Abstract
Biobanking in its various forms is an activity involving the collection of biospecimens and associated data and their storage for differing lengths of time before use. In some cases, biospecimens are immediately used, but in others, they are stored typically for the term of a specified project or in perpetuity until the materials are used up or declared to be of little scientific value. Legacy planning involves preparing for the phase that follows either biobank closure or a significant change at an operational level. In the case of a classical finite collection, this may be brought about by the completion of the initial scientific goals of a project, a loss of funding, or loss of or change in leadership. Ultimately, this may require making a decision about when and where to transfer materials or whether to destroy them. Because biobanking in its entirety is a complex endeavour, legacy planning touches on biobank operations as well as ethical, legal, financial, and governance parameters. Given the expense and time that goes into setting up and maintaining biobanks, coupled with the ethical imperative to appropriately utilize precious resources donated to research, legacy planning is an activity that every biobanking entity should think about. This article describes some of the fundamental considerations for preparing and executing a legacy plan, and we envisage that this article will facilitate dialogue to help inform best practices and policy development in the future.
Introduction
Legacy planning is the activity of planning for the transition of a biobank's physical collection and associated data, including the end of operations (and end-of-life) for a biobank. As such, legacy planning is distinct from other forms of biobank contingency planning (i.e., planning for an emergent event). The latter may involve preparing for predictable issues and circumstances (e.g., key equipment failure, failure to meet accrual targets, and so on) or unknown and unpredictable issues (e.g., disaster planning).
Many think of biobanking as a continuous effort, that is, an ongoing series of operations to collect, store, and share biospecimens and annotated data. However, there are many forms of biobanks,1 and the end of a biobank may come about as a natural part of a defined scientific project, or it may be due to a precipitous event such as the loss of funding or change/loss of leadership.2 While all of these are real and possible events, biobanks often only plan for immediate crises such as freezer failure; more complex scenarios that are equally significant are not always considered. Such issues may affect a biobank, regardless of size of collection or operational model. For a scientific project-based collection, there may be a known end date for the project's funding, yet a scientific and ethical obligation to maintain and share the biospecimens and associated data.
For a biobank storing a variety of collections over time, the uneven mechanisms for funding biobank infrastructures make the issue of loss of biobank funding all too real. In many cases, the funding for a biobank is finite and increasingly biobanks are asked to find ways to enact a sustainability strategy beyond a set period of initial biobank funding.3
Even with the growing dialogue and sharing of strategies around biobank “sustainability”4,5 and recognition of the value of having in place a sound business plan to guide a biobank's operational and capital expenses,6 the end of stable funding or leadership presents a real challenge. However, given biobanks' roles as custodians of biospecimens and data, legacy planning for the known and unknown future is not only a point of good governance but management of the transition may also be considered an ethical responsibility of the custodian biobank.
The objective of this article is to present considerations around biobank legacy planning and to define elements to be considered in creating a biobank and/or a biospecimen collection legacy plan. Given a limited literature in this area, for the purpose of this article we will focus on some universal biobank legacy planning considerations with the hope that this discussion will help to inform downstream activities, including best practices and policy development.
Background
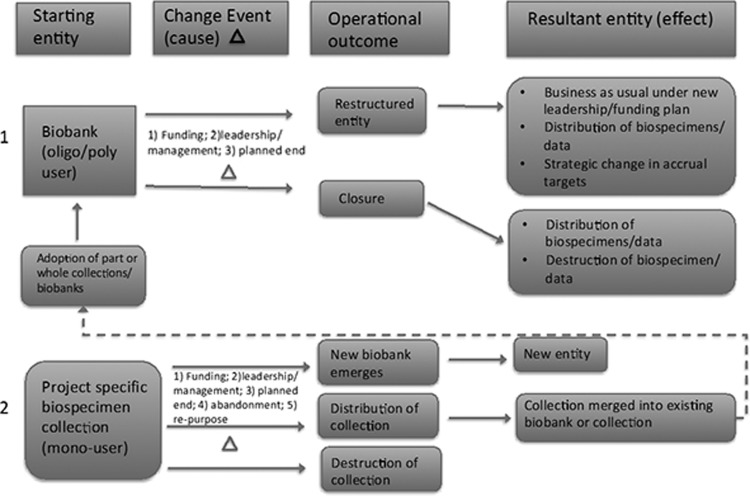
At its core, a biobank legacy plan acts to guide the who, what, when, where, why, and how biospecimens, and associated data should be distributed or destroyed following a precipitating event (e.g., loss of/change in funding or leadership) or planned end to a project. Biobanks exist in many different forms and are varied in their composition, including design, scope, funding sources, and aims,1 and a precipitating event may significantly change the biobanking entity (Fig. 1). Project-specific collections, or “mono-user” biobanks,1 may have a set start and end date to their collection. This allows for a planned end of the project and execution of the plan alongside the lifetime of the collection. In the case of more commonly recognized classical or “oligo/poly-user” biobanks,1 many initiate their collection with a plan to accrue and collect biospecimens prospectively with no specific targets and for yet-to-be defined research purposes. Such activities may be planned to continue in perpetuity. Given that such an infinite collection and storage model underlies many biobanks, changes to funding and leadership that may bring about the end of an entire biobank can be devastating. Biobanks may also over time absorb other project-specific collections/mono-user biobanks or inherit newly discovered cryptohistorical collections.
FIG. 1.
Biobank/Biospecimen collection transformation events. In example pathway 1, a large biobank may have a change event caused by the loss of funding or leadership. This event may or may not be defined by a finite endpoint. Operationally, the biobank may (a) choose to restructure itself and carry on under a new leadership/funding plan and/or it may plan to redistribute biospecimens or change strategic accrual targets or (b) close and distribute and/or destroy biospecimens and data. In example pathway 2, a small project-specific biospecimen collection may experience a change event caused by termination of funding, leadership, arrival at a planned defined endpoint, or may be abandoned (e.g., leading to an unexpected find by another researcher) or repurposed for a new unrelated study initiative. With these changes, the original collection may be the nidus for a new biobank or it may be distributed and merged into an existing collection and/or destroyed.
As noted earlier, changes in biobank funding are a reality, and biobank closure due to loss of funding has been documented.7,8 To mitigate the negative impact of funding changes, biobanks strive to use a wide range of sustainability strategies, including engaging in a business continuity plan, diversifying their funding sources, and/or using cost recovery methods.6 Given the complexity of biobanks and the unpredictability in scale and timing of serious events, planning for a legacy event will be different for each and every biobank; there is no “one-size-fits-all” solution. However, as a biobank increases in scale, maturity, and value, the importance of pre-emptive legacy planning or assignment of responsibility for developing a plan also increases.
Interestingly, Cadigan et al.9 noted that only 26% of biobanks they interviewed had a plan on what to do with their biospecimens should their biobank be terminated, even though more than 40% were greater than 10 years old.10 The “where” and “how” to transfer or destroy biospecimens involve more complex considerations beyond just the operational level and encompass many familiar biobanking themes, including ethical, legal, and societal issues (ELSI), as well as issues related to biospecimen collection parameters and quality assessment, including extent and quality of associated data. During the planning and execution phases of the legacy plan, many individuals, including institution heads (health, academic, other), Institutional Review Boards/Research Ethics Board (IRB/REBs), funding bodies, financial entities, and third party contractors, will likely need to be consulted for guidance and input, given the range of issues.
As there are currently few resources available to aid in biobank legacy planning, the authors of this article held a workshop at the International Society of Biological and Environmental Repositories (ISBER) 2015 Annual Meeting & Exhibits Phoenix, AZ, entitled “Legacy Planning for Biobanks and Biospecimen Collections.” The workshop focused on the necessary elements of Legacy Planning that biobanks/research groups can use to create their own plans.
Topics covered in the workshop included a discussion of general considerations for biobank legacy planning, including when to begin legacy planning and operational elements involved in planning; ELSI issues important to consider in legacy planning; and a case study for legacy planning, based on the NIH Genotype-Tissue Expression project (GTEx). The resulting workshop, attended by >50 individuals, delivered many of the concepts outlined in this article and was elaborated upon by an expert panel. Workshop attendees were able to address the expert panel with questions and comments.
Several topics garnered varying opinions. Of note, the audience was divided on the issue retaining all biospecimens for usage until exhaustion: while some felt there was a philosophical and ethical impetus to do so, others felt there was an economic burden in doing so. Given the lack of resolution on topics related to legacy planning, we see this workshop as a first step in engaging the biobank community at large and leading next steps in best practice and policy development.
Methods
Operational considerations
The nature of a precipitating event will influence when the legacy plan will start and end and prioritization many of the issues. Little has been discussed about national level guidelines or regulations that might direct or mandate biobank legacy plans, but some guidance may be found in local, institutional, or funding level requirements. In the United Kingdom, for example, membership in the NCRI (National Cancer Research Institute) Confederation of Cancer Biobanks (CCB) required biobanks and institutions to sign a memorandum of understanding and agree to alignment with a common set of Guiding Principles that includes “maintaining continuity.”11 Specifically, principle 4.7 states: “………….Individuals or organizations acting as custodians should have plans for maintaining continuity in such circumstances; in effect, an ‘ethical preservation order’ should exist to protect biobanked samples for the long term.” (Confederation of Cancer Biobanks).
Funding issues connected to a precipitating event fall into two categories. In the case where a leadership position has changed, funding may be tied to a previous leader or frozen until a new leader has been appointed and funding is directed to the new leader. Without funding sources that are earmarked or specifically set aside, the challenge of how to fund the process of a legacy event and dedicate resources is not straightforward. One option may be to direct a percentage of operating costs or user fee revenue to a contingency fund throughout the life cycle of the biobank to cover costs associated with closure and redistribution of the contents of a biobank.
Current and accurate financial information for the biobank or project will help to inform many key decisions required for funding transfer events. In the case where transfer of the biobank to a third party is planned, funding to facilitate the transfer of materials may be part of an overarching agreement with a predetermined third party. While each transfer event and each biobank is different, it may be anticipated that some dedicated personnel will be required to facilitate the transfer or alternative destruction processes (e.g., packing and shipping of biospecimens) and to conduct subsequent validation procedures to confirm biospecimen quality and case data integrity. Accurate and up-to-date biospecimen information (e.g., biospecimen aliquots, derivatives, products, consent status, and location) commonly captured in a biobank LIMS system is necessary to help estimate biospecimen transfer costs.
Box 1 gives three examples of biobanks that experienced different types of change events related to legacy planning and lessons learned.
BOX 1.
Three examples of legacy events involving biobanks, with options considered, and solutions selected.
Example 1. The leader of a large polyuser biobank leaves to work at another institution in the same country, but there is no one suitable locally as a replacement. The immediate options considered included stopping active operations or transfer of the collection with the leader. The solution chosen was to utilize the existing strong governance structure that included a multidisciplinary scientific review panel to determine access and to assign the new leader role to a member of this committee, with the past leader appointed as an expert advisor for a transition period.
Example 2. A local institution “discovers” a large historical tissue collection in a freezer when a senior staff member leaves. Research relevant to this collection is no longer an institutional priority. The immediate options considered included destruction, continued storage by the host department without plans for use, and seeking advice from the research ethics board regarding future use since the consent status was unclear and there was some but limited associated data. The solution approved by the research ethics board was to transfer a small subset of the collection to a local biobank for initial testing to determine potential value. This was followed by a formal transfer and permanent anonymization of the samples by the recipient biobank; anonymization was accomplished as part of the necessary process of switching storage vials.
Example 3. A biobank loses half of its funding for its ongoing work to collect clinical formalin fixed paraffin-embedded (FFPE) blocks and process matching blood specimens into the plasma and buffy coat. The immediate options considered included stopping enrollment operations and dedicating resources to maintaining the existing collection or transferring the collection to another biobank. The solution chosen was to reconfigure to maintain all operations for a period of time while new funding was sought. Reconfiguration involved maintaining consent rates, but only actively collecting FFPE blocks and blood on a selected subset of consented cases. In addition, the blood processing protocol was simplified to only store the buffy coat to reduce time, cost, and storage space requirements.
Current state assessment
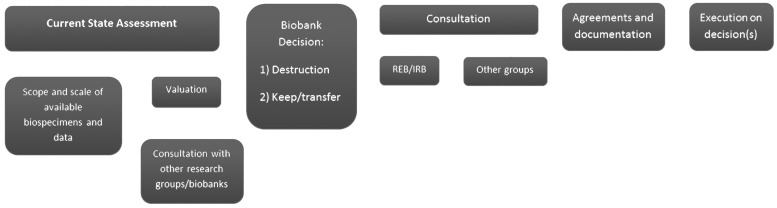
An assessment of the current state of a collection, including both the biospecimens and associated data, is best conducted by the custodian before formulating a plan for material transfer (Fig. 2). This analysis can serve as a basis for confirmation assessment by the recipient after transfer. This optimal process cannot be applied in all legacy scenarios; nevertheless, at least one assessment by the original custodian or recipient as soon as possible after transfer is important to complete. This should involve a review of the biobank to verify the elements of the collection as denoted in Box 2.
FIG. 2.
Steps in formulating a biobank legacy plan. From left to right, steps of biobank legacy planning. Current state assessment involves a detailed assessment of current collection numbers, format and location, and collection duration of biospecimens. Then, a valuation of the collection can be initiated—this includes evaluating the collection case types (disease, treatment, SOPs), matched biospecimens (Box 3) to help assess current outside market interest. Once better assessed, a biobank may have an initial decision to (1) destroy biospecimens or (2) keep and/or transfer biospecimens. This decision would be discussed with the local institutional research ethics board in consultation with other groups (e.g., funders, institutional oversight bodies, pathology departments, and so on). Once a decision has been agreed upon, agreements and documentation can be formulated and signed for further destruction or transfer activities. SOP, Standard Operating Procedures.
BOX 2.
Elements of the collection for review during current state assessment.
○ Number of biospecimens
○ Format of biospecimens
○ How and where biospecimens are stored (aliquot sizes, containers, and so on)
○ Details on previous use of biospecimens (information of freeze–thaw events, remaining aliquots, and so on)
○ How and where data are stored and any relevant linkages to other data sources
○ Format for coding and level of data privacy (coding system and anonymization or deidentification details)
○ Consent status (informed consent details or basis for a waiver of consent)
A subset review (e.g., a feasible proportion such as 1%) of randomly selected biospecimens in their containers should also be done to determine the general preservation state, nature and status of labeling, presence or absence of personal identifiers, and integrity of the containers. Similarly, a subset review should be conducted of randomly selected data sets in their current format (paper and/or electronic) to determine general organizational state, nature and status of completeness, presence or absence of personal identifiers, and the existence of backup data.
Formal statistical calculations can be used to determine sample sizes for such subset reviews; factors such as collection size, knowledge of previous events that may have affected quality or linkage within the collection, and anticipated operational error rates can be considered. However, in many cases, the goal is to determine overall collection value and integrity rather than specific quality levels and to determine if any fractions of the collection are of reduced value. In this instance, random selection is often made of a small number (e.g., 3–10 cases) from each of multiple compartments of the collection (e.g., combinations chosen by factors such as year, technician employment period, freezer location, biospecimen type) up to an empirical representative proportion (e.g., 1%) of the total collection.
Inherently, the effort required to conduct any review differs by biospecimen type (e.g., formalin fixed paraffin-embedded [FFPE] vs. frozen biospecimens) and extent of data. Therefore, the effort needed relative to the value of the collection should be estimated and apportioned accordingly. This is summarized in Table 1.
Table 1.
Effort Required for Legacy Events by Biospecimen Type
| Biospecimen type | ||
|---|---|---|
| Frozen (mechanical freezer, liquid nitrogen storage) | FFPE/other (ambient temperature storage) | |
| Legacy change management processes | ||
| Collection inventory | ||
| Biospecimen retrievala | b | c |
| Confirm number and format of biospecimens | b | c |
| Packaging and shipping | ||
| Biospecimen packaging | b | c |
| Biospecimen shipping | b | c |
| Biospecimen quality control and validation | d | d |
| Biospecimen destruction | d | d |
| Data management | d | d |
Effort is increased where biospecimens are being inventoried across multiple sites.
Estimate of typical “effort” is based on time and resources needed to complete each event.
Extensive effort.
Some effort.
Moderate effort.
FFPE, formalin fixed paraffin-embedded.
Once gathered, the custodian is able to (1) assemble a list of likely biobanks/research groups that may be interested in acquiring the transfer materials and/or (2) actively seek third parties with the necessary information to begin the discussion of the merits, feasibility, and logistics of material transfer. Likewise, the recipient may be able to determine the value of the collection and any negative effects due to the transfer.
Biospecimens and data have an inherent scientific and therefore market value, which is based on the needs and demands of a third party, the research user. The composition and nature of the collection may comprise materials representing common or rare disease types from large or specialized population cohorts. Furthermore, the collection format (e.g., formalin fixed paraffin-embedded, frozen, blood aliquots), the annotation of collection Standard Operating Procedures (SOPs), the nature of consent and documentation of consent, and the volume and the amount of available demographic and outcome data may influence the value of the collection. This valuation assessment (Box 3) may best be done by referral to an appropriate regional/national research body or biobank network for objective expert opinion.
BOX 3.
Valuation of biospecimens.
○ Common versus rare disease types
○ Large or specialized population cohorts
○ Generalized of specialized SOP used and documentation of such
○ Collection format (e.g., FFPE, frozen, blood aliquots)
○ Matched biospecimen formats (e.g., serum, FFPE, and buffy coat)
○ Volume of biospecimens
○ Age of biospecimens
○ Nature of therapeutic treatments associated with the cohort
○ Specialized therapy types
○ Amount and extent of available demographic and outcome data
○ Specialized data available (e.g., kinship, kinship outcomes)
○ Nature of consent
○ Broad versus specialized use allowed
Biospecimens and data
For biobanks that are involved in transfer of biospecimens and data either into or out of the biobank, many processes and personnel are needed to identify and retrieve biospecimens and data, assess quality, and transfer data and materials (summarized in Table 2).
Table 2.
Retrieval, Quality Assessment, and Transfer of Materials
| Retrieval | Quality Assessment | Transfer | ||||
|---|---|---|---|---|---|---|
| Components | Process | Personnel | Process | Personnel | Process | Personnel |
| Biospecimens | Locating biospecimens Pulling biospecimens from storage (freezer, shelf, box) |
Biobank administrative manager Biobank technician |
Random selection of subset biospecimens Apply QA/QC methodology |
Biobank technician Data analyst/manager |
Recoding/labeling biospecimens Packaging and shipping of Biospecimens preparing and executing required documentation (e.g., Material Transfer Agreement, general agreements, shipping logs) |
Biobank technician Administrative manager |
| Data | Identifying specimens in LIMS Identifying which data belongs to the bank and the relations between data (a) fully understanding the data sources (b) understand the history, structure, meaning of source data Identifying required system/software (a) what software is needed to process that data (e.g., format of the database (b) hardware Planning the migration of the data (a) which parts can/should be migrated/where manual input is easier (b) specifications for mapping the data |
Data manager | Record QA/QC biospecimen results Identify quality issues in data |
Biobank technician Data manager |
Migrating the data using defined migration rules (a) Start with a small sample to test Validate/test the data Follow-up and maintenance |
Data manager |
Maintaining records of biospecimen and data transfer is important and may be mandated at a local level. The details around which materials were sent, to whom, where, and when they were sent will need to be detailed along with relevant transfer process documentation, including shipping details, Material Transfer Agreements (MTAs), and other agreements. In addition, all documentation and signatures at the time of receipt of a collection may need to be held and conserved by both original and recipient institutions. These documents would most likely be necessary for IRB/REB purposes; thus, the record keeping and maintenance of a legacy collection may have ongoing associated responsibilities and linked costs.
Communication strategies
When designing and enacting a legacy plan, the biobank or managers of a project-specific collection must consider their responsibility to communicate the fate of the biospecimens and their associated data to all relevant stakeholders. This may include the donors as well as other stakeholders such as institutions, funders, recipients of biospecimens and data, and other research partners.
In many cases, donors may have originally declined to be recontacted as part of initial consent or their consent has been waived by an ethics review board acting on their behalf, and if legacy plans and future use do not pertain to a difference/change in the terms of their consent, providing notice to the donors may not be required. However, when feasible and allowable, it may be considered to be in the best interest of those who donated to provide information, which adequately explains what is happening with a collection, particularly when faced with closure, loss of funding, or transfer of collections to a different institution. Certainly, there may be a clear duty to ensure that donors maintain the ability to withdraw their biospecimens from future research use.
In the case of biobank closure and subsequent transfer of specimens, the institution originally housing the specimens may be required to make adequate efforts to provide the new host institution's contact information to the donors and their families.
In addition, given the costs associated with storing collections long term, institutions and biobanks accepting biospecimens from the execution of legacy plans should be prepared to promote the distribution of those specimens for research purposes. Tools such as the National Cancer Institute's Specimen Resource Locator (SRL) (https://specimens.cancer.gov), the CTRNet Biobank Resource Center locator (www.biobanking.org/brc/locator), or the (ISBER International Repository Locator (IRL) (www.irlocator.isber.org) can help expose these collections to researchers. Well-placed promotion and advertising of these collections can also assist in speeding their distribution, thereby offsetting the long-term costs of hosting.
New social media platforms such as Twitter, LinkedIn and Facebook have become a reliable and effective means to reach a wider researcher audience. In addition to websites, brochures, and informational mailings, these tools should form a part of the overall communication toolbox that biobanks think to use to promote the value of the biospecimens and data as a research resource. The development and implementation of a well-crafted communication strategy are an essential part of ensuring the success of a legacy plan.
ELSI considerations
Little has been written on the ethical, legal, and social issues related to biobank closure.12 The details around what would happen to biospecimens and data (e.g., where they would go, how they would be used, and whether they should be destroyed) are questions that have far reaching implications beyond the original goals of the biobank. Such implications include participants' privacy, autonomy, and dignity.7
Given the ever-changing landscape of informed consent around human biospecimens and their associated data, applying a uniform approach to dealing with all biospecimens in a biobank's collection can prove to be challenging, particularly if preparing for a legacy phase was not considered during the development of informed consent documents. The lack of appropriate legacy planning before collection, for biospecimens already accrued as well as for future collections, can place a heavy burden on biobanks as legacy events can precipitate unplanned resource intensive activities from both a financial and labor perspective. Without a doubt, the ethical, legal, and social issues related to biobanking and biobank closure raise many questions that must be adequately addressed during the legacy planning process.
The questions that stem from a few recent examples7 are relevant across the entire discipline of biobanking: What will happen to biospecimens and data? Will they be destroyed or transferred—or both? Who will guide the transfer of biospecimens and data? What ethical/legal provisions must be in place to transfer biospecimens and data? What conditions must be met or set in place for transfer of biospecimens and data? What are the necessary conditions of use, disclosure, and ethical approval process? How would a new MTA for third party use be constructed, and what are the conditions? What relationship does the new custodian have with the participants, and what is the new custodian's access to personal data? What are the governance parameters required of the new custodian? Should end-of-biobank issues be described in the informed consent process?
When developing a legacy plan, biobanks and those involved in project-specific collections should ensure that the best interests of the donors are represented in the planning process; this may require the participation of patient advocates in addition to deliberation by an IRB/REB. In cases where the transfer of biospecimens and data is desirable or required, the original consent for the biospecimens and data must be transferred along with the biospecimens themselves; a recipient institution should be bound by consent parameters that are no less restrictive than the originals. To guarantee and safeguard this, any transfer of specimens and data to a different institution should be constrained by a proper and enforceable MTA, binding the recipient institution to honor the intent of the original consent materials.
For those biobanks absorbing collections with little to no associated history, gathering the above-mentioned information may be near impossible. For example, consent details, SOPs, and standards under which the materials were collected are often not known. There may be little to no demographic or biospecimen data recorded. Making the case for absorbing such collections may be difficult and will need to be balanced between the operational, business, and scientific interests of a potential recipient biobank.
Discussion
Planning for events that seem unlikely or distant is often set aside to address more pressing operational issues. However, it is essential that biobanks consider the development of legacy plans. Such planning can be challenging, however.
For many biobanks, determining timelines to operationalize a legacy plan may not be possible at the outset of biobank operations and specific details will need to be set in place soon after a change event has been identified. Furthermore, biobanks that need to operationalize a legacy plan may struggle to find sufficient funding and resources to do so. Newly funded biobank initiatives may not even consider the legacy planning process until well into their operation. Nevertheless, having draft legacy plans in hand for the various biospecimen collections under a biobank's charge can be very helpful to taking proper and prompt actions when the need arises and for anticipating the potential needs in terms of costs and resources.
As outlined earlier, a transfer event will often require dedicated personnel to operationalize transfer of biospecimens and data. Accurately planning how many personnel are needed will be contingent directly on the scale and scope of materials to be transferred. Conversely, project-specific collections, with a clear end date, may define these activities and timelines upfront and allow the opportunity to plan the timelines for a transfer event well in advance. Transfer event resource allocation and funding can be delineated early on, and costs can be better attributed where numbers of biospecimens and data are accurately known or can be projected. Biobanks may consider that legacy plans can be initiated and updated annually with current information about the nature and number of their collections.
The considerations for executing a legacy plan described here would require a financial dedication on the part of the biobank. As biobanks are diverse in their scope and scale, one would anticipate that executing a plan would be tailored to fit the biobank purpose and available resources: that is, a monouser biobank will scale and execute a plan differently than a polyuser bank that may have the resources to roll out a multiyear closeout plan. The goal of this article is to identify critical components of legacy planning that researchers, administrators, and operational leads can consider.
We have principally focused on considerations for transfer of biospecimens, but the destruction of biospecimens and data is an alternative operational decision that biobanks must consider. In these scenarios, real costs may be associated with the destruction and disposal of biospecimens, data, and data files. Operationally, destroying specimens under the control of the biobank may be less complex than ensuring the destruction of resources that have been provided to third parties.13 From an ethical standpoint, the controlled destruction of biospecimens following consent for use by a biobank or a specific project may not be a straightforward decision12; typically, this is not a part of the conversation in the initial informed consent process, and thus, participant attitudes to destruction and disposal of biospecimens are largely unknown.
Biobanks traditionally convey to participants how biospecimens will be utilized as a part of a biobank to support biomedical research initiatives. In giving their consent, there is often an expectation that their specific donation will be used at some time in the future, and therefore, some expectations are set regarding the activities participants believe they are supporting. Biobanks may seek to include appropriate statements regarding potential destruction of donated biospecimens in their communications, privacy statements/policies, or consent documents.12 Understanding and accepting a biobank's obligation to represent and safeguard the interests of those who have donated specimens are paramount.
Under the custodianship model of biobanking, donors offer biobanks a unique personal resource—literally a piece of themselves—and in exchange, the biobank is charged with ensuring that biospecimens are collected and used in accordance with the donor wishes and those explicitly communicated in the informed consent documents.14 Furthermore, the role of custodian recognizes that donations are “gifts” to the research community and not property of the biobank or institution and that adequate and precise planning along with good governance form part of the responsibilities.14
Although biobanks retain decision-making power over the distribution of biospecimens and associated data and there exists no restrictions on the legality of destroying unwanted biospecimens, such destruction may be considered by some to be a violation of the spirit of the consent process and the ethical principle of justice. By this philosophy, tissue collected for research purposes should not be discarded, but rather should be used within the confines of the consent under which it was given, to the greatest extent possible and be utilized fully in research projects.
It is also important to note that scientific advancements may uncover new techniques to apply to different biospecimens and their products, and discarding biospecimen collections may be considered unwise. However, an opposing view would note that the cost to store biospecimens and data is enormous. Properly assessing the scientific value of a collection will be central to making decisions about destruction of biospecimens. When scientific value has been shown to be limited, and in the absence of users to utilize these materials, keeping specific cases and/or entire low value collections ultimately diverts precious funds from new biobank initiatives and from health research. In this philosophy, the destruction or culling of biospecimens may well be warranted when parameters such as quality assessment, or lack of selection and use over time, are considered.
Conclusion
While many legacy planning elements cannot be operationalized until a legacy event is imminent, this article has laid out key considerations for biobanks to consider in their planning. This does not represent an exhaustive list, and we anticipate that this article could serve as a starting point for a wider dialogue around biobank legacy planning. Going forward, we envisage further best practice and policy development around biospecimen and data transfer; end-of-biobank issues; and destruction of biospecimens in the context of donor consent, privacy, and confidentiality, as well as appropriate local and national regulations.
Acknowledgments
The authors would like to thank invited panel members, Dr. Jim Vaught and Ms. Marianna Bledsoe, for contributing their expertise and thoughts during the “Legacy Planning for Biobanks and Biospecimen Collections” workshop at the (ISBER) 2015 Annual Meeting & Exhibits Phoenix, AZ. They would also like to thank Dr. Brian Clark for his thoughtful comments and discussions on different aspects of these topics.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Watson PH, Barnes RO. A proposed schema for classifying human research biobanks. Biopreserv Biobank 2011;9:327–333 [DOI] [PubMed] [Google Scholar]
- 2.Couzin-Frankel J. The legacy Plan. Science 2010;329:135–137 [DOI] [PubMed] [Google Scholar]
- 3.Matharoo-Ball B, Thomson BJ. Nottingham Health Science Biobank: A sustainable bioresource. Biopreserv Biobank 2014;12:312–316 [DOI] [PubMed] [Google Scholar]
- 4.Watson PH, Nussbeck SY, Carter C, O'Donoghue S, Cheah S, Matzke LA, et al. A framework for biobank sustainability. Biopreserv Biobank. 2014;12:60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simeon-Dubach D, Henderson MK. Sustainability in Biobanking. Biopreserv Biobank 2013;12:287–291 [DOI] [PubMed] [Google Scholar]
- 6.Vaught J, Rogers J, Carolin T, Compton C. Biobankonomics: Developing a sustainable business model approach for the formation of a human tissue biobank. J Natl Cancer Inst Monogr 2011;2011:24–31 [DOI] [PubMed] [Google Scholar]
- 7.Caulfield T, Burningham S, Joly Y, Master Z, Shabani M, Borry P, et al. A review of the key issues associated with the commercialization of biobanks. J Law Biosci 2014;1:94–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tupasela A, Stephens N. The boom and bust cycle of biobanking—Thinking through the life cycle of biobanks. Croat Med J [Internet] 2013;54:501–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadigan RJ, Lassiter D, Haldeman K, Conlon I, Reavely E, Henderson GE. Neglected ethical issues in biobank management: Results from a U.S. study. Life Sci Soc Policy [Internet] 2013;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson GE, Cadigan RJ, Edwards TP, Conlon I, Nelson AG, Evans JP, et al. Characterizing biobank organizations in the U.S.: Results from a national survey. Genome Med [Internet] 2013;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Confederation of Cancer Biobanks: Human Research Tissue Banks/Resources/Biobanks Guiding Principles. Available at http://ccb.ncri.org.uk/wp-content/uploads/2014/03/ccb_guiding_principles_v7_01–03-12–1.pdf, accessed September3, 2015
- 12.Zawati MH, Borry P, Howard HC. Closure of population biobanks and direct-to-consumer genetic testing companies. Hum Genet 2011;130:425–432 [DOI] [PubMed] [Google Scholar]
- 13.Dhai A, Mahomed S. Biobank research: Time for discussion and debate. S Afr Med J 2013;103:225–227 [DOI] [PubMed] [Google Scholar]
- 14.Yassin R, Lockhart N, Del Riego MG, Pitt K, Thomas JW, Weiss L, et al. Custodianship as an ethical framework for biospecimen-based research. Cancer Epidemiol Biomarkers Prev 2010;19:1012–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]