Abstract
Background: Thyroid cancers are rare in the pediatric age group, and unlike in adults, few data are available regarding the clinical implication of histologic subtypes in the pediatric population. The purpose of the current study was to determine the prognostic significance of histologic subtypes of differentiated thyroid cancer (DTC) in a large series of children and adolescents followed at a single institution.
Methods: A retrospective review was conducted of all pediatric DTC patients who were treated and followed between 1988 and 2012. Sixty-two patients (median age at diagnosis 13.8 years, median age at follow-up 18 years, 77% female) were assessed. The most common subtypes included classic papillary thyroid carcinoma (PTC; 48%), diffuse sclerosing PTC (16%), and follicular variant PTC (15%); 37% were considered “high-risk” histologies based on adult criteria.
Results: In a multivariate model, only extensive extrathyroidal extension (ETE), defined as the presence of two or more microscopic foci of tumor cells ≤1 mm in size each or any foci >1 mm in size invading beyond the thyroid capsule into perithyroid soft tissue or organs, was significantly associated with extent of disease at presentation. At last follow-up, 76% of subjects had no evidence of disease, 18% had persistent disease, and 5% had recurrent/progressive disease. Event-free survival was associated with extent of disease at presentation (p = 0.01), extensive ETE at diagnosis (p < 0.01), and male sex (p = 0.01), but not histologic subtype (p = 0.20).
Conclusions: Pediatric DTC carries an excellent prognosis. Extensive ETE at diagnosis was found to be an independent predictor of extent of disease at presentation, as well as event-free survival. Unlike in the adult population, “high-risk” histologic subtypes did not independently predict extent of disease at presentation or event-free survival in this pediatric population with DTC.
Introduction
Thyroid cancers are rare in the pediatric age group, with an annual incidence from 0.4 to 0.7 cases per 100,000 children aged 0–19 years (1,2). Recent data, however, demonstrate a rising incidence of thyroid cancer in the pediatric population, with a 1.1% increase in overall incidence per year (2,3). Differentiated thyroid cancer (DTC) comprises 90–95% of all childhood thyroid cancers, with papillary thyroid carcinoma (PTC) accounting for the majority of DTC cases (4,5).
Children tend to present with more extensive disease (positive cervical lymph nodes and evidence of local or distant metastasis) and have a higher risk of recurrence compared with the adult population (6–10). Nonetheless, pediatric DTC has an excellent long-term prognosis, with 30-year survival rates of 90–99% (1,7).Treatment generally involves surgery and postoperative treatment with radioactive iodine (131I).
Some believe that the difference in the natural history and outcome between pediatric and adult PTC may be related to differences in the underlying genetics. Studies have demonstrated that BRAF mutations are commonly seen in adult PTC, while RET mutations/rearrangements predominate in the pediatric PTC population (11–14).
In adults, the histologic features of follicular cell–derived DTC are felt to have prognostic importance, and subjects are often classified as either low or high risk based in part on histologic subtyping. Certain variants of PTC (tall cell, diffuse sclerosing, solid) and poorly differentiated thyroid carcinomas (PDTC) tend to be more aggressive and carry an increased risk of recurrence and mortality, and are therefore classified as high-risk histologies, while classical and follicular variants of PTC carry a more favorable prognosis and are therefore classified as low-risk histologies (4,15–19). Given that DTC in children behaves differently from thyroid cancer in adults, it is unclear if histologic subtypes carry the same implications in children as they do in adults.
The purpose of the current study was to determine the prognostic significance of histologic subtypes of DTC in a large series of children and adolescents followed at a single institution.
Materials and Methods
Patients
This study is a retrospective review of pediatric DTC patients who were treated and followed between 1988 and 2012 at Memorial Sloan Kettering Cancer Center. One hundred and twenty-six charts were reviewed. Sixty-four patients were excluded due to original pathology slides unavailable for review (n = 57), history of exposure to neck radiation prior to thyroid cancer diagnosis (n = 2), history of prior malignancy without neck radiation (n = 2), pathology reclassified as other than DTC (n = 1), history of familial syndrome associated with thyroid cancer (n = 1), and pathology classified as minimally invasive follicular carcinoma (n = 1). The latter was excluded, since it was the only follicular carcinoma in these series, and thus no meaningful analysis of this unique category could be performed with only one case. Sixty-two patients were included in the study. Patients were divided into three groups based on the extent of their disease at presentation: N0, lymph node negative/no lymph nodes removed; N1, lymph node positive without distant metastases; or M1, presence of distant metastases. The Institutional Review Board of Memorial Sloan Kettering Cancer Center approved this study.
Treatment
All patients underwent surgical resection of their tumor. The majority of patients had their initial surgery at Memorial Sloan Kettering Cancer Center (44/62; 71.0%). Extent of lymph node surgery was classified into four different categories: none (i.e., no neck dissection, or lymph node biopsy only), unilateral neck, central compartment, and bilateral (including central compartment plus bilateral jugulodigastric dissection). Postoperative 131I treatment was administered to the majority of patients (48/62; 77.4%) following surgery. Median time to first 131I treatment was 96 days (range 8–1857 days). Initial median 131I dose was 100 mCi (range 10.3–435 mCi) or 2.0 mCi/kg (range 0.3–5.1 mCi/kg). 131I dosing was based on results of dosimetry rather than body weight, even in the youngest patients (20). 131I treatment was repeated in selected patients with persistently positive post-treatment whole-body 131I scans performed at least 12 months after their initial treatment with 131I. All patients were placed on thyroid hormone therapy (at thyrotropin [TSH] suppressive doses) following treatment. Post-treatment follow-up consisted of regularly scheduled physical examinations, thyroid function testing (TSH, free thyroxine, and thyroglobulin levels), and neck ultrasounds. Additionally, computed tomography and magnetic resonance imaging scans of the chest/neck and positron emission tomography scans were performed in selected patients. More recently, Thyrogen stimulation testing was performed in selected patients in which thyroglobulin levels were obtained pre and post administration of Thyrogen (21).
Methods
Data collected for the study included demographic information, surgical history, pathologic findings, 131I treatment history, laboratory testing, and imaging history.
A meticulous histopathology review was performed for all patients by one head and neck pathologist with a special interest in thyroid neoplasia (R.G.) to confirm the diagnosis, extent of disease, and tumor characteristics. Thyroid carcinomas were classified according to the last World Health Organization classification of endocrine tumors, except for tall-cell PTC variant and PDTC (22). PDTC was defined as a carcinoma displaying high mitotic activity (≥5 mitosis/10 high-power fields, 400×) and/or tumor necrosis, showing follicular cell differentiation at the morphologic or immunohistochemical level. Tall-cell variant was characterized as a papillary carcinoma composed of >50% tall cells. The latter was defined as having a height at least twice its width with an oncocytic cytoplasm.
The largest dimension of the carcinoma was based on review of the gross pathology report and direct microscopic measurement of the tumor on the slides. The mitotic rate of the tumor was determined by counting 10 contiguous high-power fields (400×) using an Olympus microscope (U-DO model BX-40; Olympus America, Inc., Melville, NY). Tumor necrosis was defined by a “comedo-like” appearance composed of degenerating cytoplasm and punctate, karyorrectic nuclear debris. Vascular invasion was categorized as present or absent. Invasion of the tumor capsule was defined as transcapsular penetration. A tumor was considered infiltrative if the carcinoma cells were present in between non-neoplastic thyroid follicles. Extrathyroidal extension (ETE) was defined as tumor cells invading beyond the thyroid capsule into perithyroid soft tissue or organs. ETE was subdivided into: (i) none; (ii) focal: presence of one or two microscopic foci of ETE measuring ≤1 mm each; or (iii) extensive: presence of more than two microscopic foci of ETE (≤1 mm in size each) or any foci >1 mm in size. The type of perithyroid tissue or organ invaded by the tumor was recorded (e.g., adipose tissue, skeletal muscle, recurrent nerve, trachea, and esophagus). Multicentricity was defined as the presence of two or more foci of carcinoma in the thyroid. The status of the resection margins was reported as positive (tumor present at the surgical margin) or negative (no tumor at surgical margin). The number of lymph nodes examined microscopically, as well as the number of nodes with metastatic carcinoma, were also recorded.
For the event-free survival analysis, patients were classified as having no evidence of disease (NED), persistent disease, or recurrent/progressive disease. NED was defined as a negative post-treatment whole-body 131I scan or a negative neck ultrasound, for those who did not undergo whole body scanning, performed one year following initial treatment. Persistent disease was defined as a positive post-treatment whole-body 131I scan that indicated similar or less iodine-avid disease compared to the initial scan, one year following initial treatment. Recurrent/progressive disease was defined as evidence of new or increased disease on whole-body 131I scan or biopsy-proven new disease on neck ultrasound following initial treatment.
Although thyroglobulin levels were collected on all study subjects, a decision was made not to include them in classifying outcome due to the large number of subjects with elevated antithyroglobulin antibodies and the fact that several different thyroglobulin assays were used over the time course of this study, which made direct comparisons across study subjects difficult (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy).
Statistical analysis
Data are expressed as medians (range) unless otherwise specified, with p ≤ 0.05 considered statistically significant. A chi-square test for trend in the proportion per group was performed. An ordinal regression model was used to determine the factors that jointly predict extent of disease at presentation. A log-rank test, with a landmark time of one year, was used to determine the factors associated with event-free survival. An event was defined as persistence or recurrence/progression of the disease.
Results
Patient characteristics
Patient characteristics are detailed in Table 1. Sixty-two patients with pediatric DTC were studied. The majority of patients were female (48/62; 77.4%). Median age at diagnosis was 13.8 years (range 2.7–18.9 years), and median age at last follow-up visit was 18.0 years (range 7.6–34.0 years).
Table 1.
Patient and Treatment Characteristics
| Characteristic | n = 62 | % of total |
|---|---|---|
| Median age at diagnosis, years (range) | 13.8 (2.7–18.9) | |
| Median age at last visit, years (range) | 18.0 (7.6–34.0) | |
| Median follow-up, years (range) | 4.0 (0.0–14.8) | |
| Extent of disease (n): | ||
| N0 | 20 | 32.3 |
| N1 | 31 | 50.0 |
| M1 | 11 | 17.7 |
| Sex (n): | ||
| Female | 48 | 77.4 |
| Male | 14 | 22.6 |
| Thyroidectomy: | ||
| Totala | 57 | 91.9 |
| Completion | 7 | 11.3 |
| Subtotal | 5 | 8.1 |
| Extent of lymph node surgery: | ||
| None | 24 | 38.7 |
| Unilateral neck | 17 | 27.4 |
| Bilateral neck | 17 | 27.4 |
| Central compartment | 4 | 6.5 |
| Radioiodine (131I) treatment: | ||
| Yes | 48 | 77.4 |
| >1 treatment | 22 | 35.5 |
| No | 14 | 22.6 |
Includes those who had an initial thyroidectomy as well as those with initial subtotal thyroidectomy.
All patients underwent thyroid surgery, which included a total thyroidectomy in 57 (91.9%) and a subtotal thyroidectomy in the remaining five (8.1%). Most subjects (48/62) also received at least one treatment with 131I. Twenty (32.3%) patients were classified as group N0, 31 (50%) as group N1, and 11 (17.7%) as group M1.The median number of positive nodes in those with lymph node involvement was 7 (range 1–61), with a median of 14 nodes (range 1–114) biopsied. All patients in group M1 presented with distant metastases to the lungs. One patient had additional metastases to the mediastinum, and one patient had evidence of distant metastases, despite negative lymph nodes at presentation.
Histopathology characteristics
Histopathology characteristics are shown in in Table 2. Median tumor size was 2 cm (range 0.3–8.0 cm), with the majority of tumors being <4 cm (50/62; 80.6%). Infiltration occurred in 46/62 (74.2%). However, most patients had negative tumor margins (45/62; 72.6%).
Table 2.
Histopathology Characteristics
| Characteristic | n = 62 | % of total |
|---|---|---|
| Tumor size, cm (n): | ||
| <4 | 50 | 80.6 |
| ≥4 | 12 | 19.4 |
| Tumor infiltration: | ||
| Yes | 46 | 74.2 |
| No | 16 | 25.8 |
| Margins: | ||
| Positive | 17 | 27.4 |
| Negative | 45 | 72.6 |
| Histology: | ||
| Low risk: | 39 | 62.9 |
| Classical papillary thyroid carcinoma | 39 | 48.4 |
| Follicular variant papillary thyroid carcinoma | 9 | 14.5 |
| Encapsulated | 8 | 12.9 |
| High risk: | 23 | 37.1 |
| Tall-cell papillary thyroid carcinoma | 8 | 12.9 |
| Diffuse sclerosing papillary thyroid carcinoma | 10 | 16.1 |
| Poorly differentiated carcinoma | 4 | 6.5 |
| Solid papillary thyroid carcinoma | 1 | 1.6 |
The classical PTC variant was the most common histologic subtype observed (30/62; 48.4%). Diffuse sclerosing was the second most common histologic subtype (10/62; 16.1%), followed by follicular variant (9/62; 14.5%), tall-cell variant (8/62; 12.9%), and solid variant (1/62; 1.6%; Figs. 1–3). Four (6.5%) patients fulfilled the criteria for PDTC.
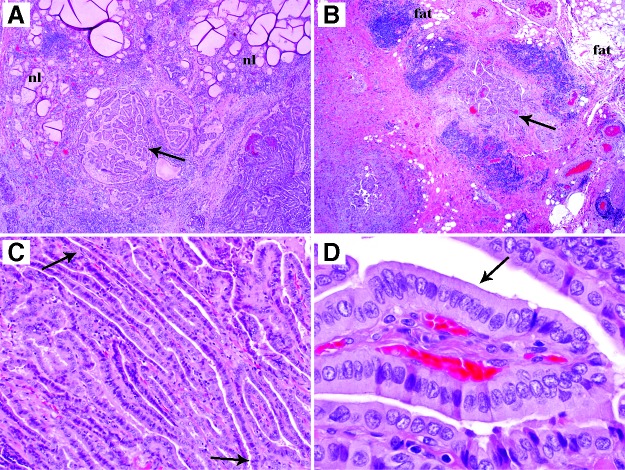
FIG. 1.
Representative microphotographs of an infiltrative papillary thyroid carcinoma, tall-cell variant (size 3.8 cm) from a 12-year-old patient with extensive extrathyroidal extension and 17 neck nodes with metastatic carcinoma. (A) Low-power view showing tumor (arrow) infiltrating in between non-neoplastic thyroid follicles (nl). (B) Low-power view depicting tumor (arrow) invading extrathyroidal adipose tissue (fat). (C) Medium-power view with arrows pointing to both ends of an elongated follicle. The latter is commonly seen in the tall-cell variant. (D) The tumor was composed of a majority of tall cells (arrow) whose height is at least twice their width (high-power view).
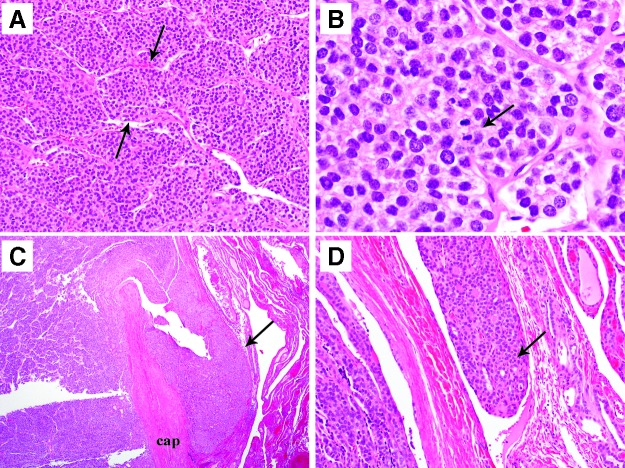
FIG. 2.
Representative microphotographs of a 4-cm encapsulated papillary carcinoma, follicular variant non-invasive from a 12.5-year-old girl with no lymph node involvement. Patient did not recur and was classified as no evidence of disease (NED) after 10 years of follow-up. (A) Low-power view showing the tumor (arrow) growing in follicles with no invasion into capsule (cap). (B) The follicles display clear, enlarged, overlapping nuclei with grooves (arrow) typical of papillary carcinoma (high-power view).
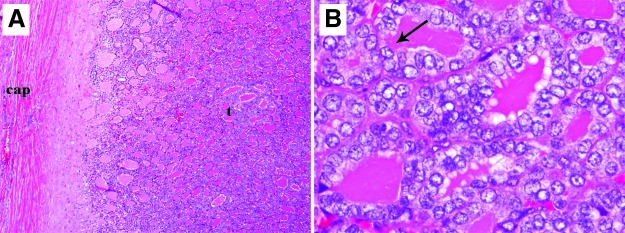
FIG. 3.
Representative microphotographs of a poorly differentiated thyroid carcinoma from a five-year-old female with encapsulated poorly differentiated thyroid carcinoma (PDTC; 2 cm in greatest size) with extensive capsular and vascular invasion. The patient did not recur and was NED after 10.4 years of follow-up. (A) Low-power view showing a solid/nested (insular) growth pattern commonly seen in PDTC. Arrows delineate a tumor nest. (B) High-power view showing a mitosis (arrow). The mitotic rate was very high (17 mitosis/10 high-power fields, 400×). (C) Low-power view showing capsular invasion. Tumor bud (arrow) has transgressed the capsule (cap). (D) Medium-power view showing vascular invasion. Tumor thrombus (arrow) is hanging in the lumen of a vessel immediately outside the tumor capsule.
Predictors of extent of disease
Predictors of extent of disease are given in Table 3. The score test from the ordinal regression model revealed that several factors predicted extent of disease at presentation: diffuse sclerosing variant (p = 0.026), male sex (p = 0.008), infiltration (p < 0.001), positive margins (p < 0.001), and extensive ETE (p < 0.001).
Table 3.
Predictors of Extent of Disease
| Univariate analysis | |
|---|---|
| Age | p = 0.40 |
| Male sex | p = 0.01 |
| Diffuse sclerosing variant | p = 0.20 |
| High-risk histology | p = 0.03 |
| Positive infiltration | p = 0.90 |
| Positive margins | p < 0.001 |
| Tumor size ≥4 cm | p < 0.001 |
| Extensive extrathyroidal extension | p < 0.001 |
The ordinal regression model was also used to determine the factors that independently predicted extent of disease at presentation. In this model, only extensive ETE was significantly associated with extent of disease at presentation. Extensive ETE was most prevalent in the M1 group (8/11; 72.7%). However, it also occurred in the N1 group (14/31; 45.2%). Extensive ETE was not present in the N0 group (0/20; 0.0%).
Follow-up and event free survival analysis
Table 4 shows the follow-up and event-free survival analysis. Fifty-five (88.7%) patients were followed for one or more years post-diagnosis. In these 55 patients, median follow-up was 5.0 years (range 1.0–14.8 years).
Table 4.
Associations Between Various Risk Factors and Outcome
| NED (n = 42) | Persistent disease (n = 10) | Recurrent/progressive disease (n = 3) | |
|---|---|---|---|
| Extent of disease: | |||
| N0 (n = 18) | 16 (88.9%) | 0 (0.0%) | 2 (11.1%) |
| N1 (n = 26) | 21 (80.8%) | 4 (15.4%) | 1 (3.8%) |
| M1 (n = 11) | 5 (45.5%) | 6 (54.5%) | 0 (0.0%) |
| Extrathyroidal extension: | |||
| None/focal (n = 35) | 31 (88.6%) | 2 (5.7%) | 2 (5.7%) |
| Extensive (n = 20) | 11 (55.0%) | 8 (40.0%) | 1 (5.0%) |
| Sex: | |||
| Male (n = 14) | 7 (50.0%) | 5 (35.7%) | 2 (14.3%) |
| Female (n = 41) | 35 (85.4%) | 5 (12.2%) | 1 (2.4%) |
| Total thyroidectomy: | |||
| Yes (n = 52) | 39 (75.0%) | 10 (19.2%) | 3 (5.8%) |
| No (n = 3) | 3 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| Bilateral neck dissection: | |||
| Yes (n = 15) | 10 (66.7%) | 4 (26.7%) | 1 (6.6%) |
| No (n = 40) | 32 (80.0%) | 6 (15.0%) | 2 (5.0%) |
| Radioiodine (131I) treatment: | |||
| Yes (n = 44) | 31 (70.5%) | 10 (22.7%) | 3 (6.8%) |
| No (n = 11) | 11 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| Histology: | |||
| Low risk (n = 32): | 24 (75.0%) | 6 (18.8%) | 2 (6.2%) |
| Classical papillary thyroid carcinoma (n = 25) | 18 (72.0%) | 6 (24.0%) | 1 (4.0%) |
| Follicular variant papillary thyroid carcinoma (n = 7) | 6 (85.7%) | 0 (0.0%) | 1 (14.3%) |
| High risk (n = 23): | 18 (78.3%) | 4 (17.4%) | 1 (4.3%) |
| Diffuse sclerosing papillary thyroid carcinoma (n = 10) | 8 (80.0%) | 2 (20.0%) | 0 (0.0%) |
| Tall-cell papillary thyroid carcinoma (n = 8) | 6 (75.0%) | 1 (12.5%) | 1 (12.5%) |
| Poorly differentiated thyroid carcinoma (n = 4) | 3 (75.0%) | 1 (25.0%) | 0 (0.0%) |
| Solid papillary thyroid carcinoma (n = 1) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) |
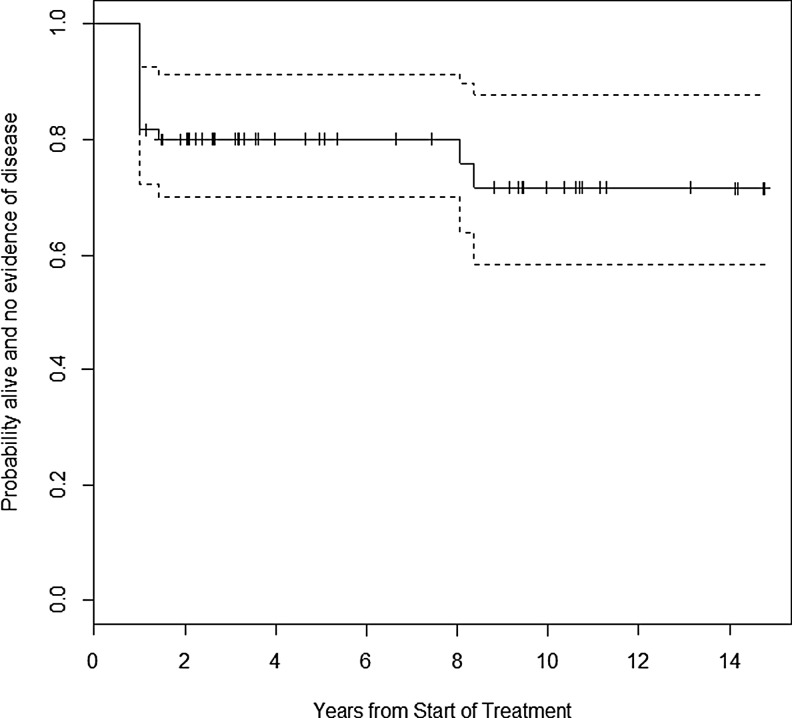
The majority of patients (42/55; 76.4%) had NED at last follow-up visit, while 10/55 (18.2%) had persistent disease and 3/55 (5.4%) had recurrent/progressive disease. There were no patient deaths from disease. The probability of remaining alive and free of disease at five years was 0.80 ([confidence interval (CI) 0.70–0.91]; Fig. 4).
FIG. 4.
Kaplan–Meier estimate of event-free survival over time.
Event-free survival was associated with extent of disease at presentation (p = 0.01), extensive ETE at diagnosis (p < 0.01), and male sex (p = 0.01). Event-free survival was not associated with histologic subtype (p = 0.20), bilateral neck dissection (p = 0.32), or total thyroidectomy (p = 0.35), though treatment with radioactive iodine showed a borderline association (p = 0.06). The majority of patients in groups N0 and N1 had NED at last follow-up visit (16/18 [88.9%] and 21/26 [80.8%], respectively). In group M1, the majority of patients had persistent disease at last follow-up visit (6/11; 54.5%). Three patients had recurrent/progressive disease (group N0, n = 2; group N1, n = 1).
A higher percentage of patients with extensive ETE at diagnosis had persistent disease at last follow-up visit (8/20; 36.8%) compared with those with no/focal ETE at diagnosis (2/35; 5.7%).
Twenty-four of 32 (75.0%) patients with low-risk histologies and 18/23 (78.3%) patients with high-risk histologies had NED at last follow-up visit. Of note, all five patients with encapsulated follicular variant PTC lacking vascular invasion had NED at last follow-up visit, which included two patients who were treated with partial thyroidectomy alone. Of those with low-risk histologies, 6/32 (18.7%) patients had persistent disease, while 2/32 (6.3%) had recurrent/progressive disease at last follow-up. Of those with high-risk histologies, 4/23 (17.4%) patients had persistent disease, while 1/23 (4.3%) patients had recurrent/progressive disease at last follow-up visit. Fifteen of 42 (35.7%) patients with NED at last follow-up visit had had recurrent/progressive disease at some point during follow-up: 7/32 (4.6%) with low-risk histologies, and 8/23 (2.9%) with high-risk histologies.
Discussion
This study confirms that pediatric DTC has an excellent prognosis, as all patients in the cohort were alive at last follow-up, with 21.0% of patients followed for ≥10 years. It was found that extensive ETE at diagnosis was an independent predictor of extent of disease at presentation. Additionally, extent of disease and ETE at presentation, as well as male sex, were associated with event-free survival. Importantly, no association was found between high-risk histologic subtype and extent of disease at presentation or event-free survival.
Prior studies in the pediatric DTC population have shown that extent of disease at presentation may predict response to initial therapy, overall prognosis, and risk of recurrence (5,23,24). Several smaller studies have shown younger age, male sex, multifocal tumors, and tumor size >2 cm to be risk factors for extent of disease at presentation (5,25–27). The present findings differ from prior pediatric studies in that no other risk factors (younger age, male sex, tumor size, infiltration, and positive margins) were found to predict extent of disease at diagnosis after controlling for extensive ETE.
The findings demonstrate a similar percentage of patients with lymph node involvement (66.1 %) and distant metastases at presentation (17.7%) compared to prior studies (25,28–30). Death from DTC did not occur, despite the fact that a majority of subjects had lymph node involvement and/or distant metastases (67.7%), in keeping with the data of others (28).
The present study found that extensive ETE was a predictor of extent of disease at diagnosis, which has previously been shown to be true in adults (31). An association was also shown between ETE and event-free survival. Persistent disease at last follow-up visit occurred more commonly in those who presented with extensive versus no/focal ETE at presentation (36.8% vs. 8.3%). NED at last follow-up visit occurred more commonly in those with no/focal versus extensive ETE at presentation (86.1% vs. 57.9%).
Studies in adults have clearly shown a distinction between low- and high-risk histologic DTC subtypes and overall prognosis. Although pediatric studies are limited, a few have also demonstrated differences between pediatric PTC subtypes and overall prognosis. Collini et al. found that solid/trabecular variants of pediatric PTC conferred a higher risk of relapse, while poorly differentiated/tall-cell variants were not associated with a worse outcome (32). Handkiewicz-Junak et al. found significantly more PTC recurrences in those with the classical versus follicular variant subtype (33).
The present data demonstrate that, in the authors' hands and following their institutional treatment protocols, so-called high-risk histologic DTC subtypes, including PDTC, were not associated with more extensive disease at presentation or inferior outcomes compared with those with low-risk subtypes. The diffuse sclerosing PTC variant was associated with more extensive disease at diagnosis on univariate analysis, which is keeping with the observation of others (17,34). However, multivariate analysis found neither diffuse sclerosing PTC variant nor high-risk histologic subtypes, as a group, to correlate with extent of disease at presentation,
Importantly, it was found that patients with encapsulated follicular variant PTC lacking vascular invasion at presentation have an excellent prognosis, similar to what has been described in adults, even following subtotal thyroidectomy (19). Therefore, patients with small, encapsulated lesions, lacking vascular invasion, may only require subtotal thyroidectomy.
A major strength of this study was that all histology was reviewed by a single pathologist using the most up-to-date criteria. While the number of subjects was relatively large for a single institution study, power to detect predictors of disease stage and outcomes was limited, given the small number of events. Thus, verification of the results will be required in larger, multicenter series. In addition, the relatively short follow-up time for many of the patients could have resulted in an underestimate of adverse outcomes.
To conclude, pediatric DTC carries an excellent prognosis. Unlike in the adult population, high-risk histologic subtypes did not independently predict extent of disease at presentation or event-free survival in this pediatric population with DTC. These findings will assist clinicians in developing treatment strategies for children and adolescents with DTC that will hopefully optimize outcomes while avoiding unnecessary and potentially toxic therapies in selected patients.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. 2009. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res 156:167–172 [DOI] [PubMed] [Google Scholar]
- 2.Holmes L, Hossain J, Opara F 2012 Pediatric thyroid carcinoma incidence and temporal trends in the USA (1973–2007): race or shifting diagnostic paradigm? ISRN Oncol 2012:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. 2014. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr 164:1481–1485 [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 5.Kiratli PO, Volkan-Salanci B, Günay EC, Varan A, Akyüz C, Büyükpamukçu M. 2013. Thyroid cancer in pediatric age group: an institutional experience and review of the literature. J Pediatr Hematol Oncol 35:93–97 [DOI] [PubMed] [Google Scholar]
- 6.Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. 2010. Long term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg 34:1192–1202 [DOI] [PubMed] [Google Scholar]
- 7.Rivkees SA, Mazzaferri EL, Verburg FA, Reiners C, Luster M, Breuer CK, Dinauer CA, Udelsman R. 2011. The treatment of differentiated thyroid cancer in children; emphasis on surgical approach and radioactive iodine therapy. Endocr Rev 32:798–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grigsby PW, Gal-or A, Michalski JM, Doherty GM. 2002. Childhood and adolescent thyroid carcinoma. Cancer 95:724–729 [DOI] [PubMed] [Google Scholar]
- 9.La Quaglia MP, Black T, Holcomb TB, Sklar CA, Azizkhan RG, Haase GM, Newman KD. 2000. Differentiated thyroid cancer; clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the Surgical Discipline Committee of the Children's Cancer Group. J Pediatr Surg 35:955–959 [DOI] [PubMed] [Google Scholar]
- 10.Waguespack SG, Francis G. 2010. Initial management and follow-up of differentiated thyroid cancer in children. J Natl Compr Canc Netw 8:1289–1300 [DOI] [PubMed] [Google Scholar]
- 11.Jarzab B, Handkiewicz-Junak D. 2007 Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens) 6:200–209 [PubMed] [Google Scholar]
- 12.Vander Poorten V, Hens G, Delaere P. 2013. Thyroid cancer in children and adolescents. Curr Opin Otolaryngol Head Neck Surg 21:135–142 [DOI] [PubMed] [Google Scholar]
- 13.Lima J, Lima J, Trovisco V, Soares P, et al. BRAF mutations are not a major event in post-Chernobyl childhood thyroid carcinomas. J Clin Endocrinol Metab 89:4267–4271 [DOI] [PubMed] [Google Scholar]
- 14.Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. 2000. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab 8:1170–1175 [DOI] [PubMed] [Google Scholar]
- 15.Ganly I, Ibrahimpasic T, Rivera M, Nixon I, Palmer F, Patel SG, Tuttle RM, Shah JP, Ghossein R. 2014. Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid 24:662–670 [DOI] [PubMed] [Google Scholar]
- 16.Ibrahimpasic T, Ghossein R, Carlson DL, Chernichenko N, Nixon I, Palmer FL, Lee NY, Shaha AR, Patel SG, Tuttle RM, Balm AJ, Shah JP, Ganly I. 2013. Poorly differentiated thyroid carcinoma presenting with gross extrathyroidal extension: 1986–2009 Memorial Sloan Kettering Cancer Center experience. Thyroid 23:997–1002 [DOI] [PubMed] [Google Scholar]
- 17.Regalbuto C, Malandrino P, Tumminia A, Le Moli R, Vigneri R, Pezzino V 2011 A diffuse sclerosing variant of papillary thyroid carcinoma: clinical and pathologic features and outcomes of 34 consecutive cases. Thyroid 21:383–389 [DOI] [PubMed] [Google Scholar]
- 18.Silver CE, Owen RP, Rodrigo JP, Rinaldo A, Devaney KO, Ferlito A. 2011. Aggressive variants of papillary thyroid carcinoma. Head Neck 33:1052–1059 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA. 2006. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer 107:1255–1264 [DOI] [PubMed] [Google Scholar]
- 20.Yeh SD, La Quaglia MP. 1997. 131I therapy for pediatric thyroid cancer. Semin Pediatr Surg 6:128–133 [PubMed] [Google Scholar]
- 21.Iorcansky S, Herzovich V, Qualey RR, Tuttlle RM. 2005. Serum thyrotropin (TSH) levels after recombinant human TSH injections in children and teenagers with papillary thyroid cancer. J Clin Endocrinol Metab 90:6553–6555 [DOI] [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer 2004 World Health Organization classification of tumours. Pathology and genetics tumours of the endocrine organs. Available at: www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf (accessed June18, 2015)
- 23.Powers PA, Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Francis GL. 2003. Tumor size and extent of disease at diagnosis predict the response to initial therapy for papillary thyroid carcinoma in children and adolescents. J Pediatr Endocrinol Metab 16:693–702 [DOI] [PubMed] [Google Scholar]
- 24.Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Svec RL, Adair C, Francis GL. 1998. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf) 49:619–628 [DOI] [PubMed] [Google Scholar]
- 25.O'Gorman CS, Hamilton J, Rachmiel M, Gupta A, Ngan BY, Daneman D. 2010. Thyroid cancer in childhood: a retrospective review of childhood course. Thyroid 20:375–380 [DOI] [PubMed] [Google Scholar]
- 26.Lazar L, Lebenthal Y, Steinmetz A, Yackobovitch-Gavan M, Phillip M. 2009. Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre-pubertal children and adolescents. J Pediatr 154:708–714 [DOI] [PubMed] [Google Scholar]
- 27.Fassina AS, Rupolo M, Pelizzo MR, Casara D. 1994. Thyroid cancer in children and adolescents. Tumori 80:257–262 [DOI] [PubMed] [Google Scholar]
- 28.Newman KD, Black T, Heller G, Azizkhan RG, Hocomb GW, Sklar C, Vlamis V, Haase GM, La Quaglia MP. 1998. Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children's Cancer Group. Ann Surg 227:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rachmiel M, Charron M, Gupta A, Hamilton J, Wherrett D, Forte V, Daneman D. 2006. Evidence-based review of treatment and follow up of pediatric patients with differentiated thyroid carcinoma. J Pediatr Endocrinol Metab 19:1377–1393 [DOI] [PubMed] [Google Scholar]
- 30.Dinauer CA, Breuer C, Rivkees SA. 2008. Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol 20:59–65 [DOI] [PubMed] [Google Scholar]
- 31.Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, Gardini G, Valcavi R, Barbieri V. 2009. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 19:707–716 [DOI] [PubMed] [Google Scholar]
- 32.Collini P, Mattavelli F, Pellegrinelli A, Barisell M, Ferrari A, Massimino M. 2006. Papillary carcinoma of the thyroid gland of childhood and adolescence: morphologic subtypes, biologic behavior and prognosis: a clinicopathologic study of 42 sporadic cases treated at a single institution during a 30-year period. Am J Surg Pathol 30:1420–1426 [DOI] [PubMed] [Google Scholar]
- 33.Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, Kukulska A, Prokurat A, Wygoda Z, Jarzab B. 2007. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med 48:879–888 [DOI] [PubMed] [Google Scholar]
- 34.Chow SM, Chan JK, Law SC, Tang DL, Ho CM, Cheung WY, Wong IS, Lau WH. 2003. Diffuse sclerosing variant of papillary thyroid carcinoma—clinical features and outcome. Eur J Surg Oncol 29:446–449 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.