Abstract
Although currently available treatment options for age-related macular degeneration (AMD) are limited, particularly for atrophic AMD, the identification of predisposing genetic variations has informed clinical studies addressing therapeutic options such as complement inhibitors and anti-inflammatory agents. To lower risk of early AMD, recommended lifestyle interventions such as the avoidance of smoking and the intake of low glycemic antioxidant-rich diets have largely followed from the identification of nongenetic modifiable factors. On the other hand, the challenge of understanding the complex relationship between aging and cumulative damage leading to AMD has fueled investigations of the visual cycle adducts that accumulate in retinal pigment epithelial (RPE) cells and are a hallmark of aging retina. These studies have revealed properties of these compounds that provide insights into processes that may compromise RPE and could contribute to disease mechanisms in AMD. This work has also led to the design of targeted therapeutics that are currently under investigation.
Keywords: age-related macular degeneration, vitamin A-aldehyde adducts, bisretinoids, retinal pigment epithelium, photoreceptor cells
Age-related macular degeneration (AMD) is a complex disorder that is predicted to have a growing impact on elderly populations. Although the cause of central vision loss in AMD is the progressive impairment of photoreceptor cells, the disease is generally thought to begin with dysfunctioning of retinal pigment epithelium (RPE) and adverse changes in subjacent Bruch’s membrane (1, 2). The early stage of the disease is typically marked by extracellular accumulations (drusen) between RPE and Bruch’s membrane. Progression to advanced disease is defined by delineated areas devoid of RPE and photoreceptor cell loss (atrophic AMD) and/or abnormal growth of blood vessels underneath RPE or within the subretinal space (neovascular AMD).
AMD Risk Factors
AMD susceptibility is influenced by multiple factors of both genetic and environmental origin. Predisposing genetic factors account for 71% of the variation in disease risk among individuals (3). Currently, 52 independently associated genetic variants at 34 loci are known to account for ∼50% of AMD heritability (4). The genes implicated by these variants belong to multiple systems some of which are associated with the complement pathway, lipid metabolism, and maintenance of extracellular matrix (5, 6). Nevertheless, many individuals carrying risk variants do not develop AMD.
Two loci, CFH (complement factor H; 1q31) and ARMS2/HTRA1 (age-related maculopathy susceptibility 2/high-temperature requirement factor A1; 10q26), make the greatest contribution to AMD risk. Variants at the ARMS2/HTRA1 and CFH loci significantly increase risk for progression to both the atrophic and neovascular forms of AMD, although the magnitude of the association of the ARMS2/HTRA1 risk allele is somewhat greater for the neovascular phenotype of late AMD, whereas CFH risk variants favor progression toward geography atrophy (5). Due to strong disequilibrium, genetic studies cannot determine whether ARMS2 or HTRA1 is the causal gene, and the functions of the genes at this locus are a matter of investigation (7, 8).
Loci near the complement genes (CFH, C2/CFB, C3, and CFI) are credited with ∼57% of the contribution of known variants to disease risk. Given the numbers of genes encoding complement system factors, together with the presence of complement factors and immune system proteins in drusen, complement dysregulation and inflammation are thought to play a major role in AMD pathogenesis (9). Accordingly, these known genetic risks have guided some of the therapeutic options for atrophic AMD that are currently under investigation (10).
Nongenetic factors such as age and smoking also make a substantial contribution to AMD risk. The strongest modifiable AMD risk factor is smoking (11, 12). Nutritional status particularly in regard to dietary antioxidants (13) and glycemic index (14) also impacts AMD risk. Intake of the antioxidant vitamins E and C and zinc together or zinc alone [Age-Related Eye Disease Study (AREDS) supplements] (15) was shown to reduce the rate of progression to advanced AMD. Although lutein and zeaxanthin are more appropriate than β-carotene for inclusion in the AREDS formulation (16), no additional benefit was derived if patients took these nutrients in addition to the AREDS supplement (17). This is also the case for the intake of ω-3 fatty acids. The benefits of AREDS nutritional supplements (vitamins E, C, and zinc) appear to be the same regardless of whether the patients carry the risk variants in CFH Y402H and/or ARMS2 A69S (18).
Efforts to unravel the pathogenesis of AMD have for many years given consideration to intracellular deposits of vitamin A-aldehydes, a prominent feature of aging RPE that unleash chronic mechanisms consistent with late-onset disease (19). Thus, the remaining sections of this article will explore biological mechanisms through which this material could modulate the development of AMD pathogenesis and address associated therapeutic implications. The interplay among these visual cycle adducts, lifetime light exposure, and oxidative stress will also be discussed in relation to AMD risk.
What Are Vitamin A-Aldehyde Adducts?
RPE cell aging is marked by intracellular accumulations of a family of autofluorescent compounds. These vitamin A aldehyde adducts have a bisretinoid structure and form by nonenzymatic reactions in photoreceptor outer segments, particularly condensations of retinaldehyde and phosphatidylethanolamine (PE). RPE phagocytosis serves to transfer bisretinoid-burdened outer segment discs to the RPE, but phagocytosis is not necessary for the formation of these fluorophores (20). The spectral characteristics of bisretinoids can account for the distinct fluorescence of RPE lipofuscin and for the fluorescence emission that is recorded noninvasively as fundus autofluorescence (AF) in clinical and experimental work (21). The AF emitted by these fluorophores is of highest intensity in the macula (22).
Bisretinoid fluorophores accumulate with age in RPE cells in all healthy eyes but form in abundance in recessive Stargardt disease (STGD1) due to mutations in the ATP-binding cassette transporter 4 (ABCA4). The toxicity of these vitamin A-aldehyde adducts is likely attributable to their propensity to photogenerate reactive oxygen species and to photodecompose into aldehyde- and dicarbonyl- (glyoxal and methylglyoxal) bearing fragments (23, 24). Evidence that proteins modified by the same dicarbonyls are detected in drusen, is indicative of a link between photodegradation of RPE lipofuscin and sub-RPE aging changes that confer risk of AMD. These damaging photodegradative processes and associated bisretinoid loss may also explain the predilection of the macula for disease. In support of this association are results showing that mice burdened with augmented bisretinoid formation monitored as elevated A2E and all-trans-retinal dimer, exhibit accentuated carbonyl-adduct deposition in Bruch’s membrane, excessive complement activation, Bruch’s membrane thickening due to basal laminar deposits, and accelerated loss of photoreceptor cells compared with WT mice (25–30). Additionally, albino Abca4−/− mice exhibit an increased susceptibility to retina light damage (31).
AMD Risk Factors That Intersect with Elements of Bisretinoid Deposition
Oxidative mechanisms are widely considered to be a major factor contributing to AMD pathogenesis (5, 32). Indeed the oxidant content of cigarette smoke may explain the impact of the latter on AMD risk (32). Numerous clinical and observation studies have demonstrated that dietary antioxidants and intake of antioxidants by supplementation reduces incidence or progression of AMD (15, 33–36). Because antioxidants can protect against AMD, and vitamins E and C have also been shown to reduce A2E photooxidation/photodegradation (37–39) (Fig. 1), the beneficial effects of antioxidant intake could, in some measure, be attributable to the suppression of photooxidative processes that are known to precede bisretinoid photodegradation. Similarly, it is significant that recent epidemiological studies (12, 40–45) and a meta-analysis (46) have supported a relationship between AMD and sunlight exposure. The potential contribution of lifetime light exposure to disease risk could be, at least partially, understood from the perspective of the cellular damage imposed by bisretinoid photodegradation.
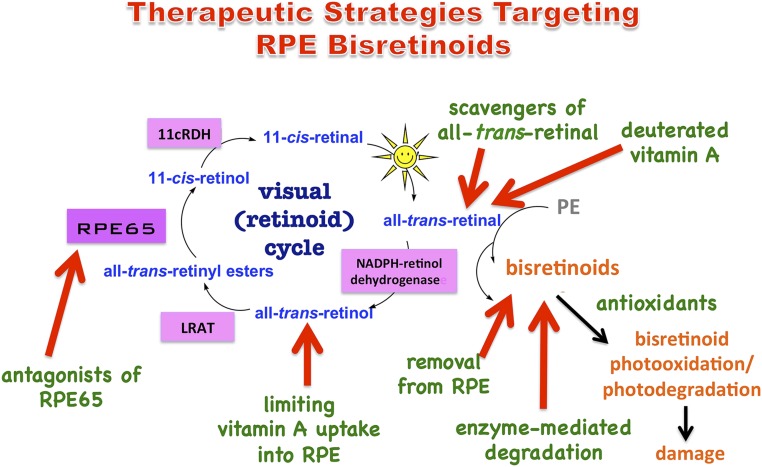
Fig. 1.
Therapeutic strategies targeting RPE bisretinoids. Bisretinoids are visual cycle adducts having a variety of structures. These fluorophores form randomly in photoreceptor cell outer segments due to reactions of retinaldehyde (all-trans- and 11-cis-retinal) with amines such as PE. Bisretinoids are transferred to RPE by phagocytosis and accumulate as lipofuscin. Vitamin A (all-trans-retinol) enters the visual cycle by uptake into RPE. On photoisomerization of 11-cis-retinal in photoreceptor cells, all-trans-retinal is released from rhodopsin and is reduced to all-trans-retinol by NADPH-dependent all-trans-retinol dehydrogenase (NADPH-retinol dehydrogenase). Within RPE, LRAT converts all-trans-retinol to all-trans-retinyl esters; the isomerase RPE65 generates 11-cis-retinol, and 11-cis-retinol is oxidized to 11-cis-retinal by 11-cis-retinol dehydrogenase (11-cRDH). Bisretinoids are photosensitizers; they also photooxidize and photodegrade, releasing damaging dicarbonyls and aldehyde-containing fragments.
Therapeutic Approaches That Target Bisretinoid: Clinical and Preclinical Studies
Limiting Vitamin A.
One approach to reducing bisretinoid formation is to limit the delivery of vitamin A (all-trans-retinol) to the RPE (Fig. 1). For delivery in serum to RPE and other tissues, retinol is held within a carrier complex of retinol binding protein 4 (RBP4) and transthyretin (TTR); uptake by RPE cells is receptor mediated (47). Fenretinide [4-hydroxy(phenyl)retinamide], a retinoid derivative, competes with retinol for binding to RBP; as a consequence systemic retinol is reduced. In preclinical studies in Abca4−/− mice, investigators demonstrated that fenretinide reduced the formation of A2E during treatment for 1 mo. Based on these data, a 2-y phase 2 double-masked, randomized, placebo-controlled multicenter trial (ClinicalTrials.gov identifier; NCT00429936) was initiated to determine safety and efficacy of oral fenretinide at 100 and 300 mg daily in subjects with geographic atrophy (GA) (48, 49). Serum RBP levels were reduced by 50% (100-mg dose) and 70% (300-mg dose). In keeping with the reduced availability of retinol, delayed dark adaptation was observed (50). Compared with placebo, significant differences in GA lesion size was not observed, and although there was a trend for reduced lesion growth rates when RBP levels dropped below 2 mg/dL, statistical significance was not achieved. Whether the effects of RBP4 antagonists on vitamin A delivery to retina are short-lived due to compensation by alternative RBP4-independent mechanisms, such as occurs in the Rbp4−/− mouse (51), is not known.
A similar platform is the focus of work testing the efficacy of the RBP4 antagonist A1120, a nonretinoid that was shown to reduce serum RBP4 levels by 75% and bisretinoid lipofuscin levels in Abca4−/− mice by 50% (52). Unlike fenretinide, A1120 does not activate retinoic acid receptors. Structural modifications made to the core of the model have improved its metabolic stability (53).
Deuterated Vitamin A.
In yet another strategy aimed at reducing bisretinoid formation, deuterium isotope replacement at the carbon 20 position of all-trans-retinol (C20-D3-vitamin A) has been designed to reduce the rate at which retinaldehyde reacts (54, 55) (Fig. 1) without slowing the visual cycle. In vitro experiments revealed several fold slower formation of the retinaldehyde adducts A2E and all-trans-retinal dimer. In addition, treatment of rats and mice with C20-D3-vitamin A administered either in the diet or by i.p. injection resulted in reduced bisretinoid. A phase 1 safety study (ClinicalTrials.gov identifier: NCT02230228) oral administration of C20-D3-vitamin A (ALK-001) in healthy volunteers has been completed. Recruitment is now ongoing for a phase 2 placebo-controlled study (ClinicalTrials.gov identifier: NCT02402660) that will examine tolerability and effects of ALK-001 in STGD1. As indicated by the authors, the efficiency with which native vitamin A is replaced by C20-D3-vitamin A remains to be determined (55). An increase in total vitamin A intake might also potentiate the drive toward bisretinoid formation (56).
Targeting RPE65.
Another approach to modulating the visual cycle for the purposes of inhibiting bisretinoid formation is exemplified by emixustat (previously known as ACU-4429; ClinicalTrials.gov identifier: NCT01802866) (57), an amine-carrying drug and derivative of all-trans-retinylamine (58). Emixustat is a retinoid mimic developed to inhibit the activity of the isomerohydrolase RPE65 (Fig. 1). RPE65 is expressed in RPE cells and serves to generate 11-cis-retinol from all-trans-retinyl esters (59). Acylation of retinylamine by lecithin:retinol acyltransferase (LRAT) may facilitate retention of the drug in RPE cells (60). In WT mice, treatment with emixustat inhibited the production of 11-cis-retinal by ∼80% (57, 61), whereas oral administration of emixustat to Abca4−/− mice for 3 mo reduced A2E in a dose-dependent manner (61). Complete arrest of RPE65 activity is not desirable as this would prevent visual pigment regeneration and create a drug effect comparable to severe retinopathy. In a multidose phase 1 trial (14 d; ClinicalTrials.gov identifier: NCT00942240) in healthy subjects and a phase 2 (90 d; NCT01002950) study of atrophic AMD, patients undergoing oral treatment with emixustat experienced dyschromatopsia and delayed dark adaptation as would be expected (62). Emixustat is currently being tested in a phase 2b/3 multicenter, randomized, double-masked, placebo-controlled dose-ranging study that is comparing its efficacy and safety with placebo for the treatment of atrophic AMD (63).
Support for a paradigm aimed at targeting RPE65 was also obtained using small molecule farnesyl-containing nonretinoid isoprenoids, one in which a ketone replaced the ester linkage (TDT) and a second that used an amide (TDH). Both antagonized the isomerase activity of RPE65 by competing with all-trans-retinyl esters. In rats and WT mice, a single injection of TDT and TDH slowed the regeneration of 11-cis-retinal after bleaching light. Chronic treatment of Abca4−/− mice with TDT had a pronounced effect on A2E accumulation; after the course of treatment, levels of this bisretinoid were the same as before treatment was begun (64).
Scavengers of All-Trans-Retinal.
To reduce the production of retinaldehyde adducts, primary amine-containing compounds are being studied for the purpose of trapping free all-trans-retinal (65) (Fig. 1). To be effective, candidate molecules should compete with PE and penetrate into outer segments at sufficient concentration. In mice carrying double null mutations in retinol dehydrogenase (Rdh8-/−) and Abca4−/−, both of which are essential to the conversion of toxic all-trans-retinal to all-trans-retinol, screening of FDA-approved drugs has revealed some that can protect against acute light-induced retinal degeneration while maintaining 11-cis-retinal levels. The Rdh8−/−/Abca4−/− mouse, under acute light exposure, provides a high throughput testing platform within which aberrant free all-trans-retinal is abundant.
Reversing Bisretinoid Accumulation.
Treatment strategies geared toward removing bisretinoid from the RPE have taken two forms (Fig. 1). Because these bisretinoid fluorophores are refractory to lysis by native lysosomal enzymes, the first approach aimed to degrade bisretinoid by delivery of exogenous enzyme (66). This design was borrowed from enzyme replacement therapies aimed at reversing lysosomal storage disease (67). As proof of principle, horseradish peroxidase (HRP) was used in noncellular and cell-based assays to demonstrate enzymatic degradation of A2E; this approach would be applicable to all of the bisretinoid visual cycle adducts as they all present with polyene side-arms. One limitation would rest with the products of bisretinoid degradation and whether they carry toxic moieties e.g., aldehydes.
Still other designs have been explored for the purpose of removing bisretinoids from RPE cells by small molecule tetrahydropyridoethers (68) or β-cyclodextrins that encase bisretinoids in hydrophobic cavities formed by seven d-glucose units for elimination (69). Additional research is required to elucidate the safety and efficacy of clearing bisretinoid not only from the RPE cells but also from the surrounding tissues.
Light Attenuation.
Light deprivation does not suppress the formation of bisretinoids (70), probably because 11-cis-retinal (the light-sensitive configuration) and all-trans-retinal both serve as bisretinoid precursors. Nevertheless, reducing light exposure can defend against damaging bisretinoid photodegradation. For instance, sunglasses that attenuate over a broad range of wavelengths or yellow lenses that reduce blue wavelengths could be expected to provide protection (71). A black contact lens that blocked >90% of light in one eye of STGD1 patients was found to reduce the progression of decreased fundus AF (72); the latter decrease is likely attributable to bisretinoid photooxidation and photodegradation.
Other Considerations
It is too early to know whether some or all of these therapeutic schemes will be effective. Although the discussion here has focused on AMD, interventions aimed at bisretinoids would also be applicable to less common diseases such as STGD1. In the latter early-onset form of macular degeneration, gene therapy promises to correct the accelerated bisretinoid deposition that is a consequence of most ABCA4 mutations (73, 74), whereas cell-based therapies aim to replace the damaged RPE (75).
Bisretinoids likely impart chronic insult that at any given time is subtle (76). Thus, therapies that target bisretinoids are probably appropriate for suppressing early and intermediate stages of cellular damage but may not be as effective for ameliorating existing disease (76). In most cases the target cell for drug delivery would be the RPE; however, scavengers of all-trans-retinal would have to gain access to photoreceptor cell outer segments. Because some of these drugs would have to be continuously active over the long term, drug delivery systems that maintain therapeutic levels of the drugs are desirable.
The choice of appropriate outcome measures is essential to the design of clinical trials. Ongoing clinical trials targeting RPE bisretinoids typically use the rate of enlargement of GA measured in color fundus or fundus AF images, as the primary end point for judging treatment efficacy. Another meaningful efficacy end point for studies addressing vitamin A-aldehyde adduct formation is the measurement of fundus AF. Retinal bisretinoids are the source of fundus AF that is imaged noninvasively by confocal laser scanning ophthalmoscopy; as such, fundus AF is suited to the definition of a biomarker (77). The introduction of protocols for quantifying fundus AF [quantitative fundus AF (qAF)] (78) also raises the possibility of acquiring qAF values as direct indicators of the response to therapeutic intervention. The robustness of this approach in the setting of clinical trials awaits testing.
Acknowledgments
This work was supported by National Eye Institute Grants EY12951 and EY024091; Foundation Fighting Blindness; and a grant from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.McLeod DS, et al. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hageman GS, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20(6):705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: Relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123(3):321–327. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Fritsche LG, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134–143. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritsche LG, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433–439. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grassmann F, et al. Clinical and genetic factors associated with progression of geographic atrophy lesions in age-related macular degeneration. PLoS One. 2015;10(5):e0126636. doi: 10.1371/journal.pone.0126636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich U, et al. Synonymous variants in HTRA1 implicated in AMD susceptibility impair its capacity to regulate TGF-β signaling. Hum Mol Genet. 2015;24(22):6361–6373. doi: 10.1093/hmg/ddv346. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, et al. Analysis of the indel at the ARMS2 3'UTR in age-related macular degeneration. Hum Genet. 2010;127(5):595–602. doi: 10.1007/s00439-010-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritsche LG, et al. Age-related macular degeneration: Genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128(3):349–358. doi: 10.1001/archophthalmol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Klein BEK, Wong TY, Tomany SC, Cruickshanks KJ. The association of cataract and cataract surgery with the long-term incidence of age-related maculopathy: The Beaver Dam eye study. Arch Ophthalmol. 2002;120(11):1551–1558. doi: 10.1001/archopht.120.11.1551. [DOI] [PubMed] [Google Scholar]
- 12.Tomany SC, Cruickshanks KJ, Klein R, Klein BEK, Knudtson MD. Sunlight and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol. 2004;122(5):750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- 13.Seddon JM, et al. Eye Disease Case-Control Study Group Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- 14.Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A. Does eating particular diets alter the risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. 2009;93(9):1241–1246. doi: 10.1136/bjo.2008.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew EY, et al. Age-Related Eye Disease Study 2 (AREDS2) Research Group Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 18.Chew EY, Klein ML, Clemons TE, Agrón E, Abecasis GR. Genetic testing in persons with age-related macular degeneration and the use of the AREDS supplements: To test or not to test? Ophthalmology. 2015;122(1):212–215. doi: 10.1016/j.ophtha.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430–1438. doi: 10.1172/JCI71029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Shabat S, et al. Biosynthetic studies of A2E, a major fluorophore of retinal pigment epithelial lipofuscin. J Biol Chem. 2002;277(9):7183–7190. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- 21.Delori FC, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36(3):718–729. [PubMed] [Google Scholar]
- 22.Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989;30(8):1691–1699. [PubMed] [Google Scholar]
- 23.Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc Natl Acad Sci USA. 2010;107(16):7275–7280. doi: 10.1073/pnas.0913112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon KD, Yamamoto K, Ueda K, Zhou J, Sparrow JR. A novel source of methylglyoxal and glyoxal in retina: Implications for age-related macular degeneration. PLoS One. 2012;7(7):e41309. doi: 10.1371/journal.pone.0041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng J, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98(1):13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 26.Kim SR, et al. Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E. Proc Natl Acad Sci USA. 2004;101(32):11668–11672. doi: 10.1073/pnas.0403499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radu RA, et al. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J Biol Chem. 2011;286(21):18593–18601. doi: 10.1074/jbc.M110.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Ueda K, Zhao J, Sparrow JR. Correlations between photodegradation of bisretinoid constituents of retina and dicarbonyl-adduct deposition. J Biol Chem. 2015;290(45):27215–27227. doi: 10.1074/jbc.M115.680363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283(39):26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda A, Golczak M, Maeda T, Palczewski K. Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina. Invest Ophthalmol Vis Sci. 2009;50(11):5435–5443. doi: 10.1167/iovs.09-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Nagasaki T, Sparrow JR. Photoreceptor cell degeneration in Abcr (-/-) mice. Adv Exp Med Biol. 2010;664:533–539. doi: 10.1007/978-1-4419-1399-9_61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handa JT. How does the macula protect itself from oxidative stress? Mol Aspects Med. 2012;33(4):418–435. doi: 10.1016/j.mam.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Age-Related Eye Disease Study Research Group SanGiovanni JP, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007;125(5):671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 34.Age-Related Eye Disease Study Group SanGiovanni JP, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. 2007;125(9):1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 35.Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2012;11:CD000254. doi: 10.1002/14651858.CD000254.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Sobrin L, Seddon JM. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparrow JR, et al. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J Biol Chem. 2003;278(20):18207–18213. doi: 10.1074/jbc.M300457200. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Gao X, Cai B, Sparrow JR. Indirect antioxidant protection against photooxidative processes initiated in retinal pigment epithelial cells by a lipofuscin pigment. Rejuvenation Res. 2006;9(2):256–263. doi: 10.1089/rej.2006.9.256. [DOI] [PubMed] [Google Scholar]
- 39.Jang YP, Zhou J, Nakanishi K, Sparrow JR. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem Photobiol. 2005;81(3):529–536. doi: 10.1562/2004-12-14-RA-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruickshanks KJ, Klein R, Klein BEK. Sunlight and age-related macular degeneration. The Beaver Dam Eye Study. Arch Ophthalmol. 1993;111(4):514–518. doi: 10.1001/archopht.1993.01090040106042. [DOI] [PubMed] [Google Scholar]
- 41.Cruickshanks KJ, Klein R, Klein BEK, Nondahl DM. Sunlight and the 5-year incidence of early age-related maculopathy: The Beaver Dam Eye Study. Arch Ophthalmol. 2001;119(2):246–250. [PubMed] [Google Scholar]
- 42.Klein BE, et al. Sunlight exposure, pigmentation, and incident age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(9):5855–5861. doi: 10.1167/iovs.14-14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang EJ, et al. Prevalence and risk factors for age-related macular degeneration in the elderly Chinese population in south-western Taiwan: The Puzih eye study. Eye (Lond) 2014;28(6):705–714. doi: 10.1038/eye.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schick T, et al. History of sunlight exposure is a risk factor for age-related macular degeneration [published online ahead of print October 5, 2015] Retina. 2015 doi: 10.1097/IAE.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 45.Fletcher AE, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol. 2008;126(10):1396–1403. doi: 10.1001/archopht.126.10.1396. [DOI] [PubMed] [Google Scholar]
- 46.Sui GY, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97(4):389–394. doi: 10.1136/bjophthalmol-2012-302281. [DOI] [PubMed] [Google Scholar]
- 47.Kawaguchi R, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315(5813):820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 48.Mata NL, Vogel R. Pharmacologic treatment of atrophic age-related macular degeneration. Curr Opin Ophthalmol. 2010;21(3):190–196. doi: 10.1097/ICU.0b013e32833866c8. [DOI] [PubMed] [Google Scholar]
- 49.Mata NL, et al. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33(3):498–507. doi: 10.1097/IAE.0b013e318265801d. [DOI] [PubMed] [Google Scholar]
- 50.Caruso RC, et al. Effects of fenretinide (4-HPR) on dark adaptation. Arch Ophthalmol. 1998;116(6):759–763. doi: 10.1001/archopht.116.6.759. [DOI] [PubMed] [Google Scholar]
- 51.Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med. 2003;24(6):421–430. doi: 10.1016/s0098-2997(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 52.Dobri N, et al. A1120, a nonretinoid RBP4 antagonist, inhibits formation of cytotoxic bisretinoids in the animal model of enhanced retinal lipofuscinogenesis. Invest Ophthalmol Vis Sci. 2013;54(1):85–95. doi: 10.1167/iovs.12-10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cioffi CL, et al. Bicyclic [3.3.0]-octahydrocyclopenta[c]pyrrolo antagonists of retinol binding protein 4: Potential treatment of atrophic age-related macular degeneration and Stargardt disease. J Med Chem. 2015;58(15):5863–5888. doi: 10.1021/acs.jmedchem.5b00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma L, Kaufman Y, Zhang J, Washington I. C20-D3-vitamin A slows lipofuscin accumulation and electrophysiological retinal degeneration in a mouse model of Stargardt disease. J Biol Chem. 2011;286(10):7966–7974. doi: 10.1074/jbc.M110.178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman Y, Ma L, Washington I. Deuterium enrichment of vitamin A at the C20 position slows the formation of detrimental vitamin A dimers in wild-type rodents. J Biol Chem. 2011;286(10):7958–7965. doi: 10.1074/jbc.M110.178640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radu RA, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008;49(9):3821–3829. doi: 10.1167/iovs.07-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiser PD, et al. Catalytic mechanism of a retinoid isomerase essential for vertebrate vision. Nat Chem Biol. 2015;11(6):409–415. doi: 10.1038/nchembio.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golczak M, Kuksa V, Maeda T, Moise AR, Palczewski K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc Natl Acad Sci USA. 2005;102(23):8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122(3):449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, et al. Expansion of first-in-class drug candidates that sequester toxic all-trans-retinal and prevent light-induced retinal degeneration. Mol Pharmacol. 2015;87(3):477–491. doi: 10.1124/mol.114.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bavik C, et al. Visual cycle modulation as an approach toward preservation of retinal integrity. PLoS One. 2015;10(5):e0124940. doi: 10.1371/journal.pone.0124940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kubota R, et al. Phase 1, dose-ranging study of emixustat hydrochloride (ACU-4429), a novel visual cycle modulator, in healthy volunteers. Retina. 2014;34(3):603–609. doi: 10.1097/01.iae.0000434565.80060.f8. [DOI] [PubMed] [Google Scholar]
- 63.Dugel PU, et al. Phase ii, randomized, placebo-controlled, 90-day study of emixustat hydrochloride in geographic atrophy associated with dry age-related macular degeneration. Retina. 2015;35(6):1173–1183. doi: 10.1097/IAE.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maiti P, et al. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry. 2006;45(3):852–860. doi: 10.1021/bi0518545. [DOI] [PubMed] [Google Scholar]
- 65.Maeda A, et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol. 2011;8(2):170–178. doi: 10.1038/nchembio.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Y, Zhou J, Fishkin N, Rittmann BE, Sparrow JR. Enzymatic degradation of A2E, a retinal pigment epithelial lipofuscin bisretinoid. J Am Chem Soc. 2011;133(4):849–857. doi: 10.1021/ja107195u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brady RO. Emerging strategies for the treatment of hereditary metabolic storage disorders. Rejuvenation Res. 2006;9(2):237–244. doi: 10.1089/rej.2006.9.237. [DOI] [PubMed] [Google Scholar]
- 68.Julien S, Schraermeyer U. Lipofuscin can be eliminated from the retinal pigment epithelium of monkeys. Neurobiol Aging. 2012;33(10):2390–2397. doi: 10.1016/j.neurobiolaging.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Nociari MM, et al. Beta cyclodextrins bind, stabilize, and remove lipofuscin bisretinoids from retinal pigment epithelium. Proc Natl Acad Sci USA. 2014;111(14):E1402–E1408. doi: 10.1073/pnas.1400530111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyer NP, et al. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: Their origin is 11-cis-retinal. J Biol Chem. 2012;287(26):22276–22286. doi: 10.1074/jbc.M111.329235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sparrow JR. Therapy for macular degeneration: Insights from acne. Proc Natl Acad Sci USA. 2003;100(8):4353–4354. doi: 10.1073/pnas.1031478100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teussink MM, et al. The effect of light deprivation in patients with Stargardt disease. Am J Ophthalmol. 2015;159(5):964–972. doi: 10.1016/j.ajo.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Kong J, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15(19):1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allocca M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118(5):1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz SD, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 76.Grassmann F, Fauser S, Weber BH. 2015. The genetics of age-related macular degeneration (AMD): Novel targets for designing treatment options? Eur J Pharm Biopharm 95(Pt B):194–202. [DOI] [PubMed]
- 77.Csaky KG, Richman EA, Ferris FL., 3rd Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49(2):479–489. doi: 10.1167/iovs.07-1132. [DOI] [PubMed] [Google Scholar]
- 78.Greenberg JP, et al. Quantitative fundus autofluorescence in healthy eyes. Invest Ophthalmol Vis Sci. 2013;54(8):5684–5693. doi: 10.1167/iovs.13-12445. [DOI] [PMC free article] [PubMed] [Google Scholar]