeled RNA was incubated with increasing concentrations of CPSF30 in 50 mM Tris, pH 8.0, 100 mM potassium chloride, 10% (vol/vol) glycerol, 100 μM ZnCl2, 2 mM DTT, and 0.1 mg/mL BSA; 5% (vol/vol) native polyacrylamide gels containing 10% (vol/vol) glycerol were used, with a 0.5× (44.5 mM) Tris-borate buffer (pH 8.0) and the gels were imaged on a GE Typhoon FLA9500.
For FA studies, measurements were taken with an ISS PC-1 spectrofluorometer configured in the L format, with an excitation wavelength/band pass of 495 nm/2 nm and an emission wavelength/bandpass of 517 nm/1 nm. A 5 nM solution of fluorescently labeled RNA in 50 mM Tris, pH 8.0, 100 mM potassium chloride with 0.2 mg/mL BSA, and 0.4 mg/mL poly-rC was added to a Spectrosil far-UV quartz window fluorescence cuvette (Starna Cells) and CPSF30 was titrated until saturation. Data were analyzed by correcting the anisotropy (r) for the change in quantum yield (Q, qfree/qbound, protein-dependent change in fluorescenc) using the following equation:
where rc is the corrected anisotropy, r0 is the anisotropy of the free fluorescein-labeled oligonucleotide and rbound is the anisotropy of the RNA–protein complex at saturation. rc was plotted against the concentration of protein. The data were best fit to a cooperative binding model using nonlinear regression (GraphPad Prism 5):
where rTc is the total, corrected anisotropy, r0 is the anisotropy of the free fluorescein-labeled oligonucleotide, rbound is the anisotropy of the RNA-protein complex at saturation, [P] is the concentration of protein, [P]1/2 is the concentration of protein at half-maximal saturation, and h is the Hill coefficient. Each data point is the average of 31 readings over 100 s, and each titration was carried out in triplicate.
Acknowledgments
This work was supported by National Science Foundation Grant CHE1306208 (to S.L.J.M.), American Heart Association/Friedreich’s Ataxia Research Alliance Grant 12PRE11720005 (to A.V.R.), American Heart Association Grant 11PRE6900008 (to B.E.Z.), NIH Grants CA102428 (to G.M.W.) and DK068139 (to T.L.S.), and NIH training Grant T32GM066706-13 (to G.D.S.). Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a directorate of the Stanford Linear Accelerator Center, National Accelerator Laboratory, and an Office of Science User Facility operated for the US Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by NIH–National Institute of General Medical Sciences (including Grant P41GM103393). Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02-98CH10886.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517620113/-/DCSupplemental.
References
- 1.Lee SJ, Michel SL. Structural metal sites in nonclassical zinc finger proteins involved in transcriptional and translational regulation. Acc Chem Res. 2014;47(8):2643–2650. doi: 10.1021/ar500182d. [DOI] [PubMed] [Google Scholar]
- 2.Michalek JL, Besold AN, Michel SL. Cysteine and histidine shuffling: Mixing and matching cysteine and histidine residues in zinc finger proteins to afford different folds and function. Dalton Trans. 2011;40(47):12619–12632. doi: 10.1039/c1dt11071c. [DOI] [PubMed] [Google Scholar]
- 3.Maret W. New perspectives of zinc coordination environments in proteins. J Inorg Biochem. 2012;111:110–116. doi: 10.1016/j.jinorgbio.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Jantz D, Amann BT, Gatto GJ, Jr, Berg JM. The design of functional DNA-binding proteins based on zinc finger domains. Chem Rev. 2004;104(2):789–799. doi: 10.1021/cr020603o. [DOI] [PubMed] [Google Scholar]
- 5.Bertini I, Decaria L, Rosato A. The annotation of full zinc proteomes. J Biol Inorg Chem. 2010;15(7):1071–1078. doi: 10.1007/s00775-010-0666-6. [DOI] [PubMed] [Google Scholar]
- 6.Laity JH, Lee BM, Wright PE. Zinc finger proteins: New insights into structural and functional diversity. Curr Opin Struct Biol. 2001;11(1):39–46. doi: 10.1016/s0959-440x(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 7.Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5(11):3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- 8.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5(1):196–201. doi: 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 9.Decaria L, Bertini I, Williams RJ. Zinc proteomes, phylogenetics and evolution. Metallomics. 2010;2(10):706–709. doi: 10.1039/c0mt00024h. [DOI] [PubMed] [Google Scholar]
- 10.Blackshear PJ, Perera L. Phylogenetic distribution and evolution of the linked RNA-binding and NOT1-binding domains in the tristetraprolin family of tandem CCCH zinc finger proteins. J Interferon Cytokine Res. 2014;34(4):297–306. doi: 10.1089/jir.2013.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Huang S, Wang X, Gu Y. Emerging roles of CCCH-type zinc finger proteins in destabilizing mRNA encoding inflammatory factors and regulating immune responses. Crit Rev Eukaryot Gene Expr. 2015;25(1):77–89. doi: 10.1615/critreveukaryotgeneexpr.2015013022. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Manley JL. The end of the message: Multiple protein-RNA interactions define the mRNA polyadenylation site. Genes Dev. 2015;29(9):889–897. doi: 10.1101/gad.261974.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millevoi S, Vagner S. Molecular mechanisms of eukaryotic pre-mRNA 3′ end processing regulation. Nucleic Acids Res. 2010;38(9):2757–2774. doi: 10.1093/nar/gkp1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore CL, Chen J, Whoriskey J. Two proteins crosslinked to RNA containing the adenovirus L3 poly(A) site require the AAUAAA sequence for binding. EMBO J. 1988;7(10):3159–3169. doi: 10.1002/j.1460-2075.1988.tb03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilmartin GM, Fleming ES, Oetjen J, Graveley BR. CPSF recognition of an HIV-1 mRNA 3′-processing enhancer: multiple sequence contacts involved in poly(A) site definition. Genes Dev. 1995;9(1):72–83. doi: 10.1101/gad.9.1.72. [DOI] [PubMed] [Google Scholar]
- 16.Keller W, Bienroth S, Lang KM, Christofori G. Cleavage and polyadenylation factor CPF specifically interacts with the pre-mRNA 3′ processing signal AAUAAA. EMBO J. 1991;10(13):4241–4249. doi: 10.1002/j.1460-2075.1991.tb05002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schönemann L, et al. Reconstitution of CPSF active in polyadenylation: Recognition of the polyadenylation signal by WDR33. Genes Dev. 2014;28(21):2381–2393. doi: 10.1101/gad.250985.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan SL, et al. CPSF30 and Wdr33 directly bind to AAUAAA in mammalian mRNA 3′ processing. Genes Dev. 2014;28(21):2370–2380. doi: 10.1101/gad.250993.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo RL, Krug RM. Influenza a virus polymerase is an integral component of the CPSF30-NS1A protein complex in infected cells. J Virol. 2009;83(4):1611–1616. doi: 10.1128/JVI.01491-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twu KY, Noah DL, Rao P, Kuo RL, Krug RM. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J Virol. 2006;80(8):3957–3965. doi: 10.1128/JVI.80.8.3957-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das K, et al. Structural basis for suppression of a host antiviral response by influenza A virus. Proc Natl Acad Sci USA. 2008;105(35):13093–13098. doi: 10.1073/pnas.0805213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.C U UniProt: A hub for protein information. Nucleic Acids Res. 2015;42(database issue):D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamir S, et al. Structure-function analysis of NEET proteins uncovers their role as key regulators of iron and ROS homeostasis in health and disease. Biochim Biophys Acta. 2015;1853(6):1294–1315. doi: 10.1016/j.bbamcr.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem. 2004;331(2):370–375. doi: 10.1016/j.ab.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 25.Schneider DJ, Roe AL, Mayer RJ, Que L., Jr Evidence for synergistic anion binding to iron in ovotransferrin complexes from resonance Raman and extended X-ray absorption fine structure analysis. J Biol Chem. 1984;259(15):9699–9703. [PubMed] [Google Scholar]
- 26.Westre TE, et al. A multiplet analysis of Fe K-Edge 1s → 3d pre-edge features of iron complexes. J Am Chem Soc. 1997;119(27):6297–6314. [Google Scholar]
- 27.Tsang HT, Batie CJ, Ballou DP, Penner-Hahn JE. X-ray absorption spectroscopy of the [2Fe-2S] Rieske cluster in Pseudomonas cepacia phthalate dioxygenase. Determination of core dimensions and iron ligation. Biochemistry. 1989;28(18):7233–7240. doi: 10.1021/bi00444a015. [DOI] [PubMed] [Google Scholar]
- 28.Sazinsky MH, et al. Characterization and structure of a Zn2+ and [2Fe-2S]-containing copper chaperone from Archaeoglobus fulgidus. J Biol Chem. 2007;282(35):25950–25959. doi: 10.1074/jbc.M703311200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbst RW, et al. Communication between the zinc and nickel sites in dimeric HypA: Metal recognition and pH sensing. J Am Chem Soc. 2010;132(30):10338–10351. doi: 10.1021/ja1005724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark-Baldwin K, et al. The limitations of X-ray absorption spectroscopy for determining the structure of zinc sites in proteins. When is a tetrathiolate not a tetrathiolate? J Am Chem Soc. 1998;120(33):8401–8409. [Google Scholar]
- 31.Rhinn H, et al. Alternative α-synuclein transcript usage as a convergent mechanism in Parkinson’s disease pathology. Nat Commun. 2012;3:1084. doi: 10.1038/ncomms2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson GM. RNA folding and RNA-protein binding analyzed by fluorescence anisotropy and resonance engery transfer. In: Geddes CD, Lakowicz JR, editors. Reviews in Fluorescence. Vol 2. Springer; New York: 2005. pp. 223–243. [Google Scholar]
- 33.Barabino SM, Hübner W, Jenny A, Minvielle-Sebastia L, Keller W. The 30-kD subunit of mammalian cleavage and polyadenylation specificity factor and its yeast homolog are RNA-binding zinc finger proteins. Genes Dev. 1997;11(13):1703–1716. doi: 10.1101/gad.11.13.1703. [DOI] [PubMed] [Google Scholar]
- 34.Chen S, et al. Structure of N-terminal domain of ZAP indicates how a zinc-finger protein recognizes complex RNA. Nat Struct Mol Biol. 2012;19(4):430–435. doi: 10.1038/nsmb.2243. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen J, Kristensen MA, Willemoës M, Nielsen FC, Christiansen J. Sequential dimerization of human zipcode-binding protein IMP1 on RNA: A cooperative mechanism providing RNP stability. Nucleic Acids Res. 2004;32(14):4368–4376. doi: 10.1093/nar/gkh754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daugherty MD, Liu B, Frankel AD. Structural basis for cooperative RNA binding and export complex assembly by HIV Rev. Nat Struct Mol Biol. 2010;17(11):1337–1342. doi: 10.1038/nsmb.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajendran KS, Nagy PD. Characterization of the RNA-binding domains in the replicase proteins of tomato bushy stunt virus. J Virol. 2003;77(17):9244–9258. doi: 10.1128/JVI.77.17.9244-9258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huelga SC, et al. Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Reports. 2012;1(2):167–178. doi: 10.1016/j.celrep.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zucconi BE, et al. Alternatively expressed domains of AU-rich element RNA-binding protein 1 (AUF1) regulate RNA-binding affinity, RNA-induced protein oligomerization, and the local conformation of bound RNA ligands. J Biol Chem. 2010;285(50):39127–39139. doi: 10.1074/jbc.M110.180182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fialcowitz-White EJ, et al. Specific protein domains mediate cooperative assembly of HuR oligomers on AU-rich mRNA-destabilizing sequences. J Biol Chem. 2007;282(29):20948–20959. doi: 10.1074/jbc.M701751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson GM, Sutphen K, Bolikal S, Chuang KY, Brewer G. Thermodynamics and kinetics of Hsp70 association with A + U-rich mRNA-destabilizing sequences. J Biol Chem. 2001;276(48):44450–44456. doi: 10.1074/jbc.M108521200. [DOI] [PubMed] [Google Scholar]
- 42.Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci USA. 2007;104(13):5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conlan AR, et al. Crystal structure of Miner1: The redox-active 2Fe-2S protein causative in Wolfram Syndrome 2. J Mol Biol. 2009;392(1):143–153. doi: 10.1016/j.jmb.2009.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Zhang L, Lai S, Ye K. Structure and molecular evolution of CDGSH iron-sulfur domains. PLoS One. 2011;6(9):e24790. doi: 10.1371/journal.pone.0024790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baxter EL, Jennings PA, Onuchic JN. Strand swapping regulates the iron-sulfur cluster in the diabetes drug target mitoNEET. Proc Natl Acad Sci USA. 2012;109(6):1955–1960. doi: 10.1073/pnas.1116369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanc B, Gerez C, Ollagnier de Choudens S. Assembly of Fe/S proteins in bacterial systems: Biochemistry of the bacterial ISC system. Biochim Biophys Acta. 2015;1853(6):1436–1447. doi: 10.1016/j.bbamcr.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Bothe JR, Alderson TR, Markley JL. Tangled web of interactions among proteins involved in iron-sulfur cluster assembly as unraveled by NMR, SAXS, chemical crosslinking, and functional studies. Biochim Biophys Acta. 2015;1853(6):1416–1428. doi: 10.1016/j.bbamcr.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, et al. The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry. 2009;48(40):9569–9581. doi: 10.1021/bi901182w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rouault TA. Iron-sulfur proteins hiding in plain sight. Nat Chem Biol. 2015;11(7):442–445. doi: 10.1038/nchembio.1843. [DOI] [PubMed] [Google Scholar]
- 50.Bak DW, Elliott SJ. Alternative FeS cluster ligands: Tuning redox potentials and chemistry. Curr Opin Chem Biol. 2014;19:50–58. doi: 10.1016/j.cbpa.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Netz DJ, Mascarenhas J, Stehling O, Pierik AJ, Lill R. Maturation of cytosolic and nuclear iron-sulfur proteins. Trends Cell Biol. 2014;24(5):303–312. doi: 10.1016/j.tcb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Landry AP, Cheng Z, Ding H. Reduction of mitochondrial protein mitoNEET [2Fe-2S] clusters by human glutathione reductase. Free Radic Biol Med. 2015;81:119–127. doi: 10.1016/j.freeradbiomed.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saouma CT, Pinney MM, Mayer JM. Electron transfer and proton-coupled electron transfer reactivity and self-exchange of synthetic [2Fe-2S] complexes: Models for Rieske and mitoNEET clusters. Inorg Chem. 2014;53(6):3153–3161. doi: 10.1021/ic403131p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landry AP, Ding H. Redox control of human mitochondrial outer membrane protein MitoNEET [2Fe-2S] clusters by biological thiols and hydrogen peroxide. J Biol Chem. 2014;289(7):4307–4315. doi: 10.1074/jbc.M113.542050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dastidar RG, et al. The nuclear localization of SWI/SNF proteins is subjected to oxygen regulation. Cell Biosci. 2012;2(1):30. doi: 10.1186/2045-3701-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chakrabarti M, Hunt AG. CPSF30 at the interface of alternative polyadenylation and cellular signaling in plants. Biomolecules. 2015;5(2):1151–1168. doi: 10.3390/biom5021151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grodick MA, Segal HM, Zwang TJ, Barton JK. DNA-mediated signaling by proteins with 4Fe-4S clusters is necessary for genomic integrity. J Am Chem Soc. 2014;136(17):6470–6478. doi: 10.1021/ja501973c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pheeney CG, Arnold AR, Grodick MA, Barton JK. Multiplexed electrochemistry of DNA-bound metalloproteins. J Am Chem Soc. 2013;135(32):11869–11878. doi: 10.1021/ja4041779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romano CA, Sontz PA, Barton JK. Mutants of the base excision repair glycosylase, endonuclease III: DNA charge transport as a first step in lesion detection. Biochemistry. 2011;50(27):6133–6145. doi: 10.1021/bi2003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee PE, Demple B, Barton JK. DNA-mediated redox signaling for transcriptional activation of SoxR. Proc Natl Acad Sci USA. 2009;106(32):13164–13168. doi: 10.1073/pnas.0906429106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boal AK, et al. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry. 2005;44(23):8397–8407. doi: 10.1021/bi047494n. [DOI] [PubMed] [Google Scholar]
- 62.Boon EM, Livingston AL, Chmiel NH, David SS, Barton JK. DNA-mediated charge transport for DNA repair. Proc Natl Acad Sci USA. 2003;100(22):12543–12547. doi: 10.1073/pnas.2035257100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poor CB, et al. Molecular mechanism and structure of the Saccharomyces cerevisiae iron regulator Aft2. Proc Natl Acad Sci USA. 2014;111(11):4043–4048. doi: 10.1073/pnas.1318869111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook JD, et al. Molecular details of the yeast frataxin-Isu1 interaction during mitochondrial Fe-S cluster assembly. Biochemistry. 2010;49(40):8756–8765. doi: 10.1021/bi1008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Randall CR, et al. X-ray absorption pre-edge studies of high-spin iron(II) complexes. Inorg Chem. 1995;34(5):1036–1039. [Google Scholar]
- 66.Bencze KZ, Kondapalli KC, Stemmler TL. Applications of Physical Methods to Inorganic and Bioinorganic Chemistry. Wiley; Chichester, UK: 2007. X-ray absorption spectroscopy; pp. 513–528. [Google Scholar]
- 67.Wang B, et al. Structure and ubiquitin interactions of the conserved zinc finger domain of Npl4. J Biol Chem. 2003;278(22):20225–20234. doi: 10.1074/jbc.M300459200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cotelesage JJ, Pushie MJ, Grochulski P, Pickering IJ, George GN. Metalloprotein active site structure determination: Synergy between X-ray absorption spectroscopy and X-ray crystallography. J Inorg Biochem. 2012;115:127–137. doi: 10.1016/j.jinorgbio.2012.06.019. [DOI] [PubMed] [Google Scholar]