Highlights
-
•
The prognosis for patients with anaplastic carcinoma of the pancreas is reported much poorer even if resected.
-
•
S-1 is more effective for anaplastic carcinoma of the pancreas.
-
•
Adjuvant chemotherapy with S-1 in patients with resected anaplastic carcinoma of the pancreas is recommended.
Keywords: Anaplastic carcinoma, Pancreatic cancer, S-1
Abstract
We herein describe the case of a 70-year-old female patient diagnosed with pancreatic carcinoma. An abdominal enhanced computed tomography scan revealed a poorly enhanced mass (17 mm × 15 mm in size) in the pancreatic head. Magnetic resonance cholangiopancreatography revealed stenosis of the main pancreatic and common bile ducts caused by a mass-neighboring cyst. Based on these findings, we performed subtotal stomach-preserving pancreaticoduodenectomy. The patient demonstrated a good postoperative course, and was discharged from our hospital in remission 49 days after the surgery. Pathological findings confirmed that it was anaplastic pancreas carcinoma (giant cell type). After the surgery, we performed S-1 adjuvant chemotherapy 100 mg/day for four weeks, repeated similarly every six weeks for a total of four courses.
We have followed this case for over 2 years so far with adjuvant chemotherapy, and no recurrence or metastasis has been revealed. Adjuvant chemotherapy with S-1 in patients with resected anaplastic carcinoma of the pancreas is also recommended as a result of Japan Adjuvant Study Group of Pancreatic Cancer 01(JASPAC-01) like the ordinary pancreatic ductal carcinomas. There is a possibility to achieve long-term survival in cases in which multidisciplinary treatment such as a curative resection and adjuvant chemotherapy are performed.
1. Introduction
Anaplastic carcinoma of the pancreas (APC) is a relatively rare type of invasive ductal carcinoma. When APCs are discovered, they tend to invade locally and metastasize to other organs, and are known to be associated with poorer prognosis than the ordinary pancreatic ductal carcinomas [1].
We herein report a case of APC associated with survival for more than 24 months after resection with S1 adjuvant chemotherapy.
2. Case report
A 70-year-old Japanese woman presented at our hospital with general fatigue that she had been experiencing for 2 weeks. The patient was admitted to the Gastroenterological Center of our hospital.
Blood chemistry analyses revealed elevated levels of hepatobiliary enzymes without T-bil.
The laboratory findings on admission were as follows: AST 143U/l,ALT189U/l,ALP 1146U/l γGTP 665U/l. An abdominal enhanced computed tomography scan revealed a poorly enhanced mass (17 mm × 15 mm) in the pancreatic head, which we diagnosed as pancreatic carcinoma.
Magnetic resonance cholangiopancreatography revealed stenosis of the main pancreatic and the common bile ducts caused by a mass-neighboring cyst (Fig. 1). Based on these findings, this patient was diagnosed with pancreatic carcinoma and was scheduled to undergo pancreaticoduodenectomy to reduce stenosis of the main pancreatic tube.
Fig. 1.
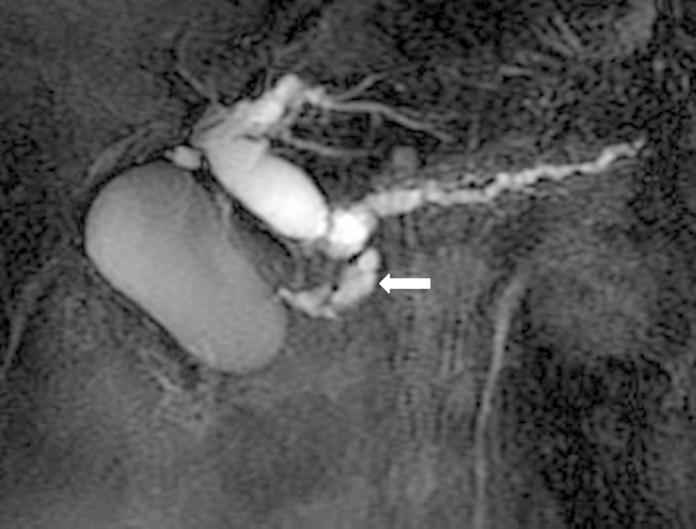
Preoperative magnetic resonance cholangiopancreatography (MRCP).
MRCP showed stenosis of the main pancreatic and the common bile ducts caused by a mass-neighboring cysts(arrow: ⟵).
We preformed subtotal stomach-preserving pancreaticoduodenectomy following diagnosis of the pancreatic carcinoma. Histological finding were Ph,TS2, mixed type, T3, CH(+), DU(−), S(−), RP(−), PV(−), A(−), PL(−), OO(−), N0, M0. PCM(−), BCM(−), DPM(−), D2,R0. This pancreatic carcinoma was classified as stage III by the General Rules for the Study of Pancreatic Cancer (The 6th edition, Revised Version). The patient demonstrated a relatively good postoperative course, and was discharged from our hospital in remission 49 days after surgery.
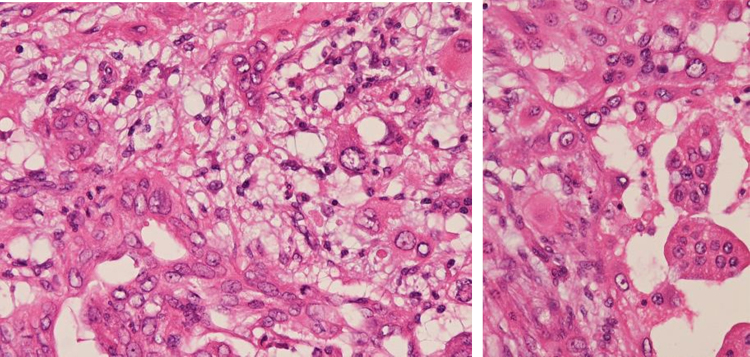
Pathological findings confirmed that it was anaplastic pancreas carcinoma (giant cell type; Fig. 2) and these giant cells were positive for cytokeratin 7 and negative for CD68, suggesting an epithelial origin (Fig. 3).
Fig. 2.
Histological appearance (hematoxylin-eosin staining, ×40).
Histological findings of the tumor showed multinuclear giant cells (bizarre giant cells) diffusely proliferated: Anaplastic carcinoma of the pancreas (giant cell type).
Fig. 3.
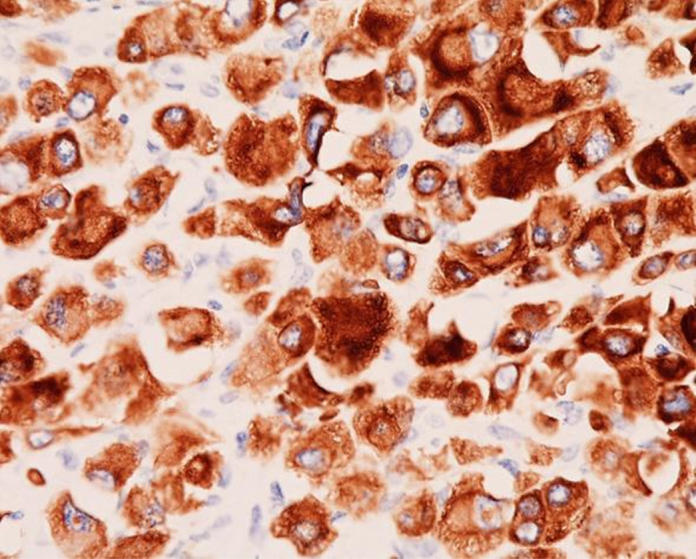
Immunohistochemical findings (staining ×40).
The mononuclear and multinuclear cells of the anaplastic carcinoma exhibited immunoreactivity for cytokeratin 7: CAM5.2 positive.
After the surgery, we performed S-1 adjuvant chemotherapy 100 mg/day for four weeks, repeated similarly every six weeks for a total of four courses, because this cancer was anaplastic pancreas carcinoma and was classified as stage III.
We have observed this case over 2 years so far with adjuvant chemotherapy and no recurrence or metastasis has been revealed.
3. Discussion
APC is an extremely rare type of pancreatic carcinoma that is characterized by extremely rapid progression and poor outcomes as compared to ordinary pancreatic ductal carcinomas; [1] in addition, APC accounts for 2–7% of all pancreatic cancers, and predominantly occurs in men [2], [3].
APC is considered to have poor prognosis, even after regression. The 3-year survival rate of patients with APC is less than 3%, with a life expectancy of 10–20 months [2], [4], [5], [6]. A variety of different subtypes of APC have been described, including spindle cell, giant cell, pleomorphic giant cell, and round cell [7]. In Japan, APC is also classified into four subtypes according to its microscopic morphology by the General Rules for the Study of Pancreatic Cancer (The 6th Edition,Revised Version): [8] giant cell, osteoclastoid, pleomorphic and spindle cell types. APC is considered as one of the pancreatic ductal carcinomas and defined since 2002 (by General Rules for the Study of Pancreatic Cancer The 5th Edition). However, we have several classifications such as those proposed by the WHO [9], AFIP [10], and the Japan Pancreas Society. Anaplastic carcinoma by Japan Pancreas Society is equivalent to undifferentiated carcinomas by WHO [9], or AFIP [10] (Table 1). On the other hand, undifferentiated carcinoma by Japan Pancreas Society is equivalent to medullary carcinoma by WHO [9], or AFIP [10], [11]. Thus, the classification of anaplastic carcinoma is much different between Japan and European countries. We investigated anaplastic carcinoma by Japan Pancreas Society though Ichushi-Web (Japan Medical Abstracts Society) from April 2002 to April 2015. Sixty-three cases were reported as APC, with the following subtypes: giant cell, 11 cases (17.5%); osteoclastoid, 20 cases (31.7%); pleomorphic, 21 cases (33.3%); and spindle cell type, 9 cases (14.3%). Most were osteoclastoid, and pleomorphic cases (65.0%), but giant cell and spindle cell cases accounted for 20% and giant cell 17.5% spindle cell 14.3%, respectively.
Table 1.
Classification of anaplastic carcinoma of the pancreas.
| General Rules for the Study of Pancreatic Cancer [8] | |
|---|---|
| The 6th Edition,Revised Version | Japan Pancreas Society |
| <WHO classification> | <AFIP classification> |
| Epithelial tumors | Epithelial Neoplasms |
| Malignant | Exocrine Neoplasms |
| Ductal adenocarcinoma | Serous neoplasms |
| Adenosquamous carcinoma | Mucinous cystic neoplasms |
| Colloid carcinoma (mucinous noncystic carcinoma) | Intraductal neoplasms |
| Hepatoid carcinoma | Pancreatic intraepithelial neoplasms |
| Medullary carcinoma | Invasive ductal carcinoma |
| Signet ring cell carcinoma | Tubular adenocarcinoma |
| Undifferentiatedcarcinoma | Adenosquamous carcinoma |
| Undifferentiated carcinoma with osteoclast like giant cells | Colloid (mucinous noncystic)adenocarcinoma |
| Hepatoid carcinoma | |
| Medullary carcinoma | |
| Signet ring cell carcinoma | |
| Undifferentiated carcinoma | |
| Anaplastic sarcomatoid carcinosarcoma | |
| Undifferentiated carcinoma with osteoclast-like giant cell | |
| Acinar cell neoplasms | |
| Bosman F.T., Carneiro F., Hurban R.G., et al.: WHO classification of Tumors of the Digestive System, Fourth edition. WHO Press, Geneva. 2010 [9] | Hruben R.H., Pitman M.B., Klistra D.S.,: Tumors of Pancreas (AFIP Atlas of Tumor Pathology Series 4) Armed Force Institute of Pathology. 2007 [10] |
Over 2-year survival was observed in 14 cases (22.2%); regarding subtypes, these included 7 osteoclastoid, 4 pleomorphic, 2 giant cell (including our case), and 1 spindle cell. Among APC subtypes, giant cell and spindle cell type cases had worse prognoses than other subtypes. Death within 12 months was observed in 29 cases (46.0%). The subtypes of these cases included 13 pleomorphic, 7 giant cell, 5 spindle cell, and 3 osteoclastoid; cases of osteoclastoid subtype seemed to be better tendency of the prognosis than other subtypes. Therefore, the prognosis for patients with APC was much poorer as reported even if resected. It is necessary to add the adjuvant-therapy such as chemotherapy or radiation therapy not only pancreatic cancer resection in order to improve this prognosis. However, according to Neoptolemos et al. [12], adjuvant chemotherapy confers a significant survival benefit in patients with resected pancreatic cancer, whereas adjuvant chemoradiotherapy has a deleterious effect on survival. Among possible adjuvant treatments for resectable pancreatic cancer, chemotherapy results in the best survival outcomes. Adjuvant treatment for resectable pancreatic cancer shows a significant survival benefit for adjuvant chemotherapy.
Gemcitabine-based chemotherapy is the famous and standard adjuvant chemotherapy for advanced pancreatic cancer. Among patients with macroscopic complete removal of pancreatic cancer, the use of adjuvant gemcitabine for 6 months compared with observation alone resulted in increased overall survival as well as disease-free survival according to the CONKO-001 Randomized Trial [13].
Recently Japan Adjuvant Study Group of Pancreatic Cancer 01(JASPAC-01) [14] phase3 study in patients with stage I–III pancreatic cancer compared treatment with S-1 vs. Gemcitabine. Toxicities were comparable in both groups. Rates of disease-free-survival at 2 years were 49% vs. 29% (HR = 0.57, 95% CI: 0.45–0.72) for S-1 vs. Gemcitabine; the corresponding rates of overall survival at 2 years were 70% vs. 53% (HR = 0.54, 99.8% CI: 0.35–0.83). A longer follow-up would be needed to find out if the disease-free survival advantage of S-1 over gemcitabine translates into an overall survival advantage for S-1. S-1 is more effective compared with gemcitabine as the adjuvant chemotherapy. However, it is unfortunate that S-1 seems to be considered for only Asian patients with pancreatic cancer.
S-1 is the key drug as the adjuvant chemotherapy with resected pancreatic cancer in any rate.
Adjuvant chemotherapy with S-1 in patients with resected APC is also recommended as a result of JASPAC-01 [14] like the ordinary pancreatic ductal carcinomas.
Actually, APC without osteoclastoid type seemed to be much poorer prognosis than other subtypes. We always administer adjuvant chemotherapy after resection of pancreatic cancer, particularly for pleomorphic, giant cell, and spindle cell subtypes.
However, we should select and add another therapy such as chemotherapy in proportion to histological types of the pancreatic ductal carcinomas. It might result in a better prognosis to perform further S-1 based chemotherapy or postoperative chemoradiation strategies undergoing curative-intent resection of pancreatic cancer. We hope to further evaluate the randomized trial such as JASPAC-01 [14] in the future. There is a possibility to achieve long-term survival in cases in which multidisciplinary treatment such as a curative resection and adjuvant chemotherapy are performed.
Ethical approval
We have gotten he ethical approval of this study by ethics committee.
Consent
We were explained to the patient and relatives, and informed consent was obtained.
Author contribution
We believe that this contribution is theoretically and practically relevant because anaplastic carcinoma of the pancreas is rare about adjuvant chemothrapy.
Guarantor
Auchor Toshikatsu Nitta.
Acknowledgment
None of the authors has any conflict of interest to declare.
References
- 1.Japan pancreas society: pancreatic cancer registry report 2007. J. Jpn. Pancreas Soc. 2007;22 [Google Scholar]
- 2.Paal E., Thompson L.D., Frommelt R.A. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann. Diagn. Pathol. 2001;5:129–140. doi: 10.1053/adpa.2001.25404. [DOI] [PubMed] [Google Scholar]
- 3.Tschang T.P., Garza-Garza R., Kissane J.M. Pleomorphic carcinoma of the pancreas: an analysis of 15 cases. Cancer. 1977;39:2114–2126. doi: 10.1002/1097-0142(197705)39:5<2114::aid-cncr2820390528>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi K., Nakamura K., Shimizu S. Pleomorphic carcinoma of the pancreas: reappraisal of surgical resection. Am. J. Gastroenterol. 1998;93:1151–1155. doi: 10.1111/j.1572-0241.1998.351_e.x. [DOI] [PubMed] [Google Scholar]
- 5.Connolly M.M., Dawson P.J., Michelassi F. Survival in 1001 patients with carcinoma of the pancreas. Ann. Surg. 1987;206:366–373. doi: 10.1097/00000658-198709000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan Z.G., Wang B. Anaplastic carcinoma of the pancreas associated with a mucinous cystic adenocarcinoma. A case report and review of the literature. JOP. 2007;18:775–782. [PubMed] [Google Scholar]
- 7.Alguacil-Garcia A., Weiland L.H. The histologic spectrum, prognosis, and histogenesis of the sarcomatoid carcinoma of the pancreas. Cancer. 1977;39:1181–1189. doi: 10.1002/1097-0142(197703)39:3<1181::aid-cncr2820390325>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Japan Pancreas Society: General Rules for the Study of Pancreatic Cancer (The 6th edition, Revised Version) August 2013.
- 9.Bosman F.T., Carneiro F., Hurban R.G. 4th edition. WHO Press; Geneva: 2010. WHO Classification of Tumors of the Digestive System. [Google Scholar]
- 10.Hruben R.H., Pitman M.B., Klistra D.S. Armed Force Institute of Pathology; 2007. Tumors of Pancreas (AFIP Atlas of Tumor Pathology Series 4) [Google Scholar]
- 11.Todaka A., Fukutomi A. Treatment for unresectable anaplastic carcinoma and undifferentiated carcinoma of the pancreas. Tan to Sui (Japan. 2012;33:669–674. (in Japanese) [Google Scholar]
- 12.Neoptolemos J.P., Stocken D.D., Friess H. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 13.Osettle Hemlet, Neuhaus Peter, Hochhaus Andreas. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer CONKO-001 randomized trial. JAMA. 2013;310(14):1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 14.Fukutomi A., Uesaka K., Boku N. JASPAC01: randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 for patients with resected pancreatic cancer. J. Clin. Oncol. 2013;31 doi: 10.1093/jjco/hym178. (suppl) 【abstract 4008】. [DOI] [PubMed] [Google Scholar]