Significance
Pupylation is a posttranslational protein modification discovered in Mycobacterium tuberculosis in which it tags proteins for degradation via the proteasome. It thus resembles eukaryotic ubiquitination. In mycobacteria, pupylation plays a role under oxidative stress and under carbon and nitrogen starvation. Intriguingly, many bacteria containing the pupylation machinery lack a proteasome, such as corynebacteria, leaving the function of this protein modification open. We show that pupylation in Corynebacterium glutamicum plays a dedicated role in iron homeostasis by targeting the iron-storage protein ferritin. Evidence is provided that pupylation triggers the disassembly of 24-meric ferritin by ARC to support the release of the stored iron without using a protease. Thus, a physiological function of pupylation was discovered for a proteasome-free bacterial species.
Keywords: iron limitation, prokaryotic ubiquitin-like protein, ATPase ARC, Corynebacterium, Mycobacterium
Abstract
The balance of sufficient iron supply and avoidance of iron toxicity by iron homeostasis is a prerequisite for cellular metabolism and growth. Here we provide evidence that, in Actinobacteria, pupylation plays a crucial role in this process. Pupylation is a posttranslational modification in which the prokaryotic ubiquitin-like protein Pup is covalently attached to a lysine residue in target proteins, thus resembling ubiquitination in eukaryotes. Pupylated proteins are recognized and unfolded by a dedicated AAA+ ATPase (Mycobacterium proteasomal AAA+ ATPase; ATPase forming ring-shaped complexes). In Mycobacteria, degradation of pupylated proteins by the proteasome serves as a protection mechanism against several stress conditions. Other bacterial genera capable of pupylation such as Corynebacterium lack a proteasome, and the fate of pupylated proteins is unknown. We discovered that Corynebacterium glutamicum mutants lacking components of the pupylation machinery show a strong growth defect under iron limitation, which was caused by the absence of pupylation and unfolding of the iron storage protein ferritin. Genetic and biochemical data support a model in which the pupylation machinery is responsible for iron release from ferritin independent of degradation.
Pupylation is a posttranslational protein modification occurring in the phylum Actinobacteria and some other bacterial lineages, such as Nitrospirae (1, 2). It resembles eukaryotic ubiquitination and was first identified in Mycobacterium tuberculosis (3). Target proteins are covalently linked to the small prokaryotic ubiquitin-like protein (Pup), which, in mycobacteria, can serve as a tag for degradation via the proteasome (3–5). The proteasomal genes prcA and prcB are encoded within the pup gene cluster of mycobacteria (6). However, several Actinobacteria harbor genes of the pupylation machinery but lack the genes encoding the proteasome, raising the question of the fate of pupylated proteins in proteasome-free species (1, 6).
The process of Pup-mediated protein degradation in mycobacteria comprises several steps. First, the 64-aa residue-protein Pup is activated via deamidation of the C-terminal glutamine residue to glutamate, catalyzed by the deamidase of Pup (Dop) (3, 7, 8). Pup is then covalently attached to target proteins by the proteasome accessory factor A (PafA). PafA catalyzes the ATP-dependent formation of an isopeptide bond between the γ-carboxyl group of Pup and the ε-amino group of a lysine residue within the target protein (8–10). Mycobacterium proteasomal AAA+ ATPase (Mpa), termed ATPase forming ring-shaped complexes (ARCs) in nonmycobacterial species, recognizes pupylated proteins, unfolds them, and directs them into the proteasome for degradation (11, 12). Besides its function as a deamidase, Dop was also shown to catalyze the depupylation of substrates (13, 14). Some species, such as members of the genera Corynebacterium and Streptomyces, encode Pup variants with a carboxyl-terminal glutamate residue, which therefore do not require the deamidation step. In these bacteria, Dop may serve exclusively as depupylase.
Hitherto, proteome-wide searches revealed several pupylated proteins (pupylomes) in a range of proteasome-bearing Actinobacteria (15–19). The proteins making up the pupylomes covered a broad spectrum of functional categories, which might be explained by a general recycling function fulfilled by pupylation. In this view, protein degradation mediated by pupylation is assumed to recycle amino acids under several stress conditions in Mycobacterium smegmatis (20). Although pupylation was shown to target proteins to proteasome-mediated degradation, not all pupylated proteins are subject to this fate (15, 21). Furthermore, the activity of the mycobacterial ATPase Mpa itself was shown to be reversibly regulated by pupylation, which renders Mpa functionally inactive (22). In view of these results, the investigation of pupylation in proteasome-lacking Actinobacteria promises new insights into its physiological role(s) and the fate of pupylated proteins.
Corynebacterium glutamicum is a member of the Actinobacteria harboring genes for the pupylation machinery (pup, dop, pafA, arc; Fig. 1A) but lacking the proteasomal genes prcAB. In a recent proteomics study, we identified 55 pupylated proteins in this species (23). C. glutamicum is a nonpathogenic Gram-positive soil bacterium, which has become a model organism for studying metabolism and regulation (24). As the physiological function of pupylation in this organism remained enigmatic, we screened for a phenotype of the Δpup mutant during growth with various carbon sources and under different stress conditions. A severe growth defect was observed only under iron limitation. Detailed studies revealed that this phenotype is caused almost exclusively by the lack of pupylation of the iron-storage protein ferritin, and we provide evidence that this could result from a defect in iron release from nonpupylated ferritin. These results disclose a distinctive role of pupylation in ferritin-mediated iron homeostasis and add another level of complexity to the control of iron homeostasis.
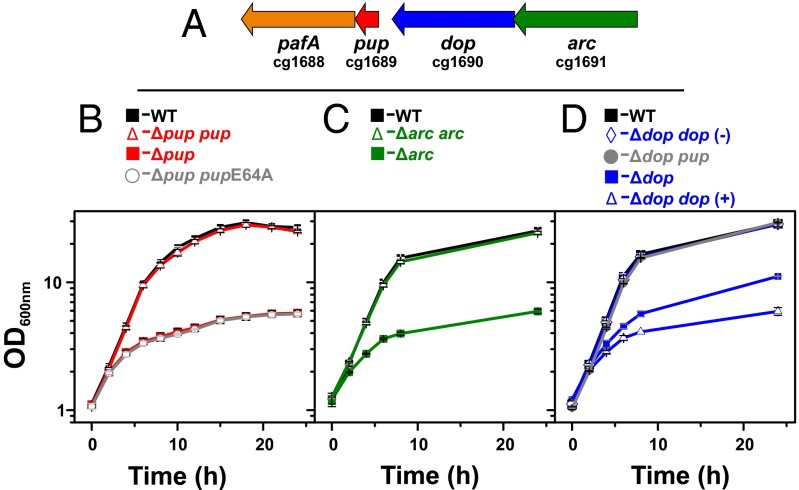
Fig. 1.
Importance of the pupylation machinery for growth of C. glutamicum ATCC 13032 under iron limitation. (A) Genetic organization of pupylation-related genes. (B–D) Growth of the WT (black squares) and pupylation-deficient mutants cultivated in glucose minimal medium under iron limitation (1 µM FeSO4). The strains are indicated at the top and represent: (B) Δpup (red squares), Δpup/pVWEx1-pup (red triangles), and Δpup/pVWEx1-pupE64A (open circles); (C) Δarc/pVWEx1 (green squares) and Δarc/pVWEx1-arc (green triangles); and (D) Δdop/pVWEx1 (blue squares) and Δdop/pVWEx1-dop in the presence (blue triangles) and absence (blue diamonds) of 1 mM IPTG, and Δdop/pVWEx1-pup (filled circles). Mean values and SDs were obtained from three independent biological replicates.
Results
Deletion of pup Results in a Growth Defect Under Iron Limitation.
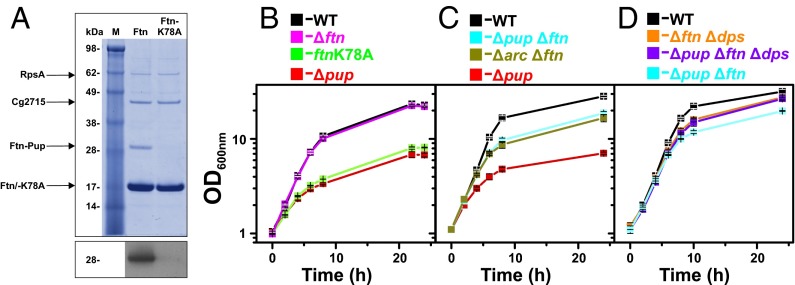
We recently identified 55 pupylated proteins, including their pupylation sites in C. glutamicum cells cultivated in a rich medium. However, pupylation was dispensable under standard cultivation conditions as a mutant strain lacking the pup gene showed no growth defect (23). To get hints on the physiological function of this posttranslational modification in C. glutamicum, we compared the growth of the Δpup mutant and WT in media with different carbon or nitrogen sources and under various stress conditions (Table S1). The two strains grew comparably under almost all tested conditions, including nitrite stress, which was shown to affect pupylation-defective mycobacteria (25). However, a severe growth defect was observed when the Δpup mutant of C. glutamicum was cultivated under iron limitation using glucose minimal medium supplemented with 1 µM FeSO4 instead of the standard 36 µM. The final biomass (measured as OD600) of the WT was four to five times higher than that of the Δpup mutant (26.6 ± 0.9 vs. 6.1 ± 0.3) in these cultures (Fig. 1B). The growth defect was observed at iron concentrations of ≤1 µM (Fig. S1) for cultures that had been precultivated under iron limitation (Fig. S2). This suggests that pupylation is particularly important for long-term adaptation to iron limitation. Based on these results, all following experiments were performed with glucose minimal medium supplemented with 1 µM FeSO4 in the preculture and main culture if not stated otherwise. Successful complementation and suppression of the growth phenotype was obtained by transformation of the Δpup mutant with the plasmid pVWEx1-pup, driving expression of native Pup using an isopropyl-β-d-thiogalactoside (IPTG)-inducible tac promoter. In contrast, transformation of the Δpup mutant with the plasmid pVWEx1-pup-E64A, encoding a pupylation-incompetent Pup-E64A variant with a C-terminal alanine residue, did not allow reversal of the growth defect (Fig. 1B). This result confirmed that the growth phenotype was indeed a result of the lack of a functional Pup protein and not caused by any secondary mutations in the Δpup mutant.
Table S1.
Growth conditions tested with C. glutamicum WT and Δpup mutant in this study
| Condition | Description |
| Carbon sources | Concentration of the carbon source in CGXII minimal medium |
| Acetate* | 2% (wt/vol) acetic acid |
| Lactate* | 100 mM sodium l-lactate |
| Gluconate* | 100 mM sodium gluconate |
| Citrate* | 50 mM trisodium citrate + 5 mM CaCl2 |
| Arabitol | 0.8% (wt/vol) d-arabitol |
| Glutamine | 70 mM l-glutamine [as sole carbon and nitrogen source: no addition of urea and (NH4)2SO4 to CGXII medium] |
| Other conditions | In CGXII minimal medium with 4% (wt/vol) glucose |
| Heat stress | Incubation at 55 °C for 5, 10, or 15 min at an OD600 of 5 followed by further incubation at 30 °C |
| Nitrosative stress | CGXII minimal medium with pH 5.5 instead of 7.0 (Mes instead of Mops buffer system) supplemented with 0, 3, or 10 mM NaNO2 using an initial OD600 of 0.1 instead of 1.0 |
| DTT* | Addition of 1 or 0.2 g/L DTT |
| Nitrogen starvation | At an OD600 of 5, cells were harvested, washed, and transferred to CGXII minimal medium with 4% (wt/vol) glucose without addition of urea and (NH4)2SO4; the number of cfu/mL was determined daily over a period of at least 3 d |
| Cu excess* | 20 µM CuSO4 added to CGXII minimal medium (total Cu2+ concentration 21.5 µM) |
| -Mn* | Medium contained trace elements solution without MnSO4 |
| -Zn* | Medium contained trace elements solution without ZnSO4 |
| -Cu* | Medium contained trace elements solution without CuSO4 |
| -Ni* | Medium contained trace elements solution without NiCl2 |
| Low Mg* | Medium contained 2.5 mg/L instead of 250 mg/L MgSO4 |
| Low Ca | Medium contained 0.1 mg/L instead of 10 mg/L CaCl2 |
| Iron concentrations | In CGXII minimal medium with 4% (wt/vol) glucose |
| High iron | Medium contained 100 µM FeSO4 instead of 36 µM FeSO4 |
| Low iron* | Medium contained 10, 5, 1, or 0.3 µM FeSO4 (medium not pretreated with chelator) |
| No iron | Medium contained trace elements solution without FeSO4; medium pretreated with Chelex-100 for 2 h before addition of trace elements solution |
Deviations to the standard cultivation conditions [CGXII medium with 4% (wt/vol) glucose] are indicated.
Cultivation conditions performed in BioLector cultivation system after precultivation in BHI complex medium. If not stated otherwise, all other cultivations were performed in shake flasks and cells were precultivated in the same medium as the main culture.
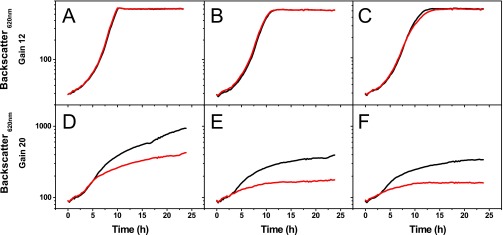
Fig. S1.
Growth of C. glutamicum WT (black) and the Δpup mutant (red) in the presence of different concentrations of Fe2+. The strains were cultivated in CGXII minimal medium with 4% (wt/vol) glucose from which the usually added 36 µM FeSO4 was omitted. Before inoculation with cells precultivated in BHI medium, the CGXII medium was supplemented with (A) 36 µM, (B) 10 µM, (C) 5 µM, (D) 1 µM, (E) 0.1 µM, or (F) 0.05 µM FeSO4. As expected, no difference was observed between cultures in E and F as a result of the contaminating iron concentration of 0.34 µM measured in the minimal medium without added FeSO4. Cultivation was performed in the BioLector system (m2p Laboratories) with 48-well flower plates, and cell density was measured as backscatter at 620 nm. The figure shows the mean values from two biological replicates that showed less than 10% deviation. Please note the different gains used for A–C vs. D–F. The gain represents the sensitivity of the detector (range, 1–100), with higher values representing a higher sensitivity.
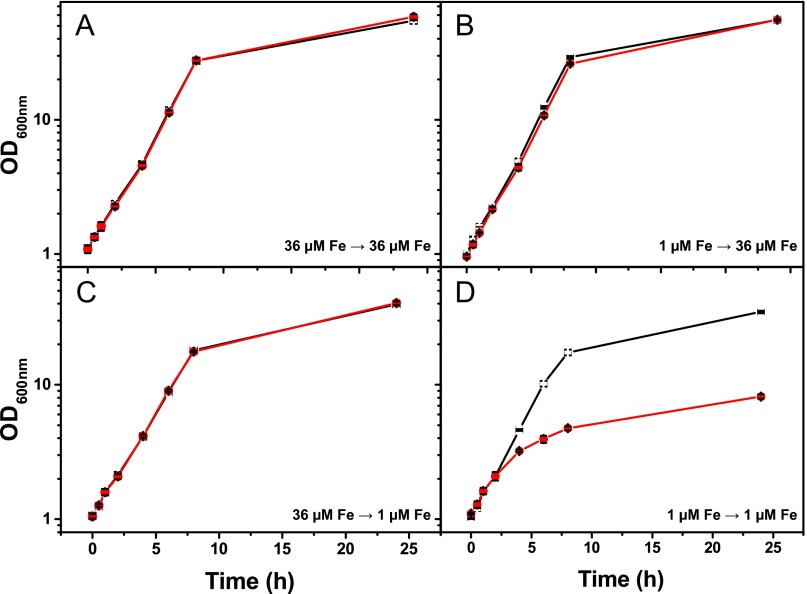
Fig. S2.
Growth of C. glutamicum WT (black squares; error bars white) and the Δpup mutant (red circles; error bars black) in media containing different iron concentrations. Cells were precultivated in CGXII minimal medium with 4% (wt/vol) glucose containing 36 µM FeSO4 (A and C) or 1 µM FeSO4 (B and D) and then transferred to CGXII medium containing 36 µM (A and B) or 1 µM (C and D) FeSO4. The figure shows mean values of three independent biological replicates. Cultivation was performed in 500-mL shake flasks containing 50 mL medium.
ARC and Dop Are Also Involved in the Adaptation to Iron Limitation.
In M. tuberculosis, pupylated proteins are recognized by the AAA+ ATPase Mpa, which unfolds Pup targets for subsequent proteasomal degradation. We therefore tested whether ARC, the homolog of Mpa in C. glutamicum, is also required for optimal growth under iron limitation. A mutant with an in-frame deletion of arc was constructed and exhibited a growth defect under iron limitation similar to the Δpup mutant, which could be reversed by plasmid-based expression of arc using plasmid pVWEx1-arc (Fig. 1C).
Besides Pup and ARC, the deamidase/depupylase Dop plays an important role during pupylation. An in-frame dop deletion mutant of C. glutamicum also showed a growth defect under iron limitation, which was, however, not as severe as the one observed for the Δpup and Δarc mutants. The Δdop mutant reached approximately 50% of the final biomass of the WT (Fig. 1D), indicating that Dop is required for optimal adaptation to iron limitation, but not as important as Pup or ARC. In contrast to the successful complementation of the Δpup and Δarc mutants, IPTG-induced overexpression of the dop gene in the Δdop strain with plasmid pVWEx1-dop resulted in an even more pronounced growth defect under iron limitation rather than in its suppression (Fig. 1D). In contrast, no growth defect caused by dop overexpression was observed under iron-replete conditions. Presumably, overexpression of dop led to an unphysiologically high depupylase activity, which antagonized pupylation and caused a growth defect similar to the one observed for the Δpup and the Δarc mutants (Fig. 1 B and C). On the contrary, when the Δdop strain carrying pVWEx-dop was cultivated in the absence of IPTG, the growth defect could be reversed, presumably because the basal tac promoter activity in the absence of IPTG allowed the synthesis of physiological Dop levels (Fig. 1D).
In summary, the results suggest that Dop is necessary for ensuring a critical level of free or recycled Pup in the cell. If this assumption holds true, artificially increased Pup levels should also result in a suppression of the growth defect of the Δdop mutant. Indeed, overexpression of pup in the Δdop strain using plasmid pVWEx1-pup led to WT-like growth under iron limitation (Fig. 1D).
The Δpup Mutant Senses a Stronger Iron Limitation than WT.
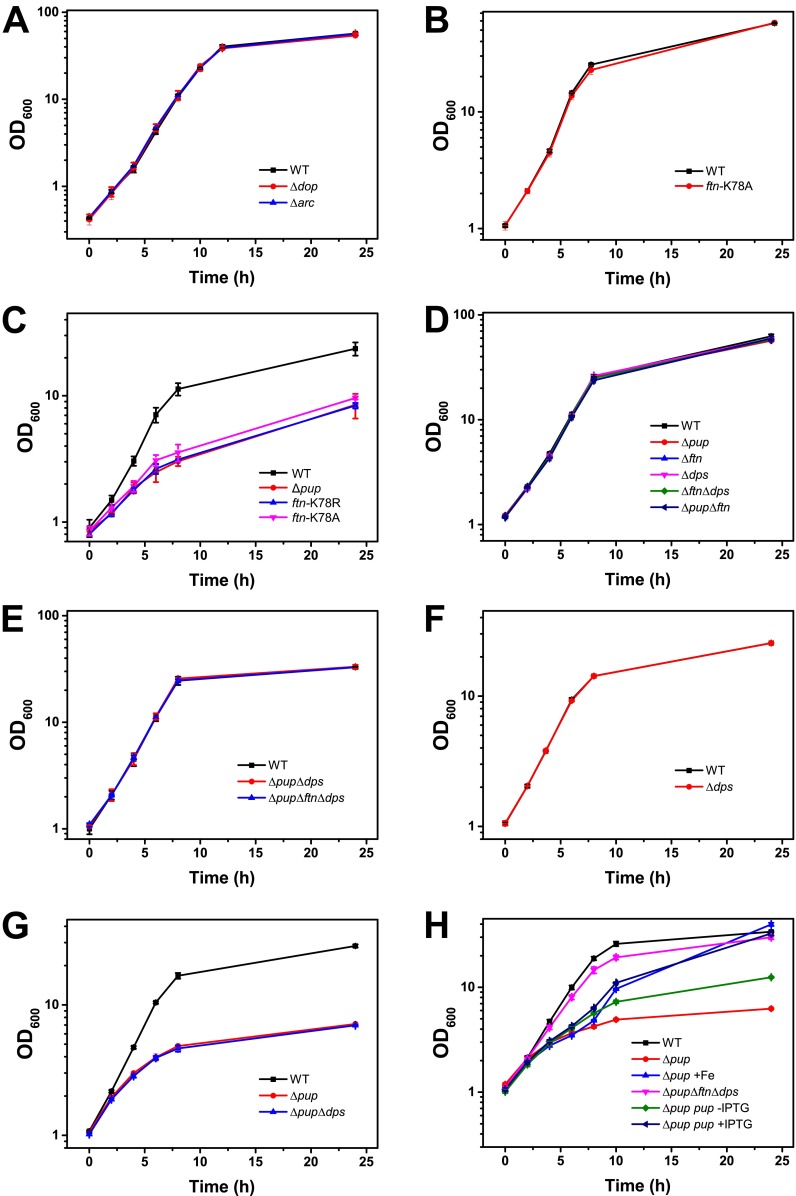
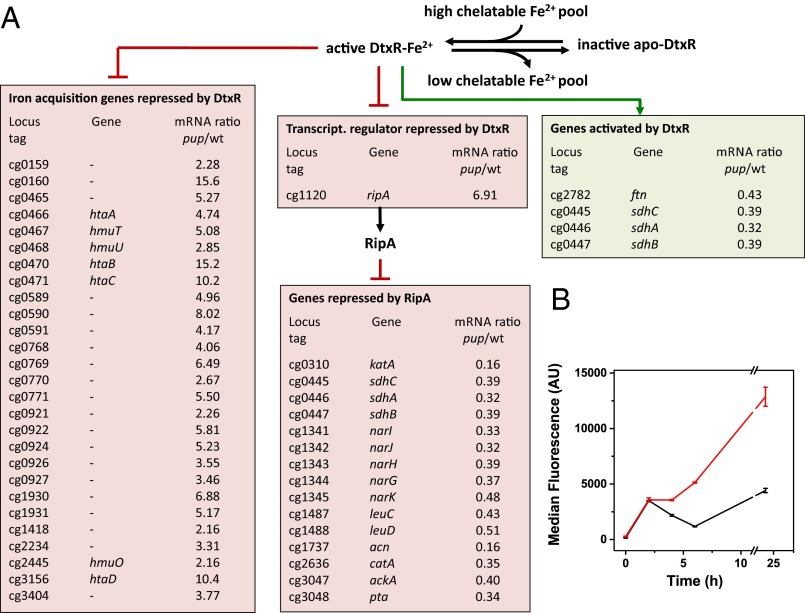
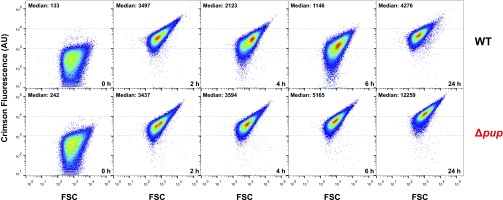
Neither the Δpup mutant (23) nor the Δarc and Δdop mutants (Fig. S3A) showed a growth defect under iron sufficiency (36 µM FeSO4), pointing to a specific role of pupylation for adaptation to iron limitation. To understand why pupylation is critical for this process, genome-wide mRNA levels of the Δpup mutant and the WT strain were compared under iron-limiting growth conditions by using DNA microarrays. The samples for RNA isolation were taken in the early exponential phase (OD600 of 3), when the growth curves of the two strains just started to diverge (Fig. 1B). Overall, 121 genes showed at least a twofold change in transcript levels in the Δpup mutant, including 33 genes (∼50%) of the DtxR regulon and the entire RipA regulon (Fig. 2A and Table S2). DtxR is the master regulator of iron homeostasis, serving as a sensor for chelatable cytosolic Fe2+, which binds to its target promoters only when complexed with Fe2+ (26). In C. glutamicum, DtxR represses 59 genes, the majority being involved in iron acquisition, and activates five genes, including ftn and dps encoding the iron-storage proteins ferritin (cg2782) and DNA-binding protein from starved cells (Dps; cg3327) (27, 28). One of the repressed genes encodes the AraC-type regulator of iron proteins, RipA, which itself represses a number of prominent iron-containing proteins under iron limitation, such as aconitase or succinate dehydrogenase, serving an analogous function as the regulatory small RNA RyhB in Escherichia coli (29). To independently confirm the DNA microarray data, we followed ripA expression in the WT and the Δpup mutant by using a plasmid-based transcriptional fusion with the autofluorescent reporter protein E2-Crimson. The cell-specific median fluorescence of 100,000 cells each was determined by flow cytometry at different points during cultivation under iron limitation. As shown in Fig. 2B, the ripA promoter was much more strongly activated in the Δpup mutant than in the WT, in agreement with the transcriptome data. These results suggest that the Δpup mutant has a lower chelatable cytosolic Fe2+ concentration than the WT, causing a shift of DtxR to the DNA binding-incompetent apo-form.
Fig. S3.
Growth of C. glutamicum WT (black squares) and the indicated C. glutamicum mutant strains in media containing different iron concentrations. Cells were cultivated in CGXII minimal medium with 4% (wt/vol) glucose containing 36 µM FeSO4 (A, B, D, and E) or 1 µM FeSO4 (C, F, G, and H). In the experiment in H, addition of 10 µM FeSO4 to a culture of the C. glutamicum Δpup mutant (blue upright triangles) 6 h after inoculation was able to abolish the growth defect. Similarly, induction of pup expression in strain C. glutamicum Δpup pVWEx1-pup (dark blue tilted triangle) by IPTG addition after 6 h improved growth compared with a control culture without IPTG (green diamonds). These results confirm that the growth defect of the Δpup mutant is caused by iron limitation and the absence of pup, respectively. The figure shows mean values of three independent biological replicates. Cultivation was performed in 500-mL shake flasks containing 50 mL medium.
Fig. 2.
(A) Influence of a pup deletion on global gene expression under iron-limitation conditions. The transcriptional network controlling iron homeostasis in C. glutamicum involves the Fe2+-sensing master regulator DtxR and the AraC-type regulator RipA. Under iron-replete conditions, DtxR is complexed with Fe2+, and only then does it bind to its target promoters. The boxes show those DtxR and RipA target genes, whose mRNA ratio was changed at least twofold in a transcriptome comparison of the Δpup mutant and the WT under iron-limitation conditions. A list of all genes showing differential expression in the Δpup mutant is given in Table S2. (B) Comparison of ripA promoter activity in C. glutamicum WT (black) and the Δpup mutant (red) using the reporter plasmid pJC1-PripA-crimson. The two strains were cultivated under iron limitation, and samples were taken after 0, 2, 4, 6, and 24 h. A total of 100,000 cells of each sample were analyzed by flow cytometry for Crimson fluorescence. Two biological replicates were performed, and error bars indicate the deviation between the samples. Scatter plots of the samples are shown in Fig. S9.
Table S2.
Results of global transcriptome analyses of C. glutamicum Δpup mutant vs. ATCC 13032 WT grown in CGXII minimal medium with 4% (wt/vol) glucose supplemented with 1 µM FeSO4
| Locus tag | Gene name | Annotation | mRNA ratio, Δpup/WT | DtxR target | RipA target |
| cg0012 | ssuR | Sulphonate sulfur utilization transcriptional regulator | 2.15 | — | — |
| cg0018 | — | Putative conserved membrane protein | 2.08 | — | — |
| cg0159 | — | Hypothetical protein | 2.28 | R | — |
| cg0160 | — | Hypothetical protein | 15.60 | R | — |
| cg0177 | — | Hypothetical protein | 3.14 | — | — |
| cg0310 | katA | Catalase | 0.16 | — | R |
| cg0405 | — | Putative ABC-type iron(III) dicitrate transporter, secreted siderophore-binding lipoprotein | 3.01 | R | — |
| cg0445 | sdhC | Succinate:menachinone oxidoreductase, cytochrome b | 0.39 | A | R |
| cg0446 | sdhA | Succinate:menachinone oxidoreductase, flavoprotein subunit | 0.32 | A | R |
| cg0447 | sdhB | Succinate:menachinone oxidoreductase, iron sulfur protein | 0.39 | A | R |
| cg0465 | — | Putative membrane protein, conserved | 5.27 | R | — |
| cg0466 | htaA | Secreted heme transport-associated protein | 4.74 | R | — |
| cg0467 | hmuT | Hemin-binding periplasmic protein precursor | 5.08 | R | — |
| cg0468 | hmuU | Hemin transport system, permease protein | 2.85 | R | — |
| cg0470 | htaB | Secreted heme transport-associated protein | 15.19 | R | — |
| cg0471 | htaC | Secreted heme transport-associated protein | 10.17 | R | — |
| cg0506 | — | Putative spermidine/putrescine/iron(III) transporter, ATPase subunit | 0.42 | — | — |
| cg0507 | — | Putative spermidine/putrescine/iron(III) transporter, permease subunit | 0.39 | — | — |
| cg0508 | — | Putative spermidine/putrescine/iron(III) transporter, substrate-binding lipoprotein | 0.39 | — | — |
| cg0543 | — | Hypothetical protein | 0.50 | — | — |
| cg0589 | — | Putative siderophore ABC transporter, ATP-binding protein | 4.96 | R | — |
| cg0590 | — | Putative siderophore ABC transporter, permease protein | 8.02 | R | — |
| cg0591 | — | Putative siderophore ABC transporter, permease protein | 4.17 | R | — |
| cg0701 | — | Putative drug/metabolite transporter, DMT superfamily | 2.08 | — | — |
| cg0737 | — | Putative high affinity ABC-type methionine transporter, substrate-binding lipoprotein | 2.25 | — | — |
| cg0755 | metY | O-Acetylhomoserine sulfhydrylase | 2.22 | — | — |
| cg0759 | prpD2 | 2-Methylcitrate dehydratase, involved in propionate catabolism | 0.33 | — | — |
| cg0760 | prpB2 | 2-Methylisocitrate lyase, involved in propionate catabolism | 0.30 | — | — |
| cg0762 | prpC2 | 2-Methylcitrate synthase, involved in propionate catabolism | 0.28 | — | — |
| cg0767 | — | Putative cytoplasmic siderophore-interacting protein | 2.23 | — | — |
| cg0768 | — | Putative iron-siderophore ABC transporter, ATP-binding protein | 4.06 | R | — |
| cg0769 | — | Putative iron-siderophore ABC transporter, permease subunit | 6.49 | R | — |
| cg0770 | — | Putative iron-siderophore ABC transporter, permease subunit | 2.67 | R | — |
| cg0771 | irp1 | Putative iron-siderophore ABC transporter, secreted siderophore-binding lipoprotein | 5.50 | R | — |
| cg0812 | dtsR1 | Acetyl-/propionyl-CoA carboxylase, β-subunit | 2.15* | — | — |
| cg0921 | — | Putative cytoplasmic siderophore-interacting protein | 2.26 | R | — |
| cg0922 | — | Putative secreted siderophore-binding lipoprotein | 5.81 | R | — |
| cg0924 | — | Putative ABC-type iron-siderophore transporter, substrate-binding lipoprotein | 5.23 | R | — |
| cg0926 | — | Putative iron-siderophore transporter, permease subunit | 3.55 | R | — |
| cg0927 | — | Putative iron-siderophore ABC transporter, permease subunit | 3.46 | R | — |
| cg0961 | — | Putative homoserine O-acetyltransferase | 0.45 | — | |
| cg1120 | ripA | Transcriptional regulator of iron proteins and repressor of aconitase, AraC family | 6.91 | R | — |
| cg1129 | aroF | 3-Deoxy-7-phosphoheptulonate synthase | 2.38 | — | — |
| cg1169 | metP | Na+:methionine symporter, SNF-family | 0.39 | — | — |
| cg1227 | ykoE | Thiamine-regulated ECF transporter for hydroxymethylpyrimidine, substrate-specific component | 6.87 | — | — |
| cg1228 | ykoD | Thiamine-regulated ECF transporter for hydroxymethylpyrimidine, duplicated ATPase component | 2.37* | — | — |
| cg1229 | ykoC | Thiamine-regulated ECF transporter for hydroxymethylpyrimidine, transmembrane component | 7.38 | — | — |
| cg1230 | — | Hypothetical protein | 2.02* | — | — |
| cg1291 | — | Putative membrane protein | 2.47 | — | — |
| cg1299 | cydD | ABC transporter, subunit II, essential for cytochrome bd oxidase assembly | 0.31 | — | — |
| cg1300 | cydB | Cytochrome bd oxidase, subunit II | 0.42 | — | — |
| cg1341 | narI | Nitrate reductase, γ-subunit, cytochrome b | 0.33 | — | R |
| cg1342 | narJ | Nitrate reductase, δ-subunit, assembly factor | 0.32 | — | R |
| cg1343 | narH | Nitrate reductase, β-subunit, iron sulfur protein | 0.39 | — | R |
| cg1344 | narG | Nitrate reductase, α-subunit | 0.37 | — | R |
| cg1345 | narK | Nitrate/nitrite antiporter | 0.48* | — | R |
| cg1418 | — | Putative secreted siderophore-binding lipoprotein | 2.16 | R | — |
| cg1419 | — | Putative Na+-dependent transporter, BASS family | 3.57 | — | — |
| cg1432 | ilvD | Dihydroxy-acid dehydratase | 0.41* | — | — |
| cg1435 | ilvB | Acetolactate synthase I, large subunit | 0.42* | — | — |
| cg1436 | ilvN | Acetolactate synthase, small subunit | 0.41 | — | — |
| cg1475 | — | Hypothetical protein | 3.25 | — | — |
| cg1476 | thiC | Thiamine biosynthesis protein ThiC | 10.08 | — | — |
| cg1487 | leuC | Isopropylmalate isomerase, large subunit | 0.43 | — | R |
| cg1488 | leuD | Isopropylmalate isomerase, small subunit | 0.51† | — | R |
| cg1514 | — | Putative secreted protein, CGP1 Region | 2.00 | — | — |
| cg1580 | argC | N-acetyl-γ-glutamyl-phosphate reductase | 2.45 | — | — |
| cg1581 | argJ | Monofunctional NAGS N-acetyl glutamate synthase | 3.43 | — | — |
| cg1582 | argB | Acetylglutamate kinase | 3.79 | — | — |
| cg1583 | argD | Acetylornithine aminotransferase | 5.09 | — | — |
| cg1584 | argF | Ornithine carbamoyltransferase | 3.29 | — | — |
| cg1585 | argR | Transcriptional repressor of arginine biosynthesis, ArgR family | 2.88 | — | — |
| cg1612 | — | Putative acetyltransferase | 0.27 | — | — |
| cg1626 | — | Hypothetical protein | 2.67 | — | — |
| cg1654 | thiD1 | Thiamine-phosphate pyrophosphorylase | 2.47 | — | — |
| cg1655 | thiM | Hydroxyethylthiazole kinase | 2.02 | — | — |
| cg1694 | recB | Exonuclease, RecB family | 0.49 | — | — |
| cg1695 | — | Putative plasmid maintenance system antidote protein | 0.40 | — | — |
| cg1701 | metH | Homocysteine methyltransferase, methionine synthase | 2.25 | — | — |
| cg1724 | meaB | Accessory protein of methylmalonyl-CoA mutase | 0.46 | — | — |
| cg1725 | mutA | Methylmalonyl-CoA mutase, α-subunit | 0.46 | — | — |
| cg1736 | — | Putative membrane protein | 0.49 | — | — |
| cg1737 | acn | Aconitase | 0.16 | — | R |
| cg1930 | — | Putative secreted hydrolase, CGP3 region | 6.88 | R | — |
| cg1931 | — | Putative secreted protein, CGP3 region | 5.17 | R | — |
| cg2181 | oppA | ABC-type peptide transport system, secreted component | 0.30 | — | — |
| cg2182 | oppB | ABC-type peptide transport system, permease component | 0.38 | — | — |
| cg2183 | oppC | ABC-type peptide transport system, permease component | 5.35 | — | — |
| cg2184 | oppD | ABC-type peptide transport system, ATPase component | 2.86 | — | — |
| cg2234 | — | Putative ABC-type iron(III) dicitrate transporter, substrate-binding lipoprotein | 3.31 | R | — |
| cg2236 | thiE | Thiamine-phosphate pyrophosphorylase | 2.14 | — | — |
| cg2237 | thiO | Putative d-amino acid oxidase flavoprotein, oxidoreductase | 2.86 | — | — |
| cg2238 | thiS | Sulfur transfer protein, involved in thiamine biosynthesis | 3.16 | — | — |
| cg2239 | thiG | Thiazole synthase, involved in thiamine biosynthesis | 3.63 | — | — |
| cg2240 | thiF | Molybdopterin biosynthesis protein MoeB, involved in thiamine biosynthesis | 3.33 | — | — |
| cg2252 | — | Hypothetical protein | 2.20 | — | — |
| cg2283 | — | Hypothetical protein | 2.82 | — | — |
| cg2348 | — | Putative secreted protein | 0.48 | — | — |
| cg2438 | — | Hypothetical protein | 0.50 | — | — |
| cg2445 | hmuO | Heme oxygenase | 2.16 | R | — |
| cg2556 | — | Putative iron-regulated membrane protein | 0.45 | — | — |
| cg2610 | — | Putative ABC-type dipeptide/oligopeptide/nickel transport system, secreted component | 0.37 | — | — |
| cg2636 | catA1 | Catechol 1,2-dioxygenase | 0.35* | — | R |
| cg2675 | — | Putative ATPase component of ABC-type transport system, contains duplicated ATPase domains | 2.28 | — | — |
| cg2676 | — | Putative ABC-type dipeptide/oligopeptide/nickel transport system, permease component | 2.96 | — | — |
| cg2677 | — | Putative ABC-type dipeptide/oligopeptide/nickel transport system, permease component | 2.58 | — | — |
| cg2782 | ftn | Ferritin | 0.43* | A | — |
| cg2837 | sucC | Succinyl-CoA synthetase, β-subunit | 0.43 | — | — |
| cg3047 | ackA | Acetate kinase | 0.40* | — | R |
| cg3048 | pta | Phosphotransacetylase, phosphate acetyltransferase | 0.34* | — | R |
| cg3107 | adhA | Zn-dependent alcohol dehydrogenase | 0.32 | — | — |
| cg3156 | htaD | Secreted heme transport-associated protein | 10.37 | R | — |
| cg3195 | — | Putative flavin-containing monooxygenase FMO | 0.24 | — | — |
| cg3216 | gntP | Gluconate permease, Gluconate:H+ symporter, GntP family | 0.45 | — | — |
| cg3266 | tnp5c | Transposase | 2.46 | — | — |
| cg3267 | — | Putative membrane protein, putative pseudogene, C-terminal fragment | 2.68 | — | — |
| cg3372 | — | Hypothetical protein | 4.52 | — | — |
| cg3373 | cyeR | Redox-sensing transcriptional regulator, ArsR family | 2.28 | — | — |
| cg3374 | cye1 | Putative NADH-dependent flavin oxidoreductase | 2.53 | — | — |
| cg3404 | — | Putative ABC-type iron(III) dicitrate transporter, substrate-binding lipoprotein | 3.77 | R | — |
| cg3431 | rnpA | Ribonuclease P | 0.48 | — | — |
Cells for RNA isolation were harvested at an OD600 of 3. The precultivation was performed under the same conditions as the main cultures. The mRNA levels of all listed genes were at least twofold up- or down-regulated (P ≤ 0.1) in three independent biological replicates including one color-swap. Differentially expressed genes were determined by quality filter using the criteria flags ≥0 and signal/noise ratio of Cy5 (F635Median/B635Median) or Cy3 (F532Median/B532Median) ≥3 (GenePix Pro-7). Genes with P values ≤0.05 were regarded as statistically significant. Gene locus tags displayed in bold indicate genes coding for pupylated proteins (23). A, activation; R, repression.
Ratios of genes with P values 0.05 < P ≤ 0.1.
In two of the three replicates, cg1488 (leuD) showed a fold change >2.
Fig. S9.
Comparison of ripA promoter activity in C. glutamicum WT and the Δpup mutant using the reporter plasmid pJC1-PripA-crimson. The two strains were cultivated under iron limitation, and samples were taken after 0, 2, 4, 6, and 24 h. A total of 100,000 cells of each sample were analyzed by flow cytometry for crimson fluorescence. The fluorescence intensities of individual cells are plotted against the forward scatter (FSC). Dotted lines were inserted at 103 and 104 arbitrary units (AU) of fluorescence to allow comparison of the two samples. The median crimson fluorescence for each sample is indicated on top of each scatter plot.
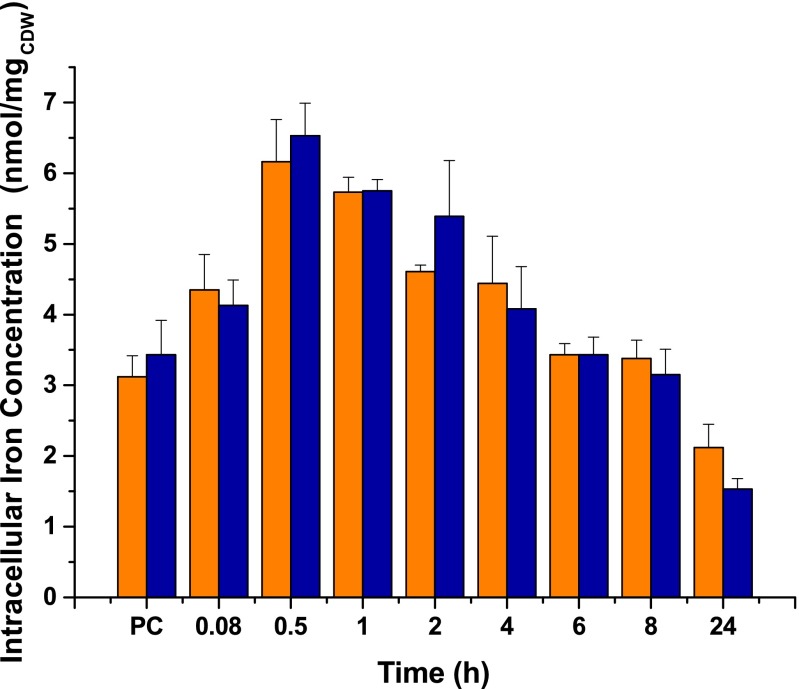
Determination of the total cellular iron content of WT and Δpup mutant by using a ferrozine-based assay revealed no significant differences between the two strains (Fig. S4). The total iron content is considered to be composed of the three pools, namely chelatable cytosolic iron, iron present in iron-dependent proteins, and iron stored in Ftn and Dps. As the prominent iron-dependent proteins repressed by RipA showed decreased mRNA levels in the Δpup strain (Fig. 2A), the low chelatable cytosolic iron concentration of this mutant revealed by the transcriptome data should be the result of increased iron content of the iron storage proteins Ftn and/or Dps.
Fig. S4.
Total intracellular iron concentrations of C. glutamicum WT (orange) and the Δpup mutant (blue) under iron starvation. Cells were precultivated in CGXII minimal medium with 4% (wt/vol) glucose containing 1 µM FeSO4 and then transferred to CGXII medium also supplemented with 1 µM FeSO4. Cell samples were taken at the end of the preculture (PC) and at the indicated time points after inoculation. Iron concentrations were determined from 1 mg cell dry weight using a colorimetric ferrozine assay. Mean values and SDs of three independent biological replicates are shown.
Ferritin Is the Key Pupylation Target for Adaptation to Iron Limitation.
The list of 55 proteins recently shown to be pupylated in C. glutamicum includes Ftn and Dps (23). Thus, the stronger iron starvation response of the Δpup strain and its growth defect under iron limitation might be related to a defective pupylation of the iron storage proteins. Because the 24-meric Ftn has a much higher iron storage capacity than the 12-mer Dps (4,500 vs. 500 iron atoms) (30), it was chosen as prime target for subsequent studies.
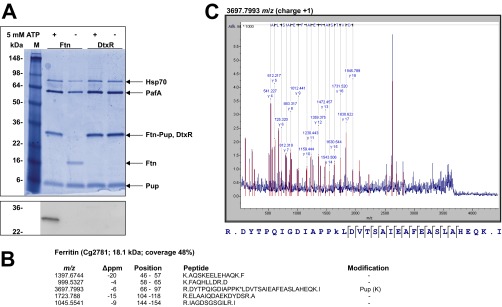
To confirm the previously detected in vivo pupylation of Ftn on K78 (23) and test for additional pupylation sites, we performed an in vitro pupylation assay using purified Ftn, Pup, and PafA (Fig. S5A). Peptide mass fingerprint analysis of trypsinized pupylated Ftn revealed five peptides (48% coverage; Fig. S5B). The one including K78 was confirmed to be pupylated using MALDI-TOF MS/MS (Fig. S5C). Ftn pupylation was additionally confirmed by immunoblot analyses using anti-Pup antiserum (Fig. S5A). As a negative control, we used DtxR, which was not found to be pupylated in our previous in vivo study (23). Also in vitro, no pupylation of DtxR was observed (Fig. S5A). To test whether K78 is the only pupylation site of Ftn in vivo, we purified His-tagged variants of Ftn and Ftn-K78A from a C. glutamicum ΔpupΔftn mutant harboring plasmid pVWEx1-pup-ftn or pVWEx1-pup-ftn-K78A, respectively. Pupylation of WT Ftn was confirmed by its apparent mass of 29 kDa, the presence of a pupylated peptide (m/z, 3,697.8) in the trypsinized protein, and immunoblotting with anti-Pup antiserum (Fig. 3A). In contrast, purified Ftn-K78A showed an apparent mass of 19 kDa, contained no detectable pupylated peptide, and was not detected by the anti-Pup antiserum (Fig. 3A).
Fig. S5.
In vitro pupylation of the iron storage protein Ftn. (A) Ftn (2 µM) was incubated for 16 h at room temperature with Pup (10 µM) and the Pup ligase PafA (0.5 µM) in the presence or absence of 5 mM ATP. As a negative control, the transcriptional regulator DtxR with a C-terminal His-tag (2 µM) was used. Aliquots of the samples were subjected to SDS/PAGE and Coomassie staining (Upper) or subsequent immunoblotting using anti-Pup antiserum (1:2,000; Lower). Hsp70 is an E. coli chaperone that coeluted with PafA during purification. (B) Result of the peptide mass fingerprint analysis of trypsinized ferritin obtained after in vitro pupylation. Five peptides were detected (48% sequence coverage), including the one covering amino acid residues 66–97 which contains K78 found to be pupylated in our previous pupylome study (23). The predicted m/z (i.e., monoisotopic mass) of the nonpupylated peptide is 3,454.7382, and the measured m/z value was 3,697.7993, corresponding to a difference of 243.0611 (expected for pupylation: 243.0855). (C) The identity of the peptide with m/z of 3,697.7993 was confirmed by MS/MS analysis by the indicated y-ion series.
Fig. 3.
(A) Requirement of K78 for in vivo pupylation of Ftn. C. glutamicum ΔpupΔftn carrying pVWEx1-pup-ftn or pVWEx1-pup-ftn-K78A was cultivated in BHI complex medium and harvested in the exponential growth phase. Crude cell extracts were subjected to Ni2+-NTA affinity chromatography, and 3.3 µg protein of each eluate was analyzed by SDS/PAGE and Coomassie staining. Pupylation was confirmed by immunoblot analysis (Lower) using polyclonal anti-Pup antiserum. Protein bands visible in the Coomassie-stained gel were excised, trypsinized, and subjected to MS analysis to determine protein identity or the pupylated lysine residue. (B–D) Growth of C. glutamicum WT and the indicated mutant strains in glucose minimal medium under iron limitation (1 µM FeSO4). The chromosomal ftn-K78A mutation prevents pupylation of Ftn. Mean values and SDs calculated from three independent biological replicates are shown.
A strain carrying a chromosomally encoded Ftn-K78A variant was constructed to test the relevance of Ftn pupylation for growth of C. glutamicum under iron limitation. Surprisingly, this strain carrying a single point mutation showed the same growth defect as the Δpup mutant under iron limitation (Fig. 3B), whereas it grew like the WT under iron-replete conditions (Fig. S3B). A Ftn-K78R strain behaved like the Ftn-K78A strain, showing that the functionality of K78 could not be replaced by R78 (Fig. S3C). In contrast to the Ftn-K78 exchange mutants, a Δftn deletion mutant grew like the WT under iron-limited and iron-replete conditions. These results strongly indicate that defective pupylation of Ftn is responsible for the growth defect of the Δpup mutant under iron limitation. To further validate this result, we cultivated the ΔpupΔftn mutant under iron limitation. In fact, this mutant grew much better than the Δpup mutant, although not as good as the WT (Fig. 3C). Similarly, the growth defect of the Δarc mutant could be largely abolished by deletion of ftn (Fig. 3C).
Pupylation of Dps Plays a Minor Role in Adaptation to Iron Limitation.
Because deletion of ftn in the Δpup mutant did not fully reverse the growth defect under iron-limitation conditions (Fig. 3C), we speculated that pupylation of Dps might be responsible for the remaining difference. Thus, we constructed the mutant strains Δdps, ΔftnΔdps, ΔpupΔdps, and ΔpupΔftnΔdps. Under iron-replete conditions, all these strains grew like the WT (Fig. S3 D and E). Under iron limitation, the strains Δdps (Fig. S3F) and Δftn (Fig. 3B) grew like the WT, whereas the double-deletion mutant ΔftnΔdps showed a slight growth defect (Fig. 3D). Importantly, the ΔpupΔftnΔdps strain grew as the ΔftnΔdps strain, suggesting that pupylation is no longer relevant under iron limitation when Ftn and Dps are absent. In contrast to the ΔpupΔftn mutant, the ΔpupΔdps mutant grew as poorly as the Δpup mutant (Fig. S3G), showing that Ftn pupylation rather than Dps pupylation is of prime importance for adaptation to iron limitation.
Evidence Against Proteolysis of Pupylated Ferritin.
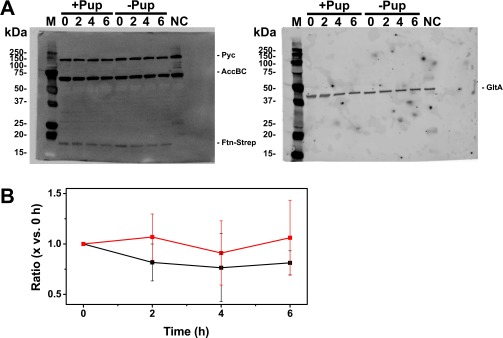
In mycobacteria, pupylated proteins are degraded by the proteasome (3, 4). C. glutamicum does not possess a proteasome but does possess the ATP-dependent proteases ClpP1P2 (31) and FtsH (32). To test whether pupylated Ftn is subject to proteolysis, we analyzed the Ftn protein levels in the WT and the Δpup mutant under iron limitation. No significant differences in the Ftn levels were detected in the two strains, indicating that pupylated Ftn is not subject to proteolysis under the conditions tested (Fig. S6).
Fig. S6.
(A) Immunoblot-based analysis of in vivo Ftn stability in the absence and presence of Pup. Strains Δftn/pVWEx1-ftn-Strep and ΔpupΔftn//pVWEx1-ftn-Strep were cultivated under iron limitation and harvested before (0 h) and 2, 4, and 6 h after addition of rifampicin (250 µg/mL) and tetracycline (100 µg/mL) to the medium. Plasmid-based expression of ftn-Strep encoding a C-terminally Strep-tagged Ftn variant under control of the IPTG-inducible tac promoter was chosen to exclude effects arising from differential expression from the native ftn promoter. The StrepTag-II did not interfere with the function of Ftn, as expression of ftn-Strep in strain ΔpupΔftn restored the growth defect under iron limitation observed for the Δpup mutant in the presence of native Ftn. Equivalent protein amounts of the different samples were subjected to SDS/PAGE and immunoblotting with StrepTactin coupled to alkaline phosphatase (1:3,500; A, Left) or polyclonal rabbit anti-GltA (citrate synthase) antiserum (1:10,000) and goat anti-rabbit Cy5-labeled antibody (1:2,500; A, Right). The two different immunoblots were performed by using the identical membrane. Three biological replicates with comparable results were performed, one of which is shown in A. The lanes of the protein molecular mass marker (marked as “M”) and the negative control (NC; C. glutamicum WT crude protein extract) are indicated. The detected bands represent pyruvate carboxylase Pyc (123.1 kDa), acetyl-CoA carboxylase AccBC (63.4 kDa), Ftn-Strep (19.1 kDa), and citrate synthase GltA (48.9 kDa). Further experimental details are provided in SI Materials and Methods. (B) Quantitative evaluation of Ftn-Strep based on the immunoblots. The Ftn-Strep level in the pupylation-competent strain (black squares) and the pupylation-defective strain (red squares) is indicated relative to the level before addition of the inhibitors. The Ftn-Strep band intensities were normalized to the respective citrate synthase controls by using ImageQuant TL (GE Healthcare). Mean values and SDs of three independent biological replicates are shown.
Discussion
In mycobacteria and streptomycetes, pupylation was shown to target proteins to proteasomal degradation and to play an important role in the response to several stress conditions, such as nitrosative stress (25), oxidative stress (19), or nitrogen and carbon starvation (20), as well as in the development of cell morphology (21). However, members of other actinobacterial genera such as Corynebacterium do not harbor a prcAB-encoded proteasome; nevertheless, they possess the genes for the pupylation machinery including Pup, Dop, PafA, and Mpa/ARC. In the present study, we found that pupylation is specifically required for adaptation to iron limitation in C. glutamicum. Interestingly, our results indicate that this process is independent from protein degradation and involves the iron-storage protein ferritin as primary target. The crucial role of ferritin pupylation for adaptation to iron limitation was demonstrated by the finding that a single chromosomal point mutation exchanging the pupylated lysine residue K78 of Ftn to alanine caused the same growth defect as the deletion of pup or arc in C. glutamicum. Furthermore, the growth defect of the Δpup and Δarc mutants could be largely abolished by additional deletion of ftn. The ARC dependency indicates that adaptation to iron limitation requires ARC-catalyzed unfolding of ferritin. However, we did not find evidence that pupylated and unfolded ferritin is subsequently degraded.
In general, ferritins, but also bacterioferritins and Dps proteins, store iron in their cavities as a ferric (Fe3+) mineral core (30). To release ferric iron for incorporation into cellular proteins, it has to be reduced to Fe2+. The process of iron release from iron-storage proteins is still under investigation. Several different mechanisms have been reported for iron release from ferritin, including spontaneous dissolution of Fe3+, lysosomal or proteasomal degradation of ferritin, and direct reduction of the ferric mineral in ferritin followed by Fe2+ release through the ferritin pores (33). Our results for C. glutamicum suggest that iron stored in ferritin cannot be easily mobilized out of the intact 24-mer in vivo without pupylation, as the transcriptome data and the results of the reporter gene fusion disclosed that the Δpup mutant shows a much stronger iron starvation response than the WT under iron-limiting conditions (Fig. 2).
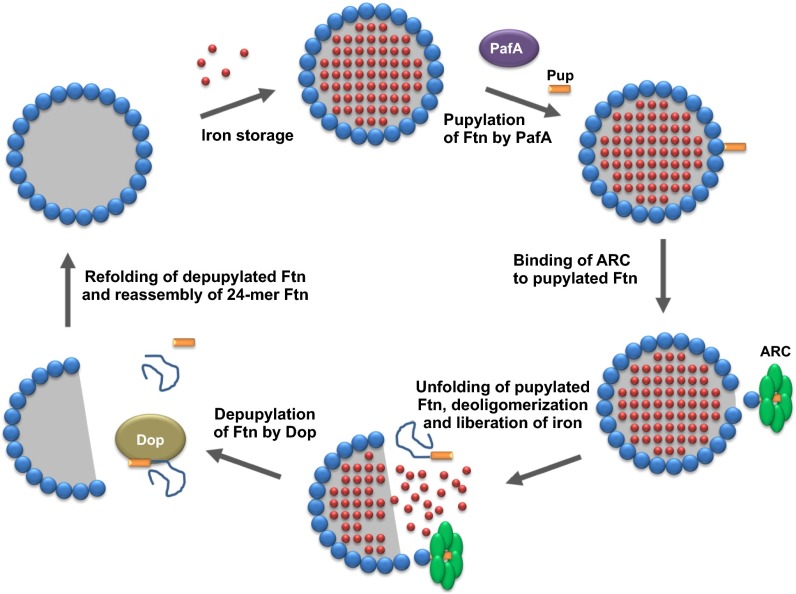
Therefore, our data support a model in which pupylation acts as a trigger for iron mobilization out of iron storage proteins, as illustrated for ferritin in Fig. 4. Pupylation of ferritin triggers ARC-catalyzed unfolding, which in turn causes partial or complete deoligomerization of the 24-meric ferritin shell. As a consequence, the ferric mineral stored in ferritin becomes accessible for reduction and dissolution, providing chelatable iron for incorporation into iron-dependent proteins. Unfolded pupylated ferritin can be depupylated by Dop and might be available for reassembling 24-meric ferritin after renaturation. In mutants lacking Pup or ARC, iron stored in ferritin is hardly accessible or is accessible at a rate too low to meet cellular demands. Under iron-sufficient conditions, such a defect has no impact on growth, as there is still enough iron available in the medium that can be taken up and used to synthesize iron-dependent proteins, such as proteins containing iron-sulfur clusters or heme. Under iron-limiting conditions, however, the absence of Pup or ARC has severe consequences, as, in this situation, the iron stored in ferritin is urgently required for essential iron-dependent proteins. Furthermore, our results suggest that the depupylase Dop is required to recycle Pup. However, Dop levels have to be carefully balanced to avoid a futile cycle of pupylation and depupylation (Fig. 1D). Growth stops when iron release from ferritin is blocked, but can be resumed when pupylation and ARC-catalyzed unfolding of ferritin become possible again. This was demonstrated by an experiment in which a delayed induction of a plasmid-borne pup gene (6 h after starting cultivation under iron-limitation conditions) could still rescue the growth defect of the Δpup mutant (Fig. S3H). Deletion of pup in the ΔftnΔdps background had no further influence on growth, indicating that pupylation is not important under iron limitation when ferritin and Dps are absent.
Fig. 4.
Model illustrating the proposed role of pupylation for iron release from ferritin. A monomer of an iron-loaded 24-meric ferritin is pupylated at K78 by the Pup ligase PafA. The pupylated ferritin monomer is recognized by the AAA+ ATPase ARC, which catalyzes the unfolding of this monomer. As a consequence, the ferritin shell is partially or completely deoligomerized, making the ferric mineral inside accessible for reduction and solubilization. The unfolded pupylated ferritin monomer is depupylated by Dop, and, after renaturation, may reassemble to form intact 24-meric ferritin shells.
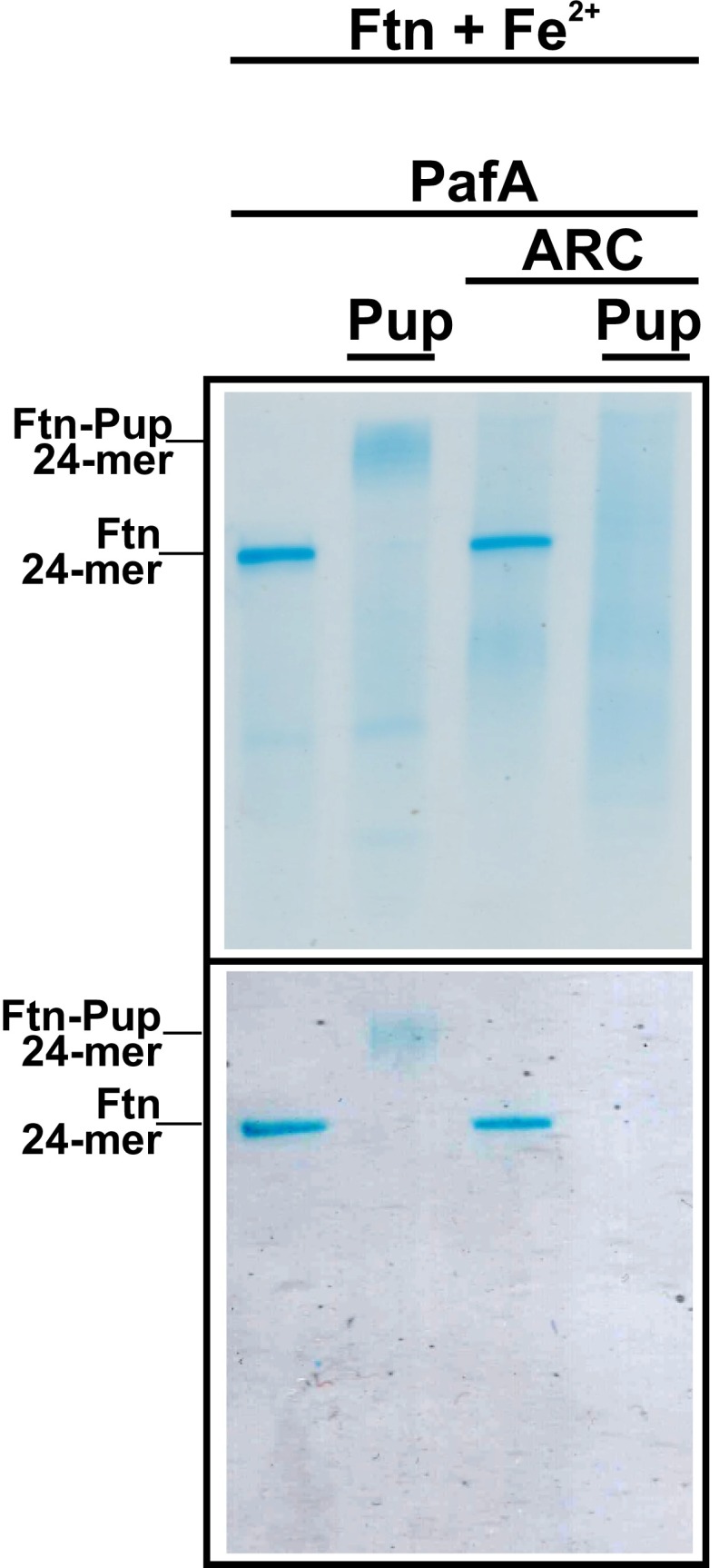
In a first attempt to demonstrate the ARC-dependent deoligomerization of pupylated 24-meric Ftn, we tried to establish an in vitro assay based on native PAGE. For this purpose, iron-loaded 24-meric Ftn was pupylated in vitro with PafA and ATP, as demonstrated by a shift of the Ftn band via Coomassie and Prussian Blue staining (Fig. S7). When incubated with purified ARC and ATP, the band of pupylated 24-meric Ftn disappeared and a broad protein smear became visible (Fig. S7). This effect was strictly dependent on pupylated Ftn and did not occur when nonpupylated 24-meric Ftn was incubated with ARC and ATP. A more detailed kinetic characterization of ARC-dependent deoligomerization and iron release from pupylated Ftn requires further studies.
Fig. S7.
In vitro pupylation and ARC treatment of 24-meric Ftn analyzed by native PAGE and staining with Coomassie (detection of proteins; Upper) or Prussian Blue (detection of ferric iron; Lower). The 24-meric Ftn was loaded with ferric iron by incubation with (NH4)2Fe(SO4)2 and pupylated in vitro. Then, ARC (3 µM) and ATP (10 mM) were added and the sample was incubated for 15 min. Control samples lacking Pup and/or ARC were treated accordingly. The presence of the four proteins in the samples is indicated at the top. Three technical replicates were performed with comparable results.
Iron homeostasis is closely interlinked with oxidative stress responses, as Fe2+ triggers the Fenton reaction leading to the formation of the extremely damaging hydroxyl radical from hydrogen peroxide (34). Iron-storage proteins, in particular Dps (35, 36), contribute to the avoidance of toxic levels of free Fe2+ within cells and are thus typical members of oxidative stress stimulons. In many bacteria, the response to oxidative stress is controlled by the H2O2-sensing transcriptional regulator OxyR (37). In contrast to E. coli, in which OxyR is an activator, C. glutamicum OxyR acts as a repressor that is inactivated by H2O2 stress (38, 39). Deletion of oxyR in C. glutamicum resulted in 3- and 12-fold up-regulation of ftn and dps expression, respectively, whereas pup and pafA were the most strongly down-regulated genes (10-fold) (38). Thus, H2O2 stress increases the levels of the iron storage proteins and, at the same time, decreases the levels of the pupylation machinery. This behavior fits to a protective role of Ftn and Dps by reduction of the free Fe2+ levels, which is enhanced by the inhibition of pupylation-triggered iron release.
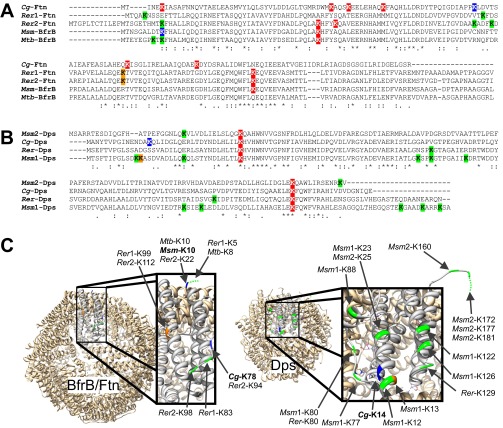
The concept that the iron release from Ftn and Dps is triggered by pupylation is likely to occur not only in corynebacteria, but also in other bacteria harboring the pupylation machinery. The ferritin homolog BfrB of M. smegmatis has been shown to be pupylated (17), and BfrB of M. tuberculosis was enriched in a pupylome study, but pupylation was not detected yet (15). Analysis of the positions of lysine residues in ferritin and Dps proteins of known crystal structure revealed that the pupylation sites currently known, namely K78 and K14 in C. glutamicum Ftn and Dps, respectively, and K10 in M. smegmatis BfrB are located on the outer surface and are thus accessible for pupylation (Fig. S8). Moreover, all analyzed actinobacterial ferritin and Dps homologs appear to possess at least one surface-exposed lysine residue that could be a target for pupylation. Further support for a role of pupylation in mycobacterial iron homeostasis comes from the observation that mpa and pafA mutants of M. tuberculosis show an increased resistance to H2O2 (25). As outlined earlier, this might be a result of an inhibition of iron release from BfrB.
Fig. S8.
Pupylation sites in the iron storage proteins ferritin and Dps. To determine the spatial positions of identified and putative pupylation sites in ferritin and Dps, amino acid sequence alignments were made to homologs of known structure, BfrB from M. tuberculosis (Mtb-BfrB, Rv3841) and Dps from Mycobacterium smegmatis (Msm1-Dps, MSMEG_6467), using ClustalO (www.ebi.ac.uk/Tools/msa/clustalo/). (A) The ferritin sequences from C. glutamicum (Cg2782), Rhodococcus erythropolis (Rer1, RER_01810 und Rer2, RER_01740), and M. smegmatis BfrB (MSMEG_6422) were aligned to Mtb-BfrB. (B) The Dps sequences from M. smegmatis (Msm2, MSMEG_3242), C. glutamicum (Cg3327), and R. erythropolis (RER_12800) were aligned to Msm1-Dps. (C) Protein structures of the 24-mer BfrB from M. tuberculosis [Protein Data Bank (PDB)_ID code 3QD8] and the 12-mer Dps from M. smegmatis (PDB ID code 1VEQ) with one of the monomers enlarged. The 3D models were exported by using Chimera (55). Based on the alignments in A and B, the positions of all lysine residues were determined in the protein structures and are indicated in colors. Lysine residues confirmed as pupylation sites are colored blue, and lysine residues located on top of helices or in loop regions that could be easily accessible for pupylation are depicted in green. Lysine residues shown in orange are located at the flanks of helices, making accessibility less likely. Lysine residues not shown in C are likely facing the interior cave of the oligomers and therefore should be inaccessible for pupylation. Lysine residues colored red in A and B are confirmed (Cg-Ftn) or assumed (Cg-Dps) not to be pupylated in C. glutamicum or inaccessible for pupylation.
Our finding that pupylation of ferritin is required for adaptation of C. glutamicum to iron limitation adds another level of complexity to the control of iron homeostasis in bacteria. Whereas regulation by transcriptional regulators such as Fur (40) or by regulatory sRNAs like RyhB (41) has been well studied, regulation at the posttranslational level has not yet been described in bacteria to the best of our knowledge. Our results raise a number of questions that demand further investigation. How many subunits of the 24-meric ferritin need to be pupylated and unfolded to make the iron from the mineral core accessible? How is iron solubilized from the mineral core in vivo? Is pupylation in C. glutamicum generally independent from degradation or are there pupylation targets that are degraded, for example, by the protease ClpP1P2? How important is pupylation-triggered iron release from ferritin for the pathogenicity of M. tuberculosis in view of the limited iron availability in the host? Obviously, the newly discovered link between pupylation and ferritin opens up new avenues for understanding the physiological role of pupylation and the mechanism of iron release from ferritin.
Materials and Methods
Bacterial Strains and Cultivation Conditions.
Bacterial strains and plasmids used in this study are listed in Table S3. C. glutamicum American Type Culture Collection (ATCC) 13032 and derivatives were cultivated at 30 °C by using brain heart infusion (BHI) medium or CGXII minimal medium containing 4% (wt/vol) glucose and 30 mg/L protocatechuate (42). To obtain iron limitation, the trace salt solution used for CGXII was prepared without iron, which was added freshly dissolved in 10 mM HCl. Detailed cultivation procedures are described in SI Materials and Methods.
Table S3.
Strains and plasmids used or constructed in this study
| Strain or plasmid | Relevant characteristics | Source |
| Strains | ||
| C. glutamicum | ||
| ATCC 13032 | WT, biotin auxotrophic | 56 |
| Δpup | WT derivative with in-frame deletion of pup (cg1689) | 23 |
| ΔpupΔftn | Δpup derivative with in-frame deletion of ftn (cg2782) | This study |
| ΔpupΔdps | Δpup derivative with in-frame deletion of dps (cg3327) | This study |
| ΔpupΔftnΔdps | ΔpupΔftn derivative with in-frame deletion of dps (cg3327) | This study |
| Δarc | Wt derivative with in-frame deletion of arc (cg1691) | This study |
| ΔarcΔftn | Δarc derivative with in-frame deletion of ftn (cg2782) | This study |
| Δdop | Wt derivative with in-frame deletion of dop (cg1690) | This study |
| Δftn | Wt derivative with in-frame deletion of ftn (cg2782) | This study |
| Δdps | Wt derivative with in-frame deletion of dps (cg3327) | This study |
| ΔftnΔdps | Δftn derivative with in-frame deletion of dps (cg3327) | This study |
| ftn-K78A | Wt derivative with exchanges A232G, A233C, G234T and T237A in ftn (cg2782) leading to a Ftn-Lys78Ala mutant | This study |
| ftn-K78R | Wt derivative with exchanges A232C, A233G, G234T and T237A in ftn (cg2782) leading to a Ftn-Lys78Arg mutant | This study |
| E. coli | ||
| DH5α | F–Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK−, mK+) phoA supE44 λ– thi-1 gyrA96 relA1 | Invitrogen |
| BL21(DE3) | F- ompT hsdSB(rB− mB−) gal dcm (DE3) | 57 |
| Plasmids | ||
| pK19mobsacB | Kanr; vector for allelic exchange in C. glutamicum (pK18 oriVE.coli sacB lacZα) | 58 |
| pK19mobsacB-Δarc | Kanr, pK19mobsacB derivative containing a 1,148-bp overlap-extension PCR product (PstI/EcoRI) which covers the flanking regions of the C. glutamicum arc gene | This study |
| pK19mobsacB-Δdop | Kanr, pK19mobsacB derivative containing a 1,278-bp overlap-extension PCR product (PstI/BamHI) which covers the flanking regions of the C. glutamicum dop gene | This study |
| pK19mobsacB-Δftn | Kanr, pK19mobsacB derivative containing a 1,253-bp overlap-extension PCR product (SalI/EcoRI) which covers the flanking regions of the C. glutamicum ftn gene | This study |
| pK19mobsacB-ftn-K78A | Kanr, pK19mobsacB derivative containing a 1,661-bp overlap-extension PCR product (SalI/EcoRI) which covers the C. glutamicum ftn gene with exchanges A232G, A233C, G234T and T237A leading to a Ftn-Lys78Ala mutant and an internal XbaI site | This study |
| pK19mobsacB-ftn-K78R | Kanr, pK19mobsacB derivative containing a 1,661-bp overlap-extension PCR product (SalI/EcoRI) which covers the C. glutamicum ftn gene with exchanges A232C, A233G, G234T and T237A leading to a Ftn-Lys78Arg mutant and an internal XbaI site | This study |
| pK19mobsacB-Δdps | Kanr, pK19mobsacB derivative containing a 1,272-bp overlap-extension PCR product (EcoRI/XbaI) which covers the flanking regions of the C. glutamicum dps gene | This study |
| pJC1-PripA-Crimson | Kanr, expression vector containing the reporter gene E2-Crimson genetically fused to the promoter sequence of ripA containing the binding site of DtxR | Heyer and Frunzke, unpublished |
| pET-TEV1 | Kanr; pET28b derivative for overexpression of genes in E. coli, adding an N-terminal decahistidine tag and a TEV protease cleavage site to the target protein (pBR322 oriVE.c., PT7, lacI) | 59 |
| pET-TEV1-pup | Kanr; pET-TEV1 derivative coding for Pup with an N-terminal decahistidine tag and a TEV protease cleavage site (NdeI/BamHI) | 23 |
| pET-TEV1-ftn | Kanr; pET-TEV1 derivative coding for Ftn with an N-terminal decahistidine tag and a TEV protease cleavage site (NdeI/BamHI) | This study |
| pET-TEV1-arc | Kanr; pET-TEV1 derivative coding for ARC with a C-terminal Strep-Tag (NcoI/XhoI) | This study |
| pET-22b | Ampr; vector for overexpression of genes in E. coli, adding a C-terminal hexahistidine tag to the target protein (pBR322 oriVE.c. PT7 lacI) | Novagen |
| pET-22b-pafA | Ampr; pET-22b derivative coding for PafA with a C-terminal hexahistidine tag (NdeI/XhoI) | 23 |
| pET24b | Kanr; vector for overexpression of genes in E. coli, adding a C-terminal hexahistidine tag to the target protein (pBR322 oriVE.c., PT7, lacI) | Novagen |
| pET24b-ftn | Kanr; pET24b derivative for overproduction of Ftn in its native form | This study |
| pET24b-dtxR-C | Kanr; pET24b derivative for overproduction of DtxR with a C-terminal histidine tag | 29 |
| pVWEx1 | Kanr, Ptac, lacIq; expression vector for C. glutamicum | 60 |
| pVWEx1-pup | Kanr; pVWEx1 derivative harboring the pup gene amplified from pET-TEV1-pup including the coding regions for the decahistidine tag and the TEV protease cleavage site (PstI/BamHI) | 23 |
| pVWEx1-pup-E64A | Kanr; derivative of pVWEx1-pup coding for a Pup protein with an Glu64Ala exchange | 23 |
| pVWEx1-Strep-pup | Kanr; pVWEx1 derivative harboring the pup gene including an N-terminal Strep-tag (PstI/BamHI) | This study |
| pVWEx1-pup-ftn | Kanr; pVWEx1-Strep-pup derivative additionally harboring the ftn gene including an N-terminal heptahistidine tag (BamHI/KpnI) | This study |
| pVWEx1-pup-ftn-K78A | Kanr; pVWEx1-Strep-pup derivative additionally harboring the ftnK78A mutein amplified from pK19mobsacB-ftnK78A including an N-terminal heptahistidine tag (BamHI/KpnI) | This study |
| pVWEx1-dop | Kanr; pVWEx1 derivative harboring the dop gene (XbaI/KpnI) | This study |
| pVWEx1-arc | Kanr; pVWEx1 derivative harboring the arc gene including an N-terminal Strep-tag (PstI/BamHI) | This study |
| pVWEx1-ftn-Strep | Kanr; pVWEx1 derivative harboring the ftn gene including a C-terminal Strep-tag (BamHI/KpnI) | This study |
Strain and Plasmid Constructions.
All oligonucleotides used in this study are listed in Table S4. Enzymes used for cloning were obtained from Thermo Fisher Scientific. Kits for plasmid DNA isolation (GeneJET Plasmid Miniprep Kit) and DNA purification (QIAquick PCR Purification Kit) were obtained from Thermo Fisher Scientific and Qiagen, respectively. Standard molecular cloning methods like DNA restriction and ligation were performed according to the manufacturers’ instructions or standard protocols (43). Competent C. glutamicum cells were obtained as described previously (44). In-frame deletion mutants of C. glutamicum and codon exchanges in chromosomal genes were created by using a two-step homologous recombination protocol (45). Detailed information on the cloning steps and plasmid constructions are provided in SI Materials and Methods.
Table S4.
Oligonucleotides used in this study
| Name | Sequence (5′→3′) | Restriction site |
| Primers for construction of pK19mobsacB-Δdop or verification of dop deletion (_check) | ||
| dop5′o | AGACTGCAGGAGGATCTGTCCAATGTCATC | PstI |
| dop5′i | CCCATCCACTAAACTTAAACAGCTTAAAAACGGTGCAGACG | — |
| dop3′o | AGAGGATCCGAACCCACATCAAGATACAAC | BamHI |
| dop3′i | TGTTTAAGTTTAGTGGATGGGGAGGGTCTTGAAAAGGAAAAC | — |
| Δdop-check-fw | TAAGAATTGCCCACGGAATC | — |
| Δdop-check-rv | TGAGACAGAGCGACAGATCC | — |
| Primers for construction of pK19mobsacB-Δarc or verification of arc deletion (_check) | ||
| arc5′o | AGACTGCAGGTGGGTGAAAAGGGCAATGTC | PstI |
| arc5′i | CCCATCCACTAAACTTAAACAAGAATTAGAGGGCGAAGAATC | — |
| arc3′o | AGAGAATTCGATCAATGCCTGAGCCAGC | EcoRI |
| arc3′i | TGTTTAAGTTTAGTGGATGGGGAATGGTCCAGGATCACTGG | — |
| Δarc-check-fw | ACTGTCCATGGCGCTGCTTC | — |
| Δarc-check-rv | ATGATGACCTGGCGGCACAC | — |
| Primers for construction of pK19mobsacB-Δftn or verification of ftn deletion (_check) | ||
| ftn5′o | AGAGTCGACAGTTACCGGTCAGACGATCC | SalI |
| ftn5′i | CCCATCCACTAAACTTAAACATGATGCGATCTTCTCGTTG | — |
| ftn3′o | AGAGAATTCCGAAGCTCGGTAAGAGACTG | EcoRI |
| ftn3′i | TGTTTAAGTTTAGTGGATGGGCTGCGCATCGACGGCGAAC | — |
| Δftn-check-fw | CCCAGCTCTTGATGTCGTTGG | — |
| Δftn-check-rv | CTGCGCGCGGCAATCGGTTC | — |
| Primers for construction of pK19mobsacB-Δdps or verification of dps deletion (_check) | ||
| dps5′o | AGAGAATTCGAACCAATGCTTCGCCCTGAG | EcoRI |
| dps5′i | TGTTTAAGTTTAGTGGATGGGTCCAGGGACTGTGTAGTTTGCCAT | — |
| dps3′o | AGATCTAGAGTCGAATCCGATGCTCATAG | XbaI |
| dps3′i | CCCATCCACTAAACTTAAACAGACGGAAACATCCAAGAGTAA | — |
| Δdps-check-fw | CACGGTAGATATTTCCCACAC | — |
| Δdps-check-rv | GCTCGCGGCGCTCATTGATG | — |
| Additional primers for construction of the chromosomal base exchanges in ftnK78A/-K78R | ||
| ftn5′iK78A | GCCTCGATAGCGGAGGTGACATCTAGAGCTGGTGGTGCAATGTCACCGATCTGTG | XbaI (internal) |
| ftn3′iK78A | CACAGATCGGTGACATTGCACCACCAGCTCTAGATGTCACCTCCGCTATCGAGGC | XbaI (internal) |
| ftn5′iK78R | GCCTCGATAGCGGAGGTGACATCTAGACGTGGTGGTGCAATGTCACCGATCTGTG | XbaI (internal) |
| ftn3′iK78R | CACAGATCGGTGACATTGCACCACCACGTCTAGATGTCACCTCCGCTATCGAGGC | XbaI (internal) |
| Primers for construction of pET-TEV1-ftn and pET24b-ftn | ||
| ftnEX-fw | AGACATATGACAATCAACGAGAAGATC | NdeI |
| ftnEX-rv | AGAGGATCCTTAGCGGGAGCCGAGTTCG | BamHI |
| Primers for construction of pET-TEV1-arc-Strep | ||
| arcEX-fw | AGACCATGGTGACCATGAGTTCACCAACTG | NcoI |
| arcStrepEX-rv | AGACTCGAGTTACTTCTCGAACTGTGGGTGGGACCAGATAACCACCTCTGCGTGAG | XhoI |
| Primers for construction of pVWEx1-Strep-pup and pVWEx1-dop | ||
| pup-fwSTREP | AGACTGCAGGAAAGGAGGATAGCAGAATGGCATGGTCCCACCCACAGTTCGAGAAGAACGCAAAGCAAACCCAAATTATGG | PstI |
| pup-rv | AGAGGATCCCTATTCGCCACCCTTTTGTACATAGG | BamHI |
| dop-fw | AGATCTAGAGAAAGGAGGATAGCAGAATGGCGCGTTTTATGGGATCG | XbaI |
| dop-rv | CAGGGTACCCTAAATAGCTTGGATGGCCG | KpnI |
| Primers for construction of pVWEx1-arc | ||
| arc-fwStrep | AGACTGCAGGAAAGGAGGATAGCAGAATGTGGTCCCACCCACAGTTCGAGAAGATGGTGACCATGAGTTCACCAACTG | PstI |
| arc-rv | AGAGGATCCTTAGATAACCACCTCTGCGTGAG | BamHI |
| Primers for construction of pVWEx1-pup-ftn, pVWEx1-pup-ftn-K78A, and pVWEx1-ftn-Strep | ||
| HISftn-fw | AGAGGATCCGAAAGGAGGATAGCAGAATGCACCACCATCACCACCATCACATGACAATCAACGAGAAGATC | BamHI |
| ftn-rv | AGAGGTACCGAATTTAGCGGGAGCCGAGTTCG | KpnI |
| ftn-fw | AGAGGATCCGAAAGGAGGATAGCAGAATGACAATCAACGAGAAGATC | BamHI |
| ftnStrep-rv | AGAGGTACCGAATTTACTTCTCGAACTGTGGGTGGGACCAGCGGGAGCCGAGTTCG | KpnI |
| Primers for sequencing of pVWEx1 or pK19mobsacB derivatives | ||
| pEKExfw | GATATGACCATGATTACGCCAAGC | — |
| pEKExrv | ACGGCGTTTCACTTCTGAGT | — |
| M13fw | TGTAAAACGACGGCCAGT | — |
| M13rv | GGAAACAGCTATGACCAT | — |
All oligonucleotides were purchased from Eurofins MWG Operon. Restriction sites for cloning purposes are underlined, and the respective enzymes denoted in the last column. cDNA sequences for overlap-extension PCR are shown in italics. Bases coding for amino acid exchanges or affinity tags are shown in bold.
DNA Microarray Analyses.
DNA microarray analyses were performed to compare the mRNA levels of the C. glutamicum Δpup mutant and its parent WT. The two strains precultivated under iron limitation were inoculated into fresh medium to an OD600 of 1.5, cultured for 2 h, and harvested on ice by centrifugation. RNA preparation, cDNA synthesis, hybridization, and data analysis were performed as described previously (46). The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database and are accessible in GEO through accession number GSE64866.
Flow Cytometry.
Analyses of each 100,000 cells of C. glutamicum WT and Δpup carrying pJC1-PripA-crimson reporter constructs was performed as described previously (47).
SI Materials and Methods
Detailed Cultivation Conditions.
For preparation of iron-limited CGXII medium, a trace salts solution lacking FeSO4 × 7 H2O was used, and a 5 mM FeSO4 × 7 H2O stock freshly dissolved in 1 M HCl was added to a final concentration of 1 µM. It has to be noted that the actual iron concentration in the minimal medium was slightly higher, as the medium had not been pretreated with chelators. The iron concentration in standard minimal medium without addition of FeSO4 was determined with a colorimetric ferrozine assay as 0.34 ± 0.03 µM, whereas it was 1.4 ± 0.1 µM in the medium supplemented with 1 µM FeSO4. Before inoculation into iron-limited culture media, cells were washed at least once with 0.9% (wt/vol) NaCl. For DNA microarray analysis, cells were harvested in the exponential growth phase at an OD600 of 3.
For growth experiments in complex medium, 20 mL BHI medium (Difco Laboratories) was inoculated with a single Corynebacterium glutamicum colony from a fresh BHI agar plate and incubated at 140 rpm overnight in an Infors Minitron shaker (Infors HT). Subsequently, main cultures (50 mL BHI medium) were inoculated with cells from the preculture to an OD600 of approximately 1. For growth in minimal media, the first preculture was grown in 5 mL BHI medium inoculated with a single colony from a fresh BHI agar plate and incubated at 170 rpm for 8 h. A total of 500 µL of this preculture were used to inoculate a second preculture in a 100-mL shake flask with 20 mL CGXII minimal medium containing 4% (wt/vol) glucose and 30 mg/L protocatechuate (42). This preculture was incubated overnight at 30 °C and 140 rpm and then used to inoculate the main culture [50 mL CGXII medium with 4% (wt/vol) glucose] to an OD600 of approximately 1. The main culture was incubated at 30 °C and 130 rpm for a maximum of 24 h.
For the experiment shown in Fig. 3A, recombinant C. glutamicum cells were cultivated in BHI medium containing 25 µg/mL kanamycin at an initial OD600 of 0.2–0.3. The gene expression was induced with 1 mM IPTG at an OD600 of 1. Cells were harvested at an OD600 of 6–8. Equal amounts of protein were subjected to SDS/PAGE and immunoblot analyses. Visible bands of the polyacrylamide gel were excised, and proteins identified by using peptide mass fingerprint analyses with MALDI-TOF MS.
Escherichia coli DH5α was grown in Lysogeny Broth (LB) medium or on LB agar plates at 37 °C. When appropriate, kanamycin was added to the media at a concentration of 25 μg/mL (C. glutamicum) or 50 μg/mL (E. coli) and carbenicillin at 100 µg/mL (E. coli). Microscale cultivation of C. glutamicum in the BioLector system (m2p-Laboratories) was carried out as described previously (48), except that cells were precultivated in BHI medium and then inoculated in the growth media described in Table S1.
Quantification of Iron.
Iron concentrations in growth media and of cells were determined as reported previously using a colorimetric ferrozine-based assay (49). For measurement of the total cellular iron content, cells corresponding to 1 mg dry weight were analyzed. Deviating from the established protocol, iron concentrations in growth media were determined by adding 30 µL of the iron detection reagent to 300 µL of the medium and measuring the absorbance at 550 nm. Defined concentrations of FeSO4 × 7 H2O (0–100 µM) dissolved in 10 mM HCl served as a standard.
Construction of Plasmids Used in This Study.
The pK19mobsacB derivatives for deletion of dop (cg1690), arc (cg1691), ftn (cg2782), and dps (cg3327) were all constructed following the same scheme. The construction of pK19mobsacB-Δdop is described here in detail as an example. Oligonucleotide pairs dop5′o/dop5′i and dop3′o/dop3′i were used to amplify ∼500 bp of the upstream and downstream sequences, respectively, flanking the dop gene in the genomic DNA of C. glutamicum WT. Oligonucleotides were constructed in such a way that the first and last 8–12 codons of dop were also amplified to generate an in-frame deletion. Oligonucleotides “xxx_o” attached restriction sites for cloning of the deletion fragment into pK19mobsacB (Table S4, right column), and oligonucleotides “xxx_i” contained a complementary sequence of 21 bp (5′-TGTTTAAGTTTAGTGGATGGG) for a subsequent overlap-extension PCR linking the upstream to the downstream sequences. The resulting fragment was digested with suitable restriction enzymes (e.g., PstI and BamHI for dop) and ligated into the pK19mobsacB plasmid digested with the same enzymes. Successful integration of the insert into the vector was checked by PCR with oligonucleotides M13fw/M13rv (Table S4), which bind up- and downstream, respectively, of the multiple cloning site of pK19mobsacB. Competent cells of C. glutamicum WT or selected single or double mutants were transformed with the resulting plasmids (Table S3). Successful deletion of the respective gene was confirmed by colony PCR by using the oligonucleotide pairs “Δxxx-check-fw” and “Δxxx-check-rv”, where xxx is to be replaced by the respective gene name (Table S4). The primers bind upstream and downstream of the regions used to amplify the flanking sequences of the gene to be deleted.
Point mutations in the chromosomal ftn gene were constructed by using a similar protocol as described earlier. Deviating from the deletion protocol, the oligonucleotide pair ftn5′i and ftn3′i was exchanged by one of the pairs ftn5′iK78A/ftn3′iK78A or ftn5′iK78R/ftn3′iK78R to generate plasmids pK19mobsacB-ftn-K78A and pK19mobsacB-ftn-K78R, respectively. In this way, the entire coding sequence of the ftn gene was amplified in addition to the up- and downstream sequences. The primer pairs “xxx_i” contained complementary sequences for overlap extension PCR, replacing the 21-bp region used for the deletion constructs. Moreover, these primers harbored nucleotide exchanges (ftn-K78A: A232G, A233C, G234T, and T237A; ftn-K78R: A232C, A233G, G234T, and T237A; T237A is a silent mutation creating an XbaI restriction site useful for subsequent screening of clones) or the coding sequence of a C-terminal Strep-tagII (WSHPQFEK) inserted before the stop codon (ftnStrep5′i/-3′i). The resulting fragments were cloned into pK19mobsacB. Then competent cells of C. glutamicum WT or the Δpup mutant were transformed with pK19mobsacB-ftn-K78A or -ftn-K78R, respectively (Table S3).
For construction of plasmid pET-TEV1-ftn, the coding sequence of ftn was amplified by PCR from chromosomal DNA of C. glutamicum WT using the oligonucleotide pair ftnEX-fw/ftnEX-rv. The resulting PCR product (504 bp) was digested with the restriction enzymes NdeI and BamHI and ligated with equally digested vector pET-TEV1. In the recombinant plasmid pET-TEV1-ftn, the encoded Ftn protein carries an N-terminal decahistidine tag as well as a TEV protease cleavage site in front of the native start codon (MGSSHHHHHHHHHHDYDIPTTENLYFQGH). This allowed purification of Ftn by Ni2+-chelate affinity chromatography and removal of the tag using TEV protease. The plasmid pET24b-ftn, which encodes the native Ftn protein, was constructed by amplification of ftn with the primer pair ftnEX-fw/ftnEX-rv and cloning into pET24b via NdeI and BamHI restriction sites. The plasmid pET-TEV1-arc-Strep was obtained by amplifying arc with primer pair arcEX-fw/arcStrepEX-rv from chromosomal C. glutamicum DNA and ligation into pET-TEV1 after digestion of both DNA molecules with restriction enzymes NcoI and XhoI. Thereby, the N-terminal polyhistidine tag and TEV cleavage site were cut out of the vector, allowing expression of arc-Strep.
All inserts for pVWEx1 derivatives were constructed by amplifying the coding sequence of the respective gene by PCR by using genomic DNA of C. glutamicum WT as template. The oligonucleotides attached a Shine-Dalgarno sequence (GAAAGGAGGATAGCAGA) directly upstream of the ATG start codon. When necessary, the native GTG start codon was exchanged to ATG. Furthermore, the oligonucleotides included restriction sites for cloning and coding sequences for N- or C-terminal affinity tags (Table S4). The constructs were digested with the restriction enzymes listed in Table S3 and ligated with pVWEx1 digested with the same enzymes. Successful integration of the insert into the vector was checked using the oligonucleotides pEKEX-fw/-rv as sequencing primers. For plasmids pVWEx1-pup-ftn and pVWEx1-pup-ftn-K78A, the ftn coding sequence was amplified from genomic DNA of C. glutamicum WT and from plasmid pK19mobsacB-ftn-K78A, respectively, using the oligonucleotide pair Hisftn-fw/ftn-rv. Oligonucleotide Hisftn-fw added a Shine-Dalgarno sequence in front of the start codon. The inserts were cloned into plasmid pVWEx1-Strep-pup resulting in an artificial pup-ftn or pup-ftn-K78A operon.
SDS/PAGE, Native PAGE, and Immunoblotting.
Protein samples were applied on Any kD or 12% Mini-PROTEAN TGX precast gels (BioRad), and SDS/PAGE was performed according to the manufacturer’s instructions. Native PAGE was performed by using 4–15% gels from the same manufacturer. Staining was performed with Coomassie (GelCode Blue Stain Reagent; ThermoFisher Scientific) or Prussian Blue (iron stain kit; Sigma Aldrich) according to the manufacturers’ instructions. For immunoblotting, StreptTactin-coupled alkaline phosphatase conjugate (IBA Life Sciences) diluted 1:3,500 or anti-Pup antiserum diluted 1:2,000 was used for detection. Anti-Pup antiserum was obtained from Pineda Antikörper-Service. Therefore, rabbits were immunized with Pup protein carrying an N-terminal decahistidine tag and TEV cleavage site purified from E. coli BL21(DE3)/pET-TEV1-pup. For detection of proteins decorated with anti-Pup antiserum, goat anti-rabbit IgG coupled to alkaline phosphatase (Sigma-Aldrich) was used as secondary antibody in a 1:20,000 dilution. Detection was performed by using NBT/BCIP stock solution (Roche Diagnostics) following the manufacturer’s instructions.
To monitor protein levels of Ftn-Strep, the strains Δftn and ΔpupΔftn harboring pVWEx1-ftn-Strep were cultivated under iron starvation as described earlier. Gene expression of ftn-Strep was induced by using 1 mM IPTG in preculture and main culture. The main cultures (500 mL iron-limited glucose minimal medium) were inoculated to an OD600 of 1.5. After 2 h of cultivation, 250 µg/mL rifampicin and 100 µg/mL tetracycline were added to inhibit transcription and translation, respectively. A total of 100 mL of each culture was harvested at 5,000 × g for 7 min at 4 °C shortly before addition of rifampicin and tetracycline, and 2, 4, and 6 h after addition. The cells were washed in resuspension buffer and stored at −20 °C. For cell disruption with a Precellys 24 homogenizer (Bertin Technologies), cells were resuspended in TE buffer (20 mM Tris, pH 8.0, 1 mM EDTA). Cell debris was removed by centrifugation at 17,300 × g for 15 min at 4 °C. The protein concentration of the crude extract was determined by using the BC-Assay kit (Interchim). A total of 20 µg of protein was then separated by SDS/PAGE for subsequent relative quantification of Ftn-Strep in the samples by using immunoblotting with StrepTactin-alkaline phosphatase conjugate and Novex AP Chemiluminescent Substrate (Life Technologies). As loading control served citrate synthase (i.e., GltA), which is not known to be subject to transcriptional regulation under iron starvation, GltA was detected by using a rabbit polyclonal anti-GltA antiserum (50) and goat anti-rabbit antibody labeled with Cy5 (Life Technologies). Cy5 fluorescence was determined by using a Typhoon Trio Variable Mode Imager (GE Healthcare). ImageQuant TL software (GE Healthcare) was used to relatively quantify immunoblot bands.
In Vitro Pupylation of 24-Meric Ferritin and ARC-Treatment.
The in vitro pupylation assay used in this study is based on a previously published protocol (8). In vitro pupylation of Ftn and DtxR (negative control) was performed according to previous studies (23). Ftn was purified analogous to Pup purification described recently (23). DtxR was purified as described previously (29). Ferritin for unfolding assays was expressed via the plasmid pET-24b-ftn in E. coli BL21(DE3) in Terrific Broth (TB) medium (51). Cells were resuspended in buffer W (100 mM Tris, pH 8.0, 100 mM NaCl, 20 mM mannitol, 1 mM DTT) and disrupted by using a Precellys 24 homogenizer. After that, the sample was centrifuged at 16,000 × g for 15 min at 4 °C. The supernatant was subsequently heated to 70 °C for 10 min and then centrifuged again at 16,000 × g for 20 min at 4 °C according to a protocol described earlier (52). The 24-meric state of Ftn was confirmed by size-exclusion chromatography, which gave a mass of 428 kDa (theoretically 433 kDa). The purified Ftn protein was diluted 1:10 in buffer Ra [100 mM Hepes, pH 6.5, 150 mM NaCl, 20 mM MgCl2, 10% (wt/vol) glycerol, 1 mM DTT] to a final concentration of 400 µM, and, after addition of 5 mM (NH4)2Fe(SO4)2, it was incubated at 4 °C overnight. Then, the solution was washed in buffer R [100 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 20 mM MgCl2, 10% (wt/vol) glycerol, 1 mM DTT] using Amicon columns with an exclusion size of 30 kDa. Before using the protein for in vitro pupylation, the solution was centrifuged for 5 min to remove insoluble material. The protein quantity was determined by using a Nano Drop ND-1000 spectrophotometer using an extinction coefficient at 280 nm of 16,960 M−1⋅cm−1 and a size of 18.066 kDa. The in vitro pupylation of 24-meric Ftn was performed in buffer R using a mixture of 30 µM Pup, 2 µM PafA, 2 µM Ftn (24-meric, corresponding to 48 µM monomeric Ftn), and 10 mM ATP. After incubation for 16 h overnight at 22 °C, the samples were incubated with 3 µM ARC and 8 mM ATP for 15 min at 22 °C, similar to an assay established previously (11). ARC was purified as a C-terminally Strep-II–tagged version. Controls without ARC and/or Pup were treated accordingly. Aliquots of the samples were applied on 4–15% native gels. Staining was performed with Coomassie Brilliant Blue for detection of proteins or with Prussian Blue for detection of stored ferric iron.
Purification of 7xHis-Ftn and 7xHis-Ftn-K78A.
Cells of strain C. glutamicum ΔpupΔftn harboring plasmid pVWEx1-pup-ftn or pVWEx1-pup-ftn-K78A (∼3.5 g wet weight each) were resuspended in buffer NN (50 mM NaH2PO4, 500 mM NaCl, adjusted with NaOH to pH 8.0) and disrupted by five passages through a French pressure cell at 172 MPa. The soluble protein fraction was obtained after two centrifugation steps as described previously (23) and subjected to immobilized metal ion affinity chromatography (IMAC) using a Ni-NTA Superflow column with 1 mL volume (Qiagen). The soluble protein fractions containing 7xHis-Ftn or 7xHis-Ftn-K78A were applied onto the IMAC column, and the eluate was applied a second time. After washing steps with NNI20 (buffer NN containing 20 mM imidazole) and NNI80 (80 mM imidazole), the His-tagged proteins were eluted with NNI300 (300 mM imidazole) in 10 × 0.5 mL fractions. The elution fractions containing protein were pooled, and the elution buffer was exchanged against NN buffer using Amicon Ultra-4 centrifugal filter devices (cutoff, 3 kDa; EMD Millipore) according to the manufacturer’s protocol. Protein concentrations were determined by measuring the absorbance at 280 nm by using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). An extinction coefficient of 16,960 M−1⋅cm−1 was used for 7xHis-Ftn (19.16 kDa).
Protein Analysis by MALDI-TOF/TOF MS/MS.
Proteins to be analyzed by MS were excised from polyacrylamide gels and in-gel trypsinized as described previously (53). For protein identification by peptide mass fingerprinting, the tryptic peptides were subjected to MALDI-TOF MS using an Ultraflex III TOF/TOF mass spectrometer (Bruker Daltonics) as described previously (54). Pupylated peptides were identified by using MALDI-TOF/TOF MS/MS in LIFT mode (Bruker Daltonics). Ionization voltage was 8.0 kV, LIFT voltage 19 kV, and reflector voltage 29.5 kV. MS/MS spectra were evaluated, allowing a mass tolerance of 0.5 Da. We regarded peptides as identified if they had been detected as a significant hit (P < 0.05) by the Mascot algorithm (v.2.2.0; Matrix Science).
Acknowledgments
We thank Susana Matamouros for carefully reading the manuscript; Antonia Heyer for providing plasmid pJC1-PripA-Crimson; Eugen Pfeifer for flow-cytometry measurements; and Meike Baumgart, Julia Frunzke, and Abigail Koch-Koerfges for discussion.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The DNA microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE64866).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514529113/-/DCSupplemental.
References
- 1.Darwin KH. Prokaryotic ubiquitin-like protein (Pup), proteasomes and pathogenesis. Nat Rev Microbiol. 2009;7(7):485–491. doi: 10.1038/nrmicro2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iyer LM, Burroughs AM, Aravind L. Unraveling the biochemistry and provenance of pupylation: A prokaryotic analog of ubiquitination. Biol Direct. 2008;3(1):45. doi: 10.1186/1745-6150-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce MJ, Mintseris J, Ferreyra J, Gygi SP, Darwin KH. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science. 2008;322(5904):1104–1107. doi: 10.1126/science.1163885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns KE, Liu WT, Boshoff HIM, Dorrestein PC, Barry CE., 3rd Proteasomal protein degradation in Mycobacteria is dependent upon a prokaryotic ubiquitin-like protein. J Biol Chem. 2009;284(5):3069–3075. doi: 10.1074/jbc.M808032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Striebel F, Imkamp F, Özcelik D, Weber-Ban E. Pupylation as a signal for proteasomal degradation in bacteria. Biochim Biophys Acta. 2014;1843(1):103–113. doi: 10.1016/j.bbamcr.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Barandun J, Delley CL, Weber-Ban E. The pupylation pathway and its role in mycobacteria. BMC Biol. 2012;10:95. doi: 10.1186/1741-7007-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imkamp F, et al. Deletion of dop in Mycobacterium smegmatis abolishes pupylation of protein substrates in vivo. Mol Microbiol. 2010;75(3):744–754. doi: 10.1111/j.1365-2958.2009.07013.x. [DOI] [PubMed] [Google Scholar]
- 8.Striebel F, et al. Bacterial ubiquitin-like modifier Pup is deamidated and conjugated to substrates by distinct but homologous enzymes. Nat Struct Mol Biol. 2009;16(6):647–651. doi: 10.1038/nsmb.1597. [DOI] [PubMed] [Google Scholar]
- 9.Cerda-Maira FA, et al. Molecular analysis of the prokaryotic ubiquitin-like protein (Pup) conjugation pathway in Mycobacterium tuberculosis. Mol Microbiol. 2010;77(5):1123–1135. doi: 10.1111/j.1365-2958.2010.07276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutter M, Damberger FF, Imkamp F, Allain FHT, Weber-Ban E. Prokaryotic ubiquitin-like protein (Pup) is coupled to substrates via the side chain of its C-terminal glutamate. J Am Chem Soc. 2010;132(16):5610–5612. doi: 10.1021/ja910546x. [DOI] [PubMed] [Google Scholar]
- 11.Striebel F, Hunkeler M, Summer H, Weber-Ban E. The mycobacterial Mpa-proteasome unfolds and degrades pupylated substrates by engaging Pup’s N-terminus. EMBO J. 2010;29(7):1262–1271. doi: 10.1038/emboj.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Darwin KH, Li H. Binding-induced folding of prokaryotic ubiquitin-like protein on the Mycobacterium proteasomal ATPase targets substrates for degradation. Nat Struct Mol Biol. 2010;17(11):1352–1357. doi: 10.1038/nsmb.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns KE, et al. “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Mol Cell. 2010;39(5):821–827. doi: 10.1016/j.molcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imkamp F, et al. Dop functions as a depupylase in the prokaryotic ubiquitin-like modification pathway. EMBO Rep. 2010;11(10):791–797. doi: 10.1038/embor.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Festa RA, et al. Prokaryotic ubiquitin-like protein (Pup) proteome of Mycobacterium tuberculosis [corrected] PLoS One. 2010;5(1):e8589. doi: 10.1371/journal.pone.0008589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poulsen C, et al. Proteome-wide identification of mycobacterial pupylation targets. Mol Syst Biol. 2010;6:386. doi: 10.1038/msb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watrous J, et al. Expansion of the mycobacterial “PUPylome”. Mol Biosyst. 2010;6(2):376–385. doi: 10.1039/b916104j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun HY, Tamura N, Tamura T. Rhodococcus prokaryotic ubiquitin-like protein (Pup) is degraded by deaminase of pup (Dop) Biosci Biotechnol Biochem. 2012;76(10):1959–1966. doi: 10.1271/bbb.120458. [DOI] [PubMed] [Google Scholar]
- 19.Compton CL, Fernandopulle MS, Nagari RT, Sello JK. Genetic and proteomic analyses of pupylation in Streptomyces coelicolor. J Bacteriol. 2015;197(17):2747–2753. doi: 10.1128/JB.00302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elharar Y, et al. Survival of mycobacteria depends on proteasome-mediated amino acid recycling under nutrient limitation. EMBO J. 2014;33(16):1802–1814. doi: 10.15252/embj.201387076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boubakri H, et al. The absence of pupylation (prokaryotic ubiquitin-like protein modification) affects morphological and physiological differentiation in Streptomyces coelicolor. J Bacteriol. 2015;197(21):3388–3399. doi: 10.1128/JB.00591-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delley CL, Striebel F, Heydenreich FM, Özcelik D, Weber-Ban E. Activity of the mycobacterial proteasomal ATPase Mpa is reversibly regulated by pupylation. J Biol Chem. 2012;287(11):7907–7914. doi: 10.1074/jbc.M111.331124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Küberl A, et al. Pupylated proteins in Corynebacterium glutamicum revealed by MudPIT analysis. Proteomics. 2014;14(12):1531–1542. doi: 10.1002/pmic.201300531. [DOI] [PubMed] [Google Scholar]
- 24.Eggeling L, Bott M, editors. 2005. Handbook of Corynebacterium glutamicum (CRC Press, Boca Raton, FL)
- 25.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302(5652):1963–1966. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 26.D’Aquino JA, Tetenbaum-Novatt J, White A, Berkovitch F, Ringe D. Mechanism of metal ion activation of the diphtheria toxin repressor DtxR. Proc Natl Acad Sci USA. 2005;102(51):18408–18413. doi: 10.1073/pnas.0500908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brune I, et al. The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genomics. 2006;7(1):21. doi: 10.1186/1471-2164-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennerhold J, Bott M. The DtxR regulon of Corynebacterium glutamicum. J Bacteriol. 2006;188(8):2907–2918. doi: 10.1128/JB.188.8.2907-2918.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wennerhold J, Krug A, Bott M. The AraC-type regulator RipA represses aconitase and other iron proteins from Corynebacterium under iron limitation and is itself repressed by DtxR. J Biol Chem. 2005;280(49):40500–40508. doi: 10.1074/jbc.M508693200. [DOI] [PubMed] [Google Scholar]
- 30.Andrews SC. The Ferritin-like superfamily: Evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim Biophys Acta. 2010;1800(8):691–705. doi: 10.1016/j.bbagen.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Engels S, Schweitzer JE, Ludwig C, Bott M, Schaffer S. clpC and clpP1P2 gene expression in Corynebacterium glutamicum is controlled by a regulatory network involving the transcriptional regulators ClgR and HspR as well as the ECF sigma factor sigmaH. Mol Microbiol. 2004;52(1):285–302. doi: 10.1111/j.1365-2958.2003.03979.x. [DOI] [PubMed] [Google Scholar]
- 32.Lüdke A, Krämer R, Burkovski A, Schluesener D, Poetsch A. A proteomic study of Corynebacterium glutamicum AAA+ protease FtsH. BMC Microbiol. 2007;7:6. doi: 10.1186/1471-2180-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honarmand Ebrahimi K, Hagedoorn PL, Hagen WR. Unity in the biochemistry of the iron-storage proteins ferritin and bacterioferritin. Chem Rev. 2015;115(1):295–326. doi: 10.1021/cr5004908. [DOI] [PubMed] [Google Scholar]
- 34.Liochev SI. The mechanism of “Fenton-like” reactions and their importance for biological systems. A biologist’s view. Interrelations between free radicals and metal ions in life processes. In: Sigel A, Sigel H, editors. Metal Ions in Biological Systems. Vol 36. Marcel Dekker; New York: 1999. pp. 1–39. [PubMed] [Google Scholar]
- 35.Almirón M, Link AJ, Furlong D, Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992;6(12B):2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 36.Zhao G, et al. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem. 2002;277(31):27689–27696. doi: 10.1074/jbc.M202094200. [DOI] [PubMed] [Google Scholar]
- 37.Choi H, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105(1):103–113. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 38.Milse J, Petri K, Rückert C, Kalinowski J. Transcriptional response of Corynebacterium glutamicum ATCC 13032 to hydrogen peroxide stress and characterization of the OxyR regulon. J Biotechnol. 2014;190:40–54. doi: 10.1016/j.jbiotec.2014.07.452. [DOI] [PubMed] [Google Scholar]
- 39.Teramoto H, Inui M, Yukawa H. OxyR acts as a transcriptional repressor of hydrogen peroxide-inducible antioxidant genes in Corynebacterium glutamicum R. FEBS J. 2013;280(14):3298–3312. doi: 10.1111/febs.12312. [DOI] [PubMed] [Google Scholar]
- 40.Ernst JF, Bennett RL, Rothfield LI. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135(3):928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massé E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci USA. 2002;99(7):4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frunzke J, Engels V, Hasenbein S, Gätgens C, Bott M. Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol Microbiol. 2008;67(2):305–322. doi: 10.1111/j.1365-2958.2007.06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Russell D. Molecular Cloning. A Laboratory Manual. 3rd Ed Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- 44.Tauch A, et al. Efficient electrotransformation of Corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr Microbiol. 2002;45(5):362–367. doi: 10.1007/s00284-002-3728-3. [DOI] [PubMed] [Google Scholar]
- 45.Niebisch A, Bott M. Molecular analysis of the cytochrome bc1-aa3 branch of the Corynebacterium glutamicum respiratory chain containing an unusual diheme cytochrome c1. Arch Microbiol. 2001;175(4):282–294. doi: 10.1007/s002030100262. [DOI] [PubMed] [Google Scholar]
- 46.Vogt M, et al. Pushing product formation to its limit: Metabolic engineering of Corynebacterium glutamicum for L-leucine overproduction. Metab Eng. 2014;22:40–52. doi: 10.1016/j.ymben.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Helfrich S, et al. Live cell imaging of SOS and prophage dynamics in isogenic bacterial populations. Mol Microbiol. 2015;98(4):636–650. doi: 10.1111/mmi.13147. [DOI] [PubMed] [Google Scholar]
- 48.Heyer A, et al. The two-component system ChrSA is crucial for haem tolerance and interferes with HrrSA in haem-dependent gene regulation in Corynebacterium glutamicum. Microbiology. 2012;158(pt 12):3020–3031. doi: 10.1099/mic.0.062638-0. [DOI] [PubMed] [Google Scholar]
- 49.Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem. 2004;331(2):370–375. doi: 10.1016/j.ab.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 50.Baumgart M, Bott M. Biochemical characterisation of aconitase from Corynebacterium glutamicum. J Biotechnol. 2011;154(2-3):163–170. doi: 10.1016/j.jbiotec.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Tartof KD, Hobbs CA. Improved media for growing plasmid and cosmid clones. Bethesda Res Lab Focus. 1987;9:12. [Google Scholar]
- 52.Hudson AJ, et al. Overproduction, purification and characterization of the Escherichia coli ferritin. Eur J Biochem. 1993;218(3):985–995. doi: 10.1111/j.1432-1033.1993.tb18457.x. [DOI] [PubMed] [Google Scholar]
- 53.Schaffer S, et al. A high-resolution reference map for cytoplasmic and membrane-associated proteins of Corynebacterium glutamicum. Electrophoresis. 2001;22(20):4404–4422. doi: 10.1002/1522-2683(200112)22:20<4404::AID-ELPS4404>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 54.Koch-Koerfges A, Kabus A, Ochrombel I, Marin K, Bott M. Physiology and global gene expression of a Corynebacterium glutamicum ΔF(1)F(O)-ATP synthase mutant devoid of oxidative phosphorylation. Biochim Biophys Acta. 2012;1817(2):370–380. doi: 10.1016/j.bbabio.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Pettersen EF, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comp Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 56.Abe S, Takayama K, Kinoshita S. Taxonomical studies on glutamic acid producing bacteria. J Gen Appl Microbiol. 1967;13:279–301. [Google Scholar]
- 57.Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 58.Schäfer A, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145(1):69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 59.Bussmann M, Baumgart M, Bott M. RosR (Cg1324), a hydrogen peroxide-sensitive MarR-type transcriptional regulator of Corynebacterium glutamicum. J Biol Chem. 2010;285(38):29305–29318. doi: 10.1074/jbc.M110.156372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters-Wendisch PG, et al. Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol. 2001;3(2):295–300. [PubMed] [Google Scholar]