Abstract
Purpose
Besides primary neurotoxicity, oxidative stress may compromise the glial immune regulation and shift the immune homeostasis toward neurodegenerative inflammation in glaucoma. We tested this hypothesis through the analysis of neuroinflammatory and neurodegenerative outcomes in mouse glaucoma using two experimental paradigms of decreased or increased oxidative stress.
Methods
The first experimental paradigm tested the effects of Tempol, a multifunctional antioxidant, given through osmotic mini-pumps for drug delivery by constant infusion. Following a 6-week treatment period after microbead/viscoelastic injection-induced ocular hypertension, retina and optic nerve samples were analyzed for markers of oxidative stress and cytokine profiles using specific bioassays. We also analyzed a redox-sensitive transcriptional regulator of neuroinflammation, namely NF-κB. The second paradigm included a similar analysis of the effects of overloaded oxidative stress on retina and optic nerve inflammation in mice knockout for a major antioxidant enzyme (SOD1−/−).
Results
Increased antioxidant capacity and decreased protein carbonyls and HNE adducts with Tempol treatment verified the drug delivery and biological function. Among a range of cytokines measured, proinflammatory cytokines, including IL-1, IL-2, IFN-γ, and TNF-α, exhibited more than 2-fold decreased titers in Tempol-treated ocular hypertensive eyes. Antioxidant treatment also resulted in a prominent decrease in NF-κB activation in the ocular hypertensive retina and optic nerve. Although pharmacological treatment limiting the oxidative stress resulted in decreased neuroinflammation, ocular hypertension–induced neuroinflammatory responses were increased in SOD1−/− mice with defective antioxidant response.
Conclusions
These findings support the oxidative stress–related mechanisms of neuroinflammation and the potential of antioxidant treatment as an immunomodulation strategy for neuroprotection in glaucoma.
Keywords: glaucoma, immunomodulation, neurodegeneration, neuroinflammation, neuroprotection, oxidative stress
Glaucomatous neurodegeneration is characterized by progressive loss of retinal ganglion cell (RGC) somas and dendrites in the retina, axons along the optic nerve, and synapses in the brain. This blinding neurodegenerative disease is commonly viewed having a multifactorial origin that includes IOP-generated mechanical and/or vascular stress,1,2 aging,3 oxidative stress,4 and genetic5/epigenetic6 risk factors. It is increasingly evident that besides primary harmful signals, secondary processes like glial dysfunction7–9 and glia-driven neuroinflammation7,9–11 may also affect the stressor-threshold determining the susceptibility of neurons for injury. Because glaucoma may progress despite IOP-lowering treatments, ongoing research aims to uncover the molecular mechanisms of neurodegeneration to thereby develop new treatment strategies for neuroprotection. With respect to growing evidence for inflammatory mechanisms in glaucoma pathogenesis,7,9–11 immunomodulatory treatments for efficient control of neuroinflammation may serve as a neuroprotection strategy against secondary injury processes.
Oxidative stress with neuroinflammatory and neurodegenerative outcomes seems to be a promising treatment target to provide immunomodulation and neuroprotection in glaucoma. Oxidative stress that increases with age appears to be an important component of the cellular processes contributing to disease progression in glaucoma (also more common in the elderly).4,12–16 Oxidative stress can inflict damage to nucleic acids, proteins, and lipids, and by acting as a second messenger or modulating the protein function by redox modifications, may serve as an early signal triggering the neuron injury.17,18 Many recent studies focusing on the immunogenic aspects of glaucoma have also supported that besides primary neurotoxic outcomes, oxidative stress may induce glial inflammatory activation, compromise immune regulation, and shift the immune homeostasis toward neurodegenerative inflammation.8 Evidently, oxidative stress can activate the receptor-mediated inflammation signaling,19,20 as well as stimulating the antigen presentation21 and complement dysregulation.22 Oxidative stress-induced signaling for neuroinflammation in glaucoma includes the stimulation of a transcriptional program for inflammatory mediators. The “redox-sensitive” master transcriptional regulator of cytokine production, namely nuclear factor-kappa B (NF-κB), is upregulated in human glaucoma23 and animal models.24 Based on the glia-specific proteomics analysis of experimental glaucoma, the major pathways mediating the glia-driven inflammatory responses in glaucoma, such as cytokine signaling,25 toll-like receptor (TLR) signaling,19 and inflammasome,23 are commonly linked to this key transcriptional regulator.26
To further determine the importance of oxidative stress in neuroinflammatory and neurodegenerative outcomes of glaucoma, this study used two experimental paradigms for analysis of the effects of decreased or increased oxidative stress in mice. In the first paradigm, we tested the effects of a pharmacological antioxidant treatment on retina and optic nerve inflammation in C57BL/6J mice with experimentally induced glaucoma. In the second paradigm, we analyzed the effects of overloaded oxidative stress in ocular hypertensive mice in which a major antioxidant enzyme, superoxide dismutase-1 (SOD1), was knocked out (SOD1−/−). Relative to ocular hypertensive controls that received the saline vehicle alone, antioxidant Tempol treatment resulted in decreased neuroinflammation in the ocular hypertensive retina and optic nerve. However, SOD1−/− mice with defective antioxidant capacity exhibited a stronger inflammatory response to ocular hypertension compared with wild-type (WT) ocular hypertensive controls. These findings support the oxidative stress-related mechanisms of neuroinflammation and the potential of antioxidant treatment as an immunomodulation strategy for neuroprotection in glaucoma.
Materials and Methods
Experimental Mouse Model of Glaucoma
Wild-type (C57BL/6J) and SOD1−/− (B6.Cg-Tg(SOD1*G93A)1Gur/J, stock #004435) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Intraocular pressure elevation was induced by anterior chamber microbead injections as originally described by Sappington et al.27 However, we applied a recently described modification,28,29 in which microbead injections are followed by injection of a viscoelastic substance to thereby push the beads into the anterior chamber angle and prevent the bead reflux when the injection cannula is removed. Briefly, a 50-μm glass cannula connected by tubing to a Hamilton syringe (Hamilton Company Reno, NV, USA).30,31 was first filled with a viscoelastic solution (10 mg/mL sodium hyaluronate), then 0.05 μL air, and finally microbeads (3 × 106 beads per μL). We injected 4 μL of the two-bead mixture (equal volumes of 6 μm and 1 μm diameter beads), then 1 μL of viscoelastic, as this protocol provides higher and more consistent IOP elevation and greater axon loss.29 The fellow eye was injected with the equivalent volume of sterile physiologic saline. Another control group included uninjected normotensive mice. The mice were maintained in a 12-hour light/dark cycle. Intraocular pressure was measured immediately before and after injection, and twice weekly thereafter using a TonoLab rebound tonometer (TioLat, Helsinki, Finland).30,31 The IOP-time integral was used to determine the cumulative IOP exposure for each mouse by calculating the area under the pressure-time curve in the ocular hypertensive eye, then subtracting this IOP-time integral from that in the normotensive fellow eye (expressed in units of mm Hg-days). To minimize the influence of IOP variability among animals, all data were analyzed in mouse eyes matched for the cumulative IOP exposure between 200 and 400 mm Hg-days (corresponds to up to 50% neuron loss). Ocular hypertension was induced in C57BL/6J and SOD1−/− mice after 5 months of age, and the animal age was less than 32 weeks at the end of the experimental period.
All animals were handled according to the regulations of the Institutional Animal Care and Use Committee, and all procedures adhered to the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Drug Administration
Mice with unilaterally induced IOP elevation received antioxidant treatment, or vehicle (saline) alone, over a period of 6 weeks. Tempol (4-hydroxytetramethylpiperidine-1-oxyl; Enzo Life Sciences, Farmingdale, NY, USA), a superoxide dismutase mimetic and peroxynitrite-derived free radical scavenger that also reduces the formation of hydroxyl radicals was used for antioxidant treatment. This multifunctional antioxidant is blood-brain barrier permeable and can readily access the intracellular compartment. Tempol (200 mg/kg/d) was given by subcutaneously implanted osmotic mini-pumps (Model 2006; Alzet, Cupertino, CA, USA) for drug delivery by constant infusion. The equivalent volume of the vehicle was similarly given in the control group. The mini-pumps that were used to deliver solution at a rate of 0.15 μL/h and provide constant infusion for 6 weeks. The treatment dose was chosen based on previously published studies of other disease models,32–34 our earlier in vitro study of RGCs,35 and in vivo dose/response experiments to provide optimum antioxidant capacity but avoid toxicity. The selected dose is far lower than the toxic or lethal doses of Tempol (LD50 = ∼2 mmol/kg given by intravenous or intraperitoneal injection36). All animals well tolerated the antioxidant treatment with no noticeable adverse effects on systemic health status. Osmotic mini-pumps were implanted at the time of microbead injection. Before implantation, pumps were primed in sterile saline at 37°C. We measured the residual volume in the pumps explanted at the end of the treatment period.
Enzyme-Linked Immunoassay (ELISA)
The protein lysates that were obtained from retina and optic nerve samples (including the optic nerve head and a 2-mm segment of the optic nerve proximal to the globe) were quantitatively analyzed for carbonyl and 4-hydroxynonenal (HNE) levels using specific competitive ELISA kits (OxiSelect Protein Carbonyl and HNE-His Adduct ELISA kits; Cell Biolabs, San Diego, CA, USA), as we previously described.21 For the assay of protein carbonyls, protein samples adsorbed to wells of a 96-well plate react with 2,4-dinitrophenylhydrazine (DNPH) in similar technical principal we previously used for analysis of retinal protein oxidation.37 For HNE assay, HNE-protein adducts present in the sample are probed with an anti-HNE-His antibody, followed by an horseradish peroxidase–conjugated secondary antibody. A multiplexed assay kit similarly based on a standard ELISA protocol (Qiagen, Valencia, CA, USA) was used to analyze retina cytokine profiles. These Multi-Analyte ELISArray Kits allow simultaneous profiling of 12 proteins (cytokines and chemokines). A specific ELISA kit was also used to analyze the DNA binding activity of NF-κB (Abcam, Cambridge, MA, USA). Within this assay kit, a specific double-stranded DNA sequence containing the NF-κB response element is immobilized onto the wells of a 96-well plate, and binding of the NF-κB in nuclear extracts can be detected by specific p65 antibody labeling. Nuclear proteins were extracted with a specific buffer containing protease and phosphatase inhibitors (Abcam). Detection sensitivity for the kits were low pg/mL to less than 10 μg/mL. All analyses included triplicated wells and negative controls. We used the positive and nonspecific binding controls provided by the kits. All concentrations were calculated from a standard curve and normalized to protein concentration.
Quantitative Western Blot Analysis
Retina and proximal optic nerve protein samples were analyzed by quantitative Western blotting that followed a methodology similar to previously described.19,22–24 Briefly, protein samples were separated on denaturing polyacrylamide gels (Bio-Rad, Hercules, CA, USA) and transferred to PVDF membranes (Bio-Rad). Following a blocking step, membranes were probed with a phosphorylation site–specific primary antibody to NF-κB subunit, p65 (phospho-Ser536) (1:500; Abcam). After a second blocking step, membranes were incubated with a secondary antibody conjugated with horseradish peroxidase (1:2000; Sigma-Aldrich Corp., St. Louis, MO, USA). Immunoreactive bands were visualized by enhanced chemiluminescence using commercial reagents (GE Healthcare, Pittsburgh, PA, USA). A beta-actin antibody (1:1000; Sigma-Aldrich Corp.) was used to reprobe the stripped immunoblots for loading and transfer control.
Immunohistochemical Analysis
Histological sections of the mouse retina were analyzed after specific immunofluorescence labeling with a monoclonal antibody to TNF-α (1:500; Abcam), as previously described.19,22–24 In addition, specific antibodies against glial fibrillary acidic protein (GFAP) or Iba1 (1:500; Santa Cruz, CA, USA) were used to identify astroglia and microglia, respectively. A mixture of Alexa Fluor 488- or 568-conjugated IgGs (1:500; Thermo Fisher Scientific, Waltham, MA, USA) was used for the secondary antibody incubation. The 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Thermo Fisher Scientific) was used for nuclear counterstaining. Slides were examined by fluorescence microscopy and images were recorded by digital photomicrography (Carl Zeiss, Thornwood, NY, USA). Negative controls were performed by replacing the primary antibody with serum or using an inappropriate secondary antibody to determine species specificity.
Neuron Counting
1 μm-thick plastic cross-sections of the optic nerve (more distal to the segment sampled for protein analysis) were used for imaging-based axon quantification in a masked fashion as previously described.24,37–39 Briefly, the optic nerves excised from enucleated eye balls were fixed and then embedded in epoxy resin. Toluidine blue–stained sections were imaged in their entirety as nonoverlapping tile images, and the captured images were analyzed for axon counts using the Zeiss/ZEN imaging software (Carl Zeiss). This methodology allows axon counts representing the entire surface area of optic nerve cross-sections. Nerve outlines were manually traced on mosaics of images, and the size and shape parameters were determined to exclude intervening glia, myelin debris, and highly degenerated axons to ensure accurate counts. After image processing and axon counting, the axon loss was expressed as a ratio of axon counts in ocular hypertensive to fellow control eye. Retinal ganglion cell counting followed a similar imaging-based methodology representing the entire whole-mounted area. Whole-mounted retinas were immunolabeled for β-III-tubulin, a neuronal lineage marker that preferentially stains tubulin-rich RGCs40–42 (1:1000; Abcam). Retinas were also double immunolabeled with an antibody to syntaxin, a marker for amacrine cells (1:1000; Abcam). After secondary antibody incubation, images were obtained at the focal plane of the RGC layer in whole-mounts, and these images were used to count β-III-tubulin+/syntaxin– neurons in a masked fashion. Most of the syntaxin+ amacrine cells that exhibited only weak to moderate labeling for β-III-tubulin were lying the outside of the immediate focal plane and were not subject to the counting process. An additional criterion for counting RGCs included a minimal somal size of 10 μm to also eliminate dying or phagocytized RGCs. Retinal ganglion cell loss was expressed as a ratio of counts in ocular hypertensive to fellow control eye.
Results
Treatment Effects on Oxidative Stress in the Ocular Hypertensive Retina and Optic Nerve
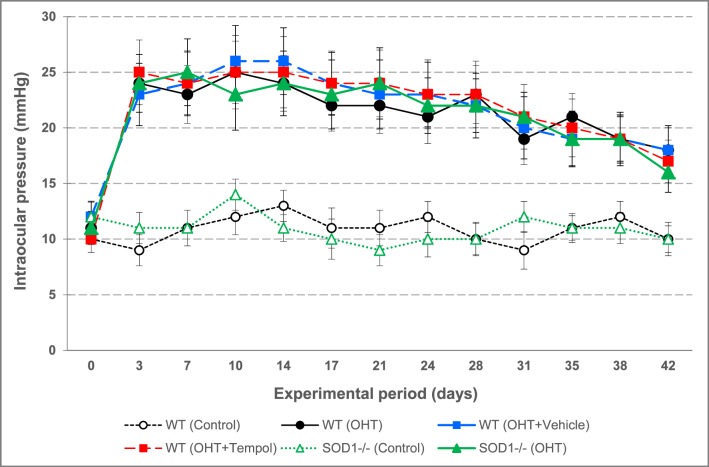
Antioxidant treatment with Tempol was started at the time of anterior chamber microbead/viscoelastic injections to induce IOP elevation in C57BL/6J mice. Another group of ocular hypertensive C57BL/6J mice received the equivalent volume of the vehicle (saline) alone. Additional groups included SOD1−/− and WT (C57BL/6J) mice with similarly induced ocular hypertension. As shown in Figure 1, Tempol treatment, or SOD1−/−, did not affect the level of IOP increase or the duration of ocular hypertension over an experimental period of 6 weeks (Mann-Whitney U test, P > 0.05).
Figure 1.
Assessment of the experimentally induced IOP elevation in mice. Shown are the IOP curves during an experimental period of 6 weeks. Control groups of C57BL/6J WT or SOD1−/− mice that received physiologic saline injection into the anterior chamber had a steady level of IOP that was maintained at an average value of 10.8 ± 1.2 mm Hg through the experimental period. However, the microbead/viscoelastic-injected mice, including both WT and SOD1−/− animals, exhibited a transient course of ocular hypertension. Also shown are the treatment groups including the WT ocular hypertensive mice that received either antioxidant Tempol or the saline vehicle alone (n = 12/group). Data are presented as mean ± SD.
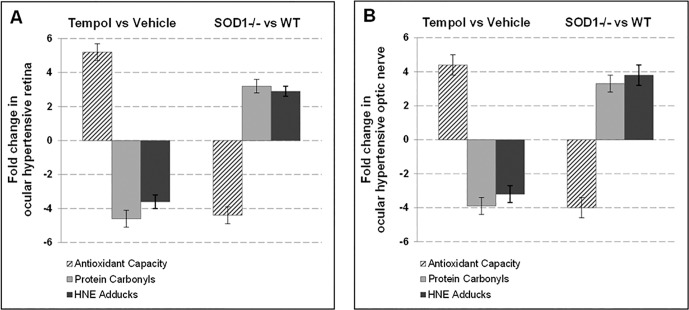
At the end of the 6-week treatment period, we measured the antioxidant capacity in retina and optic nerve samples by a specific assay and detected a significant increase in Tempol-treated ocular hypertensive samples relative to ocular hypertensive controls that were given the vehicle alone (P < 0.01). Parallel to the increased antioxidant capacity, protein carbonyls and HNE adducts exhibited a significant decrease in ocular hypertensive samples treated with Tempol (P < 0.01). Figure 2 presents our data (mean ± SD), expressed as fold-change with antioxidant treatment in ocular hypertensive mice relative to ocular hypertensive mice that received only the vehicle. These observations verified drug delivery and biological function. In contrast, ocular hypertensive retina and optic nerve exhibited decreased antioxidant capacity and increased protein oxidation in SOD1−/− mice relative to WT ocular hypertensive controls (also shown in Fig. 2; P < 0.01). Presented data represent at least six mice for each group.
Figure 2.
Assessment of treatment effects on oxidative stress. The mouse retina (A) and optic nerve (B) protein samples were analyzed for antioxidant capacity, protein carbonyls, and HNE adducts by specific assays. Presented is the fold-change (mean ± SD) in Tempol-treated versus vehicle-treated groups, or SOD1−/− versus WT controls (C57BL/6J). The antioxidant capacity was increased and the oxidative stress end products were decreased after Tempol treatment of ocular hypertensive mice relative to ocular hypertensive controls that received only the vehicle (Mann-Whitney U test; P < 0.01). In contrast, ocular hypertensive SOD1−/− mice exhibited decreased antioxidant capacity and increased protein oxidation compared with ocular hypertensive WT controls (P < 0.01). Data represent at least six mice for each group.
We next tested the neuroinflammatory and neurodegenerative outcomes of mouse glaucoma in the study groups with decreased or increased oxidative stress. Overall, our data indicated an opposing trend of responses. As detailed below, Tempol treatment limiting the oxidative stress resulted in a prominent decrease in neuroinflammation and neurodegeneration in ocular hypertensive mice (relative to ocular hypertensive mice that received the saline vehicle alone). However, ocular hypertension–induced neuroinflammatory and neurodegenerative responses were increased in SOD1−/− mice with defective antioxidant response (relative to WT ocular hypertensive controls). It should be clarified that although fold alterations are presented in the same graphs, Tempol-treated or SOD1−/− groups represent different experimental paradigms, having their specific controls (vehicle-treated, or WT, ocular hypertensive groups, respectively). Comparison of the magnitude of effects between Tempol treatment or SOD1−/− groups would not be suitable, because Tempol is a broad-spectrum antioxidant with dose-dependent outcomes, whereas the mice knockout for a specific antioxidant enzyme (SOD1−/−) may potentially exhibit compensatory changes of other superoxide dismutases or other antioxidant enzymes.
Treatment Effects on Neuroinflammatory Responses to Ocular Hypertension
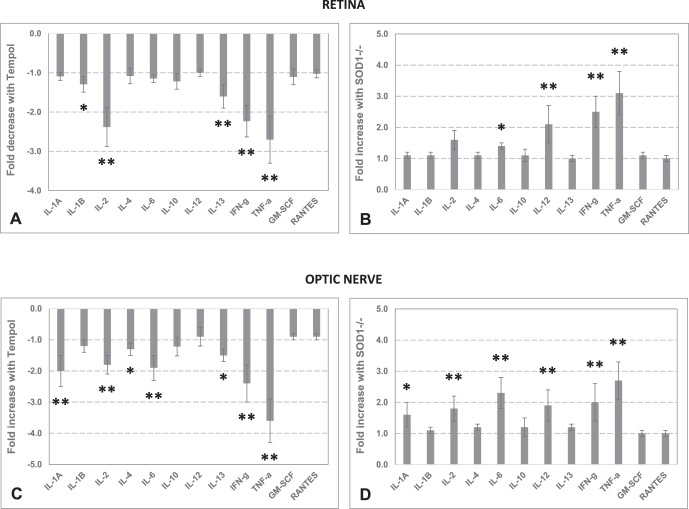
To determine the inflammatory status of the retina and optic nerve, we measured the cytokine/chemokine titers. Among a range of cytokines measured in retina samples, proinflammatory cytokines, IL-1B (Mann-Whitney U test, P = 0.03), IL-2 (P = 0.005), IFN-γ (P = 0.005), and TNF-α (P = 0.006), exhibited over 2-fold decreased titers in Tempol-treated ocular hypertensive samples compared with the ocular hypertensive controls given the vehicle alone. Optic nerve samples from Tempol-treated samples presented a similar decrease in proinflammatory cytokine production. The optic nerve cytokines exhibiting over 2-fold decreased titers with Tempol treatment, relative to the vehicle group, included IL-1A (P = 0.03), IL-2 (P = 0.005), IFN-γ (P = 0.005), and TNF-α (P = 0.005). In addition, some cytokines that may act as either proinflammatory or anti-inflammatory, including IL-13 (P = 0.006) in the retina, and IL-4 (P = 0.01), IL-6 (P = 0.006), and IL-13 (P = 0.03) in the optic nerve, exhibited decreased titers with Tempol treatment.
In contrast to a prominent decrease in cytokine response to ocular hypertension with antioxidant treatment, the retina and optic nerve samples collected from ocular hypertensive SOD1−/− mice exhibited a significant increase in cytokine production. Compared with ocular hypertensive WT controls, ocular hypertensive SOD1−/− retina exhibited an over 2-fold increase in IL-6 (P = 0.03), IL-12 (P = 0.005), IFN-γ (P = 0.005), and TNF-α (P = 0.009). The most significantly increased cytokines in the optic nerve samples from ocular hypertensive SOD1−/− mice included IL-1A (P = 0.03), IL-2 (P = 0.005), IL-6 (P = 0.005), IL-12 (P = 0.005), IFN-γ (P = 0.005), and TNF-α (P = 0.006). Bar graphs in Figure 3 show the fold-change (mean ± SD) in ocular hypertension–induced cytokine production in the retina or optic nerve with antioxidant treatment (relative to vehicle) or SOD1−/− (relative to WT).
Figure 3.
Retina and optic nerve inflammation in ocular hypertensive mice. To determine the inflammatory status of the retina and optic nerve, cytokine titers were analyzed by ELISA. The bar graphs show the fold-change (mean ± SD) in ocular hypertension–induced cytokine production. (A) Fold decrease in retina cytokine titers in Tempol-treated ocular hypertensive mice compared with ocular hypertensive controls that received only the saline vehicle. (B) Fold increase in retina cytokine titers in SOD1−/− mice with ocular hypertension compared with WT (C57BL/6J) ocular hypertensive mice. (C) Fold decrease in optic nerve cytokine titers in Tempol-treated versus vehicle groups of ocular hypertensive mice. (D) Fold increase in optic nerve cytokine titers in SOD1−/− versus WT ocular hypertensive mice. Presented data represent at least six mice per group. *P < 0.05; **P < 0.01 (Mann-Whitney U test).
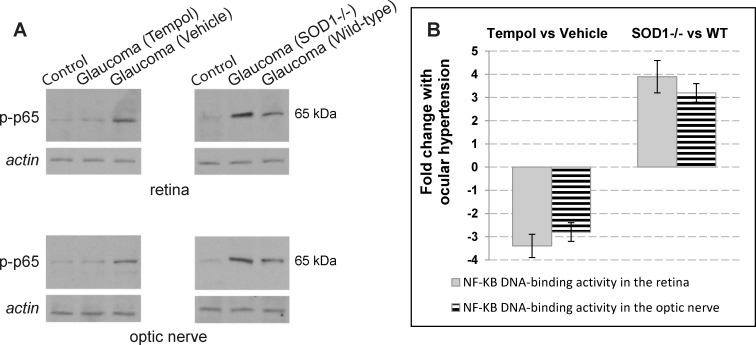
Antioxidant treatment also resulted in a prominent decrease in ocular hypertension-induced activation of NF-κB, a redox-sensitive master regulator of inflammatory mediators. Based on Western blot analysis using a phosphorylation site-specific antibody to NF-κB subunit (p65), and the NF-κB DNA binding activity assay, Tempol-treated samples of the ocular hypertensive retina and optic nerve exhibited over 3-fold decreased activation of NF-κB (relative to ocular hypertensive controls that received the vehicle alone). However, NF-κB activation was increased 3-fold in ocular hypertensive SOD1−/− mice relative to ocular hypertensive WT controls (Fig. 4). Presented data from protein analysis represent at least six mice per group.
Figure 4.
Activity of NF-KB, a transcriptional regulator of inflammation, in experimental mouse glaucoma. (A) For Western blot analysis of protein expression, retina and optic nerve protein samples were probed with a phosphorylation site-specific antibody to NF-κB subunit, p65 (p-p65). This analysis indicated that Tempol treatment of the ocular hypertensive mice (relative to ocular hypertensive controls that received the saline vehicle alone) resulted in a prominent decrease in p-p65 expression. However, SOD1−/− mice (relative to C57BL/6J WT controls) exhibited a prominent increase in ocular hypertension–induced p-p65 expression. (B) Nuclear factor–κB DNA binding activity assay similarly indicated over 3-fold decreased activity of NF-κB in Tempol-treated ocular hypertensive samples relative to ocular hypertensive controls that received only the vehicle (mean ± SD). However, NF-κB activity was significantly increased in ocular hypertensive SOD1−/− mice relative to ocular hypertensive WT controls. Presented data represent at least six mice per group.
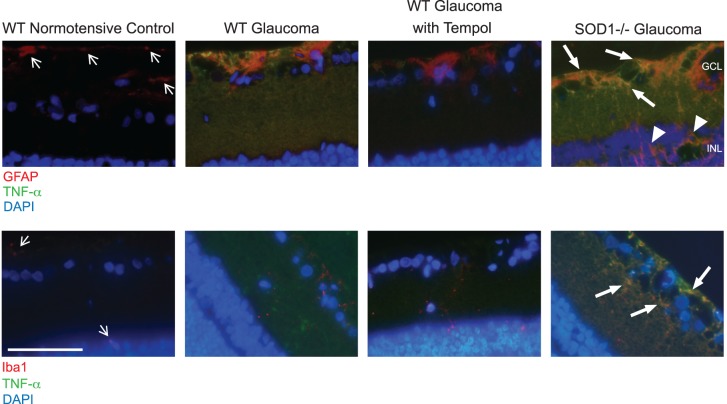
We also analyzed the extent and cellular localization of cytokine production by immunolabeling of the retina sections for an important proinflammatory cytokine, TNF-α. We detected increased immunolabeling of the ocular hypertensive retina for TNF-α that was localized to GFAP+ astroglia and Iba1+ microglia. The ocular hypertension-induced increase in glial TNF-α immunolabeling was prominently decreased after Tempol treatment compared with ocular hypertensive controls that received only the vehicle. In contrast, glial immunolabeling for TNF-α was greater in the ocular hypertensive SOD1−/− retina than WT ocular hypertensive control (Fig. 5). Data from immunohistochemical analysis represent three different samples per group.
Figure 5.
Immunohistochemical analysis of glial cytokine production in the ocular hypertensive mouse retina. Specific antibodies to astroglia (GFAP) or microglia (Iba1) markers were used to determine the retinal localization of TNF-α, an important proinflammatory cytokine. Presented include retina images from C57BL/6J WT mice with or without induced ocular hypertension, WT ocular hypertensive mice treated with Tempol, and SOD1−/− mice with induced ocular hypertension. Compared with the normotensive retina (thin arrows), hypertrophic astrocytes in the ocular hypertensive retina exhibited increased immunolabeling for GFAP (red). As seen in the image from SOD1−/− mice, the ocular hypertension–induced glial response also included GFAP immunolabeling of the Müller glia located in the inner nuclear layer (arrowheads). Besides astroglia, the glial activation response to ocular hypertension included the microglia. The weakly labeled microglia for Iba1 in the normotensive control retina (thin arrows) were mainly localized around the blood vessels in the ganglion cell (GCL) or inner nuclear (INL) layers. However, ocular hypertensive retinas exhibited increased number and Iba1 immunolabeling (red) of the microglia that were distributed throughout the inner retina. Tumor necrosis factor-α immunolabeling was not detectable in the normotensive retina; however, ocular hypertensive retinas were prominently immunolabeled for this proinflammatory cytokine (green), which was remarkably decreased after Tempol treatment. The glia in the ocular hypertensive SOD1−/− mice presented the most prominent immunolabeling for TNF-α (thick arrows). The increased TNF-α immunolabeling in the glaucomatous retina was localized to GFAP+ astroglia and Iba1+ microglia. Blue indicates nuclear DAPI staining. Data represent three different samples for each group (scale bar: 100 μm).
Treatment Effects on Ocular Hypertension–Induced Neuron Loss
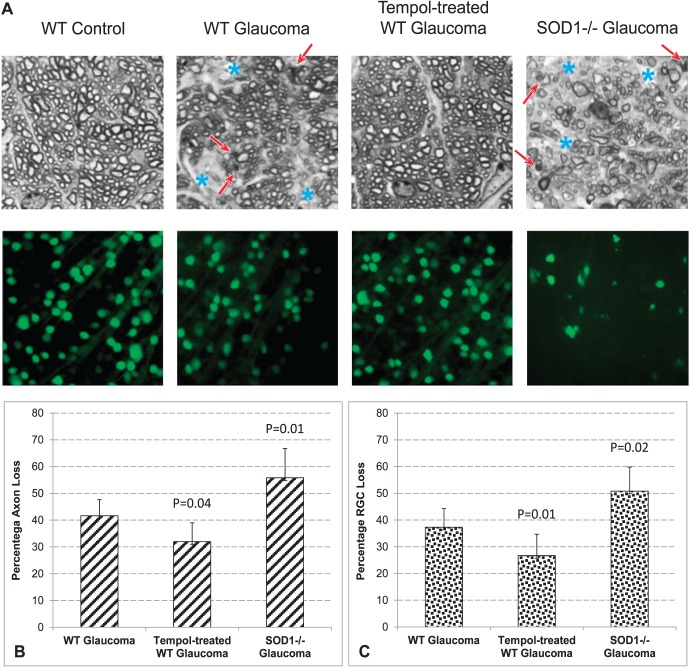
We counted optic nerve axons and RGCs in Tempol-treated versus vehicle-treated groups, and SOD1−/− versus WT groups. As shown in Figure 6, we detected approximately 23% decrease in ocular hypertension–induced axon loss (Mann-Whitney U test, P = 0.04) and 29% decrease in ocular hypertension–induced RGC loss (P = 0.01) in Tempol-treated animals relative to ocular hypertensive controls that received the vehicle alone. However, in ocular hypertensive SOD1−/− mice, axon loss increased by 34% (P = 0.008) and RGC loss increased by 36% compared with ocular hypertensive WT controls (P = 0.02). Presented data (mean ± SD) represent 12 mice per group for axon counts and at least 3 mice per group for RGC counts.
Figure 6.
Assessment of treatment effects on neuron injury. (A) Analysis of optic nerve cross-sections and whole-mounted retinas indicated prominent injury to RGC somas and axons with experimentally induced glaucoma in mice. Compared with C57BL/6J WT mice, ocular hypertension induced greater injury to neurons in SOD1−/− mice. The red arrow indicates degenerating axons and myelin debris; blue asterisk indicates the areas of prominent axon loss. Optic nerve axons were counted in cross-sections, and RGCs were counted in whole-mounted retinas after β-III-tubulin immunolabeling. To estimate the neuron loss in each mouse, axon and RGC counts in the ocular hypertensive eye were adjusted to the normotensive fellow eye. Bar graphs in (B) and (C) indicate the percentage of axon or RGC loss in (1) WT ocular hypertensive mice (relative to WT normotensive controls); (2) Tempol-treated WT ocular hypertensive mice (relative to WT ocular hypertensive controls that received only the saline vehicle); and (3) ocular hypertensive SOD1−/− mice (relative to ocular hypertensive WT controls). P values are based on Mann-Whitney U test. Presented data (mean ± SD) represent 12 mice per group for axon counts and at least 3 mice per group for RGC counts.
Discussion
Glaucomatous neurodegeneration has been recognized as having an inflammatory component,7,9 and resident astroglia and microglia in the retina and optic nerve play key roles in the phenotype that drives innate and adaptive immune responses with neurodestructive consequences.7 Based on multiple evidence, oxidative stress may signal for neuroinflammation in human glaucoma and animal models.8 Findings of this study support the oxidative stress–induced mechanisms that contribute to neuroinflammatory and neurodegenerative outcomes of glaucoma. Presented findings also point to the immunomodulatory potential of antioxidant treatment for protecting neurons from inflammatory injury during glaucomatous neurodegeneration.
To determine the importance of oxidative stress for neurodegenerative inflammation in glaucoma, this study analyzed the neuroinflammatory and neurodegenerative outcomes of mouse glaucoma using two experimental paradigms of decreased or increased oxidative stress (by pharmacological antioxidant treatment, or SOD1−/−, respectively). In the first paradigm, C57BL/6J mice with experimentally induced glaucoma received an antioxidant, Tempol. By providing protection against multiple oxidants, including superoxide, peroxynitrite, and hydroxyl radicals, Tempol treatment has the advantage of multitarget combination strategies. Our previous in vitro studies with primary cultures of isolated RGCs have also used Tempol and detected its treatment effect on RGC protection against glaucoma-related stimuli.35 This multifunctional antioxidant has been successfully used in various in vivo models of oxidative stress, prolonged the life span of normal mice,43 provided neuroprotection in brain injury models,44,45 and protected RGCs against optic nerve crush injury46 and light-induced retinal injury in rats.47,48
Evidently, one of the mechanisms by which oxidative stress stimulates neuroinflammation is the redox-sensitive transcriptional activation of inflammatory mediators by glial NF-κB. Opposing its critical roles in regulation of the neuronal survival programs,49 NF-κB activation is known to trigger inflammation and secondary neurodegenerative processes through the transcriptional activation of pro-interleukins that are later processed into active cytokines by the inflammasome.26,50–52 This redox-sensitive transcriptional program is a common regulator of the cytokine signaling,25 TLR signaling,19 and inflammasome23 that comediate the glia-driven proinflammatory processes during neurodegeneration in human glaucoma and experimental models.23,24 In the present study, we detected that antioxidant treatment of ocular hypertensive mice with Tempol, in comparison with the controls given the vehicle alone, resulted in decreased activation of NF-κB and decreased production of cytokines in the retina and optic nerve. The proinflammatory cytokines exhibiting a significant decrease in Tempol-treated ocular hypertensive tissues included the transcriptional targets of NF-κB, such as TNF-α53,54 and IFN-γ.55,56 Among the proinflammatory cytokines, TNF-α has been the most studied in the field of glaucomatous neurodegeneration.25 Increased glial production of TNF-α in the glaucomatous human retina57 and optic nerve58 has been shown to trigger pro-apoptotic caspase cascade in RGCs25,59,60 and induce neuroinflammatory responses during glaucomatous neurodegeneration.21,23,24 Besides TNF-α and IFN-γ, other proinflammatory cytokines also exhibited significant decrease after Tempol treatment of ocular hypertensive mice, which included IL-1B and IL-2 in the retina, and IL-1A and IL-2 in the optic nerve. In addition, Tempol treatment affected some cytokines that may act either proinflammatory or anti-inflammatory, including IL-13 in the retina, and IL-4, IL-6, and IL-13 in the optic nerve. It is important to note that cytokine/chemokine profiles (and different sets of receptors) determine the status of an inflammatory activation, in which dynamics of anti-inflammatory versus proinflammatory cytokines are critical for the outcome function that spans from tissue cleaning and repair to neurodegenerative inflammation.61 Our findings supporting the oxidative stress–related proinflammatory activation in this study stimulate more focused studies to illuminate the individual contribution (and interrelationship) of different cytokines in inflammatory injury to glaucomatous retina and optic nerve.
Although the pharmacological antioxidant treatment was used to study the effects of decreased oxidative stress in mouse glaucoma, a complementary experimental strategy aimed to model increased oxidative stress. Therefore, we also studied SOD1−/− mice to determine whether overloaded oxidative stress (as evident by aging) promotes neuroinflammation and neurodegeneration in experimental glaucoma. The SOD family is a major antioxidant system, and SOD1 deficiency in mice results in a phenotype that resembles accelerated aging.62 In addition to motor neuron injury,62 SOD1−/− promotes injury of retinal neurons. For example, SOD1−/− mice, compared with WT mice, have showed a greater injury to neurons in oxidative stress–induced retinal degeneration models.63 A progressive degeneration of retinal neurons in these animals has been documented by morphological and physiological criteria.64 Despite normal IOP, SOD1 deficiency has also resulted in decreased RGC counts and decreased PERG amplitude along with the elevated superoxide anions in the RGC layer.65 Regarding glaucoma, this major antioxidant enzyme has been found to be upregulated in ocular hypertensive animal models.24 In the present study of SOD1−/− mice, we detected a stronger inflammatory response to ocular hypertension (relative to WT ocular hypertensive controls) that resulted in increased injury to RGCs and optic nerve axons. Compared with WT mice, neuron counts were lower in SOD1−/− mice before IOP elevation; however, the neuron loss ratio that reflects the injury in the ocular hypertensive eye adjusted to the normotensive fellow eye supported the adverse effect of overloaded oxidative stress on ocular hypertension–induced inflammatory and neurodegenerative outcomes in SOD1−/− mice. Thus, if there is a deficiency in the endogenous antioxidant response, as in SOD1−/− (or if the generated oxidative stress overwhelms the endogenous antioxidant response), proinflammatory activation cannot be repressed and the stimulated inflammation may contribute to neurodegeneration. The increased neuroinflammation and neurodegeneration in SOD1−/− mice with experimental glaucoma support the involvement of SOD1 in oxidative stress–induced damaging outcomes. These observations warrant additional studies to expand the information; however, due to a wide spectrum of injuries detected in SOD1−/− (which may affect both neuronal and non-neuronal tissues), these mice do not present an informative model for further analysis. Likewise, the SOD1−/− group did not receive antioxidant treatment, because treatment of SOD1−/− mice (that may exhibit compensatory changes of other superoxide dismutases or other antioxidant enzymes) with Tempol (that is a multifunctional antioxidant against different reactive oxygen species) would not provide any specific information. We hope that prospective studies using tissue/cell-targeted inducible transgenic lines or locally delivered treatments (with RGC or glia-targeting vectors) should help gain detailed information about the immunomodulatory potential of different antioxidants (and the inflammatory role of different reactive oxygen species) in glaucoma.
The accumulating evidence of oxidative stress in human glaucoma includes a decreased antioxidant potential in aqueous humor66,67 and blood samples of patients with glaucoma.68 The blood samples collected from these patients also exhibit elevated levels of oxidative stress end products.69–72 Furthermore, recent proteome analysis in the glaucomatous human donor retina has indicated oxidative stress–inducing cellular events23 and increased generation of oxidation-related end products.20,22 More recent proteomics analysis of the ocular hypertensive human retina has also pointed to oxidative stress as a molecular risk factor distressing the physiological equilibrium toward glaucoma development.73 Similar to clinical or postmortem studies of human glaucoma, experimental glaucoma models exhibit a prominent oxidative stress in the retina and optic nerve, as evidenced by increased free radical production, decreased antioxidant levels, and accumulation of protein oxidation and lipid peroxidation end products.37,74–76
Given the neurodegenerative and neuroinflammatory consequences of oxidative stress in glaucoma, the oxidative stress–targeting therapeutic approaches seem highly promising to provide immunomodulation and increase neuron survival. Indeed, a number of previous studies have indicated that antioxidant treatment can decrease oxidative stress and improve neuron survival in glaucoma. For example, α-luminol treatment has prevented the age-related decreases in glutamate, glutathione, and glutamine synthetase77; α-lipoic acid treatment has limited the RGC death and dysfunction78; and coenzyme Q10 treatment has inhibited the oxidative stress–mediated mitochondrial alterations79 in DBA/2J mice with hereditary glaucoma. In rat glaucoma, treatment with the Ginkgo biloba extract (a nitric oxide scavenger), or overexpression of thioredoxins, has protected RGCs,80,81 whereas the dietary deficiency of antioxidants predispose to increased RGC loss.82 Regarding human glaucoma, there are similar reports supporting the protective effects of Ginkgo biloba extract in some patients with glaucoma.83,84 A prospective population-based study has also revealed a protective effect of the dietary intake of antioxidant nutrients on human glaucoma.85
Despite the studies focusing on antioxidant treatment effects on neuron survival, only one previous study of glaucoma has examined the antioxidant treatment responses on inflammatory outcomes, which merely focused on the in vitro analysis of pig trabecular meshwork cells. In primary cultures of trabecular meshwork cells subjected to chronic oxidative stress, chronic administration of the dietary supplement of resveratrol has prevented the increased production of reactive oxygen species and resulted in a decrease in inflammatory markers, including IL-1A, IL-6, IL-8, and ELAM-1.86 In addition, inhibition of oxidative stress by coenzyme Q10 has improved the bioenergetic function of cultured optic nerve head astrocytes.87 As far as we are aware, the presented herein is the first study testing the antioxidant treatment effects on inflammatory outcomes of experimental glaucoma. Findings of this study encourage further research to value oxidative stress as an immunomodulatory treatment target to restrain neurodegenerative inflammation, as well as primarily improving the neuron survival. Respecting the widespread aspects of oxidative stress and inflammation through different neuronal compartments in glaucoma, antioxidants can provide a widely useful treatment strategy to protect RGCs and their axons, and also manipulate the inflammatory responses of neighboring glia.
Acknowledgments
Supported in part by the National Eye Institute, Bethesda, MD, USA (1R21EY024105), and Glaucoma Research Foundation, San Francisco, CA, USA. In addition, GT is the recipient of the Homer McK. Rees Scholarship in Glaucoma Research, and an awardee of the Peacock Trusts. Supported in part also by the Research to Prevent Blindness, Inc., New York, NY, USA, by providing an unrestricted grant to the Columbia University, Department of Ophthalmology.
Disclosure: X. Yang, None; G. Hondur, None; G. Tezel, None
References
- 1. Quigley HA. Glaucoma: macrocosm to microcosm the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2005; 46: 2662–2670. [DOI] [PubMed] [Google Scholar]
- 2. Osborne NN,, Melena J,, Chidlow G,, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 2001; 85: 1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quigley HA,, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997; 38: 83–91. [PubMed] [Google Scholar]
- 4. Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006; 25: 490–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libby RT,, Gould DB,, Anderson MG,, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005; 6: 15–44. [DOI] [PubMed] [Google Scholar]
- 6. Nickells RW,, Merbs SL. The potential role of epigenetics in ocular diseases. Arch Ophthalmol. 2012; 130: 508–509. [DOI] [PubMed] [Google Scholar]
- 7. Tezel G. The role of glia mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 1001–1012. [DOI] [PubMed] [Google Scholar]
- 8. Tezel G. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp Eye Res. 2011; 93: 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tezel G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol. 2013; 13: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howell GR,, Soto I,, Zhu X,, et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest. 2012; 122: 1246–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nickells RW,, Howell GR,, Soto I,, John SW. Under pressure: cellular and molecular responses during glaucoma a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012; 35: 153–179. [DOI] [PubMed] [Google Scholar]
- 12. Alvarado J,, Murphy C,, Polansky J,, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981; 21: 714–727. [PubMed] [Google Scholar]
- 13. Levin LA. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv Ophthalmol. 1999; 43: S98–S101. [DOI] [PubMed] [Google Scholar]
- 14. Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008; 173: 339–352. [DOI] [PubMed] [Google Scholar]
- 15. Kong GY,, Van Bergen NJ,, Trounce IA,, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009; 18: 93–100. [DOI] [PubMed] [Google Scholar]
- 16. Lee S,, Van Bergen NJ,, Kong GY,, et al. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2011; 93: 204–212. [DOI] [PubMed] [Google Scholar]
- 17. Scott CJ,, Seidler EA,, Levin LA. Cell-autonomous generation of mitochondrial superoxide is a signal for cell death in differentiated neuronal precursor cells. Brain Res. 2010; 1306: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanamori A,, Catrinescu MM,, Kanamori N,, et al. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010; 133: 2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo C,, Yang X,, Kain AD,, et al. Glaucomatous tissue stress and the regulation of immune response through glial toll-like receptor signaling. Invest Ophthalmol Vis Sci. 2010; 51: 5697–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tezel G,, Luo C,, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007; 48: 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tezel G,, Yang X,, Luo C,, et al. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 2007; 48: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tezel G,, Yang X,, Luo C,, et al. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X,, Luo C,, Cai J,, et al. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci. 2011; 52: 8442–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tezel G,, Yang X,, Luo C,, Cai J,, Powell DW. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 4220–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res. 2008; 173: 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harari OA,, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010; 1207: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sappington RM,, Carlson BJ,, Crish SD,, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010; 51: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cone FE,, Gelman SE,, Son JL,, Pease ME,, Quigley HA. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res. 2010; 91: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cone FE,, Steinhart MR,, Oglesby EN,, et al. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp Eye Res. 2012; 99: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang WH,, Millar JC,, Pang IH,, Wax MB,, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005; 46: 4617–4621. [DOI] [PubMed] [Google Scholar]
- 31. Pease ME,, Cone FE,, Gelman S,, Son JL,, Quigley HA. Calibration of the TonoLab tonometer in mice with spontaneous or experimental glaucoma. Invest Ophthalmol Vis Sci. 2011; 52: 858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawada N,, Imai E,, Karber A,, Welch WJ,, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002; 13: 2860–2868. [DOI] [PubMed] [Google Scholar]
- 33. Dikalova A,, Clempus R,, Lassegue B,, et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005; 112: 2668–2676. [DOI] [PubMed] [Google Scholar]
- 34. Willett NJ,, Kundu K,, Knight SF,, et al. Redox signaling in an in vivo murine model of low magnitude oscillatory wall shear stress. Antioxid Redox Signal. 2011; 15: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tezel G,, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004; 45: 4049–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilcox CS,, Pearlman A. Chemistry and antihypertensive effects of Tempol and other nitroxides. Pharmacol Rev. 2008; 60: 418–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tezel G,, Yang X,, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005; 46: 3177–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang X,, Luo C,, Cai J,, Pierce WM,, Tezel G. Phosphorylation-dependent interaction with 14-3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008; 49: 2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tezel G,, Yang X,, Luo C,, et al. Hemoglobin expression and regulation in glaucoma: insights into retinal ganglion cell oxygenation. Invest Ophthalmol Vis Sci. 2010; 51: 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fournier AE,, McKerracher L. Tubulin expression and axonal transport in injured and regenerating neurons in the adult mammalian central nervous system. Biochem Cell Biol. 1995; 73: 659–664. [DOI] [PubMed] [Google Scholar]
- 41. Sharma RK,, Netland PA. Early born lineage of retinal neurons express class III beta-tubulin isotype. Brain Res. 2007; 1176: 11–17. [DOI] [PubMed] [Google Scholar]
- 42. Chen H,, Wei X,, Cho KS,, et al. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest Ophthalmol Vis Sci. 2011; 52: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilcox CS. Effects of Tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010; 126: 119–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kato N,, Yanaka K,, Hyodo K,, et al. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003; 979: 188–193. [DOI] [PubMed] [Google Scholar]
- 45. Deng-Bryant Y,, Singh IN,, Carrico KM,, Hall ED. Neuroprotective effects of Tempol a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008; 28: 1114–1126. [DOI] [PubMed] [Google Scholar]
- 46. Thaler S,, Fiedorowicz M,, Rejdak R,, et al. Neuroprotective effects of tempol on retinal ganglion cells in a partial optic nerve crush rat model with and without iron load. Exp Eye Res. 2010; 90: 254–260. [DOI] [PubMed] [Google Scholar]
- 47. Wang M,, Lam TT,, Fu J,, Tso MO. TEMPOL, a superoxide dismutase mimic, ameliorates light-induced retinal degeneration. Res Commun Mol Pathol Pharmacol. 1995; 89: 291–305. [PubMed] [Google Scholar]
- 48. Tanito M,, Li F,, Elliott MH,, Dittmar M,, Anderson RE. Protective effect of TEMPOL derivatives against light-induced retinal damage in rats. Invest Ophthalmol Vis Sci. 2007; 48: 1900–1905. [DOI] [PubMed] [Google Scholar]
- 49. Takahashi Y,, Katai N,, Murata T,, Taniguchi SI,, Hayashi T. Development of spontaneous optic neuropathy in NF-kappaBetap50-deficient mice: requirement for NF-kappaBetap50 in ganglion cell survival. Neuropathol Appl Neurobiol. 2007; 33: 692–705. [DOI] [PubMed] [Google Scholar]
- 50. Ogura Y,, Sutterwala FS,, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006; 126: 659–662. [DOI] [PubMed] [Google Scholar]
- 51. Hayden MS,, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008; 132: 344–362. [DOI] [PubMed] [Google Scholar]
- 52. Hanamsagar R,, Hanke ML,, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012; 33: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Collart MA,, Baeuerle P,, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990; 10: 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shakhov AN,, Collart MA,, Vassalli P,, Nedospasov SA,, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990; 171: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sica A,, Tan TH,, Rice N,, et al. The c-rel protooncogene product c-Rel but not NF-kappa B binds to the intronic region of the human interferon-gamma gene at a site related to an interferon-stimulable response element. Proc Natl Acad Sci U S A. 1992; 89: 1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sica A,, Dorman L,, Viggiano V,, et al. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997; 272: 30412–30420. [DOI] [PubMed] [Google Scholar]
- 57. Tezel G,, Li LY,, Patil RV,, Wax MB. Tumor necrosis factor-alpha and its receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001; 42: 1787–1794. [PubMed] [Google Scholar]
- 58. Yan X,, Tezel G,, Wax MB,, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000; 118: 666–673. [DOI] [PubMed] [Google Scholar]
- 59. Tezel G,, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000; 20: 8693–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tezel G,, Yang X,, Yang J,, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004; 996: 202–212. [DOI] [PubMed] [Google Scholar]
- 61. Cherry JD,, Olschowka JA,, O'Banion MK. Neuroinflammation and M2 microglia: the good the bad, and the inflamed. J Neuroinflammation. 2014; 11: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gurney ME,, Pu H,, Chiu AY,, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994; 264: 1772–1775. [DOI] [PubMed] [Google Scholar]
- 63. Dong A,, Shen J,, Krause M,, et al. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006; 208: 516–526. [DOI] [PubMed] [Google Scholar]
- 64. Hashizume K,, Hirasawa M,, Imamura Y,, et al. Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am J Pathol. 2008; 172: 1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yuki K,, Ozawa Y,, Yoshida T,, et al. Retinal ganglion cell loss in superoxide dismutase 1 deficiency. Invest Ophthalmol Vis Sci. 2011; 52: 4143–4150. [DOI] [PubMed] [Google Scholar]
- 66. Ferreira SM,, Lerner SF,, Brunzini R,, Evelson PA,, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004; 137: 62–69. [DOI] [PubMed] [Google Scholar]
- 67. Bagnis A,, Izzotti A,, Centofanti M,, Sacca SC. Aqueous humor oxidative stress proteomic levels in primary open angle glaucoma. Exp Eye Res. 2012; 103: 55–62. [DOI] [PubMed] [Google Scholar]
- 68. Gherghel D,, Mroczkowska S,, Qin L. Reduction in blood glutathione levels occurs similarly in patients with primary-open angle or normal tension glaucoma. Invest Ophthalmol Vis Sci. 2013; 54: 3333–3339. [DOI] [PubMed] [Google Scholar]
- 69. Yildirim O,, Ates NA,, Ercan B,, et al. Role of oxidative stress enzymes in open-angle glaucoma. Eye (Lond). 2005; 19: 580–583. [DOI] [PubMed] [Google Scholar]
- 70. Yuki K,, Murat D,, Kimura I,, Tsubota K. Increased serum total antioxidant status and decreased urinary 8-hydroxy-2′-deoxyguanosine levels in patients with normal-tension glaucoma. Acta Ophthalmol. 2010; 88: e259–e264. [DOI] [PubMed] [Google Scholar]
- 71. Sorkhabi R,, Ghorbanihaghjo A,, Javadzadeh A,, Rashtchizadeh N,, Moharrery M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis. 2011; 17: 41–46. [PMC free article] [PubMed] [Google Scholar]
- 72. Javadiyan S,, Burdon KP,, Whiting MJ,, et al. Elevation of serum asymmetrical and symmetrical dimethylarginine in patients with advanced glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 1923–1927. [DOI] [PubMed] [Google Scholar]
- 73. Yang X. Proteomics analysis of molecular risk factors in the ocular hypertensive human retina. 2015; 56: 5816–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moreno MC,, Campanelli J,, Sande P,, et al. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004; 37: 803–812. [DOI] [PubMed] [Google Scholar]
- 75. Ko ML,, Peng PH,, Ma MC,, Ritch R,, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005; 39: 365–373. [DOI] [PubMed] [Google Scholar]
- 76. Ferreira SM,, Lerner SF,, Brunzini R,, et al. Time course changes of oxidative stress markers in a rat experimental glaucoma model. Invest Ophthalmol Vis Sci. 2010; 51: 4635–4640. [DOI] [PubMed] [Google Scholar]
- 77. Gionfriddo JR,, Freeman KS,, Groth A,, et al. alpha-Luminol prevents decreases in glutamate, glutathione, and glutamine synthetase in the retinas of glaucomatous DBA/2J mice. Vet Ophthalmol. 2009; 12: 325–332. [DOI] [PubMed] [Google Scholar]
- 78. Inman DM,, Lambert WS,, Calkins DJ, Horner PJ. alpha-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One. 2013; 8: e65389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee D,, Shim MS,, Kim KY,, et al. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2014; 55: 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hirooka K,, Tokuda M,, Miyamoto O,, et al. The Ginkgo biloba extract (EGb 761) provides a neuroprotective effect on retinal ganglion cells in a rat model of chronic glaucoma. Curr Eye Res. 2004; 28: 153–157. [DOI] [PubMed] [Google Scholar]
- 81. Caprioli J,, Munemasa Y,, Kwong JM,, Piri N. Overexpression of thioredoxins 1 and 2 increases retinal ganglion cell survival after pharmacologically induced oxidative stress optic nerve transection, and in experimental glaucoma. Trans Am Ophthalmol Soc. 2009; 107: 161–165. [PMC free article] [PubMed] [Google Scholar]
- 82. Ko ML,, Peng PH,, Hsu SY,, Chen CF. Dietary deficiency of vitamin E aggravates retinal ganglion cell death in experimental glaucoma of rats. Curr Eye Res. 2010; 35: 842–849. [DOI] [PubMed] [Google Scholar]
- 83. Dorairaj S,, Ritch R,, Liebmann JM. Visual improvement in a patient taking ginkgo biloba extract: a case study. Explore (NY). 2007; 3: 391–395. [DOI] [PubMed] [Google Scholar]
- 84. Lee J,, Sohn SW,, Kee C. Effect of Ginkgo biloba extract on visual field progression in normal tension glaucoma. J Glaucoma. 2013; 22: 780–784. [DOI] [PubMed] [Google Scholar]
- 85. Ramdas WD,, Wolfs RC,, Kiefte-de Jong JC,, et al. Nutrient intake and risk of open-angle glaucoma: the Rotterdam Study. Eur J Epidemiol. 2012; 27: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Luna C,, Li G,, Liton PB,, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009; 47: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Noh YH,, Kim KY,, Shim MS,, et al. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013; 4: e820. [DOI] [PMC free article] [PubMed] [Google Scholar]