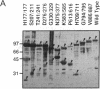
Abstract
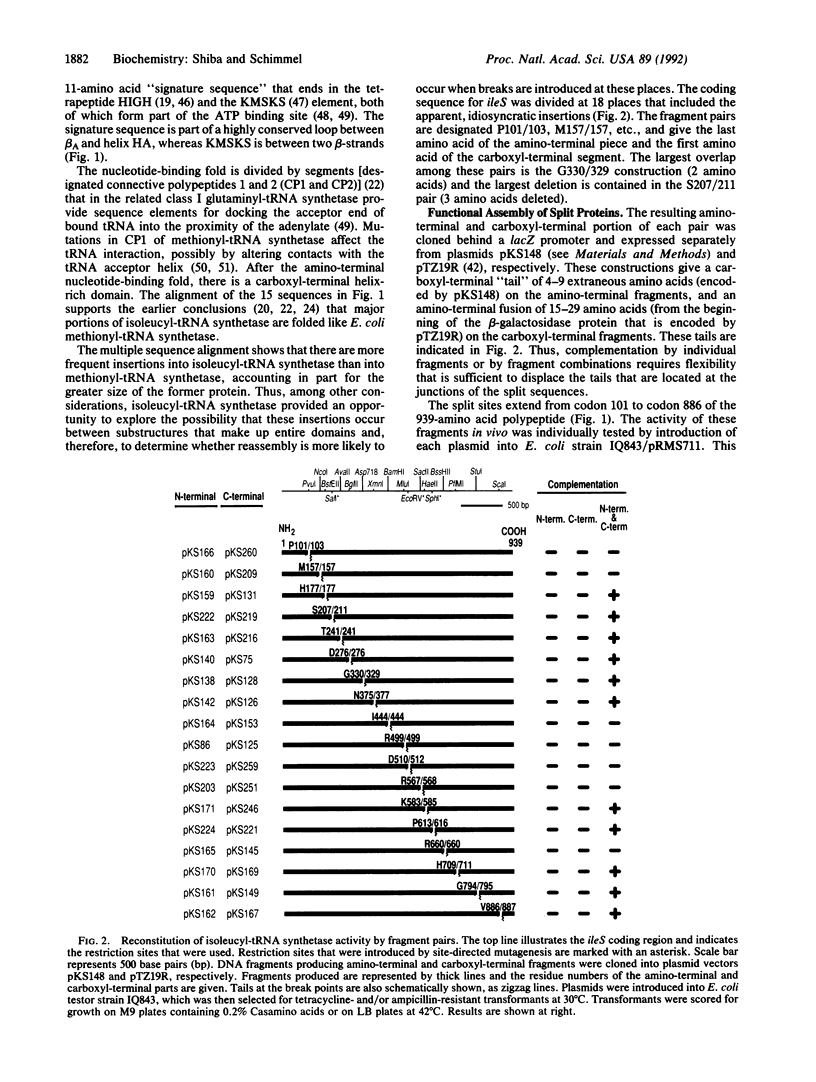
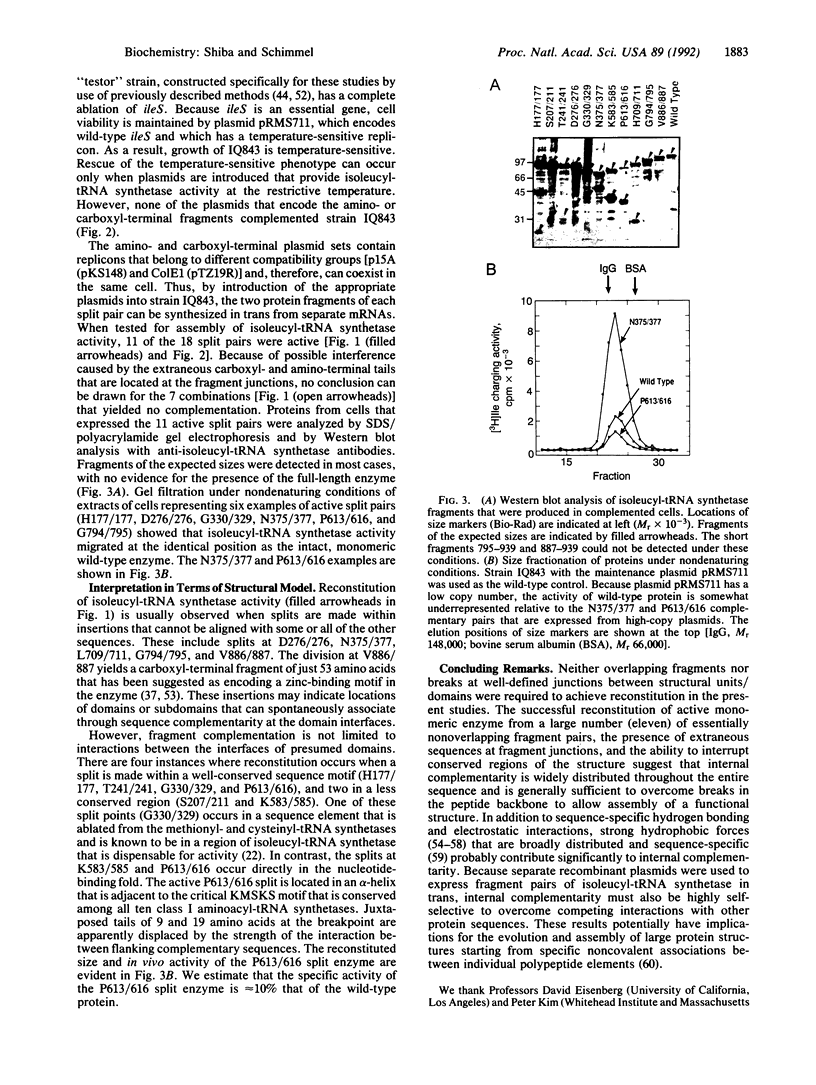
The sequence of a 939-amino acid polypeptide that is a member of the aminoacyl-tRNA synthetase class of enzymes has been aligned with sequences of 15 related proteins. This alignment guided the design of 18 fragment pairs that were tested for internal sequence complementarity by reconstitution of enzyme activity. Reconstitution was achieved with fragments that divide the protein at both nonconserved and conserved sequences, including locations proximal to or within elements believed to form critical elements of secondary structure. Structure assembly is sufficiently flexible to accommodate fusion of short segments of unrelated sequences at fragment junctions. Complementary chain packing interactions and chain flexibility appear to be widely distributed throughout the sequence and are sufficient to reconstruct large three-dimensional structures from an array of disconnected pieces. The results may have implications for the evolution and assembly of large proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benarous R., Chow C. M., RajBhandary U. L. Cytoplasmic leucyl-tRNA synthetase of Neurospora crassa is not specified by the leu-5 locus. Genetics. 1988 Aug;119(4):805–814. doi: 10.1093/genetics/119.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi E., Kaback H. R. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4325–4329. doi: 10.1073/pnas.87.11.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgford T. J., Brand N. J., Gray T. E., Fersht A. R. The valyl-tRNA synthetase from Bacillus stearothermophilus has considerable sequence homology with the isoleucyl-tRNA synthetase from Escherichia coli. Biochemistry. 1987 May 5;26(9):2480–2486. doi: 10.1021/bi00383a012. [DOI] [PubMed] [Google Scholar]
- Brunie S., Zelwer C., Risler J. L. Crystallographic study at 2.5 A resolution of the interaction of methionyl-tRNA synthetase from Escherichia coli with ATP. J Mol Biol. 1990 Nov 20;216(2):411–424. doi: 10.1016/S0022-2836(05)80331-6. [DOI] [PubMed] [Google Scholar]
- Burbaum J. J., Schimmel P. Assembly of a class I tRNA synthetase from products of an artificially split gene. Biochemistry. 1991 Jan 15;30(2):319–324. doi: 10.1021/bi00216a002. [DOI] [PubMed] [Google Scholar]
- Burbaum J. J., Starzyk R. M., Schimmel P. Understanding structural relationships in proteins of unsolved three-dimensional structure. Proteins. 1990;7(2):99–111. doi: 10.1002/prot.340070202. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C. The nature of the accessible and buried surfaces in proteins. J Mol Biol. 1976 Jul 25;105(1):1–12. doi: 10.1016/0022-2836(76)90191-1. [DOI] [PubMed] [Google Scholar]
- Chow C. M., Metzenberg R. L., Rajbhandary U. L. Nuclear gene for mitochondrial leucyl-tRNA synthetase of Neurospora crassa: isolation, sequence, chromosomal mapping, and evidence that the leu-5 locus specifies structural information. Mol Cell Biol. 1989 Nov;9(11):4631–4644. doi: 10.1128/mcb.9.11.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N. D., Lien D. C., Schimmel P. Evidence from cassette mutagenesis for a structure-function motif in a protein of unknown structure. Science. 1988 Apr 22;240(4851):521–523. doi: 10.1126/science.3282306. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Reconstitution of horse heart cytochrome c: interaction of the components obtained upon cleavage of the peptide bond following methionine residue 65. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3036–3039. doi: 10.1073/pnas.68.12.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Buchman S. R., Beychok S. Characterization of globin domains: heme binding to the central exon product. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1384–1388. doi: 10.1073/pnas.77.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardel F., Fayat G., Blanquet S. Molecular cloning and primary structure of the Escherichia coli methionyl-tRNA synthetase gene. J Bacteriol. 1984 Dec;160(3):1115–1122. doi: 10.1128/jb.160.3.1115-1122.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englisch U., Englisch S., Markmeyer P., Schischkoff J., Sternbach H., Kratzin H., Cramer F. Structure of the yeast isoleucyl-tRNA synthetase gene (ILS1). DNA-sequence, amino-acid sequence of proteolytic peptides of the enzyme and comparison of the structure to those of other known aminoacyl-tRNA synthetases. Biol Chem Hoppe Seyler. 1987 Aug;368(8):971–979. doi: 10.1515/bchm3.1987.368.2.971. [DOI] [PubMed] [Google Scholar]
- Eriani G., Dirheimer G., Gangloff J. Cysteinyl-tRNA synthetase: determination of the last E. coli aminoacyl-tRNA synthetase primary structure. Nucleic Acids Res. 1991 Jan 25;19(2):265–269. doi: 10.1093/nar/19.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Galakatos N. G., Walsh C. T. Specific proteolysis of native alanine racemases from Salmonella typhimurium: identification of the cleavage site and characterization of the clipped two-domain proteins. Biochemistry. 1987 Dec 15;26(25):8475–8480. doi: 10.1021/bi00399a066. [DOI] [PubMed] [Google Scholar]
- Ghosh G., Pelka H., Schulman L. H. Identification of the tRNA anticodon recognition site of Escherichia coli methionyl-tRNA synthetase. Biochemistry. 1990 Mar 6;29(9):2220–2225. doi: 10.1021/bi00461a003. [DOI] [PubMed] [Google Scholar]
- Gilbert W. The exon theory of genes. Cold Spring Harb Symp Quant Biol. 1987;52:901–905. doi: 10.1101/sqb.1987.052.01.098. [DOI] [PubMed] [Google Scholar]
- Gribskov M., Lüthy R., Eisenberg D. Profile analysis. Methods Enzymol. 1990;183:146–159. doi: 10.1016/0076-6879(90)83011-w. [DOI] [PubMed] [Google Scholar]
- Hall J. G., Frieden C. Protein fragments as probes in the study of protein folding mechanisms: differential effects of dihydrofolate reductase fragments on the refolding of the intact protein. Proc Natl Acad Sci U S A. 1989 May;86(9):3060–3064. doi: 10.1073/pnas.86.9.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan R. R., Taniuchi H. Formation of a biologically active, ordered complex from two overlapping fragments of cytochrome c. J Biol Chem. 1977 Feb 25;252(4):1367–1374. [PubMed] [Google Scholar]
- Holmgren A., Slabý I. Thioredoxin-C': mechanism of noncovalent complementation and reactions of the refolded complex and the active site containing fragment with thioredoxin reductase. Biochemistry. 1979 Dec 11;18(25):5591–5599. doi: 10.1021/bi00592a011. [DOI] [PubMed] [Google Scholar]
- Hou Y. M., Shiba K., Mottes C., Schimmel P. Sequence determination and modeling of structural motifs for the smallest monomeric aminoacyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):976–980. doi: 10.1073/pnas.88.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hountondji C., Blanquet S., Lederer F. Methionyl-tRNA synthetase from Escherichia coli: primary structure at the binding site for the 3'-end of tRNAfMet. Biochemistry. 1985 Feb 26;24(5):1175–1180. doi: 10.1021/bi00326a018. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Frank R., Madern D. Nucleotide sequence of Escherichia coli valyl-tRNA synthetase gene valS. Nucleic Acids Res. 1987 Nov 11;15(21):9081–9082. doi: 10.1093/nar/15.21.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Schimmel P. Deletion of an essential gene in Escherichia coli by site-specific recombination with linear DNA fragments. J Bacteriol. 1984 Aug;159(2):783–786. doi: 10.1128/jb.159.2.783-786.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal U., Rechsteiner T., Tan P. Y., Bühlmann E., Meile L., Leisinger T. Isoleucyl-tRNA synthetase of Methanobacterium thermoautotrophicum Marburg. Cloning of the gene, nucleotide sequence, and localization of a base change conferring resistance to pseudomonic acid. J Biol Chem. 1991 Jun 5;266(16):10570–10577. [PubMed] [Google Scholar]
- Jordana X., Chatton B., Paz-Weisshaar M., Buhler J. M., Cramer F., Ebel J. P., Fasiolo F. Structure of the yeast valyl-tRNA synthetase gene (VASI) and the homology of its translated amino acid sequence with Escherichia coli isoleucyl-tRNA synthetase. J Biol Chem. 1987 May 25;262(15):7189–7194. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kato I., Anfinsen C. B. On the stabilization of ribonuclease S-protein by ribonuclease S-peptide. J Biol Chem. 1969 Feb 10;244(3):1004–1007. [PubMed] [Google Scholar]
- Kellis J. T., Jr, Nyberg K., Sali D., Fersht A. R. Contribution of hydrophobic interactions to protein stability. Nature. 1988 Jun 23;333(6175):784–786. doi: 10.1038/333784a0. [DOI] [PubMed] [Google Scholar]
- Li C. H., Bewley T. A. Human pituitary growth hormone: restoration of full biological activity by noncovalent interaction of two fragments of the hormone. Proc Natl Acad Sci U S A. 1976 May;73(5):1476–1479. doi: 10.1073/pnas.73.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmerer S. W., Schimmel P. Gene for yeast glutamine tRNA synthetase encodes a large amino-terminal extension and provides a strong confirmation of the signature sequence for a group of the aminoacyl-tRNA synthetases. J Biol Chem. 1987 Aug 5;262(22):10801–10806. [PubMed] [Google Scholar]
- Matsuyama S., Kimura E., Mizushima S. Complementation of two overlapping fragments of SecA, a protein translocation ATPase of Escherichia coli, allows ATP binding to its amino-terminal region. J Biol Chem. 1990 May 25;265(15):8760–8765. [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Dardel F., Le Corre D., Blanquet S., Fayat G. Lysine 335, part of the KMSKS signature sequence, plays a crucial role in the amino acid activation catalysed by the methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1991 Feb 5;217(3):465–475. doi: 10.1016/0022-2836(91)90750-z. [DOI] [PubMed] [Google Scholar]
- Meinnel T., Mechulam Y., Le Corre D., Panvert M., Blanquet S., Fayat G. Selection of suppressor methionyl-tRNA synthetases: mapping the tRNA anticodon binding site. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):291–295. doi: 10.1073/pnas.88.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K., Kidera A., Kanehisa M. Cluster analysis of amino acid indices for prediction of protein structure and function. Protein Eng. 1988 Jul;2(2):93–100. doi: 10.1093/protein/2.2.93. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nureki O., Muramatsu T., Suzuki K., Kohda D., Matsuzawa H., Ohta T., Miyazawa T., Yokoyama S. Methionyl-tRNA synthetase gene from an extreme thermophile, Thermus thermophilus HB8. Molecular cloning, primary-structure analysis, expression in Escherichia coli, and site-directed mutagenesis. J Biol Chem. 1991 Feb 15;266(5):3268–3277. [PubMed] [Google Scholar]
- Rose G. D., Geselowitz A. R., Lesser G. J., Lee R. H., Zehfus M. H. Hydrophobicity of amino acid residues in globular proteins. Science. 1985 Aug 30;229(4716):834–838. doi: 10.1126/science.4023714. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Saint Girons I., Gilles A. M., Margarita D., Michelson S., Monnot M., Fermandjian S., Danchin A., Bârzu O. Structural and catalytic characteristics of Escherichia coli adenylate kinase. J Biol Chem. 1987 Jan 15;262(2):622–629. [PubMed] [Google Scholar]
- Shortle D., Stites W. E., Meeker A. K. Contributions of the large hydrophobic amino acids to the stability of staphylococcal nuclease. Biochemistry. 1990 Sep 4;29(35):8033–8041. doi: 10.1021/bi00487a007. [DOI] [PubMed] [Google Scholar]
- Starzyk R. M., Webster T. A., Schimmel P. Evidence for dispensable sequences inserted into a nucleotide fold. Science. 1987 Sep 25;237(4822):1614–1618. doi: 10.1126/science.3306924. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Barry R. E., Corcoran D. Tryptic conversion of cytochrome b 5 reductase to an active derivative containing two peptide chains. J Biol Chem. 1972 May 10;247(9):2768–2775. [PubMed] [Google Scholar]
- Taniuchi H., Anfinsen C. B. Simultaneous formation of two alternative enzymology active structures by complementation of two overlapping fragments of staphylococcal nuclease. J Biol Chem. 1971 Apr 10;246(7):2291–2301. [PubMed] [Google Scholar]
- Taniuchi H., Parker D. S., Bohnert J. L. Study of equilibration of the system involving two alternative, enzymically active complementing structures simultaneously formed from two overlapping fragments of staphylococcal nuclease. J Biol Chem. 1977 Jan 10;252(1):125–140. [PubMed] [Google Scholar]
- Toth M. J., Schimmel P. Internal structural features of E. coli glycyl-tRNA synthetase examined by subunit polypeptide chain fusions. J Biol Chem. 1986 May 25;261(15):6643–6646. [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Kurkulos M., Repetto B. Homology of yeast mitochondrial leucyl-tRNA synthetase and isoleucyl- and methionyl-tRNA synthetases of Escherichia coli. J Biol Chem. 1988 Jan 15;263(2):850–856. [PubMed] [Google Scholar]
- Tzagoloff A., Vambutas A., Akai A. Characterization of MSM1, the structural gene for yeast mitochondrial methionyl-tRNA synthetase. Eur J Biochem. 1989 Feb 1;179(2):365–371. doi: 10.1111/j.1432-1033.1989.tb14562.x. [DOI] [PubMed] [Google Scholar]
- Walter P., Gangloff J., Bonnet J., Boulanger Y., Ebel J. P., Fasiolo F. Primary structure of the Saccharomyces cerevisiae gene for methionyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1983 May;80(9):2437–2441. doi: 10.1073/pnas.80.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster T., Tsai H., Kula M., Mackie G. A., Schimmel P. Specific sequence homology and three-dimensional structure of an aminoacyl transfer RNA synthetase. Science. 1984 Dec 14;226(4680):1315–1317. doi: 10.1126/science.6390679. [DOI] [PubMed] [Google Scholar]
- Wrubel W., Stochaj U., Sonnewald U., Theres C., Ehring R. Reconstitution of an active lactose carrier in vivo by simultaneous synthesis of two complementary protein fragments. J Bacteriol. 1990 Sep;172(9):5374–5381. doi: 10.1128/jb.172.9.5374-5381.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]