Significance
Water sustains photosynthesis and growth of land plants, but it must be transported from the soil to leaves under high tension. Drying soil leads to an increase in water tension, exposing plants to the problem of breakage of the water column, causing embolisms that cut off water supply, leading to tissue death during drought. The ability of leaves to resist embolism formation is a key adaptive axis in plant evolution, and yet the process itself has never been visualized in the leaf venation. We describe a new optical method that allows the evolution and spread of embolism in the entire leaf network to be mapped, thus revealing general rules in the sequence of leaf vein transport failure.
Keywords: embolism, drought, xylem, vein, leaf
Abstract
The intricate patterns of veins that adorn the leaves of land plants are among the most important networks in biology. Water flows in these leaf irrigation networks under tension and is vulnerable to embolism-forming cavitations, which cut off water supply, ultimately causing leaf death. Understanding the ways in which plants structure their vein supply network to protect against embolism-induced failure has enormous ecological and evolutionary implications, but until now there has been no way of observing dynamic failure in natural leaf networks. Here we use a new optical method that allows the initiation and spread of embolism bubbles in the leaf network to be visualized. Examining embolism-induced failure of architecturally diverse leaf networks, we found that conservative rules described the progression of hydraulic failure within veins. The most fundamental rule was that within an individual venation network, susceptibility to embolism always increased proportionally with the size of veins, and initial nucleation always occurred in the largest vein. Beyond this general framework, considerable diversity in the pattern of network failure was found between species, related to differences in vein network topology. The highest-risk network was found in a fern species, where single events caused massive disruption to leaf water supply, whereas safer networks in angiosperm leaves contained veins with composite properties, allowing a staged failure of water supply. These results reveal how the size structure of leaf venation is a critical determinant of the spread of embolism damage to leaves during drought.
One of the most striking features of leaves is the network of veins that function as microfluidic circuits responsible for importing water and nutrients and exporting sugars. These microfluidic circuits supply water to tissues engaged in photosynthesis (1), thus governing the rate of water and CO2 exchange between plants and the atmosphere (2, 3). Despite the essential function of the leaf vasculature, its integrity is constantly under threat of failure because of the uncontrolled air embolization of the network when water stress exceeds a critical threshold value (4). Hence, leaf networks have evolved under competing selective pressures to simultaneously maximize efficiency (in terms of flow and construction cost) and safety (from embolism disruption) of water transport within the leaf (5). Major evolutionary transitions have affected the transport efficiency of the leaf vein network (6, 7), but little is known about adaptation of leaf networks to avoid catastrophic failure by air embolism.
High water tension in the water-transporting xylem cells of plants originates in drying soils and, according to the “air-seeding” hypothesis, is responsible for pulling air into the continuous xylem water column through submicron “pit” pores in the thin, porous membranes that separate neighboring xylem conduits (8). Typically, the closure of stomatal valves on the leaf surface dramatically slows the pace of plant dehydration before damage to the xylem begins (9), but without rain, drying soil will ultimately cause water tension to exceed the air-seeding threshold (4). At this critical tension, the capillary forces that prevent air from bursting into the xylem through microscopic pores in the pit membranes are exceeded, causing a bubble to invade the water-filled lumen and expand rapidly to form an air embolism that blocks the xylem conduit. These embolisms are theoretically able to propagate between interconnected conduits (which range in length from microns to meters) by traversing pit membranes between xylem conduits, although this process has only been visualized in wood (10). Subsequent blockage of xylem cells by air embolisms progressively reduces water transport, further increasing xylem tension in a positive feedback loop that can cause catastrophic failure of the xylem vasculature unless plants are rewatered (11). Progress in understanding the dynamics of embolism spread using traditional hydraulic methods to quantify the effect of drought-induced embolism formation on plant function have been hampered by the presence of high xylem hydraulic tension, which makes intrusive measurements prone to exogenous embolism formation during manipulation (12). Recent techniques such as X-ray and magnetic resonance imaging have provided new insights into when and where embolisms form in stems (10, 13, 14), but a lack of resolution, and the fact that leaves are damaged by ionizing radiation, currently prevents this technique from being used to investigate the spread of embolism through the leaf water transport network. The resultant knowledge gap is significant not only because of the critical role of water transport failure in determining plant mortality during drought (15) but also because the dominant leaf vascular architectures found in modern land plants are the product of >400 million years of evolutionary selection and should therefore represent diverse solutions for building failsafe supply networks (16). The properties of these leaf vascular supply networks may have applications to a wide range of large-scale man-made networks for fluid delivery (water, gas, oil, and other fluids), energy distribution (17) (electrical current), data communication (internet, landline, and cellular telephone) (18), or urban architecture. Here we develop a simple technique that provides the first opportunity, to our knowledge, to visualize where embolism damage initiates in leaf networks and how it propagates as leaves are exposed to water stress. Our aim was to use this new optical technique to discover the basic rules of network failure in leaves and to determine whether species with markedly different network architecture perform differently in terms of preserving network connection under imposed stress.
To capture embolism events, we recorded rapid changes in light transmission through the venation as leaves were allowed to desiccate while attached to branches. Leaf veins are composed of tight bundles of water-filled xylem tubes, relatively transparent to light (Fig. 1 and Fig. S1). These tubes are segmented by membranes that temporarily stop gas interface propagation. When embolism occurs in these xylem cells, they immediately fill with air, changing the refractive properties of the venation as the result of an increase in the number of air–water interfaces (19). By recording images at a relatively high frequency (one image every 30 s) on a low-magnification microscope or slide scanner (see SI Methods for details), we were able to distinguish rapid, embolism-induced changes in light transmission, from slower changes in light transmission resulting from processes such as changing leaf density as specimens desiccated. This technique allowed us to produce what are, to our knowledge, the first ever temporally resolved maps of xylem embolism in leaves exposed to water stress over many hours or days (Fig. 1 and Movies S1–S2).
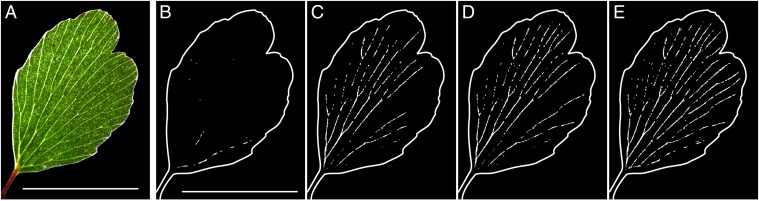
Fig. 1.
Digital representation of embolism recorded optically during leaf drying in the fern Adiantum. (A) Leaf illuminated by transmitted light. (Scale bar, 5 mm.) (B–E) The result of image analysis, showing the progression of embolism after (B) 2 h 5 min, (C) 4 h 10 min, (D), 6 h 15 min, and (E) 8 h 20 min. The darkening of pixels between successive images (30 s apart) by embolism is detected by image difference. If the darkening exceeds a threshold, these pixels are assigned as white (see Movies S1 and S2 for further explanation).
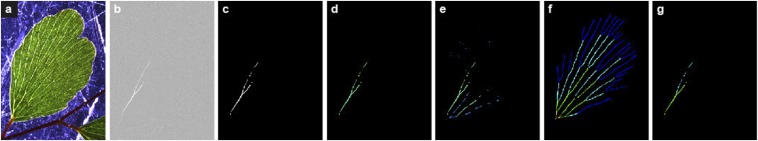
Fig. S1.
Image analysis on Adiantum (same sample presented in Fig. 1 and Fig. 2 A and B). (A) Original experimental image, photographed with transmitted light illumination. (B) Subtraction between two consecutive images separated by 30 s, here showing a large embolism. (C) A threshold on this image allows detection of events. (D) Coloration of the event according to time elapsed. (E) Cumulated sum of the embolisms, colored according to the time elapsed. (F) Manual coloring of the orders in Adiantum; each order corresponds to a color. (G) Coloring corresponding to the orders applied on the event detected on C, using map F. See also Movie S2 for an animated illustration.
By severing the contact between branches and soil, we were able to simulate the onset of severe drought conditions during a relatively short period. On the initiation of drying, leaves attached to branches were observed to resist any embolism for variable periods ranging from 30 min (in the fern Adiantum capillus-veneris) to 70 h (in the woody angiosperm Eucalyptus globulus). After this quiescent period, large embolism events were observed to spread through the vascular network, continuing until leaf water potentials reached levels causing cellular damage, whereupon embolism was no longer observed. We have recently shown that these embolism events in leaf veins are responsible for the decline in leaf vein water transport function that occurs during severe water deficit after the closure of stomata and immediately before leaf death (9). Here we used the technique of image subtraction (SI Methods) to map the dynamics of embolism spread within the leaf xylem network (Fig. 2). These spatial observations of leaf embolism in situ enabled us to determine whether there were “weak links” in the leaf vein network that always embolized early, or whether the process of embolism spread was entirely random. We were also able to assess the performance of different vein network architectures under exposure to high water tension generated by acute leaf water stress.
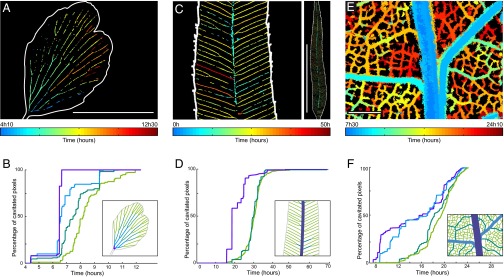
Fig. 2.
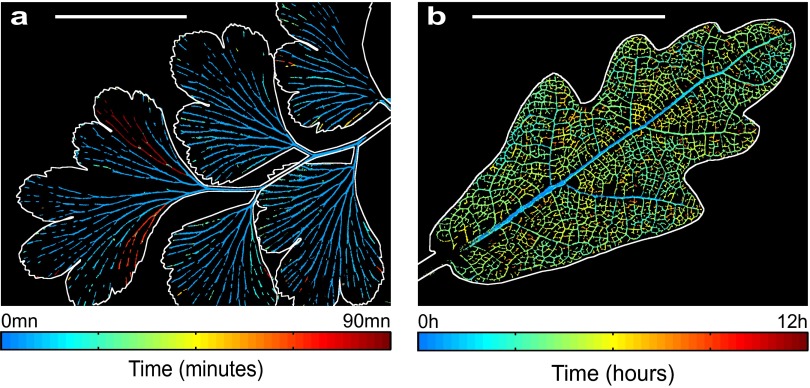
Spatiotemporal evolution of embolism formation in leaves exposed to acute water stress. (A) Adiantum: progression map of embolism in a single pinna. (Scale bar, 5 mm.) (B) Progression of embolism for different vein orders. (Inset) Definition of the colors of each order. (C and D) Embolism map for the fern Pteris and progression of emboli for the whole leaf; the orders are here defined in the usual way from the petiole. (Left) Zoom. (Right) Whole leaf. (Scale bar, 50 mm.) (E and F) The same for Quercus. Colors represent the first embolism. See Fig. S4B for the progression of embolism in a whole Quercus robur leaf. (Scale bar, 1 mm.)
From these first observations of drying leaves, we discovered that embolism invades the network by abrupt steps (Movie S3), contrary to the smooth physiological flow of sap observed in veins (20). This is expected because according to the “air-seeding” hypothesis, air should only break into a water-filled vessel when the water tension exceeds a threshold that cannot be contained by capillary forces in the walls or membranes of individual xylem vessels (21). The progressive invasion of air into xylem conduits indicates that a range of thresholds for air entry into xylem conduits exists in the xylem network of a single leaf. The size of these embolism steps was highly variable, with the first embolism events generally propagating further than those occurring later in the drying cycle (Fig. 1). A strong, size-dependent pattern was observed in vein diameter, such that a typical embolism sequence begins in the largest veins and progresses into smaller veins (Fig. 2). These observations were common to all vein networks sampled across phylogenetically and architecturally diverse group of fern and angiosperm species (Fig. 3), suggesting that a gradient in xylem vulnerability to embolism is present within leaves. We found that widespread embolism of minor veins often occurred after the bulk of the larger vein orders were embolized, and thus deactivated (Fig. 2 B, D, and F). In contrast to previous studies (22), our observations show that major veins are the first conduits to become damaged by water stress. This pattern of drying from the leaf interior is contrary to that observed in inert porous materials, which initiate embolism from the periphery (23).
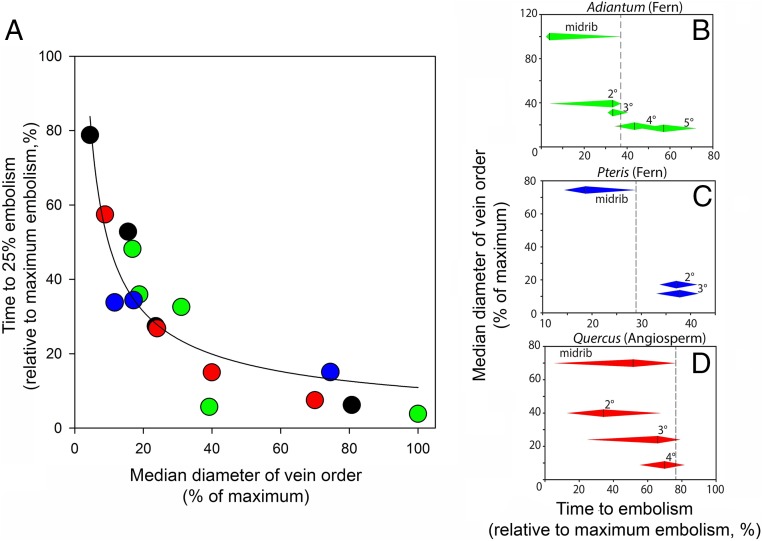
Fig. 3.
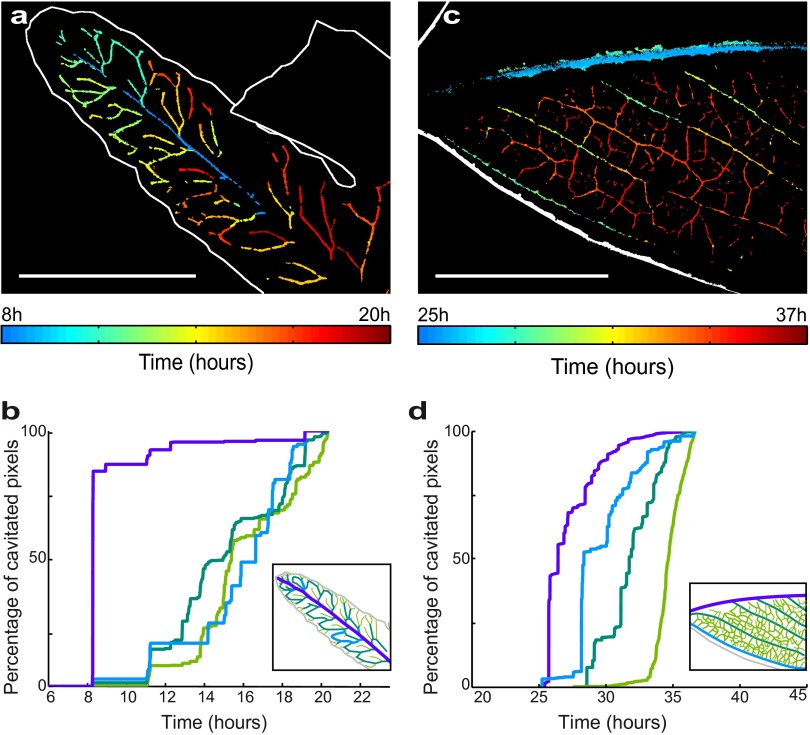
Normalized plot showing that within each leaf, the larger vein diameters are the first to embolize. (A) Relative vein sizes (in each species, the sizes of vein orders are expressed relative to the largest vein size) and time to embolism (in each species, the time for 25% embolism of each vein order is expressed relative to the time to 99% embolism of all veins) are plotted for two ferns (Adiantum, green; Pteris, blue) and two angiosperms (Quercus, red; Eucalyptus, black). The median vein diameter D of the considered order is normalized by Dmax, the maximum vein diameter in the corresponding leaf. The time T25 to reach 25% of vein order embolism is normalized using (T25 − Tmin)/(Tmax − Tmin), where Tmin and Tmax are times for 1% and 99% embolism in the whole leaf, respectively). A best-fit regression with the equation T25 = k/D is fitted to all points. (B–D) Overlap between vein orders in terms of timing of embolism is shown for species with contrasting vein architectures (note that axes are inverted compared with A to emphasize functional overlap between veins). The dotted line shows the point in time where the midrib is completely cavitated, and leaves are no longer able to function. Diamonds for each vein order extend between times for 25th–75th percentile embolism for the particular vein order.
In all species, we observed a consistent relationship between relative embolism vulnerability (as represented by time to reach 25% of total embolism in each vein order) and vein size in different vein orders (Fig. 3). The form of this relationship was close to T = k/D (where T = time to 25% embolism and D = vein order diameter). Relating this timing of embolism to the properties of the xylem conduits, which are bundled together to form the leaf veins (24), is simplified by the observation of a consistent scaling relationship between mean conduit diameter and vein diameter in leaves (25). Thus, if the sizes (d) of air-seeding pores in xylem membranes [through which air enters a conduit during embolism (21)] are proportional to conduit size (26), and the water tension (P) in the xylem is a linear function of drying time, then an inverse proportionality between T and D would be predicted by the Young-Laplace equation that states an inverse proportionality between P and d. Given that a gas–liquid interface presents a surface tension σ, this interface can seed an embolism through an opening of size d by curving its shape with a curvature radius d/2, which requires a tension −P = 2σ/(d/2), according to the Young-Laplace equation. The observed relationship between vein size and resistance to embolism within leaves supports the idea of an efficiency–safety trade-off within the leaf network, whereby larger conduits that transport water more efficiently are more exposed to loss of function by embolism. Thus, the scaling between conduit size and vulnerability within each leaf appears to mirror the larger-scale pattern observed among species from diverse plant lineages (27).
In terms of function, it was surprising to observe a progression of damage from larger upstream (supply) nodes to smaller downstream (delivery) nodes during exposure to water stress, because this is counterintuitive in terms of maintaining a functional vein network poststress. Early cavitation of upstream venation means downstream venation becomes redundant once disconnected from the water source (in this case, the stem of the plant), leading to catastrophic desiccation unless the connection can be restored. Nevertheless, we found that the same size–vulnerability scaling rules operated in all species measured, despite the fact that other xylem anatomical features such as conduit length are very different among leaves (28). It should be noted that the absolute relationships between embolism vulnerability and leaf vein size are only likely to be conserved within species (9), but the within-leaf scaling pattern among diverse lineages observed here appears to be a general rule.
Network architecture was observed to play a major role in the propagation of embolism. Within our small sample of fern and angiosperm species, we found three types of network architecture that produced contrasting patterns of embolism, leading to contrasting characteristics of network safety. We have classified these architectures according to the presence or absence of hierarchy in veins (i.e., the presence of several classes, or vein orders, with a clear distinction in size with respect to each other) and their reticulation (i.e., reconnections of veins after branching points).
SI Methods
Plant Material.
Five species were chosen to encompass the broad range of network architectures in vascular plants. Leaves of fern species were all grown in a nonheated glasshouse at the University of Tasmania in Hobart, Australia, whereas angiosperms were collected from adult trees growing on the grounds. Experiments were conducted between October 2014 and March 2015. Samples were collected from well-watered plants early in the morning to ensure low levels of native embolism. Branches were collected in the morning half an hour before the experiment, at the most. To prevent an invasion of air from the cut edge of the plant, vessel lengths were measured by air-injection, and branches were cut more than two times longer than the longest vessel observed entering the petiole. This required very long branches in Eucalyptus (2 m). All measurements were made on leaves at the distal ends of these cut branches.
Recordings.
Except for Pteris, samples were examined under a stereomicroscope microscope to capture the tiny changes in the refraction index on the leaves resulting from embolism events. Adiantum (shown in Figs. 1 and 2 A and B), Quercus, Eucalyptus, and Goniophlebium samples were imaged using a stereomicroscope (Leica M205A) or a 4-Mpixel CCD Camera (Allied Vision Technology, Model Pike 421B) with a macro lens. Frames were captured every 15, 30, and 60 s, depending on the rate of leaf drying. Leaves of the fern Pteris were too large to fit under the microscope, so they were scanned using a transparent film scanner (Canon 8800F) every 2 min. In all cases, leaves were fixed in position using adhesive tape to prevent any movement between frames. Image sequences were terminated when embolism was no longer observed in the vein network.
Image Analysis.
Step 1: image difference.
If necessary, we first converted the color image sequences to 8-bit grayscale. In the original recording (Fig. S1A), the change of color resulting from the air invasion appears as a darker tone and is barely visible. To highlight changes in light transmission, we used ImageJ (NIH) to subtract successive pairs of images, such that identical regions of the images would yield a black result, whereas regions of change would be highlighted as white (Fig. S1B). If necessary, we enhanced the contrast of this processed sequence of images.
Step 2: localizing embolisms.
We used ImageJ to detect the embolism events from the previous images sequence. To increase the difference between noise and the embolism events, we applied a smoothing filter. This filter replaces each pixel with the average of the neighboring 3 × 3-pixel region. Next we carefully thresholded the image sequence by choosing a threshold that kept the maximum of events and minimized noise. If necessary, we cleaned the residual noise, using Analyze Particles, and removed small artifacts (Fig. S1C).
Because of the thickness of the midrib in Eucalyptus experiments, we first had to perform two separate thresholds for the leaf and the midrib to capture the less visible events in the midrib, and then recombine the sequences.
Step 3: spatiotemporal embolism maps.
We sought to quantify both the location and timing of embolism in the leaf vein network by creating color-coded maps from the processed images sequences of each species. First, we colored the thresholded image sequence with a color corresponding to the time elapsed (Fig. S1D), using the software MATLAB (Mathworks). Next, we overlaid images onto a single projection (Fig. S1E), retaining the color from the first embolism, where multiple changes were observed in single pixels.
Step 4: quantifying embolism in vein orders.
We used the projection of all embolism events as a template for the full vein network. These network templates were manually colored with a different color for each order to obtain a mask of orders (Fig. S1F).
Different vein orders were defined as follows: For ferns, orders are defined from the extremities, increasing as each bifurcation. In the case of two veins with different orders reconnecting, we increase the lowest order by one. In the case of Pteris and Goniophlebium, we also differentiated the midrib from the rest of the leaf. For angiosperms, orders are defined the usual way from the petiole. For Quercus, we counted the midrib (1°), primary branching side veins (2°), and larger (3°) and smaller (4°) minor venation. For Eucalyptus, we counted the midrib (1°), marginal veins, primary branching side veins (2°), and tertiary venation (3°). Inserts in Fig. 2 B, D, and E and in Fig. S2 B and D show the differentiation of vein orders.
Fig. S2.
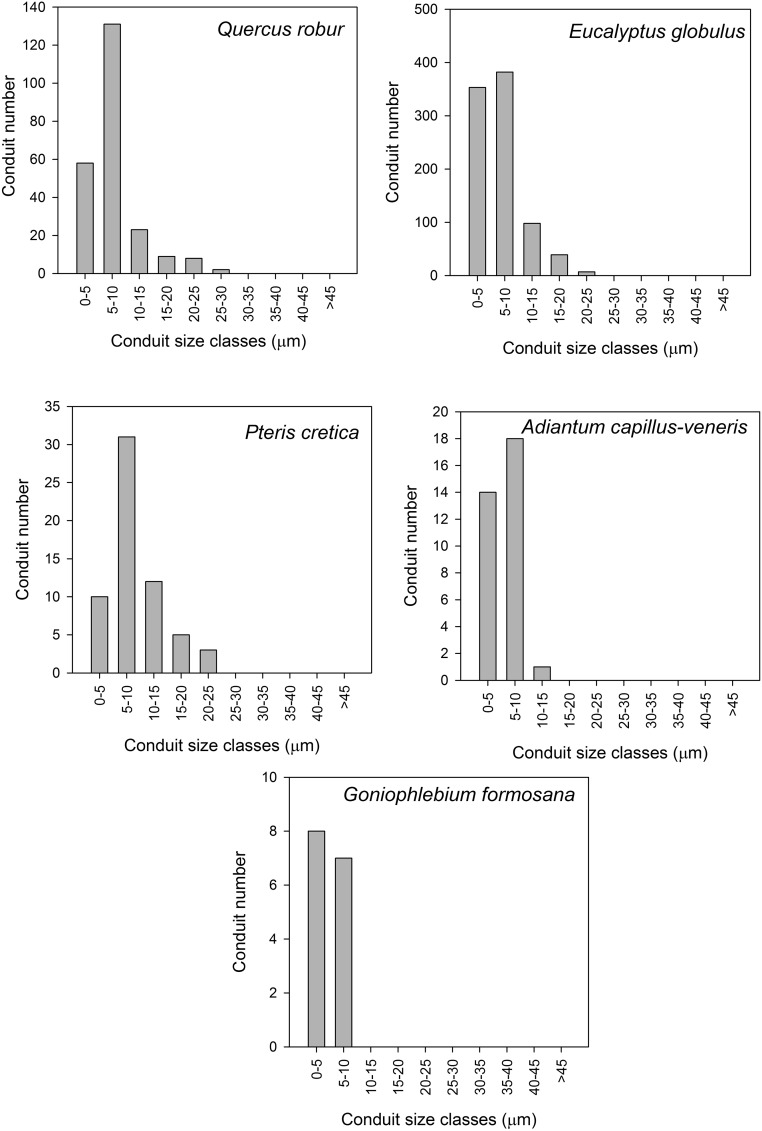
Conduit size histograms for the single largest vein in the leaf of each species measured.
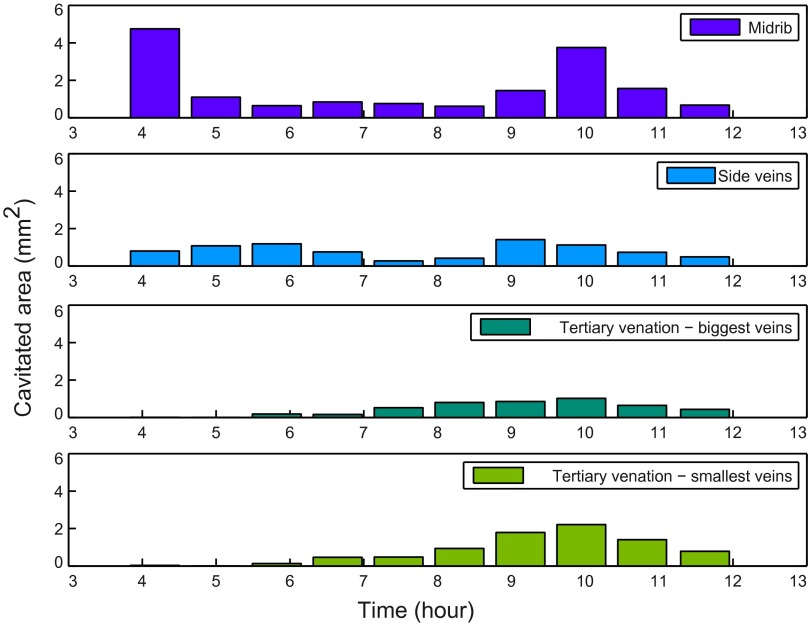
We multiplied the thresholded image sequence by the order-network using ImageJ, so the events, initially white, adopted the color of the order. For each image, we counted the number of pixels in each order by counting the number of pixel of the corresponding color. Fig. S5 shows this result in a histogram form. We also calculated the cumulative pixels depending on time as shown in Fig. 2 B, D, and E and Fig. S2 B and D).
Fig. S5.
Histogram of cavitated area versus time for different orders in Quercus robur. Data from the same leaf as Fig. 2F, but showing actual cavitated area rather than relative embolism in different orders.
Step 5: size of veins.
Using ImageJ, we measured the size of the veins on the first image of the recording for Adiantum, Pteris, and Goniophlebium. For Quercus and Eucalyptus, because of the thickness of the leaves, we measured the size of veins from images of the leaf after bleaching and staining postembolism. Thus, leaves were soaked in household bleach until transparent and then washed and stained with toluidine blue, which stains the lignin walls of the xylem cells. Cross sections of the primary vein supplying each leaf were made, using a microtome with a freezing stage. These sections were stained with toluidine blue to identify xylem cells and photographed at 10× magnification on a compound microscope. All xylem conduits >10 µm were counted.
Nonhierarchical, Nonreticulate Venation (as in Adiantum: Fern; Fig. 2 A and B)
This represents the earliest type of leaf vasculature observed in the first vascular land plants (29), and today only in gymnosperms, ferns, and lycophyte relatives of early-diverging tracheophyte clades. This category describes topologies in which veins are typically linear, tapering and bifurcating without reconnections. Because of the tapering in venation from leaf base to tip, we observed that embolism always initiated in the source node of the network and progressed to the tip. In Adiantum, the largest vein contained few xylem conduits with a narrow size range (Fig. S2), leading to rapid loss of function during embolism because distal veins were immediately severed from the supply node (Figs. 2 A and B and 3B). This network type was highly prone to the formation of very large embolism events that deactivated entire pinnae in single embolism events (Figs. S3B and S4).
Fig. S3.
Embolism maps and progression of embolism for Goniophlebium formosana (A and B), a fern with reticulate venation but weak hierarchical organization, leading to early embolism of the midrib relative to minor veins. In contrast, the angiosperm Eucalyptus globulus (C and D) has strong hierarchical organization with overlapping embolism profiles for different orders, preserving vascular function during embolism. For Goniophlebium, the orders are defined from the extremities (like Adiantum), and the midrib is defined separately. (Scale bars, 10 mm.)
Fig. S4.
Embolism in whole leaves. (A) Catastrophic failure in a frond of Adiantum. An embolism quickly invades the quasi-totality of the vein network in 3 s. (Scale bar, 10 mm.) (B) Embolism map for a whole Quercus leaf. A high degree of spatial homogeneity in the timing of minor vein embolism can be seen, likely because of an overlap in the range of vulnerability present in larger vein orders. (Scale bar, 100 mm.)
Hierarchical, Nonreticulate Venation (as in Pteris: Fern)
Common among ferns, but never occurring in flowering plants, this topology also describes networks with linear tapering veins, but vein sizes are disjunct, leading to “major” and “minor” vein size classes. In the fern Pteris, we found that embolism in the large midrib occurred before in the minor veins, and that there was no overlap in the size or the embolism sequence of these different orders (Fig. 2 C and D). As a result, embolism in minor veins occurred randomly along the length of the leaf, but most minor veins were cut off from water supply (by midrib embolism) before they reached their embolism threshold (Fig. 3B).
Hierarchical, Reticulate Network (as in Quercus, Eucalyptus: Angiosperms)
This type of venation network is present in all modern angiosperms, but also in some fern species. Veins typically branch unequally, leading to loopiness and reconnection between neighboring veins and among vein orders. In our sample leaves, we found considerable overlap in the embolism profiles of all vein orders (Figs. 2 E and F, 3D, and Fig. S3 C and D), probably because of the large number and size range of xylem vessels that made up major veins. Of particular note was the fact that the midrib maintained a degree of functionality throughout much of the embolism of the network (Table S1 and Fig. S5). The extended functionality of the midrib, in combination with the reticulation of vein orders, explains the observation that embolism of minor veins occurred homogeneously across the leaf surface despite being preceded by large embolism events in the major veins.
Table S1.
Systematic and leaf-specific data for the five species sampled
| Species | Group | Family | Leaf/Pinna size (cm2) | Conduits in 1° vein |
| Adiantum capillus veneris | Fern | Pteridaceae | 0.29 | 33 |
| Pteris cretica | Fern | Pteridaceae | 9.98 | 58 |
| Goniophlebium formosanum | Fern | Polypodiaceae | 0.49 | 15 |
| Eucalyptus globulus | Angiosperm tree | Myrtaceae | 47.55 | 879 |
| Quercus robur | Angiosperm tree | Fagaceae | 28.20 | 231 |
The sum of xylem conduits in the base of the midrib in each leaf or pinna is shown for xylem conduits >3 µm.
The three patterns described here span the range of evolved possibilities among leaf vascular supply networks, yet the general principal of size-dependent scaling of network nodes with respect to embolism vulnerability remained consistent throughout. Thus, we propose that this rule is conserved among vascular plants and that it provides a reliable means to predict the behavior of leaf networks during water stress, based on their size structure. These vein size structures are extremely diverse among leaves, yet they appear to be conserved within species (30), thus providing critical information about leaf adaptation to water stress in both living and fossil plant species (31).
Ultimately, the progression of embolism within the leaf network must be a combination of the vein network topology and the structure of the xylem conduits that make up the leaf veins (Fig. S2). Although our data suggest significant relationships between conduit size and embolism vulnerability within leaves, it is not expected that a general relationship exists between species. Furthermore, it is unknown whether differences in embolism propagation observed in the sampled fern and angiosperm leaves occurred as a result of peculiarities of the fern xylem (an absence of vessels in leaves) or because of size distributions of conduits between leaf network nodes. Despite these uncertainties, the general size-dependent pattern of embolism spread within leaf veins remained consistent among species, thus providing a strong foundation for future study.
In terms of maintaining supply under water stress, the reticulate networks represented by Quercus and Eucalyptus performed better than the fern species (even compared with ferns with reticulate venation; Fig. S3 A and B and Movie S4), in the sense that minor veins failed randomly whereas the rest of the network retained some connection to the source node (the petiole). Minor veins tended to embolize only once, presumably terminating hydraulic connection to the downstream leaf tissue (Movie S4). The fact that these minor veins are the last to lose function means that as long as some nonembolized conduits remain in the larger veins, the supply of water to the mesophyll can continue until the minor veins fail. The result is a gradual decline, rather than a catastrophic decline in water transport efficiency, as larger (major) veins begin to embolize during acute water stress (9). Thus, the dense reticulation of the minor venation in angiosperms (which appears at first glance to be an inefficient redundancy) represents an efficient means of bypassing local disruption in major veins and avoiding rapid and catastrophic hydraulic failure when leaves are drought stressed (32). In addition, if it is assumed that the production of embolism resistance comes at a cost in terms of construction (33) and transport efficiency (26, 34), then the optimal network architecture occurs when there is overlap in the vulnerability of different nodes (thus avoiding both catastrophic failure and excessive redundancy). Overlapping vulnerability of network nodes in Quercus and Eucalyptus was no doubt aided by the composite structure of the larger veins (Fig. S2) in these species, which contained hundreds of connected xylem conduits spanning a large range of sizes and, presumably, vulnerabilities (Table S1 and Fig. S2). A degree of hydraulic isolation between conduits seems to prevent embolisms from spreading immediately between neighboring conduits, particularly in the radial direction (35, 36). In artificial networks, the failure of major nodes is the source of a major disruption; the lesson we learn from these natural networks it that a gradual decay of the main nodes, instead of an on–off transition, is the key to extended functionality.
The simplicity of the imaging approach we describe here brings questions about hydraulic failure within the reach of any research group with access to basic imaging tools. Thus, our study provides the departure point for a new generation of research into the evolution and function of biological networks in leaves and in roots, where high transparency will also allow quantification of xylem embolism.
Supplementary Material
Acknowledgments
P.M. acknowledges financial support from the University of Tasmania for a visiting scholar program and European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007-2013) ERC Grant Agreement Bubbleboost 614655.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522569113/-/DCSupplemental.
References
- 1.Sack L, Holbrook NM. Leaf hydraulics. Annu Rev Plant Biol. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- 2.Boyce CK, Zwieniecki MA. Leaf fossil record suggests limited influence of atmospheric CO2 on terrestrial productivity prior to angiosperm evolution. Proc Natl Acad Sci USA. 2012;109(26):10403–10408. doi: 10.1073/pnas.1203769109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodribb TJ, Feild TS, Jordan GJ. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007;144(4):1890–1898. doi: 10.1104/pp.107.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:19–38. [Google Scholar]
- 5.Hacke UG, Sperry JS, Wheeler JK, Castro L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006;26(6):689–701. doi: 10.1093/treephys/26.6.689. [DOI] [PubMed] [Google Scholar]
- 6.Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc Biol Sci. 2009;276(1663):1771–1776. doi: 10.1098/rspb.2008.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodribb TJ, Feild TS. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol Lett. 2010;13(2):175–183. doi: 10.1111/j.1461-0248.2009.01410.x. [DOI] [PubMed] [Google Scholar]
- 8.Jansen S, Choat B, Pletsers A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am J Bot. 2009;96(2):409–419. doi: 10.3732/ajb.0800248. [DOI] [PubMed] [Google Scholar]
- 9.Brodribb TJ, et al. Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytol. 2016;209(4):1403–1409. doi: 10.1111/nph.13846. [DOI] [PubMed] [Google Scholar]
- 10.Brodersen CR, et al. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiol. 2013;161(4):1820–1829. doi: 10.1104/pp.112.212712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyree MT, Sperry JS. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress?: Answers from a model. Plant Physiol. 1988;88(3):574–580. doi: 10.1104/pp.88.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ. 2013;36(11):1938–1949. doi: 10.1111/pce.12139. [DOI] [PubMed] [Google Scholar]
- 13.Choat B, Brodersen CR, McElrone AJ. Synchrotron X-ray microtomography of xylem embolism in Sequoia sempervirens saplings during cycles of drought and recovery. New Phytol. 2015;205(3):1095–1105. doi: 10.1111/nph.13110. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg U, et al. Grapevine petioles are more sensitive to drought induced embolism than stems: Evidence from in vivo MRI and microCT observations of hydraulic vulnerability segmentation. Plant Cell Environ. December 9, 2016 doi: 10.1111/pce.12688. [DOI] [PubMed] [Google Scholar]
- 15.Sperry JS, Love DM. What plant hydraulics can tell us about responses to climate-change droughts. New Phytol. 2015;207(1):14–27. doi: 10.1111/nph.13354. [DOI] [PubMed] [Google Scholar]
- 16.Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang D-U. Complex networks: Structure and dynamics. Phys Rep. 2006;424(4–5):175–308. [Google Scholar]
- 17.Albert R, Albert I, Nakarado GL. Structural vulnerability of the North American power grid. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69(2 Pt 2):025103. doi: 10.1103/PhysRevE.69.025103. [DOI] [PubMed] [Google Scholar]
- 18.Cohen R, Erez K, ben-Avraham D, Havlin S. Resilience of the internet to random breakdowns. Phys Rev Lett. 2000;85(21):4626–4628. doi: 10.1103/PhysRevLett.85.4626. [DOI] [PubMed] [Google Scholar]
- 19.Ponomarenko A, et al. Ultrasonic emissions reveal individual cavitation bubbles in water-stressed wood. J R Soc Interface. 2014;11(99):20140480. doi: 10.1098/rsif.2014.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katifori E, Szöllosi GJ, Magnasco MO. Damage and fluctuations induce loops in optimal transport networks. Phys Rev Lett. 2010;104(4):048704. doi: 10.1103/PhysRevLett.104.048704. [DOI] [PubMed] [Google Scholar]
- 21.Cochard H, Cruiziat P, Tyree MT. Use of positive pressures to establish vulnerability curves : Further support for the air-seeding hypothesis and implications for pressure-volume analysis. Plant Physiol. 1992;100(1):205–209. doi: 10.1104/pp.100.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salleo S, Lo Gullo MA, Raimondo F, Nardini A. Vulnerability to cavitation of leaf minor veins: Any impact on leaf gas exchange? Plant Cell Environ. 2001;24(8):851–859. [Google Scholar]
- 23.Vincent O, Sessoms DA, Huber EJ, Guioth J, Stroock AD. Drying by cavitation and poroelastic relaxations in porous media with macroscopic pores connected by nanoscale throats. Phys Rev Lett. 2014;113(13):134501. doi: 10.1103/PhysRevLett.113.134501. [DOI] [PubMed] [Google Scholar]
- 24.Tyree MT, Zimmermann MH. Xylem Structure and the Ascent of Sap. Springer; Berlin: 2002. [Google Scholar]
- 25.Coomes DA, Heathcote S, Godfrey ER, Shepherd JJ, Sack L. Scaling of xylem vessels and veins within the leaves of oak species. Biol Lett. 2008;4(3):302–306. doi: 10.1098/rsbl.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lens F, et al. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 2011;190(3):709–723. doi: 10.1111/j.1469-8137.2010.03518.x. [DOI] [PubMed] [Google Scholar]
- 27.Brodersen C, Jansen S, Choat B, Rico C, Pittermann J. Cavitation Resistance in Seedless Vascular Plants: The Structure and Function of Interconduit Pit Membranes. Plant Physiol. 2014;165(2):895–904. doi: 10.1104/pp.113.226522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comstock JP, Sperry JS. Tansley Review No. 119. Theoretical Considerations of Optimal Conduit Length for Water Transport in Vascular Plants. New Phytol. 2000;148(2):195–218. [Google Scholar]
- 29.Boyce CK, Knoll AH. Evolution of developmental potential and the multiple independent origins of leaves in Paleozoic vascular plants. Paleobiology. 2002;28(1):70–100. [Google Scholar]
- 30.Hickey LJ. Classification of the architecture of dicotyledonous leaves. Am J Bot. 1973;60(1):17–33. [Google Scholar]
- 31.Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. Evolution and function of leaf venation architecture: A review. Ann Bot (Lond) 2001;87(5):553–566. [Google Scholar]
- 32.Bejan A. Constructal tree network for fluid flow between a finite-size volume and one source or sink. Rev Gen Therm. 1997;36(8):592–604. [Google Scholar]
- 33.Hacke U, Sperry JS, Pockman WT, Davis SD, McCulloch A. Trends in wood density and structure are linked to the prevention of xylem implosion by negative pressure. Oecologia. 2001;126(4):457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- 34.Pittermann J, et al. The relationships between xylem safety and hydraulic efficiency in the Cupressaceae: The evolution of pit membrane form and function. Plant Physiol. 2010;153(4):1919–1931. doi: 10.1104/pp.110.158824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlquist S. Vessel grouping in dicotyledon wood: Significance and relationship to imperforate tracheary elements. Aliso. 1984;10(4):505–525. [Google Scholar]
- 36.Brodersen CR, et al. Xylem vessel relays contribute to radial connectivity in grapevine stems (Vitis vinifera and V. arizonica; Vitaceae) Am J Bot. 2013;100(2):314–321. doi: 10.3732/ajb.1100606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.