Abstract
Here we reported the first case that enhancing self-assembling ability boosts anti-cancer efficacy through simple and minimal modification of C-terminal of a D-tripeptide. By only 2% change of the molecular weight, this facile approach increases the inhibitory activity over an order of magnitude (IC50 from 431 to 23 μM) towards an osteosarcoma cell line.
The ideal cancer therapeutics should selectively kill cancer cells without harming normal cells. Current chemotherapy for cancer treatment, however, still falls short of that goal. For example, cisplatin (CDDP), a well-established clinical anticancer drug, causes myelosuppression.1 Another extensively used anticancer drug, paclitaxel, exhibits toxic effect on the bone marrow.2 The recent success in immunotherapy relies on the actions of antibodies,3 has yet to be extended to the tumors that express low level cancer specific antigens.4 These limitations call for paradigm-shifting approaches for the development innovative chemotherapeutics of cancer treatment. To meet this need, we and others are developing anticancer nanomedicine based on the enzyme-instructed supramolecular assemblies of small molecules,5, 6–9 which represents a new approach that departs from the current dogma of tight and specific ligand-receptor interactions. For example, the assemblies of small molecules even can inhibit cancer cells selectively.10, 11 These encouraging results have led to the development of enzyme-instructed self-assembly (EISA),12 which selectively generates assemblies of small molecules (e.g., small peptide derivatives6, 9 or carbohydrate derivatives8) in-situ on cancer cells for killing the cancer cells. Despite these advancements, the concentrations of those molecules required for inhibiting cancer cells still are quite high (e.g., 300–500 μM).6–8 Thus, there is a need of an effective strategy to increase the efficacy of self-assembling molecules for cancer inhibition.
Because it is the in-situ formation of supramolecular assemblies to kill cancer cells,11, 12 a logical approach for increasing the inhibitory efficacy is to enhance the self-assembling ability of the building blocks. Although elongating the peptides improves the self-assembling ability of peptides,12 it adds up the molecular weight, which thwarts the purpose of reducing the therapeutic dosage. Thus, we choose to use a neutral small functional group replacing the carboxylic acid group at the C-terminal of the peptides for enhancing the self-assembling ability of the peptides (Fig. 1). Despite the simplicity of this approach, it receives little attention for designing self-assembling molecules.13 The results of this work reveal that such a simple and minimal modification of C-terminal of a D-tripeptide significantly enhance the self-assembling ability of the peptide, which increases their efficacy of cancer cell inhibition over an order of magnitude, even results in a IC50 to be comparable to that of cisplatin and more potent than carboplatin towards an osteosarcoma cell line.14, 15 Using different C-terminal groups, we further confirm the correlation between self-assembling ability and the inhibitory activity of the D-tripeptides. Moreover, this modification preserves the exceptional selectivity of enzyme-instructed self-assembly for inhibiting cancer cells. In addition, we confirm that the supramolecular assemblies on the cancer cells induce cell necroptosis, a recently defined modality of cell death.16 This work illustrates a facile strategy to explore self-assembling molecules for selectively killing cancer cells via enzymatic reactions.
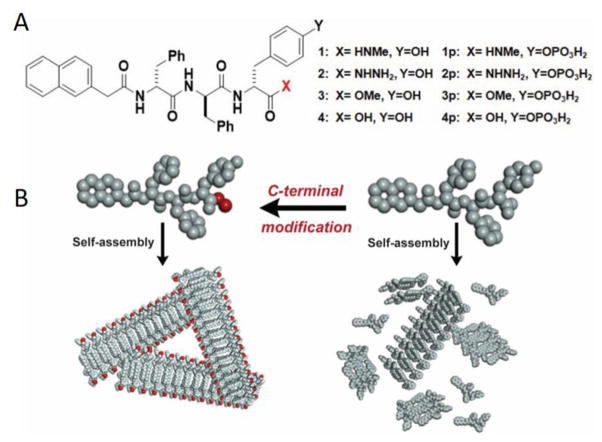
Fig. 1.
A) Molecular structures of self-assembling short peptides 1, 2, 3 and 4 and the corresponding precursors 1p, 2p, 3p and 4p that are enzyme substrates; B) Illustration of C-terminal modification enhancing self-assembly.
To prove the concept that enhancing self-assembling ability boosts inhibitory efficacy, we choose a naphthyl-capped D-tripeptide derivative (4)6 as the parent compound. As shown in Fig. 1, we select N-methylacetamine (–CONHMe), acetohydrazide (-CONHNH2), and methylacetate (–COOMe) groups to replace the carboxylic acid terminal of 4 to result in D-tripeptide derivatives 1, 2, and 3, respectively. Based on that phosphatase-instructed self-assembly of 4 selectively kills cancer cells,6–8, 17 we choose to phosphorylate the D-Tyr in 1, 2, 3 and 4 to generate the corresponding precursors, 1p, 2p, 3p and 4p. Being different in structural and chemical properties, these C-terminal groups provide a set of representative molecules to validate the molecular design in Fig. 1. From 4p to 1p, 2p, and 3p, the molecular weights increase less than 2%. According to those structures, we combine solid phase peptide synthesis (SPPS) and solution phase synthesis to produce 1p, 2p, 3p, and 4p in fair yields (Scheme S1).
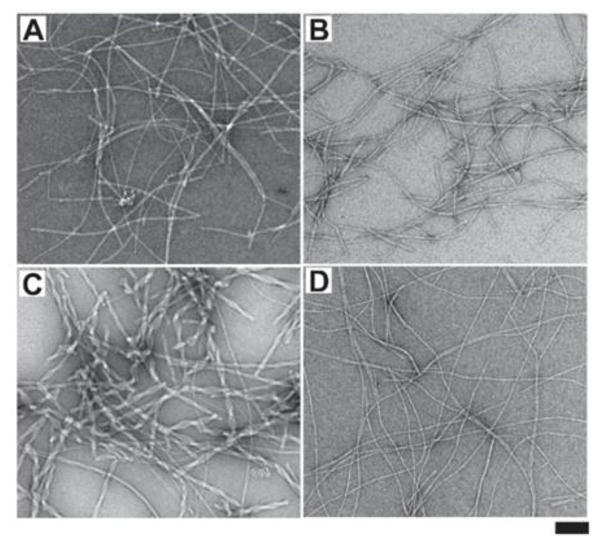
After obtaining the precursors, we examine the self-assembly ability of those four D-peptide derivatives in water via enzymatic dephosphorylation of their corresponding precursors. Using hydrogelation as a simple assay to test the molecular self-assembly in water,12 we find that, at the concentration of 1 mM and pH 7.4, all the four precursors form transparent solutions. After the addition of alkaline phosphatase (ALP, 3U/mL), the solution of 1p or 2p turns into a hydrogel, the solution of 3p forms a suspension, and the solution of 4p becomes more viscous (Fig. S5). This result suggests that 1, 2, and 3 possess higher self-assembling ability than 4 does. Transmission electron microscopy (TEM) reveals that, after their enzymatic formation, 1, 2, 3 and 4 self-assembles to result in nanofibers with the diameter of 10.6±2, 9.7±2, 17.4±2 and 6.2±2 nm, respectively (Fig. 2). Compared with 1, 2, and 3, 4 yields much thinner but uniform nanofibers, further indicating that 4 has relatively weaker self-assembly ability. In addition, 3 self-assembles to form bundles of short nanofibrils, which is consistent with the suspension after adding ALP in the solution of 3p. These results confirm the higher self-assembling abilities of 1, 2, and 3 than that of 4.
Fig. 2.
TEM images of the nanostructures formed by adding ALP (3 U/mL) into the aqueous solution (1 mM, pH7.4) of (A) 1p, (B) 2p, (C) 3p, and (D) 4p after 24 hrs, respectively (scar bar 100 nm).
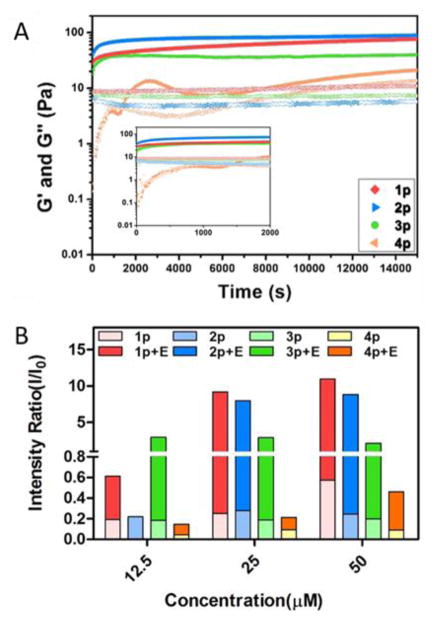
To further examine the enhancement of self-assembling ability, we use rheometer to compare the viscoelastic properties of hydrogels formed by 1, 2, 3 and 4 upon adding ALP in the solutions of their corresponding precursors (0.5 wt%). As shown in Fig. 3A, the addition of ALP into the solution of 4p causes the storage modulus (G′) to intersect with loss modulus (G″) at around 0.4 hours, indicating the gelation point of 4. However, immediately after mixing ALP with the solution of 1p, 2p or 3p, G′ is greater than G″ (i.e., G′ > G″ at t=0), indicating that 1, 2, or 3 already reaches its gelation point immediately after mixing. These rheological results confirm that minimal C-terminal modification of 4 boosts the self-assembling ability of the resulting short peptides. To further compare the self-assembling abilities of 1, 2, 3, and 4 below their gelation concentrations, we use static light scattering (SLS) to assess the self-assembly of those D-tripeptide derivatives before and after the enzymatic dephosphorylation. As shown in Fig. 3B, the addition of ALP into the solution of each precursor results in significant increase of SLS signals, indicating enzyme-instructed self-assembly. The increases of the SLS signals depends on the concentrations of the precursors. At 12.5 μM, SLS suggests the self-assembling abilities follow the order of 3 > 1 > 4. At 25 μM, the SLS signals of 3p and 3 change little, suggesting that 3 starts to precipitate, agreeing with the observation of suspension after adding ALP to the solution of 3p (vide supra); SLS signals of 2 increases more than that of 4 does, but still less than that of 1. This result indicates that the self-assembling abilities follow the order of 3 > 1 > 2 > 4. At 50 μM, the SLS signal of 1p starts increasing, indicating 1p starts to aggregates. Besides confirming that C-terminal modification increases the self-assembling ability of 4, these results reveal the characteristics of each C-terminal motif in the process of enzyme-instructed self-assembly, which is consistent with the trend of IC90 values (Fig. 4B).
Fig. 3.
A) Storage modulus (G′-solid symbol) and loss modulus (G″-open symbol) as a function of time for 0.5 wt % of 1p, 2p, 3p and 4p catalyzed by ALP (0.05 U/mL) at pH7.4. Inset: enlarged time sweep between 0 to 2000 seconds; B) Intensity of static light scattering (SLS) of the solutions of 1p, 2p, 3p and 4p (12.5–50 μM) before and after adding ALP (2 U/mL) for 24 hours at different concentrations in pH7.4 PBS buffer (light scattering angle = 30 degree).
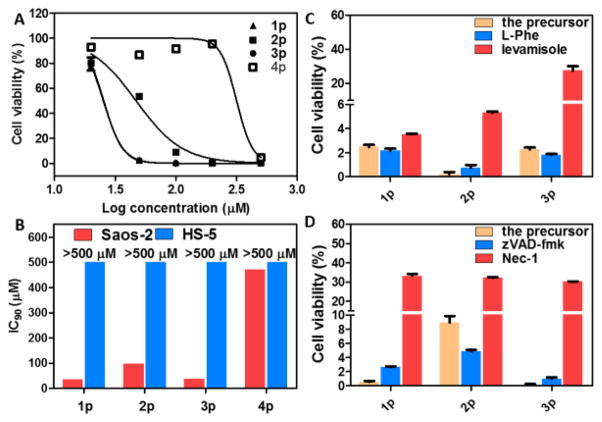
Fig. 4.
A) Cell viability (log-dose response curves) for sarcoma osteogenic (Saos-2) cells treated with 1p, 2p, 3p or 4p. B) IC90 values (48 h) of 1p, 2p, 3p, and 4p against Saos-2 and HS-5 cells. C) The cell viability of Saos-2 cells treated with 1p (50 μM), 2p (200 μM), or 3p (50 μM) in the presences of different ALP inhibitors (L-Phe and levamisole). Cells were treated for 48h; [L-Phe] = 1 mM; [levamisole] = 1 mM. D) The cell viability of Saos-2 cells treated with 1p, 2p, or 3p (100 μM) in the presence of a pan-caspase inhibitor (zVAD-fmk) or a necroptosis inhibitor (Nec-1). Cells were treated for 48h; [zVAD-fmk] = 45 μM; [Nec-1] = 50 μM. All cell viability test are done in triplicate (n = 3).
Using sarcoma osteogenic (Saos-2) cells that overexpress alkaline phosphatase as a model for human cancer cells and human marrow stromal cells (HS-5) as a model for normal human cells, we test the anticancer efficacy and selectivity of 1p, 2p, 3p and 4p. While 4p only inhibits Saos-2 cells at a high concentration (i.e., about 500 μM), 1p, 2p or 3p inhibits Saos-2 at the concentration of 50 μM and above (Fig. 4A and Fig. S6). The IC90 values of 1p, 2p, 3p and 4p against Saos-2 cells are 36 μM, 97 μM, 37 μM, and 470 μM respectively (Fig. 4B), demonstrating that the C-terminal modification lower the IC90 against Saos-2 cells over an order of magnitude (from 470 μM of 4p to 36 μM of 1p). Since HS-5 hardly overexpress ALP, 1p, 2p, 3p and 4p all show limited cytotoxicity (i.e., IC50 > 260μM (Fig. S7) and IC90 > 500 μM) on HS-5 cells. Moreover, the IC50 of 1p (32 μM, 72 h) against Saos-2 is only about one-sixth of the IC50 of carboplatin (198 μM, 72 h),15 confirming that 1p can exhibit a higher anticancer efficacy than that of a clinical used drug. Moreover, our study reveals that, at pH 8.0 and because of the hydrolysis of ester bond, the inhibitory activity of 3p decreases, but the activity of 1p remains (Fig. S8). These results agree with the self-assembling abilities of the D-tripeptides (Fig. 3B), thus validating C-terminal modification as an effective approach for developing enzyme-instructed self-assembly of short peptides for selectively inhibiting cancer cells.
To establish the essential role of enzymatic self-assembly of the D-tripeptide for their inhibitory activities, we co-incubate ALP inhibitors with the precursors during cell viability assay. We use two kinds of ALP inhibitors: levamisole for selectively inhibiting the activity of tissue-nonspecific alkaline phosphatase (ALPL),18 and L-phenylalanine (L-Phe) selectively for placental alkaline phosphatase (ALPP).19 As shown in Fig. 4C and Fig. S9, levamisole increases the cell viability of the Saos-2 cells treated by 1p (50 μM), 3p (50 μM), or 2p (200 μM), while L-Phe is unable to rescue the cells. These results agree with previous reports that levamisole largely inhibit the enzyme activity of phosphatase in Saos-2 cells,20 confirming the importance of enzyme reaction in forming localized supramolecular assemblies of the D-tripeptide for cancer inhibition. In addition, with the help of Congo red—a dye for beta-sheet like, self-assembled nanofibers, we are able to visualize the nanofibers mainly formed in pericellular space of Saos-2 cells after treating the cells with the precursors 1p, 2p or 3p (Fig. S11). This imaging results support localized enzyme-instructed self-assembly. Using a pan-caspase inhibitor (zVAD-fmk)21 and a necroptosis inhibitor (Nec-1)22, we further evaluate the modality of the death of Saos-2 cells resulted from the self-assemblies of the D-tripeptides (Fig. 4D and Fig. S10). Notably, Nec-1 significantly protects Saos-2 cells from the death caused by 1p, 2p and 3p, suggesting that the cell death largely involve necroptosis. On the other hand, zVAD-fmk only slightly protects cells from 1p (20 μM), 2p (50 μM), and 3p (20 μM), indicating that the death of Saos-2 cells less relies on apoptosis when the precursor concentrations are relatively low.
In conclusion, this work illustrates C-terminal modification of short peptides as an effective approach to modulate the self-assembly of the peptides, thus providing a new dimension for exploring enzyme-instructed self-assembly for controlling the fate of cell. Moreover, the approach illustrated here should be useful for increasing the self-assembling ability of other self-assembling peptides and peptide conjugates.23 In addition, this work illustrates that a simple control of molecular structures can have profound impact on multiple length scales, from nanometers to microns, which would help explore emergent properties of supramolecular assemblies in cellular environment, a new frontier of supramolecular chemistry. Because cell death caused by 2p/2 differs from that of 1p/1 or p/3, different C-terminal modifications likely result in subtle differences in supramolecular assemblies. This observation warrants further investigation since it may provide new insights on the use of supramolecular assemblies for inhibiting cancer cells.
Supplementary Material
Acknowledgments
This work was partially supported by NIH (CA142746) and W. M. Keck Foundation. ZF thanks the Dean’s fellowship from Brandies University.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Vonhoff DD, Schilsky R, Reichert CM, Reddick RL, Rozencweig M, Young RC, Muggia FM. Cancer Treat Rep. 1979;63:1527–1531. [PubMed] [Google Scholar]
- 2.Kohn EC, Sarosy G, Bicher A, Link C, Christian M, Steinberg SM, Rothenberg M, Adamo DO, Davis P, Ognibene FP, Cunnion RE, Reed E. J Natl Cancer Inst. 1994;86:18–24. doi: 10.1093/jnci/86.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelderman S, Schumacher TNM, Haanen JBAG. Mol Oncol. 2014;8:1132–1139. doi: 10.1016/j.molonc.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Yang C, Wang L, Kong D, Zhang Y, Yang Z. Chem Commun. 2011;47:4439–4441. doi: 10.1039/c1cc10506j. [DOI] [PubMed] [Google Scholar]; Gao Y, Kuang Y, Guo ZF, Guo ZH, Krauss IJ, Xu B. J Am Chem Soc. 2009;131:13576–13577. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]; Yang ZM, Xu KM, Guo ZF, Guo ZH, Xu B. Adv Mater. 2007;17:3152–3156. [Google Scholar]; Zhou J, Du X, Yamagata N, Xu B. J Am Chem Soc. 2016 doi: 10.1021/jacs.5b13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuang Y, Shi J, Li J, Yuan D, Alberti KA, Xu Q, Xu B. Angew Chem Int Ed. 2014;53:8104–8107. doi: 10.1002/anie.201402216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, Du X, Yuan D, Zhou J, Zhou N, Huang Y, Xu B. Biomacromolecules. 2014;15:3559–3568. doi: 10.1021/bm5010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pires RA, Abul-Haija YM, Costa DS, Novoa-Carballal R, Reis RL, Ulijn RV, Pashkuleva I. J Am Chem Soc. 2015;137:576–579. doi: 10.1021/ja5111893. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka A, Fukuoka Y, Morimoto Y, Honjo T, Koda D, Goto M, Maruyama T. J Am Chem Soc. 2015;137:770–775. doi: 10.1021/ja510156v. [DOI] [PubMed] [Google Scholar]
- 10.Kuang Y, Xu B. Angew Chem Int Ed Engl. 2013;52:6944. doi: 10.1002/anie.201302658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuang Y, Long MJ, Zhou J, Shi J, Gao Y, Xu C, Hedstrom L, Xu B. J Biol Chem. 2014;289:29208–29218. doi: 10.1074/jbc.M114.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du X, Zhou J, Shi J, Xu B. Chem Rev. 2015;115:13165–13307. doi: 10.1021/acs.chemrev.5b00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Wang H, Wang L, Wang J, Kong D, Yang Z. J Am Chem Soc. 2009;131:11286–11287. doi: 10.1021/ja9042142. [DOI] [PubMed] [Google Scholar]; Zhao F, Gao Y, Shi J, Browdy HM, Xu B. Langmuir. 2011;27:1510–1512. doi: 10.1021/la103982e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tardito S, Isella C, Medico E, Marchio L, Bevilacqua E, Hatzoglou M, Bussolati O, Franchi-Gazzola R. J Biol Chem. 2009;284:24306–24319. doi: 10.1074/jbc.M109.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marley K, Helfand SC, Edris WA, Mata JE, Gitelman AI, Medlock J, Seguin B. BMC Vet Res. 2013:9. doi: 10.1186/1746-6148-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linkermann A, Green DR. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Nat Rev Mol Cell Biol. 2014;15:134–146. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 17.Du X, Zhou J, Wu L, Sun S, Xu B. Bioconjugate Chem. 2014;25:2129–2133. doi: 10.1021/bc500516g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozlenkov A, Le Du MH, Cuniasse P, Ny T, Hoylaerts MF, Millan JL. J Bone Miner Res. 2004;19:1862–1872. doi: 10.1359/JBMR.040608. [DOI] [PubMed] [Google Scholar]
- 19.Hoylaerts MF, Manes T, Millan JL. Biochem J. 1992;286:23–30. doi: 10.1042/bj2860023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray E, Provvedini D, Curran D, Catherwood B, Sussman H, Manolagas S. J Bone Miner Res. 1987;2:231–238. doi: 10.1002/jbmr.5650020310. [DOI] [PubMed] [Google Scholar]
- 21.Slee EA, Zhu HJ, Chow SC, MacFarlane M, Nicholson DW, Cohen GM. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kammuller ME, Seinen W. Int J Immunopharmacol. 1988;10:997–1010. doi: 10.1016/0192-0561(88)90047-1. [DOI] [PubMed] [Google Scholar]; Degterev A, Huang ZH, Boyce M, Li YQ, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan JY. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 23.Jiang T, Vail OA, Jiang Z, Zuo X, Conticello VP. J Am Chem Soc. 2015;137:7793–7802. doi: 10.1021/jacs.5b03326. [DOI] [PubMed] [Google Scholar]; Liang C, Ni R, Smith JE, Childers WS, Mehta AK, Lynn DG. J Am Chem Soc. 2014;136:15146–15149. doi: 10.1021/ja508621b. [DOI] [PubMed] [Google Scholar]; Sathaye S, Zhang H, Sonmez C, Schneider JP, MacDermaid CM, Von Bargen CD, Saven JG, Pochan DJ. Biomacromolecules. 2014;15:3891–3900. doi: 10.1021/bm500874t. [DOI] [PMC free article] [PubMed] [Google Scholar]; Smith DJ, Brat GA, Medina SH, Tong D, Huang Y, Grahammer J, Furtmueller GJ, Oh BC, Nagy-Smith KJ, Walczak P, Brandacher G, Schneider JP. Nat Nanotechnol. 2016;11:95–102. doi: 10.1038/nnano.2015.238. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sun Z, Li Z, He Y, Shen R, Deng L, Yang M, Liang Y, Zhang Y. J Am Chem Soc. 2013;135:13379–13386. doi: 10.1021/ja403345p. [DOI] [PubMed] [Google Scholar]; Cui H, Cheetham AG, Pashuck ET, Stupp SI. J Am Chem Soc. 2014;136:12461–12468. doi: 10.1021/ja507051w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Foster JS, Zurek JM, Almeida NMS, Hendriksen WE, le Sage VAA, Lakshminarayanan V, Thompson AL, Banerjee R, Eelkema R, Mulvana H, Paterson MJ, van Esch JH, Lloyd GO. J Am Chem Soc. 2015;137:14236–14239. doi: 10.1021/jacs.5b06988. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang X, Chu X, Wang L, Wang H, Liang G, Zhang J, Long J, Yang Z. Angew Chem, Int Ed. 2012;51:4388–4392. doi: 10.1002/anie.201108612. [DOI] [PubMed] [Google Scholar]; He M, Li J, Tan S, Wang R, Zhang Y. J Am Chem Soc. 2013;135:18718–18721. doi: 10.1021/ja409000b. [DOI] [PubMed] [Google Scholar]; Yu Z, Tantakitti F, Yu T, Palmer LC, Schatz GC, Stupp SI. Science. 2016;351:497–502. doi: 10.1126/science.aad4091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.