Summary
It is paramount that any child or adolescent with a suspected disorder of sex development (DSD) is assessed by an experienced clinician with adequate knowledge about the range of conditions associated with DSD. If there is any doubt, the case should be discussed with the regional DSD team. In most cases, particularly in the case of the newborn, the paediatric endocrinologist within the regional team acts commonly as the first point of contact. This clinician should be part of a multidisciplinary team experienced in management of DSD and should ensure that the affected person and parents have access to specialist psychological support and that their information needs are comprehensively addressed. The underlying pathophysiology of DSD and the strengths and weaknesses of the tests that can be performed should be discussed with the parents and affected young person and tests undertaken in a timely fashion. Finally, in the field of rare conditions, it is imperative that the clinician shares the experience with others through national and international clinical and research collaboration.
Introduction and development of guidance
Disorders of sex development (DSD) are a wide range of conditions with diverse features and pathophysiology1 that most often present in the newborn or the adolescent. Affected newborns usually present with atypical genitalia, whereas adolescents present with atypical sexual development during the pubertal years. These clinical situations can often be difficult to manage, particularly in those cases where the sex of rearing is uncertain. Developing a logical and pragmatic plan for investigations whilst establishing a dialogue and building rapport with the affected child and the parents is central to the initial approach and ongoing management.
The consensus reached in Chicago in 2005 on the general principles of managing patients with DSD represented a historic milestone for international and multidisciplinary collaboration in this area.2, 3 Some areas of care, such as the initial approach to evaluating the infant or young person with suspected DSD, were not covered in detail as that was beyond the scope of the exercise. As guidance on the initial evaluation of a complex condition is often coloured by local provision of health care, it was felt in 2009 that reaching a consensus at a national, UK level was the most effective means of proceeding further. A UK DSD taskforce was formed in March that year under the auspices of the UK Society for Endocrinology, and it was agreed that the remit of this group would be to concentrate on guidance on the initial evaluation process and the diagnostic approach rather than the clinical management of the condition once a provisional or definitive diagnosis has been reached.4 This guidance would be aimed at a range of clinical professionals who encounter newborns and adolescents with DSD, and its purpose would not be simply to act as a manual but to harmonize good clinical practice. Stakeholder professional societies and clinical professionals who could represent these societies were identified and approached to join the taskforce, as were members of two patient support/advocacy groups (Appendix /). These taskforce members took responsibility for individual sections and based their opinion on observational studies and expert opinion, and the whole taskforce considered each draft. After completion, the guidelines were subject to open external review by the involved professional societies and their members as well as patient group representatives. After this period of open consultation of over 3 months, comments were reviewed and most suggestions were incorporated. All members of the taskforce approved the final draft of the guidance that was first published in 2011.4 This revision of the guidance which was initiated in 2014 has taken a similar path as described above.
The multidisciplinary team
Optimal care for infants and adolescents with DSD requires an experienced multidisciplinary team (MDT) that should be accessible through regional centres. The team may exist as a clinical network with links between more than one specialist centre. As a minimum standard, the clinical team should include specialists in endocrinology, surgery and/or urology, clinical psychology/psychiatry, radiology, nursing and neonatology. Ideally, discussions with the family are led by one professional. In most situations, particularly in the case of the newborn, the paediatric endocrinologist assumes the role of clinical lead and oversees the timely involvement of other members of the team. Other team members should be discouraged from providing results as soon as they are received. For infants with suspected DSD, this team should develop a plan for clinical management with respect to diagnosis, sex assignment and management options. The lead clinician should process this information, co‐ordinate further support and take responsibility for sharing the information with the parents so that informed decisions can be reached in a timely manner. The process of informing parents, children and young people of the various investigations and results should be documented such that the whole MDT team is aware of the status of new or ongoing conversations with the family. In addition, the clinical team should have links to a wider MDT which consists of specialists from adult endocrinology, plastic surgery, gynaecology, clinical genetics, clinical biochemistry, adult clinical psychology and social work and, if possible, to a clinical ethics forum5 (Table 1). However, the core composition of the team for each affected person and family will vary according to DSD type, family need or preference, local resources, developmental context and location as well as the age of the person. The parents and the young person should be informed of the range of support that is available from the MDT and should be provided with contact details of these personnel. Ongoing communication with the family's primary care physician is important, and consent issues in relation to sharing information outside of the hospital setting should be discussed with parents and young person. The team has a responsibility to educate other healthcare staff and should have a forum to meet regularly, in the context of a clinic and an educational meeting where the team can review and discuss its own performance. Audit of clinical activity, research studies, building collaborative working partnerships with other DSD teams and attendance at joint clinics and education events are crucial if knowledge and information sharing are to be optimized across MDT teams. Transfer of care for the adolescent should be organized with the multidisciplinary team operating in an environment comprising specialists with experience in adolescent care.6
Table 1.
The clinical members of the MDT and their roles in providing care to the patient and the parents
| Role | |
|---|---|
| Neonatologist or General Paediatrician | Initial explanation |
| Management of the unwell child | |
| Initiation of first‐line investigations | |
| Seek advice from paediatric subspecialist (endocrine or surgical) with an interest in DSD | |
| Paediatric Endocrinologista | Detailed explanation over multiple visits |
| Management of the unwell child | |
| Interpreting first‐line investigations and planning second‐line investigations | |
| Organize timely and appropriate involvement of other members of MDT | |
| Act as the link between the parents and MDT | |
| Initiate and monitor long‐term medical therapy such as steroid or sex steroid therapy | |
| Paediatric Radiologist | Interpret and often perform ultrasound scans in the newborn |
| Judge the reliability of ultrasound scans in the newborn esp. when the results may influence sex assignment | |
| Paediatric Urologista | Assessment of external anatomy |
| Explanation of the anatomy and results of imaging | |
| Explanation of pros and cons of reconstructive surgery | |
| Develop a plan for complex imaging (other than pelvic ultrasound) and further assessment of the anatomy | |
| Perform procedures such as laparoscopy, biopsy, reconstructive surgery and gonadectomy | |
| Organize timely and appropriate involvement of other members of MDT | |
| Paediatric Specialist Nursea | Provide general support to the patient and parents in addition to that provided by other members of the MDT |
| Arrange specialist investigations | |
| Liaise with the rest of the DSD team, including the clinical psychologist | |
| Clinical Psychologista | Provide specialist support to parents soon after birth |
| Provide support to the growing up child and the parents | |
| Develop an individualized plan for each family | |
| Guide the MDT on timing and tempo of explanation of the condition to the older child and adolescent | |
| Clinical Endocrine Biochemista | Facilitate timely analysis of samples |
| Provide specialist support and interpretation of results | |
| Guide subsequent biochemical tests | |
| Facilitate storage of samples for analysis at a later stage | |
| Clinical Geneticista | Facilitate timely analysis of karyotype |
| Closer involvement in the child with dysmorphic features | |
| Oversee the process of genetic analysis | |
| Facilitate storage of samples for analysis at a later stage | |
| Genetic counselling | |
| Gynaecologista | Availability at an early stage to discuss future outcome and map long‐term care pathway in the affected girl |
| Discuss issues related to sexual function, reproductive function and surgery | |
| Assess the understanding and review the diagnosis | |
| Assess the need for psychology support in the adolescent girl | |
| Initiate and monitor long‐term sex steroid therapy | |
| Perform examination, investigative and therapeutic procedures in the adolescent girl | |
| Oversee vaginal dilator training with specialist nurse | |
| Adult Endocrinologist | Investigate and manage the adolescent presenting for the first time after the age of 16 years |
| Liaise with other members of the MDT | |
| Act as the link between the patient and MDT | |
| Initiate and monitor long‐term medical therapy such as steroid or sex steroid therapy | |
| Act as the transition link for adolescents under paediatric care |
Professionals that are core members of the MDT who should meet regularly to discuss cases in a clinic setting.
Psychological support for the affected person and family
Early psychological input, provided by a specialist clinical psychologist with experience of supporting people with DSD and their parents, will allow those affected to examine and understand their early emotional reactions as well as explore present and future worries, adjust to the period of uncertainty during the diagnosis process and facilitate inclusion in informed decision‐making about themselves or their child.7, 8, 9, 10, 11 The clinical psychologist is also well placed to assess the level of support the family needs, assess and facilitate the bonding of the parents with the newborn and, in the case of the young person, perform an assessment of gender identity, when appropriate. As a minimum, the parents of every newborn with suspected DSD where there has been a delay in sex assignment should be offered clinical psychology input. In addition, all adolescents with a newly diagnosed DSD or existing DSD requiring medical or surgical attention should be routinely offered clinical psychology input in addition to any support offered to their parents or wider family. The point of transfer from paediatric to adult services offers an ideal opportunity for a standardized assessment of the need for future clinical psychology input.
Discussions with parents and young people need to occur on multiple occasions in a quiet and peaceful setting, with enough time for the family and MDT to develop a shared understanding of investigations, results, diagnosis, management and the value of ongoing psychological support for both themselves and/or their child. The pace at which information is shared should be set by the family, and issues of confidentiality should be discussed and respected.12 Parents’ and young people's initial recollections of conversations with professionals may have long‐lasting effects on them and their relationship with their child and health professionals.13 The use of phrases such as ‘differences’ or ‘variations’ in sex development may help to introduce the concept of the range of variation that may occur in sex development. The acronym, DSD, lends itself to be used as a phrase that includes ‘differences and disorders of sex development’ and, as such, may be more acceptable to the young person and their parents.14 The team should be cognizant of the needs of the parents who cannot speak English fluently. It is also possible that for rare dialects and languages, the interpreter may originate from the same community. A record of early discussions, either as audiotapes or as a letter, which is shared between the parents and other immediate members of the MDT and the general practitioner may be helpful. Use of drawings and written material during discussion and a list of websites and support groups are useful aids for families.
Parents and young people need to be aware that the management of the condition will require a step‐wise approach that first targets short‐term goals and then more distant goals that achieve optimal long‐term well‐being.15 It is very likely that families’ decisions will be shaped by their own expectations, experiences, and their understanding of sex and gender roles within the religious and cultural context of their own social networks. Some parents may consider early genital surgery as a mechanism that could possibly protect their child from the risk of future stigma.16 This will require a thorough discussion with several members of the MDT team including the clinical psychologist, surgeons, gynaecologist and nurses so that the parents are fully informed of the controversies around undertaking or withholding early genital surgery.17, 18 Parents and young people will need support and guidance about how to share news following the birth of their child/diagnosis and manage the social challenges they may face at this time.12, 19 Members of the MDT should also be aware of how their own values and beliefs are played out in consultation with the family.
The role of the support group
Support groups can provide ongoing support to parents and the affected individual, including opportunities to gather and explore information, promote autonomy, and build knowledge and self‐confidence regarding the diagnosis of DSD. For parents, gathering, using and questioning information will shape their understanding as they often act as the advocate for their child or young person and therefore need to be fully informed about DSD practice, short and long‐term outcomes of treatments, and health risks and psychological challenges for their child. Support groups can provide a range of such information via websites and newsletters as well as through phone helplines and group meetings for both families and professionals.13, 20 They can also work in collaboration with the MDT to help families as well as affected people in seeking appropriate medical care and improve patients’ understanding of their condition as well as the reasons for medical therapy.7 Alongside the formal psychological support provided by the specialist clinical psychologist, support groups can also offer invaluable peer support to families and individuals affected by DSD. By being in touch with others with a similar condition and belonging to a support group, people can gain a sense of empowerment and the whole experience may also normalize a condition that may have previously been perceived as a source of stigma.21 Healthcare professionals rely on support groups for guidance on the development of healthcare strategy as well as for providing the opportunity to interact with affected people at national support group meetings and conferences.22 Contact details of national support groups and web resources such as AIS Support Group (aissg.org), CAH Support Group (livingwithcah.com) and dsdfamilies.org should be supplied as routine as part of any written information. Whilst such groups and resources are not subject to a standard process of national accreditation in the UK, the co‐involvement of local clinical experts as advisors has allowed these groups to function within the framework of clinical practice in the UK. However, it is also possible that families may prefer to talk to local families affected in a similar way and the remit of regional services should include the creation of a local pool of helpers and locally organized support and education days. Some parents may not wish to access support groups or meet other families, and the members of the MDT should also be aware and over time review these wishes.
Which newborn should be investigated and how extensively?
It is generally accepted that investigations are necessary in those cases where the appearance of the genitalia is so ambiguous that sex assignment is not possible at birth or the appearance is not consistent with any prenatal genetic tests. However, the extent of genital ambiguity may depend on the expertise of the observer and prior to presentation to a clinical expert, the label of ambiguous genitalia is often assigned to newborns where the most appropriate sex of rearing is not immediately clear to those present at the child's birth. The birth prevalence of atypical genitalia may be as high as 1 in 300 births,23 but the birth prevalence of a condition that may lead to true genital ambiguity on expert examination may be as low as 1 in 5000 births.24
Besides those whose genitalia are truly ambiguous, in the clinical situation, infants can often be divided into those who are apparently a boy with atypical genitalia and those who are apparently a girl with atypical genitalia. However, it is very important to bear in mind that the same girl with congenital adrenal hyperplasia may present as an apparent girl with clitoromegaly or an apparent boy with bilateral undescended testes. When evaluating these infants, the clinical features of the external genitalia that require examination include the presence of gonads in the labioscrotal folds, the fusion of the labioscrotal folds, the size of the phallus and the site of the urinary meatus on the phallus, although the real site of the urinary meatus may, sometimes, only become clear on surgical exploration.25 These external features can be individually scored to provide an aggregate score, the external masculinization score (EMS)26 (Fig. 1). Routine systematic examination of 423 consecutive, apparently healthy, term newborn boys revealed that 412 (98%) had the maximum EMS of 12, 10 had an EMS of 11 and only 1 of 423 had an EMS of less than 11.26 In boys with atypical genitalia, a chromosomal anomaly may be present in approximately 3% of those with isolated cryptorchidism, 7% of those with hypospadias and 13% of those with a combination of cryptorchidism and hypospadias.27 In infants with proximal hypospadias (penoscrotal, scrotal, perineal), detailed biochemical and molecular studies performed over a decade ago revealed a likely cause in about 30% of cases28 and based on current reports of genetic analysis in XY DSD, a genetic cause may be identified in about 40% of cases.29 With advances in biochemical and molecular techniques, it is unclear whether the diagnostic yield is even greater now.
Figure 1.
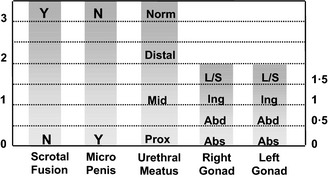
Calculating the external masculinization score (EMS) provides an objective aggregate score of the extent of masculinization of the external genitalia Each individual feature of the genitalia (phallus size, labioscrotal fusion, site of the gonads and location of urethral meatus) can be individually scored to provide a score out of 12. Microphallus refers to a phallus below the male reference range. L/S, labioscrotal; Ing, inguinal; Abs, abdominal or absent on examination.
Infants with suspected DSD who require further clinical evaluation and need to be considered for investigation by a specialist should include those with isolated perineal hypospadias, isolated micropenis, isolated clitoromegaly, any form of familial hypospadias and those who have a combination of genital anomalies with an EMS of less than 11. This will avoid unnecessary detailed investigations of boys with isolated glandular or mid‐shaft hypospadias and boys with unilateral inguinal testis. In approximately 25% of affected cases, DSD is part of a complex condition30 and the coexistence of a systemic metabolic disorder, other malformations or dysmorphic features, would lower the threshold for investigation as would a family history of consanguinity, stillbirths, multiple miscarriages, fertility problems, genital abnormalities, hernias, delayed puberty, genital surgery, unexplained deaths and the need for steroid replacement. In addition, maternal health and drug exposure during pregnancy and the pregnancy history itself may hold key information. Knowledge of birthweight in XY DSD is very helpful and as highlighted by a recent report that showed a lower yield of mutations in AR in those cases who had a lower birthweight for gestation.31
In all infants with ambiguous genitalia and/or bilateral impalpable gonads, a first tier of investigations should be undertaken to define the sex chromosomes and delineate, by pelvic ultrasound, the internal genitalia and exclude life‐threatening congenital adrenal hyperplasia (CAH) – the commonest cause of ambiguous genitalia of the newborn. This first tier should, therefore, also include plasma glucose, serum 17OH‐progesterone (17OHP) and serum electrolytes. Serum 17OHP is usually unreliable before the age of 36 h, and in the salt‐losing form of CAH, serum electrolytes usually do not become abnormal before day 4 of life. The results of PCR or FISH analysis using Y‐ and X‐specific markers should be available within one working day, and the 17OHP results should be available with a maximum of two working days in all specialist DSD centres. In situations where the level of suspicion of CAH is very high and the infant needs immediate steroid replacement therapy, further serum samples should be collected and stored before starting therapy. These should be of a sufficient volume to assess 17OHP, testosterone, androstenedione and, possibly, renin activity or concentration, in that order of priority. At least one spot or 24‐h urine sample (at least 5 ml) for a urine steroid profile should be collected before starting therapy. The results of these initial investigations shall often dictate the second tier of investigations.
In an infant with impalpable gonads, a karyotype of 46,XX, a significantly elevated serum 17OHP and the presence of a uterus makes congenital adrenal hyperplasia (CAH) due to 21‐hydroxylase deficiency very likely. A urine steroid profile can confirm this diagnosis and can also identify other rare forms of CAH, which may also be associated with a raised 17OHP in the newborn such as 11β‐hydroxylase deficiency and 3β‐hydroxysteroid dehydrogenase deficiency. In infants with sex chromosomes other than 46,XX, a second tier of investigations is necessary to determine the presence of testes and the adequacy of androgen production and action. These tests include measurement of serum anti‐Müllerian hormone (AMH), the hCG stimulation test, urinalysis for proteinuria, further detailed imaging and laparoscopy. Confirmation of a specific diagnosis will often require further biochemical identification of a defect in the androgen biosynthesis pathway and detailed genetic analysis.
Which adolescent should be investigated and how extensively?
The initial assessment in an affected adolescent should not only start the process of diagnosis but should also be used to develop a rapport with the patient. In adolescents with an existing DSD, transferring to adult services is an opportunity to review the diagnosis and consider further investigations. An appropriate hospital setting is very important for the sensitive management of complex conditions with full access to the necessary medical, nursing and psychological care. Whilst the explanation of the diagnosis to the patient and the family is critical, this needs to be performed sensitively and carefully and expert psychological input is essential over a period of time.
Adolescents may typically present with a suspected DSD in three ways – a girl with primary amenorrhoea (with or without breast development), a girl who virilizes at puberty or a boy with pubertal delay (Fig. 2). The potential psychological impact of examinations and medical photography should be considered and for the adolescent, a thorough physical examination by a surgeon and a gynaecologist, depending on the sex of the adolescent, may only be appropriate under an anaesthetic.32
Figure 2.
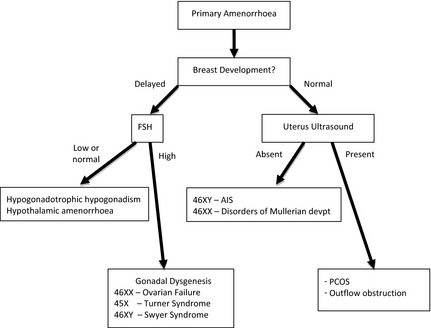
Approach to investigating adolescent girls with primary amenorrhoea.
In girls with primary amenorrhoea, investigations should be considered at the age of 14 years if there is no pubertal development and at 16 years if other aspects of puberty, particularly breast development, have progressed normally. History should include a family history and an assessment of coexisting chronic disease, exercise and weight changes. Physical examination should include measurement of blood pressure, height and weight and assessment of secondary sexual characteristics including clitoral enlargement. Vaginal examination to assess vaginal length is rarely indicated if imaging is informative and should be clearly explained and performed by a gynaecologist. An initial investigation screen should comprise measurements of serum electrolytes, LH, FSH, prolactin, TSH, FT4, SHBG, androstenedione, oestradiol, testosterone and transabdominal pelvic ultrasound by a sonographer who has experience of ultrasonography in children/adolescents and is aware of normative data. Raised gonadotrophins or an absent uterus in the presence of normal breast development is the indication for a karyotype.
The appearance of clitoromegaly and hirsutism at puberty in the presence of primary amenorrhoea is a classical presentation of two 46,XY DSDs: 17β‐hydroxysteroid dehydrogenase type 3 deficiency and 5α‐reductase type 2 deficiency. It is less typical of partial androgen insensitivity syndrome (PAIS) which is usually associated with ambiguous genitalia at birth. In all these conditions, Müllerian structures will not be detectable. Also, in partial gonadal dysgenesis, alterations in steroidogenic factor‐1 (SF‐1/NR5A1) and ovotesticular DSD, the mild clitoromegaly that may have been present at birth, may have been overlooked but becomes a more prominent feature at adolescence. The differential diagnosis would also include congenital adrenal hyperplasia and androgen‐secreting tumours of the ovary or adrenal gland; in all these cases, Müllerian structures are present. Investigations include serum measurements of LH, FSH, DHEAS, SHBG, androstenedione, testosterone, dihydrotestosterone and 17OHP. A 24‐h urine collection for urinary steroid profile will confirm 5α‐reductase type 2 deficiency, CAH or adrenocortical tumour. A pelvic ultrasound will assess the presence of a uterus and determine the need for a karyotype.
Although the commonest cause of delayed puberty is constitutional delay, all boys with delayed puberty who are over the age of 14 years should be assessed. Overweight boys need careful examination so that a buried penis is not mistaken for micropenis. Rarely, PAIS, a disorder of testosterone biosynthesis or mild forms of testicular dysgenesis, can present in this age group, especially if there is a history of hypospadias repair or orchidopexy. Investigations include a bone age and serum measurements of LH, FSH, testosterone and prolactin. For those with raised gonadotrophins, karyotype should be performed to exclude disorders such as Klinefelter's syndrome (47,XXY and variants) or 45,X/46,XY mosaicism.
The role of the clinical geneticist
Establishing a specific molecular diagnosis is helpful in the clinical management of cases and in offering accurate genetic counselling for the family. In those cases, where a clear steroidogenic defect has been identified biochemically, targetted single‐gene analysis will confirm the diagnosis in most cases. However, in 46,XY DSD with no clear abnormality of steroidogenesis, the yield from diagnostic genetic testing has often been poor, costly and limited, with less than 50% of affected cases having an identified genetic alteration. With the advent of genomic medicine, the ability to better diagnose, predict and treat disease is anticipated to transform many aspects of care.32 For individuals with a DSD, its utility may reside in ending diagnostic uncertainty.
Vast improvements in genetic sequencing technology combined with a huge reduction in costs has provided a stimulus for change, with next‐generation sequencing and whole‐genome‐and‐exome sequencing (WGES) becoming available in clinical practice (Fig 3).33 Diagnostic DNA laboratories are moving from single‐gene sequencing (sequential analysis) to next‐generation sequencing assays (parallel testing), designed to sequence multiple DSDs genes on a targeted panel in one analysis or whole‐exome sequencing with predetermined filters that target DSD genes.29 A targeted panel is advantageous as it yields high‐quality coverage of the genes of interest, whilst minimizing the risk of incidental findings. For example, a panel of thirty‐two 46, XX DSD and 46, XY DSD genes (to complement CYP21A2 testing) with a turnaround time of 80 days has been developed in one UK Genetic Regional Laboratory.34 Currently, most targeted panels interrogate 5–200 genes, in comparison to the 3000 known genes currently covered by whole‐exome sequencing.35
Figure 3.
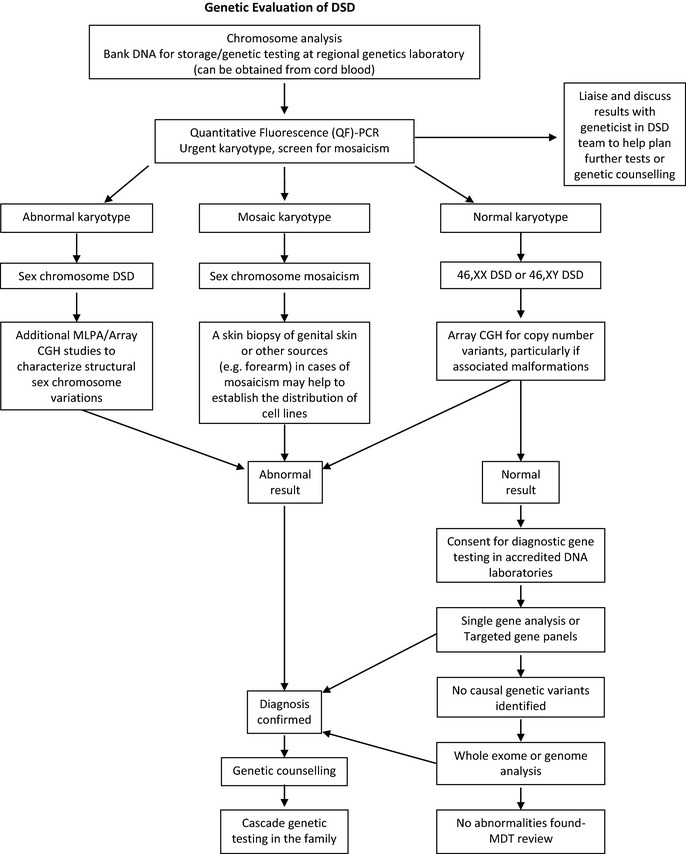
Role of the clinical genetics service within the specialist DSD team. MLPA, mutation ligation‐dependent probe amplification; CGH, comparative genomic hybridisation.
The clinical geneticist at the specialist DSD centre can evaluate complex genetic syndromes and advise which genetic testing technique is appropriate and cost‐effective for each clinical situation, once urgent karyotype testing and copy number variant analysis have been completed (Fig 3).36 With the advent of targeted panels and WGES, more extensive biochemical and radiological investigations might be reserved until answers from analysis are obtained, with the potential to avoid further costly investigations.29 However, there are challenges in bringing WGES into routine practise, which is expected to become mainstream in the next 5 years.37 Pretest counselling needs to be broader, to cover all potential test outcomes, whilst explaining the limitations of this approach. In addition, in line with international recommendations, patients and parents should be informed about the possibility of unsolicited findings of medically relevant disease variants.38 It is, therefore, recommended that procedures such as WGES should only currently be considered following informed consent and as part of an ethics approved study.
Close involvement of the clinical genetics service can ensure that the MDT covers all aspects of genetic counselling including provision of information to the family, the mode of inheritance of the disorder and the choices or options available for dealing with this risk. Established links with the clinical genetics service are also useful when considering prenatal testing or interventions such as steroid therapy in CAH (Fig. 3). As the scope for prenatal noninvasive diagnosis using free‐floating foetal DNA becomes more realistic, the close involvement of the clinical geneticist at a very early stage in at‐risk pregnancies will become even more important.39
Assessment of anatomy
Examination and assessment by a paediatric surgeon with experience of DSD is critically important in the affected newborn. Combining expert physical examination with endoscopic visualization and radiological assessment can provide information on the location and state of the gonads, the urogenital sinus and Müllerian structures. During this initial assessment, the anatomy and drainage of the renal tract should also be assessed.40, 41
Ultrasonography is the first‐line imaging modality and includes analysis in the adrenals, kidneys, pelvis, inguinal regions and scrotum where appropriate. In the neonate, the uterus, ovaries and adrenals should be easily identifiable, but the reliability is child and operator dependent. It should also be borne in mind that the presence of a uterine structure does not guarantee later function and intra‐abdominal testes and streak gonads are difficult to identify ultrasonography. In the adolescent, it is sometimes difficult to confirm the presence of a prepubertal uterus by ultrasonography and there may be a place for repeat imaging after a 6‐month course of oestrogen. Magnetic resonance imaging (MRI) should be reserved for cases where ultrasonography has failed to delineate the relationship of the Müllerian structures and where there are abnormalities of the urinary tract. High‐resolution MRI should include the pelvis and perineum and use high‐resolution T2 with and without fat saturation and T1 in three planes where possible. MRI can identify extra‐abdominal ectopic testes and the presence of the spermatic cords, but is of less value in trying to define the presence and character of intra‐abdominal testes or streak gonads. In adolescents, MRI can delineate structural anomalies such as hydrometrocolpos or hydronephrosis and identify secretory tumours, but its value in identifying premalignant changes in retained testes remains questionable.42 In general, T1 imaging of the upper abdomen in adolescents is not required unless there is an adrenal mass in which case contrast enhancement shall also be required.43, 44
Nowadays, the ‘genitogram’ is not routinely performed for diagnostic purposes. It has been superseded by endoscopic examination of the genital tract (genitoscopy), which provides a more detailed and thorough assessment. However, at the time of surgery, stents can be accurately placed in various structures to allow a more focused radiological examination. These investigations need to provide information on the length of the urogenital sinus, the associated Müllerian structures and the relationship of the urethra and its sphincter. In 46,XX DSD, genitoscopy can assess drainage of both the bladder and Müllerian structures and provide a detailed assessment of the urogenital anatomy. In 46,XY DSD, endoscopic examination can be used to identify any Müllerian remnants that arise from the posterior urethra.
Genitoscopy can be augmented by laparosocopy, but this is not necessary in all cases of DSD. It is a very effective method of visualizing the internal sex organs and facilitates direct inspection, biopsy or excision of intra‐abdominal gonads. However, as laparoscopy can only visualize intraperitoneal structures, Müllerian remnants deep within the pelvis or closely attached to the bladder may not be seen. In 46,XY DSD, laparoscopy is clearly indicated in all infants with impalpable testes where the gonads need to be identified and brought down to the scrotum if possible. Laparoscopy can also be used in adolescents who present with a DSD. However, MRI may be a more suitable first‐line investigation for defining the anatomy. It should be borne in mind that it is possible that in the absence of prior oestrogen exposure, a uterus may be difficult to identify on ultrasound, MRI and even laparoscopy and in a case of raised suspicion, a trial of oestrogen therapy would be advisable.
Steroid measurement and its interpretation
Steroid hormone analysis is a vital component of the biochemical evaluation, but the method of analysis can have a significant impact on the result.45 Analysis is most often performed by nonextraction or nonchromatographic (direct) immunoassays on automated platforms, and these are subject to concerns of analytical specificity.46, 47 Liquid chromatography linked with tandem mass spectrometry (LC‐MS/MS) allows multiple analyte analysis from a single sample whilst maintaining analytical specificity.48 Thus, in cases of DSD, plasma or serum steroids should be measured by either LC‐MS/MS or immunoassays after organic solvent extraction. As these are more labour intensive, there may be an impact on the turnaround time for results and on clinical decision‐making. Close communication between the clinical and biochemistry personnel within the DSD team is vital to enable correct interpretation of laboratory results and awareness that results should be available in a timely manner.
Urinary steroid profile (USP) analysis by gas chromatography mass spectrometry (GC‐MS) provides qualitative and quantitative data on excretion of steroid metabolites. It is ideal for detecting altered steroid metabolites, especially in cases of CAH where the activity of a combination of steroidogenic enzymes can produce unusual metabolites, which can cross‐react in traditional serum assays.49 The diagnosis of rarer forms of CAH such as P450 oxidoreductase deficiency (PORD) is best established using urinary GC‐MS analysis as it allows for concurrent determination of all adrenal‐derived steroid metabolites.50, 51 As gonadotrophins, androgens and precursors fluctuate markedly over the first few months of life and may lead to a diagnostically blind window, there is a place to consider an early neonatal collection as well as further samples at a later stage. A urine sample can be frozen and stored for many years and may help with a review of the diagnosis at a later stage. USP is not appropriate for suspected cases of 5α‐reductase type 2 deficiency until after 3 months of age as diagnostic pairs of 5β‐to 5α‐reduced metabolites are not detectable until then.
Normally, infants, particularly, boys, have significant changes in steroid and other endocrine hormone concentrations during the first 100 days of birth.46, 52 In boys, serum testosterone and DHT may initially be high at birth but decline to less than 1 nmol/l or undetectable, respectively. Concentrations then rise from around day 30 after birth to peak at day 70 before declining to normal prepubertal concentrations.51 These normal variations may influence the interpretation of sex steroid and gonadotrophin measurements as well as the results of the hCG stimulation test. Furthermore, the actual value for the hormone concentration will vary depending on the assay methodology.
Serum Anti‐Müllerian Hormone (AMH)
AMH, also known as Müllerian inhibiting substance (MIS), is strongly expressed in Sertoli cells from the time of testicular differentiation to puberty and to a much lesser degree in granulosa cells from birth to menopause and is widely used nowadays to assess ovarian reserve.53 Published information on circulating AMH concentrations have to be interpreted with caution due to differences in the way immunoassays are standardized and the units used for measurement. It is therefore important to liaise with the specialist clinical biochemist to ensure appropriate reference ranges are used for interpretation. In boys, AMH is detectable at birth at a much higher circulating concentrations than in girls and these concentrations rise over infancy before gradually declining at puberty. Therefore, up to date, age, sex and method‐related reference ranges are necessary for interpretation.54 In male neonates, levels that are close to the lower end of the normal range should be repeated later in infancy as they should rise further in boys with normal testes. As summarized in Table 2, measurement of AMH is a powerful tool to assess Sertoli cell activity in children with suspected DSD and may also have a diagnostic utility in conditions associated with androgen deficiency or insensitivity 54, 55 (Table 2). The discriminant value of AMH value in cases of bilateral anorhcia is so high that an undetectable AMH in such a case may avoid the need for invasive surgical exploration.56
Table 2.
Interpretation of serum AMH concentration in DSD
| Serum AMH | Testicular tissue | Interpretation |
|---|---|---|
| Undetectable or very low | Absent | 46,XX CAH |
| Complete gonadal dysgenesis | ||
| PMDS due to AMH gene defect | ||
| Congenital anorchia | ||
| Within female age‐related reference range | Usually absent | 46,XX CAH |
| Complete gonadal dysgenesis | ||
| Dysgenetic testes or ovotestes | ||
| Below male or above female age‐related reference range | Present | Dysgenetic testes |
| Ovotestes | ||
| Within male age‐related reference range | Usually normal | Non‐specific XY,DSD Hypogonadotrophic hypogonadism |
| PMDS due to AMH‐R defect | ||
| 46,XX testicular DSD | ||
| Ovotestes | ||
| Above male age‐related reference range | Present | AIS esp. CAIS |
| 5α‐reductase deficiency | ||
| Testosterone biosynthetic defect | ||
| Leydig cell hypoplasia |
The human chorionic gonadotrophin (hCG) stimulation test
Stimulation with hCG allows the identification of functioning testicular tissue as well as biosynthetic defects in testosterone synthesis (Fig. 4). However, it is an invasive test that should only be performed as a second‐line investigation after discussion with the paediatric endocrinologist in the regional DSD team. Most protocols for hCG stimulation in the UK use intramuscular hCG 1000–1500 units on three consecutive days.57 This can be followed by further hCG stimulation with 1500 units on 2 days per week for the following 2 weeks. In young infants and adolescents, 3 days of hCG stimulation may be sufficient,58 and in the very young infant with an intrinsically active gonadal axis, an hCG stimulation test may not be necessary if serial blood samples show raised serum testosterone concentrations. A testosterone response to hCG may be labelled as normal if absolute testosterone concentrations reach a level that is above the upper limit of the normal prepubertal range, or rise by more than twice the baseline value.55, 57, 58, 59 As a minimum, other androgens that should be assessed include dihydrotestosterone (DHT) and androstenedione. For these two metabolites, the post‐hCG, day 4 sample is more important than the pre‐hCG sample on day 1. If there are sufficient samples to analyse on day 4, there does not seem to be any additional benefit of analysing a sample for these two metabolites on day 22.58 The day 22 sample that is collected for testosterone measurement should be stored and can be used to measure DHT or androstenedione if a sufficient sample was not available at day 4.
Figure 4.
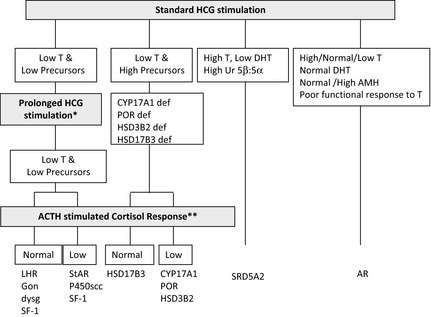
Interpretation of the results of the hCG stimulation test when investigating XY DSD and pointers for consideration of prolonged hCG stimulation and ACTH stimulation. Prolonged hCG stimulation test should be considered in those cases where there is a poor testosterone response to a standard hCG stimulation test. Synacthen stimulation test should be considered in those cases who show a poor testosterone response to hCG stimulation. 46,XY children with lipoid CAH due to a StAR defect or P450scc deficiency due to CYP11A1 defect will have female genitalia and present in a salt‐losing state in the first days or weeks of life before Synacthen performed.
In routine cases of XY DSD, a persistently low AMH may have a high predictive value for a low hCG‐stimulated testosterone concentration, but a normal AMH has a low predictive value for a normal hCG‐stimulated testosterone value.54 These relationships between the two variables do not apply to cases of persistent Müllerian duct syndrome where there is an intrinsic defect of AMH or the AMH receptor. There is no evidence that a urine steroid profile or a serum AMH checked after hCG stimulation has any added diagnostic value. In the presence of a poor testosterone response following hCG stimulation, assessment of adrenal function by a standard short Synacthen stimulation test should be considered in all cases. There is less experience as well as a lower demand for a corresponding test to assess ovarian tissue, but recent reports of ovarian hormones following stimulation with FSH need to be explored further.60, 61 There is currently insufficient evidence to recommend that everybody with XY DSD should have a Synacthen stimulation test, but clinicians should be aware of the clear association between some forms of DSD and primary adrenal insufficiency and should consider thorough assessment of adrenal function in those diagnoses where an association has already been described and in those with any clinical suspicion of adrenal insufficiency, especially those with low steroid precursors on USP.
XX DSD
46,XX DSD can be classified into disorders of ovarian development, conditions with androgen excess and other syndromes, which are often associated with other developmental abnormalities.
Congenital adrenal hyperplasia (CAH) is the commonest cause of 46,XX DSD with ambiguous genitalia in the neonatal period or early infancy and is characterized by androgen excess and a variable alteration in glucocorticoid and mineralocorticoid function and a specific profile of steroid hormones.62, 63 This profile can identify the enzyme defects including deficiency of 21α‐hydroxylase (90–95% of cases), 11β‐hydroxylase (4–8% of cases), 3β‐hydroxysteroid dehydrogenase type 2 (rare) and P450 oxidoreductase (unknown prevalence). P450 oxidoreductase deficiency (PORD) biochemically manifests as apparent combined CYP17A1 and CYP21A2 deficiency, sometimes also resembling CYP19A1 (aromatase) deficiency. Unlike other forms of CAH, PORD is characterized by increased androgen concentrations only during the prenatal and early neonatal period, but rapidly develop sex hormone deficiency. Further details of these enzyme defects are outlined in Table 3.
Table 3.
Characteristics of 46, XX DSD due to androgen excess
| Inheritance and Gene | Genitalia | Wolffian duct derivatives | Mullerian duct derivatives | Gonads | Typical signs and symptoms | Hormone profile | |
|---|---|---|---|---|---|---|---|
| 21‐hydroxylase def | Autosomal Recessive, CYP21A2 | Ambiguous | Absent | Normal | Ovary | Severe adrenal insufficiency in infancy ± salt loss; moderate to severe androgenization at birth |
Decreased cortisol and/or mineralocorticoids. Increased 17‐hydroxyprogesterone, 21‐deoxycortisol, androstenedione, testosterone, and/or plasma renin (activity) |
| 11β‐hydroxylase def | Autosomal Recessive, CYP11B1 | Ambiguous | Absent | Normal | Ovary | Adrenal insufficiency in infancy; moderate to severe androgenization at birth; arterial hypertension often developing at different ages |
Decreased cortisol, corticosterone, aldosterone, and or plasma renin (activity) Increased 11‐deoxycortisol, 11‐deoxycorticosterone, androstenedione, testosterone |
| 3β‐hydroxysteroid dehydrogenase II def | Autosomal Recessive, HSD3B2 | Commonly clitoromegaly or mild virilization can also be normal | Absent | Normal | Ovary | Severe adrenal insufficiency in infancy ± salt loss, androgenization during childhood and puberty, premature pubarche | Increased concentrations of Δ5 C21‐ and C19‐ steroids, 17 hydroxypregnenolone and DHEA suppressible by dexamethasone |
| P450 oxidoreductase def | Autosomal Recessive, POR | Ambiguous or normal female | Absent | Normal | Ovary |
Variable androgenization at birth and puberty, glucocorticoid deficiency, features of skeletal malformations. Maternal androgenization during pregnancy onset second trimester possible |
Combined P450c17 and P450c21 insuff, normal or low cortisol with poor response to ACTH stim, elevated 17‐hydroxyprogesterone, testosterone, progesterone and corticosterone; low oestradiol. |
| P450 aromatase def | Autosomal Recessive, CYP19A1 | Ambiguous | Absent | Normal | Ovary | Delayed bone age, development of ovarian cysts during infancy, childhood and puberty. Maternal androgenization during pregnancy | High androgens in cord blood, androgens may stay elevated or normalize soon after birth |
46,XX DSD also includes disorders of gonadal development including 46,XX ovotesticular DSD and 46,XX testicular DSD. 46,XX ovotesticular DSD commonly presents at birth with ambiguous genitalia and progressive virilization during puberty. In contrast, individuals with 46,XX testicular DSD usually have a male phenotype and absent Müllerian structures and are often diagnosed after karyotype analysis during work‐up for infertility. In 46,XX testicular DSD, about 80–90% of patients will have Y chromosomal material including a translocated SRY gene, which is only rarely detected in 46,XX ovotesticular DSD. In other cases of 46,XX testicular DSD, duplications of the SOX9 gene and mutations of the RSPO1 gene have been described. In those with a suspicion of 46,XX ovotesticular DSD, functional testing will require detection of testicular and ovarian tissue by a combination of biochemical testing, imaging and surgical exploration.
Disorders of Müllerian development are another group of 46,XX DSD and in these cases, ovarian function is usually normal but often associated with cloacal anomalies and other characteristic malformations. Although most cases of Müllerian development disorders are not associated with androgen excess, the presence of the latter, particularly in the adolescent, should alert the clinician to a possible abnormality of the WNT4 gene.
XY DSD with low testosterone and low precursors
The differential diagnosis of 46,XY DSD associated with low testosterone and low precursors includes: high defects in steroid synthesis (steroidogenic acute regulatory (StAR) protein, P450 side chain cleavage(scc) enzyme/CYP11A1, sometimes Smith‐Lemli‐Optiz/DHCR7); LH receptor defects (LHCGR); and partial and complete forms of gonadal (testicular) dysgenesis (Table 4).
Table 4.
Characteristics of 46, XY disorders of sex development
| Inheritance and Gene | Genitalia | Wolffian duct derivatives | Mullerian duct derivatives | Gonads | Typical features | Hormone profile | |
|---|---|---|---|---|---|---|---|
| Leydig cell hypoplasia | Autosomal Recessive, LH/HCGR | Female, hypospadias or micropenis | Hypoplastic | Absent | Testes | Underandrogenization with variable failure of sex hormone production at puberty | Low T and DHT, elevated LH and FSH, exaggerated LH response to LHRH, poor T and DHT response to hCG stimulation |
| Lipoid CAH | Autosomal Recessive, StAR | Female, rarely ambiguous or male | Hypoplastic or normal | Absent | Testes | Severe adrenal insufficiency in infancy with salt loss, failure of pubertal development, rare cases associated with isolated glucocorticoid deficiency | Usually deficient of glucocorticoids, mineralocorticoids and sex steroids |
| P450SCC def | Autosomal Recessive, CYP11A1 | Female, rarely ambiguous or hypospadias | Hypoplastic or normal | Absent | Testes | Severe adrenal insufficiency in infancy with salt loss ranging to milder adrenal insufficiency with onset in childhood | Usually deficient of glucocorticoids, mineralocorticoids and sex steroids |
| 3β‐hydroxysteroid dehydrogenase II def | Autosomal Recessive, HSD3B2 | Ambiguous, hypospadias | Normal | Absent | Testes | Severe adrenal insufficiency in infancy ± salt loss, poor androgenization at puberty with gynaecomastia | Increased concentrations of Δ5 C21‐ and C19‐ steroids, 17 hydroxypregnenolone and DHEA suppressible by dexamethasone |
| Combined 17α‐hydroxylase/17,20‐lyase def | Autosomal Recessive, CYP17A1 | Female, ambiguous, hypospadias or micropenis | Absent or hypoplastic | Absent | Testes | Absent or poor virilization at puberty, gynaecomastia, hypertension | Decreased T, increased LH and FSH, increased plasma deoxycorticosterone, corticosterone and progesterone, decreased plasma renin activity, low renin hypertension with hypokalaemic alkalosis |
| Isolated 17,20‐lyase def | Autosomal Recessive, CYP17A1, usually affecting key redox domains, alternatively caused by cytochrome b5 mutations (CYB5) | Female, ambiguous or hypospadias | Absent or hypoplastic | Absent | Testes | Absent or poor androgenization at puberty, gynaecomastia | Decreased T, DHEA, androstenedione and oestradiol, abnormal increase in plasma 17‐hydroxyprogesterone and 17‐hydroxypregnenolone, increased LH and FSH, increased ratio of C21‐deoxysteroids to C19‐steroids after hCG stim |
| P450 oxidoreductase def | Autosomal Recessive, POR | Ambiguous, hypospadias or normal male | Absent or hypoplastic | Absent | Testes | Variable androgenization at birth and puberty, glucocorticoid deficiency, features of skeletal malformations. Maternal androgenization during pregnancy onset second trimester possible | Combined P450c17 and P450c21 insuff, normal or low cortisol with poor response to ACTH stim, elevated 17‐hydroxyprogesterone, T low |
| 17β‐hydroxysteroid dehydrogenase type 3 def | Autosomal Recessive HSD17B3 | Female, ambiguous, blind vaginal pouch | Present | Absent | Testes | Androgenization at puberty, gynaecomastia variable | Increased plasma estrone, decreased ratio of testosterone/androstenedione and oestradiol after hCG stim, increased FSH and LH |
| 5α‐reductase‐2 def | Autosomal Recessive SRD5A2 | Ambiguous, micropenis, hypospadias, blind vaginal pouch | Normal | Absent | Testes | Decreased facial and body hair, no temporal hair recession, prostate not palpable | Decreased ratio of 5α/5β C21‐ and C19‐ steroids in urine, increased T/DHT ratio beforeand after hCG stim, modest increase in LH, decreased conversion of T to DHT in vitro |
| CAIS | X‐linked Recessive AR | Female with blind vaginal pouch | Often present depending on mutation type | Absent or vestigial | Testes | Scant or absent pubic and axillary hair, breast development and female body habitus at puberty, primary amenorrhoea | Increased LH and T, increased oestradiol, FSH levels normal or slightly increased, resistance to androgenic and metabolic effects of T (may be normal in some cases) |
| PAIS | X‐linked Recessive AR | Ambiguous with blind vaginal pouch, isolated hypospadias, normal male with infertility (mild) | Often normal | Absent | Testes | Decreased to normal axillary and pubic hair, facial and body hair, gynaecomastia common at puberty | Increased LH and T, increased oestradiol, FSH levels may be normal or slightly increased, partial resistance to androgenic and metabolic effects of T |
DHT, dihydrotestosterone; FSH, follicle‐stimulating hormone; hCG, human chorionic gonadotropin; LH, luteinising hormone; T, testosterone; DHEA, dehydroepiandrosterone; ACTH, adrenocorticotropin hormone.
Of note, complete or partial combined 17α‐hydroxylase/17,20‐lyase deficiency (CYP17A1) may also present with ‘low testosterone and low precursors’ if DHEAS and androstenedione are the only intermediates measured. The actual diagnosis can be reached by the assessment of adrenal function by measuring ACTH, ACTH‐stimulated cortisol, PRA, DOC, corticosterone, aldosterone, measurement of Δ5 (pregnenolone, 17OHPreg) and Δ4 (progesterone, 17OHP) precursors or urine steroid analysis. Isolated 17,20‐lyase deficiency, cytochrome b5 deficiency and PORD might also be diagnosed by this approach. Proximal blocks (StAR, P450scc) in the pathway affect steroidogenesis in the adrenal gland as well as the developing gonad.
LH receptor defects (‘Leydig cell hypoplasia’) typically result in elevated basal LH, hyperresponsive LH to GnRH stimulation, low precursors and testosterone, and impaired androgen response to hCG stimulation. No Müllerian structures will be present, and adrenal function is normal. A spectrum of phenotypes has been reported including ambiguous genitalia and micropenis. In some cases, basal LH may not be elevated at times when the HPG axis is quiescent (6 months to late childhood).
In complete gonadal dysgenesis (‘Swyer syndrome’), affected people will usually have a female phenotype with intra‐abdominal streak gonads and a risk of tumour development.2 In some situations, ovotestes or even undifferentiated gonadal tissue may be found.64, 65, 66 Müllerian structures are usually present due to impaired AMH secretion in early foetal life. Androgens and their precursors will be low, LH elevated, depending on age, and a poor or absent testosterone response to hCG stimulation is seen. AMH concentrations will be low or undetectable, and adrenal function is usually normal unless the underlying defect is in steroidogenic factor‐1 (NR5A1) or related adrenal or gonadal factors.
Partial gonadal (testicular) dysgenesis can present with a spectrum of phenotypes ranging from clitoromegaly, to ambiguous genitalia or severe hypospadias. Müllerian structures may or may not be present, and testes of variable size and architecture are present along the path of descent. The biochemical profile is similar to complete gonadal dysgenesis, but generally less severe. If mild degrees of clitoromegaly in infancy are overlooked, a 46,XY child with partial gonadal dysgenesis may first present at puberty with progressive androgenization. Genetic analysis and associated features may be useful in defining the molecular aetiology of some forms of gonadal dysgenesis.67 This group of conditions are also associated with a risk of tumour development, which may be related to the extent of androgenization of the external genitalia in the XY child.68
XY DSD with low testosterone and high steroid precursors
46,XY DSD with low testosterone and increased precursors can be caused by several variants of CAH, namely by 17α‐hydroxylase (CYP17A1) deficiency, PORD and 3β‐hydroxysteroid dehydrogenase type 2 (3βHSD2) deficiency, caused by inactivating mutations in the corresponding genes CYP17A1, POR and HSD3B2, respectively. In addition, 46,XY DSD with low testosterone and increased precursors can typically be found in individuals affected by 17β‐hydroxysteroid dehydrogenase type 3 (17βHSD3) deficiency, caused by HSD17B3 mutations (Table 4).
Deficiency of CYP17A1 leads to CAH in about 1% of cases of 46,XY DSD. Characteristically, affected individuals present with a female genitalia and low DHEA, androstenedione and testosterone. There is an increase in mineralocorticoid synthesis, and although there may be cortisol deficiency, this is rarely manifested, as corticosterone can also bind and activate the glucocorticoid receptor. In PORD, sex steroids are characteristically low, sometimes low normal, while pregnenolone and progesterone and their metabolites accumulate, as expression of the combined block of CYP21A2 and CYP17A1 activities. Although there is often a relative preponderance of mineralocorticoid over glucocorticoid metabolites in affected cases, hypertension only manifests in adolescence or later. Although baseline glucocorticoid secretion is usually sufficient, in the majority of cases, the stress response to ACTH is significantly impaired, requiring at least stress dose hydrocortisone cover or permanent glucocorticoid replacement. 3β‐HSD2 (also termed Δ4‐Δ5 isomerase) deficiency invariably leads to glucocorticoid deficiency as well as a variable degree of mineralocorticoid deficiency, and its characteristic features are outlined in Table 4. 17β‐HSD3 deficiency is responsible for the conversion of androstenedione to testosterone in the gonad and has no effect on adrenal steroidogenesis. Plasma steroids characteristically show increased androstenedione levels, while testosterone levels are concurrently low, particularly after hCG stimulation. However, a low testosterone to androstenedione ratio may also occur in cases of gonadal dysgenesis and the reliability of a low ratio in identifying 17β‐HSD3 deficiency is unclear. In urine, the typical finding is an increase in the androgen (and androstenedione) metabolites, androsterone (An) and etiocholanolone (Et), but it is unclear whether this applies across all age groups.49
XY DSD with normal testosterone, normal precursors and low DHT
The type 2 isoenzyme of 5α‐reductase type 2 (SRD5A2) is highly expressed in androgen‐sensitive tissues69 and converts testosterone to the more potent androgen, dihydrotestosterone (DHT) required for the development of external male genitalia. At birth, the external appearance of the genitalia of an infant with SRD5A2 deficiency can range from a completely female phenotype to a range of hypospadias severity or, rarely, isolated micropenis. A positive family history is often present in this autosomal recessive condition. In serum, the testosterone:DHT ratio following hCG stimulation often exceeds 30:1, but there are several reports of cases with a lower ratio.70 In infants over 3–6 months, the defect should be easily identifiable simply on a urine sample, which shows a decreased ratio for 5α:5β‐reduced C21 and C19 steroids and thus can be reached in a child who had early gonadectomy. Early diagnosis of this condition is important as the affected infant may need sex reassignment if initially raised as a girl, and definitive diagnosis in a highly suspicious case may require access to a diagnostic genetics service with a quick turnaround time. In the infant is raised as a boy, application of topical DHT cream may be a method of assessing the potential of the genitalia to virilize over the longer term.
XY DSD with normal testosterone, normal precursors and normal DHT
A defect in androgen signalling is most likely due to dysfunction of the androgen receptor (AR) and mutations resulting in a complete lack of function of the AR cause complete androgen insensitivity syndrome (CAIS).71 This presents in the newborn infant as a discordance between a female phenotype and a prenatal karyotype of 46,XY, as a postnatal check because of a positive family history or as inguinal swellings in a girl. CAIS usually presents in adolescence as primary amenorrhoea with normal breast development. The presence of pubic hair is often reported in CAIS and should not be used to exclude the diagnosis. Mutations that result in some residual AR function and varying degrees of androgenization cause partial androgen insensitivity syndrome (PAIS). Although children with AIS typically have normal testosterone and DHT response to hCG stimulation and a normal urinary steroid profile, some demonstrate a poor response to hCG stimulation.57, 72 The serum AMH concentration is normal or may even be elevated. LH levels are increased in the face of normal or elevated serum testosterone, reflecting a state of androgen resistance.55 A family history of X‐linked inheritance is informative although one‐third of cases are the result of spontaneous new mutations.
A functional assessment of androgen sensitivity may include assessing the clinical effect of a short course of testosterone or dihydrotestosterone applied on the phallus or by the effect of systemic testosterone following hCG stimulation. However, there is no consensus on the choice of androgen, dosage, method of administration, timing and duration of treatment as well as the definition of a satisfactory response in the growth of the phallus. Androgen sensitivity can be also assessed by measuring change in sex hormone‐binding globulin (SHBG), an androgen‐responsive protein that normally decreases following androgen exposure. This fall in SHBG is absent in CAIS, variable in PAIS 73 and difficult to interpret in young infants who have highly variable circulating SHBG. AR analysis may reveal a mutation in over 90% of cases with a CAIS phenotype, but only 20% of cases with a PAIS phenotype 74 and androgen receptor‐binding studies are not necessary for routine diagnosis of AIS. A number of newborns with XY DSD are loosely labelled as ‘PAIS’ when no conclusive biochemical or genetic abnormalities are identified in gonadal function, androgen synthesis or androgen action. The term PAIS should solely be used in the context of a molecular confirmation of a mutation in AR.
Networks and registers for clinical care, audit and research
It is unrealistic to expect that every major clinical centre can possess a comprehensive, multidisciplinary DSD team as outlined earlier. Furthermore, in many cases, care at a local hospital with regular telephone or videolink consultation with the regional centre may be more appropriate for reasons of both convenience and necessity (for example, adrenal crisis in CAH). For the less complex case of hypospadias, immediate multidisciplinary input may not be necessary and initial discussion and explanation of the condition with the parents does not require urgent transfer of the baby at an emotionally sensitive period. Similarly, some investigations can also be performed at local centres that are affiliated to a regional centre. It is, however, important that all personnel who may be involved in the care of an affected person have access to the regional DSD team and have the opportunity to develop themselves professionally. Some regions have overcome these hurdles with the development of a managed clinical network (www.sdsd.scot.nhs.uk). A service model such as this ensures the provision of an equitable state‐of‐the‐art service for all affected children and adolescents in a region. A formal organization allows a structured referral pathway within the region as well as beyond and provides the infrastructure for better long‐term care of the patient as close to home as possible. A network also facilitates the creation of nationally agreed protocols for the care of the affected newborn, setting and monitoring of national standards of care, rational utilization of other services such as clinical genetics and clinical biochemistry and provides a forum for education and professional development.
Research and audit are vital for the management of DSD, and clinical networks have a strong potential to drive these activities with the development of care standards including patient experience data and peer‐observation of clinical care provision. The 2005 Consensus Workshop on DSD stressed the need for the creation and maintenance of a database in centres of expertise. Clinical audit systems that collect information on clinical activity and outcome should be an integral component of national specialist services. Such databases may exist at a less formal level in many other regional centres, and until recently have lacked international uniformity and the ability to cross‐talk. A web‐based register has been approved by the UK National Research Ethics Service as a multicentre research database, which does not require any further local research approvals but does require the approval of the patient or parent.75 This web‐based register, www.I-DSD.org, which was initially supported by the European Society for Paediatric Endocrinology and an EUFP7 framework grant is currently funded by the MRC and has the potential to address many unanswered questions about long‐term outcome in these rare conditions. Such registers of patients can also facilitate the development of local circles of patients and parents with similar conditions who can support each other.
Conclusion
The rationale for investigating a newborn or an adolescent with a suspected DSD may include the need to determine the sex of rearing, to anticipate early medical problems, to explain the aetiology to the young person and the parents of an affected newborn, to support them psychologically in assimilating this knowledge and, finally, to develop a management plan that leads to optimal long‐term outcome. A rational and empathic approach that relies on the skills and knowledge of the experts within the MDT is essential for achieving these goals. An unequivocal diagnosis confirmed by biochemical and genetic means remains elusive in many cases of DSD, particularly XY DSD. The stepwise approach to reaching the final diagnosis needs to be explained to parents, and the most important goals of the initial period of assessment should be to support the affected person and the parents, assign a sex of rearing and exclude the possibility of any early medical problems.
Acknowledgements
This document is dedicated to the memory of Professor Mike Wallace who sadly passed away in the final stages of development of the original guidelines. The members of the UK DSD working group are grateful for the original support from the Society for Endocrinology and the British Society of Paediatric Endocrinology & Diabetes. Syed Faisal Ahmed is currently supported by a partnership grant from the MRC (1100236). John C. Achermann is supported by a Senior Fellowship in Clinical Science from the Wellcome Trust (098513), and Wiebke Arlt is supported by a programme grant from the MRC (0900567). Syed Faisal Ahmed and Nils Krone are also on the management committee of BMBS COST Action 1303, DSDnet.
Appendix 1.
Authors and Members of the Working Group
| S. Faisal Ahmed | British Society of Paediatric Endocrinology & Diabetes, Society for Endocrinology, Chair & Corresponding Author |
| John C. Achermann | British Society of Paediatric Endocrinology & Diabetes, Society for Endocrinology |
| Wiebke Arlt | Society for Endocrinology |
| Adam Balen | British Society for Paediatric & Adolescent Gynaecology |
| Gerry Conway | Society for Endocrinology |
| Zoe Edwards | Chartered Member of the British Psychological Society |
| Sue Elford | CLIMB CAH Support Group |
| Ieuan A. Hughes | British Society of Paediatric Endocrinology & Diabetes, Society for Endocrinology |
| Louise Izatt | British Society for Genetic Medicine, Clinical Genetics Society |
| Nils Krone | British Society of Paediatric Endocrinology & Diabetes, Society for Endocrinology |
| Harriet Miles | British Society of Paediatric Endocrinology & Diabetes |
| Stuart O'Toole | British Association of Paediatric Urologists |
| Les Perry | Association for Clinical Biochemistry, Society for Endocrinology |
| Caroline Sanders | Royal College of Nursing |
| Margaret Simmonds | AIS Support Group |
| Andrew Watt | British Society of Paediatric Radiology |
| Debbie Willis | Society for Endocrinology |
References
- 1. Biason‐Lauber, A. (2010) Control of sex development. Best Practice & Research. Clinical Endocrinology & Metabolism, 24, 163–186. [DOI] [PubMed] [Google Scholar]
- 2. Hughes, I.A. , Houk, C. , Ahmed, S.F. et al (2006) Consensus statement on management of intersex disorders. Archives of Disease in Childhood, 91, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasterski, V. , Prentice, P. & Hughes, I.A. (2010) Consequences of the Chicago consensus on disorders of sex development (DSD): current practices in Europe. Archives of Disease in Childhood, 95, 618–623. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed, S.F. , Achermann, J.C. , Arlt, W. et al (2011) UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clinical Endocrinology, 75, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brain, C.E. , Creighton, S.M. , Mushtaq, I. et al (2010) Holistic management of DSD. Best Practice & Research. Clinical Endocrinology & Metabolism, 24, 335–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liao, L.M. , Tacconelli, E. , Wood, D. et al (2010) Adolescent girls with disorders of sex development: A needs analysis of transitional care. Journal of Pediatric Urology, 6, 609–613. [DOI] [PubMed] [Google Scholar]
- 7. Karzakis, K. (2009) Fixing Sex: Intersex, Medical Authority, and Lived Experience. 1st edn. Duke University Press, Durham, USA. [Google Scholar]
- 8. Karkazis, K. , Tamar‐Mattis, A. & Kon, A.A. (2010) Genital surgery for disorders of sex development: implementing a shared decision‐making approach. JPEM, 23, 789–806. [DOI] [PubMed] [Google Scholar]
- 9. Abusaad, F.E. & Elmasri, Y.M. (2011) Effect of counseling sessions on coping strategies and anxiety among parents of children with ambiguous genitalia. Journal of American Science, 7, 674–682. [Google Scholar]
- 10. Crissman, H.P. , Warner, L. , Gardner, M. et al (2011) Children with disorders of sex development: a qualitative study of early parental experience. International Journal of Pediatric Endocrinology, 2011, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liao, L.‐M. , Simmonds, M. (2014) A values‐driven and evidence‐based health care psychology for diverse sex development. Psychology and Sexuality, 5, 83–101. [Google Scholar]
- 12. Duguid, A. , Morrison, S. , Robertson, A. et al (2007) The psychological impact of genital anomalies on the parents of affected children. Acta Paediatrica, 96, 348–352. [DOI] [PubMed] [Google Scholar]
- 13. Cull, M.L. & Simmonds, M. (2010) Importance of support groups for intersex (disorders of sex development) patients, families and the medical profession. Sexual Development, 4, 310–312. [DOI] [PubMed] [Google Scholar]
- 14. Davis, G. (2014) The Power in a name: diagnostic terminology and diverse experiences. Psychology & Sexuality, 5, 15–27. [Google Scholar]
- 15. Magritte, E. (2012) Working together in placing the long term interests of the child at the heart of the DSD evaluation. Journal of Pediatric Urology, 8, 571–575. [DOI] [PubMed] [Google Scholar]
- 16. Sanders, C. , Carter, B. & Goodacre, L. (2012) Parents need to protect: influences, risks and tensions for parents of prepubertal children born with ambiguous genitalia. Journal of Clinical Nursing, 21, 3315–3323. [DOI] [PubMed] [Google Scholar]
- 17. Nelson, P.A. , Caress, A.L. , Glenny, A.M. et al (2012) Doing the “right” thing: how parents experience and manage decision‐making for children's ‘normalising’ surgeries. Social Science and Medicine, 74, 796–804. [DOI] [PubMed] [Google Scholar]
- 18. Wiesemann, C. , Ude‐Koeller, S. , Sinnecker, G.H. et al (2010) Ethical principles and recommendations for the medical management of differences of sex development (DSD)/intersex in children and adolescents. European Journal of Pediatrics, 169, 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liao, L.M. (2003) Learning to assist women born with atypical genitalia: journey through ignorance, taboo and dilemma. Journal of Reproductive and Infant Psychology, 21, 229–238. [Google Scholar]
- 20. GrApSIA and Audí, L. (2014) ‘Past experiences of adults with disorders of sex development’ In: Hiort O., Ahmed S.F. eds. Understanding Differences and Disorders of Sex Development (DSD) Endocr Dev. Karger, Basel, vol 27, pp 138–148 (DOI: 10.1159/000363639) [DOI] [PubMed] [Google Scholar]
- 21. Bartlett, Y.K. & Coulson, N.S. (2011) An investigation into the empowerment effects of using online support groups and how this affects health professional/patient communication. Patient Education and Counselling, 83, 113–119. [DOI] [PubMed] [Google Scholar]
- 22. Creighton, S.M. , Minto, C.L. , Liao, L.M. et al (2004) Meeting between experts: evaluation of the first UK forum for lay and professional experts in intersex. Patient Education and Counseling, 54, 153–157. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed, S.F. , Dobbie, R. , Finlayson, A.R. et al (2004) Regional & temporal variation in the occurrence of genital anomalies amongst singleton births, 1988–1997 Scotland. Archives of Disease Childhood, 89, F149–F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thyen, U. , Lanz, K. , Holterhus, P.M. et al (2006) Epidemiology and initial management of ambiguous genitalia at birth in Germany. Hormone Research, 66, 195–203. [DOI] [PubMed] [Google Scholar]
- 25. Vidal, I. , Gorduza, D.B. , Haraux, E. et al (2010) Surgical options in disorders of sex development with ambiguous genitalia. Best Practice & Research. Clinical Endocrinology & Metabolism, 24, 311–324. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed, S.F. , Khwaja, O. & Hughes, I.A. (2000) Clinical features and gender assignment in cases of male undermasculinisation: the role for a masculinisation score. BJU International, 85, 120–124. [DOI] [PubMed] [Google Scholar]
- 27. Moreno‐Garcia, M. & Miranda, E.B. (2002) Chromosomal anomalies in cryptorchidism and hypospadias. Journal of Urology, 168, 2170–2172. [DOI] [PubMed] [Google Scholar]
- 28. Boehmer, A.L.M. , Nijman, R.J.M. , Lammers, B.A.S. et al (2001) Etiological studies of severe or familial hypospadias. Journal of Urology, 165, 1246–1254. [PubMed] [Google Scholar]
- 29. Ahmed, S.F. , Bashamboo, A. , Lucas‐Herald, A. et al (2013) Understanding the genetic aetiology in patients with XY DSD. British Medical Bulletin, 106, 67–89. [DOI] [PubMed] [Google Scholar]
- 30. Cox, K. , Bryce, J. , Jiang, J. et al (2014) Novel associations in disorders of sex development: findings from the I‐DSD Registry. Journal of Clinical Endocrinology and Metabolism, 99, E348–E355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lek, N. , Miles, H. , Bunch, T. et al (2014). Low frequency of androgen receptor gene mutations in 46 XY DSD and fetal growth restriction. Archives of Disease in Childhood, 99, 358–361. [DOI] [PubMed] [Google Scholar]
- 32. Creighton, S. , Alderson, J. , Brown, S. et al (2002) Medical photography: ethics, consent and the intersex patient. BJU International, 89, 67–71. [DOI] [PubMed] [Google Scholar]
- 33. Biesecker, L.G. & Green, R.C. (2014) Diagnostic clinical genome and exome sequencing. New England Journal of Medicine, 370, 2418–2425. [DOI] [PubMed] [Google Scholar]
- 34. Hughes, L. , Cole, T. , Krone, N. et al (2013) Development of a next generation sequencing panel for Disorders of Sexual Development. P165, Abstract from BSGM Sept 2013.
- 35. Amor, D.J. (2015) Future of whole genome sequencing. Journal of Paediatric Child Health, 51, 251–254. [DOI] [PubMed] [Google Scholar]
- 36. Ledig, S. , Hiort, O. , Scherer, G. et al (2010) Array‐CGH analysis in patients with syndromic and non‐syndromic XY gonadal dysgenesis: evaluation of array CGH as diagnostic tool and search for new candidate loci. Human Reproduction, 25, 2637–2646. [DOI] [PubMed] [Google Scholar]
- 37. PHG foundation briefing note: Clinical whole genome analysis. Setting the right standards for clinical genome analysis. Published July 2014 (released 1/8/14)
- 38. van El, C.G. , Cornel, M.C. , Borry, P. et al (2013) ESHG Public and Professional Policy Committee. Whole‐genome sequencing in health care. Recommendations of the European Society of Human Genetics. European Journal of Human Genetics, 21(Suppl 1), S1–S5. [PMC free article] [PubMed] [Google Scholar]
- 39. New, M.I. , Tong, Y.K. , Yuen, T. et al (2014) Noninvasive prenatal diagnosis of congenital adrenal hyperplasia using cell‐free fetal DNA in maternal plasma. Journal of Clinical Endocrinology and Metabolism, 99, E1022–E1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chertin, B. , Koulikov, D. , Alberton, J. et al (2006) The use of laparoscopy in intersex patients. Pediatric Surgery International, 22, 405–408. [DOI] [PubMed] [Google Scholar]
- 41. Denes, F.T. , Cocuzza, M.A. , Schneider‐Monteiro, E.D. et al (2005) The laparoscopic management of intersex patients: the preferred approach. BJU, 95, 863–867. [DOI] [PubMed] [Google Scholar]
- 42. Nakhal, R.S. , Hall‐Craggs, M. , Freeman, A. et al (2013) Evaluation of retained testes in adolescent girls and women with complete androgen insensitivity syndrome. Radiology, 268, 153–160. [DOI] [PubMed] [Google Scholar]
- 43. Chavhan, G.B. , Parra, D.A. , Oudjhane, K. et al (2008) Imaging of ambiguous genitalia: classification and diagnostic approach. Radiographics, 28, 1891–1904. [DOI] [PubMed] [Google Scholar]
- 44. Wright, N.B. , Smith, C. , Rickwood, A.M. et al (1995) Imaging children with ambiguous genitalia and intersex states. Clinical Radiology, 50, 823–829. [DOI] [PubMed] [Google Scholar]
- 45. Tomlinson, C. , Wallace, A.M. & Ahmed, S.F. (2004) Erroneous testosterone assay causing diagnostic confusion in a newborn infant with intersex anomalies. Acta Paediatrica, 93, 1004–1005. [DOI] [PubMed] [Google Scholar]
- 46. Tomlinson, C. , Macintyre, H. , Dorrian, C.A. et al (2004) Testosterone measurements in early infancy. Archives of Disease in Childhood. Fetal and Neonatal Edition, 89, F558–F559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Albrecht, L. & Styne, D. (2007) Laboratory testing of gonadal steroids in children. Pediatric Endocrinology Reviews, 5(Suppl 1), 599–607. [PubMed] [Google Scholar]
- 48. Nakamoto, J. & Fuqua, J.S. (2007) Laboratory assays in pediatric endocrinology: common aspects. Pediatric Endocrinology Reviews, 5(Suppl 1), 539–554. [PubMed] [Google Scholar]
- 49. Krone, N. , Hughes, B.A. , Lavery, G.G. et al (2010) Gas chromatography/mass spectrometry (GC/MS) remains a pre‐eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). Journal of Steroid Biochemistry and Molecular Biology, 22, 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shackleton, C. , Marcos, J. , Malunowicz, E.M. et al (2004) Biochemical diagnosis of Antley‐Bixler syndrome by steroid analysis. American Journal of Medical Genetics, 128A, 223–231. [DOI] [PubMed] [Google Scholar]
- 51. Krone, N. , Reisch, N. , Idkowiak, J. et al (2012) Genotype‐phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Journal of Clinical Endocrinology and Metabolism, 97, E257–E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kulle, A.E. , Riepe, F.G. , Melchior, D. et al (2010) A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age‐ and sex‐specific reference data. Journal of Clinical Endocrinology and Metabolism, 95, 2399–2409. [DOI] [PubMed] [Google Scholar]
- 53. Teixeira, J. , Maheswaran, S. & Donahoe, P.K. (2001) Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocrine Reviews, 22, 657–674. [DOI] [PubMed] [Google Scholar]
- 54. Lindhardt Johansen, M. , Hagen, C.P. , Johannsen, T.H. et al (2013) Anti‐müllerian hormone and its clinical use in pediatrics with special emphasis on disorders of sex development. Int J Endocrinol, 2013, 198698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ahmed, S.F. , Keir, L. , McNeilly, J. et al (2010) The concordance between serum anti‐mullerian hormone and testosterone concentrations depends on duration of hCG stimulation in boys with disorders of sex development. Clinical Endocrinology, 72, 814–819. [DOI] [PubMed] [Google Scholar]
- 56. Teo, A.Q. , Khan, A.R. , Williams, M.P. et al (2013) Is surgical exploration necessary in bilateral anorchia? Journal of Pediatric Urology, 9, e78–e81. [DOI] [PubMed] [Google Scholar]
- 57. Ahmed, S.F. , Cheng, A. & Hughes, I.A. (1999) Biochemical evaluation of the gonadotrophin‐gonadal axis in androgen insensitivity syndrome. Archives of Disease in Childhood, 80, 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dixon, J.R. , Wallace, A.M. , O'Toole, S. et al (2007) Prolonged human chorionic gonadotrophin (hCG) stimulation as a tool for investigating and managing undescended testes. Clinical Endocrinology, 67, 816–821. [DOI] [PubMed] [Google Scholar]
- 59. Segal, T.Y. , Mehta, A. , Anazodo, A. et al (2009) Role of gonadotropin‐releasing hormone and human chorionic gonadotropin stimulation tests in differentiating patients with hypogonadotropic hypogonadism from those with constitutional delay of growth and puberty. Journal of Clinical Endocrinology and Metabolism, 94, 780–785. [DOI] [PubMed] [Google Scholar]
- 60. Steinmetz, L. , Rocha, M.N. , Longui, C.A. et al (2009) Inhibin A production after gonadotropin stimulus: a new method to detect ovarian tissue in ovotesticular disorder of sex development. Hormone Research, 71, 94–99. [DOI] [PubMed] [Google Scholar]
- 61. Rosencrantz, M.A. , Wachs, D.S. , Coffler, M.S. et al (2010) Comparison of inhibin B and estradiol responses to intravenous FSH in women with polycystic ovary syndrome and normal women. Human Reproduction, 25, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Krone, N. , Dhir, V. , Ivison, H.E. et al (2007) Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clinical Endocrinology (Oxf), 66, 162–172. [DOI] [PubMed] [Google Scholar]
- 63. Shackleton, C.H. (2008) Genetic disorders of steroid metabolism diagnosed my mass spectrometry In: Blau N., Duren M., Gibson K.M. eds. Laboratory Guide to the Methods in Biochemical Genetics, 1st edn Springer, Berlin Heidelberg, 549–605. [Google Scholar]
- 64. Cameron, F.J. , Hageman, R.M. , Cooke‐Yarborough, C. et al (1996) A novel germ line mutation in SOX9 causes familial campomelic dysplasia and sex reversal. Human Molecular Genetics, 5, 1625–1630. [DOI] [PubMed] [Google Scholar]
- 65. Harley, V.R. , Clarkson, M.J. & Argentaro, A. (2003) The molecular action and regulation of the testis‐determining factors, SRY (sex‐determining region on the Y chromosome) and SOX9 [SRY‐related high‐mobility group (HMG) box 9]. Endocrine Reviews, 24, 466–487. [DOI] [PubMed] [Google Scholar]
- 66. Biason‐Lauber, A. , Konrad, D. , Meyer, M. et al (2009) Ovaries and female phenotype in a girl with 46, XY karyotype and mutations in the CBX2 gene. American Journal of Human Genetics, 84, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lin, L. , Achermann, J.C. (2008) Steroidogenic factor‐1 (SF‐1, Ad4BP, NR5A1) and disorders of testis development. Sexual Development, 2, 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cools, M. , Pleskacova, J. , Stoop, H. et al (2011) Mosaicism Collaborative Group. Gonadal pathology and tumor risk in relation to clinical characteristics in patients with 45, X/46, XY mosaicism. Journal of Clinical Endocrinology and Metabolism, 96, 1171–1180. [DOI] [PubMed] [Google Scholar]
- 69. Zhu, Y.S. & Imperato‐McGinley, J.L. (2009) 5alpha‐reductase isozymes and androgen actions in the prostate. Annals of the New York Academy of Sciences, 1155, 43–56. [DOI] [PubMed] [Google Scholar]
- 70. Maimoun, L. , Philibert, P. , Cammas, B. et al (2011) Phenotypical, biological, and molecular heterogeneity of 5α‐reductase deficiency: an extensive international experience of 55 patients. Journal of Clinical Endocrinology and Metabolism, 96, 296–307. [DOI] [PubMed] [Google Scholar]
- 71. Lubahn, D.B. , Brown, T.R. , Simental, J.A. et al (1989) Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proceedings of the National Academy of Sciences of the United States of America, 86, 9534–9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bouvattier, C. , Carel, J.C. , Lecointre, C. et al (2002) Postnatal changes of T, LH, and FSH in 46, XY infants with mutations in the AR gene. Journal of Clinical Endocrinology and Metabolism, 87, 29–32. [DOI] [PubMed] [Google Scholar]
- 73. Belgorosky, A. & Rivarola, M.A. (1985) Sex hormone binding globulin response to testosterone. An androgen sensitivity test. Acta Endocrinologica (Copenh), 109, 130–138. [DOI] [PubMed] [Google Scholar]
- 74. Ahmed, S.F. , Cheng, A. , Dovey, L.A. et al (2000) Clinical features, androgen receptor binding and mutational analysis in 278 reported cases of the androgen insensitivity syndrome. Journal of Clinical Endocrinology and Metabolism, 85, 658–665. [DOI] [PubMed] [Google Scholar]
- 75. Ahmed, S.F. , Rodie, M. , Jiang, J. et al (2010) The European DSD Registry – A Virtual Research Environment. Sexual Development, 4, 192–198. [DOI] [PubMed] [Google Scholar]


