Highlights
-
•
Hemangiopericytoma is known to occur in any anatomical site, especially the extremities and retroperitoneum.
-
•
Primary surgical resection is the treatment of choice. There is no benefit of radiation or systemic chemotherapy.
-
•
Angiogenic inhibitors represent promising systemic therapeutic concepts for Hemangiopericytoma.
Keywords: Hemangioma pericytoma, Mesenchymal tumor, Greater omentum
Abstract
Introduction
Hemangiopericytoma (HPC) has been first described in 1942 by Stout as a tumor originating from the capillary surrounding pericytes. It is known to occur in any anatomical site, especially the extremities and retroperitoneum.
Presentation of case
We describe a case of a 24 year old patient presenting with lower abdominal pain due to a tumor of the greater omentum, the patient was treated by conventional laparotomy with tumor resection and the histological evaluation confirmed the diagnosis Hemangiopericytoma/Solitary fibrous tumor (HPC/SFT). The patient has regularly followed-up with periodic imaging for the last 4 years, with no recurrences.
Discussion and conclusion
According to our knowledge, HPC rarely develops in the greater omentum, only 20 cases were described in the literature. Primary surgical resection is the treatment of choice. There is no benefit of radiation or systemic chemotherapy. Angiogenic inhibitors represent promising systemic therapeutic concepts.
1. Introduction
Hemangiopericytoma (HPC) is a tumor first described in 1942 by Stout as a tumor originating from the capillary-surrounding pericytes [1]. It is known to occur in any anatomical site, especially the skeletal muscles, subcutaneous tissue of the lower limbs and retroperitoneum [2]. HPC is most commonly prevalent amongst individuals between 20 and 70 years, with the median age in the 40s [2]. HPC of the greater omentum is a rare condition, according to our knowledge; only 20 cases have been published till the current date (PubMed, National Library of Medicine, Bethesda MD, USA). This following article describes a case of HPC/SFT of the greater omentum with discussion of the literature and the value of different therapeutic options.
2. Presentation of case
A 25-year-old male patient presenting with lower abdominal pain was referred to our surgical department. The patient had no nausea or vomiting, no complaints of irregular bowel movements or blood in stool. The patient reported no previous abdominal complaints or interventions, apart from lower leg procedures as a result of an injury seven years ago. Abdominal examination revealed a slightly hyper-peristaltic bowel, with a soft consistency and a palpable moveable mass in the upper abdomen, though no sign of peritonitis. Ultrasound showed a solid intra-abdominal tumor lying above the bladder. Abdominal CT-scan showed a hypervascularized solid centrally inhomogeneous tumor measuring 5.7 × 8.3 × 6.8 cm in the left lower quadrant lateral to the bladder, with an emphasized vascular supply originating from the left gastoepiploic vessels (Fig. 1). Thorax CT-scan revealed no tumor. The patient was considered for surgical laparotomy and tumor resection, which was carried out successfully.
Fig. 1.
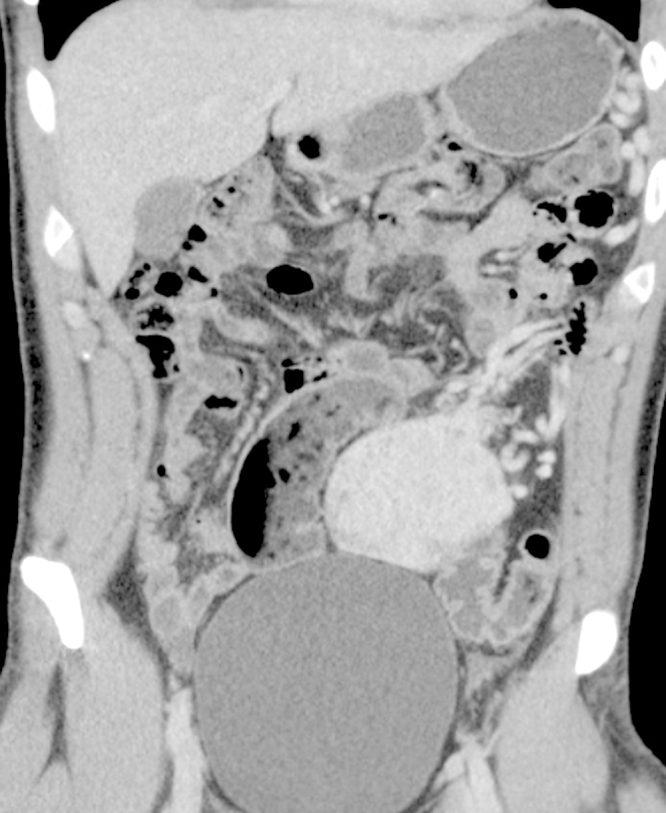
Abdominal CT-scan showing a hypervascularized solid centrally inhomogeneous tumor measuring 5.7 × 8.3 × 6.8 cm in the left lower quadrant with an emphasized vascular supply originating from the left gastoepiploic vessels.
Intraoperative findings showed a tumor within the greater omentum, mainly supplied by the left gastroepiploic vessels (Fig. 2). No peritoneal or organ metastasis were seen. The tumor was removed en bloc with an omental root containing the supplying vessels and was sent for histological evaluation, which showed alternating hypocellular and hypercellular areas and a focal myxoid change (Fig. 3). The round to spindle-shaped nuclei had little cytoplasm and dispersed chromatin. The tumor was rich in partly hyalinised branching vessels. Mitotic counts reached 7 mitoses per 10 high-power fields. In the immunohistochemical analysis, the tumor showed a consistent positivity for CD34 and CD99 with negativity for AE1/3, Desmin, CD31, CD117, DOG1 and S100. Proliferation rate assessed by MIB1 staining was 5%. These findings confirm hemangiopericytoma as a diagnosis and the resection margin was free of tumor. The patient recovered with no complications and was discharged on the seventh postoperative day. The patient has regularly followed-up with periodic imaging for the last 4 years, with no recurrences.
Fig. 2.
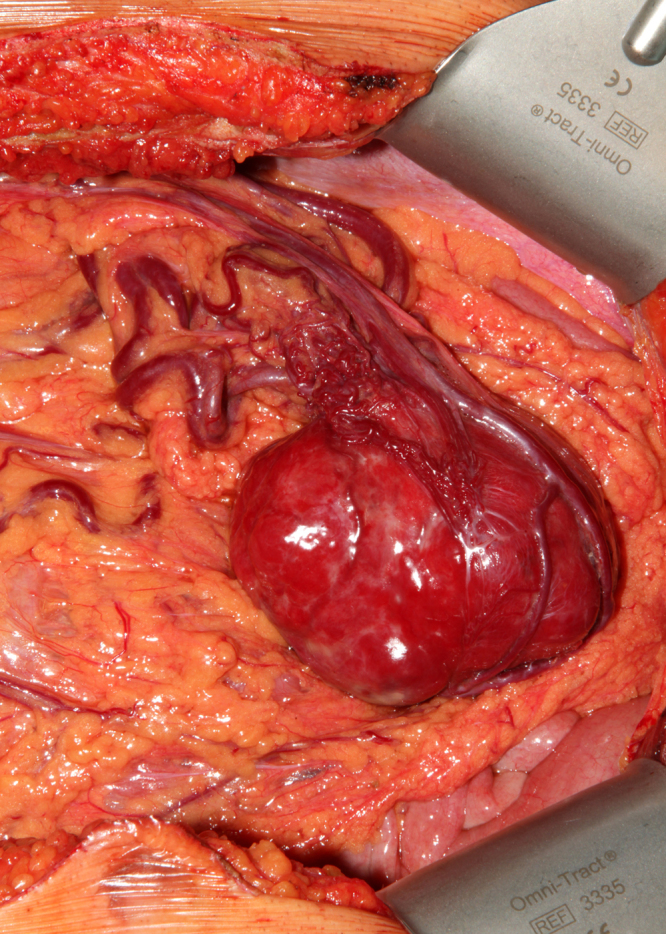
Inraoperative image showing the tumor within the greater omentum, mainly supplied by the left gastroepiploic vessels.
Fig. 3.
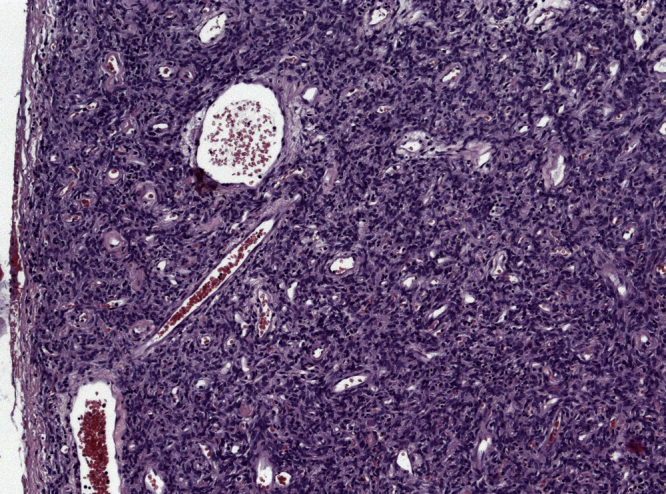
Microscopic examination demonstrating numerous partly hyalinised branching vessels (stag-horn appearance) in the high population of spindle-shaped tumor cells. H&E, × 100.
3. Discussion and conclusion
Stout described several histological features of HPC in which the perivascular cells had different appearences [1]. Stout originally acknowledged the fact that the term Haemangiopericytoma was given in reference to Zimmermann’s pericytes, which became questioned due to the scientific basis of the assumption [3]. Furthermore, studies have shown that only up to one third of HPCs show specific ultrastructural [4], [5] or immunhistochemical features of pericytic origin [6], [7]. As a consequence, the term solitary fibrous tumor SFT is being frequently utilized to embrace all HPC-like lesions [8].
According to the literature, HPC/SFT has a favorable long-term outcome after primary surgical resection [2], [9]. HPC of the greater omentum has shown to recur at local or distant sites in several cases in the literature [10]. Enzinger and Smith suggested HPC malignancy criteria to be large tumor size ( > 5 cm), increased mitotic rate (≥4 mitotic figures/10HPF), high cellularity, presence of pleomorphic tumor cells and presence of haemorrhage and necrosis [2]. Still, the behavior of lesions lacking the mentioned criteria cannot be predicted [8]. Kaneko et al. concluded that a tumor size of more than 20 cm is an unfavorable prognostic factor of greater omental HPC [10], though due to the limited number of cases on which the conclusion was made as well as the different possible entities of HPC/SFT, we believe that prognostic factors of omental HPC/SFT are to be seen as those of other regions.
Primary surgical resection is the treatment of choice, whenever possible [9], [11]. Literature reports showed no significant benefit of radiation [12] or systemic chemotherapy [13], [14], although a few responses have been reported. Due to the rich vascular characteristics of these tumors, angiogentic inhibitors have emerged as promising systemic therapeutic concepts [15].
Conflicts of interest
The authors declare that there are no conflicts of interest.
Funding
We report no involvement of sponsors.
Ethical approval
The case report was approved by the local ethic committee at the university of Tuebingen. IEC-Project-number: 737/2015A.
Consent
Written informed consent was obtained from the patient and is available upon request. No patient identifying material was used in this manuscript.
Author contributions
Data collection: Rami Archid, Carl Christoph Schneider, Patrick Adam, Ahmed Othman, Derek Zieker; data analysis: Rami Archid, Patrick Adam, Ahmed Othman, Alfred Königsrainer; writing and revising of the final version of the manuscript: Rami Archid, Carl Christoph Schneider, Patrick Adam, Ahmed Othman, Derek Zieker, Alfred Königsrainer.
Guarantor
MD Rami Archid, University Hospital Tuebingen, Germany, Hoppe-Seyler-Str. 3, 72076-Tuebingen, Germany.
Acknowledgments
The work has been reported in line with the CARE criteria.
References
- 1.Stout A.P., Murray M.R. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann. Surg. 1942;116(1):26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enzinger F.M., Smith B.H. Hemangiopericytoma. an analysis of 106 cases. Hum. Pathol. 1976;7(1):61–82. doi: 10.1016/s0046-8177(76)80006-8. [DOI] [PubMed] [Google Scholar]
- 3.Stout A.P. Hemangiopericytoma; a study of 25 cases. Cancer. 1949;2(6):1027–1054. doi: 10.1002/1097-0142(194911)2:6<1027::aid-cncr2820020609>3.0.co;2-r. illust. [DOI] [PubMed] [Google Scholar]
- 4.Dardick I., Hammar S.P., Scheithauer B.W. Ultrastructural spectrum of hemangiopericytoma: a comparative study of fetal, adult, and neoplastic pericytes. Ultrastruct. Pathol. 1989;13(2–3):111–154. doi: 10.3109/01913128909057440. [DOI] [PubMed] [Google Scholar]
- 5.Battifora H. Hemangiopericytoma: ultrastructural study of five cases. Cancer. 1973;31(6):1418–1432. doi: 10.1002/1097-0142(197306)31:6<1418::aid-cncr2820310618>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Schurch W., Skalli O., Lagace R., Seemayer T.A., Gabbiani G. Intermediate filament proteins and actin isoforms as markers for soft-tissue tumor differentiation and origin.III. Hemangiopericytomas and glomus tumors. Am. J. Pathol. 1990;136(4):771–786. [PMC free article] [PubMed] [Google Scholar]
- 7.Porter P.L., Bigler S.A., McNutt M., Gown A.M. The immunophenotype of hemangiopericytomas and glomus tumors, with special reference to muscle protein expression: an immunohistochemical study and review of the literature. Mod. Pathol. 1991;4(1):46–52. [PubMed] [Google Scholar]
- 8.Gengler C., Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48(1):63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 9.Spitz F.R., Bouvet M., Pisters P.W., Pollock R.E., Feig B.W. Hemangiopericytoma: a 20-year single-institution experience. Ann. Surg. Oncol. 1998;5(4):350–355. doi: 10.1007/BF02303499. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko K., Shirai Y., Wakai T., Hasegawa G., Kaneko I., Hatakeyama K. Hemangiopericytoma arising in the greater omentum: report of a case. Surg. Today. 2003;33(9):722–724. doi: 10.1007/s00595-003-2559-6. [DOI] [PubMed] [Google Scholar]
- 11.Espat N.J., Lewis J.J., Leung D., Woodruff J.M., Antonescu C.R., Shia J. Conventional hemangiopericytoma: modern analysis of outcome. Cancer. 2002;95(8):1746–1751. doi: 10.1002/cncr.10867. [DOI] [PubMed] [Google Scholar]
- 12.Galanis E., Buckner J.C., Scheithauer B.W., Kimmel D.W., Schomberg P.J., Piepgras D.G. Management of recurrent meningeal hemangiopericytoma. Cancer. 1998;82(10):1915–1920. [PubMed] [Google Scholar]
- 13.Beadle G.F., Hillcoat B.L. Treatment of advanced malignant hemangiopericytoma with combination adriamycin and DTIC: a report of four cases. J. Surg. Oncol. 1983;22(3):167–170. doi: 10.1002/jso.2930220306. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain M.C., Glantz M.J. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery. 2008;63(4):720–726. doi: 10.1227/01.NEU.0000325494.69836.51. [DOI] [PubMed] [Google Scholar]
- 15.Park M.S., Araujo D.M. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr. Opin. Oncol. 2009;21(4):327–331. doi: 10.1097/CCO.0b013e32832c9532. [DOI] [PubMed] [Google Scholar]


