Abstract
Purpose
Human limbal palisade of Vogt is an ideal model for studying and practicing regenerative medicine due to their accessibility. Nonresolving inflammation is a common manifestation of limbal stem cell deficiency, which is the major cause of corneal blindness, and presents as a threat to the success of transplanted limbal epithelial stem cells. Clinical studies have shown that the efficacy of transplantation of limbal epithelial stem cells can be augmented by transplantation of cryopreserved human amniotic membrane (AM), which exerts anti-inflammatory, antiscarring, and antiangiogenic action to promote wound healing.
Methods
Review of published data to determine the molecular action mechanism explaining how AM exerts the aforementioned therapeutic actions.
Results
From the water-soluble extract of cryopreserved AM, we have biochemically purified one novel matrix component termed heavy chain (HC)-hyaluronan (HA)/pentraxin 3 (PTX3) as the key relevant tissue characteristic responsible for the aforementioned AM's efficacy. Heavy chain–HA is a complex formed by a covalent linkage between HA and HC1 of inter-α-trypsin inhibitor (IαI) by tumor necrosis factor-stimulated gene-6 (TSG-6). This complex may then be tightly associated with PTX3 to form HC-HA/PTX3 complex. Besides exerting an anti-inflammatory, antiscarring, and antiangiogenic effects, HC-HA/PTX3 complex also uniquely maintains limbal niche cells to support the quiescence of limbal epithelial stem cells.
Conclusions
We envision that HC-HA/PTX3 purified from AM can be used as a unique substrate to refine ex vivo expansion of limbal epithelial stem cells by maintaining stem cell quiescence, self-renewal and fate decision. Furthermore, it can also be deployed as a platform to launch new therapeutics in regenerative medicine by mitigating nonresolving inflammation and reinforcing the well-being of stem cell niche.
Keywords: anti-inflammation, antiangiogenesis, antiscarring, amniotic membrane, heavy chain, hyaluronan, inter-α-inhibitor, limbus, stem cells, stem cell niche, umbilical cord
Stem cells (SCs) with extensive proliferative potential of giving rise to one or more differentiated cell types are common in early mammalian embryos. By adulthood, such SCs are dispersed and kept in a unique anatomic location (niche) of each self-renewing tissue where they continue to maintain quiescence, while performing remarkable and relentless self-renewal to replenish the SC population lost to progeny production and ensuring proper fate decision. Cumulative evidence reveals that nonresolving inflammation is a common threat of a number of degenerative diseases. It remains elusive how chronic inflammation might pose as a threat to the well-being of SCs. Although SCs hold considerable promise for the treatment of a number of diseases, it is not clear whether SCs can still perform the expected task when transplanted to the tissue that manifests nonresolving inflammation. Inasmuch as we wish to deploy the potential of SC-based therapies in regenerative medicine, we still face the challenge of achieving sufficient numbers of adult tissue-specific SCs via ex vivo expansion while maintaining the stemness. This major obstacle is due to our lack of better understanding of how quiescence, self-renewal, and fate decision of adult somatic SCs are controlled in the in vivo native niche let alone in an in vitro environment. This review appraises the knowledge and experience gathered from the studies of corneal epithelial stem cells at the limbus in the last three decades. By focusing on the close relationship between “inflammation” and “regeneration,” we summarize our research effort in identifying the tissue characteristics relevant to human amniotic membrane (AM) explaining how cryopreserved AM controls inflammation and promotes wound healing. In the end, we also lay down key needs and opportunities that may guide others in identifying better therapeutic strategies in treating corneal blindness caused by limbal SC deficiency in the future.
Unique Limbal Model of Corneal Epithelial Stem Cells and Their Niches
Among all adult epithelial tissues, the model of the corneal epithelium is most unique in having its SCs located at the basal epithelial layer of the limbus (between the cornea and the conjunctiva) in a special anatomic structure termed “palisades of Vogt,” while its transient amplifying cells (TACs; i.e., the immediate progeny of SC) are located in both limbal and corneal basal epithelia.1,2 This location is more accessible than other epithelial tissues, rendering the cornea the prime location for studying the aforementioned questions and for practicing regenerative medicine.
Corneal Diseases With Limbal Stem Cell Deficiency
Full regeneration of the entire corneal epithelium is expected when limbal SCs are intact and healthy. Nevertheless, conjunctival epithelial cells migrate onto the corneal surface when limbal SCs are partially3 or totally4,5 damaged, leading to a pathologic state termed limbal SC deficiency (LSCD). Limbal SC deficiency carries the hallmark of conjunctivalization (i.e., the corneal surface is covered by an ingrowing conjunctival epithelium containing goblet cells as first illustrated by impression cytology).6 The process of conjunctivalization is invariably associated with destruction of the basement membrane, emergence of superficial vascularization, and chronic inflammation and scarring.3,5,7–9 Patients inflicted with LSCD suffer from a severe loss of vision and annoying irritation, and are poor candidates for conventional corneal transplantation. It remains unclear how chronic inflammation perpetuates, if not triggers, LSCD.
Corneal Surface Reconstruction by Limbal Stem Cell Transplantation
As conventional corneal transplantation cannot satisfactorily reconstruct corneal surfaces with extensive LSCD, new surgical strategies have been devised by transplanting limbal SCs from an autologous (autograft) or allogeneic source (allograft; for reviews of different surgical procedures see Refs. 10–12). When total LSCD involves only one eye (unilateral), the damaged corneal surface can be effectively reconstructed by conjunctival limbal autograft.13 To reduce the potential risk to the patient's donor eye, the first option is to perform limbal SC allograft, where an allogeneic (not patient's own) source of limbal SC are derived from either HLA-matched living donors14–16 or nonmatched cadavers.15,17–19 The second option is to perform oral mucosal graft as a limbal surrogate20 or ex vivo expansion of limbal epithelial stem cells,21 especially for eyes where transplantation of allogeneic limbal SCs has failed or is not feasible.22,23 The third option is to perform amniotic membrane transplantation (AMT) as an adjunctive therapy to promote the success of transplanting autologous24 and allogeneic25 limbal SCs for treating total limbal deficiency. In the former case, AMT has allowed the donor site in the fellow eye to be reduced to 60° limbal arc length.24 Recently, Sangwan et al.26 and others27 have devised “simple limbal epithelial transplantation (SLET)” as an alternative to conjunctival limbal autograft and ex vivo expansion of limbal epithelial SCs. Simple limbal epithelial transplantation subdivides the limbal biopsy into tissue fragments as a source of regeneration when placed on the limbal deficient cornea that is covered by AM as a graft without26 or with27 another AM as a bandage. Although the exact action mechanism remains unknown, clinical success indicates that AM helps expand residual or transplanted limbal SCs in vivo.
What Is Amniotic Membrane?
Anatomically, the AM is the innermost membrane enwrapping the fetus in the amniotic cavity and extends from the fetal membrane (i.e., encompassing both the AM and the chorion) to the placental proper and the umbilical cord (UC), of which the latter connects the placenta and the fetus. Histologically, the AM consists of a simple epithelium, a basement membrane, and an avascular stroma. The stromal layer of the AM can be further subdivided into compact, fibroblast, and spongy layers.28 The AM in the UC has a thicker stroma, which is also avascular and primarily composed of a viscous, glycosaminoglycan rich Wharton's Jelly. Physiologically, the integrity of the AM dictates the well-being of the fetus during development. Developmentally, the fertilized egg first forms the blastocyte, which then develops into the inner and outer cell mass, of which the latter further differentiates into the trophoectoderm. Subsequently, the inner cell mass develops into the fetus, the AM and the UC, while the trophoectoderm turns into the chorion and the decidua. Hence, both the AM and the UC share the same cellular origin as the fetus. The AM's barrier function is not only “physical” but also “biological.” During pregnancy, the maternal immune system is challenged by the presence of the fetus, which must be tolerated despite being semiallogeneic. Although one such “biological” barrier function resides at the decidua level where decidual macrophages contribute to fetal tolerance and are involved in several other processes required for a successful pregnancy, it remains unclear whether AM also plays a key role in supporting the homeostatic and tolerant immune milieu required for a successful pregnancy.
Differences in Adult and Fetal Wound Healing
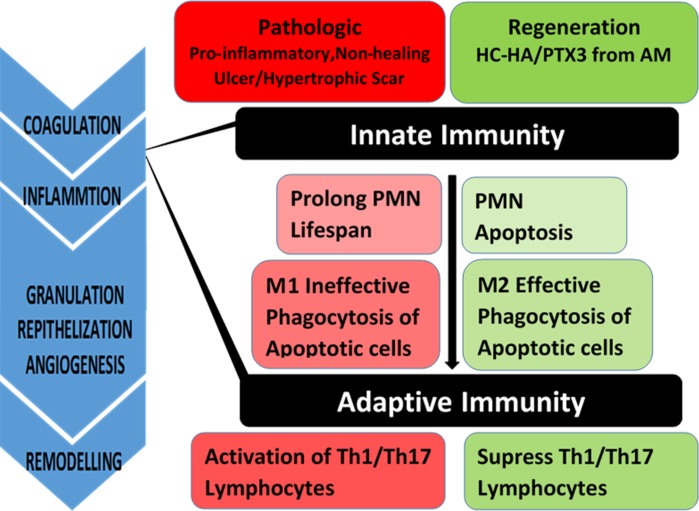
Adult wound healing is heralded by inflammation, which can be subdivided into two major phases that involve cellular infiltration by polymorphonuclear neutrophils (PMNs), macrophages, and lymphocytes derived from innate and adaptive immune responses, respectively (Fig. 1). Polymorphonuclear neutrophils, first arriving at the scene, will eventually undergo apoptosis due to their short life span. These apoptotic neutrophils are removed by M2 macrophages via phagocytosis, resulting in the restoration and maintenance of anti-inflammatory and immune-tolerogenic milieu.29 On the contrary, under pathological states when there is a wider extent of injury/wound and PMN infiltration, which together with a prolonged lifespan result in additional collateral damage. This may then lead to a significant delay of PMN apoptosis or emergence of PMN necrosis, which exacerbates inflammation and activates M1 macrophages that are ineffective in phagocytic clearance of apoptotic neutrophils.30,31 Collectively, these pathological states lead to prolonged inflammation that is the hallmark of a number of diseases.32–35 M1 macrophages are also professed to activate Th1 and Th17 lymphocytes that play a key role in allogeneic rejection and autoimmune dysregulation, respectively.36 (Fig. 1) A lack of transition from M1 to M2 macrophages is a hallmark of nonhealing skin wounds.30,37 A significant increase of epidermal Langerhans cells (i.e., a special type of macrophages),38 the presence of activated T lymphocytes (CD3+, HLA DR', CD25′),39 and a high amount of TGF-β1 during the proliferative phase of wound healing40 are characteristic of hypertrophic scars.
Figure 1.
Nonresolving inflammation is correlated to progression from innate to adaptive immune responses. Under normal circumstances, infiltrating PMNs leads to apoptosis and apoptotic PMNs are cleared by M2 macrophages. Under pathological states, prolonged PMN infiltration delays their apoptosis. This leads to and together with delayed phagocytic clearance of apoptotic PMNs by M1 macrophages activates Th1 or Th17 lymphocytes of the adaptive immune response leading to nonhealing chronic wounds or ulcers. HC-HA/PTX3 purified from AM facilitates PMN apoptosis, polarizes M2 macrophages, and suppresses lymphocyte activation.
As a contrast, the fetal wound healing is characteristically known as “scarless.”41,42 It has been known that following injury to the embryo, the inflammatory response (by virtue of a less than mature immune system) is less marked and differs in terms of the types and number of inflammatory cells that enter the wound43 and diminished IL-6 and IL-8 production.44,45 Besides downregulation of proinflammatory responses, there is downregulation of proscarring response in fetal wound healing.46,47
Search for Relevant Tissue Characteristics in Amniotic Membrane
We thus speculate that AM contributes to the fetal immune-tolerance state and the scarless fetal wound healing during pregnancy by delivering anti-inflammatory and antiscarring action, and to modulate alloreactive immune activation. As a first step to strengthen this hypothesis, it is important to identify the molecule(s) that is responsible for AM's anti-inflammatory action, which has been demonstrated in a number of studies where transplanted cryopreserved AM induces apoptosis of neutrophils,48,49 monocytes, and macrophages50; reduces infiltration of neutrophils,48,49 macrophages,51,52 and lymphocytes53; and promotes polarization of M2 macrophages.54 We first showed that the aforementioned anti-inflammatory action exerted by cryopreserved AM is retained in the water-soluble AM extract (AME) prepared from cryopreserved AM Specifically, we have shown that human AME can induce apoptosis of IFN-γ, lipopolysaccharide (LPS), and IFN-γ/LPS-activated but not resting macrophages.55,56 AME also downregulates expression of M1 macrophage markers such as TNF-α, IL-6, CD86, and MHC II while upregulating M2 macrophage markers such as cytokine IL-10.56
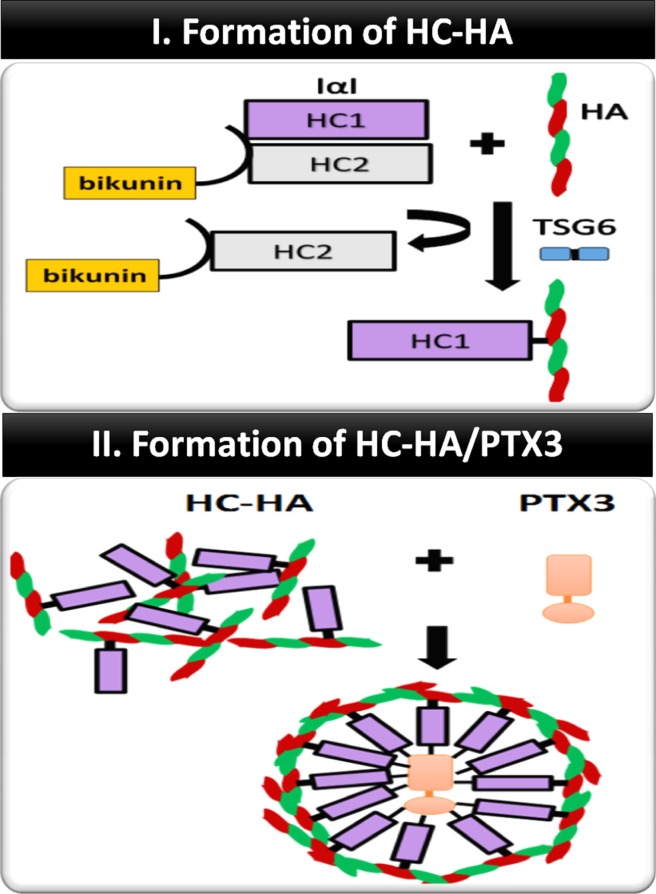
Following the work of identifying the heavy chain (HC)-hyaluronan (HA)/pentraxin 3 (PTX3) complex as the key component in the cumulus-oocyte complex surrounding the ovulated oocyte to ensure fertilization,57,58 our laboratory was the first reporting that the biosynthetic pathway used for ovulation also takes place in the AM. In short, we have purified the HC-HA/PTX3 complex from AME by two successive runs of ultracentrifugation in a CsCl gradient in the presence of 4M guanidine HCl.59,60 The biosynthetic process of HC-HA/PTX3 involves the following two steps (Fig. 2): the first is to from HC-HA complex via tumor necrosis factor-stimulated gene-6 (TSG-6), which is an enzyme that catalyzes the covalent (ester bond) transfer of HCs from inter-α-trypsin inhibitor (IαI) to HA.61–64 IαI contains two HCs (i.e., HC1 and HC2) and a light chain termed bikunin jointed a chondroitin sulfate chain and is present in the blood after being secreted by the liver.65–70 We have demonstrated that the HC-HA/PTX3 complex purified from AM consists of HMW HA (>3000 kDa) covalently linked with HC1 and tightly bound PTX3, but not HC2, bikunin, and TSG-6. Unlike the cumulus-oocyte complex, the source of IαI is endogenously produced by AM epithelial cells and stromal cells but not derived from the liver, and the expression of TSG-6 and PTX3 is constitutive (i.e., without relying on proinflammatory cytokines).60,71 Similar to ovulation,58,72 the second step is to from the HC-HA/PTX complex by tight association of the HC-HA complex with PTX3.
Figure 2.
Formation of HC-HA/PTX3. IαI is composed of two heavy chains (HC1 and HC2) covalently linked to bikunin via a chondroitin sulfate. HCs from IαI are covalently transferred to HMW HA to form HC-HA complex via the catalytic action of TSG-6. PTX3 octamers are tightly associated with the HC-HA complex via binding with HCs.
Anti-Inflammatory Effect of HC-HA/PTX3
As stated above, PMNs are among the first recruited to engulf pathogens and damaged tissues before their eventual apoptosis. Delayed neutrophil apoptosis will lead to chronic inflammation, which is the hallmark of many diseases.34,35 We have reported that water-soluble HC-HA/PTX3, but not HA, significantly promotes apoptosis of freshly-isolated neutrophils after activated by fMLP or LPS but sparing resting neutrophils.73 Similarly, water-soluble HC-HA/PTX3, but not HA, does-dependently promotes apoptosis of LPS-activated, IFN-γ–activated, or IFN-γ/LPS-activated, but not resting macrophages.56,59,73 Clearance of apoptotic neutrophils by M2 macrophages is essential to resolve inflammation.74–76 We noted that both water-soluble and substrate (plastic)-immobilized HC-HA/PTX3, but not HA, promotes phagocytosis of apoptotic neutrophils by resting and LPS-activated macrophages, respectively. Therefore, HC-HA/PTX3 suppresses proinflammatory responses of neutrophils and macrophages involved in innate immune responses (Fig. 1).
Macrophages, besides undergoing classical M1 activation (e.g., by IFN-γ and/or LPS) to express high levels of proinflammatory cytokines (e.g., IL-12, IL-23, and TNF-α) and activate Th1 and Th17 lymphocytes,36 can also be polarized toward M2 activation (e.g., by IL-4/IL-13 or immune complex), which express a low level of IL-12 but a high level of anti-inflammatory IL-10, to activate Treg lymphocytes.77 Polarization of M2 macrophages promotes wound healing and resolves inflammation.78–80 We have recently reported that immobilized HC-HA/PTX3 promotes polarization of LPS- or IFN-γ/LPS-activated macrophages toward M2 phenotype.73,81 These data show that HC-HA/PTX3 can further downregulate the innate immune responses and extends its reach against adaptive immune responses by polarizing M2 macrophages (Fig. 1).
Because HC-HA/PTX3 polarizes M2 macrophages,73 and because macrophages are at the cross-road bridging innate immune responses and adaptive immune responses, we speculate that the anti-inflammatory effect of HC-HA/PTX3 in innate immune responses may also be extended to modulate adaptive immune responses. CD4+ T cells become activated by contacting with antigen presenting cells presenting the peptide antigen through MHC II to proliferate rapidly and differentiate into Th1, Th2, Th17, or Treg.82–85 Th1 cells secrete IFN-γ and IL-2 to enhance proinflammatory responses.86,87 These responses can be downregulated by Tregs, which is activated by M2 macrophages.77 To test the aforementioned hypothesis, we have reported that water-soluble HC-HA/PTX3, but not HA, suppresses activation of CD4+ T cells isolated from murine lymph nodes and spleens via ligation with α-CD3/α-CD28 regarding proliferation and production of Th1 cytokines (IFN-γ, IL-2) and promotes significant expansion of CD25+/FOXP3+ T cells.81 These data indicate that HC-HA/PTX3 also extends its action toward adaptive immune responses by directly suppressing Th1 cells while promoting the expansion of Tregs (Fig. 1).
To demonstrate that the aforementioned anti-inflammatory actions of HC-HA/PTX3 can downregulate both innate and adaptive immune responses in vivo, we performed corneal allograft transplantation in mice treated with HC-HA/PTX3 and assessed the allograft survival. Our results showed that subconjunctival injection of HC-HA/PTX3 dose-dependently prolonged the corneal allograft survival.81 Collectively, the above data shed a new light in how this novel matrix, present from ovulation58,88 to pregnancy,59,60,71,73 might contribute to the development of fetal immune tolerance during pregnancy.
Antiscarring Effect of HC-HA/PTX3
Although anti-inflammatory effects can indirectly lead to antiscarring effects, experimental evidence also shows that the AM stroma has a direct antiscarring effect. Previously, we have reported that expression of TGF-β1 to 3 and TGF-βR2 transcripts (using Northern blot) is downregulated in human corneal fibroblasts and human limbal and conjunctival fibroblasts cultured on the stromal side of cryopreserved AM (CAM).89,90 This direct antiscarring effect also explains why AM implanted into the corneal stromal pocket reduces myofibroblast differentiation elicited by invading epithelial cells in a rabbit model,91 and why corneal haze is reduced in excimer laser-induced keratectomy in rabbits.48,49,92,93 We subsequently reported that water-soluble AME induces cell aggregation and prevents expression of α-smooth muscle actin (α-SMA) by myofibroblasts.94 Human95 and mouse96 keratocytes seeded on the stromal side of cryopreserved AM maintain their normal phenotype without eliciting nuclear translocation of pSmad2/3 even if they were exposed to serum or TGF-β1. Water-soluble HC-HA/PTX3, but not HA, suppresses the TGF-β1 promoter activity of human corneal fibroblasts.59
Antiangiogenic Effect of HC-HA/PTX3
Besides reduction of inflammation and scarring, AM transplanted corneal surfaces also show reduced vascularization.97 This antiangiogenic action has also been exploited during corneal surface reconstruction in conjunction with transplantation of corneal epithelial stem cells from the limbus.25,98,99 Previously, a soluble AM extract prepared by boiling and homogenization was shown to prevent angiogenesis in a rat model of corneal neovascularization induced by alkali burn and by suppressing viability and tube formation of cultured human umbilical vein endothelial cells (HUVEC).100 Besides the aforementioned anti-inflammatory and antiscarring actions, we have also reported that HC-HA/PTX3 suppresses HUVEC viability more significantly than HA and AM stromal extract, and such suppression is not mediated by CD44.101 HC-HA/PTX3 also causes HUVEC to become small and rounded with a decrease in spreading and filamentous actin.101 Without promoting cell detachment or death, HC-HA/PTX3 dose-dependently inhibited proliferation and was 100-fold more potent than HA.101 Migration triggered by VEGF and tube formation were also significantly inhibited by HC-HA/PTX3.101
HC-HA/PTX3 Maintains Limbal Niche Cell Phenotype for Supporting SC Quiescence
Inasmuch as the limbal niche is easy to access and plays an important role, the progress in understanding how it regulates limbal SCs considerably lags behind other SC model systems. We have embarked on this challenge by discovering a new method of isolating entire limbal epithelial SCs together with their niche cells (NCs) from the human limbus based on digestion with collagenase.102 The isolated niche cells (LNCs) are closely associated with limbal basal epithelial progenitor cells (LEPCs), pancytokeratin (PCK) negative but vimentin positive, as small as 5 μm in diameter, and heterogeneously express SC markers such as Oct4, Sox2, Nanog, Rex1, Nestin, N-cadherin, SSEA4, and CD34.102,103 Isolated niche cells can then be effectively expanded on coated Matrigel in modified embryonic stem cell medium (MESCM) up to 12 passages with angiogenesis104 and mesenchymal stem cells105 potentials. Upon being reseeded in three-dimensional (3D) Matrigel, expanded LNCs revert back their phenotype with overexpression of the aforementioned embryonic SC (ESC) markers. Taking advantage of this advance, we have established an in vitro model system in 3D Matrigel, in which reunion between a single LEPC and a single LNC leads to sphere formation through the SDF-1/CXCR4 chemokine axis.106 A close contact with LNCs endows LEPCs with better clonal growth on 3T3 fibroblast feeder layers.102 and prevents LEPCs from adopting the corneal fate decision.103,105 Both bone morphogenic protein (BMP) and Wnt signaling control stem cells in bulge/dermal papilla, intestinal crypt, and bone marrow. Our study showed that balancing acts between Wnt signaling and BMP signaling exist not only within LEPCs but also between LEPCs and LNCs to regulate clonal growth of LEPCs. In 3D Matrigel, the resultant sphere exhibits inhibition of corneal fate decision and marked clonal growth of LEPCs, of which the latter is correlated with activation of canonical Wnt signaling.107 Using immobilized HC-HA/PTX3, we noted that the resultant spheres exhibited similar suppression of the corneal fate decision but upregulation of quiescence markers including nuclear translocation of phosphorylated Bmi-1, and negligible clonal growth of LEPCs.108 This outcome was correlated with the suppression of canonical Wnt but activation of noncanonical (PCP) Wnt signaling as well as BMP signaling in both LEPCs and LNCs. The activation of BMP signaling in LNCs was pivotal because nuclear translocation of pSmad1/5/8 was prohibited in hLEPCs when reunioned with mLNCs of conditionally deleted Bmpr1a;Acvr1 DCKO mice. Furthermore, ablation of BMP signaling in LEPCs led to upregulation of cell cycle genes, downregulation of Bmi-1, nuclear exclusion of phosphorylated Bmi-1, and marked promotion of the clonal growth of LEPCs. Hence, HC-HA/PTX3 uniquely upregulates BMP signaling in LNCs, which leads to BMP signaling in LEPCs to achieve quiescence, helping explain how AMT is clinically useful as a matrix for both in vivo26,27 and ex vivo109 expansion of limbal epithelial stem cells and to treat corneal blindness caused by LSCD.
Key Needs and Opportunities
The aforementioned progresses help us identify the following key needs and opportunities in practicing regenerative medicine in the model of limbal epithelial SCs. Firstly, we should recognize that nonresolving inflammation is a common threat of diverse causes of LSCD leading to corneal blindness and against the success of transplanted limbal epithelial SCs. Hence, it is necessary to understand how inflammation becomes “nonresolving” and what pathological processes can threaten the well-being of limbal epithelial SCs. For the former question, one promising direction is to focus on the polarization of M1 macrophages to M2 macrophages as a pivotal step to abort progression of inflammation. For the latter question, we propose to use the said in vitro reconstituted niche to study how various “inflammatory” insults may alter the function of limbal epithelial SCs. Such research pursuits can be facilitated by judging the maintenance of the normal limbal NC phenotype as a small round shape expressing “ESC markers” and the close SC-NC contact as pre-requisites in maintaining quiescence, self-renewal and corneal fate decision of limbal epithelial SCs. Secondly, by realizing the causative role of nonresolving inflammation in LSCD, we should appreciate the importance of controlling such inflammation in order to promote the function of limbal epithelial SCs toward regeneration. One effective strategy that has been successfully deployed clinically to control inflammation is transplantation of cryopreserved AM. Our cumulative research has identified the HC-HA/PTX3 complex as the key relevant tissue characteristic of the AM to validate its anti-inflammatory and antiscarring clinical efficacies.59,60,73,81,101 Because all conventional anti-inflammatory agents such as glucocorticosteroids, nonsteroid anti-inflammatory agents, cyclosporine/tarcolimus, or various humanized antibodies, target at a specific action of one particular type of inflammatory/immune cells, the anti-inflammatory action of the HC-HA/PTX3 complex stands out as a unique class as it exerts broad anti-inflammatory actions by targeting at PMNs, macrophages, and lymphocytes extending from innate to adaptive immune responses. Thirdly, we have learned that the function of limbal epithelial SCs is dictated by a close interaction with limbal NCs as disclosed in the said in vitro limbal niche reconstituted by reunion between limbal epithelial progenitors and limbal NCs. Hence, such an in vitro reconstituted limbal niche is an important approach to study how such intercellular interactions might lead to the control of quiescence, self-renewal, and fate decision of limbal epithelial SCs. For the time being, our research has discovered that clonal expansion is governed by the activation of canonical Wnt signaling promoted in 3D Matrigel,107 while quiescence is controlled by the activation of canonical BMP signaling promoted in HC-HA/PTX3.108 Because the HC-HA/PTX3 also exerts desirable anti-inflammatory and antiscarring actions, its capability of maintaining quiescence of limbal epithelial SCs strongly suggests its clinical usefulness in restoring the limbal niche as another new strategy of restoring limbal SC population in the treatment of LSCD. Further studies are also necessary to characterize how extracellular HC-HA/PTX3 might exert these actions regarding the receptor binding and the elicited signaling pathways. These studies may also unravel a new engineering strategy of a surgical graft containing limbal epithelial SCs for correcting LSCD but also directly modulating in vivo limbal niche under the threat of nonresolving inflammation. Consequently, we envision that the HC-HA/PTX3 complex as a novel matrix can be formulated as a platform technology to launch much other therapeutics to aid regenerative medicine in the future.
Acknowledgments
Supported by Research Grants EY06819, EY017497, EY021045, and EY022502 from National Eye Institute, National Institutes of Health, Bethesda, Maryland, United States, a research grant from TissueTech, Inc., and an unrestricted grant from Ocular Surface Research & Education Foundation, Miami, Florida, United States.
Disclosure: S.C.G. Tseng, Tissue Tech, Inc. (I, E), P
References
- 1. Schermer A,, Galvin S,, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986; 103: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lavker RM,, Tseng SC,, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004; 78: 433–446. [DOI] [PubMed] [Google Scholar]
- 3. Chen JJ,, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991; 32: 2219–2233. [PubMed] [Google Scholar]
- 4. Kruse FE,, Chen JJY,, Tsai RJF,, Tseng SC. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990; 31: 1903–1913. [PubMed] [Google Scholar]
- 5. Huang AJ,, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991; 32: 96–105. [PubMed] [Google Scholar]
- 6. Puangsricharern V,, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995; 102: 1476–1485. [DOI] [PubMed] [Google Scholar]
- 7. Chen JJ,, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990; 31: 1301–1314. [PubMed] [Google Scholar]
- 8. Dua HS,, Forrester JV. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol. 1990; 110: 646–656. [DOI] [PubMed] [Google Scholar]
- 9. Dua HS,, Saini JS,, Azuara-Blanco A,, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000; 48: 83–92. [PubMed] [Google Scholar]
- 10. Holland EJ,, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996; 15: 549–556. [PubMed] [Google Scholar]
- 11. Tseng SC. Regulation and clinical implications of corneal epithelial stem cells. Mol Biol Rep. 1996; 23: 47–58. [DOI] [PubMed] [Google Scholar]
- 12. Grueterich M,, Espana EM,, Romano A,, Touhami A,, Tseng SC. Surgical approaches for limbal stem cell deficiency. Contemporary Ophthalmology. 2002; 1: 1–8. [DOI] [PubMed] [Google Scholar]
- 13. Kenyon KR,, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989; 96: 709–722. [DOI] [PubMed] [Google Scholar]
- 14. Kenyon KR,, Rapoza PA. Limbal allograft transplantation for ocular surface disorders. Ophthalmology. 1995; 102(Suppl);101–102. [DOI] [PubMed]
- 15. Tan DTH,, Ficker LA,, Buckley RJ. Limbal transplantation. Ophthalmology. 1996; 103: 29–36. [DOI] [PubMed] [Google Scholar]
- 16. Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996; 94: 677–743. [PMC free article] [PubMed] [Google Scholar]
- 17. Tsai RJ,, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994; 13: 389–400. [DOI] [PubMed] [Google Scholar]
- 18. Tsubota K,, Toda I,, Saito H,, Shinozaki N,, Shimazaki J. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995; 102: 1486–1496. [DOI] [PubMed] [Google Scholar]
- 19. Theng JT,, Tan DT. Combined penetrating keratoplasty and limbal allograft transplantation for severe corneal burns. Ophthalmic Surg Lasers. 1997; 28: 765–768. [PubMed] [Google Scholar]
- 20. Liu J,, Sheha H,, Fu Y,, Giegengack M,, Tseng SC. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. 2011; 152: 739–747. e731. [DOI] [PubMed] [Google Scholar]
- 21. Nishida K,, Yamato M,, Hayashida Y,, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004; 351: 1187–1196. [DOI] [PubMed] [Google Scholar]
- 22. Kolli S,, Ahmad S,, Mudhar HS,, Meeny A,, Lako M,, Figueiredo FC. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells. 2014; 32: 2135–2146. [DOI] [PubMed] [Google Scholar]
- 23. Burillon C,, Huot L,, Justin V,, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012; 53: 1325–1331. [DOI] [PubMed] [Google Scholar]
- 24. Meallet MA,, Espana EM,, Grueterich M,, Ti SE,, Goto E,, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003; 110: 1585–1592. [DOI] [PubMed] [Google Scholar]
- 25. Tseng SC,, Prabhasawat P,, Barton K,, Gray T,, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998; 116: 431–441. [DOI] [PubMed] [Google Scholar]
- 26. Sangwan VS,, Basu S,, MacNeil S,, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012; 96: 931–934. [DOI] [PubMed] [Google Scholar]
- 27. Amescua G,, Atallah M,, Nikpoor N,, Galor A,, Perez V. Modified Simple Limbal Epithelial Transplantation Using Cryopreserved Amniotic Membrane for Unilateral Limbal Stem Cell Deficiency. Am J Ophthalmol. 2014; 158: 469–475. [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi A,, Sugiyama K,, Li W,, Tseng SC. In vivo laser confocal microscopy findings of cryopreserved and fresh human amniotic membrane. Ophthalmic Surg Lasers Imaging. 2008; 39: 312–318. [DOI] [PubMed] [Google Scholar]
- 29. Fadok VA,, Bratton DL,, Konowal A,, Freed PW,, Westcott JY,, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998; 101: 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koh TJ,, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011; 13: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khanna S,, Biswas S,, Shang Y,, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010; 5: e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haringman JJ,, Gerlag DM,, Zwinderman AH,, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005; 64: 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lumeng CN,, Bodzin JL,, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lutgens E,, Lievens D,, Beckers L,, et al. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010; 207: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woollard KJ,, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010; 7: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krausgruber T,, Blazek K,, Smallie T,, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011; 12: 231–238. [DOI] [PubMed] [Google Scholar]
- 37. Sindrilaru A,, Peters T,, Wieschalka S,, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011; 121: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niessen FB,, Schalkwijk J,, Vos H,, Timens W. Hypertrophic scar formation is associated with an increased number of epidermal Langerhans cells. J Pathol. 2004; 202: 121–129. [DOI] [PubMed] [Google Scholar]
- 39. Castagnoli C,, Trombotto C,, Ondei S,, et al. Characterization of T-cell subsets infiltrating post-burn hypertrophic scar tissues. Burns. 1997; 23: 565–572. [DOI] [PubMed] [Google Scholar]
- 40. Akaishi S,, Ogawa R,, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008; 71: 32–38. [DOI] [PubMed] [Google Scholar]
- 41. Rolfe KJ,, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol. 2012; 2012: 698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Larson BJ,, Longaker MT,, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010; 126: 1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cowin AJ,, Holmes TM,, Brosnan P,, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001; 11: 424–431. [PubMed] [Google Scholar]
- 44. Liechty KW,, Adzick NS,, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000; 12: 671–676. [DOI] [PubMed] [Google Scholar]
- 45. Liechty KW,, Crombleholme TM,, Cass DL,, Martin B,, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998; 77: 80–84. [DOI] [PubMed] [Google Scholar]
- 46. Sullivan KM,, Lorenz HP,, Meuli M,, Lin RY,, Adzick NS. A model of scarless human fetal wound repair is deficient in transforming growth factor beta. J Pediatr Surg. 1995; 30: 198–202. [DOI] [PubMed] [Google Scholar]
- 47. Olutoye OO,, Yager DR,, Cohen IK,, Diegelmann RF. Lower cytokine release by fetal porcine platelets: a possible explanation for reduced inflammation after fetal wounding. J Pediatr Surg. 1996; 31: 91–95. [DOI] [PubMed] [Google Scholar]
- 48. Park WC,, Tseng SC. Modulation of acute inflammation and keratocyte death by suturing, blood and amniotic membrane in PRK. Invest Ophthalmol Vis Sci. 2000; 41: 2906–2914. [PubMed] [Google Scholar]
- 49. Wang MX,, Gray TB,, Parks WC,, et al. Corneal haze and apoptosis is reduced by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cat Refract Surg. 2001; 27: 310–319. [DOI] [PubMed] [Google Scholar]
- 50. Shimmura S,, Shimazaki J,, Ohashi Y,, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001; 20: 408–413. [DOI] [PubMed] [Google Scholar]
- 51. Bauer D,, Wasmuth S,, Hermans P,, et al. On the influence of neutrophils in corneas with necrotizing HSV-1 keratitis following amniotic membrane transplantation. Exp Eye Res. 2007; 85: 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heiligenhaus A,, Bauer D,, Meller D,, Steuhl KP,, Tseng SC. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001; 42: 1969–1974. [PubMed] [Google Scholar]
- 53. Bauer D,, Wasmuth S,, Hennig M,, Baehler H,, Steuhl KP,, Heiligenhaus A. Amniotic membrane transplantation induces apoptosis in T lymphocytes in murine corneas with experimental herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2009; 50: 3188–3198. [DOI] [PubMed] [Google Scholar]
- 54. Bauer D,, Hennig M,, Wasmuth S,, et al. Amniotic membrane induces peroxisome proliferator-activated receptor-gamma positive alternatively activated macrophages. Invest Ophthalmol Vis Sci. 2012; 53: 799–810. [DOI] [PubMed] [Google Scholar]
- 55. Li W,, He H,, Kawakita T,, Espana EM,, Tseng SC. Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Exp Eye Res. 2006; 82: 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. He H,, Li W,, Chen SY,, et al. Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci. 2008; 49: 4468–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhuo L,, Yoneda M,, Zhao M,, et al. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem. 2001; 276: 7693–7696. [DOI] [PubMed] [Google Scholar]
- 58. Salustri A,, Garlanda C,, Hirsch E,, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004; 131: 1577–1586. [DOI] [PubMed] [Google Scholar]
- 59. He H,, Li W,, Tseng DY,, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-a-inhibitor (HC•HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009; 284: 20136–20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang S,, He H,, Day AJ,, Tseng SC. Constitutive expression of inter-alpha-inhibitor (IalphaI) family proteins and tumor necrosis factor-stimulated gene-6 (TSG-6) by human amniotic membrane epithelial and stromal cells supporting formation of the heavy chain-hyaluronan (HC-HA) complex. J Biol Chem. 2012; 287: 12433–12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rugg MS,, Willis AC,, Mukhopadhyay D,, et al. Characterization of complexes formed between TSG-6 and inter-alpha-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J Biol Chem. 2005; 280: 25674–25686. [DOI] [PubMed] [Google Scholar]
- 62. Fulop C,, Szanto S,, Mukhopadhyay D,, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003; 130: 2253–2261. [DOI] [PubMed] [Google Scholar]
- 63. Jessen TE,, Odum L. Role of tumour necrosis factor stimulated gene 6 (TSG-6) in the coupling of inter-alpha-trypsin inhibitor to hyaluronan in human follicular fluid. Reproduction. 2003; 125: 27–31. [DOI] [PubMed] [Google Scholar]
- 64. Sanggaard KW,, Sonne-Schmidt CS,, Jacobsen C,, et al. Evidence for a two-step mechanism involved in the formation of covalent HC x TSG-6 complexes. Biochemistry. 2006; 45: 7661–7668. [DOI] [PubMed] [Google Scholar]
- 65. Enghild JJ,, Thogersen IB,, Pizzo SV,, Salvesen G. Analysis of inter-alpha-trypsin inhibitor and a novel trypsin inhibitor pre-alpha-trypsin inhibitor, from human plasma. Polypeptide chain stoichiometry and assembly by glycan. J Biol Chem. 1989; 264: 15975–15981. [PubMed] [Google Scholar]
- 66. Odum L. Inter-alpha-trypsin inhibitor and pre-alpha-trypsin inhibitor in health and disease. Determination by immunoelectrophoresis and immunoblotting. Biol Chem Hoppe Seyler. 1990; 371: 1153–1158. [PubMed] [Google Scholar]
- 67. Salier JP,, Rouet P,, Raguenez G,, Daveau M. The inter-a-inhibitor family: from structure to regulation. Biochem J. 1996; 315: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mizushima S,, Nii A,, Kato K,, Uemura A. Gene expression of the two heavy chains and one light chain forming the inter-alpha-trypsin-inhibitor in human tissues. Biol Pharm Bull. 1998; 21: 167–169. [DOI] [PubMed] [Google Scholar]
- 69. Blom AM,, Morgelin M,, Oyen M,, Jarvet J,, Fries E. Structural characterization of inter-alpha-inhibitor. Evidence for an extended shape. J Biol Chem. 1999; 274: 298–304. [DOI] [PubMed] [Google Scholar]
- 70. Zhuo L,, Hascall VC,, Kimata K. Inter-alpha-trypsin inhibitor a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004; 279: 38079–38082. [DOI] [PubMed] [Google Scholar]
- 71. Zhang S,, Zhu YT,, Chen SY,, He H,, Tseng SC. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J Biol Chem. 2014; 289: 13531–13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Inforzato A,, Jaillon S,, Moalli F,, et al. The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens. 2011; 77: 271–282. [DOI] [PubMed] [Google Scholar]
- 73. He H,, Zhang S,, Tighe S,, Son J,, Tseng SC. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem. 2013; 288: 25792–25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aderem A,, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999; 17: 593–623. [DOI] [PubMed] [Google Scholar]
- 75. Underhill DM,, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012; 12: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Murray PJ,, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011; 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hadis U,, Wahl B,, Schulz O,, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011; 34: 237–246. [DOI] [PubMed] [Google Scholar]
- 78. Mosser DM,, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gordon S,, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010; 32: 593–604. [DOI] [PubMed] [Google Scholar]
- 80. Sica A,, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012; 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. He H,, Tan Y,, Duffort S,, Perez VL,, Tseng SC. In vivo downregulation of innate and adaptive immune responses in corneal allograft rejection by HC-HA/PTX3 complex purified from amniotic membrane. Invest Ophthalmol Vis Sci. 2014; 55: 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ayliffe W,, Alam Y,, Bell EB,, McLeod D,, Hutchinson IV. Prolongation of rat corneal graft survival by treatment with anti-CD4 monoclonal antibody. Br J Ophthalmol. 1992; 76: 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. He YG,, Ross J,, Niederkorn JY. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Invest Ophthalmol Vis Sci. 1991; 32: 2723–2728. [PubMed] [Google Scholar]
- 84. Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007; 32: 1005–1016. [DOI] [PubMed] [Google Scholar]
- 85. Yamada J,, Kurimoto I,, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci. 1999; 40: 2614–2621. [PubMed] [Google Scholar]
- 86. Paul WE,, Seder RA. Lymphocyte responses and cytokines. Cell. 1994; 76: 241–251. [DOI] [PubMed] [Google Scholar]
- 87. Dong C,, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000; 2: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Scarchilli L,, Camaioni A,, Bottazzi B,, et al. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem. 2007; 282: 30161–30170. [DOI] [PubMed] [Google Scholar]
- 89. Lee SB,, Li DQ,, Tan DT,, Meller DC,, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000; 20: 325–334. [PubMed] [Google Scholar]
- 90. Tseng SC,, Li DQ,, Ma X. Suppression of transforming growth factor-beta isoforms TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999; 179: 325–335. [DOI] [PubMed] [Google Scholar]
- 91. Choi TH,, Tseng SC. In vivo and in vitro demonstration of epithelial cell-induced myofibroblast differentiation of keratocytes and an inhibitory effect by amniotic membrane. Cornea. 2001; 20: 197–204. [DOI] [PubMed] [Google Scholar]
- 92. Choi YS,, Kim JY,, Wee WR,, Lee JH. Effect of the application of human amniotic membrane on rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998; 17: 389–395. [DOI] [PubMed] [Google Scholar]
- 93. Woo HM,, Kim MS,, Kweon OK,, Kim DY,, Nam TC,, Kim JH. Effects of amniotic membrane on epithelial wound healing and stromal remodelling after excimer laser keratectomy in rabbit cornea. Br J Ophthalmol. 2001; 85: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li W,, He H,, Chen YT,, Hayashida Y,, Tseng SC. Reversal of myofibroblasts by amniotic membrane stromal extract. J Cell Physiol. 2008; 215: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Espana EM,, He H,, Kawakita T,, et al. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Science. 2003; 44: 5136–5141. [DOI] [PubMed] [Google Scholar]
- 96. Kawakita T,, Espana EM,, He H,, et al. Keratocan expression of murine keratocytes is maintained on amniotic membrane by downregulating TGF-beta signaling. J Biol Chem. 2005; 280: 27085–27092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim JC,, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 1995; 9: 32–46. [DOI] [PubMed] [Google Scholar]
- 98. Tsubota K,, Satake Y,, Kaido M,, et al. Treatment of severe ocular surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999; 340: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 99. Tsai RJF,, Li LM,, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000; 343: 86–93. [DOI] [PubMed] [Google Scholar]
- 100. Jiang A,, Li C,, Gao Y,, et al. In vivo and in vitro inhibitory effect of amniotic extraction on neovascularization. Cornea. 2006; 25: S36–S40. [DOI] [PubMed] [Google Scholar]
- 101. Shay E,, Khadem JJ,, Tseng SC. Efficacy and limitation of sutureless amniotic membrane transplantation for acute toxic epidermal necrolysis. Cornea. 2010; 29: 359–361. [DOI] [PubMed] [Google Scholar]
- 102. Chen SY,, Hayashida Y,, Chen MY,, Xie HT,, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011; 17: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xie HT,, Chen SY,, Li GG,, Tseng SC. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012; 53: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li GG,, Chen SY,, Xie HT,, Zhu YT,, Tseng SC. Angiogenesis potential of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012; 53: 3357–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li GG,, Zhu YT,, Xie HT,, Chen SY,, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Science. 2012; 53: 5686–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xie HT,, Chen SY,, Li GG,, Tseng SC. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011; 29: 1874–1885. [DOI] [PubMed] [Google Scholar]
- 107. Han B,, Chen SY,, Zhu YT,, Tseng SC. Integration of BMP/Wnt signaling to control clonal growth of limbal epithelial progenitor cells by niche cells. Stem Cell Res. 2014; 12: 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen SY,, Han B,, Zhu YT,, et al. HC-HA/PTX3 purified from amniotic membrane promotes bmp signaling in limbal niche cells to maintain quiescence of limbal epithelial progenitor/stem cells [published online ahead of print July 7, 2015]. Stem Cells. doi: http://dx.doi.org/10.1002/stem.2091. [DOI] [PubMed]
- 109. Tseng SC,, Chen SY,, Shen YC,, Chen WL,, Hu FR. Critical appraisal of ex vivo expansion of human limbal epithelial stem cells. Curr Mol Med. 2010; 10: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]