Significance
Depressive and manic phases in bipolar disorder show opposite constellations of affective, cognitive, and psychomotor symptoms. These may be related to disbalance between large-scale networks, such as the default-mode (DMN) and sensorimotor network (SMN) that are involved in these functions. The variability of resting-state signal amplitude—an index of neuronal activity—of large-scale networks and their balances was investigated in bipolar disorder. The DMN/SMN balance was tilted toward the DMN in depression (characterized by excessive focus on internal thought contents and psychomotor inhibition) and toward the SMN in mania (characterized by excessive focus on external environmental contents and psychomotor overexcitement). Accordingly, the contrasting symptoms of depression and mania may be related to opposite spatiotemporal patterns in the resting-state structure.
Keywords: bipolar disorder, neuronal variability, default mode network, sensorimotor network
Abstract
Depressive and manic phases in bipolar disorder show opposite constellations of affective, cognitive, and psychomotor symptoms. At a neural level, these may be related to topographical disbalance between large-scale networks, such as the default mode network (DMN) and sensorimotor network (SMN). We investigated topographical patterns of variability in the resting-state signal—measured by fractional SD (fSD) of the BOLD signal—of the DMN and SMN (and other networks) in two frequency bands (Slow5 and Slow4) with their ratio and clinical correlations in depressed (n = 20), manic (n = 20), euthymic (n = 20) patients, and healthy controls (n = 40). After controlling for global signal changes, the topographical balance between the DMN and SMN, specifically in the lowest frequency band, as calculated by the Slow5 fSD DMN/SMN ratio, was significantly increased in depression, whereas the same ratio was significantly decreased in mania. Additionally, Slow5 variability was increased in the DMN and decreased in the SMN in depressed patients, whereas the opposite topographical pattern was observed in mania. Finally, the Slow5 fSD DMN/SMN ratio correlated positively with clinical scores of depressive symptoms and negatively with those of mania. Results were replicated in a smaller independent bipolar disorder sample. We demonstrated topographical abnormalities in frequency-specific resting-state variability in the balance between DMN and SMN with opposing patterns in depression and mania. The Slow5 DMN/SMN ratio was tilted toward the DMN in depression but was shifted toward the SMN in mania. The Slow5 fSD DMN/SMN pattern could constitute a state-biomarker in diagnosis and therapy.
Bipolar disorder (BD) type I is a debilitating psychiatric disease with recurrent episodes of depression and mania, characterized by opposite constellations of psychopathological symptoms (1, 2). Typically, depression is characterized by mood biased toward negative affect, cognitive symptoms with thought internally focused: that is, self-focused (which manifests in ruminations) and inhibited psychomotor behavior (which manifests in psychomotor retardation). In contrast, most commonly mania presents mood biased toward positive affect, cognitive symptoms with thought externally focused: that is, environment-focused (which manifests in flight of ideas/distractibility) and excited psychomotor behavior (which manifests in psychomotor agitation) (1–7). The neural basis underlying such co-occurrence of psychopathological symptoms with opposing constellations in depressive and manic phases of BD, however, remains unclear.
Affect, thought, and psychomotor functions can be related to distinct neural networks in the brain’s resting state. One central network is the default-mode network (DMN), which was first defined as a group of brain areas consistently showing decrease from baseline state during task-related activity and is indicative of an organization within the brain’s intrinsic ongoing activity (8, 9). Although showing strong intranetwork functional connectivity, the DMN is also related to other networks, including the sensorimotor (SMN) (10), salience (SN) (11, 12), and central executive (CEN) (12, 13) networks with the relationships between networks being either positive (i.e., correlating) or negative (i.e., anticorrelating). The DMN is involved in affective regulation and internal thoughts (14–16), showing major changes in psychiatric illnesses, such as BD (17–21), and major depressive disorder (22–24). At the same time, the DMN may be related to psychomotor behavior through its relationship with the sensorimotor network (10). Because of the co-occurrence of alterations in affect, thought, and psychomotor behavior in BD, based on existing evidence one could hypothesize abnormal relationships—topographical patterns in the balances between these networks—especially between the DMN and SMN (10) (considering the central clinical role of psychomotor disturbances in BD) (25, 26). Although recent findings in other psychiatric diseases, such as schizophrenia and unipolar depression (3, 4, 27–29), highlight the need to consider global signal power and variance (30, 31), as well as the relationships between different networks (such as DMN–CEN and DMN–SN) (11, 12, 32–34), this remains unclear in BD and its various phases. The relationships between networks concern the topographical patterns in signal power and variance across brain regions, as distinguished from global signal power and variance (31). A recent study demonstrated normal global signal power and variance in BD patients (31). This, however, leaves open changes in the topographical patterns—specifically the balance between networks—and their relationship to the opposite psychopathological symptom constellations in bipolar depression and mania.
Resting-state networks and their relationships have been investigated in BD by using functional connectivity (FC) (17–21), which provides information on the spatial structure of neural networks, importantly contributing to a better understanding of the relationship between the activity of different brain regions and how they interact via networks in different states (35). In addition to FC, which mainly targets the spatial dimension, the variability of the amplitude of neural activity, which implies a strong temporal dimension, has recently been investigated to characterize the resting state in the healthy brain (36–40). Variability is operationalized as the SD of blood-oxygen level-dependent signal, as well as the amplitude, or fractional amplitude, of low-frequency fluctuations (ALFF and fALFF) (40). Analogous to fALFF in respect to ALFF, fractional SD (fSD) is a normalized index of SD and can provide a more specific measure of variability of neuronal oscillatory phenomena with decreased sensitivity to artifacts (35, 41). fSD as a variability measure has been shown to link directly to neuronal activity implicated in the neuronal processing of incoming stimuli and neuronal outputs, thus underscoring its physiological relevance (40, 42–45). Neuronal variability was found to be altered in Alzheimer disease (46, 47), brain injury (48), vegetative state (49), anesthesia (50), and schizophrenia (51, 52). Together, these findings suggest high neurophysiological and neuropsychiatric relevance of neuronal variability as an index of neural activity, which remains to be investigated in BD and its phases.
Using functional MRI (fMRI), neuronal variability can be investigated in the range of low-frequency oscillations (0.01–0.10 Hz), which are typically used for the analyses of resting-state activity (such as FC) (35, 53, 54). Interestingly, variability in the low-frequency range appears to be strongest along the midline structures associated with the DMN (35, 55). Recently, the low-frequency oscillations were further subdivided into two frequency bands in the healthy brain: Slow5 (0.01–0.027 Hz) is strongest in the anterior DMN and Slow4 (0.027–0.073 Hz) is strongest throughout the basal ganglia and thalamus (35, 46, 51, 53, 56–59). Significant alterations in variability, in Slow5 SD especially, were found in disorders of consciousness, such as vegetative states (49) and anesthesia (50). This remains to be investigated in BD and its phases.
The general aim of the present study is to investigate resting-state variability in fSD Slow5 and Slow4 frequencies in both global brain activity and its topographical patterns, particularly in the relationship between networks during the depressive, manic, and euthymic phases of a specific and selective sample of severe BD type I and a smaller independent BD type I sample that served to replicate our findings. Our specific aims are to investigate: (i) the global signal variance and, especially, the topographical pattern or balance of normalized variability (fSD) between the DMN and other networks in the various phases of BD (i.e., depressive, manic, and euthymic phases) and in healthy controls (HC); (ii) fSD in the DMN and SMN (and in others networks) in Slow5 and Slow4 in the various subgroups, as explorative analysis; and (iii) the correlations between the variability of the networks’ ratios, which show significant differences between subgroups and clinical parameters (i.e., depression and mania rating scales). Considering the opposing constellations of affective, cognitive, and psychomotor symptoms in the different bipolar phases, we hypothesized opposing topographical patterns—increased or decreased ratio—specifically between the DMN and SMN fSD in Slow5 in depressive and manic patients, as well as divergent correlations of fSD DMN/SMN ratio in Slow5 with depressive and manic symptoms. See Supporting Information for a more extensive background, detailed hypotheses, and analyses overview.
Results
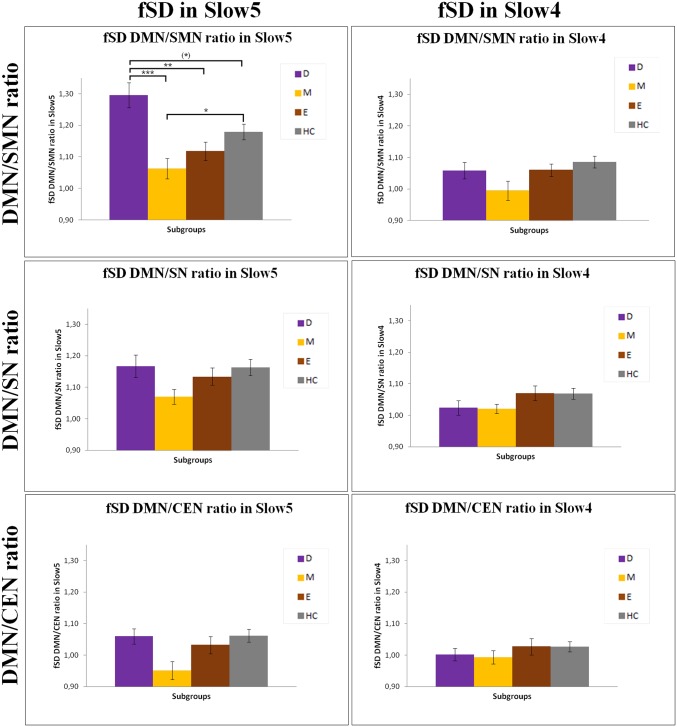
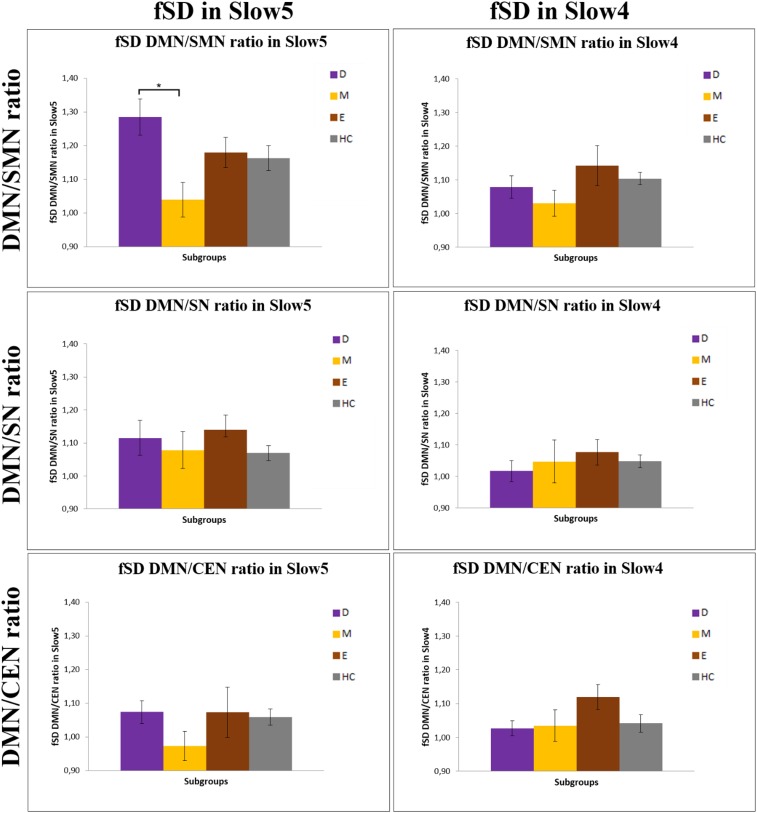
First, we investigated the global signal variance for which no significant difference in both Slow5 and Slow4 between BD and healthy subjects (t = 1.101 and P = 0.274; t = −0.050 and P = 0.960, respectively) was found. Similarly, no difference was found among the depressive, manic, and euthymic subgroups (F = 1.21 and P = 0.310; F = 0.13 and P = 0.942, respectively). We then calculated the ratio between the fSD of the DMN and the other three networks (as normalized by global signal variance); that is, the SMN, SN, and CEN in Slow5 and in Slow4 for BD patients in the different phases of illness and HC. The 2 (frequencies) × 4 (subgroups) ANOVA of the fSD DMN/SMN ratio showed a significant interaction between the frequency bands and subgroups (F = 6.43, P = 0.001). A significant main effect of the Slow5 fSD DMN/SMN ratio between the various subgroups was found (F = 5.78, P = 0.001), but no significant effect between the two frequency bands was detected (F = 2.96, P = 0.089). In contrast, there were no significant differences between subgroups for the fSD DMN/SN (interaction between frequency bands and subgroups: F = 1.78, P = 0.156; interaction between frequency bands: F = 1.98, P = 0.172; main effect: F = 1.96, P = 0.124) and fSD DMN/CEN (interaction between frequency bands and subgroups: F = 2.00, P = 0.118; interaction between frequency bands: F = 0.52, P = 0.470; main effect: F = 2.69, P = 0.050) ratios. As a result of these findings, we investigated, by using the post hoc analyses, the differences in the Slow5 fSD DMN/SMN ratio between all subgroups. A significant increase in Slow5 fSD DMN/SMN ratio in depressed patients compared with manic (P = 0.000) and euthymic patients (P = 0.006), as well as a tendency to a significant increase in depressed patients compared with HC (P = 0.085) was found. In contrast, the Slow5 fSD DMN/SMN ratio was significantly decreased in manic patients compared with HC (P = 0.040) (Figs. 1 and 2). Unlike in the depressive and manic phases, patients in the euthymic phase, as well as BD overall, did not show any significant difference in the Slow5 fSD DMN/SMN ratio compared with HC. Testing for Slow4, we found no significant difference of fSD for the DMN/SMN ratio in all comparisons in the post hoc analyses (Fig. 1). We found similar results by using a different DMN template. We controlled the specificity of our findings on the Slow5 fSD DMN/SMN ratio and found no significant differences between the subgroups in any of the tested variables: the fSD of DMN/SMN, DMN/SN, and DMN/CEN ratios in Slow3 and Slow2, and the SD of the same ratios in Slow5 and Slow4, as well as the DMN–SMN FC, DMN–SN FC, and DMN–CEN FC in Slow5 and Slow4 (Supporting Information).
Fig. 1.
The DMN/SMN, DMN/SN, and DMN/CEN ratios in fSD Slow5 and Slow4 in the various subgroups. Results of the ANOVA and Games–Howell post hoc test of fSD of the DMN/SMN, DMN/SN, and DMN/CEN ratios in Slow5 and Slow4 between the various subgroups. Corrected *P < 0.05, **P < 0.01, ***P < 0.001. D, depressive patients; E, euthymic patients; HC, healthy controls; M, manic patients.
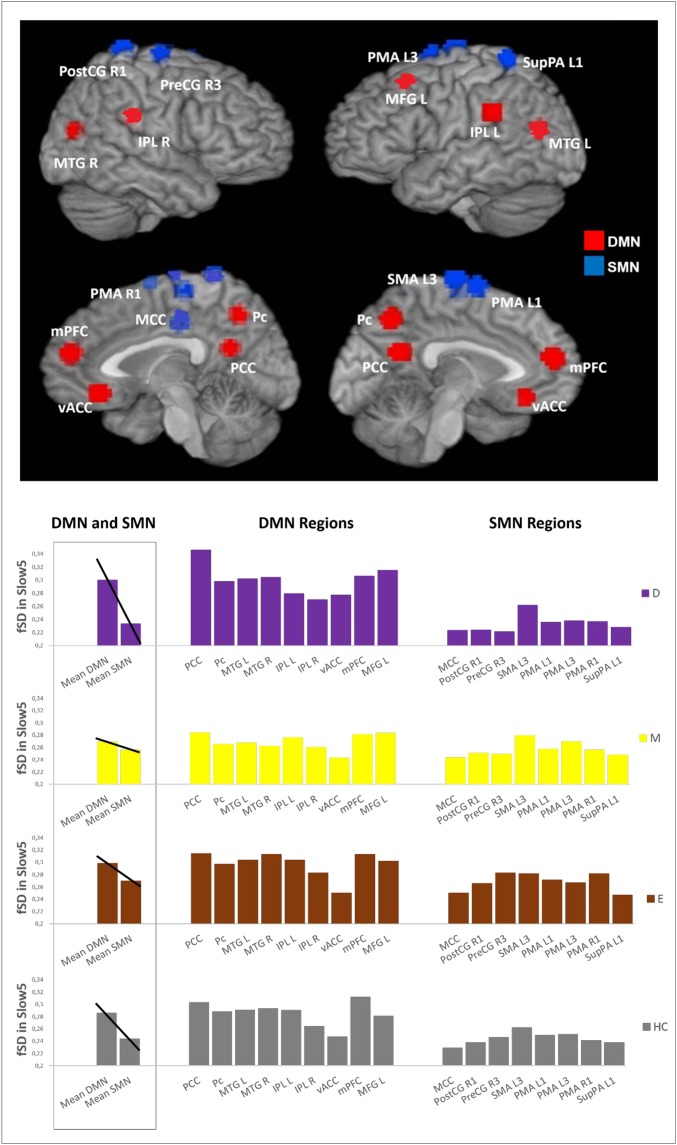
Fig. 2.
Differences in the DMN/SMN balance in the various subgroups. The Upper part of the figure is a global view of the DMN (red) and SMN (blue) regions. The Lower Left of the figure is the mean of the fSD values in Slow5 of the DMN and SMN, together with a visual trend of the balance between the DMN and SMN, for each subgroup. The Lower Right of the figure is the mean fSD values in Slow5 of the various regions belonging to the DMN and SMN, for each subgroup. D, depressive patients; E, euthymic patients; HC, healthy controls; IPL L, inferior parietal lobule left; IPL R, inferior parietal lobule right; M, manic patients; MCC, middle cingulate cortex; MFG L, middle frontal gyrus left; mPFC, medial preFrontal cortex; MTG L, middle temporal gyrus left; MTG R, middle temporal gyrus right; Pc, precuneus; PCC, posterior cingulate cortex; PMA L1, premotor area left; PMA L3, premotor area left; PMA R1, premotor area right; PostCG R1, postcentral gyrus right; PreCG R3, precentral gyrus right; SMA L3, supplementar motor area left; SupPA L1, superior parietal area left; vACC, ventral anterior cingulate cortex.
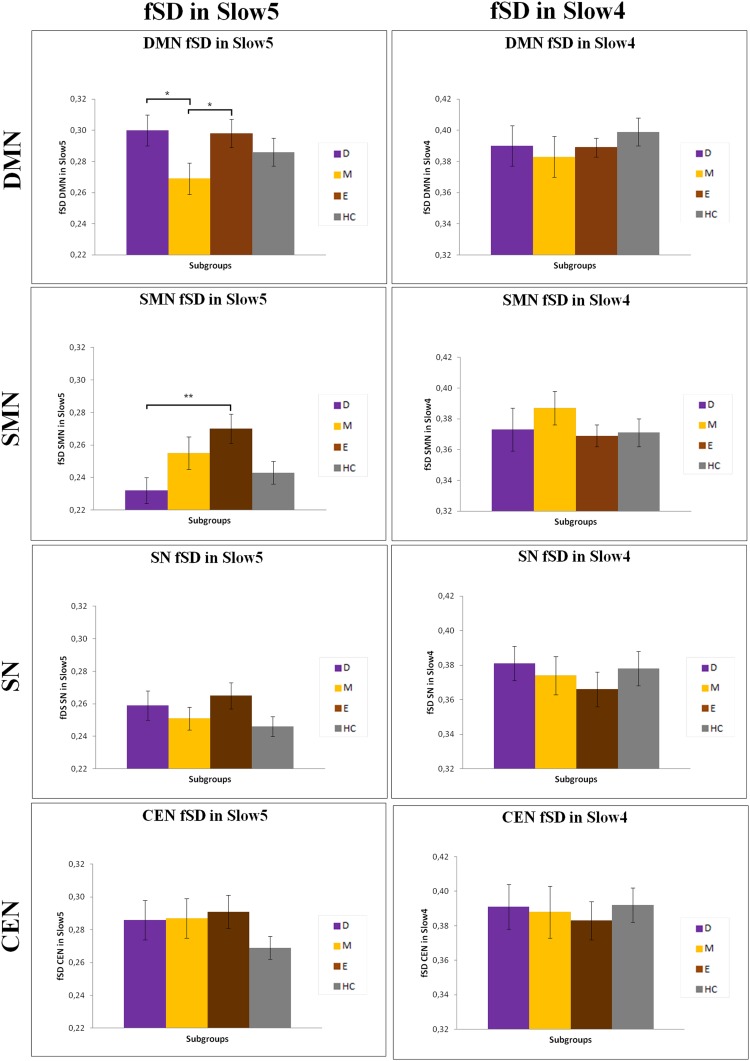
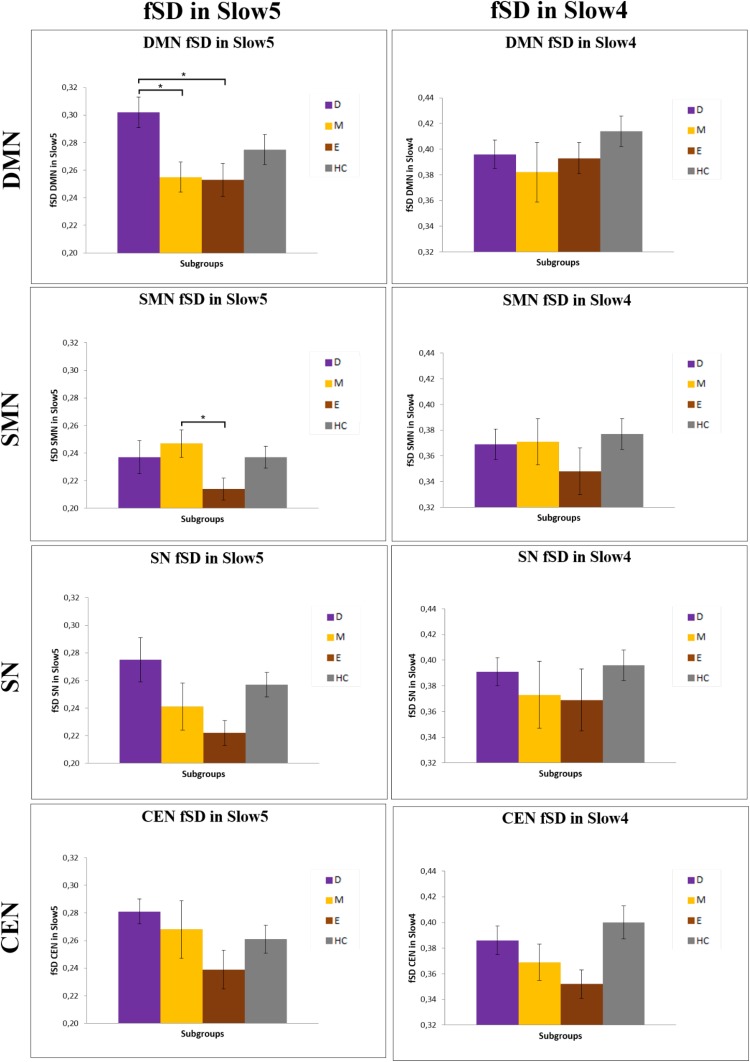
We investigated, as explorative analysis, fSD within the different networks in Slow5 and Slow4 in the various subgroups. We mainly found significant differences in Slow5 fSD in the DMN and SMN of patients during the depressed and manic phases (Fig. S1 and Table S1). In particular, we found an increase in the Slow5 fSD in the DMN with a decrease in the Slow5 fSD in the SMN of depressed patients compared with manic and euthymic patients, respectively. In contrast, the Slow5 fSD in the DMN was decreased in manic compared with euthymic patients.
Fig. S1.
Differences in DMN, SMN, SN, and CEN fSD Slow5 and Slow4 in the various subgroups. Mean values of fSD in Slow5 and Slow4 of DMN, SMN, SN, and CEN in the various subgroups; *P < 0.05, **P < 0.01. D, depressive patients; E, euthymic patients; HC, healthy controls; M, manic patients.
Table S1.
Variability in the different phases of BD and HC
| Study group | fSD | |
| Slow5 | Slow4 | |
| Depression | ||
| DMN | 0.300 (0.047) | 0.390 (0.058) |
| SMN | 0.233 (0.034) | 0.373 (0.062) |
| SN | 0.259 (0.042) | 0.381 (0.045) |
| CEN | 0.286 (0.058) | 0.391 (0.059) |
| Mania | ||
| DMN | 0.269 (0.045) | 0.383 (0.061) |
| SMN | 0.256 (0.046) | 0.387 (0.053) |
| SN | 0.251 (0.034) | 0.374 (0.050) |
| CEN | 0.287 (0.054) | 0.388 (0.067) |
| Euthymia | ||
| DMN | 0.298 (0.042) | 0.389 (0.031) |
| SMN | 0.269 (0.045) | 0.369 (0.034) |
| SN | 0.265 (0.039) | 0.366 (0.045) |
| CEN | 0.291 (0.048) | 0.383 (0.052) |
| HC | ||
| DMN | 0.286 (0.059) | 0.399 (0.059) |
| SMN | 0.244 (0.051) | 0.371 (0.063) |
| SN | 0.246 (0.042) | 0.378 (0.067) |
| CEN | 0.269 (0.049) | 0.392 (0.063) |
Mean values (±SD) of the fSD of the various networks in Slow5 (0.01–0.027 Hz) and Slow4 (0.027–0.073 Hz) in the various subgroups of BD patients and HC.
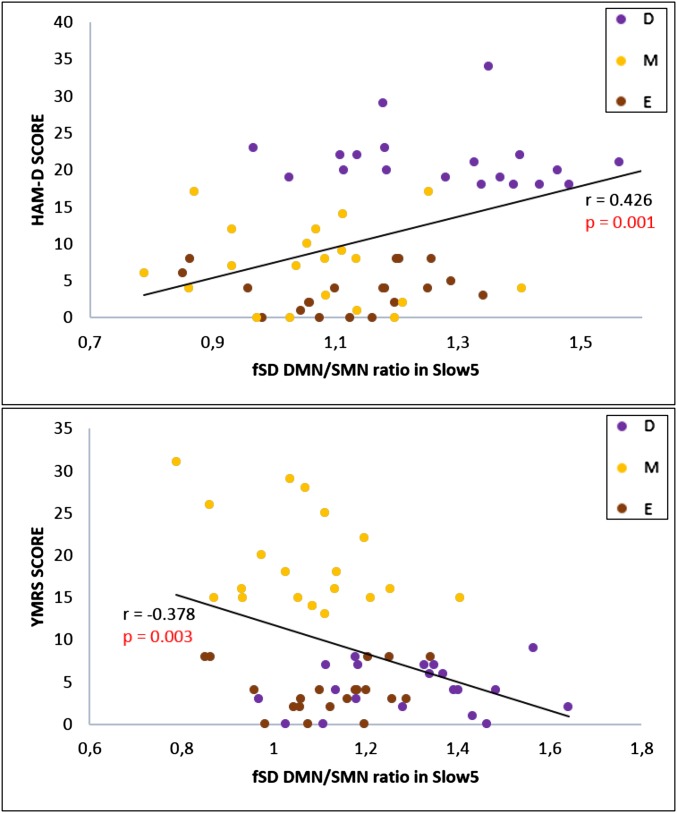
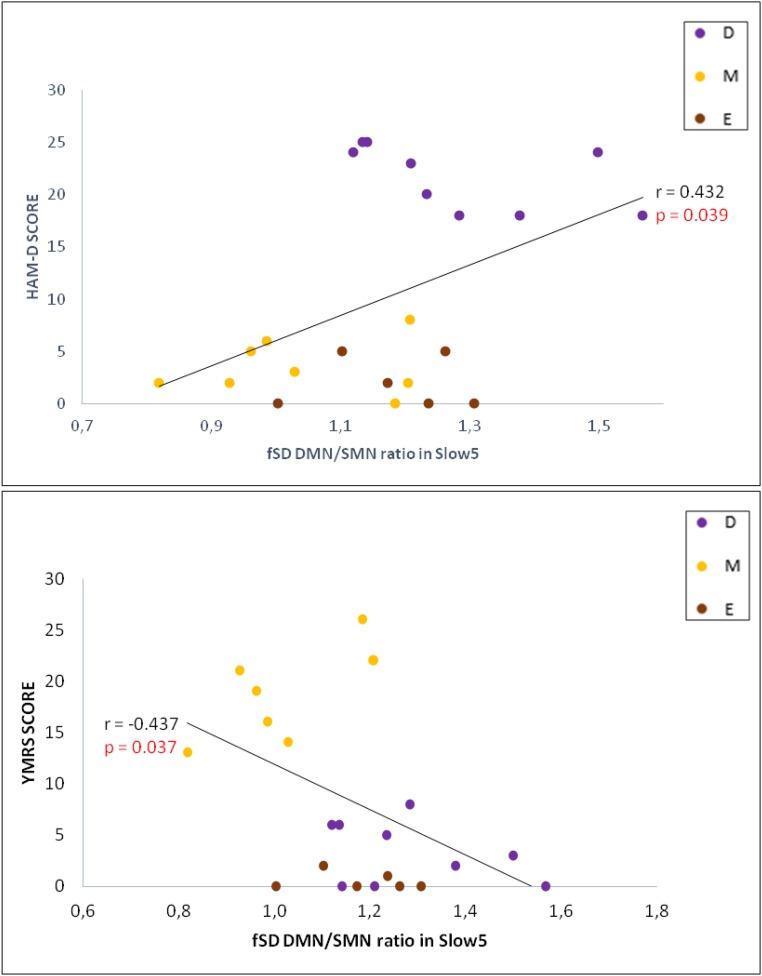
With regard to clinical correlations in BD patients, the fSD of the DMN/SMN ratio in Slow5 was found to be positively correlated (after bootstrapping) with the Hamilton depression scale (HAM-D) total score [r = 0.426; P = 0.001; confidence interval (CI): 0.203∼0.597], and negatively correlated with the Young mania rating scale (YMRS) total score (r = −0.378; P = 0.003; CI: −0.564 ∼ −0.146) (Fig. 3 and Supporting Information). Finally, our exploratory receiver operator characteristic (ROC) analysis revealed an area under the curve value of 0.83 for the Slow5 fSD DMN/SMN ratio, indicating good predictive ability for the depressed and manic phases of BD.
Fig. 3.
Clinical correlations. Pearson correlation (after bootstrapping) between fSD of the DMN/SMN ratio in Slow5 and the HAM-D and YMRS in BD. D, depressive patients; E, euthymic patients; M, manic patients.
Finally, we confirmed our findings in a replication study on an independent BD sample, and on follow-up data (Supporting Information).
Discussion
Our main findings are the following: (i) After controlling for global signal changes, the balance between the DMN and SMN specifically in the lowest frequency band, as calculated by the Slow5 fSD DMN/SMN ratio, was significantly increased in depression, whereas the same ratio was significantly decreased in mania. Having replicated these findings in an independent BD sample, this finding suggests topographical changes in slow-frequency variance between the DMN and SMN in the various phases of BD. (ii) Slow5 variability was increased in the DMN and decreased in the SMN in depressed patients, whereas the opposite pattern was observed in mania. (iii) The Slow5 fSD DMN/SMN ratio correlated positively with clinical scores of depressive symptoms and negatively with those of mania.
To date, there are only few studies on neuronal variability concerning healthy subjects and some pathological conditions, such as brain injury and vegetative state (36–39, 48, 49). To the best of our knowledge, this is the first study on the topographical patterns of neuronal variability in BD concerning the main resting-state networks—the DMN, SMN, SN, and CEN—and their balances in the various phases of illness, the depressive, manic, and euthymic phases. We first confirmed findings from a recent study (31) that found no differences in global signal variance between bipolar and healthy participants. Our findings go further by demonstrating changes in global signal variance neither in Slow5 and Slow4 in BD nor in any of the subgroups. Most importantly, we found significant differences in the relationships between the DMN and SMN (i.e., DMN/SMN Slow5 fSD ratio) in the depressive and manic phases. Depressed patients showed an abnormally increased ratio, but it was abnormally decreased in manic patients. In contrast, we did not find any difference in the variability balance between other networks—the DMN/SN or DMN/CEN—nor in Slow4, in either depressive or manic patients. This finding suggests an opposite spatiotemporal topographical pattern in the Slow5 variability balance between the DMN and SMN, which may, therefore, be central in distinguishing between depressive and manic phases.
Our exploratory results also show that the variability of neuronal activity is altered in the DMN (and in the SMN) in Slow5, in the active phases of BD. In particular, DMN Slow5 variability increases in depression and decreases in mania. The opposite pattern is seen in Slow5 variability in the SMN, with a decrease in depression especially. Taken together, these data suggest: first, a special role of DMN and SMN variability and particularly their balance in distinguishing depressive and manic phases; and second, a relevance of resting-state variability in slow-frequency ranges, Slow5 especially, with contrasting changes in depressive and manic phases. Because these changes were observed only in depressive and manic states, not in euthymic patients, one may tentatively consider an abnormality in Slow5 fSD as a state—rather than trait—marker of BD.
The relationship between the Slow5 DMN/SMN ratio and depressive and manic states is further supported by our correlation findings. We found significant and contrasting correlations of the DMN/SMN Slow5 fSD ratio with the HAM-D total score (positive correlation) and the YMRS total score (negative correlation) in the total BD sample. This finding further strengthens the link between the contrasting topographical patterns in the DMN/SMN Slow5 fSD ratio in depression and mania on the one hand, and their opposite clinical symptoms on the other. This was further, although tentatively, supported by our ROC analysis, which showed values higher than 0.80 in predicting the depressive or manic phase. If confirmed in a larger sample, the DMN/SMN Slow5 fSD ratio may be considered a diagnostic marker of BD depression and mania, including their opposite constellations of affect, thought, and psychomotor alterations.
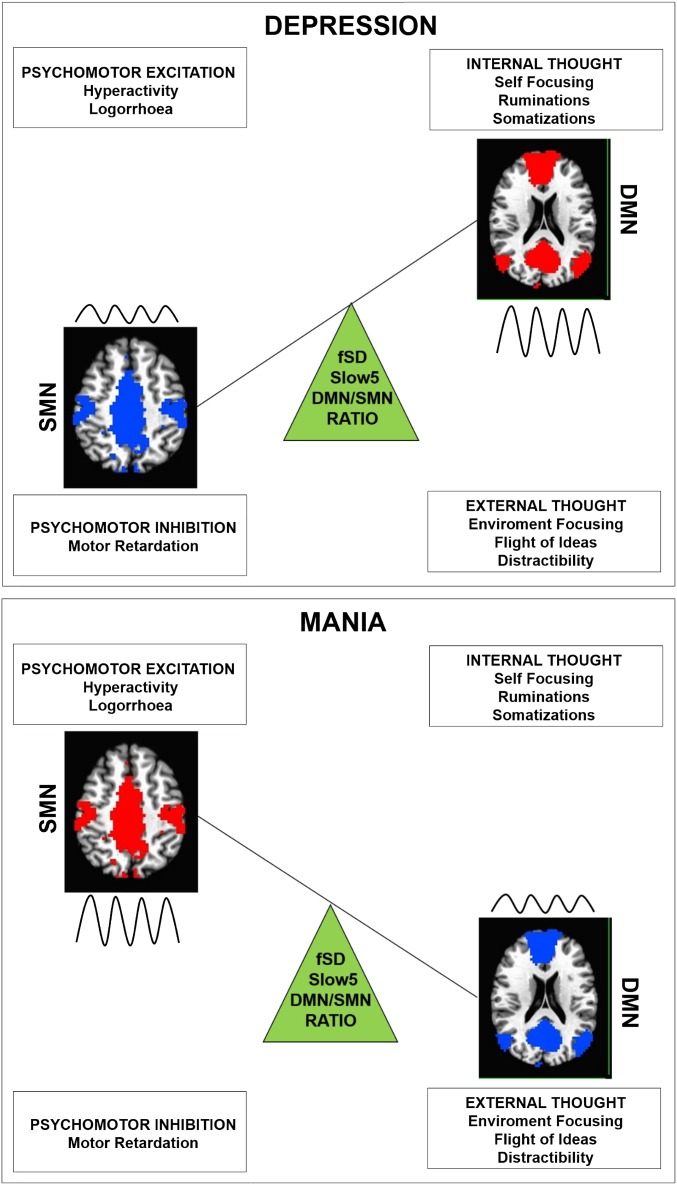
In sum, the baseline Slow5 variability may be abnormally altered in the topographical pattern or balances between networks, primarily involving the DMN and its relationship with the SMN. Accordingly, major functional and structural alterations were found in the anterior DMN (17, 19–21). The ultraslow frequency band, Slow5, was interestingly found to be more dominant, especially in the ventromedial prefrontal cortices; that is, the anterior cortical midline structures, which are central to the DMN (35, 56). The DMN/SMN abnormal topographical resting-state pattern may affect all subsequent neuronal processing of both input and outputs, leading to the opposing constellations of affective, cognitive, and psychomotor symptoms in depression and mania. How and why does the abnormal balancing of the DMN/SMN Slow5 fSD ratio lead to such contrasting clinical symptom patterns, as are seen in depression and mania (Fig. 4)?
Fig. 4.
Schema of DMN/SMN disbalance in depression and mania. The changes in the fSD of the DMN/SMN ratio in Slow5 (green triangle). The model represents the hypothetical relationship between changes in DMN/SMN balance and the most typical clinical presentation of BD depression and mania. Changes of the relative weight of the lower frequency Slow5 band (wave) could affect the balance between different resting-state networks, in the various phases of BD. In depression, the increase of the ratio could tilt the network disbalance toward the DMN (red and higher amplitude of the wave) at the expense of the SMN (blue and lower amplitude of the wave), which may lead to internal thought (focused on internal contents at the expense of the external contents) and psychomotor inhibition. In mania, the decrease of the ratio could tilt the network disbalance toward the SMN (red and higher amplitude of the wave) at the expense of the DMN (blue and lower amplitude of the wave), which may lead to external thought (focused on external contents at the expense of the internal contents) and psychomotor excitation.
Our findings show that in depression the network Slow5 variability balance is tilted toward the DMN at the expense of the SMN. The DMN has been shown to be involved in self-related processing and internal mental states (60–62), as well as was found to be hyperactive in bipolar and unipolar depression (22–24, 63). Increased variability, particularly in Slow5 fSD, may thus lead to increased internal thoughts and self-referential processing, manifesting clinically in ruminations and increased self-focus (3, 7). At the same time, depressed patients exhibit a decrease in movements and action and are often withdrawn from their environment, showing psychomotor retardation and decreased environment-focus (61, 62), which, at the neuronal level, may be closely related to their decreased variability in the SMN. Taken together, the topographical pattern with increased Slow5 variability in the DMN and decreased Slow5 variability in SMN may result in an excessive focus on internal thought contents at the expense of external environmental contents (as related to increased variability in DMN) with inhibition in psychomotor behaviors (as related to decreased variability in SMN) (3).
The opposite appears to occur in mania. During this phase, the Slow5 variability network balance is tilted toward the SMN at the expense of the DMN. External environmental contents related to both sensory and motor functions predominate over internal thought contents, resulting in decreased self-related processing—as manifest in decreased self-focus and internal thoughts—and excessive sensorimotor recruitment, as manifest in increases in both perceptual distraction and motor behavior. Taken together, the topographical pattern with decreased Slow5 variability in the DMN and increased Slow5 variability in the SMN may result in an excessive focus on external environmental contents at the expense of internal thought contents (as related to decreased variability in DMN), with overexcitement in psychomotor behaviors (as related to increased variability in SMN). Accordingly, the contrasting symptoms seen in depression and mania may be related to opposite spatial topographical patterns (DMN and SMN) in the resting state’s temporal structure (variability as indexed by Slow5 fSD), reflecting what has been recently described as “spatiotemporal psychopathology” (3, 4).
The main limitation of the study is medication confounds, because almost all of the patients in our sample were undergoing pharmacotherapy. Furthermore, our sample consisted of patients at varying stages of the disease. However, when investigated, the medication load and duration of illness did not correlate with the fSD in DMN/SMN ratio in Slow5, suggesting the absence of major effects of these clinical factors on the investigated parameters (Supporting Information).
In conclusion, our findings demonstrate a specific abnormal topographical resting-state pattern in the balance between the DMN and SMN infra-slow signal variance, which, in turn, may affect all subsequent neuronal processing of both input and outputs leading to the opposing constellations of affect, thought, and psychomotor disturbances during the active depressive and manic phases of BD. If confirmed in larger samples, this may serve as a biomarker in the diagnosis and therapy of BD, further improving understanding of the relationship between the spatiotemporal structure of intrinsic brain activity and behavioral correlates.
Materials and Methods
The study consisted of a specific and selective sample of 60 severe BD type I patients (20 depressed, 20 manic, and 20 euthymic) on their current medication regiment, and 40 HC. The Ethics Committee of San Martino Hospital approved the study, and written informed consent was obtained from all participants. After controlling for global signal variance, we calculated the balances (i.e., ratio) between networks (DMN, SMN, SN, and CEN) fSD in Slow5 and Slow4, and investigated potential differences between subgroups. We then explored the single networks fSD differences and investigated potential clinical correlations. Finally, we performed additional analyses of control and explorative analyses on an independent BD sample and follow-up data. For a detailed description of samples, acquisition parameters, processing, and all neuroimaging and statistical analyses, see Supporting Information, including Figs. S1–S4 and Tables S1–S5.
Fig. S4.
Clinical correlations in the independent sample. Pearson correlation (after bootstrapping) between fSD of the DMN/SMN ratio in Slow5 and the HAMD and YMRS in BD. D, depressive patients; E, euthymic patients; M, manic patients.
Table S5.
Variability in the different phases of BD and HC of the independent sample
| Study group | fSD | |
| Slow5 | Slow4 | |
| Depression | ||
| DMN | 0.302 (0.033) | 0.396 (0.033) |
| SMN | 0.237 (0.036) | 0.369 (0.037) |
| SN | 0.275 (0.050) | 0.391 (0.033) |
| CEN | 0.281 (0.028) | 0.386 (0.033) |
| Mania | ||
| DMN | 0.255 (0.033) | 0.382 (0.066) |
| SMN | 0.247 (0.030) | 0.371 (0.053) |
| SN | 0.241 (0.048) | 0.373 (0.075) |
| CEN | 0.268 (0.059) | 0.369 (0.040) |
| Euthymia | ||
| DMN | 0.253 (0.029) | 0.393 (0.030) |
| SMN | 0.214 (0.020) | 0.348 (0.046) |
| SN | 0.222 (0.023) | 0.369 (0.059) |
| CEN | 0.239 (0.035) | 0.352 (0.027) |
| HC | ||
| DMN | 0.275 (0.047) | 0.414 (0.054) |
| SMN | 0.237 (0.035) | 0.377 (0.052) |
| SN | 0.257 (0.041) | 0.396 (0.052) |
| CEN | 0.261 (0.045) | 0.400 (0.056) |
Mean values (±SD) of the fSD of the various networks in Slow5 (0.01–0.027 Hz) and Slow4 (0.027–0.073 Hz) in the various subgroups of BD patients and HC.
Background
In recent years, a growing number of neurobiological changes have been detected in psychiatric illnesses, such as affective disorders (22–24, 63–65). Neuroimaging studies have reported abnormal functional connectivity (FC) in resting-state networks, especially the default mode network (DMN), and in particular in its anterior midline regions, both in bipolar disorder (BD) in all phases (17, 18) and in manic (mainly hypoconnectivity in DMN) (19) or euthymic phases (20, 21). Furthermore, the DMN has been characterized by abnormal resting-state hyperactivity and deficit in deactivation during task in bipolar depression (63), as well as in unipolar depression, consistently (22–24). Although data concerning other networks are still lacking in BD, the little evidence there is has shown altered connectivity between the anterior cingulate and other areas of the salience network (SN), such as the ventrolateral prefrontal cortex, and a loss of anticorrelation between the medial and dorsolateral prefrontal cortex in mania (66), and a reduction in FC within the central executive network (CEN) in psychotic BD in acute states (67). Another network implicated in BD is the sensorimotor network (SMN), which was found to be hyperconnected in mania (68).
In addition to FC, the variability of the amplitude of neural activity has recently been investigated to characterize the resting state in the healthy brain (36–40). It has been proposed that the variability of neuronal activity is central for “tonic” activity of the brain (fluctuating, ongoing activity), which provides the substrate for the brain’s default activity; “phasic” or stimulus-driven activity appears to operate on the basis of existing tonic activity to permit specific behaviors (40). Thus, a moderate increase of variability in a neural system can enhance the detection of weak signals, allowing subthreshold neurons to fire and facilitating outputs (40, 42–45). Therefore, increases or decreases in variability can be associated with higher or lower complexity in spatial and temporal domains of functional networks, and can affect the subsequent neuronal processing of incoming stimuli and neuronal outputs (40). Neuronal variability has been shown to be central to both resting-state and task-evoked activity in the healthy brain, related to development and aging—lower variability in infancy, higher variability in adulthood, lower variability in old age—and directly correlated with cognitive performance (37–40). Moreover, neuronal variability was found to be altered in various neuropsychiatric diseases: mainly decreased variability in Alzheimer disease (46, 47), brain injury (48), vegetative state (49), anesthesia (50), and schizophrenia, although an increase in variability was found in young, medication-naïve, and symptomatic schizophrenics (51, 52). In addition, global resting-state variance has recently been shown to be abnormal in schizophrenia but normal in bipolar patients (31).
Neuronal variability can be investigated in the frequency domain across multiple frequency bands (35, 53), which have been linked to different neuronal processes and physiological functions (53, 69). In particular, low-frequency oscillations (0.01–0.10 Hz) primarily detected within gray matter are thought to reflect cyclic modulation of long-distance neuronal synchronization; the low-frequency bands are those typically used for the analysis of resting-state activity, such as FC (35, 53, 54). Low-frequency oscillations appear to originate directly from neuronal activity because blood-oxygen level-dependent (BOLD) low-frequency oscillations were found to be directly related to infra-slow fluctuations (<0.1 Hz), as observed in neuronal activity in EEG (70). Moreover, low-frequency oscillations have demonstrated the highest amplitudes along the midline structures associated with the DMN, which seems to reflect a physiological baseline for the brain (35, 55). During a task, low-frequency oscillations decrease both within the DMN and in task-activated regions (such as premotor areas), suggesting a redistribution of resources associated with different functional states (35, 71, 72). Furthermore, low-frequency oscillations were further subdivided recently into different frequency bands—Slow5 (0.01–0.027 Hz) and Slow4 (0.027–0.073 Hz)—in the healthy brain (35, 46, 51, 53, 57–59). Interestingly, the ultraslow frequency band, Slow5, is strongest in the anterior cortical midline structures, which are central to the DMN (35, 56), whereas Slow4 is strongest throughout the basal ganglia, thalamus, and several sensorimotor regions (35). In contrast, higher-frequency bands, such as Slow3 (0.073–0.198 Hz) and Slow2 (0.198–0.25 Hz), were primarily restricted to white matter (35) and can be affected by physiological variables, like respiration and aliased cardiac signals (35, 73). A distinct contribution of the different frequency bands has been shown in various neuropsychiatric diseases, such as greater alterations in Slow5 in vegetative states (49), anesthesia (50), and in BD (with regard to FC) (17). Despite the early stage of these findings compared with higher-frequency bands (53), they suggest the functional relevance of different infra-slow frequency bands in both healthy and pathological brains.
Analyses Overview
To begin with, our study focused on the analyses of global variance. A recent study observed increased global variance in schizophrenia but normal variance in BD patients (31). Based on these results, we predicted normal global SD in BD generally and, extending the previous findings, in the various subgroups of depression, mania, and euthymia. After excluding global changes in SD, we analyzed the fSD (as normalized index of SD, controlling for global effects) (74, 75) of the ratio between the DMN and the SMN, SN, and CEN (as indices of the balances between the DMN and other networks activity) in the various subgroups. We analyzed these ratios within the low-frequency fluctuations, specifically in Slow5 and Slow4, to detect potential contributions of the infra-slow frequency band. We hypothesized that there exists an abnormal relationship of the DMN, which represents the physiological baseline and tonic activity of the brain and is involved in affective and thought disturbances, with the SMN especially—considering the central clinical role of psychomotor disturbances in BD—in both mania and depression in opposing ways. Based on our previous findings on abnormal resting-state FC in Slow5 in BD, as well as the primary role of Slow5 rather than Slow4 in DMN activity, we hypothesized that the fSD abnormalities occur particularly in Slow5 rather than Slow4.
Second, we explored potential fSD alterations in the single networks themselves (DMN, SMN, SN, and CEN) to investigate if the potential changes depend on specific networks or on the balance itself. We hypothesized that fSD changes in BD and its phases occur in the DMN and specifically in Slow5, and possibly in the SMN (in the same frequency band).
Third, we correlated the relationship between networks, the ratio, with clinical measures of both depression and manic symptoms in our BD sample. We hypothesized that the relationship between the DMN and SMN fSD in Slow5 especially correlate with both depressive and manic symptoms in opposing ways. This therefore might account for the opposite constellations of cognitive and sensorimotor symptoms of BD.
SI Materials and Methods
Participants and Clinical Assessment.
Participants were admitted to the in- and out-patient service of the Psychiatric Clinic at the University of Genoa (Istituto di Recovero e Cura a Carattere Scientifico Azienda Ospedaliera Universitaria San Martino, Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, and Maternal and Child Health, Section of Psychiatry) between 2013 and 2015. The study comprised of 60 bipolar patients (42 females, 18–60 y old, 20 in depressive, 20 in manic, and 20 in euthymic phases) and 40 HC (Table S2). The mean age did not show any significant difference between the various sample subgroups (F = 0.23, P = 0.871). The Ethics Committee of San Martino Hospital approved the study, and written informed consent was obtained from all participants.
Table S2.
Subject demographic and clinical information
| Demographic | BD | HC | |||
| BD TOT | Manic BD | Depressed BD | Euthymic BD | ||
| Sample size n (%) | 60 (100) | 20 (33.3) | 20 (33.3) | 20 (33.3) | 40 (100) |
| Age (y) mean (SD) | 44.7 (11.2) | 46 (12) | 44.9 (10.9) | 43.1 (11) | 43.9 (12.8) |
| Female n (%) | 42 (70) | 17 (85) | 13 (65) | 12 (60) | 26 (65) |
| Age of onset mean (SD) | 25 (10.8) | 25 (12.3) | 25.3 (9.8) | 24.6 (10.9) | — |
| Duration of illness mean (SD) | 19.9 (11.6) | 21.7 (14.5) | 19.5 (10.8) | 18.2 (9) | — |
| HAM-D mean (SD) | — | 7 (5.4) | 21.5 (4) | 3.6 (2.8) | — |
| YMRS mean (SD) | — | 19 (5.7) | 4.2 (2.8) | 3.9 (2.7) | — |
| Mood stabilizers n (%) | 52 (86.7) | 16 (80) | 18 (90) | 18 (90) | — |
| Lithium n (%) | 16 (26.7) | 4 (20) | 5 (25) | 7 (35) | — |
| Valproate n (%) | 26 (43.3) | 10 (50) | 8 (40) | 8 (40) | — |
| Other antiepileptic drugs n (%) | 23 (38.3) | 5 (25) | 9 (45) | 9 (45) | — |
| Antidepressants n (%) | 22 (36.7) | 2 (10) | 11 (55) | 9 (45) | — |
| SRI n (%) | 10 (16.7) | 1 (5) | 6 (30) | 3 (15) | — |
| Tricyclic antidepressants n (%) | 6 (10) | 0 (0) | 4 (20) | 2 (10) | — |
| Duals and other antidepressants n (%) | 10 (16.7) | 1 (5) | 4 (20) | 5 (25) | — |
| Antipsychotics n (%) | 34 (56.7) | 14 (70) | 12 (60) | 9 (45) | — |
| Atypical n (%) | 29 (48.3) | 10 (50) | 13 (65) | 6 (30) | — |
| Typical n (%) | 7 (11.7) | 5 (25) | 1 (5) | 1 (5) | — |
| Benzodiazepines n (%) | 20 (33) | 5 (25) | 7 (35) | 8 (40) | — |
| Unmedicated n (%) | 2 (3.3) | 1 (5) | 0 (0) | 1 (5) | — |
SRI, serotonin reuptake inhibitors.
Each participant was evaluated with standardized structured or semistructured clinical instruments to obtain information on clinical and diagnostic features, course of illness, family history, and actual and past pharmacotherapy. The instruments are: the Mini International Neuropsychiatric Interview (MINI) (76), the Structured Clinical Interview for Axis-I Disorders/Patient edition (SCID-I/P) (77), the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (78), the Structured Interview for Mood Disorder–Revised (SIMD-R) (79), the Hamilton depression scale (HAM-D) with 17 items (80), and the Young mania rating scale (YMRS) (81). General, physiologic, pathologic, and psychopathologic history was also investigated.
Inclusion criteria were as follows: diagnosis of BD type I according to the Diagnostic and Statistical Manual for Mental Disorders–Fourth Edition (DSM-IV) criteria (1) assessed by the SCID-I/P (for manic, depressed, and euthymic patients); score of HAM-D with 17 items ≥ 18 or score of YMRS ≥ 13 (81) (for manic and depressed patients); HAM-D score < 8 and YMRS score < 8 for euthymic patients; age between 18 and 60; and an ability to provide written informed consent. Exclusion criteria were as follows: diagnoses of schizophrenia, mental retardation, dementia, or other cognitive disorders; history of severe or decompensated somatic diseases, neurological diseases (stroke, cerebral vascular malformations, or epilepsy), previous head injury with loss of consciousness (for 5 or more minutes); current alcohol and substance abuse (during the preceding 3 mo); history of alcohol or substance dependence; history of abuse of synthetic or new drugs; pregnancy and lactation; left-handedness; the inability to undergo an MRI examination (claustrophobia, metal implants, and so forth); previous treatment with electroconvulsive therapy, chemotherapy, or brain radiotherapy. Healthy controls did not meet the DSM-IV criteria for psychiatric disorders, either at the time of study participation or in the past; they had a HAM-D score < 8 and a YMRS score < 8; they also met the same exclusion criteria indicated for patients.
In our sample, all of the bipolar patients (except for two participants) were taking medications, including mood stabilizers (total: n = 52, 86.7%; lithium: n = 16, 26.7%; valproate: n = 26, 43.3%; other antiepileptic drugs: n = 23, 38.3%), antidepressants (total: n = 22, 36.7%; serotonin reuptake inhibitors: n = 10, 16.7%; tricyclic antidepressants: n = 6, 10%; duals and others antidepressants: n = 10, 16.7%), antipsychotics (total: n = 34, 56.7%; atypical antipsychotics: n = 29, 48.3%; typical antipsychotics: n = 7, 11.7%), and benzodiazepines (total: n = 20, 33%) (Table S2).
Overall, our BD sample was composed by a selective and specific group of BD-I patients without major comorbidities (see exclusion criteria). Comorbidities consisted of panic disorder (n = 4), social phobia (n = 3), generalized anxiety disorder (n = 2), obsessive-compulsive disorder (n = 5), somatoform disorders (n = 3), and bulimia (n = 5). Anxiety comorbidities, as well as medication exposure, are typical in severe and mostly hospitalized BD type I patients in active phases, of which our sample is representative.
fMRI Data Acquisition.
Images were acquired using a 1.5-T GE scanner with a standard head coil. Foam pads were used to reduce head motion and scanner noise. fMRI scanning was carried out in the dark, with participants explicitly instructed to keep their eyes closed, to relax, and to move as little as possible. Functional images were collected by using a gradient echo Echo Planar Imaging (EPI) sequence sensitive to BOLD contrast (TR/TE = 2,000/30 ms, flip angle = 90°, FOV = 24 cm). Whole-brain volumes were acquired in 33 contiguous 4-mm-thick transverse slices, with a 1-mm gap and 3.75 × 3.75-mm2 in-plane resolution. For each participant, fMRI scanning lasted 6 min and acquired a total of 150 scans.
In addition, 3D T1-weighted anatomical images were acquired for all participants in a sagittal orientation by means of a 3D-SPGR sequence (TR/TE = 11.5/5 ms, IR = 500 ms, flip angle = 8°, FOV = 25.6 cm) with an in-plane resolution of 256 × 256, and slice thickness of 1 mm.
Data Analysis.
Preprocessing steps were implemented in AFNI (https://afni.nimh.nih.gov/afni) (82). The first two volumes of each functional time series were discarded. The remaining functional images were slice-timing–corrected and aligned (head motion correction). Each participant’s motion was assessed by means of translation/rotation, and an exclusion criterion (translation > 3 mm, rotation > 3°; in each direction) was set. All participants selected for this study showed head motion of less than 1 mm. The T1 anatomical images of all participants were normalized to the Talairach space. Resting-state data, masked with the T1 images, were then spatially transformed into the Talaraich space (83), resampled to 3 × 3 × 3 mm3, and spatially smoothed (6 mm). The estimated head motion and the mean time series from the white matter and the cerebrospinal fluid were used as covariates in the correlation computation (13, 75). On the basis of recent findings in healthy subjects (35, 53), the data, after detrending, were then filtered with two separate bands within the standard range of 0.01–0.10 Hz, which is thought to reflect mainly neuronal fluctuations (58, 59, 84): Slow5 (0.01–0.027 Hz) and Slow4 (0.027–0.073 Hz). These frequencies are not affected by physiological variables, like respiration and aliased cardiac signals, that fall in the other ranges (Slow3 and Slow2) (35, 73).
Resting-State Analysis and Clinical Correlations.
Resting-state data analysis was performed using AFNI (https://afni.nimh.nih.gov/afni) (82).
First, the global signal variance (31) was calculated to account for possible global changes in all subsequent analyses. Specifically, we calculated the SD of BOLD signal changes in the resting state—a measure of variability—in Slow5 and Slow4, respectively. The ratio of SD for each frequency band of the entire range (0–0.25 Hz) was then calculated, thus obtaining the fSD in Slow5 and Slow4 as a measure of frequency-specific contributions of total variability. The global mean of fSD in Slow5 and Slow4 was extracted across voxels in the whole brain. Because the global mean did not reveal significant differences in BD and subgroups, the subject-level voxel-wise fSD maps were standardized into subject-level z-score maps per brain volume by subtracting the mean voxel-wise fSD obtained for the entire brain (global mean of fSD), then dividing by the SD across voxels (31, 51, 74, 75). Next, according to the literature, spherical regions of interest (ROIs) with a radius of 6 mm were placed in the Talairach coordinates of all cortical nodes of each network: the DMN (85), the SMN (86), the SN, and the CEN (33) (Table S3). We extracted the fSD in Slow5 and Slow4 from all of the nodes. We then calculated the mean fSD of all nodes within each resting-state network, followed by the ratio between the mean of fSD of the DMN and SMN, the DMN and SN, and the DMN and CEN in both frequency bands (i.e., Slow5 and Slow4).
Table S3.
Resting-state network coordinates
| DMN | SMN | SN | CEN | ||||
| Anato. Reg. | x y x | Anato. Reg. | x y x | Anato. Reg. | x y x | Anato. Reg. | x y x |
| PCC | 4 52 22 | MCC | −9 22 39 | SACC R | −6 −23 26 | DLPFC R | −46 −45 11 |
| Pc | 4 58 44 | PostC R1 | −16 43 69 | SACC L | 6 −19 27 | DLPFC L | 34 −45 3 |
| MTG L | 42 66 18 | PreC R3 | −22 19 65 | VLPFC/In R | −42 −9 −11 | PLat R | −38 52 43 |
| MTG R | −46 66 16 | SMA L3 | 7 2 65 | VLPFC/In L | 40 −17 -11 | PLat L | 48 44 46 |
| IPL L | 56 36 28 | PMA L1 | 12 15 62 | STG R | −63 37 7 | ITG R | −57 53 −11 |
| IPL R | −52 28 24 | PMA L3 | 8 17 70 | STG L | 61 15 8 | ||
| vACC | −2 −32 -8 | PMA R1 | −8 25 60 | DLPFC R | −30 −48 18 | ||
| mPFC | 2 −50 18 | Psup L1 | 25 48 62 | DLPFC L | 38 −51 7 | ||
| MFG L | 26 −16 44 | FP L | 24 −55 6 | ||||
Coordinates of regions for the resting-state networks (Tailarach space). Anato. Reg., anatomical region; CEN, central executive network; DLPFC L, dorsolateral prefrontal cortex left; DLPFC R, dorsolateral prefrontal cortex right; DMN, default mode network; FP L, frontal pole left; IPL L, inferior parietal lobule left; IPL R, inferior parietal lobule right; ITG R, inferior temporal gyrus right; LatPA L, lateral parietal area left; LatPA R, lateral parietal area right; MCC, middle cingulate cortex; MFG L, middle frontal gyrus left; mPFC, medial prefrontal cortex; MTG L, middle temporal gyrus Left; MTG R, middle temporal gyrus right; Pc, precuneus; PCC, posterior cingulate cortex; PMA L1, premotor area left; PMA L3, premotor area left; PMA R1, premotor area right; PostCG R1, postcentral gyrus right; PreCG R3, precentral gyrus right; SACC L, supragenual anterior cingulate cortex left; SACC R, supragenual anterior cingulate cortex right; SMA L3, supplementar motor area left; SMN, sensoriMotor network; SN, salience network; STG L, superior temporal gyrus left; STG R, superior temporal gyrus right; SupPA L1, superior parietal area left;; vACC, ventral anterior cingulate cortex; VLPFC/In L, ventrolateral prefrontal cortex/insula left; VLPFC/In R, ventrolateral prefrontal cortex/insula right.
The fSD DMN/SMN ratios in Slow5 and Slow4 were entered in a 2 (frequency bands Slow5 and Slow4) × 4 (subgroups) mixed between-within subjects ANOVA, with age as a covariate, to detect differences in the DMN/SMN ratio in the various subgroups (depressed, manic, and euthymic patients) and HC. The same analysis was conducted on the others two network balances, the fSD DMN/SN and fSD DMN/CEN ratios, to control the specificity of the DMN/SMN ratio (Bonferroni correction was carried out for multiple comparisons). The networks ratio that was significantly different between subgroups was entered into a post hoc Games–Howell test to detect differences in the variability between the various subgroups (i.e., depressed, manic, and euthymic patients and HC). All results were thresholded at a corrected P value of 0.05.
We also controlled the eventual findings by using a different DMN template (87) that, unlike the one above, included the insula, in all of the previously described analyses.
To control the specificity of the Slow5 or Slow4 fSD findings in large-scale networks, we tested the fSD parameters in other frequency bands—Slow3 (0.073–0.198 Hz), Slow2 (0.198–0.25 Hz), and the combined Slow5 and Slow4 band (Slow5/4, 0.01–0.073 Hz)—as well as other BOLD signal parameters: SD (nonfractional rather than fractional SD) and FC between networks. Thus, the data were filtered within Slow3, Slow2, and Slow5/4. With regard to Slow3 and Slow2, it is important to note that these frequencies can be affected by physiological variables, such as respiration and aliased cardiac signals (35, 73). Then, we extracted the fSD in these frequency bands from all of the nodes and calculated the mean fSD of all nodes within each resting-state network. Next, the ratio between the mean of fSD of the DMN and SMN, the DMN and SN, and the DMN and CEN, was calculated in the different frequency ranges. Analogously, we performed the same analyses by using SD in Slow5 and Slow4. Regarding the FC analyses, the seed reference time-series of each network was obtained by averaging the fMRI time-series of all voxels within all ROIs of the different networks, in Slow5 and Slow4. Correlation analyses were carried out between the seed reference region of the DMN and the other networks: SMN, SN, and CEN. The correlation coefficients were then transformed to z-values by means of the Fisher r-to-z transformation to improve normality for group-level t tests. Thus, the FC between the different networks—DMN and SMN, DMN and SN, DMN and CEN—were obtained in Slow5 and Slow4. Each calculated parameter was entered into a one-factorial (factor groups: four subgroups) ANOVA, with age as a covariate, to detect differences in these variables in the various subgroups: depressed, manic, and euthymic patients and HC. All results were thresholded at a P value of 0.05.
Because head motion, especially micromotion, can affect the fSD parameter and may be phase-specific (manic patients could move more than depressed patients), we checked this issue further in various ways. Head-motion parameters themselves were entered in an ANOVA to detect differences between the various subgroups. Then, a correlation analysis was performed between head-motion parameters and fSD DMN/SMN ratio in Slow5. Finally, the fSD DMN/SMN ratio in Slow5 was entered in an ANOVA using head motion as a covariate.
Second, we conducted an exploratory analysis on the single networks to investigate if the changes depend on specific networks or on the balance itself. The fSD values of the mean in each network (DMN, SMN, SN, and CEN) in each frequency band (Slow5 and Slow4) were entered into a two-sample t test to compare group-level differences in the variability between each subgroup: depressed vs. manic, depressed vs. euthymic, depressed vs. HC, manic vs. euthymic, manic vs. HC, and euthymic vs. HC. The results were thresholded at P < 0.05.
Third, a Pearson correlation analysis was performed between fSD of the DMN/SMN ratio in Slow5 and clinical variables, specifically the HAM-D total score and YMRS total score, in BD patients. Considering that the subgroup definition may confound our results, a partial correlation controlling for subgroup status was performed between fSD of the DMN/SMN ratio in Slow5 and clinical scales. The results were thresholded at corrected P < 0.05 (Bonferroni correction was carried out for multiple comparisons and Bootstrap correction was carried out to detect outliers). Moreover, as exploratory analysis, a Pearson correlation was performed between fSD of the DMN/SMN ratio in Slow5 and the following clinical variables in BD patients: the four factors of HAM-D [“anxiety” (psychic anxiety, somatic anxiety, agitation, and hypochondriasis), “depression” (depressed mood, retardation, loss of interests, guilt, and suicide ideation), “insomnia” (initial, middle, and delayed insomnia), and “somatic” (general somatic symptoms, gastrointestinal somatic symptoms, libido loss, and weight loss)]; and the three factors of YMRS [“irritable mania” (irritability, increased motor activity/energy, and aggressive behavior), “elated mania” (elated mood, language abnormalities/thought disorder, increased sexual interest, and poor insight), and “psychotic mania” (alteration of thought content, impaired self-care, poor sleep, and speech abnormalities)] (88, 89). Finally, an ROC curve was constructed and the area under the curve was obtained for fSD of the DMN/SMN ratio in Slow5 in the depressed and manic patients.
All comparisons and correlation analyses were performed by means of IBM SPSS Statistics Version 19 for Windows.
SI Results
We confirmed our findings on the fSD DMN/SMN ratio in Slow5 by using a different DMN template (87). Specifically, the 2 × 4 ANOVA of the fSD DMN/SMN ratio showed a significant interaction between the frequency bands and subgroups (F = 5.32, P = 0.002). A significant main effect of the Slow5 fSD DMN/SMN ratio between the various subgroups (F = 5.43, P = 0.002) was found, whereas no significant effect between the two frequency bands was detected (F = 0.92, P = 0.338). In contrast, there were no significant main effect between the subgroups for the other two ratios, the fSD DMN/SN (F = 1.62, P = 0.18) and the fSD DMN/CEN (F = 1.90, P = 0.13) ratios. The post hoc analyses on the Slow5 fSD DMN/SMN ratio showed a significant increase in depressed compared with manic (P = 0.001), and euthymic patients (P = 0.011) and HC (P = 0.027). Testing for Slow4, we found no significant difference of fSD for the DMN/SMN ratio in all comparisons in the post hoc analyses.
We controlled the specificity of our findings on the Slow5 fSD DMN/SMN ratio and found no significant difference between the various subgroups in any of the tested variables. Specifically, there were no differences between subgroups in the fSD DMN/SMN ratio in Slow3 (F = 0.26, P = 0.848) and Slow2 (F = 0.74, P = 0.531), fSD DMN/SN ratio in Slow3 (F = 0.47; P = 0.701) and Slow2 (F = 0.31, P = 0.814), and fSD DMN/CEN ratio in Slow3 (F = 0.40, P = 0.747) and Slow2 (F = 0.81, P = 0.487). The fSD DMN/SMN ratio in Slow5/4 showed significant differences between subgroups (F = 4.27, P = 0.007). However, when the same variable was analyzed in Slow5 and Slow4 separately, a significant difference was found in Slow5 (F = 7.91, P = 0.000) but not in Slow4 (F = 2.49, P = 0.065). Furthermore, neither the fSD DMN/SN ratio (F = 1.68, P = 0.175) nor fSD DMN/CEN ratio (F = 2.14, P = 0.100) in Slow5/4 was significantly different between subgroups. Taken together, these findings confirm our initial results, in which we conducted a 2 × 4 factorial analysis (one factor frequency band with Slow5 and Slow4; one factor group with the four subgroups). With regard to SD, we found no significant differences between subgroups in the SD DMN/SMN ratio in Slow5 (F = 1.52, P = 0.212) and Slow4 (F = 1.91, P = 0.133), SD DMN/SN ratio in Slow5 (F = 1.84, P = 0.144) and Slow4 (F = 0.55, P = 0.647), and SD DMN/CEN ratio in Slow5 (F = 2.25, P = 0.087) and Slow4 (F = 1.82, P = 0.148). Finally, we found no significant differences between subgroups in the DMN-SMN FC in Slow5 (F = 2.54, P = 0.060) and Slow4 (F = 0.26, P = 0.853), DMN-SN FC in Slow5 (F = 1.51, P = 0.215) and Slow4 (F = 0.08, P = 0.968), and DMN-CEN FC in Slow5 (F = 0.44, P = 0.725) and Slow4 (F = 0.91, P = 0.436).
With regard to head motion, no significant differences were found between subgroups (F = 0.62, P = 0.601). Head-motion parameters did not correlate with fSD DMN/SMN ratio in Slow5 (r = −0.134, P = 0.178). Furthermore, the fSD DMN/SMN ratio in Slow5 remained significantly different between subgroups, even if head motion was used as a covariate (F = 7.51, P = 0.000). These results suggest that head motion has not confounded our findings.
With regard to clinical correlations in BD patients, the fSD of the DMN/SMN ratio in Slow5 was found to be positively correlated (after bootstrapping) with the HAM-D total score (r = 0.426, P = 0.001; CI: 0.203∼0.597), and negatively correlated with the YMRS total score (r = −0.378, P = 0.003; CI: −0.564 ∼ −0.146) (Fig. 3). Furthermore, partial correlation analysis confirmed a positive correlation of the ratio with the HAM-D total score (r = 0.455, P = 0.000; CI: 0.254∼0.634) and a negative correlation with the YMRS total score (r = −0.443, P = 0.000; CI: −0.625 ∼ −0.255). The HAM-D and YMRS total scores did not show a significant correlation in BD (P > 0.05). Furthermore, in our exploratory analysis on the subscales, fSD of the DMN/SMN ratio in Slow5 was positively correlated with the somatic factor of the HAM-D (r = 0.275, P = 0.033), but not with anxiety, depression, and insomnia factors; the same neuronal measures was negatively correlated with the elated mania factor (r = −0.310; P = 0.016), psychotic mania factor (r = −0.386, P = 0.002), and irritable mania factor (r = −0.290, P = 0.024).
Limitations
The main limitation of the present study was the possible confounding effects of medication. Indeed, almost all of the bipolar patients in our sample were taking medications, including mood stabilizers, antipsychotics, antidepressants, and benzodiazepines, which could interfere with the BOLD signal. Following recent suggestions and standards, we examined the potential impact of the psychotropic medication load—the number and dosage of different medications—on fSD in BD (90). This was done by converting antipsychotics into chlorpromazine dose-equivalents (91), mood stabilizers into lithium dose equivalents (91), antidepressants into imipramine dose-equivalents (91), and benzodiazepines into diazepam dose-equivalents (92). We then used the codes 0, 1, 2, and 3 to indicate no medication, and dose-equivalents below, equal, or above the mean effective daily dose, respectively (93). We generated a composite measure of the medication load by summing all individual medication codes for each category and each individual BD patient (90). We investigated the potential impact of medications on imaging data by correlating the resulting pharmacological load with fSD in DMN/SMN activity ratio in Slow5. The medication load did not correlate with this measure (r = 0.057, P = 0.668). Then, to further control for an eventual effect of pharmacotherapy on the fSD DMN/SMN ratio in Slow5, we compared this variable by using a t test for each medication class (mood stabilizers, antidepressants, benzodiazepines, and antipsychotics), between those patients who were in treatment with the respective drug and those who were not. We found no differences between patients who were in treatment with mood stabilizers (n = 52) and patients who were not (n = 8) (t = 1.34, P = 0.184), between patients who were in treatment with antidepressants (n = 22) and patients who were not (n = 38) (t = 0.71, P = 0.477), between patients who were in treatment with benzodiazepines (n = 20) and patients who were not (n = 40) (t = −0.02, P = 0.980), as well as between patients who were in treatment with antipsychotics (n = 34) and patients who were not (n = 26) (t = −0.27, P = 0.786). Psychotic features can be related to changes in fMRI data (20). However, it is worth noting that in our sample patients with psychotic features were poorly represented (n = 6; four were manic and two were depressed) compared with patients without psychotic features (n = 54). Also, antipsychotics were prescribed primarily to patients in our sample for affective symptoms according to the current and standard guidelines for BD. Moreover, the fSD DMN/SMN ratio in Slow5 was not significantly different between those patients with psychotic features and those without (t = −1.84, P = 0.070). Finally, the fSD DMN/SMN ratio in Slow5 was entered in ANOVAs by using medication load and each drug class separately as covariates in the BD sample. The difference in this ratio between subgroups remained significant despite controlling for medication load (F = 10.52, P = 0.000), mood stabilizers (F = 13.40, P = 0.000), antidepressants (F = 11.00, P = 0.000), benzodiazepines (F = 11.02, P = 0.000), and antipsychotics (F = 11.00, P = 0.000). The result suggests that this resting-state parameter was not greatly affected by pharmacotherapy.
Furthermore, age, duration of illness, and number of episodes may represent a confounding factor. Our sample consisted of patients at different age and stages of the disease. To assess the potential role of these clinical factors, we correlated fSD in the DMN/SMN ratio in Slow5 with age, with duration of illness, and with number of episodes. No significant correlations were observed (r = −0.076 and P = 0.566; r = −0.159 and P = 0.238; r = 0.062 and P = 0.639, respectively). Then, to further control for an eventual effect of duration of illness on the fSD DMN/SMN ratio in Slow5, we compared this variable by using a t test between patients with short illness duration vs. those with long illness duration. We found no differences between patients who had less than 1 y of illness (n = 5) and patients who had more (n = 55) (t = −0.97, P = 0.332), between patients who had less than 5-y of illness (n = 12) and patients who had more (n = 48) (t = −1.13, P = 0.260), between patients who were at the first episode (n = 6) and patients who were not (n = 54) (t = −0.89, P = 0.373), between patients who had fewer than five episodes in a lifetime (n = 25) and patients who had more (n = 35) (t = 0.68, P = 0.494). These data suggested the absence of major effects of these clinical factors on the resting-state parameter.
In our BD sample there was a high representation of women (∼70%). Because BD type I is thought to be gender equivalent (50–50% M–F), we controlled for an eventual effect of gender on the fSD DMN/SMN ratio in Slow5, and the t test between male (n = 18) and female (n = 42) in the BD sample revealed no significant differences in this parameter (t = 1.42, P = 0.159) (as well as no differences in the HC sample; t = –1.66, P = 0.103), suggesting no major gender effect in our findings.
The images were acquired by using a 1.5-T scanner with a 1-mm gap between slices. Therefore, we focalized only on cortical nodes.
Finally, our main study concerned different patients in the various phases of illness. Although we confirmed our findings in an independent BD sample and some follow-up data (see Replication Study, below), the comparisons of the various phases of illness in the same patients should be addressed in future longitudinal studies on larger samples.
Replication Study
Replication Study in an Independent Sample of BD Patients.
To preliminarily validate our findings on the fSD Slow5 DMN/SMN ratio in the depressed and manic phases of BD, we conducted the analyses on a smaller independent sample of BD patients and HC.
Materials and Methods.
The independent sample was recruited at the in- and out-patient service of the Psychiatric Clinic at the University of Genoa, and consisted of 23 bipolar patients (14 females, 18–60 y old, 9 in depressive, 8 in manic, and 6 in euthymic phases; all under medications) and 18 HC (Table S4).The Ethics Committee of San Martino Hospital approved the study, and written informed consent was obtained from all new participants. As per the main study, each new participant was evaluated with standardized structured or semistructured clinical instruments (i.e., MINI, SCID-I/P, SCID-II, SIMD-R, HAM-D, YMRS) and met the same inclusion and exclusion criteria (see above).
Table S4.
Subject demographic and clinical information of the independent sample
| Demographic | BD | HC | |||
| BD TOT | Manic BD | Depressed BD | Euthymic BD | ||
| Sample size n (%) | 23 (100) | 8 (34.7) | 9 (39.1) | 6 (26) | 18 (100) |
| Age mean (SD) | 46.8 (8.6) | 47.8 (8.8) | 43.6 (8.0) | 50.1 (8.9) | 29.6 (5.4) |
| Female n (%) | 14 (60.8) | 4 (50) | 5 (55.5) | 5 (83.3) | 12 (66.6) |
| Age of onset mean (SD) | 30.7 (7.8) | 29.1 (8.5) | 31.8 (7.8) | 31.1 (8.0) | — |
| Duration of illness mean (SD) | 16.9 (13.0) | 18.7 (15.2) | 11.7 (9.9) | 19.0 (14.4) | — |
| HAM-D mean (SD) | — | 3.5 (2.6) | 21.6 (3.1) | 2.0 (2.4) | — |
| YMRS mean (SD) | — | 19.1 (4.4) | 3.3 (3.0) | 0.5 (0.8) | — |
| Mood stabilizers n (%) | 20 (86.9) | 8 (100) | 7 (77.7) | 5 (83.3) | — |
| Lithium n (%) | 6 (26) | 2 (25) | 1 (11.1) | 3 (50) | — |
| Valproate n (%) | 13 (56.5) | 6 (75) | 5 (55.5) | 2 (33.3) | — |
| Other antiepileptic drugs n (%) | 2 (8.6) | 0 (0) | 2 (22.2) | 0 (0) | — |
| Antidepressants n (%) | 8 (34.7) | 0 (0) | 5 (55.5) | 3 (50) | — |
| SRI n (%) | 6 (26) | 0 (0) | 4 (44.4) | 2 (33.3) | — |
| Tricyclic antidepressants n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | — |
| Duals and other antidepressants n (%) | 3 (13) | 0 (0) | 1 (11.1) | 2 (33.3) | — |
| Antipsychotics n (%) | 14 (60.8) | 5 (62.5) | 7 (77.7) | 2 (33.3) | — |
| Atypical n (%) | 11 (47.8) | 3 (37.5) | 6 (66.6) | 2 (33.3) | — |
| Typical n (%) | 3 (13) | 2 (25) | 1 (11.1) | 0 (0) | — |
| Benzodiazepines n (%) | 6 (26) | 2 (25) | 3 (33.3) | 1 (16.6) | — |
| Unmedicated n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | — |
SRI, serotonin reuptake inhibitors.
Functional (EPI) and structural (3D-SPGR) images were acquired using the same scanner (1.5-T GE) and the same parameters applied in the main study (TR/TE = 2000/30 ms, flip angle = 90°, FOV = 24 cm; TR/TE = 11.5/5 ms, IR = 500 ms, flip angle = 8°, FOV = 25.6 cm, respectively; see above for the details).
Preprocessing and resting-state data analyses were conducted using AFNI, exactly with the same procedures of the main study (see above for a systematic description). Thus, after the standard preprocessing (slice timing, alignment, normalization, smoothing and regression out of motion, white matter and cerebrospinal fluid, and detrending), the data were filtered within Slow5 and Slow4. All participants selected showed head motion of less than 1 mm. Then, the variability of BOLD signal was extracted and the mean values of fSD were calculated for DMN, SMN, SN, and CEN, as well as for their ratios (DMN/SMN, DMN/SN, and DMN/CEN) in Slow5 and in Slow4 (by using the same spherical ROIs) (Table S3).
The fSD DMN/SMN ratios in Slow5 and Slow4 (as well as the fSD DMN/SN and fSD DMN/CEN ratios in Slow5 and Slow4) were entered in 2 (Slow5 and Slow4) × 4 (subgroups) mixed between-within subjects ANOVAs, with age as a covariate (Bonferroni correction was carried out for multiple comparisons). The networks ratio that was significantly different between subgroups was entered into a post hoc Games–Howell test. All results were thresholded at a corrected P value of 0.05. To further show similarity in results between this independent sample and the main sample of BD patients, we also compared the groups from both samples with each other with regard to the fSD Slow5 DMN/SMN ratio. Then, the fSD mean values of each network (DMN, SMN, SN, and CEN) in each frequency band (Slow5 and Slow4) were entered into two-sample t tests. Finally, a Pearson correlation analysis was performed between fSD of the DMN/SMN ratio in Slow5 and clinical variables (i.e., HAM-D and YMRS total scores) in BD patients, as well as potential confounders (i.e., medication load, calculated as previously described) and illness duration.
Results.
The 2 × 4 ANOVA of the fSD DMN/SMN ratio showed a significant difference between the various subgroups (F = 3.93, P = 0.016). In contrast, there were no significant differences between subgroups for the fSD DMN/SN (F = 0.22, P = 0.879) and fSD DMN/CEN (F = 2.50, P = 0.074) ratios. Then, the post hoc analyses revealed a significant increase in Slow5 fSD DMN/SMN ratio in depressed patients compared with manic (corrected P = 0.023), confirming the major finding of the study on the main sample (Fig. S2). Testing for Slow4, we confirmed no significant differences of fSD for the DMN/SMN ratio in all comparisons in the post hoc analyses. Furthermore, no significant differences in the fSD Slow5 DMN/SMN ratio were found between manic patients of the independent sample and those of the main sample (mean value = 1.039 ± 0.144 and 1.063 ± 0.146, respectively, t = −0.33, P = 0.701); between depressed patients of the independent sample and those of the main sample (mean value = 1.285 ± 0.163 and 1.296 ± 0.180, respectively, t = −0.16, P = 0.870); between euthymic patients of the independent sample and those of the main sample (mean value = 1.180 ± 0.112 and 1.118 ± 0.133, respectively, t = 1.02, P = 0.315); and between HC of the independent sample and those of the main sample (mean value = 1.163 ± 0.160 and 1.179 ± 0.163, respectively, t = −0.33, P = 0.739).
Fig. S2.
The DMN/SMN, DMN/SN, and DMN/CEN ratios in fSD Slow5 and Slow4 in the various subgroups of the independent sample. Results of the ANOVA and Games–Howell post hoc test of fSD of the DMN/SMN, DMN/SN, and DMN/CEN ratios in Slow5 and Slow4 between the various subgroups. Corrected *P < 0.05; D, depressive patients; E, euthymic patients; HC, healthy controls; M, manic patients.
Then, the single-networks analyses revealed an increase in the Slow5 fSD in the DMN in depressed patients compared with manic (t = 2.87, P = 0.012) and euthymic patients (t = 2.88, P = 0.013), as well as an increase in the Slow5 fSD in the SMN in manic patients compared with euthymic patients (t = 2.23, P = 0.045) (Fig. S3 and Table S5).
Fig. S3.
Differences in DMN, SMN, SN, and CEN fSD Slow5 and Slow4 in the various subgroups of the independent sample. Mean values of fSD in Slow5 and Slow4 of DMN, SMN, SN, and CEN in the various subgroups; *P < 0.05. D, depressed patients; E, euthymic patients; HC, healthy controls; M, manic patients.
Finally, with regard to clinical correlations in BD patients, the fSD of the DMN/SMN ratio in Slow5 was found to be positively correlated (after bootstrapping) with the HAM-D total score (r = 0.432, P = 0.039; CI: 0.120∼0.686), and negatively correlated with the YMRS total score (r = −0.437, P = 0.037; CI: −0.696 ∼ −0.155) (Fig. S4). In contrast, no significant correlations were found between the fSD Slow5 DMN/SMN ratio and medication load (r = −0.050, P = 0.822) or illness duration (r = −0.112, P = 0.610).
These results preliminary confirmed the validity of a reduction in the manic phase and an increase in the depressive phase of the fSD Slow5 DMN/SMN ratio in BD.
Intrasubject Comparison.
To confirm the changes of fSD Slow5 DMN/SMN ratio across depressive and manic phases of BD, we preliminarily investigated this parameter at the intrasubject level in follow-up data of resting-state fMRI acquisitions of some bipolar subjects of the main sample.
We have since collected a follow-up scan of nine bipolar patients (six females; mean age = 45.7 ± 8.8; rescanned after a mean period of 9.1 ± 3.3 mo). Two manic subjects at T0 were rescanned in euthymia at T1 and one euthymic subject (T0) was rescanned in manic phase (T1); three depressed patients at T0 were rescanned in euthymic phase at T1; two euthymic and one manic subjects (T0) were rescanned in the same phase (T1). Exactly the same protocol as described for the main study (i.e., clinical assessment, imaging acquisition, preprocessing and resting-state data analyses, and fSD data extraction), was carried on for each follow-up subject. Then, the fSD Slow5 DMN/SMN ratio was calculated and entered in paired t tests to compare this parameter across different phases in the same subject.
The fSD of the DMN/SMN ratio in Slow5 was found to be lower in the manic phase (mean value = 1.032 ± 0.064) compared with the euthymic phase (mean value = 1.197 ± 0.030) of the same patients (n = 3; t = −4.66, P = 0.043), although it was found to be higher in the depressive phase (mean value = 1.271 ± 0.083) compared with the euthymic phase (mean value = 1.173 ± 0.099) of the same patients (n = 3; t = 9.98, P = 0.010).
This preliminary intrasubject analysis further confirmed the different DMN/SMN disbalance in mania and depression found in the main sample at an intersubject level.
Reliability Analysis on Healthy Subjects.
One question that arises is whether the measures used are suitably reliable in the different frequency bands (35). To test if this is the case we calculated our measures of interest (fSD in Slow5 and Slow4 for the DMN and SMN) from a dataset with scans at different time points from the same participants and calculated the intraclass coefficients (ICC) for each measure. In more detail, we obtained an openly available fMRI resting-state dataset in which 25 healthy participants were scanned on three occasions (NYU CSC TestRetest: www.nitrc.org/projects/nyu_trt/): baseline (T1), 5–11 mo after T1 (T2), and 30–45 min after T2 (T3). The basic acquisition settings were: 197 contiguous EPI functional volumes; TR = 2 s, voxel size = 3 × 3 × 3 mm. We then applied the same preprocessing procedures to these data as were used in our main analysis before calculating the fSD in the relevant frequency bands from out DMN and SMN ROIs (see Table S3 for the regions used). The ICC for fSD values at T1, T2, and T3 were then calculated for each frequency band and each network separately. In the DMN, the ICC for Slow5 was 0.639 (P = 0.002) and for Slow4 was 0.683 (P = 0.000). In the SMN the ICC for Slow5 was 0.735 (P = 0.000) and for Slow4 was 0.684 (P = 0.000). Good ICC values (ICC > 0.5) were found in both networks and in both frequency bands, suggesting that the measurements used in our main analysis are likely to be suitably reliable.
Acknowledgments
The authors thank Prof. Gianluigi Mancardi for the access to the MRI Unit (University of Genoa). G.N. is supported in part by the EJLB-Canadian Institutes of Health Research, Michael Smith Foundation, the Canadian Institutes of Health Research, and the Brain and Mind Research Institute of the University of Ottawa.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517558113/-/DCSupplemental.
References
- 1. American Psychiatrich Association (2013) Diagnostic and Statistical Manual for Mental Disorders. 5th ed. (DSM-5). (American Psychiatrich Association, Washington, DC)
- 2.Kraepelin E. Clinical Psychiatry. Macmillan; New York: 1902. [Google Scholar]
- 3.Northoff G. Spatiotemporal psychopathology I: No rest for the brain's resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. J Affect Disord. 2016;190:854–866. doi: 10.1016/j.jad.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Northoff G. Spatiotemporal Psychopathology II: How does a psychopathology of the brain's resting state look like? Spatiotemporal approach and the history of psychopathology. J Affect Disord. 2016;190:867–879. doi: 10.1016/j.jad.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Minassian A, et al. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. 2010;120(1-3):200–206. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souery D, et al. Depression across mood disorders: Review and analysis in a clinical sample. Compr Psychiatry. 2012;53(1):24–38. doi: 10.1016/j.comppsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19(9):966–977. doi: 10.1038/mp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, et al. Multisensory competition is modulated by sensory pathway interactions with fronto-sensorimotor and default-mode network regions. J Neurosci. 2015;35(24):9064–9077. doi: 10.1523/JNEUROSCI.3760-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jilka SR, et al. Damage to the salience network and interactions with the default node network. J Neurosci. 2014;34(33):10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulden N, et al. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 13.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. Am Psychol. 2000;55(11):1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- 15.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magioncalda P, et al. Functional connectivity and neuronal variability of resting state activity in bipolar disorder—Reduction and decoupling in anterior cortical midline structures. Hum Brain Mapp. 2015;36(2):666–682. doi: 10.1002/hbm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171(3):189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ongür D, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anticevic A, et al. Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr Bull. 2015;41(1):133–143. doi: 10.1093/schbul/sbu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meda SA, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci USA. 2014;111(19):E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheline YI, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm S, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4):932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- 24.Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34(4):592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Angst J, et al. Evidence-based definitions of bipolar-I and bipolar-II disorders among 5,635 patients with major depressive episodes in the Bridge Study: Validity and comorbidity. Eur Arch Psychiatry Clin Neurosci. 2013;263(8):663–673. doi: 10.1007/s00406-013-0393-4. [DOI] [PubMed] [Google Scholar]
- 26.Cassano GB, et al. The role of psychomotor activation in discriminating unipolar from bipolar disorders: A classification-tree analysis. J Clin Psychiatry. 2012;73(1):22–28. doi: 10.4088/JCP.11m06946. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Northoff G. Is schizophrenia a spatiotemporal disorder of the brain’s resting state? World Psychiatry. 2015;14(1):34–35. doi: 10.1002/wps.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Curr Top Med Chem. 2012;12(21):2404–2414. doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- 30.Deco G, Kringelbach ML. Great expectations: Using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84(5):892–905. doi: 10.1016/j.neuron.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Yang GJ, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci USA. 2014;111(20):7438–7443. doi: 10.1073/pnas.1405289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carhart-Harris RL, et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull. 2013;39(6):1343–1351. doi: 10.1093/schbul/sbs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo XN, et al. The oscillating brain: Complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zang YF, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30(14):4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci. 2011;31(12):4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrett DD, McIntosh AR, Grady CL. Brain signal variability is parametrically modifiable. Cereb Cortex. 2014;24(11):2931–2940. doi: 10.1093/cercor/bht150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrett DD, et al. Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neurosci Biobehav Rev. 2013;37(4):610–624. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou QH, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basalyga G, Salinas E. When response variability increases neural network robustness to synaptic noise. Neural Comput. 2006;18(6):1349–1379. doi: 10.1162/neco.2006.18.6.1349. [DOI] [PubMed] [Google Scholar]
- 43.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9(4):292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lugo E, Doti R, Faubert J. Ubiquitous crossmodal stochastic resonance in humans: Auditory noise facilitates tactile, visual and proprioceptive sensations. PLoS One. 2008;3(8):e2860. doi: 10.1371/journal.pone.0002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7(12):553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Han Y, et al. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fMRI study. Neuroimage. 2011;55(1):287–295. doi: 10.1016/j.neuroimage.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 47.Xi Q, et al. Spontaneous brain activity in mild cognitive impairment revealed by amplitude of low-frequency fluctuation analysis: A resting-state fMRI study. Radiol Med (Torino) 2012;117(5):865–871. doi: 10.1007/s11547-011-0780-8. [DOI] [PubMed] [Google Scholar]
- 48.Raja Beharelle A, Kovačević N, McIntosh AR, Levine B. Brain signal variability relates to stability of behavior after recovery from diffuse brain injury. Neuroimage. 2012;60(2):1528–1537. doi: 10.1016/j.neuroimage.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Z, et al. The self and its resting state in consciousness: An investigation of the vegetative state. Hum Brain Mapp. 2014;35(5):1997–2008. doi: 10.1002/hbm.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Z, et al. Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum Brain Mapp. 2014;35(11):5368–5378. doi: 10.1002/hbm.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoptman MJ, et al. Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr Res. 2010;117(1):13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernández A, Gómez C, Hornero R, López-Ibor JJ. Complexity and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:267–276. doi: 10.1016/j.pnpbp.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 54.Balduzzi D, Riedner BA, Tononi G. A BOLD window into brain waves. Proc Natl Acad Sci USA. 2008;105(41):15641–15642. doi: 10.1073/pnas.0808310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage. 2009;48(3):515–524. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee TW, Northoff G, Wu YT. Resting network is composed of more than one neural pattern: An fMRI study. Neuroscience. 2014;274:198–208. doi: 10.1016/j.neuroscience.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 57.Xue SW, Li D, Weng XC, Northoff G, Li DW. Different neural manifestations of two slow frequency bands in resting functional magnetic resonance imaging: A systemic survey at regional, interregional, and network levels. Brain Connect. 2014;4(4):242–255. doi: 10.1089/brain.2013.0182. [DOI] [PubMed] [Google Scholar]
- 58.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 59.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 60.Northoff G, et al. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Northoff G. Unlocking the Brain. Oxford Univ Press; New York: 2014. [Google Scholar]
- 62.Northoff G. How is our self altered in psychiatric disorders? A neurophenomenal approach to psychopathological symptoms. Psychopathology. 2014;47(6):365–376. doi: 10.1159/000363351. [DOI] [PubMed] [Google Scholar]
- 63.Liu CH, et al. Regional homogeneity within the default mode network in bipolar depression: A resting-state functional magnetic resonance imaging study. PLoS One. 2012;7(11):e48181. doi: 10.1371/journal.pone.0048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fountoulakis KN, Giannakopoulos P, Kövari E, Bouras C. Assessing the role of cingulate cortex in bipolar disorder: Neuropathological, structural and functional imaging data. Brain Res Brain Res Rev. 2008;59(1):9–21. doi: 10.1016/j.brainresrev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Savitz JB, Rauch SL, Drevets WC. Clinical application of brain imaging for the diagnosis of mood disorders: The current state of play. Mol Psychiatry. 2013;18(5):528–539. doi: 10.1038/mp.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chai XJ, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker JT, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu M, et al. Altered affective, executive and sensorimotor resting state networks in patients with pediatric mania. J Psychiatry Neurosci. 2013;38(4):232–240. doi: 10.1503/jpn.120073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31(3):377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 70.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105(41):16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duff EP, et al. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum Brain Mapp. 2008;29(7):778–790. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 73.Cordes D, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 74.Gotts SJ, et al. The perils of global signal regression for group comparisons: A case study of autism spectrum disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saad ZS, et al. Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33, quiz 34–57. [PubMed] [Google Scholar]
- 77.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79(2):163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 78.First MB, et al. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Version 2.0. Biometrics Research Deprtment, New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- 79.Cassano GB, et al. Psychopathology, temperament, and past course in primary major depressions. 2. Toward a redefinition of bipolarity with a new semistructured interview for depression. Psychopathology. 1989;22(5):278–288. doi: 10.1159/000284608. [DOI] [PubMed] [Google Scholar]
- 80.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 82.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 83.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- 84.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 85.Laird AR, et al. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Göttlich M, et al. Altered resting state brain networks in Parkinson’s disease. PLoS One. 2013;8(10):e77336. doi: 10.1371/journal.pone.0077336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 88.Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62(1):123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 89.Hanwella R, de Silva VA. Signs and symptoms of acute mania: A factor analysis. BMC Psychiatry. 2011;11:137. doi: 10.1186/1471-244X-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baldessarini RJ. Chemotherapy in Psychiatry. Pharmacologic Basis of Treatments of Major Mental Illness. 3rd Ed Springer; New York: 2013. [Google Scholar]
- 92.Arana GW, Rosenbaum JF. 2000. Handbook of Psychiatric Drug Therapy, 4th Ed (Lippincott, Williams and Wilkins, Philadelphia, PA)
- 93.Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol. 2004;24(2):192–208. doi: 10.1097/01.jcp.0000117422.05703.ae. [DOI] [PubMed] [Google Scholar]