Summary
Older adult patients (≥60 years) with acute myeloid leukaemia (AML) are generally considered to be poor‐risk and there is limited information available regarding risk stratification based on molecular characterization in this age group, particularly for the double‐mutant CEBPA (CEBPA DM) genotype. To investigate whether a molecular favourable‐risk genotype can be identified, we investigated CEBPA, NPM1 and FLT3 status and prognostic impact in a cohort of 301 patients aged 60 years or more with intermediate‐risk cytogenetics, all treated intensively. Overall survival (OS) at 1 year was highest in the 12 patients (4%) that were CEBPA DM compared to the 76 (28%) with a mutant NPM1 and wild‐type FLT3 (NPM1 MUT FLT3 WT) genotype or all other patients (75%, 54%, 33% respectively), with median survival 15·2, 13·6 and 6·6 months, although the benefit was short‐term (OS at 3 years 17%, 29%, 12% respectively). Combination of the CEBPA DM and NPM1 MUT FLT3 WT genotype patients defined a molecular group with favourable prognosis (P < 0·0001 in multivariate analysis), with 57% of patients alive at 1 year compared to 33% for all other patients. Knowledge of genotype in older cytogenetically intermediate‐risk patients might influence therapy decisions.
Keywords: acute myeloid leukaemia, molecular prognostication, CEBPA genotype, NPM1 and FLT3 genotype
In recent decades there have been considerable improvements in the long‐term outlook for younger adult patients with acute myeloid leukaemia (AML) (Burnett et al, 2011). Current therapy is risk‐adapted, based predominantly on cytogenetics and molecular characterization (Dohner et al, 2010; Dohner & Gaidzik, 2011; Ofran & Rowe, 2013), and consolidation of first remission by allogeneic transplantation is not usually considered in patients with either good‐risk cytogenetics or a favourable mutation profile, defined as either mutant for nucleophosmin 1 and lacking a fms‐like tyrosine kinase 3 internal tandem duplication (NPM1 MUT FLT3 WT) or double mutant for CCAAT/enhancer binding protein‐α (CEBPA DM) (Cornelissen et al, 2012; O'Donnell et al, 2012). These two mutational categories are almost totally mutually exclusive (Green et al, 2010). In our study of younger patients, the presence of an NPM1 MUT FLT3 WT genotype was associated with a higher complete remission (CR) rate and a lower relapse rate, both contributing to improved survival (Gale et al, 2008). The presence of a CEBPA DM genotype was associated with a non‐significantly higher CR rate and a significantly lower relapse rate, leading to improved long‐term survival (Green et al, 2010).
However, the median age of AML at diagnosis approximates 70 years (Derolf et al, 2009), so that the majority of patients are considered to be elderly (≥60 years), and the improvements seen in the prognosis of younger patients have not been matched by improvements in this older age group (Derolf et al, 2009; Burnett et al, 2011; Thein et al, 2013). This is attributable to both biological factors (e.g. co‐morbidities, poor performance status, pharmacokinetics and pharmacodynamics) and disease‐related factors (e.g. adverse cytogenetic and molecular aberrations, multidrug resistance and antecedent haematological disorders) (Pollyea et al, 2011; Ossenkoppele & Lowenberg, 2015).
There is limited information concerning risk stratification in the older compared to younger patients, partly because all older patients have been considered as poor‐risk. The reasons for risk stratification in the older age group, however, are different from those in younger patients. In the older patient fit enough to receive intensive therapy, there is a growing consensus that more intensive therapy, similar to that used in younger patients, results in prolonged survival (Derolf et al, 2009; Oran & Weisdorf, 2012), and the quality of life is probably no worse than in those receiving best supportive care or non‐intensive therapy (Alibhai et al, 2015). This does not imply that living with AML is not extremely difficult for older patients, and some informed patients might choose not to receive life‐extending therapy. One of the factors to be considered in making this decision is how long patients are likely to live if they elect to receive intensive therapy, and prognostic stratification in the elderly is clearly relevant to this issue.
There is some data suggesting a better outcome, at least in the short‐ to medium‐term, in those older patients with intermediate‐risk (IR) cytogenetics and an NPM1 MUT or NPM1 MUT FLT3 WT genotype, although this largely manifests as increased duration of survival rather than cure (Buchner et al, 2009; Becker et al, 2010; Lazenby et al, 2014), and may be limited to those ≤65 years of age (Ostronoff et al, 2015). There is very little data on the impact of CEBPA DM specifically in the older age group. We have therefore determined the impact on survival of the NPM1, FLT3 and CEBPA mutation status in a cohort of 301 patients aged 60 years or more with IR cytogenetics who received intensive therapy. We first examined the impact of the presence of a CEBPA DM genotype and then considered the outcome of the combined NPM1 MUT FLT3 WT and CEBPA DM subgroup (considered as the favourable mutational profile in younger patients) compared to those with any other genotype.
Methods
Patients and mutation analysis
Genomic DNA was available from diagnostic samples of 301 (45%) of the 662 patients aged ≥60 years with IR cytogenetics and entered on the UK Medical Research Council (MRC) AML11 trial between 1990 and 1998. Median age was 67 years (range, 60–85). Compared to the 361 patients with IR cytogenetics that were not included in the study, there was no difference in age, sex or type of leukaemia (de novo/secondary), CR rate or overall survival (OS), but patients studied were more likely to have a higher presenting white blood cell count (WBC) (Table SI). Ethical approval for the trial was obtained from participating institution's ethics review committees and patients gave informed consent. FLT3, NPM1 and CEBPA screening were performed as previously described (Gale et al, 2008; Green et al, 2010).
Therapy, clinical endpoints and statistical methods
Details of the trial protocol have been published elsewhere (Goldstone et al, 2001). CR was defined as a normocellular bone marrow (BM) containing <5% blasts and showing evidence of normal maturation of other marrow elements. Persistence of myelodysplastic features did not preclude the diagnosis of CR. OS was the time from trial entry to death. For patients achieving CR, relapse‐free survival (RFS) was the time from the date of first CR to an event (death in first CR or relapse) and cumulative incidence of relapse (CIR) was the incidence of relapse after CR, with death in CR as a competing risk.
Mantel‐Haenszel and chi‐squared tests were used to test for differences in demographic and clinical data by genotype. Kaplan–Meier curves were constructed for survival data and compared by means of the log‐rank test. Surviving patients were censored on 9 August, 2010, with follow‐up complete for 98% of patients. Median follow‐up for survival was 16·1 years (range, 13·7–19·5 years). Multivariate Cox models were used to analyse CIR and OS, adjusting for age, secondary leukaemia, WBC, performance status and molecular genotype. Models were fitted using forward selection, with variables added to the model if they had a P value, derived using the deviance statistic, of <0·05. Odds ratios (OR) or hazard ratios (HR) and 95% confidence intervals (CI) are quoted for endpoints. In all cases a ratio of <1 indicates benefit. All P values are two‐tailed.
Results
Patient characteristics according to CEBPA genotype
Details of the cohort studied are shown in Table 1. Overall, 28 patients (9%) were CEBPA MUT, 16 (57%; 5% of total cohort) had a single mutation (CEBPA SM) and 12 (43%; 4%) were CEBPA DM (Fig 1, Table SII). All CEBPA DM patients had mutations that would be predicted to lead to complete loss of normal C/EBP‐α activity, with N‐terminal mutations leading to production of the p30 isoform or frameshift or nonsense mutations leading to a truncated protein and/or C‐terminal mutations disrupting the DNA binding or leucine zipper domains. There was no significant difference between CEBPA DM, CEBPA SM and CEBPA WT patients in age, sex, type of leukaemia, WBC and incidence of either FLT3 ITD or NPM1 MUT, although it should be noted that no patient with a CEBPA DM had the NPM1 MUT FLT3 WT genotype (P = 0·02) (Table 1).
Table 1.
Characteristics of the patients studied according to CEBPA genotype
| Parameter | CEBPA WT (n = 273) | CEBPA SM (n = 16) | CEBPA DM (n = 12) | WT versus Single versus Double |
|---|---|---|---|---|
| Age, years | 0·19b | |||
| 60–64 | 92 (34%) | 6 (38%) | 7 (58%) | |
| 65–69 | 96 (35%) | 5 (31%) | 3 (25%) | |
| ≥70 | 85 (31%) | 5 (31%) | 2 (17%) | |
| Median (range) | 67 (60–85) | 66 (60–75) | 63 (60–74) | |
| Sex | 0·5a | |||
| Female | 117 (43%) | 8 (50%) | 6 (50%) | |
| Male | 156 (57%) | 8 (50%) | 6 (50%) | |
| Performance Status | 0·8a | |||
| WHO 0 | 105 (38%) | 8 (50%) | 4 (33%) | |
| WHO 1 | 121 (44%) | 5 (31%) | 7 (58%) | |
| WHO 2 | 18 (7%) | 1 (6%) | 0 | |
| WHO 3 | 23 (8%) | 1 (6%) | 1 (8%) | |
| WHO 4 | 6 (2%) | 1 (6%) | 0 | |
| Diagnosis | 0·5a | |||
| De Novo | 202 (74%) | 12 (75%) | 10 (83%) | |
| Secondary | 71 (26%) | 4 (25%) | 2 (17%) | |
| WBC, ×109/l | 0·14b | |||
| 0–9·9 | 90 (33%) | 5 (31%) | 2 (17%) | |
| 10–49·9 | 91 (34%) | 4 (25%) | 6 (50%) | |
| 50–99·9 | 53 (20%) | 3 (19%) | 1 (8%) | |
| ≥100 | 37 (14%) | 4 (25%) | 3 (25%) | |
| Median (range) | 26·6 (0·3–513·0) | 30·5 (1·8–349) | 40·2 (4·2–301) | |
| FLT3 ITD | 0·8a | |||
| WT | 206 (75%) | 9 (56%) | 11 (92%) | |
| Mutant | 67 (25%) | 7 (44%) | 1 (8%) | |
| NPM1 MUT | 0·3a | |||
| WT | 157 (58%) | 5 (31%) | 11 (92%) | |
| Mutant | 116 (42%) | 11 (69%) | 1 (8%) | |
| NPM1 MUT FLT3 WT | 76 (28%) | 7 (44%) | 0 (0%) | 0·02c |
| Other | 197 (72%) | 9 (56%) | 12 (100%) | |
DM, double mutant; SM, single mutant; ITD, internal tandem duplication; MUT, mutant; WT, wild‐type; WBC, white blood cell count; WHO, World Health Organization.
Test for trend.
Spearman correlation.
Fisher's exact test.
Figure 1.
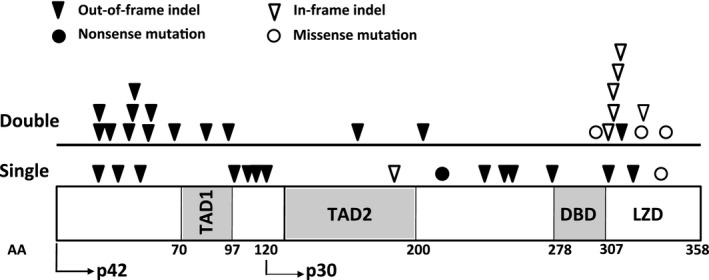
Location and type of mutation detected in CEBPA‐single mutant and CEBPA‐double mutant patients. Amino acids (AA) encoding the transactivation domains (TAD1 and TAD2), DNA‐binding domain (DBD) and leucine zipper domain (LZD) and the ATG start site for the p30 isoform are indicated.
Response to therapy and outcome of patients with CEBPA mutations
There was no evidence of a benefit in CEBPA SM patients, where response to therapy and outcome were either the same or worse than CEBPA WT patients (Table 2). CEBPA DM patients had a higher CR rate than CEBPA WT patients (75% vs. 59%). Although a relatively large difference, this was not significant in multivariate analysis adjusting for baseline characteristics (OR = 0·33, CI = 0·08–1·38; P = 0·12), which is not unexpected as the number of such cases is small (n = 12) (Table 2). CIR was lower in the CEBPA DM patients compared to the CEBPA WT patients, being 44% vs. 55%, respectively, at 1 year and 67% vs. 73% at 3 years (Fig 2A), but again this did not achieve statistical significance (P = 0·4 for CEBPA DM versus all others). Short‐term survival was improved for CEBPA DM patients (median 471 d for CEBPA DM and 248 d for CEBPA WT), although the benefit was lost by 3 years when the OS was the same (17% vs. 18%) (Fig 2B). In multivariate analysis, there was a trend for a better OS in the CEBPA DM patients when compared to other patients (HR = 0·57, CI = 0·31–1·08; P = 0·08).
Table 2.
Outcome data according to CEBPA genotype
| Outcome | CEBPA WT (n = 273) | CEBPA SM (n = 16) | CEBPA DM (n = 12) | CEBPA WT versus CEBPA SM versus CEBPA DM OR or HR (95% CI), P‐value | CEBPA DM versus not OR or HR (95% CI), P‐value | ||
|---|---|---|---|---|---|---|---|
| Univariate | aMultivariate | Univariate | aMultivariate | ||||
| CR/CRi | 59% | 56% | 75% | 0·79 (0·46–1·37), P = 0·4 | 0·71 (0·39–1·27), P = 0·2 | 0·51 (0·16–1·66), P = 0·3 | 0·33 (0·08–1·38), P = 0·12 |
| 30‐d mortality | 20% | 25% | 8% | 0·82 (0·42–1·57), P = 0·5 | 0·76 (0·38–1·51), P = 0·4 | 0·53 (0·15–1·84), P = 0·3 | 0·35 (0·05–2·60), P = 0·3 |
| 3‐year OS | 18% | 6% | 17% | 0·92 (0·71–1·19), P = 0·5 | 0·87 (0·67–1·13), P = 0·3 | 0·75 (0·45–1·27), P = 0·3 | 0·57 (0·31–1·08), P = 0·08 |
| 3‐year RFS | 21% | 11% | 22% | 0·95 (0·69–1·31), P = 0·8 | 0·93 (0·68–1·29), P = 0·7 | 0·83 (0·43–1·59), P = 0·6 | 0·74 (0·36–1·55), P = 0·4 |
| 3‐year CIR | 73% | 89% | 67% | 0·96 (0·68–1·35), P = 0·8 | 0·94 (0·66–1·34), P = 0·7 | 0·75 (0·37–1·52), P = 0·4 | 0·68 (0·29–1·58), P = 0·4 |
95% CI, 95% confidence intervals; CIR, cumulative incidence of relapse; CR, complete remission; CRi, complete remission with incomplete haematological recovery; DM, double mutant; HR, hazard ratio; OR, odds ratio; OS, overall survival; RFS, relapse‐free survival; SM, single mutant; WT, wild‐type.
Adjusted for age, secondary leukaemia, white blood cell count, performance status, FLT3 and NPM1 genotype.
Figure 2.
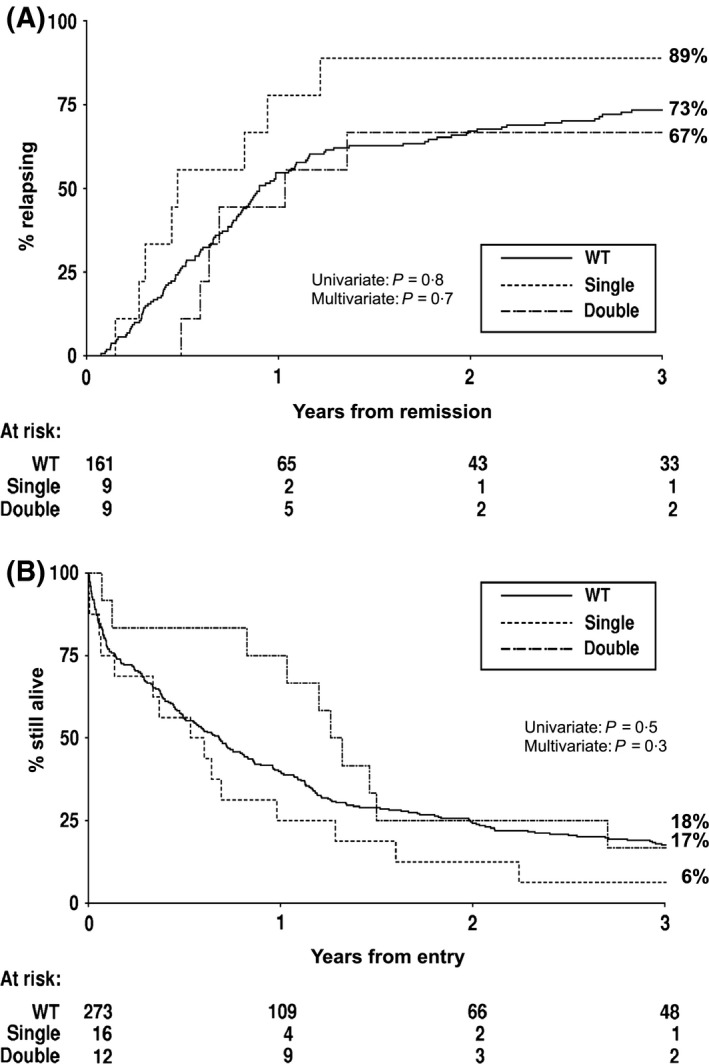
Kaplan–Meier curves stratified according to CEBPA genotype. (A) Cumulative incidence of relapse. (B) Overall survival. WT, wild type.
Comparison of response to therapy and outcome in patients with CEBPADM, NPM1MUTFLT3WT and Other genotypes
Although the CEBPA DM genotype appeared to be associated with only a limited benefit in outcome, the above analysis is likely to be influenced by the presence of good‐risk NPM1 MUT FLT3 WT patients in the non‐CEBPA DM group, which would attenuate any difference between CEBPA DM patients and those with a poor‐risk genotype. Overall, 83 patients (28%) had an NPM1 MUT FLT3 WT genotype and it was mutually exclusive with a CEBPA DM genotype. We therefore divided the patients into three groups, NPM1 MUT FLT3 WT patients, CEBPA DM patients, and all other patients (i.e. those with an NPM1 WT or NPM1 MUT FLT3 ITD genotype), hereafter called ‘Other’ genotypes. The CR rate in patients with an NPM1 MUT FLT3 WT genotype was 71% compared to 75% in the CEBPA DM patients and 54% in those with ‘Other’ genotypes (Table 3). NPM1 MUT FLT3 WT or CEBPA DM patients had a lower CIR at 1 year than the remaining patients (44%, 56% and 62% respectively). However, this difference was less apparent by 3 years (66%, 67% and 78% respectively) and, although it was statistically significant in univariate analysis (P = 0·01 for the 3‐way comparison), it did not retain significance after adjustment for other factors (P = 0·3) (Fig 3A). Survival at 1 year was highest in the CEBPA DM group, but this then fell towards the level of the patients in the ‘Other’ genotype category and showed no difference by 2 years (Fig 3B). Even so, multivariate analysis showed that the OS was significantly better in the CEBPA DM patients than in the ‘Other’ genotypic group (HR = 0·52, CI = 0·28–0·97, P = 0·04). Similarly, OS was significantly better in the NPM1 MUT FLT3 WT group compared to the ‘Other’ genotype group (P < 0·0001 for univariate analysis; P = 0·002 for multivariate analysis). Median survival was 13·6, 15·2 and 6·6 months, respectively, in the NPM1 MUT FLT3 WT, CEBPA DM and ‘Other’ groups.
Table 3.
Outcome data comparing the CEBPA DM and NPM1 MUT FLT3 WT favourable‐risk groups
| Outcome | CEBPA DM (n = 12) | NPM1 MUT FLT3 WT (n = 83) | Others (n = 206) | CEBPA DM versus NPM1 MUT FLT3 WT | CEBPA DM versus Others | NPM1 MUT FLT3 WT versus Others | |||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | aMultivariate | Univariate | aMultivariate | Univariate | aMultivariate | ||||
| CR/CRi | 75% | 71% | 54% | ||||||
| OR (95% CI) | 0·84 (0·22–3·14) | 0·83 (0·20–3·57) | 0·43 (0·13–1·37) | 0·39 (0·10–1·57) | 0·43 (0·13–1·37) | 0·39 (0·10–1·57) | |||
| P | 0·8 | 0·8 | 0·15 | 0·18 | 0·15 | 0·18 | |||
| 30‐d mortality | 8% | 18% | 21% | ||||||
| HR (95% CI) | 0·53 (0·13–2·23) | 0·33 (0·04–2·70) | 0·51 (0·15–1·79) | 0·33 (0·05–2·46) | 0·51 (0·15–2·79) | 0·33 (0·05–2·46) | |||
| P | 0·4 | 0·3 | 0·3 | 0·3 | 0·3 | 0·3 | |||
| OS | |||||||||
| 1 year | 75% | 54% | 33% | ||||||
| 3 years | 17% | 29% | 12% | ||||||
| HR (95% CI) | 1·10 (0·57–2·12) | 0·93 (0·47–1·84) | 0·64 (0·40–1·04) | 0·52 (0·28–0·97) | 0·64 (0·40–1·04) | 0·52 (0·28–0·97) | |||
| P | 0·8 | 0·8 | 0·07 | 0·04 | 0·07 | 0·04 | |||
| RFS | |||||||||
| 3 years | 22% | 31% | 14% | ||||||
| HR (95% CI) | 1·18 (0·53–2·61) | 1·04 (0·47–2·30) | 0·70 (0·38–1·27) | 0·66 (0·32–1·37) | 0·70 (0·38–1·27) | 0·66 (0·32–1·37) | |||
| P | 0·7 | 0·9 | 0·2 | 0·3 | 0·2 | 0·3 | |||
| CIR | |||||||||
| 1 year | 56% | 44% | 62% | ||||||
| 3 years | 67% | 66% | 78% | ||||||
| HR (95% CI) | 1·01 (0·42–2·42) | 0·84 (0·34–2·06) | 0·65 (0·34–1·27) | 0·59 (0·25–1·36) | 0·65 (0·34–1·27) | 0·59 (0·25–1·36) | |||
| P | 1·0 | 0·7 | 0·2 | 0·2 | 0·2 | 0·2 | |||
95% CI, 95% confidence intervals; CIR, cumulative incidence of relapse; CR, complete remission; CRi, complete remission with incomplete haematological recovery; DM, double mutant; HR, hazard ratio; MUT, mutant; OR, odds ratio; OS, overall survival; RFS, relapse‐free survival; WT, wild‐type.
Adjusted for age, secondary leukaemia, white blood cell count and performance status.
Figure 3.
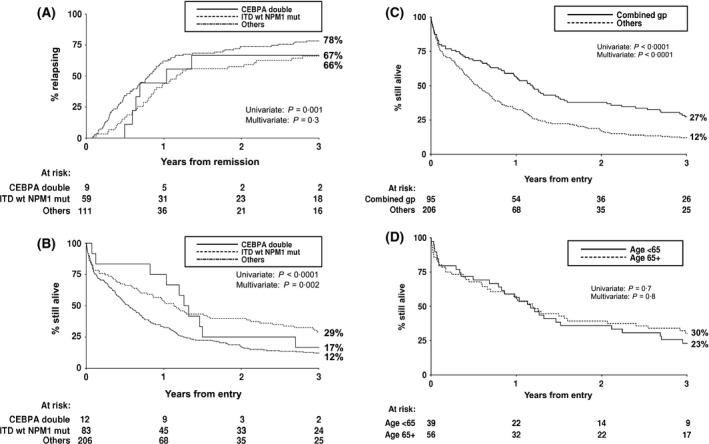
Kaplan–Meier curves stratified according to CEBPA DM and NPM1 MUT FLT3 WT genotype. (A) Cumulative incidence of relapse and (B) overall survival in the three genotype groups. (C) Overall survival for the combined favourable‐risk CEBPA DM and NPM1 MUT FLT3 WT group compared with all other patients. (D) Overall survival stratified according to age in the favourable‐risk group. CEBPA double, CEBPA DM genotype; ITD wt NPM1 mut, NPM1 MUT FLT3 WT genotype.
As outcome was broadly comparable for the CEBPA DM and NPM1 MUT FLT3 WT patients, they were combined into a favourable‐risk group, as in younger patients, together comprising 32% of the patients in this study. OS was very significantly better in this combined group than the ‘Other’ genotypes group, 57% vs. 33% at 1 year, and 27% vs. 12% at 3 years (HR = 0·50, CI = 0·38–0·65, P < 0·0001 in multivariate analysis) (Fig 3C). Median survival was 14·3 months in the favourable‐risk group compared to only 6·6 months in the remainder. There was no evidence that survival in the favourable‐risk group differed according to age: OS at 3 years was 30% for the 56 patients (59%) aged >65 years compared to 23% for those aged <65 years (HR = 0·92, CI = 0·59–1·44; P = 0·7) (Fig 3D), median survival 14·3 and 14·0 months, respectively.
Discussion
In younger adult AML patients the presence of a CEBPA DM genotype is associated with better response to treatment and improved long‐term outcome (Green et al, 2010; Taskesen et al, 2011). It is sometimes assumed that this is also true in older patients (Ossenkoppele & Lowenberg, 2015), but this has never been formally demonstrated, with such results only presented within a much wider age range of patients (Renneville et al, 2009; Wouters et al, 2009; Dufour et al, 2010; Fasan et al, 2014). The study presented here is the first to report on the impact of a CEBPA DM genotype specifically in patients ≥60 years of age. The incidence of 4% CEBPA DM in the present cohort was similar to the 5% incidence reported in our study of younger patients with IR cytogenetics (Green et al, 2010), and is consistent with other studies where age and double/single mutant status have been given (Dufour et al, 2010; Marcucci et al, 2012). A CEBPA DM genotype was associated with improved short‐term survival that was significant in multivariate analysis when compared to patients without either a CEBPA DM or NPM1 MUT FLT3 WT genotype. It is clear that this improvement is relatively short‐lived and does not equate to a high cure rate.
Older patients with an NPM1 MUT FLT3 WT genotype have already been shown to have an improved 1‐year OS (Buchner et al, 2009; Lazenby et al, 2014; Ostronoff et al, 2015), and this was confirmed in the present cohort. The combined favourable‐risk genotypic group reported here, of either a CEBPA DM or an NPM1 MUT FLT3 WT genotype, accounted for 32% of all the patients investigated, although it must be acknowledged that this cohort was restricted to patients deemed fit enough to receive intensive chemotherapy. Even in this favourable‐risk genotypic group only 57% of the patients were alive at 1 year and the corollary of identifying a favourable group is that, by default, an unfavourable‐risk group is also identified. In this cohort of patients, of those without a favourable‐risk genotype, nearly 50% had died by 6 months and only 12% were alive at 3 years.
Although the cohort presented here was treated two decades ago, there has been very little progress in the intervening years in improving outcome in this age group and the findings are still likely to apply. This disease‐related information needs to be integrated with other patient‐related information, including co‐morbidities, but for some patients it may influence the decision of whether or not to receive intensive therapy. Therefore consideration should be given to offering molecular screening as part of the diagnostic work‐up for older patients with IR cytogenetics. Nevertheless, even for the more favourable‐risk group, there remains an undoubted need to develop novel therapeutic strategies for older patients with AML.
Author contributions
Glenda J. Dickson performed research, analysed data and wrote the paper. Sophia Bustraan and Akbar Ali performed research. Robert K. Hills performed statistical analysis. Anthony H. Goldstone and Alan K. Burnett were the chief investigators of the trial. David C. Linch designed the study and wrote the paper. Rosemary E. Gale designed the study, analysed data and wrote the paper. All authors approved the final version of the manuscript.
Conflicts of interest
The authors have no competing conflicts of interest to declare.
Supporting information
Table SI. Characteristics of UK AML11 trial patients aged ≥60 years and with IR cytogenetics that were excluded and included in the molecular investigation.
Table SII. Details of the mutations identified in the CEBPA MUT cases.
Acknowledgements
This work was supported by Leukaemia and Lymphoma Research, UK, and was undertaken at UCLH/UCL, which receives a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme.
References
- Alibhai, S.M. , Breunis, H. , Timilshina, N. , Brignardello‐Petersen, R. , Tomlinson, G. , Mohamedali, H. , Gupta, V. , Minden, M.D. , Li, M. , Buckstein, R. & Brandwein, J.M. (2015) Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. Journal of Geriatric Oncology, 6, 262–271. [DOI] [PubMed] [Google Scholar]
- Becker, H. , Marcucci, G. , Maharry, K. , Radmacher, M.D. , Mrozek, K. , Margeson, D. , Whitman, S.P. , Wu, Y.Z. , Schwind, S. , Paschka, P. , Powell, B.L. , Carter, T.H. , Kolitz, J.E. , Wetzler, M. , Carroll, A.J. , Baer, M.R. , Caligiuri, M.A. , Larson, R.A. & Bloomfield, C.D. (2010) Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene‐ and microRNA‐expression signatures: a Cancer and Leukemia Group B study. Journal of Clinical Oncology, 28, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner, T. , Berdel, W.E. , Haferlach, C. , Haferlach, T. , Schnittger, S. , Muller‐Tidow, C. , Braess, J. , Spiekermann, K. , Kienast, J. , Staib, P. , Gruneisen, A. , Kern, W. , Reichle, A. , Maschmeyer, G. , Aul, C. , Lengfelder, E. , Sauerland, M.C. , Heinecke, A. , Wormann, B. & Hiddemann, W. (2009) Age‐related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. Journal of Clinical Oncology, 27, 61–69. [DOI] [PubMed] [Google Scholar]
- Burnett, A. , Wetzler, M. & Lowenberg, B. (2011) Therapeutic advances in acute myeloid leukemia. Journal of Clinical Oncology, 29, 487–494. [DOI] [PubMed] [Google Scholar]
- Cornelissen, J.J. , Gratwohl, A. , Schlenk, R.F. , Sierra, J. , Bornhauser, M. , Juliusson, G. , Racil, Z. , Rowe, J.M. , Russell, N. , Mohty, M. , Lowenberg, B. , Socie, G. , Niederwieser, D. & Ossenkoppele, G.J. (2012) The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated‐risk adapted approach. Nature Reviews, 9, 579–590. [DOI] [PubMed] [Google Scholar]
- Derolf, A.R. , Kristinsson, S.Y. , Andersson, T.M. , Landgren, O. , Dickman, P.W. & Bjorkholm, M. (2009) Improved patient survival for acute myeloid leukemia: a population‐based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood, 113, 3666–3672. [DOI] [PubMed] [Google Scholar]
- Dohner, H. & Gaidzik, V.I. (2011) Impact of genetic features on treatment decisions in AML. American Society of Hematology. Education Program, 2011, 36–42. [DOI] [PubMed] [Google Scholar]
- Dohner, H. , Estey, E.H. , Amadori, S. , Appelbaum, F.R. , Buchner, T. , Burnett, A.K. , Dombret, H. , Fenaux, P. , Grimwade, D. , Larson, R.A. , Lo‐Coco, F. , Naoe, T. , Niederwieser, D. , Ossenkoppele, G.J. , Sanz, M.A. , Sierra, J. , Tallman, M.S. , Lowenberg, B. & Bloomfield, C.D. (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behlaf of the European LeukemiaNet. Blood, 115, 453–474. [DOI] [PubMed] [Google Scholar]
- Dufour, A. , Schneider, F. , Metzeler, K.H. , Hoster, E. , Schneider, S. , Zellmeier, E. , Benthaus, T. , Sauerland, M.C. , Berdel, W.E. , Buchner, T. , Wormann, B. , Braess, J. , Hiddemann, W. , Bohlander, S.K. & Spiekermann, K. (2010) Acute myeloid leukemia with biallelic CEBPA gene mutations and normal karyotype represents a distinct genetic entity associated with a favorable clinical outcome. Journal of Clinical Oncology, 28, 570–577. [DOI] [PubMed] [Google Scholar]
- Fasan, A. , Haferlach, C. , Alpermann, T. , Jeromin, S. , Grossman, V. , Eder, C. , Weissmann, S. , Dicker, F. , Kohlmann, A. , Schindela, S. , Kern, W. , Haferlach, T. & Schnittger, S. (2014) The role of different genetic subtypes of CEBPA mutated AML. Leukemia, 28, 794–803. [DOI] [PubMed] [Google Scholar]
- Gale, R.E. , Green, C. , Allen, C. , Mead, A.J. , Burnett, A.K. , Hills, R.K. & Linch, D.C. (2008) The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood, 111, 2776–2784. [DOI] [PubMed] [Google Scholar]
- Goldstone, A.H. , Burnett, A.K. , Wheatley, K. , Smith, A.G. , Hutchinson, R.M. & Clark, R.E. (2001) Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood, 98, 1302–1311. [DOI] [PubMed] [Google Scholar]
- Green, C.L. , Koo, K.K. , Hills, R.K. , Burnett, A.K. , Linch, D.C. & Gale, R.E. (2010) Prognostic significance of CEBPA mutations in a large cohort of younger adult patients with acute myeloid leukemia: impact of double CEBPA mutations and the interaction with FLT3 and NPM1 mutations. Journal of Clinical Oncology, 28, 2739–2747. [DOI] [PubMed] [Google Scholar]
- Lazenby, M. , Gilkes, A.F. , Marrin, C. , Evans, A. , Hills, R.K. & Burnett, A.K. (2014) The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non‐intensively: a study of 1312 patients in the UK NCRI AML16 trial. Leukemia, 28, 1953–1959. [DOI] [PubMed] [Google Scholar]
- Marcucci, G. , Metzeler, K.H. , Schwind, S. , Becker, H. , Maharry, K. , Mrozek, K. , Radmacher, M.D. , Kohlschmidt, J. , Nicolet, D. , Whitman, S.P. , Wu, Y.Z. , Powell, B.L. , Carter, T.H. , Kolitz, J.E. , Wetzler, M. , Carroll, A.J. , Baer, M.R. , Moore, J.O. , Caligiuri, M.A. , Larson, R.A. & Bloomfield, C.D. (2012) Age‐related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. Journal of Clinical Oncology, 30, 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, M.R. , Abboud, C.N. , Altman, J. , Appelbaum, F.R. , Arber, D.A. , Attar, E. , Borate, U. , Coutre, S.E. , Damon, L.E. , Goorha, S. , Lancet, J. , Maness, L.J. , Marcucci, G. , Millenson, M.M. , Moore, J.O. , Ravandi, F. , Shami, P.J. , Smith, B.D. , Stone, R.M. , Strickland, S.A. , Tallman, M.S. , Wang, E.S. , Naganuma, M. & Gregory, K.M. (2012) Acute myeloid leukemia. Journal of the National Comprehensive Cancer Network, 10, 984–1021. [DOI] [PubMed] [Google Scholar]
- Ofran, Y. & Rowe, J.M. (2013) Genetic profiling in acute myeloid leukaemia – where are we and what is its role in patient management. British Journal of Haematology, 160, 303–320. [DOI] [PubMed] [Google Scholar]
- Oran, B. & Weisdorf, D.J. (2012) Survival for older patients with acute myeloid leukemia: a population‐based study. Haematologica, 97, 1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele, G. & Lowenberg, B. (2015) How I treat the older patient with acute myeloid leukemia. Blood, 125, 767–774. [DOI] [PubMed] [Google Scholar]
- Ostronoff, F. , Othus, M. , Lazenby, M. , Estey, E. , Appelbaum, F.R. , Evans, A. , Godwin, J. , Gilkes, A. , Kopecky, K.J. , Burnett, A. , List, A.F. , Fang, M. , Oehler, V.G. , Petersdorf, S.H. , Pogosova‐Agadjanyan, E.L. , Radich, J.P. , Willman, C.L. , Meshinchi, S. & Stirewalt, D.L. (2015) Prognostic significance of NPM1 mutations in the absence of FLT3‐internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UK National Cancer Research Institute/Medical Research Council report. Journal of Clinical Oncology, 33, 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollyea, D.A. , Kohrt, H.E. & Medeiros, B.C. (2011) Acute myeloid leukaemia in the elderly: a review. British Journal of Haematology, 152, 524–542. [DOI] [PubMed] [Google Scholar]
- Renneville, A. , Boissel, N. , Gachard, N. , Naguib, D. , Bastard, C. , de Botton, S. , Nibourel, O. , Pautas, C. , Reman, O. , Thomas, X. , Gardin, C. , Terre, C. , Castaigne, S. , Preudhomme, C. & Dombret, H. (2009) The favorable impact of CEBPA mutations in patients with acute myeloid leukemia is only observed in the absence of associated cytogenetic abnormalities and FLT3 internal duplication. Blood, 113, 5090–5093. [DOI] [PubMed] [Google Scholar]
- Taskesen, E. , Bullinger, L. , Corbacioglu, A. , Sanders, M.A. , Erpelinck, C.A. , Wouters, B.J. , van der Poel‐van de Luytgaarde, S.C. , Damm, F. , Krauter, J. , Ganser, A. , Schlenk, R.F. , Lowenberg, B. , Delwel, R. , Dohner, H. , Valk, P.J. & Dohner, K. (2011) Prognostic impact, concurrent genetic mutations, and gene expression features of AML with CEBPA mutations in a cohort of 1182 cytogenetically normal AML patients: further evidence for CEBPA double mutant AML as a distinctive disease entity. Blood, 117, 2469–2475. [DOI] [PubMed] [Google Scholar]
- Thein, M.S. , Ershler, W.B. , Jemal, A. , Yates, J.W. & Baer, M.R. (2013) Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer, 119, 2720–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters, B.J. , Lowenberg, B. , Erpelinck‐Verschueren, C.A. , van Putten, W.L. , Valk, P.J. & Delwel, R. (2009) Double CEBPA mutations, but not single CEBPA mutations, define a subgroup of acute myeloid leukemia with a distinctive gene expression profile that is uniquely associated with a favorable outcome. Blood, 113, 3088–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Characteristics of UK AML11 trial patients aged ≥60 years and with IR cytogenetics that were excluded and included in the molecular investigation.
Table SII. Details of the mutations identified in the CEBPA MUT cases.


