Abstract
Juan Su, Zhanquan Li, Sen Cui, Linhua Ji, Hui Geng, Kexia Chai, Xiaojing Ma, Zhenzhong Bai, Yingzhong Yang, Tana Wuren, Ri-Li Ge, and Matthew T. Rondina. The local HIF-2α/EPO pathway in the bone marrow is associated with excessive erythrocytosis and the increase in bone marrow microvessel density in chronic mountain sickness. High Alt Med Biol. 16:318–330, 2015.—Aim: Chronic mountain sickness (CMS) is characterized by excessive erythrocytosis, and angiogenesis may be involved in the pathogenesis of this disease. The bone marrow niche is the primary site of erythropoiesis and angiogenesis. This study was aimed at investigating the associations of the levels of hypoxia-inducible factors (HIFs), erythropoietin (EPO), and erythropoietin receptor (EPOR), as well as microvessel density (MVD) in the bone marrow with CMS.
Results: A total of 34 patients with CMS and 30 control subjects residing in areas at altitudes of 3000–4500 m were recruited for this study. The mRNA and protein expression of HIF-2α and EPO in the bone marrow cells was significantly higher in the CMS patients than in the controls. Moreover, changes in HIF-2α expression in CMS patients were significantly correlated with EPO and hemoglobin levels. In contrast, the expression of mRNA and protein expression of HIF-1α and EPOR did not differ significantly between the CMS and control patients. Increased MVD was observed in the bone marrow of the patients with CMS and it was significantly correlated with hemoglobin.
Conclusions: Bone marrow cells of CMS patients may show enhanced activity of the HIF-2α/EPO pathway, and EPO may regulate the erythropoiesis and vasculogenesis through autocrine or/and paracrine mechanisms in the bone marrow niche. The increased MVD in the bone marrow of CMS patients appears to be involved in the pathogenesis of this disease.
Key Words: : bone marrow cells, chronic, erythropoietin, hypoxia-inducible factors, microvessel density, mountain sickness
Introduction
Chronic mountain sickness (CMS) is a clinical syndrome caused by maladaptation to chronic exposure to a high-altitude hypoxic environment and occurs among some individuals in high-altitude regions worldwide. Currently, 140 million people live permanently at altitudes of more than 2500 m (Penaloza and Arias-Stella, 2007). The prevalence of CMS was found to be increased by 10% in the adult population living at altitudes of more than 2500 m in the Peruvian Andes (León-Velarde et al., 1994), and the prevalence of this disease in Chinese Han men who migrated to the Qinghai–Tibetan plateau (altitude of 3700–5000 m) was increased by 17.8% (Jiang et al., 2014). CMS is characterized by excessive erythrocytosis (EE) and hypoxemia (León-Velarde et al., 2003, 2005). Chronic high-altitude hypoxia is a key pathological mechanism for the development of this disease.
Hypoxia-inducible factors (HIFs) control a wide spectrum of tissue-specific and systemic hypoxia responses. HIFs are heterodimers comprising one of three major oxygen-labile HIF-α subunits (HIF-1α, HIF-2α, and HIF-3α) and a constitutive HIF-1β subunit, which together form the HIF-1α, HIF-2α, and HIF-3α transcriptional complexes, respectively (Wang et al., 1995). Of the three α-subunits, HIF-1α and HIF-2α are the most studied. Little information is available about HIF-3α, which plays an inhibitory role in certain contexts (Makino et al., 2001). HIF-1α has been described as the master regulator of hypoxic responses and the crucial node in ensuring survival during hypoxic stress (Wang et al., 1995). Moreover, levels have been measured in white blood cells (WBCs) and correlations have been identified between increased HIF-1α expression in WBCs and CMS (Appenzeller et al., 2006).
In comparison, HIF-2α was initially identified as the endothelial PAS domain protein 1 (EPAS1) and hence has been traditionally considered to have a more specialized function than HIF-1α (Tian et al., 1997). While HIF-1α is ubiquitously expressed, HIF-2α expression is more restricted, found largely within cardiomyocytes, hepatocytes, type II pneumocytes, glial cells, renal cortical interstitial cell, and bone marrow stromal cells (Rosenberger et al., 2002; Wiesener et al., 2003; Ben-Shoshan et al., 2008). The localization of HIF-2α within bone marrow cells suggests that it may be a critical factor in hypoxia responses (Wiesener et al., 2003). HIF-1α and HIF-2α play divergent, but complementary, roles during hypoxic response in tissues under both physiological and pathophysiological conditions. Studies of families in the idiopathic EE registry revealed the presence of mutations in the HIF-2α-coding, but not HIF-1α-coding, sequence (Gordan et al., 2007; Gale et al., 2008; Martini et al., 2008; Percy et al., 2008a, 2008b; Furlow et al., 2009). Genetic variants of the HIF2α gene have been associated with high-altitude dwellers who seem to be protected from CMS (Beall et al., 2010; Simonson et al., 2010; Yi et al., 2010).
Nevertheless, while the bone marrow niche is known to be one of the primary sites of erythropoiesis, the expression and role of HIFs in the bone marrow of patients with CMS have not been studied previously.
Erythropoietin (EPO) is an essential glycoprotein that facilitates the maturation of red blood cells from erythroid progenitors and mediates erythropoiesis. EPO also mediates nonerythroid processes such as angiogenesis, neuroprotective properties, and immune regulation (Jaquet et al., 2002; Bahlmann et al., 2004; Wang et al., 2004; Lifshitz et al., 2010). EPO is a classic example of a hypoxia-inducible gene and both HIF-1α and HIF-2α regulate the expression of the EPO gene. While in adult organisms, the kidney produces around 90% of systemic EPO, hypoxia-induced EPO transcripts have also been found in the liver, brain, spleen, lung, and bone marrow tissue (Yeo et al., 2008; Weidemann and Johnson, 2009). EPO is also produced by erythroid progenitors (Stopka et al., 1998; Fandrey, 2004) and osteoblasts (Rankin et al., 2012) in the local bone marrow. It can also regulate erythropoiesis through autocrine or paracrine mechanisms (Sato et al., 2000). Upon ligand binding, the erythropoietin receptor (EPOR), which lacks intrinsic catalytic function and is hypoxia inducible (Chin et al., 2000; Yoon et al., 2006), may contribute to the increased sensitivity of response to EPO (Beleslin-Cokic et al., 2004).
Previous studies examining EPO polymorphisms, EPOR, and EE failed to show any evidence of a major monogenic contribution of the loci tested in response to EE in CMS patients (Mejía et al., 2005). Although the variation in serum EPO levels does not explain the striking variation in hemoglobin at high altitudes, EPO in the serum usually shows high expression in patients with elevated hemoglobin (Hb) levels (León-Velarde et al., 1991). Recently, whole-genome sequencing revealed significantly higher SENP1 expression in individuals with CMS than in those without (Zhou et al., 2013; Cole et al., 2014). SENP1 has also been shown to regulate EPO production by regulating the stability of HIF during hypoxia (Cheng et al., 2007; Yu et al., 2010). Therefore, there is reason to believe that EPO, especially EPO in the bone marrow niche, might play an important role in the pathophysiology of CMS.
Plasma-soluble EPOR levels are known to be decreased in CMS (Villafuerte et al., 2014), but the changes in EPOR in bone marrow cell membrane (mEPOR), which are a direct response to EPO, have not previously been studied in CMS. Hence, whether the increased expression of mEPOR in CMS leads to the development of EE is still unclear.
It is well established that angiogenesis and vasculogenesis are part of the physiological response to hypoxia. Angiogenesis might be a compensatory process in high-altitude residents that occurs to alleviate hypoxia in microcirculation and it may also be involved in the pathophysiology of CMS (Appenzeller et al., 2003; Ge et al., 2011; Buroker et al., 2012, 2013; Espinoza et al., 2014). The bone marrow is the primary site of angiogenesis and is a readily accessible tissue for the investigation of angiogenesis. Angiogenesis is commonly estimated in terms of the concentration of vessels and is expressed as microvessel density (MVD) in the bone marrow. Nevertheless, to the best of our knowledge, little research has been performed on the changes in bone marrow MVD in patients with CMS.
This study was aimed at evaluating the association of EE with the expression of HIF-1α, HIF-2α, EPO, and EPOR, and MVD in the bone marrow of CMS patients. We hypothesized that the bone marrow cells of CMS patients might show enhanced activity of EPO/EPOR through increase in HIF-1α and HIF-2α. Thus, EPO/EPOR might regulate erythropoiesis through autocrine or/and paracrine mechanisms in the bone marrow niche. We further hypothesized that MVD would be increased in the bone marrow of CMS patients, which contributes to the pathogenesis of the disease.
Materials and Methods
Subjects
The research protocol was approved by the human subject protection committee at the Qinghai University Affiliated Hospital. Informed consent was obtained from each subject. Thirty-four patients with CMS (men; Han Chinese; age range, 35–60 years) and 30 control subjects (men; Han Chinese; age range, 33–59 years) participated in this study. All the participants were residing at altitudes of 3000–4500 m in the Qinghai province for 5–20 years. The control subjects were healthy patients without any chronic diseases, who were undergoing elective orthopedic surgery to remove remotely placed internal fixation rods. None of the participants had a history of respiratory or cardiovascular diseases, such as chronic obstructive pulmonary disease, pulmonary infection, asthma, shunt, valvular disease, congenital heart disease, or hypertensive heart disease. All participants first underwent blood routine testing and the Qinghai CMS score was calculated as described previously (León-Velarde et al., 2005) for determining the presence of CMS. Arterial O2 saturation (SaO2) was measured by pulse oximetry (YX302 Pulse Oximeter, Yuyue-Jiangsu, China).
Assessment of CMS
A complete clinical examination was performed for each participant. The presence and severity of CMS were assessed using the Qinghai CMS score, which is based on Hb levels and the following symptoms: breathlessness, palpitations, sleep disturbance, cyanosis, dilatation of veins, paresthesia, headache, and tinnitus. Each item was scored on a scale of 0–3 according to the severity. Hb level was dichotomized as either 0 or 3 points with a cutoff of 210 g/L for males and 190 g/L for females. All the participants were males, who were considered to have CMS if Hb levels were ≥210 g/L and the CMS score was >5; Hb level of <210 g/L or a CMS score of <5 was considered to indicate that the participant was a control.
Blood sampling and assay
Blood samples were drawn between 7 and 10 AM to avoid variations in serum EPO due to circadian rhythm and EPO levels in the circulating blood were determined. For these measurements, 5 mL of blood was drawn from the brachial vein, collected into a serum separator tube, and centrifuged at 3000 rpm for 15 minutes. The separated serum samples were stored on dry ice during transport to Xining, where they were further analyzed.
Bone marrow sampling and assay
All the CMS patients (n = 34) underwent conventional bone marrow biopsy and bone marrow puncture of the posterior superior iliac spine. Samples from the control subjects (n = 30) were drawn by bone surgery during orthopedic surgery. Fifteen milliliters of the bone marrow fluid was collected into one serum separator tube and two tubes containing ethylene diamine tetraacetic acid (EDTA). The bone marrow tissues were fixed with formalin. All the bone marrow samples were transported on dry ice to Xining within 24 hours of collection, and then analyzed within 1 hour after arrival.
The bone marrow supernatant was harvested for measuring the EPO levels. Ficoll lymphocyte solution was used to isolate bone marrow mononuclear cells (BMMNCs) for flow cytometry and real-time RT-polymerase chain reaction (PCR) analysis. The fixed bone marrow tissues were embedded in paraffin after EDTA decalcification and alcohol dehydration.
Flow cytometry
For flow cytometry, 1 × 106 cells were collected, washed with phosphate-buffered saline (PBS), and then fixed with 4% paraformaldehyde for 20 minutes. After the addition of 0.1% Triton X-100 (2 mL) for permeabilization, the cells were stained for 40 minutes at 4°C with DyLight488-conjugated mouse anti-human HIF-2α IgG (Novus Biologicals, Littleton, CO), FITC-conjugated mouse anti-human EPO IgG (Millipore, Billerica, MA), or the appropriate isotype control antibodies. The washed cells were then analyzed and the percentage of positive cells was determined using FACSCalibur (CellQuest Version 3.2.1) software.
HIF-1α and EPOR levels were measured by indirect flow cytometry. The BMMNCs were collected and washed, and then incubated with anti-EPOR (mouse IgG antibody; Abcam, Cambridge, United Kingdom) for 40 minutes at 4°C, washed again, and stained with the R-phycoerythrin-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 40 minutes at 4°C. The washed cells were analyzed on a flow cytometer.
The cells were fixed (20 minutes, 4% paraformaldehyde), washed with PBS, and permeabilized (20 minutes, 0.1% Triton X-100), and then stained with an anti-HIF-1α antibody (rabbit IgG antibody; Abcam) for 40 minutes at 4°C. After washing, the cells were again incubated for 40 minutes at 4°C with the R-phycoerythrin-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories). BMMNCs incubated only with the secondary antibody were used as negative controls. The washed cells were then analyzed on a flow cytometer.
Real-time PCR
Real-time quantitative PCR was used to determine the expression of HIF-1α, HIF-2α, EPO, EPOR mRNA, and 18S rRNA (housekeeping gene) in the BMMSCs. Total RNA was isolated using Trizol Reagent (Life Technologies, Carlsbad, CA). cDNA was generated from 2 μg of total RNA using Quant Reverse Transcriptase (TIANGEN, Beijing, China). The cDNA obtained was then amplified using the SYBR Green PCR Master Mix (Life Technologies) in an Applied Biosystems 7500 Real-Time PCR System. Two-step real-time PCR was performed as follows: 95°C for 2 minutes, 60°C for 1 minute extension, and detection, for 40 cycles. The specific primers used were as follows: HIF-1α (forward: 5′-ATCCATGTGACCAT GAGGAAATG-3′; reverse: 5′-TCGGCTAGTTAGGGTACACTTC-3′), HIF-2α (forward: 5′-CCTTTGATGCCGGACAAG-3′; reverse: 5′-GGGACTGAGGCAGATGG-3′), EPO (forward: 5′-TCATCTGTGACAGCCGAGTC-3′; reverse: 5′-TTTGGTGTCTGGGACAGTGA-3′), EPOR (forward: 5′-AC CTTGTGGTATCTGACTCTGG-3′; reverse: 5′-GAGTAGGGGCCATCGG ATAAG-3′) and 18S rRNA (forward: 5′-GAGGATGAGGTGGAACGTGT-3′; reverse: 5′-GGACCTGGCTGTATTTTCCA-3′). Data were analyzed using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Immunohistochemical evaluation
The primary antibodies used were as follows: mouse anti-human HIF-1α monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human HIF-2α polyclonal antibody (Santa Cruz Biotechnology), rabbit anti-human EPO polyclonal antibody (Santa Cruz Biotechnology), rabbit anti-human EPOR polyclonal antibody (Santa Cruz Biotechnology), and mouse anti-human CD34 monoclonal antibody (Maixin, Fuzhou, China). Immunohistochemical assays were performed on the paraffin-embedded sections. The 5-μm-thick sections were deparaffinized in xylene, rehydrated in graded alcohol, and then boiled in EDTA (pH 9.0) for 20 minutes. Endogenous biotin was blocked using the Biotin Block Kit (Maixin), according to the manufacturer's specifications. After blocking with normal goat serum, all the sections were incubated with the primary antibodies overnight at 4°C. Negative controls were prepared by replacing the specific primary antibodies with PBS. The sections were washed with PBS, and then incubated with the appropriate biotin-labeled goat anti-rabbit or goat anti-mouse IgG secondary antibodies (Maixin) for 30 minutes at 37°C. The sections were then incubated with streptavidin-biotin-peroxidase (Maixin) for 40 minutes at 37°C, after which the slides were developed using diaminobenzidine chromogen (Maixin) for 10 minutes and counterstained with hematoxylin. Positive cells were scored according to the staining intensity as follows: 0, colorless; 1, light buffy; 2, heavy buffy; and 3, brown. Scores based on the percentage of positive bone marrow cells were as follows: 0, no positive cells; 1, 1%–25%; 2, 26%–50%; 3, 51%–75%; and 4, 76%–100%. The results were analyzed by the sum of the scores obtained based on staining intensity and the percentage of positive cells as follows: 0–1 (−); 2–3 (+); 4–5 (++); and >5 (+++).
MVD was determined using immunohistochemical staining for CD34 as a marker for neovessel endothelium in accordance with published studies (Korkolopoulou et al., 2003). The slides were examined at low-power magnification (100×) to identify the area with the highest vascular density within the bone marrow. Three fields with the greatest number of blood vessels for each slide under 200× magnification were examined by two pathologists blinded to the study group, and the fields with the highest MVD were selected for counting. The average of the three fields was recorded as the MVD level for each subject.
ELISA
The concentration of EPO in the serum and bone marrow supernatant was measured using an enzyme-linked immunosorbent assay (Uscn Life Science, Inc., Wuhan, China). The measurable range of the EPO assay was 6.25–400 pg/mL and the intra-assay and interassay coefficients of variation were <10% and <12%, respectively.
Statistical analysis
All calculations were performed using the SPSS statistical software version 19.0 (SPSS, Inc., Chicago, IL). Data of the normal distributions were tested using the Student's t-test, while the data of the non-normal distributions were tested using the Mann–Whitney U test. Non-normally distributed data were log transformed before the statistical correlation and relationships between the variables were analyzed by calculating the Pearson product–moment correlation. Following this, stepwise regression analysis was performed to determine the correlation between the various components and Hb levels. Spearman's correlation coefficients were calculated for the non-normally distributed data that could not be log transformed. The chi-square test was applied for the comparison of ratios. All tests were two-sided and p ≤ 0.05 was considered to indicate statistical significance.
Results
General characteristics
The general characteristics of the study subjects are shown in Table 1. The two groups were similar in terms of age, height, and body–mass index. As expected, SaO2 was markedly lower, while hemoglobin, hematocrit, and erythrocyte counts were significantly higher in patients with CMS than in the control subjects. Heart rate, blood pressure, leukocyte, and platelet counts were similar between the two groups. Based on Qinghai CMS scoring guidelines, 10 subjects had mild CMS, 16 had moderate CMS, and 8 had severe CMS.
Table 1.
Characteristics of the Participants
| Characteristics | CMS patients (n = 34) | Control subjects (n = 30) | p |
|---|---|---|---|
| Age, years | 46.6 ± 8.9 | 43.2 ± 11.0 | 0.196 |
| Height, cm | 173.4 ± 5.8 | 169.5 ± 5.2 | 0.143 |
| Body–mass index, kg/m2 | 24.6 ± 2.1 | 22.9 ± 1.7 | 0.071 |
| Heart rate, beats/min | 80.9 ± 8.6 | 81.4 ± 7.5 | 0.886 |
| Systolic blood pressure, mmHg | 124.5 ± 15.5 | 113.6 ± 7.5 | 0.089 |
| Diastolic blood pressure, mmHg | 81.3 ± 10.3 | 72.9 ± 9.5 | 0.075 |
| Hemoglobin, g/dL | 22.3 ± 1.5 | 15.2 ± 1.5 | <0.001 |
| Hematocrit, % | 68.1 ± 4.5 | 54.3 ± 4.0 | <0.001 |
| Erythrocytes, ×1012/L | 7.1 ± 0.6 | 5.3 ± 0.3 | <0.001 |
| Leukocyte, ×109/L | 4.9 ± 1.8 | 4.3 ± 1.9 | 0.448 |
| Platelet, ×109/L | 109.3 ± 59.8 | 134.9 ± 77.1 | 0.370 |
| SaO2, % | 85.7 ± 6.6 | 90.6 ± 1.4 | 0.014 |
| CMS score | 10 (8–15) | 2 (0–3) | <0.001 |
Data are presented as mean ± SD unless specified otherwise.
CMS, chronic mountain sickness; SaO2, arterial O2 saturation.
The mRNA expression of HIF-2α and EPO was higher in the BMMNCs of CMS patients than in those of the controls
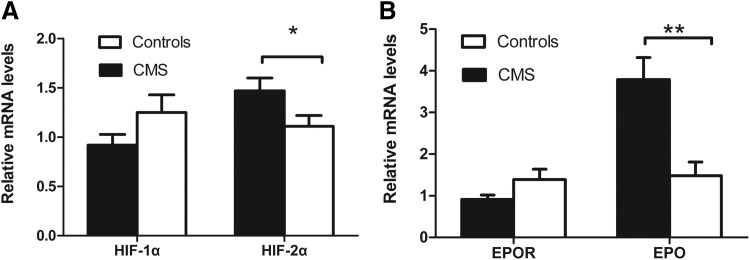
The mRNA expression of HIF-1α, HIF-2α, EPO, and EPOR was assayed using real-time quantitative PCR. In BMMNCs of CMS patients, the mRNA levels of both HIF-2α and EPO were 1.83-fold (p < 0.05) and 2.95-fold (p < 0.001) higher, respectively, in the CMS patients than in the controls, while HIF-1α and EPOR mRNA levels were similar between the two groups (0.74- and 0.75-fold higher, respectively; p > 0.05) (Fig. 1).
FIG. 1.
mRNA expression of HIF-2α and EPO, as measured using real-time quantitative polymerase chain reaction, was higher in the BMMNCs of CMS patients than in the controls. (A) mRNA expression of HIF-1α and HIF-2α. (B) mRNA expression of EPO and EPOR. Data are presented as mean ± SEM. The statistical differences from the controls were calculated using the t-test after log transformation. *p < 0.05. **p < 0.001. BMMNC, bone marrow mononuclear cell; CMS, chronic mountain sickness; EPO, erythropoietin; EPOR, erythropoietin receptor; HIF, hypoxia-inducible factor.
The percentage of HIF-2α-positive cells in the BMMNCs of CMS patients was higher than that in the BMMNCs of the controls
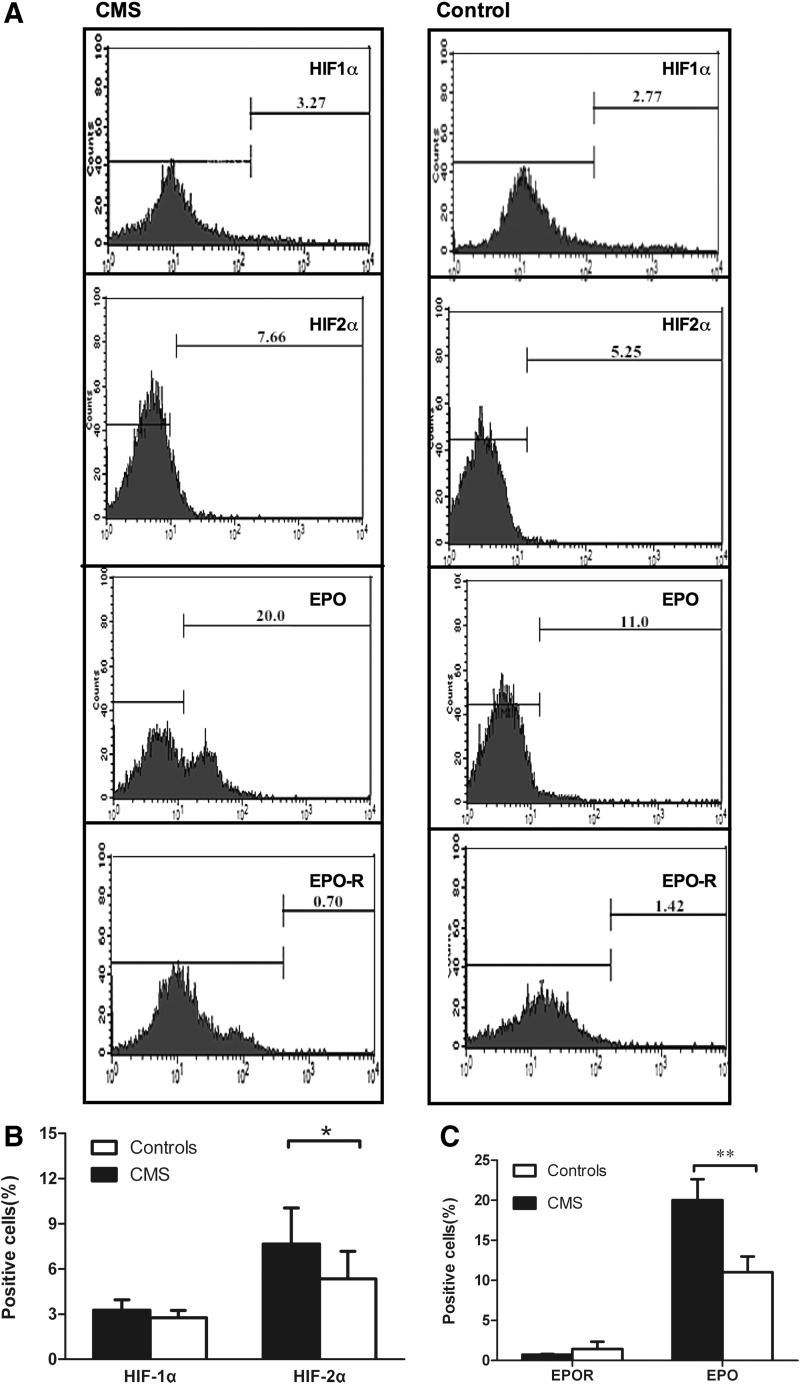
We determined whether the increased HIF-2α levels in the bone marrow niche were associated with EE in CMS. Based on the results of the flow cytometry, the percentage of HIF-2α-positive cells, but not that of HIF-1α-positive cells, was significantly higher in the BMMNCs of CMS patients than in the controls (p < 0.05; Fig. 2A, B). Furthermore, the percentage of EPO-positive cells in CMS patients was also significantly higher in the BMMNCs of the CMS patients than in those of the controls (p < 0.01), while the percentage of EPOR-positive cells in the BMMNCs was similar in the two groups (Fig. 2A, C).
FIG. 2.
Cells positive for HIF-2α and EPO were higher in the BMMNCs of CMS patients, as determined by flow cytometry. (A) The protein expression of HIF-1α, HIF-2α, EPO, and EPOR in CMS patients versus the controls. Numbers shown in each plot are means of the percentage of positive cells. (B) Contents of the HIF-1α and 2α subunits. (C) Contents of EPO and EPOR. Data are presented as mean ± SEM. The statistical differences from the controls were calculated using the t-test after log transformation. *p < 0.05. **p < 0.001.
The increase in the percentage of HIF-2α-positive cells was associated with an alteration of EPO and Hb levels in the BMMNCs
The percentage of HIF-2α-positive cells was positively correlated with Hb levels, both in the entire study population (r = 0.305, p = 0.013) and more strongly in the CMS patients (r = 0.462, p = 0.006) (Fig. 3A). Similarly, the percentage of HIF-2α-positive cells and EPO-positive cells was significantly correlated in the entire study population (r = 0.424, p < 0.001) and in the patients with CMS (r = 0.342, p = 0.043) (Fig. 3B). Furthermore, the percentage of HIF-1α-positive cells and HIF-2α-positive cells was significantly correlated in the CMS patients (r = 0.351, p = 0.042) and in the entire study population (r = 0.357, p = 0.006). Nevertheless, the percentage of HIF-1α-positive cells in the BMMNCs from the CMS patients did not significantly differ from those in the controls (Fig. 2B) and there was no correlation between HIF-1α and Hb levels in either the CMS patients (r = −0.007, p = 0.956) or the entire study population (r = −0.023, p = 0.896). Neither HIF-1α levels nor HIF-2α levels correlated with EPOR in either the CMS patients or the entire study population.
FIG. 3.
Relationship between (A) percentage of HIF-2α-positive cells and Hb levels, (B) percentage of HIF-2α-positive cells and EPO-positive cells, and (C) percentage of EPO-positive cells and Hb levels in the BMMNCs of the 34 patients with CMS (+) and 30 control subjects (•).
Moreover, the percentage of EPO-positive cells and Hb levels significantly and positively correlated in the CMS patients (r = 0.347, p = 0.043) and in the entire population (r = 0.453, p < 0.001) (Fig. 3C), whereas there was no correlation between EPOR and Hb levels in the CMS patients (r = −0.107, p = 0.546) or in the entire study population (r = 0.067, p = 0.593).
The expression of HIF-2α and EPO proteins was higher in the bone marrow tissues of CMS patients than in the controls
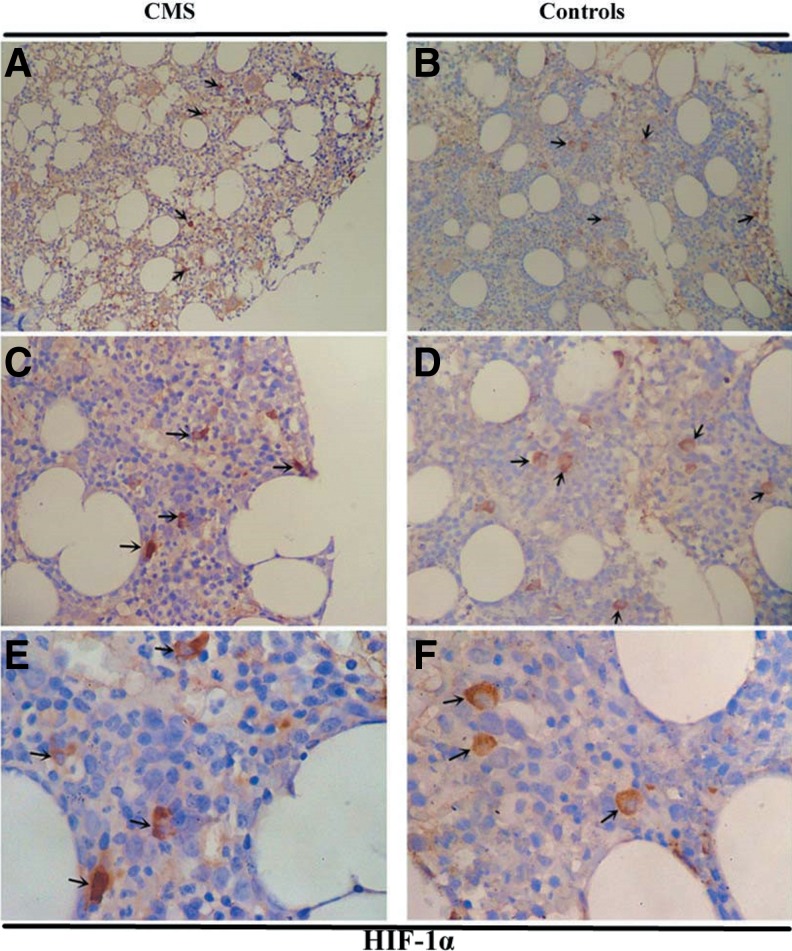
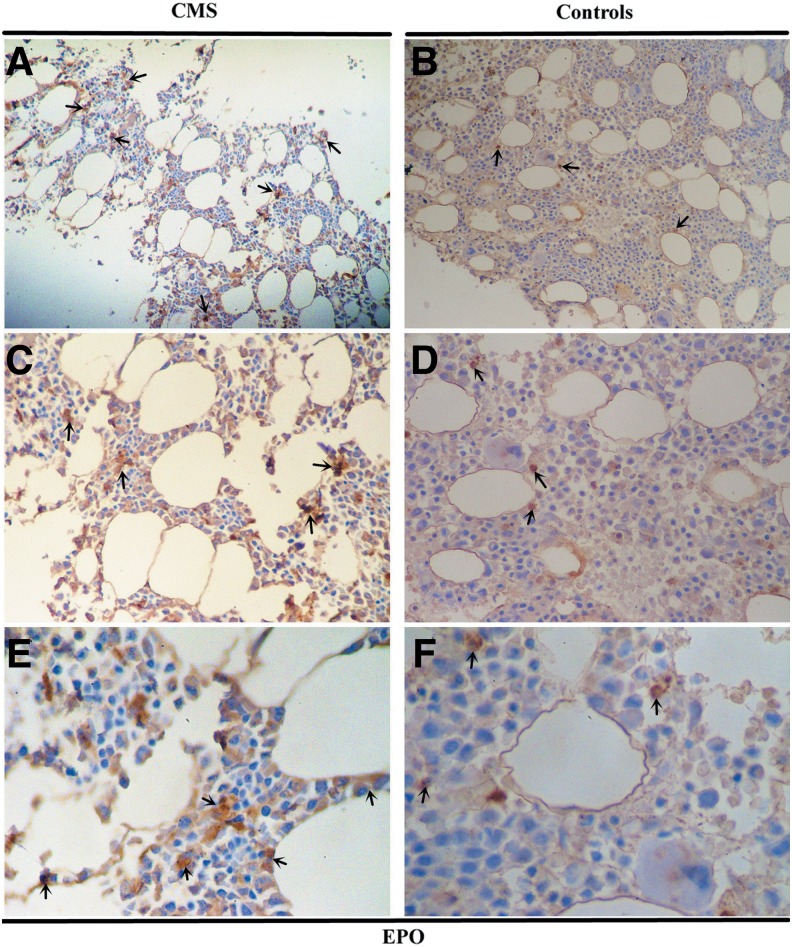
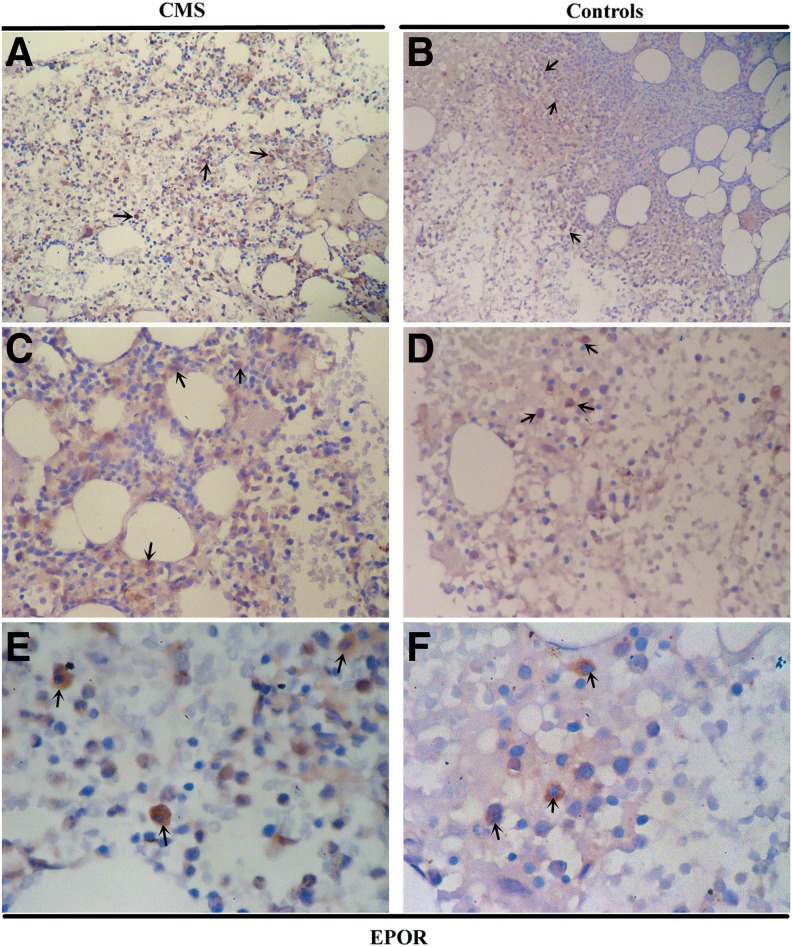
The protein expression of HIF-1α, HIF-2α, EPO, and EPOR in the bone marrow tissues was examined by immunohistochemistry (IHC) (Fig. 4–7 and Table 2). As shown in Figure 4 and Figure 5, HIF-1α and HIF-2α appeared to localize within the nucleus and cytoplasm in the bone marrow tissues with staining patterns that were widely distributed in the cellular regions of the bone marrow. The expression of HIF-2α was significantly higher in the CMS patients than in the controls (p = 0.004), while there was no difference in the HIF-1α expression between the two groups (Figs. 4 and 5 and Table 2). In the bone marrow tissues, EPO was present diffusely, localizing to the cytoplasm in bone marrow cells, while EPOR localized to both the cytoplasm and the cytomembrane. EPO expression by IHC was significantly increased in CMS (p < 0.001) (Figs. 6 and 7 and Table 2).
FIG. 4.
HIF-1α IHC in the bone marrow of CMS patients and the controls. Magnification: (A, B) ×100; (C, D) ×200; (E, F) ×400. IHC, immunohistochemistry. Arrows indicate the HIF-1α-positive cells.
FIG. 5.
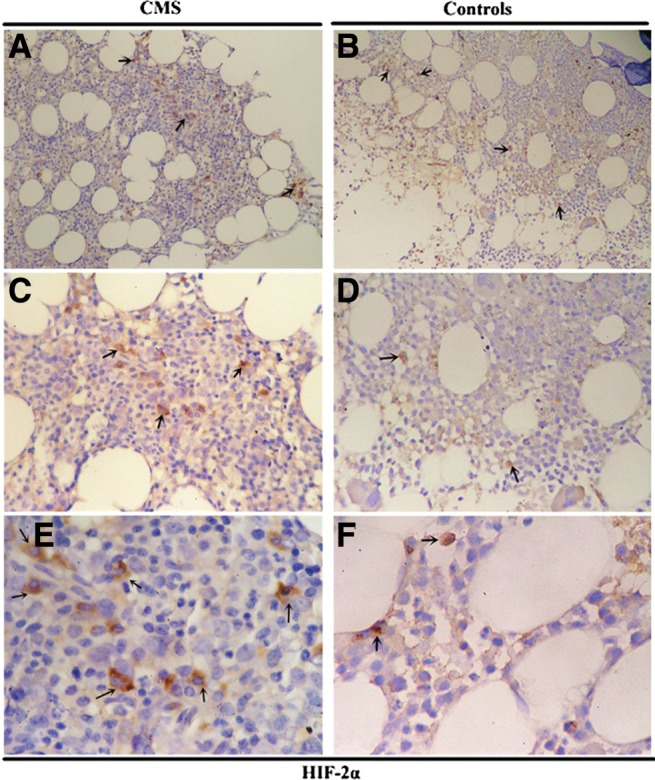
HIF-2α IHC in the bone marrow of CMS patients and the controls. Magnification: (A, B) ×100; (C, D) ×200; (E, F) ×400. Arrows indicate the HIF-2α-positive cells.
FIG. 6.
EPO IHC in the bone marrow of CMS patients and the controls. Magnification: (A, B) ×100; (C, D) ×200; (E, F) ×400. Arrows indicate the EPO-positive cells.
FIG. 7.
EPOR IHC in the bone marrow of CMS patients. Magnification: (A, B) ×100; (C, D) ×200; (E, F) ×400. Arrows indicate the EPOR-positive cells.
Table 2.
Immunohistochemical Analyses for the Various Parameters Between CMS Patients and the Controls
| CMS patients, n = 34 (%) | Controls, n = 30 (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive cells | Positive cells | ||||||||
| − | + | ++ | +++ | − | + | ++ | +++ | p | |
| HIF-1α | 17 (50.0) | 6 (17.8) | 10 (29.4) | 1 (2.9) | 17 (56.7) | 3 (10.0) | 9 (30.0) | 1 (3.3) | 0.563 |
| HIF-2α | 4 (11.8) | 5 (14.7) | 18 (52.9) | 7 (20.6) | 7 (23.3) | 13 (43.3) | 8 (26.7) | 2 (6.6) | 0.004 |
| EPO | 4 (11.8) | 4 (11.8) | 7 (20.6) | 19 (55.9) | 10 (33.3) | 10 (33.3) | 8 (26.7) | 2 (6.7) | < 0.001 |
| EPOR | 4 (11.8) | 14 (41.2) | 5 (14.7) | 11 (32.4) | 2 (6.67) | 10 (33.3) | 8 (26.7) | 10 (33.3) | 0.064 |
The expression of HIF-1α, HIF-2α, EPO, and EPOR in the bone marrow tissues, as determined by IHC; data were compared using the chi-square test.
EPO, erythropoietin; EPOR, erythropoietin receptor; HIF, hypoxia-inducible factor.
Increased protein expression of HIF-2α and EPO was associated with Hb levels in the bone marrow tissues
Spearman analyses indicated a significant positive correlation between HIF-2α and Hb levels in the CMS patients (ρ = 0.690, p = 0.019) and in the entire population (ρ = 0.649, p = 0.001). HIF-2α expression also correlated with EPO levels in CMS patients (ρ = 0.692, p < 0.001) and in the entire study population (ρ = 0.649, p < 0.001). HIF-1α and HIF-2α expression was also positively correlated, both in CMS patients and in the entire population (ρ = 0.553, p = 0.006; ρ = 0.663, p < 0.001, respectively). There was no significant correlation between HIF-1α and either Hb or EPO levels in CMS patients (ρ = 0.384, p = 0.070; ρ = 0.319, p = 0.094, respectively). Spearman analyses showed a significant positive correlation between EPO and Hb in CMS patients (ρ = 0.534, p = 0.009) and the entire population (ρ = 0.534, p = 0.009). There was no correlation between EPOR and Hb either in CMS patients (ρ = 0.144, p = 0.513) or the entire population (ρ = 0.243, p = 0.172).
Increased MVD was involved in the pathological changes in CMS and associated with the alteration of EPO and Hb levels
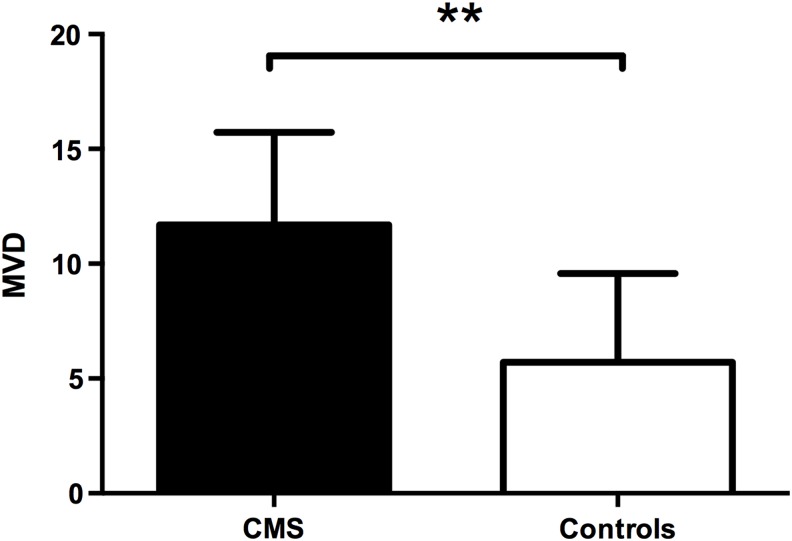
CD34 expression and MVD were assessed in the bone marrow tissues by IHC (Fig. 8). CD34-positive vascular endothelial cells were identified by brown staining. The MVD values in the bone marrow tissues from CMS patients were significantly higher than those in the controls (14.54 ± 8.84 vs. 8.45 ± 2.98, p = 0.004, Fig. 9). There was also a positive correlation between MVD and Hb levels both in the CMS patients (ρ = 0.413, p = 0.050) and in the entire study population (ρ = 0.703, p < 0.001). In the CMS patients, both HIF-1α and HIF-2α expression levels were correlated with MVD (ρ = 0.456, p = 0.029; ρ = 0.600, p = 0.002, respectively); furthermore, EPO expression was also correlated with MVD (ρ = 0.435, p = 0.028). Similar results were also obtained in the entire study population (ρ = 0.609, p < 0.001 and ρ = 0.726, p < 0.001 for HIF-1α and HIF-2α, respectively; and ρ = 0.447, p = 0.010 for EPO). There was no correlation between EPOR and MVD (ρ = 0.232, p = 0.519).
FIG. 8.
MVD in the local bone marrow tissue of the CMS patients and the controls. Magnification: (A, B) ×100; (C, D) ×200; (E, F) ×400. MVD, microvessel density. Arrows indicate the microvessel in bone marrow tissue.
FIG. 9.
MVD in the local bone marrow tissue was significantly higher in the CMS patients than in the controls. Data are presented as mean ± SED. The statistical differences were calculated using the t-test. **p < 0.01.
The local high EPO concentration in the bone marrow played a role in EE through an autocrine/paracrine mechanism in CMS
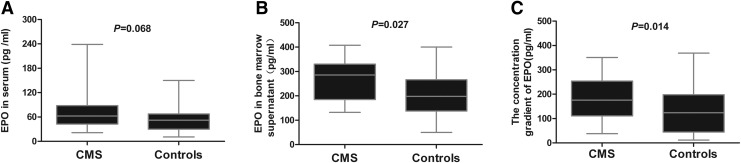
The concentration of EPO in the serum and bone marrow supernatant was measured using ELISA (Fig. 10). EPO concentration in the serum was similar between the two groups (p = 0.068) (Fig. 10A), whereas it was significantly higher in the bone marrow supernatant of the CMS patients than in the controls (p = 0.027) (Fig. 10B). To determine changes in EPO concentration locally in the bone marrow, the concentration gradient of EPO in the bone marrow and serum was calculated as described previously (Abali et al., 2002). The concentration gradient of EPO was found to be significantly higher in the CMS patients than in the controls (p = 0.014) (Fig. 10C).
FIG. 10.
EPO concentration in the bone marrow was significantly higher in the CMS patients than in the control subjects. (A) EPO concentrations in the serum, (B) EPO concentrations in the bone marrow supernatants. (C) EPO concentration gradient in the bone marrow and the serum. The statistical differences from the controls were calculated using the Mann–Whitney U test.
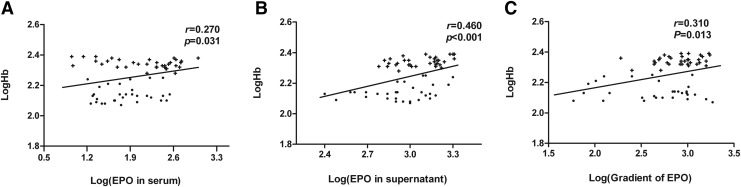
EPO concentration in the serum was not correlated with Hb levels in the CMS patients (r = 0.331, p = 0.054), but a positive correlation was found in the entire study population (r = 0.270, p = 0.031) (Fig. 11A). EPO concentrations in the bone marrow supernatants too correlated with Hb levels in the CMS patients and in the entire study population (r = 0.397, p = 0.020; r = 0.460, p < 0.001, respectively) (Fig. 11B). Significant correlations were found between the concentration gradient of EPO and the Hb levels both in the CMS patients (r = 0.349, p = 0.043) and in the entire study population (r = 0.310, p = 0.013) (Fig. 11C).
FIG. 11.
Relationship between (A) EPO concentration in the serum and Hb levels, (B) EPO concentration in the bone marrow supernatant and Hb levels, and (C) EPO concentration gradient and Hb levels of the 34 patients with CMS (+) and the 30 control subjects (•).
Since the EPO concentration in the serum and bone marrow supernatant, the gradient concentration, the percentages of EPO-positive cells, and the percentages of HIF-2α-positive cells would present multicollinearity when included in the same model, stepwise regression analysis was performed. The independent variables in the multiple regression model that had significant influence on Hb levels were found to be the concentration gradient of EPO (β = 0.487, p < 0.001), which showed the highest regression coefficient, followed by the percentage of EPO-positive cells (β = 0.284, p = 0.014). EPO concentrations in the serum and the bone marrow supernatant and the percentages of HIF-2α-positive cells had little influence on Hb levels (β = 0.214, p = 0.063; β = 0.175, p = 0.173; β = 0.189, p = 0.092, respectively) in this adjusted model.
Discussion
In this study, we identified several important aspects of how changes in the bone marrow niche occur in patients with CMS. Patients with CMS show an upregulation of the HIF-2α/EPO pathway and increase in bone marrow MVD. We hypothesize that the upregulation of these pathways mediates the pathological changes in CMS likely through induction of EE and angiogenesis.
CMS is a multifactorial disease caused by maladaptation to chronic exposure to a high-altitude hypoxic environment. HIFs play a central role in the regulation of oxygen homeostasis and regulate EPO gene expression, both of which are essential for adaptive erythropoiesis and angiogenesis. However, the pattern of mechanisms underlying the changes in HIF-1α and HIF-2α within the bone marrow (the primary site of erythropoiesis) remains unknown. To our knowledge, this is the first study to show a significant upregulation of HIF-2α levels in the bone marrow niche of CMS patients.
HIF-1α and HIF-2α have similar protein structures while also having distinct target genes and mechanisms of regulation. HIF-1α levels have been identified to be increased in WBCs of CMS patients (Appenzeller et al., 2006). Researchers have previously proposed genetic variants of HIF-2α to be associated with Hb levels in Tibetan natives living in high-altitude areas (Beall et al., 2010; Simonson et al., 2010; Yi et al., 2010). Oxygen tensions in the bone marrow tissue are significantly lower than the ambient oxygen tensions in healthy humans and HIFs are the important regulators that help maintain hematopoiesis (Carreau et al., 2011). The hypoxic bone marrow microenvironment would be more severe in a highlander. Therefore, changes in HIF-1α and HIF-2α levels in the bone marrow niche might be involved in the pathogenesis of CMS.
We indeed found that the expression of HIF-2α was higher in the bone marrow niche of CMS patients than in the controls, while HIF-1α expression was similar between the two groups. In a previous study, HIF-1α was found to be the initial responder to hypoxia and its activity was found to be increased in severe hypoxia (<0.1% O2). On the other hand, HIF-2α has been known to play a more sustained role during chronic hypoxia and mild or physiological hypoxia (<5% O2) (Holmquist-Mengelbier et al., 2006; Koh et al., 2011). We speculate that HIF-1α in the bone marrow niche might be initially active in the hypoxic microenvironment in people living in high-altitude areas. Furthermore, HIF-1α facilitates O2 delivery and cellular adaptation to hypoxia by stimulating a wide spectrum of biological processes. Through these processes, the concentration of O2 might be maintained at a suitable level so that HIF-2α would be continuously active and stabilized, which would improve the MVD and Hb levels to allow the cells to adapt to hypoxia. In some cases of maladaptation to chronic high-altitude hypoxia, excessive activation of HIF-2α would lead to EE and excessive angiogenesis in the bone marrow niche. It has been suggested that HIF-2α is an important control factor in patients with CMS.
We found HIF-1α and HIF-2α expression to be significantly correlated with one another in the bone marrow in the CMS patients as well as in the entire study population, yet only HIF-2α was found to be correlated with Hb levels or EPO expression. The activity of HIF-2α would follow that of HIF-1α because of the increased concentration of O2 and the prolonged duration of hypoxia because of which HIF-1α and HIF-2α levels are significantly correlated with one another in the bone marrow. Although HIF-1α was originally proposed to promote the hypoxic induction of EPO expression in vitro (Semenza et al., 1992; Haase, 2013), HIF-2α has now emerged as the main regulator of EPO expression and erythropoiesis in vivo (Warnecke et al., 2004; Scortegagna et al., 2005; Kapitsinou et al., 2010). Our data also revealed that EPO levels in the bone marrow significantly correlated with HIF-2α levels, but not with HIF-1α levels. Although HIF-1 and HIF-2 recognize the same consensus target gene promoters, their role in hypoxic gene induction is apparently nonredundant and it remains unclear as to how HIF-1 and HIF-2 determine their respective targets. Our findings suggest that HIF-2α might preferentially be involved in the regulation of EPO expression within the bone marrow niche and the subsequent EE associated with CMS.
Vasculogenesis and angiogenesis are also part of the physiological responses to hypoxia, and angiogenesis is mediated mainly by HIF-1α and HIF-2α (Dachs et al., 1997; Blancher et al., 2000). HIF-1α and HIF-2α perform complementary functions in pathophysiological angiogenesis (Skuli et al., 2012). HIF-1α plays a dominant role during vasculogenesis occurring under intense and early hypoxia, while HIF-2α is a control checkpoint for the later stages of vascular remodeling and maturation under mild hypoxic conditions (Adams and Alitalo, 2007; Skuli et al., 2009, 2012). Bone marrow stromal cells (BMSCs) participate in postnatal angiogenesis and HIF-2α appears to play a greater role in the regulation of BMSC-induced angiogenic responses than HIF-1α (Ben-Shoshan et al., 2008). Consistent with these prior reports, we found that MVD and HIF-2α expression was significantly higher in the CMS patients than in the controls, while HIF-1α expression did not differ between the two groups. However, the levels of both HIF-1α and HIF-2α correlated with MVD in the bone marrow tissue. These findings suggest HIF-2α to be the primary regulator driving increased angiogenesis in the bone marrow niche of CMS patients. We speculate that although the activation of HIF-2α would be dominant in CMS because of chronic and continuous exposure to high-altitude hypoxia, HIF-1α plays a crucial role in the vasculogenesis. Both HIF-1 and HIF-2 drive overlapping functions, which might be the reason for the correlation of their expression with MVD in the bone marrow tissue. Further studies to elucidate the underlying mechanisms are necessary.
Erythropoiesis is primarily regulated by EPO. Given the EE in CMS, previous research was focused on the changes in serum EPO levels (León-Velarde et al., 1991; Mejía et al., 2005). However, we focused on the bone morrow niche and attempted to determine the changes in EPO expression in the bone marrow of patients with CMS. The protein and mRNA expression levels of EPO were increased in CMS patients. Although the concentrations of EPO in the serum showed no difference between the CMS patients and the controls, the percentage of cells positive for EPO, the EPO concentrations in the bone marrow, and the EPO concentration gradient of the CMS patients were significantly higher compared with the controls. Moreover, in a multiple regression analysis, the EPO concentration gradient and the percentage of cells positive for EPO were found to have significant influence on Hb levels. Hence, we suggested that EPO in the bone marrow niche was indeed involved in the development of CMS and that the increased expression of EPO in the bone marrow may act as an important regulator of EE in CMS through autocrine or/and paracrine mechanisms.
EPOR is expressed most abundantly in colony-forming unit erythroid (CFU-E) and forms a homodimer that triggers phosphorylation upon stimulation. The circulating EPO concentrations are similar between CMS patients and healthy people living in high-altitude regions (León-Velarde et al., 1991). Therefore, we hypothesized that EPOR expression is increased to promote EPO signal transduction in the bone marrow of CMS patients. Although we did not find any significant difference between EPOR expression in the bone marrow of the CMS patients and the controls, EPOR forms a homodimer and triggers phosphorylation upon stimulation. Whether the increased levels of phosphorylation of EPOR lead to the increased sensitivity of the EPO signal in CMS is worth investigating in future studies.
It is well established that regulation of erythropoiesis and vasculogenesis is an adaption to hypoxia in the healthy body. Hematopoietic and endothelial cell lineages share common progenitors and EPO affects the proliferation and differentiation of bone marrow-derived or umbilical cord blood-derived endothelial progenitor cells, which in turn promote angiogenesis (Bahlmann et al., 2004; Bennis et al., 2012). EPO exhibits the same angiogenic effect on endothelial cells from human adult myocardial tissue as vascular endothelial growth factor, which is a key mediator of angiogenesis (Jaquet et al., 2002). Hypoxia upregulates local EPO expression in the retina and contributes to intravitreal neovascularization (Morita et al., 2003; Chen et al., 2009). Building on these published results, we now show that increased EPO expression in the bone marrow of CMS patients also correlates with MVD. Taken together, these findings suggest that EPO in the bone marrow niche may be involved in the increased angiogenesis and contributes to the development of CMS.
During clinical examinations, we have frequently observed that patients with CMS exhibit an erythemic facial color with marked congestion of the mucosa and conjunctiva as a result of the formation of new vessels; however, whether CMS is associated with changes in bone marrow MVD and whether these changes correlate with EE in CMS have not previously been examined. In this study, we show that MVD in the bone marrow is higher in patients with CMS than in the controls and the increase in the MVD significantly correlates with Hb levels. The hematopoietic function of the bone marrow depends on the support and regulation of the hematopoietic microenvironment, of which bone marrow microvessels are a fundamental structure. We speculate that on the one hand, the increase in MVD might be a partly compensatory process to help alleviate hypoxia, but, on the other hand, excessive angiogenesis might change the normal morphology and structure of the bone marrow tissue under chronic hypoxic conditions. Due to this, the original bone marrow niche might be reconstructed, which might affect its function, eventually leading to a degree of maladaptation to hypoxia and the occurrence of CMS.
Conclusions
Our findings indicate that the upregulation of the HIF-2α/EPO pathway within the bone marrow niche of CMS patients may regulate EE and angiogenesis. Increased MVD in the bone marrow of CMS patients appears to be involved in the pathogenesis of this disease. We further suggest that the hypoxia-induced angiogenesis might contribute to changes in the structure and function of the bone marrow, thus resulting in the development of CMS.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30960149), The National Natural Science Foundation of China (No. 31160219), and The Program of International S&T Cooperation of Qinghai Province, China (No. 2015-HZ-810).
Author Disclosure Statement
No competing financial interests exist.
References
- Abali H, Haznedaroglu IC, Goker H, Celik I, Ozatli D, Koray Z, and Caglar M. (2002). Circulating and local bone marrow renin-angiotensin system in leukemic hematopoiesis: Preliminary evidences. Hematology 7:75–82 [DOI] [PubMed] [Google Scholar]
- Adams RH, and Alitalo K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8:464–478 [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Minko T, Pozharov V, Bonfichi M, Malcovati L, Gamboa J, and Bernardi L. (2003). Gene expression in the Andes; relevance to neurology at sea level. J Neurol Sci 207:37–41 [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Minko T, Qualls C, Pozharov V, Gamboa J, Gamboa A, and Wang Y. (2006). Gene expression, autonomic function and chronic hypoxia: Lessons from the Andes. Clin Auton Res 16:217–222 [DOI] [PubMed] [Google Scholar]
- Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, and Fliser D. (2004). Erythropoietin regulates endothelial progenitor cells. Blood 103:921–926 [DOI] [PubMed] [Google Scholar]
- Beall C, Cavalleri G, Deng L, Elston R, Gao Y, Knight J, Li C, Li J, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, and Zheng YT. (2010). Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Nat Acad Sci USA 107:11459–11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beleslin-Cokic BB, Cokic VP, Yu X, Weksler BB, Schechter AN, and Noguchi CT. (2004). Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 104:2073–2080 [DOI] [PubMed] [Google Scholar]
- Bennis Y, Sarlon-Bartoli G, Guillet B, Lucas L, Pellegrini L, Velly L, Blot-Chabaud M, Dignat-Georges F, Sabatier F, and Pisano P. (2012). Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost 10:1914–1928 [DOI] [PubMed] [Google Scholar]
- Ben-Shoshan J, Schwartz S, Luboshits G, Maysel-Auslender S, Barzelay A, Polak-Charcon S, Tzahor E, Barshack I, Barak A, Levkovitch-Verbin H, Keren G, and George J. (2008). Constitutive expression of HIF-1α and HIF-2α in bone marrow stromal cells differentially promotes their proangiogenic properties. Stem Cells 26:2634–2643 [DOI] [PubMed] [Google Scholar]
- Blancher C, Moore JW, Talks KL, Houlbrook S, and Harris AL. (2000). Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res 60:7106–7113 [PubMed] [Google Scholar]
- Buroker NE, Ning XH, Zhou ZN, Li K, Cen WJ, Wu XF, Zhu WZ, Scott CR, and Chen SH. (2012). AKT3, ANGPTL4, eNOS3, and VEGFA associations with high altitude sickness in Han and Tibetan Chinese at the Qinghai-Tibetan Plateau. Int J Hematol 96:200–213 [DOI] [PubMed] [Google Scholar]
- Buroker NE, Ning XH, Zhou ZN, Li K, Cen WJ, Wu XF, Zhu WZ, Scott CR, and Chen SH. (2013). VEGFA SNPs and transcriptional factor binding sites associated with high altitude sickness in Han and Tibetan Chinese at the Qinghai-Tibetan Plateau. J Physiol Sci 63:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, and Kieda C. (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15:1239–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, and Smith LEH. (2009). Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci 50:1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Kang X, Zhang S, and Yeh ET. (2007). SUMO-specific protease 1 is essential for stabilization of HIF1α during hypoxia. Cell 131:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, Yu X, Beleslin-Cokic B, Liu C, Shen K, Mohrenweiser HW, and Noguchi CT. (2000). Production and processing of erythropoietin receptor transcripts in brain. Brain Res Mol Brain Res 81:29–42 [DOI] [PubMed] [Google Scholar]
- Cole AM, Petousi N, Cavalleri GL, and Robbins PA. (2014). Genetic variation in SENP1 and ANP32D as predictors of chronic mountain sickness. High Alt Med Biol 15:497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ, and Harris AL. (1997). Targeting gene expression to hypoxic tumor cells. Nat Med 3:515–520 [DOI] [PubMed] [Google Scholar]
- Espinoza JR, Alvarez G, León-Velarde F, Preciado HF, Macarlupu JL, Rivera-Ch M, Rodriguez J, Favier J, Gimenez-Roqueplo AP, and Richalet JP. (2014). Vascular endothelial growth factor-A is associated with chronic mountain sickness in the Andean population. High Alt Med Biol 15:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandrey J. (2004). Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol 286:R977–R988 [DOI] [PubMed] [Google Scholar]
- Furlow PW, Percy MJ, Sutherland S, Bierl C, McMullin MF, Master SR, Lappin TR, and Lee FS. (2009). Erythrocytosis-associated HIF-2α mutations demonstrate a critical role for residues C-terminal to the hydroxylacceptor proline. J Biol Chem 284:9050–9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale DP, Harten SK, Reid CD, Tuddenham EG, and Maxwell PH. (2008). Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2α mutation. Blood 112:919–921 [DOI] [PubMed] [Google Scholar]
- Ge RL, Mo VY, Januzzi JL, Jin G, Yang Y, Han S, Wood MJ, and Levine BD. (2011). B-type natriuretic peptide, vascular endothelial growth factor, endothelin-1 and nitric oxide synthase in chronic mountain sickness. Am J Physiol Heart Circ Physiol 300:H1427–H1433 [DOI] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, and Simon MC. (2007). HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11:335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. (2013). Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 27:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, and Påhlman S. (2006). Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell 10:413–423 [DOI] [PubMed] [Google Scholar]
- Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, and Kuck KH. (2002). Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res 64:326–333 [DOI] [PubMed] [Google Scholar]
- Jiang CH, Chen J, Liu FY, Luo YJ, Xu G, Shen HY, Gao YQ, and Gao WX. (2014). Chronic mountain sickness in Chinese Han males who migrated to the Qinghai-Tibetan plateau: Application and evaluation of diagnostic criteria for chronic mountain sickness. BMC Public Health 14:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, and Haase VH. (2010). Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116:3039–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MY, Lemos R, Jr., Liu X, and Powis G. (2011). The hypoxia-associated factor switches cells from HIF-1α-to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res 71:4015–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkolopoulou P, Viniou N, Kavantzas N, Patsouris E, Thymara I, Pavlopoulos PM, Terpos E, Stamatopoulos K, Plata E, Anargyrou K, Androulaki A, Davaris P, and Yataganas X. (2003). Clinicopathologic correlations of bone marrow angiogenesis in chronic myeloid leukemia: A morphometric study. Leukemia 17:89–97 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Arregui A, Vargas M, Huicho L, and Acosta R. (1994). Chronic mountain sickness and chronic lower respiratory tract infections. Chest 106:151–155 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, and Zubieta-Calleja G. (2005). Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 6:147–157 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, McCullough RG, McCullough RE, and Reeves JT; CMS Consensus Working Group. (2003). Proposal for scoring severity in chronic mountain sickness (CMS). Background and conclusions of the CMS Working Group. Adv Exp Med Biol 543:339–354 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Monge C, Vidal A, Carcagno M, Criscuolo M, and Bozzini CE. (1991). Serum immunoreactive erythropoietin in high altitude natives with and without excessive erythrocytosis. Exp Hematol 19:257–260 [PubMed] [Google Scholar]
- Lifshitz L, Tabak G, Gassmann M, Mittelman M, and Neumann D. (2010). Macrophages as novel target cells for erythropoietin. Haematologica 95:1823–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, and Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, and Poellinger L. (2001). Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414:550–554 [DOI] [PubMed] [Google Scholar]
- Martini M, Teofili L, Cenci T, Giona F, Torti L, Rea M, Foà R, Leone G, and Larocca LM. (2008). A novel heterozygous HIF2AM535I mutation reinforces the role of oxygen sensing pathway disturbances in the pathogenesis of familial erythrocytosis. Haematologica 93:1068–1071 [DOI] [PubMed] [Google Scholar]
- Mejía OM, Prchal JT, León-Velarde F, Hurtado A, and Stockton DW. (2005). Genetic association analysis of chronic mountain sickness in an Andean high-altitude population. Haematologica 90:13–19 [PubMed] [Google Scholar]
- Morita M, Ohneda O, Yamashita T, Takahashi S, Suzuki N, Nakajima O, Kawauchi S, Ema M, Shibahara S, Udono T, Tomita K, Tamai M, Sogawa K, Yamamoto M, and Fujii-Kuriyama Y. (2003). HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J 22:1134–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza D, and Arias-Stella J. (2007). The heart and pulmonary circulation at high altitudes: Healthy highlanders and chronic mountain sickness. Circulation 115:1132–1146 [DOI] [PubMed] [Google Scholar]
- Percy MJ, Beer PA, Campbell G, Dekker AW, Green AR, Oscier D, Rainey MG, van Wijk R, Wood M, Lappin TR, McMullin MF, and Lee FS. (2008a). Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood 111:5400–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, and Lee FS. (2008b). A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med 358:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E, and Giaccia AJ. (2012). The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, and Eckardt KU. (2002). Expression of hypoxia-inducible factor-1alpha and −2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13:1721–1732 [DOI] [PubMed] [Google Scholar]
- Sato T, Maekawa T, Watanabe S, Tsuji K, and Nakahata T. (2000). Erythroid progenitors differentiate and mature in response to endogenous erythropoietin. J Clin Invest 106:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, and Garcia JA. (2005). HIF-2 alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 105:3133–3140 [DOI] [PubMed] [Google Scholar]
- Semenza GL, and Wang GL. (1992). A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff C, Yun H, Qin G, Witherspoon D, Bai Z, Lorenzo F, Xing J, Jorde LB, Prchal JT, and Ge R. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329:72–75 [DOI] [PubMed] [Google Scholar]
- Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, and Keith B. (2009). Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood 114:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, Schenkel S, Yodh AG, Keith B, and Simon MC. (2012). Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes. J Clin Invest 122:1427–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopka T, Zivny JH, Stopkova P, Prchal JF, and Prchal JT. (1998). Human hematopoietic progenitors express erythropoietin. Blood 91:3766–3772 [PubMed] [Google Scholar]
- Tian H, Mcknight SL, and Russer DW. (1997). Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11:72–82 [DOI] [PubMed] [Google Scholar]
- Villafuerte FC, Macarlupú JL, Anza-Ramírez C, Corrales-Melgar D, Vizcardo-Galindo G, Corante N, and León-Velarde F. (2014). J Appl Physiol 117:1356–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jing BH, Rue EA, and Semenza GL. (1995). Hypoxia-inducible factor 1 is a basic-helix- loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92:5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, and Chopp M. (2004). Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 35:1732–1737 [DOI] [PubMed] [Google Scholar]
- Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, and Eckardt KU. (2004). Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: Erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J 18:1462–1464 [DOI] [PubMed] [Google Scholar]
- Weidemann A, and Johnson RS. (2009). Nonrenal regulation of EPO synthesis. Kidney Int 75:682–688 [DOI] [PubMed] [Google Scholar]
- Wiesener MS, Jürgensen JS, Rosenberger C, Scholze CK, Hörstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, and Eckardt KU. (2003). Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 17:271–273 [DOI] [PubMed] [Google Scholar]
- Yeo EJ, Cho YS, Kim MS, and Park JW. (2008). Contribution of HIF-1alpha or HIF-2alpha to erythropoietin expression: In vivo evidence based on chromatin immunoprecipitation. Ann Hematol 87:11–17 [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J, and Wang J. (2010). Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, and Prchal JT. (2006). Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem 281:25703–25711 [DOI] [PubMed] [Google Scholar]
- Yu L, Ji W, Zhang H, Renda MJ, He Y, Lin S, Cheng EC, Chen H, Krause DS, and Min W. (2010). SENP1-mediated GATA1 de SUMOylation is critical for definitive erythropoiesis. J Exp Med 207:1183–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, Zhao HW, Yin Y, Du Y, Guo L, Cao R, Wang Y, Jin X, Huang C, Jia W, Cao D, Guo G, Gamboa JL, Villafuerte F, Callacondo D, Xue J, Liu S, Frazer KA, Li Y, Bafna V, and Haddad GG. (2013). Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet 93:452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]