Significance
Cytotoxic T lymphocyte antigen-4 (CTLA-4), up-regulated by activated conventional T cells and constitutively expressed by Foxp3+ regulatory T cells, is essential for immunological self-tolerance. Because of early fatality in germ-line CTLA-4–deficient mice, the role in adulthood remains unclear. We abrogated CTLA-4 expression in adult mice and compared their phenotype with congenitally CTLA-4–deleted mice. We found that the two modes of CTLA-4 deficiency differed in resulting autoimmune disease phenotype and severity. Additionally, although CTLA-4–deficient mice had more severe collagen-induced arthritis, they were protected against peptide-induced experimental autoimmune encephalomyelitis (EAE). However, onset of protein-induced EAE was only delayed. These results taken together indicate that CTLA-4 deficiency affects both central and peripheral tolerance and Treg cell-mediated suppression.
Keywords: CTLA-4, autoimmunity, tolerance, regulatory T cells, Foxp3
Abstract
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is essential for immunological (self-) tolerance, but due to the early fatality of CTLA-4 KO mice, its specific function in central and peripheral tolerance and in different systemic diseases remains to be determined. Here, we further examined the role of CTLA-4 by abrogating CTLA-4 expression in adult mice and compared the resulting autoimmunity that follows with that produced by congenital CTLA-4 deficiency. We found that conditional deletion of CTLA-4 in adult mice resulted in spontaneous lymphoproliferation, hypergammaglobulinemia, and histologically evident pneumonitis, gastritis, insulitis, and sialadenitis, accompanied by organ-specific autoantibodies. However, in contrast to congenital deficiency, this was not fatal. CTLA-4 deletion induced preferential expansion of CD4+Foxp3+ Treg cells. However, T cells from CTLA-4–deficient inducible KO mice were able to adoptively transfer the diseases into T cell-deficient mice. Notably, cell transfer of thymocytes de novo produced myocarditis, otherwise not observed in donor mice depleted in adulthood. Moreover, CTLA-4 deletion in adult mice had opposing impacts on induced autoimmune models. Thus, although CTLA-4–deficient mice had more severe collagen-induced arthritis (CIA), they were protected against peptide-induced experimental autoimmune encephalomyelitis (EAE); however, onset of protein-induced EAE was only delayed. Collectively, this indicates that CTLA-4 deficiency affects both central and peripheral tolerance and Treg cell-mediated suppression.
Cytotoxic T lymphocyte antigen-4 (CTLA-4), a member of the CD28 family, is a vital coinhibitory molecule, up-regulated by activated conventional T cells (Tconv cells) and constitutively expressed by Foxp3+ Treg cells (1–3). Germ-line haploinsufficiency of CTLA-4 causes lymphoproliferation and autoimmunity in humans (4, 5), and CTLA-4 polymorphisms are associated with several human autoimmune-disorders, including Graves disease (6), systemic lupus erythematosus (7), type I diabetes (8), and rheumatoid arthritis (6, 9). Moreover, anti–CTLA-4 antibody (Ab) treatment enhances tumor immunity in metastatic melanoma patients (10) but also elicits autoimmunity (11, 12). In mice, Ctla4 germ-line deficiency produces a lymphoproliferative disorder hallmarked by multiorgan lymphocyte infiltrations, especially in the heart and pancreas, that is lethal 3–4 wk after birth (13, 14). Moreover, CTLA-4 deficiency specifically in Foxp3+ Treg cells is sufficient to cause lymphoproliferation and autoimmune diseases including myocarditis, which is fatal at around 8 wk of age. It also leads to enhanced tumor immunity in vivo and abrogated suppressive function induced by allo-antigen in vitro. These findings collectively indicate CTLA-4 as a key molecule for Treg cell-mediated suppression (15). However, recent reports have shown that ctla-4–deficient Treg cells exert effective suppression in mouse models of colitis and experimental autoimmune encephalomyelitis (EAE), whereas they failed to be suppressive in an adoptive transfer model of type I diabetes (16–19). These apparently conflicting results suggest that the role of CTLA-4 might vary depending on disease condition.
CTLA-4 has been shown to control immune activation via both cell autonomous and nonautonomous pathways (20). Autonomous mechanisms include among others internal signaling that leads to cell cycle arrest (21, 22), as well as competition with CD28 for interaction with CD80 and CD86 on antigen presenting cells (APCs) (23, 24). In contrast, several studies show that CTLA-4–sufficient T cells, primarily Treg cells, can keep other T cells inert (1, 25–28). This nonautonomous CTLA-4–mediated regulation is at least partly dependent on control of the antigen presenting capacity of the APCs by CD80 and CD86 modulation (29, 30) or by induction of the immune suppressive agent kynurenine (31). Apart from peripheral tolerance, it is controversial whether CTLA-4 has a role in central tolerance and selection of thymic T cells and Foxp3+ Treg cells. Some studies have not detected any differences (19, 32), whereas others have reported an impact on negative selection (27, 33–35). CTLA-4 has also been suggested to affect thymic quantitative output of Foxp3+ Treg cells and to broaden the T-cell receptor (TCR) repertoire of Tconv cells in TCR transgenic mice specific for myelin basic protein (17, 36). Taken together, the function of CTLA-4 is complex and likely operates by different mechanisms on different levels of tolerance via different cell populations. Hence better-defined ways to study CTLA-4 in vivo are needed to elucidate its multiple roles.
To this end, we bred mice in which ctla-4 can be conditionally deleted by tamoxifen treatment in adulthood, thus circumventing the critical period shortly after birth when murine T cells migrate and populate the periphery. In contrast to a recent report by Paterson et al. (18), who used a similar way of CTLA-4 deletion but saw no overt disease, we found that CTLA-4 deletion in adult mice rapidly induced aberrant immune activation, multiorgan lymphocyte infiltration, and auto-antibody production, but only mice born with CTLA-4 deficiency developed myocarditis and succumbed to fatal pancreatitis. Furthermore comparison of protein-induced EAE or collagen-induced arthritis (CIA) with peptide-induced EAE revealed opposing effects of CTLA-4 deletion in adulthood. Collectively, our results show that abrogation of CTLA-4 expression in adult mice induces autoimmune diseases in otherwise unmanipulated mice, that CTLA-4 has a role in regulating both central and peripheral tolerance, and that its function in autoimmune diseases differs depending on underlying disease-specific mechanisms.
Results
CTLA-4 Depletion in Adult Mice Produces Lymphoproliferation and Autoimmunity.
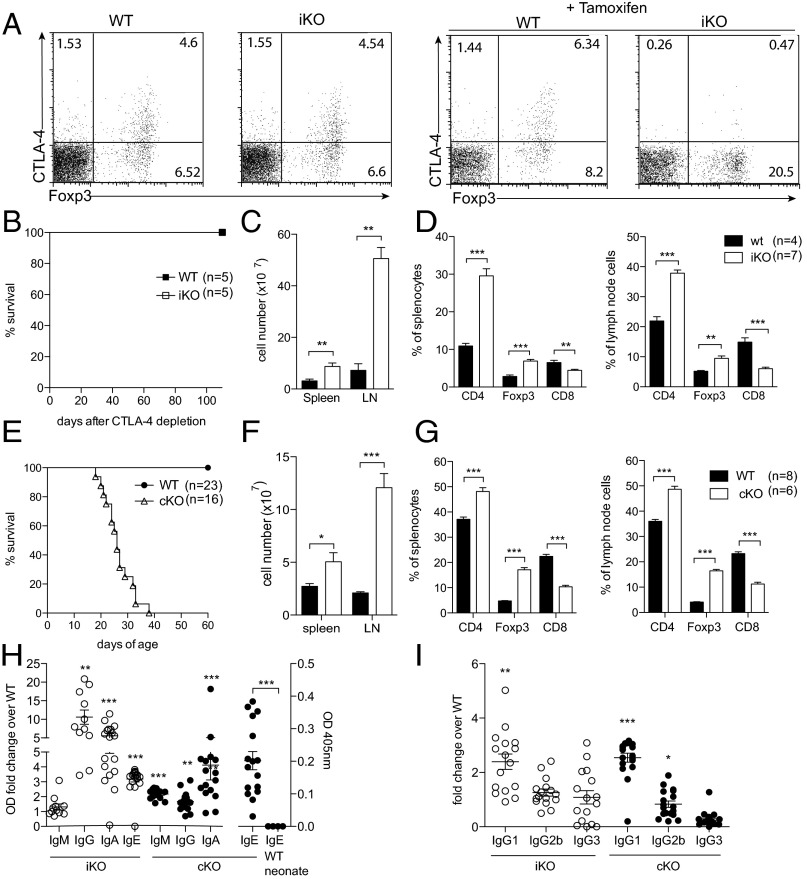
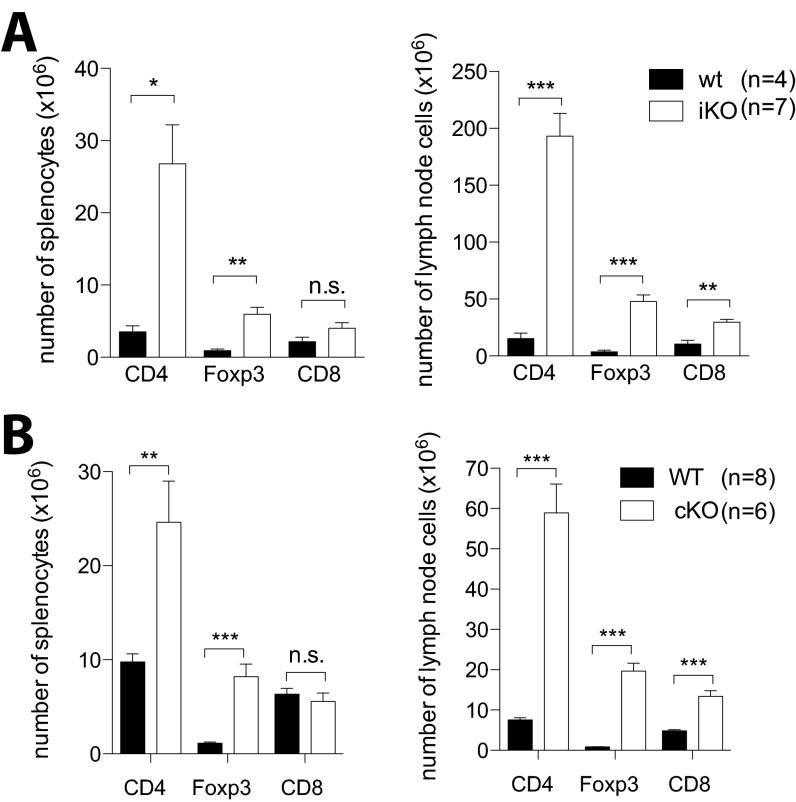
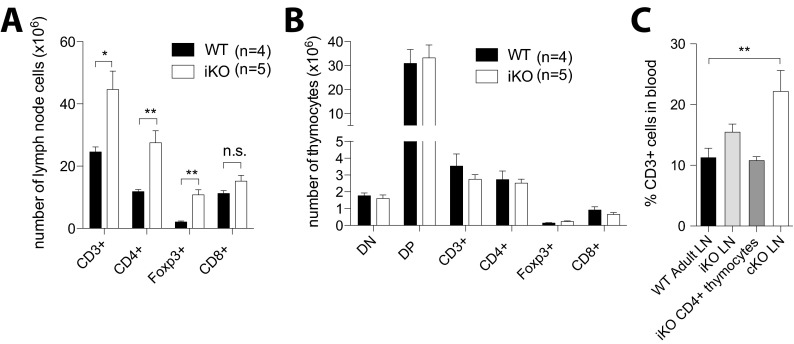
To study CTLA-4 in a mature adult, yet naïve immune system, we crossed mice with a floxed Ctla-4 gene (i.e., CTLA-4fl/fl) (15) to mice possessing the tamoxifen-inducible Cre recombinase gene at the Rosa26 locus (i.e., Rosa26Cre/ERT2+). Before Cre recombinase induction by tamoxifen, CTLA-4 levels were equivalent between Rosa26Cre/ERT2+/−CTLA-4fl/fl mice, hereafter designated CTLA-4 inducible knockout (iKO) mice, and Rosa26Cre/ERT2−/−CTLA-4fl/fl mice, hereafter called WT littermates, both on the C57BL/10.Q genetic background. To induce CTLA-4 deletion, 6- to 8-wk-old mice were treated with tamoxifen three times (days 0, 1, and 5). After the second injection, CTLA-4 expression was reduced by 60% in Foxp3+ Treg cells; complete and permanent CTLA-4 depletion was achieved by day 6 in all T cells, whereas CTLA-4 levels and cell composition in WT controls were unaffected (Fig. 1A). Because germ-line Ctla-4 KO mice succumb to a fatal lymphoproliferative syndrome before weaning age, iKO mice were monitored for long-term survival (Fig. 1B). The treated iKO mice survived more than 100 d, whereas congenital CTLA-4 KO mice produced by crossing CD4Cre mice with CTLA-4fl/fl mice (hereafter called cKO mice) had to be killed around 21 d of age due to a wasting disease (Fig. 1 B and E). However, both iKO and cKO mice were found to suffer from massive lymphadenopathy and splenomegaly (Fig. 1 C and F). Lymphoproliferation was mainly due to an increase in CD4+ T-cell and CD4+Foxp3+ Treg cell frequencies and absolute numbers, whereas CD8+ T cells decreased in frequency but not in absolute numbers (Fig. 1 D and G and Fig. S1).
Fig. 1.
CTLA-4 deletion in adult mice produces lymphoproliferation and autoimmunity similar to congenital CTLA-4 deficiency. (A) Representative examples of CTLA-4 levels in WT and iKO mice before and after complete tamoxifen treatment day 6. (B) Survival of iKO mice and WT littermate controls after tamoxifen treatment. (C) Absolute cell numbers in spleen and lymph nodes of iKO and WT littermate controls 8 wk after CTLA-4 depletion. (D) Frequency of CD8+, CD4+, and Foxp3+ T-cell subsets in spleen and lymph nodes of iKO and WT mice after 8 wk of CTLA-4 depletion. (E) Survival of cKO mice and littermate controls after birth. (F) Absolute cell numbers in spleen and lymph nodes of 20- to 22-d-old cKO mice and WT littermate controls. (G) Frequency of CD8+, CD4+, and Foxp3+ T-cell subsets in spleen and lymph nodes of 20- to 22-d-old cKO mice and WT littermate controls. (H) Total IgM, IgG, IgE, and IgA levels in serum of cKO and iKO mice depicted as relative OD fold change over respective WT littermate control except for IgE in cKO mice depicted as OD only because it was not detectable in corresponding WT littermate controls. (I) IgG subclasses depicted as relative OD fold change over control. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Fig. S1.
CTLA-4 deletion in adult mice produces lymphoproliferation and autoimmunity similar to congenital CTLA-4 deficiency. (A) Absolute numbers of CD4+, Foxp3+, and CD8+ T-cell subsets in spleen and lymph nodes of iKO and WT mice after 8 wk of CTLA-4 depletion. (B) Absolute numbers of CD4+, Foxp3+, and CD8+ T-cell subsets in spleen and lymph nodes of 20- to 22-d-old cKO mice and WT littermate controls. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Measurement of serum Ig titers revealed 4- to 10-fold increased levels of IgG, IgA, and IgE, but no increase in IgM in iKO mice, whereas cKO mice had significantly elevated serum titers of IgM, IgG, and IgA but only very low levels of IgE (Fig. 1H). In both iKO and cKO mice, the change in IgG titer was mainly due to increase in IgG1, although a trend toward increased IgG3 in iKO and IgG1 and IgG2b in cKO mice was also seen (Fig. 1I).
Taken together, CTLA-4 deficiency in adult mice produces immunological anomalies such as lymphoproliferation and hyper-immunoglobulinemia, which are similar to congenital and germ-line CTLA-4 deficiency but with some critical differences in phenotype and severity as reflected in different survivals.
Spontaneous Development of Autoimmune Diseases in iKO Mice.
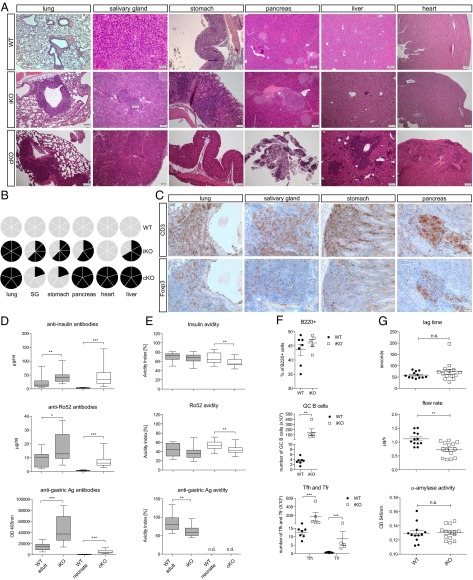
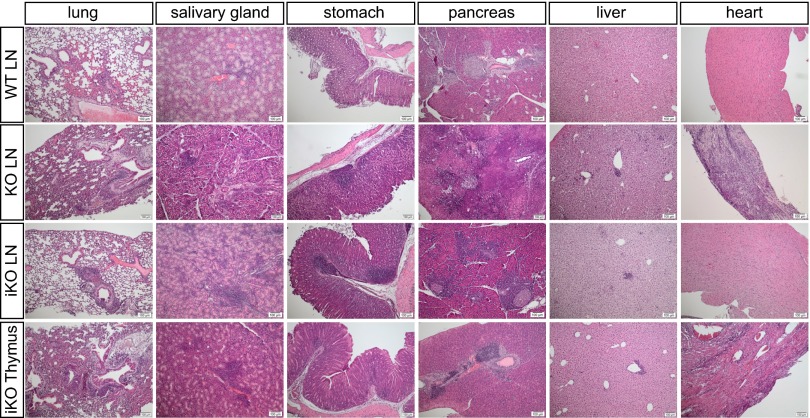
To further investigate the difference between iKO and cKO mice, a detailed pathologic analysis was carried out. Eight weeks after CTLA-4 deletion, iKO mice, but not WT littermate controls, frequently had substantial focal lymphocytic infiltrations around the bronchioles in the lung and also in the salivary gland, pancreatic β-islets (insulitis), and the stomach (Fig. 2 A and B), although heart and kidney were not affected. In contrast, all analyzed cKO mice had heart pathology, and in addition, showed severe destruction of the endocrine and exocrine pancreas (Fig. 2 A and B), which was likely a main cause of the fatal wasting disease observed in cKO mice. Thus, both iKO and cKO mice suffer from pathologies but in distinct sets of organs, which has impact on survival rate.
Fig. 2.
Spontaneous development of autoimmunity in iKO mice. (A) H&E stainings of representative organ sections of WT mice (Top; n = 6), iKO mice 8 wk after CTLA-4 depletion (Middle; n = 6), and cKO newborns at 16 d of age (Bottom; n = 5). (B) Cake diagrams depicting organ infiltrations in all WT (Top), iKO (Middle), and cKO (Bottom) mice. Each cake piece represents one mouse and filled pieces symbolize observed infiltrations. (C) Immunohistochemistry on representative paraffin sections from 8-wk depleted iKO mice stained with anti-CD3 (Upper) and anti-Foxp3 antibody (Lower). Sequential sections are shown and n = 6. (D) Total auto-antibody levels against insulin, Ro52, and gastric Ag in serum of 19- to 22-d-old cKO and >14-wk-old iKO mice and littermate controls. (E) Calculated avidity index of antibodies against insulin, Ro52, and gastric Ag. Tested serum was from 16- to 25-d-old cKO neonates (n = 16) and from 8- to 13-wk depleted iKO mice (n = 18) with age-matched littermate controls (n = 10 WT adult, n = 16 WT neonate). (F) Lag time, saliva flow rate, and amylase activity in iKO mice and WT controls after pilocarpine injection. (G) Frequency of B220+ B cells (Top), germinal center (GC) B cells (Middle), and T follicular helper (Tfh) and T follicular regulatory (Tfr) T cells (Bottom) in spleens of iKO mice and WT littermate controls 4 wk after CTLA-4 depletion. GC B cells were defined as B220+CD38−PNA+, Tfh as CD19−CD4+CXCR5+ICOS+Foxp3−, and Tfr as CD19−CD4+CXCR5+ICOS+Foxp3+. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
More detailed immunohistochemical analysis of affected organs of iKO mice 8 wk after CTLA-4 depletion revealed infiltrations of CD3+ T cells together with Foxp3+ Treg cells (Fig. 2C). Moreover, both CD3+ T cells and Foxp3+ Treg cells could be detected from the earliest stages of organ infiltration, which occurred in lung, salivary gland, and pancreas 2 wk after full CTLA-4 depletion, followed by stomach infiltration 1 wk later (Table S1). Hence, Tconv cells and Treg cells appeared to start to migrate from the lymphoid organs into tissue simultaneously.
Table S1.
Kinetics of organ infiltration in iKO mice
| Week | Affected/total | |||||
| Lung | SG | Stomach | Pancreas | Liver | Heart | |
| 1 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| 2 | 2/3 | 2/3 | 0/3 | 2/3 | 0/3 | 0/3 |
| 3 | 2/3 | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| 4 | 2/3 | 2/3 | 2/3 | 3/3 | 0/3 | 0/3 |
| 8 | 6/6 | 4/6 | 4/6 | 3/5 | 0/3 | 0/3 |
Six- to 8-wk-old mice were depleted of CTLA-4, and organs were analyzed for the presence of lymphocytic infiltrations after 1, 2, 4, and 8 wk. Shown is the number of affected mice out of total mice for each analyzed organ. SG, salivary gland.
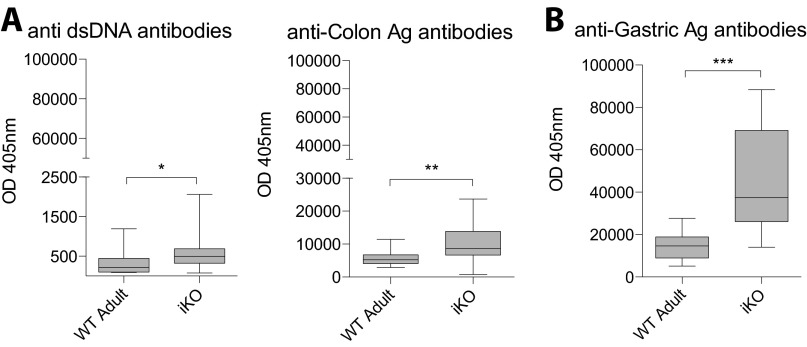
In accordance with the routinely found lymphocytic infiltrations in various organs in iKO and cKO mice, we detected significantly elevated levels of auto-Ab against insulin, gastric antigen, and Ro52 [i.e., tripartite motif-containing protein 21 (TRIM21)], an auto-antigen associated with Sjögren’s syndrome could be readily detected in the sera of both iKO and cKO mice (Fig. 2D). Interestingly, although auto-Ab titers were higher in iKO and cKO animals compared with littermate controls, their avidity remained either unchanged or decreased (Fig. 2E). Notably, anti-dsDNA and anti-colon lysate antibodies, which represent organs with no apparent pathology, were also detected in iKO mice. However, these levels were far below those of insulin, Ro52, and gastric antigen (Fig. S2). CTLA-4 has been shown to directly regulate germinal center responses by influencing T follicular helper (Tfh) and regulatory (Tfr) populations, and indeed, iKO mice had significantly increased numbers of germinal center B cells, Tfh, and Tfr in the spleen 4 wk after depletion, whereas total B-cell frequencies were not yet affected (Fig. 2F).
Fig. S2.
Spontaneous development of autoimmunity in iKO mice. (A) iKO mice do not suffer from lymphocytic infiltrations in kidneys or colon but total auto-antibody levels against dsDNA and colon Ag are elevated in serum of 8-wk depleted iKO mice compared with WT littermate controls (n = 10 WT adult, n = 18 iKO). As a comparison for the difference in titers between infiltrated vs. noninfiltrated organs, (B) auto-antibody levels against gastric Ag are shown. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Finally, as sialadenitis, elevated auto-Ab against Ro52, and dry mouth are all signs of human Sjögren’s syndrome and the first two were observed in iKO mice, we investigated salivary gland function using pilocarpine, which enhances saliva secretion. Although neither lag time nor salivary α-amylase activity was altered, the salivary flow rate was significantly reduced in iKO mice (Fig. 2G).
Thus, both iKO and cKO mice developed histologically evident autoimmune diseases accompanied by specific autoantibodies and functional deficits of affected organs.
Activation of Treg Cells and Tconv Cells by CTLA-4 Deletion.
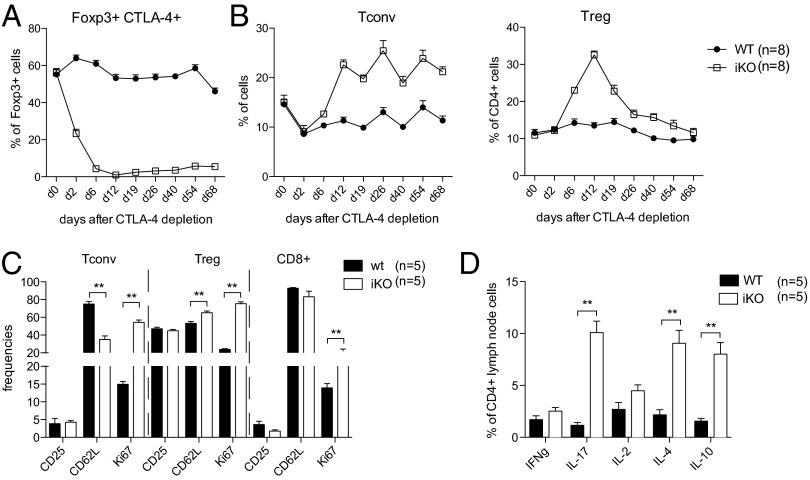
To investigate the earliest events that result from loss of CTLA-4 in vivo, we analyzed the frequency of Tconv cells and Treg cells in the blood after tamoxifen administration. As mentioned above, our depletion strategy resulted in a gradual reduction of CTLA-4 levels (Fig. 3A). By day 2, CTLA-4 levels on Treg cells were decreased by ∼60–80%, but Tconv cell and Treg cell frequencies were unaffected (Fig. 3B). However, by day 6 when CTLA-4 was fully deleted, Foxp3+ Treg cells had already increased twofold, and at the peak, day 12, up to 30% of total CD4+ T cells expressed Foxp3. Thereafter, Treg cell frequencies rapidly decreased in blood and with time approached normal levels (Fig. 3B). These results were mirrored in lymph nodes (LNs) and spleen, although here Treg cell levels remained continuously elevated (Fig. S3). Interestingly, Tconv cells showed delayed proliferation kinetics compared with Treg cells and did not increase in frequency until after day 6 in blood and peripheral lymphoid organs (Fig. 3B and Fig. S3). The early expansion of Treg cells compared with Tconv cells resulted in a dramatic change in ratio in favor of Treg cells. Notably, CTLA-4 depletion led to a rapid and general immune activation as Tconv cells had an activated phenotype (CD25+CD62Llow) and were highly proliferative (Ki67+) already after 1 wk of full loss of CTLA-4. This activation was less pronounced in CD8+ T cells, whereas Treg cells were slightly more activated compared with WT controls and significantly more proliferative (Fig. 3C and Fig. S4 for gating strategy). Additionally, iKO mice had elevated frequencies of IL-2, IL-4, and IL-17 but not IFNγ-producing CD4+ cells in LNs (Fig. 3D and Fig. S4 for gating strategy). This pattern of T-cell activation, proliferation, and cytokine production was still visible 8 wk after depletion (Fig. S5). In summary, loss of CTLA-4 in adult mice led to a preferential expansion of Treg cells, which was, however, not sufficient to circumvent activation of Tconv cells and proinflammatory cytokine production.
Fig. 3.
Activation of Treg and Tconv cells by CTLA-4 depletion in adulthood. (A) Frequency of CTLA-4+ Treg cells in blood of iKO and WT littermate controls before, during, and after depletion of CTLA-4. (B) Frequencies of Tconv (Left) and Foxp3+Treg cells (Right) in blood of iKO and WT littermate mice before, during, and after CTLA-4 depletion. (C) Flow cytometric analysis of T-cell activation markers in lymph nodes 1 wk after full CTLA-4 depletion in iKO mice and WT littermate controls. (D) Intracellular cytokine production by lymph node CD4+ T cells after stimulation with PMA/Ionomycin, 1 wk after full CTLA-4 depletion in iKO mice and WT littermate controls. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Fig. S3.
Activation of Treg and Tconv cells by CTLA-4 depletion in adulthood. (A) Frequency of CTLA-4+ Treg cells in LN of iKO and WT littermate controls after depletion of CTLA-4. (B) Frequencies of Tconv cells (Left) and Foxp3+ Treg cells (Right) in LNs of iKO and WT littermate mice after CTLA-4 depletion. (C) Frequency of CTLA-4+ Treg cells in spleen of iKO and WT littermate controls after depletion of CTLA-4. (D) Frequencies of Tconv cells (Left) and Foxp3+Treg cells (Right) in spleen of iKO and WT littermate mice after CTLA-4 depletion. n = 3–5 mice per group and time point. Error bars represent mean ± SEM.
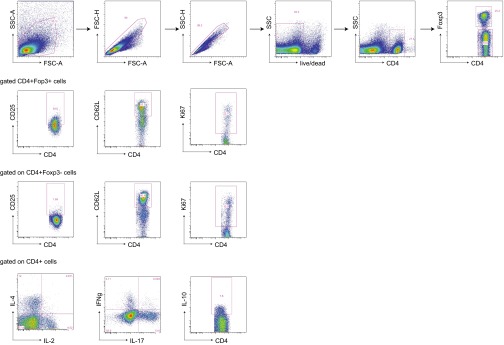
Fig. S4.
Gating strategy for gating of T-cell populations, activation markers, and cytokines.
Fig. S5.
Spontaneous T-cell activation in iKO mice after 8 wk of depletion. (A) Flow cytometric analysis of T-cell activation markers in lymph node 8 wk after full CTLA-4 depletion in iKO mice and littermate controls. (B) Intracellular cytokine production by lymph node CD4+ T cells after stimulation with PMA/Ionomycin, 8 wk after full CTLA-4 depletion in iKO mice and littermate controls. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
CTLA-4–Deficient Thymocytes and Peripheral T Cells Cause Different Pathologies.
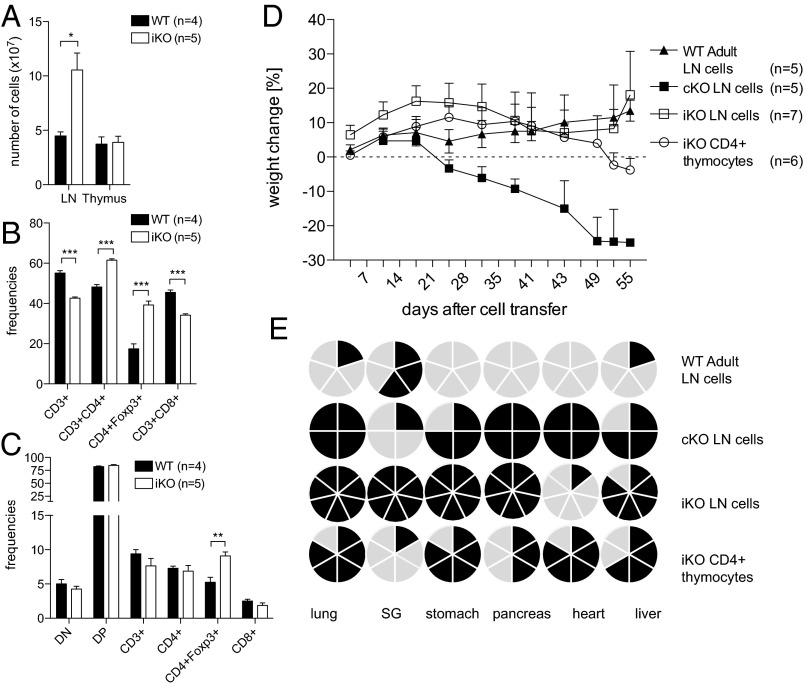
The observed difference in organ pathology between iKO and cKO mice suggests differences in the antigen specificity of T cells. However, differences in age and related events in immune homeostasis could also be contributing factors. To exclude this possibility and investigate potential differences in the TCR repertoire, we transferred T cells from cKO, iKO, or WT littermate donors into TCRβ−/− recipients. Cells from iKO and WT donors were harvested 1 wk after complete loss of CTLA-4. At this time point, iKO LNs were already significantly enlarged, whereas total thymocyte numbers were unaffected (Fig. 4A). CTLA-4 deficiency increased the frequencies of CD4+ Tconv cells and Foxp3+ Treg cells, whereas CD3+ and CD8+ populations decreased in ratio, although absolute numbers were either increased or not affected (Fig. 4B and Fig. S6A). In iKO thymi, no changes occurred apart from an increase in Foxp3+ Treg cell frequency, which was not reflected in absolute numbers (Fig. 4C and Fig. S6B). On transfer into TCRβ−/− recipients, T-cell repopulation was similar between all groups, but 3 wk after transfer, the recipients of cKO LN cells started to rapidly lose body weight and eventually had to be killed (Fig. 4D and Fig. S6C). Recipients of WT or iKO LN cells either increased or maintained their weight, whereas recipients of iKO thymocytes began to lose body weight toward the end of the experiment (Fig. 4A). Histopathological analysis 8 wk after transfer (or on humane scarification in case of cKO), revealed that the recipients of either cKO or iKO LN cells recapitulated lymphocyte infiltrations in the same distinct set of affected organs described in donors [Figs. 4E (see Fig. S7 for representative images) and 2B]. Importantly, iKO LN recipients did not develop any sign of heart pathology, whereas recipients of cKO LN cells developed severe myocarditis. Strikingly, iKO thymocyte recipients also suffered from myocarditis but not sialadenitis, a phenotype more similar to that of cKO LN recipients than of iKO LN recipients (Fig. 4E). These results indicate that the inflammatory destruction of various organs in iKO and cKO mice are T cell-mediated autoimmune diseases and that the differences in phenotype and severity are not exclusively due to mere age-related homeostatic differences. The fact that thymic and peripheral T cells from the same iKO donor caused different pathologies could suggest differences in thymic selection affecting the TCR repertoire and function of Tconv cells and/or Treg cells, although age-related developmental differences can also not be fully excluded.
Fig. 4.
T-cell transfer into TCRβKO recipients recapitulates the distinct organ infiltration pattern seen in iKO and cKO donors. (A) Absolute cell numbers in lymph nodes and thymus in iKO donors and WT littermates 1 wk after loss of CTLA-4. Frequencies of T-cell subsets in lymph nodes (B) and thymus (C) of iKO and WT mice 1 wk after CTLA-4 depletion. (D) Weight change in percent of initial weight of TCRβKO recipients after i.v. cell transfer of 30 million total LN cells from either 16-d-old cKO neonates, >6-wk-old iKO donors, or 1.3–3.4 million purified CD4+ thymocytes from iKO donors. As a control, 30 million total LN cells from >4-wk-old WT littermate controls were transferred. (E) Cake diagrams depicting organ infiltrations in recipient mice. Each cake piece represents one mouse and filled pieces symbolize observed infiltrations. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Fig. S6.
T-cell transfer into TCRβKO recipients recapitulates the distinct organ infiltration pattern seen in iKO and cKO donors. Absolute numbers of T-cell subsets in LNs (A) and thymus (B) of iKO and WT mice 1 wk after CTLA-4 depletion. (C) CD3 T-cell repopulation in blood of TCRβKO recipients 19 d after i.v. cell transfer of 30 million total LN cells from 16-d-old cKO neonates or 14 d after transfer of 30 million total LN cells from >6-wk-old iKO donors or 1.3–3.4 million purified CD4+ thymocytes from iKO donors. As a control, 30 million total LN cells from >4-wk-old WT littermate controls were transferred (n = 5–7 mice per group). Statistical comparisons were made against the WT adult LN group. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Fig. S7.
Lymphocytic infiltrations in TCRβKO recipients 8 wk after transfer of iKO or cKO donor cells. H&E staining of representative paraffin-embedded organ sections of TCRbKO recipients 8 wk after i.v. transfer of 30 million total LN cells from either 16-d-old cKO neonates (second row), >7-wk-old iKO donors (third row), or 1.3–3.4 million purified CD4+ thymocytes from iKO donors (fourth row). As a control, 30 million total LN cells from >7-wk-old WT littermate controls were transferred (first row).
Loss of CTLA-4 in Adult Mice Enhances Incidence and Severity of CIA.
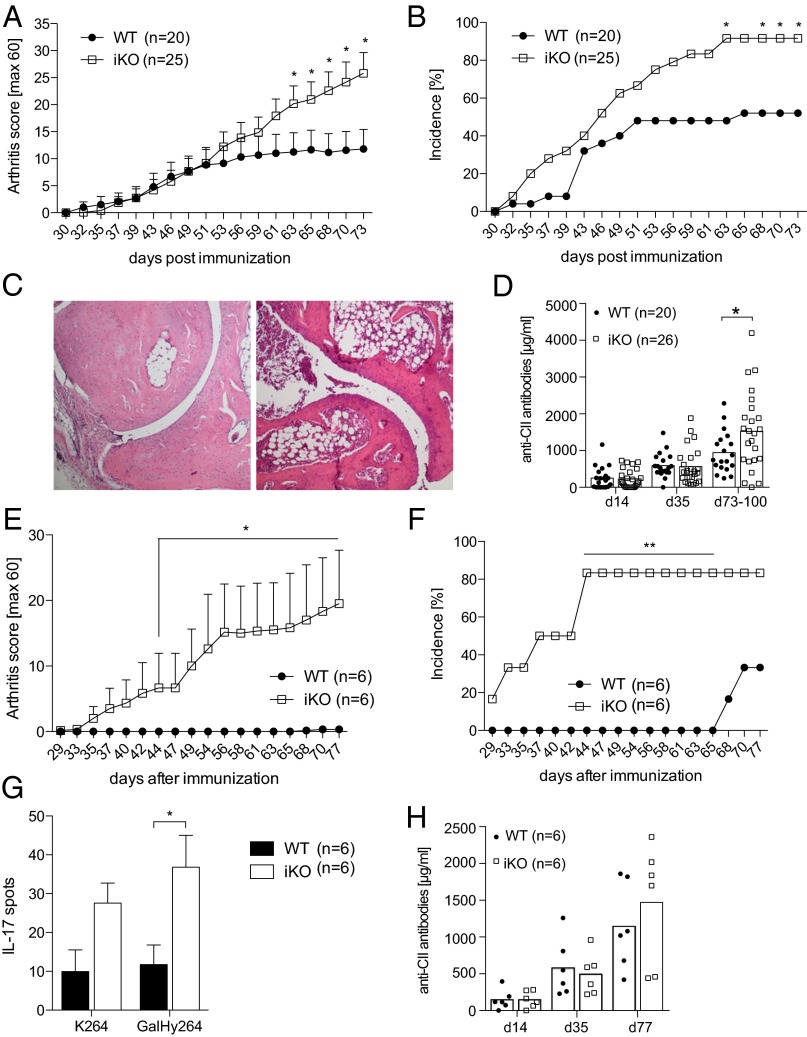
Genetic association studies have confirmed a role for CTLA-4 in rheumatoid arthritis (RA) (9, 37–39), and CTLA-4-Ig is an effective treatment of RA (40), as well as in the CIA model (41). We therefore tested the outcome of CTLA-4 depletion in adulthood on the development of CIA. Loss of CTLA-4 before immunization with heterologous rat collagen type II (CII) led to a significant exacerbation of CIA severity, as well as an increase in incidence (Fig. 5 A and B). This exacerbation was evident in macroscopic disease/clinical score, as well as in histological analysis of articular joints (Fig. 5C). Furthermore, iKO mice had increased titers of anti-CII Abs compared with controls late in disease progression (Fig. 5D). Interestingly, loss of CTLA-4 was sufficient to confer disease susceptibility in the absence of the booster immunization typically required for disease development in WT littermates (Fig. 5 E and F). Increased disease susceptibility in the absence of a boost was reflected by an enhanced T-cell response toward the dominant T-cell epitope (GalHyK264) in CIA as measured by IL-17A ELISPOT analysis, whereas the anti-CII Ab response was not significantly affected (Fig. 5 G and H). Hence, loss of CTLA-4 in adulthood confers enhanced disease susceptibility to CIA.
Fig. 5.
Depletion of CTLA-4 in adulthood enhances severity and susceptibility to collagen-induced arthritis. (A) Mean arthritis score and (B) incidence of iKO and littermate controls immunized with CII day 7 after tamoxifen treatment was initiated. Mice were boosted 35 d later. (C) Representative images of one WT (Left) and one iKO (Right) joint section stained with H&E, 25× magnification. (D) Total anticollagen antibody titers in serum. (E) Mean arthritis score and (F) incidence of iKO mice and littermate controls without booster immunization. (G) IL-17 T-cell response against CII259-273 peptides determined by ELISPOT from draining lymph node cells of immunized but not boosted mice with unmodified CII peptide (K264), CII peptide glactocylated on hydroxylysine at position 264 (GalHyK264), or Concaviline A (ConA). (H) Total anticollagen antibodies in serum of immunized but not boosted mice. A–D show data from three pooled independent experiments, and error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Loss of CTLA-4 in Adult Mice Protects from Peptide- but Not Protein-Induced EAE.
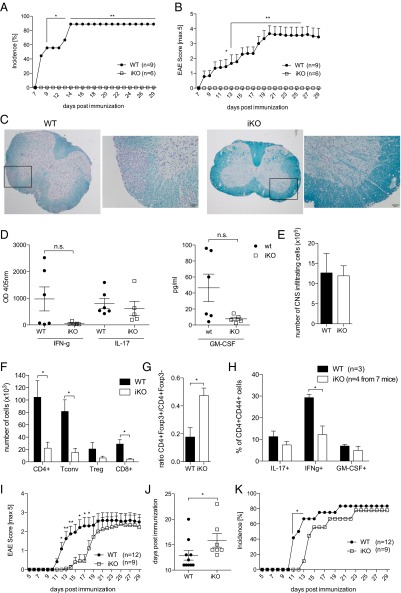
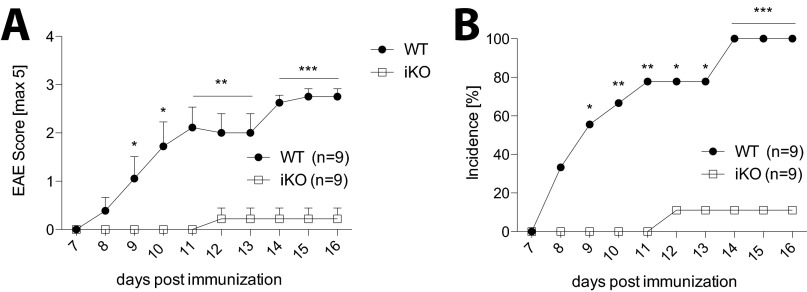
We found that iKO mice were completely protected from myelin oligodendrocyte glycoprotein79–96 (MOG79–96)-induced EAE (Fig. 6A–C for demyelination), which is in accordance with the recent result obtained by Paterson at al., who showed that mice depleted of CTLA-4 in adulthood were resistant to MOG35–55-induced EAE (18). Proinflammatory cytokines in general and GM-CSF in particular are crucial for development of EAE (42). We found that purified draining LN CD4+ T cells from resistant iKO mice did not significantly differ in their IFNγ, IL-17, or GM-CSF response to MOG79–96 compared with susceptible WT littermate controls (Fig. 6D). Notably, iKO mice had similar numbers of total CNS infiltrating cells as controls but a lower frequency of T cells among these (Fig. 6 E and F). However, WT and iKO mice had similar percentages of Treg cells among the infiltrating CD4+ cells, which resulted in a more than twofold increase in the Treg cell/Tconv cell ratio in the CNS of protected iKO mice (Fig. 6G). Similar to the CNS draining LNs, there was no significant difference in GM-CSF or IL-17 produced by CD4+ CNS infiltrating T cells; however, IFNγ was reduced in the CNS of iKO mice (Fig. 6H).
Fig. 6.
Depletion of CTLA-4 in adulthood leads to resistance to MOG79–96 peptide but not MOG1–125 protein-induced EAE. (A) Clinical scores and (B) incidence of iKO mice and littermate controls immunized with MOG79–96 peptide day 7 after tamoxifen treatment was initiated. (C) Representative images of one WT (Left) and one iKO (Right) spinal cord section stained with Luxol Fast Blue and Cresyl violet, 25× magnification or 200× magnification for the depicted inlet. (D) Cytokine production in supernatants of purified and restimulated CD4+ T cells pooled from draining lymph nodes and spleen on day 36 of MOG79–96 EAE. (E) Absolute numbers of total CNS infiltrating cells. (F) Absolute numbers of CNS infiltrating CD4+, CD4+Foxp3− Tconv, CD4+Foxp3+ Treg, and CD8+ cells, (G) ratio of Tconv/Treg, and (H) intracellular cytokine production in iKO mice and littermate controls 14 d after EAE induction. (I) Clinical scores, (J) day of onset, and (K) incidence of MOG1-125 protein induced EAE in iKO mice and WT controls. A and B show a representative experiment of four independent experiments, and G–I show data from two pooled independent experiments. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
Because loss of CTLA-4 in adulthood had opposing effects in EAE and CIA, we went on to elucidate possible reasons. We first investigated whether the difference in temporal disease course could explain the contrasting results, by depleting CTLA-4 4 wk before induction of MOG79–96 EAE. However, iKO mice were still protected from EAE in this setting (Fig. S8). We next used MOG1–125 protein for EAE immunization, which has been shown to induce disease in a B cell–dependent fashion (43). B cells are critical in CIA but less so in peptide-induced EAE, although they can modulate disease severity (43, 44). Strikingly, although disease was significantly delayed, iKO mice immunized with MOG1–125 protein were not protected from EAE, and incidence and severity were the same as in WT littermates (Fig. 6 I–K).
Fig. S8.
iKO mice are protected from MOG79-96 peptide EAE also when CTLA-4 is depleted 4 wk before disease induction. CTLA-4 was depleted in iKO mice, and 4 wk later, MOG79-96 peptide EAE was induced. With this scheme, iKO mice have a similar day of EAE disease onset after loss of CTLA-4 as in CIA. (A) Clinical scores and (B) incidence are shown. Error bars represent mean ± SEM. Differences were considered statistically significant with a P value of <0.05 (*), <0.01 (**), or <0.001 (***).
These results taken together demonstrate that depletion of CTLA-4 in adulthood enhances autoimmune disease in some models such as CIA and prevents it in other models, for example, peptide-induced EAE. Furthermore, the deletion also produces a difference in EAE susceptibility between immunization with MOG protein or peptide, suggesting a possible regulatory effect of CTLA-4 on B-cell responses or changes in TCR repertoire in CTLA-4–deficient mice.
Discussion
CTLA-4 is critical for immune homeostasis, and studies in recent years have suggested a much broader function than originally thought, including both cell intrinsic signaling on activated effector T cells and Foxp3+ Treg cell-mediated suppression (20, 45, 46). However, the rapid and fatal condition of germ-line ctla-4–deficient mice has hampered studies aimed to reveal the full spectra of CTLA-4–dependent tolerance mechanisms in vivo. We used an inducible Cre system and separated the role of CTLA-4 in T-cell development from that of the adult mature immune system. As a result, we found that animals devoid of CTLA-4 from birth vs. those depleted of CTLA-4 in adulthood developed separate signatures of organ pathology. Similar to previous studies in CTLA-4 KO mice, CD4-dependent CTLA-4 deficiency led to a more aggressive condition that involved both exocrine and endocrine pancreatic tissue and the heart (13, 14). CTLA-4 deletion in adult mice did not lead to myocarditis but instead resulted in a condition related to human Sjögren’s syndrome. The fact that multiorgan pathology was readily detected in iKO mice is in contrast to a recent study with a similar experimental system that reported no overt disease (18). To fully explain this discrepancy is difficult, but possible reasons include differences in genetic background and genetic modifications as the studies use two independently generated CTLA-4fl/fl lines and Cre recombinase expression is driven by two different promotors, which could affect the CTLA-4 depletion efficiency. Another difference can be found in MHC class II haplotype as in this study iKO mice expressed H-2q instead of H-2b. In any event, severe lymphoproliferation and expansion of Foxp3+ Treg cells was detected in both studies.
As lymphoproliferation is also evident in CTLA-4 cKO mice, this alone does not explain the different signatures of organ pathology in iKO vs. cKO mice but indicates that a degree of organ specificity is operating. Indeed, it has been shown that CTLA-4 KO mice have T cells specific for the pancreas (47). Moreover, both the fact that we detected organ-specific autoantibodies and that T cells could transfer organ pathology suggest T cell-mediated autoimmunity. Interestingly, although peripheral T cells from iKO mice did not infiltrate the heart, thymocytes of the same donor did, which could point to a role for CTLA-4 in thymic selection. Whether CTLA-4 deficiency primarily affects Tconv cell or Foxp3+ Treg cell development or both is not yet clear. However, it has been shown that CTLA-4 deficiency affects both conventional T cells and Treg cells by altering TCR Vα and Jα segments and the CDR3α composition in TCR transgenic mice (36). In support, overexpression of CTLA-4 was shown to favor self-skewing of the Treg cell TCR repertoire as clonal deletion in response to endogenous superantigens was reduced (48). Hence, lack of CTLA-4 signal may result in T cells with lower TCR avidities, which is at variance with the dogma of high-affinity autoreactive T cells causing autoimmune disease. However, this might only be true for a fraction of immunization induced autoreactive T cells, whereas the great majority of endogenous autoreactive CD4 T cells are of low affinity (49–51). Taken together, our results suggest that the natural T-cell repertoire might be affected if CTLA-4 is absent during thymic selection and that this operates in addition to the well-established role of CTLA-4 in peripheral tolerance. It should, however, be noted that the maturation status of the peripheral immune system at the time of depletion likely also affects organ pathology. That is, T-cell transfer equaled out the effect of homeostatic expansion between neonatal and adult CTLA-4–deficient individuals but not necessarily the inherent status of other age-dependent peripheral tolerance mechanisms. Thus, although signs of myocarditis were completely absent in mice depleted of CTLA-4 at 6–8 wk of age and myocarditis was seen in CTLA-4 cKO neonates, minor heart pathology was detected in some samples from mice depleted at 4 wk of age, although it was not fatal. Again, as there likely are age-dependent differences in thymic self-antigen processing that result in distinct Treg cell populations at different ages (52), further studies are needed to sort out the specific contributions of central vs. peripheral tolerance.
To better understand the role of CTLA-4 in peripheral tolerance, iKO mice were subjected to the induced autoimmune models of CIA and EAE. Interestingly and opposite to earlier studies that used anti–CTLA-4 blocking Ab, we found that iKO mice were protected against peptide-induced EAE (53–55). Similar results were recently obtained in a comparable study, which may indicate that the way in which CTLA-4 is manipulated matters (18). This study showed that lack of CTLA-4 specifically on Treg cells led to an increased Treg cell proliferation and up-regulation of suppression related molecules like IL-10 and LAG-3. Moreover, expansion of Treg cell numbers has previously been shown to prevent spontaneous EAE in CTLA-4–deficient TCR transgenic mice (17). In line with this, we detected an increase in Foxp3+ Treg cells and elevated IL-10 production in iKO mice. Furthermore, we discovered reduced numbers of CNS-infiltrating cells but no significant difference in IL-17 and GM-CSF production both important in EAE. These findings indicate that Tconv cells were primed but prevented from migrating to the CNS, which is different from Paterson et al. that detected similar numbers of CNS infiltrating T cells. CTLA-4–deficient Treg cells, in particular in vivo activated ones, can clearly use alternative mechanisms to suppress immune responses as they can suppress anti-CD3 Ab-induced T-cell proliferation to the same degree as WT Treg cells (15, 18). That CTLA-4 is nonetheless needed for optimal function is shown by their failure to suppress mixed lymphocyte reactions (15). In any event it seems clear that CTLA-4 deficiency can lead to protection against peptide-induced EAE, which is likely related to increased Treg cell frequency and their use of alternative suppressive mechanisms. Importantly, however, this protection was not applicable to protein-induced EAE or CIA. Collectively this indicates that the local milieu might be important but also that the antigen itself and the immune cells involved are of significance for the function of different Treg cell suppressive mechanisms. For example, it is interesting to note that CIA and protein-induced EAE have a different dependency on B cells compared with peptide-induced EAE (43, 44, 56), especially because CTLA-4 expressed by Treg cells is shown to be involved in restraining Tfh cells (57–59) and iKO mice have increased germinal center formation, antibody, and autoantibodiy levels. Future studies are, however, needed to specifically determine the role of CTLA-4 on B-cell responses and in EAE and CIA development.
Taken together our data show that CTLA-4 can operate on different levels of immune tolerance and that this may have dramatically different outcomes depending on the maturation status of the immune system. In addition, we demonstrate that CTLA-4 deletion can have paradoxical effects in different models of autoimmunity, which likely depends not only on the local milieu but also on the specific immune reactions involved in disease pathology. The fact that CTLA-4 is expressed by more than one cell population and exists in several isoforms, likely with different roles, creates additional complexity. This complexity, however, also generates possibilities for further improved future therapies.
Materials and Methods
Mice.
CTLA-4 floxed (15) and Rosa26Cre/ERT2+ mice (Jackson Laboratory) were backcrossed to C57BL/10Q for at least six generations. Offspring of these colonies was intercrossed to obtain Cre+/wtCTLA-4fl/fl (iKO) and Crewt/wtCTLA-4fl/fl (WT) genotypes. To generate CTLA-4 cKO mice, CTLA-4fl/fl mice were crossed with CD4Cre mice [Tg(Cd4-cre)1Cwi; Jackson Laboratory]. All mice were maintained under specific-pathogen-free conditions. All experimental animal procedures were approved by the local ethics committee (Stockholms djurförsöksetiska nämnd).
CTLA-4 Depletion.
To induce CTLA-4 depletion, mice were i.p. injected with 4 mg tamoxifen in corn oil [+5% (vol/vol) ethanol] on days 0, 1, and 5. Complete depletion was achieved by day 6.
Antibodies.
The following reagents were purchased from Biolegend: anti-CD3 (145-2C11), anti-CD4 (H129.19), anti-CTLA-4 (UC10-4B9), anti-CD25 (7D4), anti-CD62L (MEL-14), anti-Ki67 (B56), anti-CD8 (53-6.7), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-B220 (RA3-6B2), anti-MHCII (2G9), anti–IL-10 (JES5-16E3), anti-IFNγ (R46A2), anti–IL-2 (JES6-5H4), anti–IL-4 (11B11), and anti–IL-17 (TC1118H10.1). Foxp3 (FJK-16s) was purchased from eBioscience.
Intracellular Cytokine Staining.
Spleen and LN cell suspensions from iKO and WT littermate controls were stimulated with 50 ng/mL 12-myristate 13-acetate (PMA), 250 ng/mL ionomycin, and 10 µg/mL brefeldin A for 4 h in vitro. Then, surface antigens were stained, and cells were fixed and permeabilized according to the manufacturer’s instructions (eBioscience) and stained for Foxp3, Ki67, and intracellular cytokines.
Serology.
Serum levels of IgA (Southern Biotech), IgE (BD), IgG (Jackson ImmunoResearch), IgG1, IgG2b, IgG3 (all Southern Biotech), and auto-antibodies specific for insulin, Ro52, or gastric antigen were determined by ELISA. Human recombinant insulin was purchased from Sigma Aldrich, human recombinant Ro52 was a kind gift from M. Wahren–Herlenius (Karolinska Institutet, Stockholm), and gastric antigen was prepared as described previously (60).
The avidity of auto-Ab was determined by incubating the ELISA plates with 1 M NaSCN for 15 min before the addition of the detection antibody. The avidity index (AI) was calculated as
Anticollagen II titers were determined by coating ELISA plates (NUNC) with 10 µg rat CII and serum diluted 1:10,000 or 1:50,000. Bound antibodies were detected with HRP-conjugated rat anti-mouse Ig κ antibody (Southern Biotech) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) substrate (Roche). The absorbance was read at 405 nm (Synergy-2). Pooled serum from immunized mice with known concentration was used as a standard.
IL-17A ELISPOT.
A total of 5 × 105 draining LN cells per well were plated in ELISPOT plates (#MSIPS4W10) coated with anti–IL-17 (TC11-18H10, 5 µg/mL) and stimulated with 20 µg/mL K264 or GalHyK264 peptide or 3 µg/mL ConA for 24 h. Bound cytokines were detected with biotinylated anti–IL-17 (TC11-8H4.1; 1 µg/mL) and alkaline phosphatase-conjugated streptavidin. Plates were developed with BCIP/NBT (Sigma-Aldrich), and wells were scanned and analyzed with ImmunoSpot software (Cellular Technology Ltd.).
Assessment of Cytokines in Supernatant.
CD4+ T cells were purified from draining LN and spleen (Dynabeads Untouched Mouse CD4 Cells; Invitrogen) of MOG79–96 immunized animals and stimulated with 2 µg/mL MOG79–96 peptide for 72 h. ELISA plates (NUNC) were coated with anti-IFNγ (R46-A2) or anti–IL-17 (TC11-18H10), and supernatants were added. Bound cytokines were detected by bio-anti-IFNγ (AN-18) or bio-anti–IL-17 (TC11-8H4) followed by Eu-labeled streptavidin (PerkinElmer). Eu3+ was measured using dissociation-enhanced time-resolved fluorometry (excitation 360/40 and emission 620/40; Synergy-2). GM-CSF was assessed with DuoSet ELISA according to the manufacturer’s instructions (R&D Systems).
Isolation of CNS Infiltrating Cells.
Animals were anesthetized with ketamine (50 mg/kg) and medetomidine (1 mg/kg) and perfused from the heart with 20 mL PBS. Brain and spinal cord were removed, passed through a 70-µm filter followed by a 40-µm filter (Corning). The resulting cell pellet was taken up in 20 mL 30% (vol/vol) Percoll, carefully overlayed with 20 mL 70% (vol/vol) Percoll (GE Healthcare), and centrifuged at 900 × g for 20 min at 4 °C without brake. CNS infiltrating cells were collected from the interphase and washed with PBS.
Histology.
Fixed [4% (wt/vol) PFA] organs were dehydrated, mounted in paraffin, sectioned (5 µm), and stained with H&E. Joints were first decalcified in formic acid for 2 d. For immunohistochemistry, sections were rehydrated, demasked using citrate and heat, and stained with purified antibody against CD3 (CD3-12; AbD Serotec), Foxp3 (FJK-16s; eBioscience), or B220 (RA3-6B2; Biolegend). Detection was done using a biotinylated secondary antibody and the VECTASTAIN Elite ABC Kit (Vectorlabs) followed by visualization with DAB.
For detection of CNS demyelination, mice were perfused via the heart before the collection of brain and spinal cord. Sections were stained with 0.1% Luxol fast blue overnight at 56 °C, differentiated in 0.05% lithium carbonate, and counterstained with 0.1% Cresyl echt Violet for 30 s. In this way, myelin is stained in blue and neurons in violet.
Test of Salivary Gland Function.
Mice were anesthesized (Ketalar/Domitor) and injected i.p. with 1.79 µg pilocarpine, and time from injection until appearance of first drop of saliva (lag time) was measured. Saliva was collected with a pipette for a period of 2 min. α-Amylase activity was analyzed using a DNS assay as described previously (61).
T-Cell Transfer.
iKO donor mice (>6 wk old) were treated with tamoxifen on days 0, 1, and 5, and LNs and thymus were harvested on day 12. WT (>4 wk old) and cKO (16 d old) donor LNs were taken without prior treatment. Single cell suspensions were generated, and 30 million unpurified LN cells or 1.3–3.4 million purified CD4+ cells (Dynabeads Untouched Mouse CD4 Cells; Invitrogen) were i.v. injected into TCRβKO recipients.
CIA Induction and Evaluation.
CIA was induced as previously described. In short, animals were intradermal (i.d.) injected with an emulsion of 100 µg rat collagen type II (CII) (purified from the Swarm rat chondrosarcoma, as previously described) (62) in 50 µL Complete Freund's Adjuvant. Thirty-five days later, mice received a booster injection of 50 µg rCII in 25 µL Incomplete Freund's Adjuvant. Arthritis was monitored macroscopically, i.e., each red and swollen toe or knuckle equals 1 point and each red and swollen ankle or wrist equals 5 points, resulting in a maximum score of 60 points per mouse. All experiments were scored blinded.
EAE Induction and Evaluation.
EAE was induced in C57BL/10.Q mice by an i.d. injection of 100 µg MOG79–96 protein in 50 µL CFA and an i.v. injection of 400 ng pertussis toxin the same day plus 48 h later. Mice were monitored daily for clinical signs of EAE and scored on a scale of 0–5: 0 = no disease, 0.5 = weak tail, 1 = limp tail, 2 = limp tail and hind limp weakness, 3 = hind limp paralysis, 4 = hind and fore limp paralysis, 5 = moribund or dead. Half points were given when only one limp out of a set was affected.
Statistical Analysis.
GraphPad Prism software was used for statistical analysis. For pairwise comparisons, either the Student unpaired two-tailed t test or Mann–Whitney test was used depending on whether the data followed a Gaussian distribution or not. For more than two groups, one-way ANOVA or the Kuskal–Wallis test was used.
Acknowledgments
We thank Prof. Marie Wahren-Herlenius for Ro52 protein and control serum; Dr. Markus Hoffmann for control serum for anti-DNA antibody ELISA; Robert Harris for MOG1-125 protein; James B. Wing and Susann Winter for critical reading of the manuscript; and Kristina Palstro, Carlos Palestro, Evelina Wernersson, and Sanna Eklund for excellent animal care. This work was funded by Swedish Research Council Grants 522-2009-2548 (to K.W.) and 521-2010-2894 (to R.H.); Åke Wibergs Stiftelse Grants 367990049, 924563126, and 940219686 (to K.W.); Pr. Nanna Svartz Stiftelse (K.W.); the Swedish Society of Medicine (K.W.); Ulla och Gustaf af Ugglas Stiftelse (K.W.); Swedish Rheumatic Association (K.W.); King Gustav V 80th birthday foundation (K.W. and R.H.); the Swedish Strategic Science Foundation (R.H.); Knut and Alice Wallenberg Foundation Grant KAW2010.0148 (to R.H.); the European Union Innovative Medicine Initiative (EU IMI) project BeTheCure and EU FP7 Neurinox projects (R.H.); grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (26253030); and Core Research for Evolutional Science and Technology from Japan Science and Technology Agency Grant 15652235 (to S.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603892113/-/DCSupplemental.
References
- 1.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 4.Kuehn HS, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert D, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouki T, et al. CTLA-4 gene polymorphism at position 49 in exon 1 reduces the inhibitory function of CTLA-4 and contributes to the pathogenesis of Graves’ disease. J Immunol. 2000;165(11):6606–6611. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- 7.Barreto M, et al. Evidence for CTLA4 as a susceptibility gene for systemic lupus erythematosus. Eur J Hum Genet. 2004;12(8):620–626. doi: 10.1038/sj.ejhg.5201214. [DOI] [PubMed] [Google Scholar]
- 8.Marron MP, et al. Insulin-dependent diabetes mellitus (IDDM) is associated with CTLA4 polymorphisms in multiple ethnic groups. Hum Mol Genet. 1997;6(8):1275–1282. doi: 10.1093/hmg/6.8.1275. [DOI] [PubMed] [Google Scholar]
- 9.Tang M-J, Zhou Z-B. Association of the CTLA-4 +49A/G polymorphism with rheumatoid arthritis in Chinese Han population. Mol Biol Rep. 2013;40(3):2627–2631. doi: 10.1007/s11033-012-2349-6. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvat TZ, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37(5):499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 14.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 15.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 16.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177(7):4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhagen J, et al. Enhanced selection of FoxP3+ T-regulatory cells protects CTLA-4-deficient mice from CNS autoimmune disease. Proc Natl Acad Sci USA. 2009;106(9):3306–3311. doi: 10.1073/pnas.0803186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paterson AM, et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med. 2015;212(10):1603–1621. doi: 10.1084/jem.20141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt EM, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182(1):274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 20.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32(9):428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183(6):2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KM, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282(5397):2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 23.Linsley PS, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185(3):393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachmann MF, Köhler G, Ecabert B, Mak TW, Kopf M. Cutting edge: Lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163(3):1128–1131. [PubMed] [Google Scholar]
- 26.Tivol EA, Gorski J. Re-establishing peripheral tolerance in the absence of CTLA-4: Complementation by wild-type T cells points to an indirect role for CTLA-4. J Immunol. 2002;169(4):1852–1858. doi: 10.4049/jimmunol.169.4.1852. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi S, et al. In vivo overexpression of CTLA-4 suppresses lymphoproliferative diseases and thymic negative selection. Eur J Immunol. 2005;35(2):399–407. doi: 10.1002/eji.200324746. [DOI] [PubMed] [Google Scholar]
- 28.Corse E, Allison JP. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J Immunol. 2012;189(3):1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 29.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105(29):10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qureshi OS, et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 32.Chambers CA, Cado D, Truong T, Allison JP. Thymocyte development is normal in CTLA-4-deficient mice. Proc Natl Acad Sci USA. 1997;94(17):9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner DH, Jr, et al. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/CTLA-4 costimulatory interactions with B7-1/B7-2. J Exp Med. 1996;184(5):1631–1638. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cilio CM, Daws MR, Malashicheva A, Sentman CL, Holmberg D. Cytotoxic T lymphocyte antigen 4 is induced in the thymus upon in vivo activation and its blockade prevents anti-CD3-mediated depletion of thymocytes. J Exp Med. 1998;188(7):1239–1246. doi: 10.1084/jem.188.7.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buhlmann JE, Elkin SK, Sharpe AH. A role for the B7-1/B7-2:CD28/CTLA-4 pathway during negative selection. J Immunol. 2003;170(11):5421–5428. doi: 10.4049/jimmunol.170.11.5421. [DOI] [PubMed] [Google Scholar]
- 36.Verhagen J, et al. CTLA-4 controls the thymic development of both conventional and regulatory T cells through modulation of the TCR repertoire. Proc Natl Acad Sci USA. 2013;110(3):E221–E230. doi: 10.1073/pnas.1208573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elshazli R, Settin A, Salama A. Cytotoxic T lymphocyte associated antigen-4 (CTLA-4) +49 A>G gene polymorphism in Egyptian cases with rheumatoid arthritis. Gene. 2015;558(1):103–107. doi: 10.1016/j.gene.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 38.Torres-Carrillo N, et al. The -319C/+49G/CT60G haplotype of CTLA-4 gene confers susceptibility to rheumatoid arthritis in Mexican population. Cell Biochem Biophys. 2013;67(3):1217–1228. doi: 10.1007/s12013-013-9640-6. [DOI] [PubMed] [Google Scholar]
- 39.Li X, et al. Polymorphisms in the CTLA-4 gene and rheumatoid arthritis susceptibility: A meta-analysis. J Clin Immunol. 2012;32(3):530–539. doi: 10.1007/s10875-012-9650-y. [DOI] [PubMed] [Google Scholar]
- 40.von Kempis J, et al. Use of abatacept in rheumatoid arthritis. Swiss Med Wkly. 2012;142:w13581. doi: 10.4414/smw.2012.13581. [DOI] [PubMed] [Google Scholar]
- 41.Knoerzer DB, Karr RW, Schwartz BD, Mengle-Gaw LJ. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995;96(2):987–993. doi: 10.1172/JCI118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Codarri L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 43.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999;29(11):3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Svensson L, et al. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. Eur J Immunol. 2002;32(7):1939–1946. doi: 10.1002/1521-4141(200207)32:7<1939::AID-IMMU1939>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 45.Walker LSK, Sansom DM. Confusing signals: Recent progress in CTLA-4 biology. Trends Immunol. 2015;36(2):63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker LSK. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ise W, et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11(2):129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi T, et al. Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proc Natl Acad Sci USA. 2013;110(23):E2116–E2125. doi: 10.1073/pnas.1307185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hood JD, Zarnitsyna VI, Zhu C, Evavold BD. Regulatory and T effector cells have overlapping low to high ranges in TCR affinities for self during demyelinating disease. J Immunol. 2015;195(9):4162–4170. doi: 10.4049/jimmunol.1501464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gebe JA, et al. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur J Immunol. 2003;33(5):1409–1417. doi: 10.1002/eji.200323871. [DOI] [PubMed] [Google Scholar]
- 51.Sabatino JJ, Jr, Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J Exp Med. 2011;208(1):81–90. doi: 10.1084/jem.20101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348(6234):589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hurwitz AA, Sullivan TJ, Krummel MF, Sobel RA, Allison JP. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73(1-2):57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 54.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157(4):1333–1336. [PubMed] [Google Scholar]
- 55.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: A negative regulator of autoimmune disease. J Exp Med. 1996;184(2):783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111(3):521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Wang CJ, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA. 2015;112(2):524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winter S, et al. Manifestation of spontaneous and early autoimmune gastritis in CCR7-deficient mice. Am J Pathol. 2011;179(2):754–765. doi: 10.1016/j.ajpath.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. [Google Scholar]
- 62.Smith BD, Martin GR, Miller EJ, Dorfman A, Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]